Abstract
We have shown that physiological levels of Ca2+-calmodulin (Ca2+CaM; 50–100 nM) activate cardiac ryanodine receptors (RyR2) incorporated into bilayers and increase the frequency of Ca2+ sparks and waves in cardiac cells. In contrast, it is well known that Ca2+CaM inhibits [3H]ryanodine binding to cardiac sarcoplasmic reticulum. Since the [3H]ryanodine binding technique does not reflect the effects of Ca2+CaM on RyR2 open probability (Po), we have investigated, using the reversible ryanoid, ryanodol, whether Ca2+CaM can directly influence the binding of ryanoids to single RyR2 channels independently of Po. We demonstrate that Ca2+CaM reduces the rate of ryanodol association to RyR2 without affecting the rate of dissociation. We also find that ryanodol-bound channels fluctuate between at least two distinct subconductance states, M1 and M2, in a voltage-dependent manner. Ca2+CaM significantly alters the equilibrium between these two states. The results suggest that Ca2+CaM binding to RyR2 causes a conformation change to regions of the channel that include the ryanoid binding site, thereby leading to a decrease in ryanoid association rate and modulation of gating within the ryanoid/RyR2 bound state. Our data provide a possible explanation for why the effects of Ca2+CaM at the single-channel level are not mirrored by [3H]ryanodine binding studies.
Introduction
Ryanodine binds with high affinity to intracellular Ca2+-release channels known as ryanodine receptors (RyR) (1,2). The binding of ryanodine causes RyR channels to open with high probability to a characteristic reduced-conductance open state (3). Ryanodine dissociates so slowly that once a channel enters the ryanodine-induced subconductance state, this effect is irreversible within the timescale of a single-channel experiment (3,4). It is thought that ryanodine can only gain access to its binding site when the channel opens (5–7). This property of ryanodine binding, together with the specific, high-affinity and essentially irreversible nature of binding to RyR has led to the use of [3H]ryanodine binding as a tool to predict whether an agent will increase or decrease the activity of RyR channels. In general, agents that increase the open probability (Po) of RyR tend to stimulate [3H]ryanodine binding to membrane fractions containing RyR channels, whereas ligands that reduce RyR Po tend to inhibit [3H]ryanodine binding. On this basis, it is usually assumed that a change in the level of [3H]ryanodine binding to a population of membrane vesicles reflects only on the Po of the population of channels. In the course of our study into the interactions between calmodulin (CaM) and cardiac RyRs (RyR2), we now show that this assumption may not always be valid.
CaM is a Ca2+-binding protein that binds tightly to many proteins, including RyR. Both the Ca2+-bound form (Ca2+CaM) and the Ca2+-free form (apoCaM) bind to cardiac and skeletal (RyR1) isoforms of RyR (8–11). Studies published by many groups clearly demonstrate that Ca2+CaM causes dose-dependent, partial inhibition of [3H]ryanodine binding to isolated cardiac and skeletal sarcoplasmic reticulum (SR) vesicles (8,12–15), suggesting that Ca2+CaM inhibits the opening of RyR channels. However, our recent studies demonstrate that physiological levels of Ca2+CaM actually activate single RyR2 channels incorporated into bilayers. The free CaM concentration in a cardiac cell is estimated at 50–75 nM (16), and we have shown that such low levels of Ca2+CaM markedly increase RyR2 Po (17). It is important to note that this is not an artifact of the reconstitution system, since experiments performed in cardiac myocytes demonstrate that 50 nM CaM also activates RyR2 in situ, increasing the frequency of Ca2+ sparks and Ca2+ waves in cardiac myocytes in a CaM kinase II-independent manner (17).
A possible explanation for why [3H]ryanodine binding studies do not mirror changes in RyR2 single-channel behavior is that Ca2+CaM, in addition to modulating Po, may also affect the actual binding of ryanoid compounds to their sites on RyR2. To examine the kinetics of ryanodine binding at the single-channel level is impractical because of the slow dissociation rate of ryanodine; only a single ryanodine-induced modification event per experiment can be observed, and demodification cannot be monitored because of the short-lived nature of the bilayer experiment. An alternative is to use a reversible ryanoid. The behavior of reversible ryanoids at the single-channel level has provided detailed information about the interactions of this group of compounds with RyR2 and has been used to predict the effects of ryanodine (18–20). We have therefore used the reversible ryanodine analog, ryanodol, to investigate whether Ca2+CaM alters ryanoid interactions with RyR2. Our results demonstrate that Ca2+CaM slows the rate of association of ryanodol with RyR2 without affecting the rate of dissociation. Ca2+CaM also alters RyR2 gating within the ryanodol-bound state. Ca2+CaM-mediated changes in ryanoid binding may explain why [3H]ryanodine binding is inhibited by Ca2+CaM and therefore why the [3H]ryanodine binding studies do not predict how Ca2+CaM will modulate RyR2 single-channel function or Ca2+ release in cardiac cells.
Materials and Methods
Preparation of heavy SR membrane vesicles and purified RyR2 channels
Heavy SR membrane vesicles (containing native RyR2 channels) were prepared from sheep cardiac muscle as previously described (21). SR vesicles were frozen rapidly in liquid nitrogen and stored at −80°C. SR vesicles were solubilized with 3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate (CHAPS) and reconstituted into unilamellar liposomes as previously described (21).
[3H]ryanodine binding
SR protein (50–100 μg/ml) was incubated at 37°C in 250 mM KCl, 20 mM HEPES, and 100 μM free Ca2+, pH 7.2, with 5 nM [3H]ryanodine for 90 min. After incubation with CaM, samples were diluted with 5 ml of ice-cold buffer and filtered through Whatman (Maidstone, UK) GF-B filters. Filters were further washed with buffer and counted in 10 ml aqueous counting scintillant the next day. Nonspecific binding was determined from incubations to which 5 μM unlabeled ryanodine had been added. All incubations were performed in triplicate.
Bilayer methods
Vesicles of heavy SR or liposomes containing purified RyR2 were fused with planar phosphatidylethanolamine (PE) lipid bilayers as previously described (21). Vesicles were fused in a fixed orientation such that the cis chamber corresponded to the cytosolic space and the trans chamber to the SR lumen. The trans chamber was held at ground and the cis chamber at potentials relative to ground. After fusion of native RyR2 (in heavy SR vesicles), the cis chamber was perfused with 250 mM HEPES, 125 mM Tris, and 10 μM free Ca2+, pH 7.2. The trans chamber was perfused with 250 mM glutamic acid, 10 mM HEPES, pH 7.2, with Ca(OH)2 (free [Ca2+] ∼50 mM). After fusion of purified RyR2 channels with the bilayer, cis and trans chambers were perfused with 250 mM KCl, 20 mM HEPES, and 100 μM free Ca2+, pH 7.2.
The free [Ca2+] and pH of all solutions were measured at 22°C using a Ca2+ electrode (93-20, Orion, Boulder, CO) and Ross-type pH electrode (81-55, Orion), as described previously (21).
Data acquisition and analysis
Single-channel recordings were made on digital audiotape. Native RyR2 recordings were filtered at 1 kHz (−3 dB) and digitized at 20 kHz using Pulse (HEKA Elektronik, Lambrecht/Pfalz, Germany). Purified RyR2 recordings were filtered at 3 kHz and digitized at 20 kHz unless otherwise stated. Under these conditions, events lasting <0.2 ms were not fully resolved. Po was determined over 3 min of continuous recording, unless otherwise stated, using the method of 50% threshold analysis (22). For display of channel traces, native RyR2 recordings were filtered at 600 Hz and purified RyR2 at 2 kHz unless otherwise stated.
After addition of ryanodol, channel Po was taken as the Po in the unmodified state only and was monitored over at least 6 min of recording. The rate of association of ryanoids with RyR channels is known to depend on Po, and Po was therefore clamped to a high level (0.84–0.95) by 20 mM caffeine. Membranes were either clamped at +20 mV or, more typically, switched between +60 mV and −60 mV to encourage ryanodol-induced modifications and demodifications, as described in Results (see Fig. 4). In all experiments, bilayers containing only a single actively gating channel were used. Ryanodol association with RyR2 channels caused modification to channel gating such that current fluctuations between two modified subconductance states (M1 and M2) were observed. The probability of dwelling in each of these states was calculated by 50% threshold analysis (22). Measurements of current amplitude were made using the WinEDR program (developed by John Dempster, University of Strathclyde, Glasgow, Scotland) by manually assessing the closed and open current levels using cursors.
Figure 4.
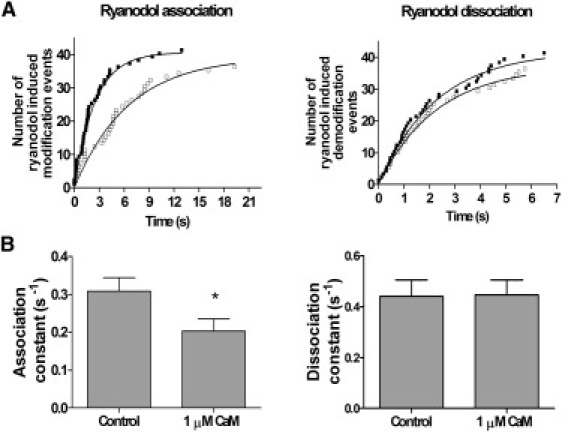
CaM modulation of rates of ryanodol association to and dissociation from RyR2. (A) Data from a single representative channel. Cumulative plots of the measured times to ryanodol-induced modification (left) and demodification (right) are shown. Data in the absence (solid squares) and presence (open squares) of 1 μM cytosolic CaM are shown. Measured times to ryanodol-induced modification were fitted according to the equation y = total number of modification events (1 − exp(K)t), where t = time. The relationship starts at zero and ascends to the total number of modification events with a rate constant K, equivalent to the rate of association of ryanodol to RyR2. The control rate of ryanodol association is 0.401, with half-time 1.729 s. In the presence of 1 μM Ca2+CaM, the rate of ryanodol association is 0.152, with a half-time of 4.566 s. Measured times to demodification back to normal channel gating were fitted according to the equation y = total number of demodification events (1 − exp(K)t). The relationship starts at zero and ascends to the total number of demodification events with a rate constant K, equivalent to the rate of dissociation of ryanodol from RyR2. The control rate of ryanodol dissociation is 0.447, with half-time 1.59 s. In the presence of 1 μM Ca2+CaM, the rate of ryanodol dissociation is 0.437, with a half-time of 1.59 s. (B) Mean data illustrating the rates of ryanodol association to (left) and dissociation from (right) RyR2. Mean ± SE for n = 8; ∗P < 0.05.
Synthesis of ryanodol
Ryanodol was obtained from hydrolysis of ryanodine as previously described (23). Briefly, ryanodine (2 mg) was dissolved in an aqueous solution containing 0.5 M KOH (2 ml) and incubated for 2 h at 98°C. To ensure full hydrolysis, the mixture was allowed to reach room temperature overnight. Thin-layer chromatography on silica gel plates (Rfryanodine = 0.69, Rfryanodol = 0.57 in 8.5:1.5 CHCl3/MeOH) and mass spectrometry indicated that no ryanodine was left in the mixture. Subsequently, the solvent was evaporated and the remaining powder was rediluted in water to a final [ryanodol] of 10 mM. This solution was titrated with PIPES to pH 7.
Chemicals and solutions
Ryanodine was purchased from Calbiochem (Nottingham, UK), CaM from SIGMA (Gillingham, UK), [3H]ryanodine from Amersham (Little Chalfont, UK), and PE from Avanti Polar Lipids (Alabaster, AL). Standard chemicals were purchased as best available grade from SIGMA or BDH (Poole, UK).
Statistics
Results are shown as mean ± SE. Where appropriate, Student's t-test was used to assess the difference between mean values. A p value of <0.05 was taken as significant. Where comparison of three or more groups was required, we used analysis of variance followed by Dunnett's test.
Results
Comparing Ca2+CaM effects on [3H]ryanodine binding and single-channel gating
To rule out the possibility that differences in membrane preparation might account for differences between [3H]ryanodine binding and single-channel studies, we used the same heavy-SR membrane preparations for both types of experiment. Fig. 1 A shows how Ca2+CaM affects [3H]ryanodine binding to cardiac heavy SR. The free [Ca2+] was maintained at 100 μM to maintain CaM in the fully Ca2+-bound conformation (24). CaM caused a dose-dependent partial inhibition of binding to ∼70% of the control, with maximal inhibition occurring at ∼4 μM. Similar effects have been reported by many other groups with both cardiac and skeletal SR (8,9,12,25,26). In contrast, even though the same SR membrane preparations were used and the same free [Ca2+] was maintained (100 μM), Ca2+CaM activated RyR2 channels at low, physiological concentrations. Fig. 1 B shows an example of channel activation with 50 nM Ca2+CaM. High levels of Ca2+CaM (≥500 nM) were able to reverse the activation caused by 50 nM Ca2+CaM, but average Po levels were not lowered below control levels (17). Fig. 1 C shows the typical effect of a high level of Ca2+CaM (1 μM), demonstrating that Po is not lowered, even though the [3H]ryanodine binding data predicts channel inhibition at this dose (Fig. 1 A).
Figure 1.
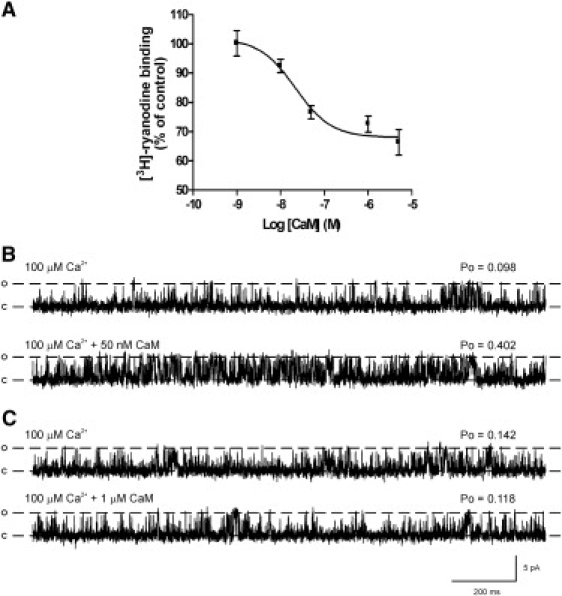
Effects of Ca2+CaM on [3H]ryanodine binding to heavy SR and on the gating of native RyR2 channels reconstituted from the same SR preparations. (A) Dose-dependent effects of Ca2+CaM on [3H]ryanodine binding to isolated heavy SR. The mean ± SE is shown for n = 4–8. Where not shown, error bars are within the symbols. (B and C) Typical single-channel experiments using the same heavy SR preparation as in A, illustrate the activating effects of 50 nM Ca2+CaM (B) and show that 1 μM Ca2+CaM does not markedly alter Po (C). The holding potential was 0 mV. Ca2+ was the permeant ion. Cytosolic free [Ca2+] was maintained at 100 μM throughout. O and C are the open and closed channel levels, respectively.
There is, therefore, a major discrepancy between the single-channel experiments (and cellular studies (17)) and the [3H]ryanodine binding assays. Inhibition of [3H]ryanodine binding suggests that Ca2+CaM inhibits the Po of RyR2, yet the single-channel experiments indicate that this is not the predominant effect of Ca2+CaM on RyR2 function.
We therefore used the reversible ryanoid, ryanodol, to investigate whether Ca2+CaM binding to RyR2 altered the subsequent binding of ryanoids in a manner independent of Po changes. We used purified RyR2 channels, so that the experiments could be performed in symmetrical K+ solutions to enable flexible voltage-clamp protocols. We used 1 μM Ca2+CaM, because this is an optimum dose for inhibition of [3H]ryanodine binding, yet it does not significantly alter channel Po compared to controls (an increase in the Po itself causes an increase in the rate of association of ryanoids (19) and could invalidate the results).
Effects of ryanodol
It has been reported that ryanoid association and dissociation rates are dependent on voltage (19,27). We also found that the rate of association of ryanodol to RyR2 was increased with increasingly positive holding potentials, and that its dissociation from RyR2 was increased with increasingly negative holding potentials. Initially, we voltage-clamped at +20 mV to encourage as many ryanodol-induced modification and demodification events as possible within the lifetime of a single-channel experiment. Fig. 2 shows a typical example of a purified RyR2 channel, held at +20 mV, after addition of 40 μM ryanodol. In agreement with our experiments on native RyR2 channels (17), 1 μM CaM did not affect the Po of purified RyR2. We recorded for 6 min before adding CaM and then for a further 6 min in the presence of 1 μM CaM. During this time, we saw only two ryanodol-induced modifications before adding CaM but no modifications after adding CaM. For clarity, the figure shows only 2 min of consecutive recordings before and after adding CaM, capturing only a single ryanodol-induced modification (Fig. 2, arrow; note appearance of a modified conductance level) and a single demodification event (Fig. 2, asterisk; note reversal to normal gating). The experiment suggests that CaM reduces the frequency of ryanodol-induced modifications, but it is clear that this experimental protocol does not permit a sufficient number of ryanodol-induced modification and demodification events to determine whether CaM can affect ryanodol rates of association and dissociation. We therefore changed the experimental protocol to increase the frequency of ryanodol-induced modification and demodification events.
Figure 2.
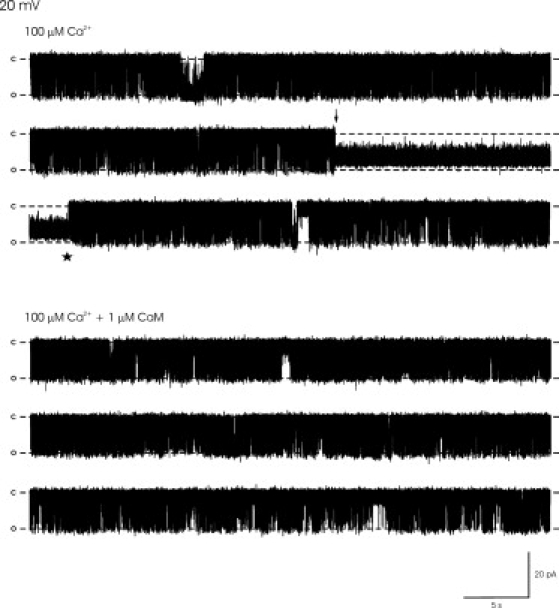
Effects of Ca2+CaM on the gating of a representative purified RyR2 channel in the presence of ryanodol. (Top) Gating of a single channel in the presence of 40 μM cytosolic ryanodol, showing 2 min of consecutive recording at a holding potential of +20 mV. Cytosolic free [Ca2+] was maintained at 100 μM. The Po averaged over 6 min was 0.10. The arrow marks the point of transition into the ryanodol-modified subconductance open state, and the asterisk indicates the demodification transition, where the channel reverts back to normal channel gating. (Bottom) The effect of adding 1 μM CaM in the continued presence of 40 μM ryanodol, illustrating 2 min of consecutive recording. The Po averaged over 6 min was 0.16. K+ was the permeant ion and channel openings are downward deflections. O and C are the open and closed channel levels, respectively.
Effects of Ca2+CaM on ryanodol association and dissociation
The rate of association of ryanoids to RyR2 increases with increasing Po (19). Consequently, we used 20 mM caffeine (and symmetrical 100 μM free Ca2+) to ensure a constant high Po. Under these conditions, Po could be reproducibly clamped within a narrow Po range and, again, as shown in Fig. 3 A, 1 μM CaM did not significantly alter Po.
Figure 3.

Effect of Ca2+CaM on purified RyR2 Po and the voltage-switching protocol for monitoring ryanodol association and dissociation from RyR2. (A) Histogram of Po before (control) and after addition of 1 μM Ca2+CaM at +60 mV, demonstrating that Po is unchanged (a necessary prerequisite for the experiments, since increasing Po will increase the ryanodol association rate). Mean ± SE for n = 8. (B) The voltage-switching protocol. The bilayer was voltage-clamped at +60 mV to encourage ryanodol association with RyR2 and then switched to −60 mV to encourage dissociation. The arrows mark the times of modification to the ryanodol-induced subconductance state and demodification back to normal channel gating. Each time the holding potential was switched to +60 mV, the time to the ryanodol-induced modification (dotted line) was measured. Values were then plotted in a cumulative fashion (Fig. 4) to determine the rate of association of ryanodol to RyR2. Each time the holding potential was switched to −60 mV, the time to demodification back to normal channel gating (dashed line) was measured. Values were then plotted in a cumulative fashion (Fig. 4) to determine the rate of dissociation of ryanodol from RyR2. In each experiment, 25–40 modification and demodification events were induced before and after CaM addition. O, C, and M refer to the fully open and closed channel levels and the ryanodol-induced modified open state, respectively.
To increase the number of modification and demodification events, we introduced a voltage-switching protocol. Fig. 3 B illustrates how we voltage-clamped at +60 mV to encourage ryanodol binding to RyR2, then switched to −60 mV to encourage ryanodol dissociation. This allowed us to record 25–40 ryanodol-induced modifications within an 8- to 12-min recording period. After each voltage switch to +60 mV, we measured the time to the ryanodol-modified conductance state. After each voltage switch to −60 mV, we measured the time taken for reversal (demodification) to normal RyR2 channel gating. The times to modification and the times to demodification were then plotted in a cumulative fashion (Fig. 4 A) to allow estimation of the rates of ryanodol association to and dissociation from RyR2 in the presence and absence, respectively, of 1 μM Ca2+CaM. The mean rate constants obtained from eight experiments plotted in this manner are shown in Fig. 4 B. Ca2+CaM significantly delayed the onset of ryanodol-induced modifications (ryanodol-induced modifications were less frequent); thus, Ca2+CaM reduces the rate of association of ryanodol to RyR2. In contrast, CaM appeared to have no effect on the rate of dissociation of ryanodol from RyR2.
Gating of RyR2 within the ryanodol-induced modified state
As described above, ryanodol association with RyR2 causes the channel to gate in a reduced-conductance open state. Fig. 5 shows that within the ryanodol-induced modified conductance state there are clear fluctuations between at least two different subconductance levels, M1 and M2, which are ∼66% and 30%, respectively, of the full conductance state (see Fig. 7 D for absolute values). The channel tends to reside predominantly in the M1 state at both positive and negative holding potentials (see Fig. 5 A). In Fig. 5 B, an extended time base shows the M1-M2 fluctuations more clearly. Fluctuations to the M2 level are very brief. Although some events obviously occur at the M2 level, there are other events that are too brief to be fully resolved, and it is therefore possible that there are other, unresolved ryanodol-induced subconductance levels.
Figure 5.
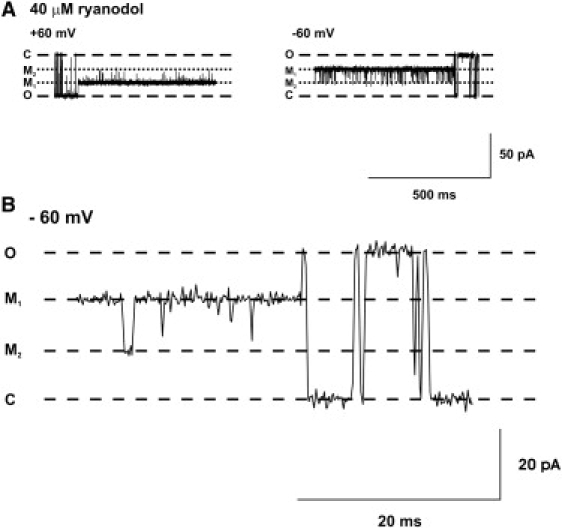
Gating within the ryanodol-induced modified state. (A) Typical examples of RyR2 gating within the ryanodol-induced modified state at holding potentials of +60 mV (left) and −60 mV (right). After modification of channel function by ryanodol, the channel gates between at least two clearly defined conductance levels labeled M1 and M2. (B) An expanded time base shows the M1↔M2 transitions more clearly. O and C refer to the fully open and closed channel levels, respectively.
Figure 7.
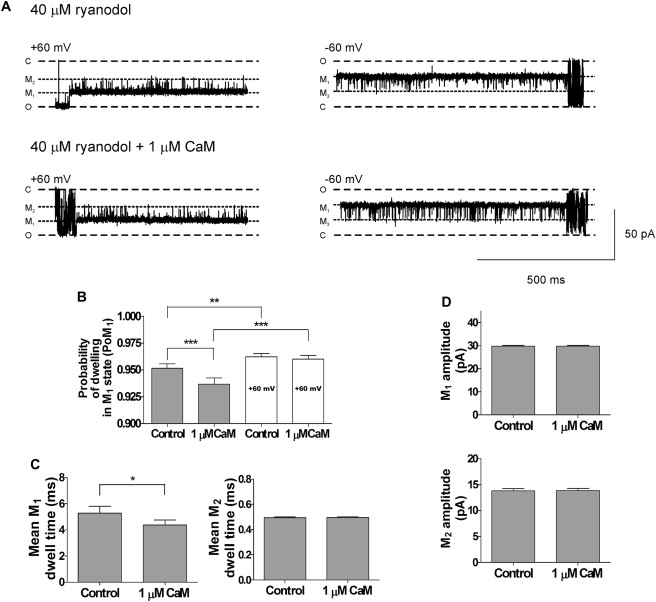
Effects of Ca2+CaM on RyR2 gating within the ryanodol-induced modified states. (A) Typical examples of the gating transitions between M1 and M2 at +60 mV and −60 mV in the absence (upper traces) and presence (lower traces) of 1 μM Ca2+CaM. O, C, M1, and M2 refer to the fully open and closed channel levels and modified levels, respectively. (B) Histograms of the probability of dwelling in the M1 state (PoM1) at −60 mV (gray bars) and +60 mV (white bars) in the absence and presence of 1 μM Ca2+CaM. (C) Mean M1 and M2 dwell times at −60 mV in the absence and presence of 1 μM Ca2+CaM. (D) Histograms of the current amplitudes of the M1 and M2 states in the absence and presence of 1 μM Ca2+CaM at −60 mV. Mean ± SE for n = 8; ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
The traces shown in Fig. 5 A indicate that there are more frequent transitions to the M2 level at −60 mV than at +60 mV. It is not surprising, therefore, that the probability of the channel dwelling in the M1 state (PoM1) is significantly lower at −60 mV than at +60 mV (see Fig. 6 A). The histograms in Fig. 6 B illustrate that the mean dwell times in M1 are significantly shorter at −60 mV than at +60 mV, whereas there is little change in the mean M2 dwell times. Thus, whereas transitions from M1 to M2 are voltage-dependent, transitions from M2 to M1 do not appear to be voltage-dependent. Switching from +60 mV to −60 mV decreases PoM1 by increasing the frequency of the transitions to the M2 state without significantly changing the duration of the events in the M2 state.
Figure 6.
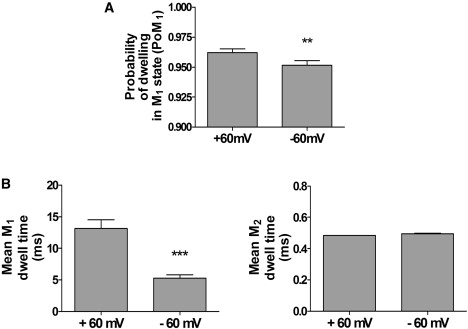
Voltage-dependent transitions between M1 and M2. (A) Histogram showing the probability of dwelling in the M1 state (PoM1) at +60 mV and −60 mV. (B) Histograms showing mean dwell times in the M1 and M2 states at both +60 mV and −60 mV. Mean ± SE for n = 8; ∗∗P < 0.01; ∗∗∗P < 0.001.
We then investigated whether the gating of the channel within the ryanodol-modified state could also be affected by Ca2+CaM. Fig. 7 A shows a typical example of the gating between M1 and M2 at +60 mV and −60 mV before (upper) and after (lower) addition of CaM (1 μM). Ca2+CaM had no significant effect on transitions between M1 and M2 at +60 mV, but it increased the frequency of M1 to M2 transitions at −60 mV. The histogram in Fig. 7 B confirms this and shows that Ca2+CaM decreased PoM1 at −60 mV but not at +60 mV. The mean M1 dwell times were significantly reduced without any effect on the mean M2 dwell times (Fig. 7 C). Thus, the underlying mechanism for this change is an increase in the frequency of transitions from M1 to M2. Ca2+CaM had no effect on the current amplitudes of either M1 or M2 (Fig. 7 D).
Transition mechanisms into and out of the ryanodol-modified state
The preferred route of transition into the ryanodol-modified state appears to be from the fully open state to the M1 state (>96% of transitions). An example of this type of transition is shown in Fig. 8 A; ∼4% of transitions appeared to occur from the fully open state directly into the M2 state and, as expected, since it is thought that ryanoids can bind only to open channels, we observed no transitions from the closed state into either the M1 or M2 states. Ca2+CaM produced no substantial changes in the relative percentages of modifications that occurred directly to the M1 level (or M2 level), as shown in the histogram. We were surprised to find, however, that in the presence of Ca2+CaM, occasional transitions directly from the closed state to the modified state were observed. An example of this type of transition is shown in Fig. 8 B. Changing the filtering level from 2 to 8 kHz and expanding the time base illustrates this type of transition more clearly.
Figure 8.
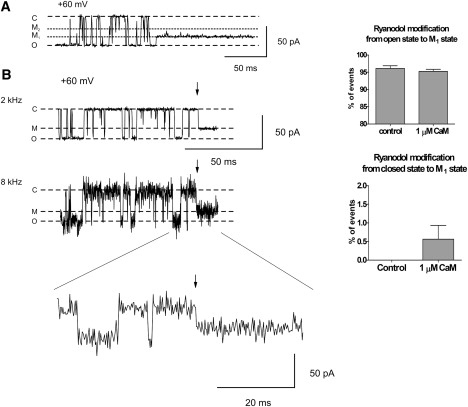
Mechanisms of ryanodol association with RyR2. (A) The trace shows a typical example of the most common route into the ryanodol-modified state; entry from the open state into M1. The histogram to the right demonstrates that CaM did not alter the percentage of the total events modifying by this route. Mean ± SE for n = 8. (B) A typical example of the apparent ryanodol association to RyR2 directly from the closed state is illustrated. The same portion of trace is filtered at 2 and 8 kHz. The lowest trace shows the ryanodol modification event on an expanded timescale. Arrows mark the time of ryanodol association. O, C, and M refer to the fully open and closed channel levels and the modified level, respectively. The histogram shows the percentage of total association events that occur directly from the closed state in the absence and presence of 1 μM Ca2+CaM. Mean ± SE for n = 8.
The most common type of ryanodol dissociation (or demodification) event was from the M1 state to the fully open state (88%). A typical example is shown in Fig. 9 A, and the histogram to the right of the trace demonstrates that Ca2+CaM significantly reduced the number of these transitions. Approximately 9% of dissociation transitions were from the M2 state to the fully open state, but this frequency was not affected by Ca2+CaM. However, as shown in Fig. 9 B, Ca2+CaM significantly increased the number of transitions from the M1 state to the closed state.
Figure 9.
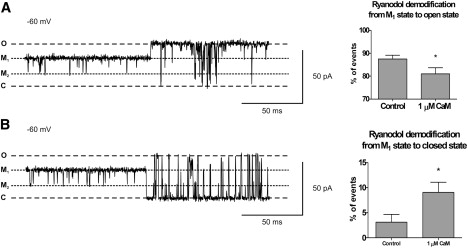
Mechanism of ryanodol dissociation and the effect of Ca2+CaM. (A and B) Representative examples of the dissociation of ryanodol from the M1 state to the fully open state (A) and the fully closed state (B). O, C, M1, and M2 refer to the fully open and closed channel levels and the modified levels, respectively. To the right of the traces, histograms show the percentage of the total events for each type of dissociation in the absence and presence of 1 μM Ca2+CaM. Mean ± SE for n = 8; ∗P < 0.05.
Discussion
In this study, we have demonstrated that Ca2+CaM causes marked changes to the mechanisms by which the reversible ryanoid, ryanodol, interacts with RyR2. A crucial finding is that Ca2+CaM slows the rate of association of ryanodol to RyR2 without changing the rate of dissociation from RyR2. We also show that the gating of the ryanodol/RyR2 complex is significantly different in the presence of Ca2+CaM.
Over the past 20 years, our understanding of the effects of Ca2+CaM on RyR channel function has depended heavily on the use of [3H]ryanodine binding assays (8,9,14,26,28). Yet we now show that [3H]ryanodine binding studies and single-channel experiments provide conflicting information even when the same SR membrane vesicle preparation is used for both studies, ruling out the possibility that the disparity could be explained by differences between our preparation of SR membrane vesicles and those of others. We observe dose-dependent inhibition of [3H]ryanodine binding to cardiac SR by Ca2+CaM a level ∼70% of controls (Fig. 1). Our data correspond well with data from previously published work (8,12,14) and, by conventional thinking, suggest that Ca2+CaM reduces RyR2 Po (ryanoids are thought to bind only to open channels, so stimulation of [3H]ryanodine binding is expected with agents that increase RyR Po and inhibition of [3H]ryanodine binding is expected with RyR2 inhibitors). Our experiments with ryanodol now provide a possible explanation for the discrepancy between [3H]ryanodine binding studies and single-channel experiments; Ca2+CaM slows the rate of association of ryanodol with RyR2. The most likely explanation for this effect is that the binding of Ca2+CaM to RyR2 causes a conformational change that extends to the ryanoid binding site, reducing the affinity of the site for ryanoids. This would be expected to inhibit [3H]ryanodine binding to cardiac SR.
For the ryanodol experiments in this study, since the rate of association of ryanoids to RyR2 is strongly increased by increasing Po (19,28), it was very important to 1), maintain Po at a high level to achieve an adequate number of modification events for the analysis; and 2), use a concentration of Ca2+CaM that does not influence Po. Since 1 μM Ca2+CaM did not alter Po, we can be sure that the observed effects of Ca2+CaM on ryanodol-induced gating kinetics were independent of Po changes.
In addition to reducing the rate of ryanodol association, Ca2+CaM also changes channel gating subsequent to ryanodol binding; the equilibrium between the M1 and M2 states is shifted and the mechanism by which the channel exits the ryanodol-modified state is affected. These gating changes demonstrate that Ca2+CaM induces conformational changes that alter the nature of the RyR2/ryanoid complex. Fig. 10 shows a simplified model of the main RyR2 gating states observed in the presence of ryanodol and identifies the transitions affected by Ca2+CaM. The frequency of the gating transitions is approximated by the thickness of the transition arrows (relative values are given in Table 1). The M1 state is the ryanodol-bound state in which the channel usually dwells. Transitions into the M2 state are voltage-dependent, since a negative holding potential drives more frequent transitions to this level. When RyR2 is bound to Ca2+CaM, more transitions from the M1 to the M2 state also occur (Fig. 7). Ca2+CaM slows the association of ryanodol to RyR2 (Fig. 4) and therefore must inhibit O→M1 transitions since they are the main route (96% of transitions) for entry into the ryanodol-modified state, but it also reduces the frequency of M1→O transitions (Fig. 9 A). Although there are frequent transitions from M1 to M2, only rarely do M2↔O transitions occur (∼4% and 10% of the total number of modification and demodification events, respectively). On no occasion were M2↔C transitions observed.
Figure 10.
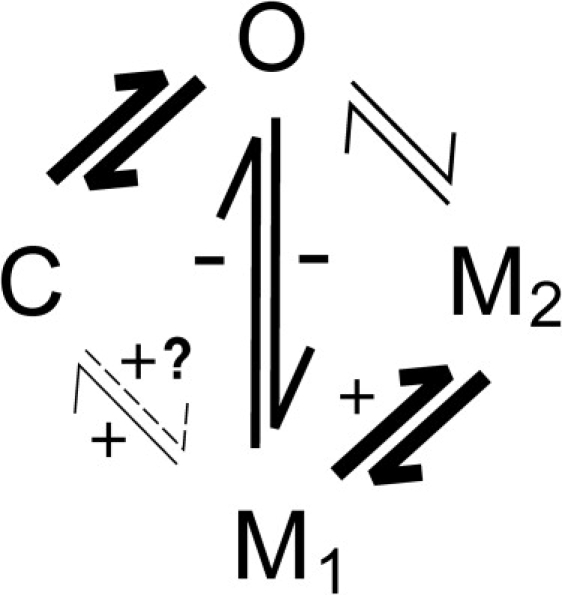
Ryanodol gating model illustrating the transitions affected by Ca2+CaM. A simplified model of RyR2 gating in the presence of ryanodol is shown to illustrate the Ca2+CaM-sensitive transitions. The thickness of the lines indicates (approximately) the relative frequency with which the transitions occur (see table below for absolute values). The plus and minus signs denote Ca2+CaM-dependent increases and decreases, respectively, in the frequency of the transition. The overall frequency of ryanodol-induced modifications was reduced in the presence of Ca2+CaM (Fig. 4), but also altered was the relative frequency of the different types of transition (see Figs. 7–9 and Table 1).
Table 1.
Frequency of gating transitions
| Type of transition |
Percent of total number of modification events |
|
|---|---|---|
| Control |
After Ca2+CaM |
|
| Modification | ||
| O→M1O→M2C→M1 | 96 ± 0.774 ± 0.770 | 95 ± 0.584 ± 0.540.56 ± 0.37 |
| Demodification | ||
| M1→OM2→OM1→C | 88 ± 1.69 ± 1.73 ± 1.6 | 81 ± 2.7∗10 ± 29 ± 2∗ |
Total number of ryanodol-induced modifications recorded was 314 for controls and 318 after Ca2+CaM. The total number of demodification events recorded was 314 for controls and 318 after Ca2+CaM. (n = 8; ∗P < 0.05).
It has always been reported that ryanodine and other ryanoids can only bind to the open state of the channel (5,19,30), and this underlies our understanding of why agents that increase or decrease the Po of RyR channels should stimulate or inhibit, respectively, [3H]ryanodine binding to RyR. Although extremely rare, our single-channel recordings indicate that after addition of Ca2+CaM, ryanodol induces C→M1 transitions. In the presence of Ca2+CaM, we also observe a significant increase in the frequency of M1→C transitions (Fig. 9 B). This does not necessarily imply that ryanodol can bind to or dissociate from the closed-channel state. Our results are limited by the resolution of the single-channel events and it is possible that in these instances, ryanodol binds to the open channel but the channel then closes before entering the conformational state that gives rise to the subconductance state. In a similar way, the M1→C transitions may not mark dissociation of ryanodol from RyR2. Dissociation may occur once the channel opens.
When a single ryanoid molecule binds to its site on RyR2, the conformation of the channel is altered so that ion flux through the pore is affected. This gives rise to the characteristic ryanoid-modified subconductance states (30,31). For ryanodol, when a single molecule binds to RyR2, the ryanodol/RyR2 complex gates between two conformations that we have termed M1 (the predominant state) and M2. In line with data on other reversible ryanoids, we show that the equilibrium between M1 and M2 is voltage-dependent (19,29). Ca2+CaM does not appear to alter the type of channel conformation formed by the ryanodol/RyR2 complex, since the conductance values for M1 and M2 were indistinguishable in the presence and absence of Ca2+CaM. Instead, Ca2+CaM increased the likelihood of the channel dwelling in the M2 conformation and also affected the preferred mechanism by which the channel exited the ryanodol-modified state. Would these changes be expected to affect the rate of ryanodol dissociation from RyR2, and, if so, why do we observe no change in the rate of dissociation of ryanodol in the presence of Ca2+CaM? Since 88% of ryanodol dissociation events involve transitions from the M1 state, it might be expected that stabilization of the M2 state would reduce the dissociation rate. Yet although Ca2+CaM does cause more frequent transitions to the M2 level, the rate of dissociation is not significantly altered. A possible reason for this is that CaM also significantly increases the frequency of dissociation events that occur following the M1→C transitions, which (at −60 mV) may balance the effect on M1→M2 transitions.
Other mechanisms may also contribute to the differences between the single-channel and [3H]ryanodine binding studies. In the bilayer experiment, we can control exactly the solutions (for example, the osmolarity, ionic concentrations, pH, and free [Ca2+]) on both sides of the bilayer. In [3H]ryanodine binding experiments, we cannot control conditions inside the vesicles, therefore, experimental conditions that will impact upon RyR2 function, such as the luminal [Ca2+], the amount of calsequestrin, and the luminal environment, are unknown. In addition, the time courses of single-channel and [3H]ryanodine binding studies are necessarily different. Since the conditions in the two types of experiments are different, single-channel and [3H]ryanodine binding data are unlikely to provide identical answers.
In summary, our results indicate that Ca2+CaM binding to RyR2 significantly alters ryanoid interactions with RyR2. Interpreting [3H]ryanodine binding data therefore becomes complex, since it is unlikely that Ca2+CaM-induced changes in [3H]ryanodine binding result purely from changes in RyR2 Po. The Ca2+CaM-induced decrease in the ryanodol association rate that we report is consistent with the reduction in [3H]ryanodine binding observed with Ca2+CaM. We have focused on the interactions between Ca2+CaM and RyR2, but much of the published work describing the functional effects of CaM on the skeletal isoform, RyR1, depends on data from [3H]ryanodine binding studies. It would be interesting, therefore, to examine how CaM affects ryanoid binding to RyR1, particularly since apoCaM is reported to increase [3H]ryanodine binding to RyR1 but have little effect on RyR2.
Our study has implications beyond simply detailing how Ca2+CaM affects ryanoid binding to RyR2, and it indicates that caution should be used when interpreting [3H]ryanodine binding experiments. The [3H]ryanodine binding assay is an extremely useful tool, providing a simple method by which to estimate changes in RyR Po for populations of channels. On the other hand, there is implicit acceptance that it reports only on channel Po, and since this may not be true, as we have proved for Ca2+CaM, erroneous conclusions can be drawn. [3H]ryanodine binding assays should therefore always be used in conjunction with other methods for investigating RyR function.
Acknowledgments
Many thanks to William Welch for helpful advice and discussion. We are also grateful to Samantha Pitt and Elisa Venturi for many valuable comments on earlier versions of the manuscript.
This study was funded by the British Heart Foundation.
References
- 1.Sutko J.L., Airey J.A. Ryanodine receptor Ca2+ release channels: does diversity in form equal diversity in function. Physiol. Rev. 1996;76:1027–1071. doi: 10.1152/physrev.1996.76.4.1027. [DOI] [PubMed] [Google Scholar]
- 2.Sutko J.L., Airey J.A., Welch W., Ruest L. The pharmacology of ryanodine and related compounds. Pharmacol. Rev. 1997;49:53–98. [PubMed] [Google Scholar]
- 3.Rousseau E., Smith J.S., Meissner G. Ryanodine modifies conductance and gating behaviour of single Ca2+ release channel. Am. J. Physiol. 1987;253:C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- 4.Buck E., Zimanyi I., Abramson J.J., Pessah I.N. Ryanodine stabilizes multiple conformational states of the skeletal muscle calcium release channel. J. Biol. Chem. 1992;267:23560–23567. [PubMed] [Google Scholar]
- 5.Lindsay A.R.G., Tinker A., Williams A.J. How does ryanodine modify ion-handling in the sheep cardiac sarcoplasmic reticulum Ca2+-release channel? J. Gen. Physiol. 1994;104:425–447. doi: 10.1085/jgp.104.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu A., Diaz-Munoz M., Hawkes M.J., Brush K., Hamilton S.L. Ryanodine as a probe for the functional state of the skeletal muscle sarcoplasmic reticulum calcium release channel. Mol. Pharmacol. 1990;37:735–741. [PubMed] [Google Scholar]
- 7.Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J. Biol. Chem. 1986;261:6300–6306. [PubMed] [Google Scholar]
- 8.Balshaw D.M., Xu L., Yamaguchi N., Pasek D.A., Meissner G. Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor) J. Biol. Chem. 2001;276:20144–20153. doi: 10.1074/jbc.M010771200. [DOI] [PubMed] [Google Scholar]
- 9.Rodney G.G., Williams B.Y., Strasburg G.M., Beckingham K., Hamilton S.L. Regulation of RYR1 activity by Ca2+ and calmodulin. Biochemistry. 2000;39:7807–7812. doi: 10.1021/bi0005660. [DOI] [PubMed] [Google Scholar]
- 10.Rodney G.G., Moore C.P., Williams B.Y., Zhang J.Z., Krol J. Calcium binding to calmodulin leads to an N-terminal shift in its binding site on the ryanodine receptor. J. Biol. Chem. 2001;276:2069–2074. doi: 10.1074/jbc.M008891200. [DOI] [PubMed] [Google Scholar]
- 11.Smith J.S., Rousseau E., Meissner G. Calmodulin modulation of single sarcoplasmic reticulum Ca2+-release channels from cardiac and skeletal muscle. Circ. Res. 1989;64:352–359. doi: 10.1161/01.res.64.2.352. [DOI] [PubMed] [Google Scholar]
- 12.Hill A.P., Kingston O., Sitsapesan R. Functional regulation of the cardiac ryanodine receptor by suramin and calmodulin involves multiple binding sites. Mol. Pharmacol. 2004;65:1258–1268. doi: 10.1124/mol.65.5.1258. [DOI] [PubMed] [Google Scholar]
- 13.Tripathy A., Xu L., Mann G., Meissner G. Calmodulin activation and inhibition of skeletal muscle Ca2+ release channel (ryanodine receptor) Biophys. J. 1995;69:106–119. doi: 10.1016/S0006-3495(95)79880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fruen B.R., Bardy J.M., Byrem T.M., Strasburg G.M., Louis C.F. Differential Ca2+ sensitivity of skeletal and cardiac muscle ryanodine receptors in the presence of calmodulin. Am. J. Physiol. 2000;279:C724–C733. doi: 10.1152/ajpcell.2000.279.3.C724. [DOI] [PubMed] [Google Scholar]
- 15.Moore C.P., Rodney G., Zhang J.Z., Santacruz-Toloza L., Strasburg G. Apocalmodulin and Ca2+ calmodulin bind to the same region on the skeletal muscle Ca2+ release channel. Biochemistry. 1999;38:8532–8537. doi: 10.1021/bi9907431. [DOI] [PubMed] [Google Scholar]
- 16.Wu X., Bers D.M. Free and bound intracellular calmodulin measurements in cardiac myocytes. Cell Calcium. 2006;41:353–364. doi: 10.1016/j.ceca.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigalas C., Bent S., Kitmitto A., O'Neill S., Sitsapesan R. Ca2+-calmodulin can activate and inactivate cardiac ryanodine receptors. Br. J. Pharmacol. 2009;156:794–806. doi: 10.1111/j.1476-5381.2008.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanna B., Welch W., Ruest L., Sutko J.L., Williams A.J. The interaction of a neutral ryanoid with the ryanodine receptor channel provides insights into the mechanisms by which ryanoid binding is modulated by voltage. J. Gen. Physiol. 2000;116:1–9. doi: 10.1085/jgp.116.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanna B., Welch W., Ruest L., Sutko J.L., Williams A.J. Interactions of a reversible ryanoid (21-amino-9α-hydroxy-ryanodine) with single cardiac ryanodine receptor channels. J. Gen. Physiol. 1998;112:55–69. doi: 10.1085/jgp.112.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanna B., Welch W., Ruest L., Sutko J.L., Williams A.J. The interaction of an impermeant cation with the sheep cardiac RyR channel alters ryanoid association. Mol. Pharmacol. 2006;69:1990–1997. doi: 10.1124/mol.105.021659. [DOI] [PubMed] [Google Scholar]
- 21.Sitsapesan R., Montgomery R.A.P., MacLeod K.T., Williams A.J. Sheep cardiac sarcoplasmic reticulum calcium release channels: modification of conductance and gating by temperature. J. Physiol. 1991;434:469–488. doi: 10.1113/jphysiol.1991.sp018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colquhoun D., Sigworth F.J. Fitting and statistical analysis of single-channel recording. In: Sakmann B., Neher E., editors. Single-Channel Recording. Plenum; New York and London: 1983. pp. 191–263. [Google Scholar]
- 23.Deslongchamps P., Belanger A., Berney D.J.F., Borschberg H.J., Brousseau R. The total synthesis of (+)-ryanodol. Part II. Model studies for rings B and C of (+)-anhydroryanodol. Preparation of a key pentacyclic intermediate. Can. J. Chem. 1990;68:115–192. [Google Scholar]
- 24.Burger D., Cox J.A., Comte M., Stein E.A. Sequential conformational changes in calmodulin upon binding of calcium. Biochemistry. 1984;23:1966–1971. [Google Scholar]
- 25.Fruen B.R., Black D.J., Bloomquist R.A., Bardy J.M., Johnson J.D. Regulation of the RYR1 and RYR2 Ca2+ release channel isoforms by Ca2+-insensitive mutants of calmodulin. Biochemistry. 2003;42:2740–2747. doi: 10.1021/bi0267689. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton S.L., Serysheva I., Strasburg G.M. Calmodulin and excitation-contraction coupling. News Physiol. Sci. 2000;15:281–284. doi: 10.1152/physiologyonline.2000.15.6.281. [DOI] [PubMed] [Google Scholar]
- 27.Tinker A., Sutko J.L., Ruest L., Deslongchamps P., Welch W. Electrophysiological effects of ryanodine derivatives on the sheep cardiac sarcoplasmic reticulum calcium-release channel. Biophys. J. 1996;70:2110–2119. doi: 10.1016/S0006-3495(96)79777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chugun A., Sato O., Takeshima H., Ogawa Y. Mg2+ activates the ryanodine receptor type 2 (RyR2) at intermediate Ca2+ concentrations. Am. J. Physiol. Cell Physiol. 2007;292:C535–C544. doi: 10.1152/ajpcell.00275.2006. [DOI] [PubMed] [Google Scholar]
- 29.Tanna B., Welch W., Ruest L., Sutko J.L., Williams A.J. Voltage-sensitive equilibrium between two states within a ryanoid-modified conductance state of the ryanodine receptor channel. Biophys. J. 2005;88:2585–2596. doi: 10.1529/biophysj.104.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tinker A., Sutko J.L., Ruest L., Welch W., Airey J.A. Electrophysiological effects of ryanodine derivatives on the sheep cardiac Ca2+-release channel. Biophys. J. 1994;66:A315. doi: 10.1016/S0006-3495(96)79777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porta M., Diaz-Sylvester P.L., Nani A., Ramos-Franco J., Copello J.A. Ryanoids and imperatoxin affect the modulation of cardiac ryanodine receptors by dihydropyridine receptor Peptide A. Biochim. Biophys. Acta. 2008;1778:2469–2479. doi: 10.1016/j.bbamem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]


