Abstract
Sarcoplasmic reticulum (SR) Ca2+ release in striated muscle is mediated by a multiprotein complex that includes the ryanodine receptor (RyR) Ca2+ channel and the intra-SR Ca2+ buffering protein calsequestrin (CSQ). Besides its buffering role, CSQ is thought to regulate RyR channel function. Here, CSQ-dependent luminal Ca2+ regulation of skeletal (RyR1) and cardiac (RyR2) channels is explored. Skeletal (CSQ1) or cardiac (CSQ2) calsequestrin were systematically added to the luminal side of single RyR1 or RyR2 channels. The luminal Ca2+ dependence of open probability (Po) over the physiologically relevant range (0.05–1 mM Ca2+) was defined for each of the four RyR/CSQ isoform pairings. We found that the luminal Ca2+ sensitivity of single RyR2 channels was substantial when either CSQ isoform was present. In contrast, no significant luminal Ca2+ sensitivity of single RyR1 channels was detected in the presence of either CSQ isoform. We conclude that CSQ-dependent luminal Ca2+ regulation of single RyR2 channels lacks CSQ isoform specificity, and that CSQ-dependent luminal Ca2+ regulation in skeletal muscle likely plays a relatively minor (if any) role in regulating the RyR1 channel activity, indicating that the chief role of CSQ1 in this tissue is as an intra-SR Ca2+ buffer.
Introduction
Fast changes in cytosolic free Ca2+ drive the contractile machinery in cardiac and skeletal muscle (1). This Ca2+ is released from the sarcoplasmic reticulum (SR) by specialized SR Ca2+ release channels called ryanodine receptors (2). The skeletal (RyR1) and cardiac (RyR2) channels are differentially regulated, but both operate within a multiprotein complex that mediates the process called excitation-contraction (EC) coupling (1,2). In both skeletal and cardiac muscle, calsequestrin (CSQ) is associated with the intra-SR surface of the RyR channel (3,4). The role of CSQ in skeletal and cardiac EC coupling is currently a topic of debate.
One CSQ role is that of an intra-SR Ca2+ buffer. The need for a Ca2+ buffer inside the SR of striated muscle is clear, and CSQ likely provides much of the needed buffer power (5). It can, because CSQ is a low-affinity and high-capacity Ca2+-binding protein (6–11). Many Ca2+ ions (∼50 mol/mole protein) can bind to a CSQ molecule with a KD of ∼1–2 mM (10,12); see (14). Cardiac muscle contains one CSQ isoform, CSQ2 (13,14). Fast-twitch skeletal muscle fibers have another isoform (CSQ1), whereas slow-twitch skeletal muscle fibers contain both isoforms (15). The two CSQ isoforms are similar proteins, but the C-terminus of CSQ2 contains additional acidic residues and consensus phosphorylation sites (16).
Another CSQ role involves regulation of the RyR channel (14,17–24). The general concept involves CSQ-dependent luminal Ca2+ regulation where Ca2+ unbinding from CSQ (as intra-SR Ca2+ levels fall during SR Ca2+ release) leads to inhibition of the RyR channel (terminating Ca2+ release). However, the cardiac and skeletal muscles of CSQ knockout (KO) animals have nearly normal RyR regulation until those muscles are functionally stressed or challenged (7,15,25). The implication is that loss of CSQ is well compensated for in the KO animals, and/or CSQ-dependent luminal Ca2+ regulation is not essential to normal muscle function. This does not diminish its potential importance during periods of high activity or disease. In either case, CSQ-dependent RyR regulation clearly warrants further investigation. Here, CSQ-dependent regulation of single RyR channels is explored in RyR and CSQ isoform swapping studies.
Methods
Details about the chemicals/drugs used in this study, as well as the statistical analysis applied, can be found in the Supporting Material.
Production of recombinant and isolation of native CSQ
The wild-type CSQ2 construct was generated as previously described (12,26). Purification was done by phenyl-Sepharose purification either in-column or in-batch. The CSQ2 protein was also isolated from adult rabbit hearts using established procedures (27). The CSQ1 protein was isolated from adult rabbit skeletal muscle using published procedures (28). Protein was quantified according to standard procedures (29).
In vitro binding assay
Heavy SR microsomes were isolated from rabbit hearts and skeletal muscle (30). Solubilized junctional SR vesicles (skeletal and cardiac) were diluted 10-fold in 20 mM Tris-HCl, 1 mM dithiothreitol fortified with protease inhibitor, to reduce the high salt and detergent concentrations. The final concentrations of detergents were 0.2% for TRITON and 0.3% for CHAPS, respectively. The solubilization protocols used are those described previously for cardiac and skeletal muscle (31,32). Western blot analysis demonstrated that measurable junctin and triadin protein levels were present in the solubilized SR samples used and that these proteins were not degraded. Solubilized membranes were centrifuged at 105,000 × g in a Beckman (Fullerton, CA) Airfuge for 1 h. The supernatant was precleared with either GST-affinity beads or T7-affinity beads for 2 h at 4°C to eliminate nonspecific binding and then incubated with either GST-CSQ1 or T7-CSQ2 in the suitable buffer for 20 h at 4°C in the presence of either 1 mM EGTA or 1 mM CaCl2. Bound proteins were eluted by boiling in the SDS sample buffer, and subjected to SDS-PAGE (33) in 10% polyacrylamide gels. After electrophoretic separation, proteins were either stained with Coomassie blue or transferred onto nitrocellulose membranes. Western blots were probed with the Sh33 antitriadin antibody or sc-3367 antijunctin antibodies.
Single RyR channel isolation
SR vesicles were prepared from adult rat ventricle and leg skeletal muscle according to published methods (34), with minor modifications. Briefly, the muscle was cut into pieces (10–30 g) and homogenized in a buffer solution containing NaCl 0.9%, and 10 mM Tris-maleate, pH 7.2. The homogenate was then centrifuged for 25 min (3000 × g). The supernatant was centrifuged again at a higher speed for 25 min (20,000 × g). The resulting supernatant was filtered through cheesecloth and centrifuged yet again for 1 h (100,000 × g). The pellet was then resuspended in a small amount of the buffer solution containing 300 mM sucrose. Small samples were flash-frozen for later use.
Single channel recording
Artificial lipid bilayers contained a 5:4:1 mixture (50 mg/ml in decane) of phosphatidylethanolamine, phosphatidylserine, and phosphatidylcholine. Bilayers were formed across a 100-μm hole in a 12-μm thick Teflon partition that separated two baths. One bath (cis) was virtually grounded and initially contained a HEPES-Tris solution (250 mM HEPES and 120 mM Tris, pH 7.4). The other bath (trans) contained a 10 to 53 mM Ca-HEPES solution. After a stable bilayer was formed, 500 mM CsCl and 5–15 μg SR vesicles were added to the cis bath while stirring. After channel activity was observed, solutions in both compartments (volume 1 ml) were exchanged at a rate of 4 ml/min (for 5 min) to establish the desired recording conditions. In our hands, the cis bath always contained the cytosolic side of the RyR channel (14,35).
Single RyR channels in the bilayer were stripped of endogenous CSQ using a process applied by us previously (14). This process is analogous to that applied by other groups (28,36). Briefly, CSQ was stripped (dissociated) from single RyR channel using a high luminal Ca2+ (10–53 mM) wash lasting at least 15 min. Note that the cytosolic side of the channel was never subjected to the high-Ca2+ salt wash and thus this process did not “salt off” any cytosolic RyR-protein partners (e.g., FKBP) that may be present. Biochemical confirmation of CSQ dissociation is impossible, since CSQ stripping was done at the single-channel level. In some studies, 5 or 10 μg/ml of CSQ was added to the luminal bath after the CSQ stripping process. This yielded a CSQ concentration of ∼100–200 nM and would have a negligible effect on free Ca2+ levels. Such small amounts of CSQ have been successfully used in single RyR channel studies (14,28,36). To provide some context, the intra-SR CSQ concentrations in cardiac and skeletal muscle cells are thought to be ∼100 and 600 μM, respectively (1,37).
The standard recording conditions were as follows (unless specified differently). The cytosolic (cis) solution contained 0.75 μM free Ca2+ (buffered using BAPTA and DiBromoBAPTA) and 250 mM HEPES-Tris (pH 7.4). The required buffer mixture was calculated using the WinMAXC 2.05 program (Stanford University, Stanford, CA) and all Ca2+ buffered solutions were verified by Ca2+ electrode. The luminal solution contained 0.01, 0.05, 0.25, 0.5, or 1 mM free Ca2+, 250 mM HEPES-Tris (pH 7.4), and 100 mM Cs+. The Cs+ assured that ample charge carrier was always present. The holding potential was 0 mV, so the net current was in the lumen-to-cytosol direction and carried by a mixture of ions (Cs+ and Ca2+). A published RyR permeation model (38) indicates that the Ca2+ component of the net current was ∼0.01 pA or ∼0.2 pA with 0.05 or 1 mM luminal Ca2+ present. Data acquisition and analysis was done using pClamp software (Axon, Union City, CA). Single RyR channel recordings were sampled at 10 kHz and filtered at 1 kHz. Dwell times, open probability (Po), and burst properties were defined using the traditional methods. Burst detection criteria required that a burst contain five events or more, and a Poisson Surprise (PS; see below) value of ≥18 (see the Supporting Material).
Results
Binding studies
The RyR channel operates within a protein complex that includes the proteins triadin (TD) and junctin (JC), and CSQ-dependent RyR regulation is thought to involve the TD and/or JC proteins (1). Affinity binding studies were done to assess CSQ interaction with these proteins. Affinity binding results between CSQ1 or CSQ2 and the cardiac and skeletal isoforms of TD or JC are shown in Fig. 1 A. The blots shown illustrate the three positive interactions found: cardiac TD bound to CSQ2; and skeletal TD and JC bound to CSQ1. No binding was observed for any other possible binding pairs (see summary in Fig. 1 A, table). Note that cardiac TD and JC did not bind CSQ1 and skeletal TD and JC did not bind CSQ2. If a CSQ-JC-TD interaction is required for CSQ-dependent RyR regulation, this predicts that the function of cardiac and skeletal RyR-TD-JC complexes will depend on which CSQ isoform is present.
Figure 1.
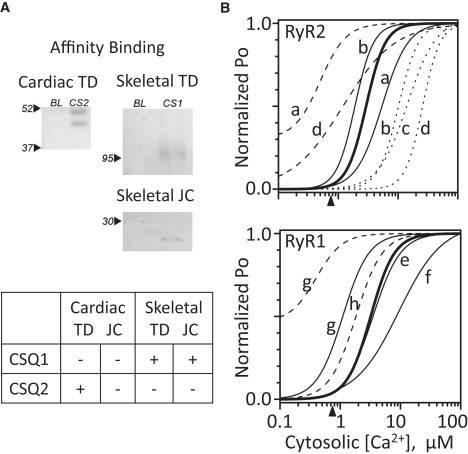
CSQ affinity binding and cytosolic Ca2+ dependence. (A) Positive affinity binding results of the skeletal and cardiac CSQ isoforms (CSQ1 and CSQ2, respectively) to skeletal and cardiac triadin (TD) or junctin (JC) are shown (n = 2–4 for each experimental condition). Negative binding results are not shown. Cardiac and skeletal TD are 45 and 95 kDa, respectively. Cardiac and skeletal JC are 21 and 16 kDa, respectively. 25 μg GST-CS1, 15 μg T7-CS2, 100 μg of the solubilized skeletal fraction, and 200 μg of the solubilized cardiac SR fraction were used. Twice the amount of solubilized cardiac protein was used, because JC levels are known to be lower in cardiac muscle (31). (B) The thick solid curve reflects the cytosolic Ca2+ sensitivity of the RyR1 (lower) and RyR2 channels (upper) tested. Arrowheads (abscissa) indicate the 0.75 μM cytosolic Ca2+ mark. Thin curves represent published results by several laboratories ((20,25,49,68–72), labeled a–h). Solid curves indicate channels activated by cytosolic Ca2+ only (no cytosolic Mg2+ or ATP present). Dashed curves indicate channels activated by cytosolic ATP (no Mg2+ present). Dotted curves indicate channels where both cytosolic Mg2+ (1 mM free) and ATP (5 mM total) were present. All curves were normalized to the maximum Po (PMAX). The minimum Po (PMIN) was zero unless specified otherwise. The Hill coefficients (η) used are given below. a, Laver et al. (20): RyR2 results with 100 μM luminal Ca2+; Ca-only KD = 5.4 μM (PMAX = ∼0.54, η = 2) and ATP-only KD = 0.5 μM (PMAX = ∼0.66, PMIN = ∼0.2, η = 2). b, Zoghgbi et al. (70): RyR2 results with 50 mM luminal Ca2+; Ca-only KD = 1.9 μM (PMAX = ∼0.8, η = 2.8) and MgATP KD = 12.2 μM (PMAX = ∼0.8, η = 2.6); c, Xu and Meissner (24): RyR2 results with 4 μM luminal Ca2+; MgATP KD = 14.4 μM (PMAX = ∼0.9, η = 1.8); d, Gyorke and Gyorke (18): RyR2 results with 20 μM luminal Ca2+; ATP-only KD = 1.6 μM (PMAX = ∼0.27, η = 1) and MgATP KD = 25.8 μM (PMAX = ∼0.25, η = 3.7); e, Meissner (2002): Refit RyR1 results; Ca-only KD = 3.5 μM (PMAX = ∼0.22, η = 1.9); f, Sárközi et al. (72): RyR1 results with 50 μM luminal Ca2+; Ca-only KD = 9.4 μM (PMAX = ∼0.35, η = 1.2); g, Laver et al. (20): Results with 1 mM luminal Ca2+; ATP-only KD = 0.4 μM (PMAX = ∼0.86, PMIN = ∼0.40, assumed η = 2) and Ca-only KD = 1.1 μM (PMAX = ∼0.55, assumed η = 2). h, Tripathy and Meissner (1996): Refit RyR1 results with 0.045 μM luminal Ca2+; ATP-only KD = 1.8 μM (PMAX = ∼0.9, η = 2).
Channel recording
A common single RyR channel recording condition was utilized to facilitate cardiac and skeletal regulatory comparison. Single RyR1 and RyR2 channel function were defined with a single cytosolic agonist present (0.75 μM free Ca2+). Fig. 1 B illustrates the rationale for selecting this cytosolic free Ca2+ level. Thin solid lines represent the cytosolic Ca2+ sensitivities of single RyR channels reported by different groups. The cytosolic Ca2+ sensitivity of the RyR2 and RyR1 channels tested here is represented by thick solid lines (10 μM luminal Ca2+ present). The arrowhead on the abscissa marks the 0.75 μM cytosolic free Ca2+ point. Dashed lines represent the published Ca2+ sensitivities of the channels when cytosolic ATP (no Mg2+) is present. The presence of ATP results in channels that are exceptionally sensitive to cytosolic Ca2+. The dotted lines represent reported RyR2 Ca2+ sensitivities when a physiological level of cytosolic MgATP is present. Note that physiological MgATP makes channels less Ca2+-sensitive compared to when ATP is absent (solid lines). Thus, the common 0.75 μM Ca2+ only recording condition used here 1), minimally activates both channels (Po < 0.05); 2), does not make the channels exceptionally Ca2+-sensitive; and 3), represents an activating Ca2+ level likely encountered by these channels in cells. To avoid having millimolar free Mg2+ present to act as a competing charge carrier and/or RyR/CSQ regulatory ligand, we did not use physiological MgATP (for most studies). This also avoids complications associated with the differential ATP sensitivities of the RyR1 and RyR2 channels (2,40–42).
The sample single-channel recordings are shown in Fig. 2. For the recordings in Fig. 2 A, there was 1 mM luminal free Ca2+ present, and the top recordings were made from single RyR2 (left) and RyR1 (right) channels before (Prewash) and after CSQ was stripped from the channel (No CSQ). The third recording (CSQ2) is the same channel after exogenous CSQ2 protein (5 μg/ml) was added to the luminal bath. The lower two recordings (from different channels) reflect the activity of stripped channels before (No CSQ) and after CSQ1 (5 μg/ml) was added to the luminal bath. For the RyR2 channel, stripping CSQ2 reduced the Po, and replacing it with either CSQ2 or CSQ1 returned the Po to the starting level. Note that the Po of the prewashed RyR2 channel is higher (∼0.35) than that predicted for CSQ2-free channels with 0.75 μM cytosolic Ca2+, as shown in Fig. 1 B. This elevated RyR2 Po is expected if CSQ is present, considering the CSQ-dependent shift in cytosolic Ca2+ reported previously by our group (14). Fig. 2 B shows the activity of a CSQ2-associated RyR2 channel (left) and a CSQ1-associated RyR1 channel at two luminal Ca2+ concentrations (1 and 0.05 mM). The change in luminal Ca2+ substantially reduced the RyR2 Po but evoked little change in RyR1 activity. Also, the recordings in Fig. 2 B were selected to illustrate the inherent bursting nature of single RyR channel activity (43–45). Bursts are temporally grouped openings separated by a relatively long closed period (see Methods). Bursts are underlined and were evident in both the RyR2 and RyR1 recordings.
Figure 2.
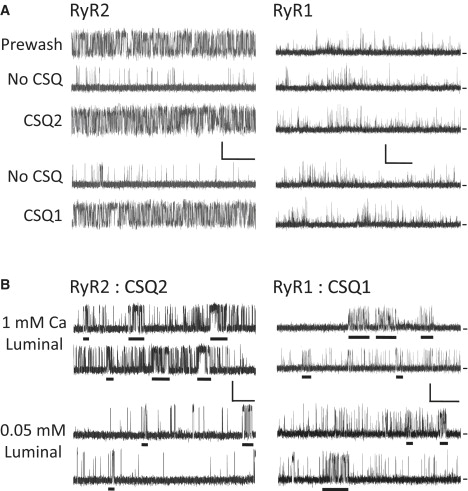
CSQ1- and CSQ2-dependent luminal Ca2+ regulation of RyR2 and RyR1 channels. Sample recordings are shown with open events as upward deflections. Closed currents are marked at right. Holding potential was 0 mV and the cytosolic free Ca2+ was 0.75 μM. Scale bars, 2 pA and 1 s. (A) RyR2 and RyR1 recordings before the CSQ stripping process (Prewash), before CSQ addition (No CSQ), and after addition of 5 μg/ml CSQ2 or CSQ1 to the luminal bath solution are shown. The luminal Ca2+ was 1 mM. Each panel contains recordings from a different channel. (B) Selected recordings from a CSQ2-associated RyR2 channel (right) and a CSQ1-associated RyR2 channel (left) in the presence of 1 mM or 0.05 mM luminal Ca2+. Recordings were selected to illustrate bursting behavior (not overall Po). Sample open-event bursts are underlined. The luminal solution contained 5 μg/ml CSQ.
Channel analysis
Open and closed dwell times of CSQ2-associated RyR2 channels and CSQ1-associated RyR1 channels were measured with 1 or 0.05 mM luminal Ca2+ present. Mean ± SE open and closed time constants determined from exponential fitting of log dwell time histograms are presented in Table 1. Open dwell time histograms were fit assuming two open-time components. Closed dwell times were fit assuming three closed-time components. The relative proportion of open or closed events for each time constant is also presented. Two significant differences (bold print) were detected. The decrease in luminal Ca2+ significantly (p < 0.05) increased one of the RyR2 closed time constants and its proportion. No significant differences in any RyR1 dwell time properties were detected.
Table 1.
Dwell time analysis
| RyR2/CSQ2 | OT1 | OT2 | CT1 | CT2 | CT3 | |
|---|---|---|---|---|---|---|
| Dwell time (ms) | 1 mM Ca | 0.89 ± 0.06 | 3.8 ± 0.6 | 1.3 ± 0.2 | 13.0 ± 4.8 | 69 ± 18 |
| 0.05 mM Ca | 0.96 ± 0.05 | 2.9 ± 0.3 | 3.2 ± 0.8 | 31.8 ± 4.6 | 135 ± 50 | |
| Percentage of events |
1 mM Ca | 67 ± 4 | 32 ± 4 | 43 ± 13 | 47 ± 5 | 10 ± 9 |
| 0.05 mM Ca | 54 ± 20 | 43 ± 20 | 19 ± 3 | 65 ± 4 | 15 ± 2 | |
| RyR1: CSQ1 | OT1 | OT2 | CT1 | CT2 | CT3 | |
| Dwell time (ms) | 1 mM Ca | 1.1 ± 0.45 | 8.3 ± 2.9 | 2.0 ± 0.2 | 12.6 ± 1.6 | 124 ± 30 |
| 0.05 mM Ca | 0.9 ± 0.3 | 8.0 ± 2.9 | 2.9 ± 0.7 | 18.7 ± 7 | 297 ± 139 | |
| Percentage of events | 1 mM Ca | 83 ± 3.8 | 16 ± 3.8 | 57 ± 9.8 | 35 ± 7.8 | 8 ± 2 |
| 0.05 mM Ca | 84 ± 6 | 16 ± 6 | 47 ± 8 | 37 ± 6 | 16 ± 4 | |
Single RyR2 channels were associated with native CSQ2. Single RyR1 channels were associated with native CSQ1. Luminal free Ca2+ concentrations are listed in the table. The cytosolic free Ca2+ was always 0.75 μM. Exponential fitting of log dwell-time histograms were fit assuming two open dwell times (OT1 and OT2) and three closed dwell times (CT1–CT3). Analysis was done on 12 different channels, and each value (mean ± SE) was determined from a minimum of three channels. Student's t-test was used to evaluate differences between values in a pair (values in bold print indicate a significance of p < 0.05).
The CSQ sensitivities of single RyR1 and RyR2 channel mean ± SE open event frequency, mean open time (MOT), mean closed time (MCT), number of bursts (per 3-min period), events per burst, and burst duration are presented in Table 2. In the table, “Native” refers to CSQ that was isolated from adult tissue and “Recomb” to CSQ expressed in and isolated from bacteria. Values within a column were statistically compared to the uppermost value in each column (i.e., CSQ-associated channels). RyR2 event frequency and number of bursts were significantly different (p < 0.05; bold print) when CSQ was removed (No CSQ). No significant differences were detected in any of these RyR2 channel properties when native CSQ2 was exchanged for recombinant CSQ2 or native CSQ1. For RyR1 channels (Table 2, lower), no significant differences were detected when CSQ1 was removed or replaced with CSQ2.
Table 2.
Single channel properties
| RyR2 | Individual event properties |
Burst properties |
||||
|---|---|---|---|---|---|---|
| Frequency (s−1) | MOT (ms) | MCT (ms) | Number | Event/burst | Duration (ms) | |
| Native CSQ2 | 133 ± 19 | 17 ± 9.0 | 15 ± 10 | 41.7 ± 12.8 | 43.7 ± 3.6 | 492 ± 101 |
| No CSQ | 71 ± 16 | 2.5 ± 1.5 | 103 ± 57 | 9.4 ± 3.0 | 33.9 ± 4.8 | 502 ± 93 |
| Recomb CSQ2 | 138 ± 17 | 11 ± 7.7 | 18 ± 6 | 52.5 ± 15.6 | 47.9 ± 5.4 | 565 ± 96 |
| Native CSQ1 | 129 ± 19 | 12 ± 4.9 | 23 ± 8 | 42.4 ± 8.3 | 40.1 ± 4.9 | 571 ± 121 |
| RyR1 | Individual event properties | Burst properties | ||||
| Frequency (s−1) | MOT (ms) | MCT (ms) | Number | Event/burst | Duration (ms) | |
| Native CSQ1 | 77 ± 32 | 2.9 ± 1.2 | 313 ± 99 | 13.7 ± 7.3 | 57.8 ± 7.2 | 492 ± 103 |
| No CSQ | 74 ± 20 | 2.6 ± 0.6 | 268 ± 98 | 12.4 ± 3.2 | 42.2 ± 7.3 | 365 ± 122 |
| Native CSQ2 | 99 ± 19 | 2.6 ± 0.7 | 224 ± 76 | 15.8 ± 5.0 | 47.5 ± 9.9 | 568 ± 148 |
Native, CSQ isolated from adult tissue; Recomb, CSQ produced in bacteria; MOT, mean open time; MCT, mean closed time; PS, Poisson Surprise. Values represent mean ± SE of four to six different channels. All data were collected with 1 mM luminal Ca2+ present. Student's t-test was used to evaluate differences between the uppermost value and other values within each column (values in bold print indicate a significance of p < 0.05).
Fig. 3 A shows histograms of intraburst Po of CSQ2-associated RyR2 channels (left) or CSQ1-associated RyR1 channels (right) at 1 and 0.05 mM luminal Ca2+. Open bars represent the 1 mM luminal Ca2+ condition. The hatched distribution represents the 0.05 mM luminal Ca2+ condition. For RyR2 channels, the change in luminal Ca2+ resulted in fewer bursts with lower intraburst Po. In contrast, the number of bursts and intraburst Po values of RyR1 channels were similar at the two luminal Ca2+ levels. Mean intraburst Po values (± SE) are listed, and RyR2, but not RyR1, intraburst Po was statistically different (p < 0.01; bold print) with reduced luminal calcium. The RyR1 results presented in Tables 1 and 2 and Fig. 3 A are consistent with the absence of obvious changes in RyR1 activity illustrated by the recordings shown in Fig. 2. Likewise, the significant differences found in RyR2 number of events and burst frequency upon CSQ removal (Table 2), as well as the closed dwell time (Table 1) and intraburst Po, as luminal Ca2+ changes is consistent with the clear RyR2 gating changes shown in Fig. 2.
Figure 3.
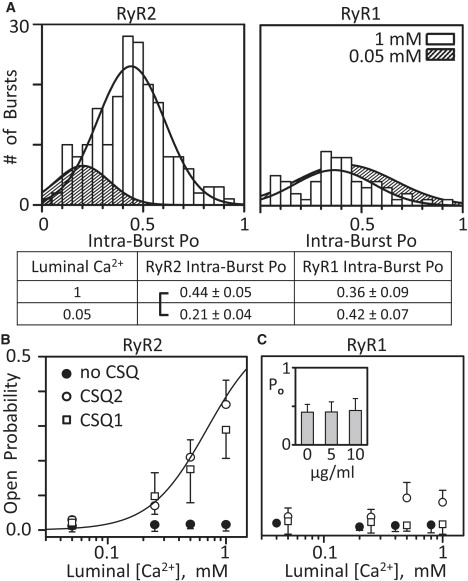
Intraburst open probability and luminal Ca2+ sensitivity of RyR2 and RyR1 channels. (A) Bursts with a minimum of five events/burst were detected in RyR2 (left) and RyR1 (right) recordings, and the intraburst Po was determined for each burst. Histograms of the intraburst Po, fit by a Gaussian distribution, at either 1 mM (open bars) or 0.05 mM (hatched area; bars not shown) luminal Ca2+ are shown. Luminal solution contained 5 μg/ml CSQ2 (left) and CSQ1 (right). Mean intraburst Po values (± SE) are shown below the graphs (bracket; p < 0.01; t-test). Results generated from five RyR2 and five RyR1 channels. (B) Summary RyR2 Po results. Native CSQ2 (open circles) or CSQ1 (squares) was added (5 μg/ml) to the luminal side of CSQ stripped channels. Solid circles are the results with no CSQ present. The line represents the luminal Ca2+ sensitivity of RyR2 channels before CSQ was stripped (taken from Qin et al. (14)). The cytosolic free Ca2+ was 0.75 μM (no Mg or ATP present) and points are mean ± SE of six to eight channels. (C) Summary RyR1 Po results. Symbols are as in B. Solid circles are shifted to avoid symbol overlap. (Inset) RyR1 Po as a function of 0, 5, and 10 μg/ml CSQ1 concentration, with average (± SE) Po values of 0.42 ± 0.10, 0.43 ± 0.13, and 0.45 ± 0.15, respectively. Here, the cytosolic solution contained 20 μM free Ca2+, 1 mM free Mg2+, and 5 mM total ATP. The luminal solution contained 100 Cs-methanesulfonate, 1 mM Ca2+, and 1 mM Mg2+. These results are from four RyR1 channels.
The mean Po values of RyR2 channels when no CSQ (solid circles), CSQ2 (open circles), or CSQ1 (open squares) is present are shown in Fig. 3 B. The Po of CSQ-free channels was always low (near zero) over the range of luminal Ca2+ concentrations tested (0.05 to 1 mM), a range that spans the luminal Ca2+ levels the channels are likely to see in cells (46–48). The Po of CSQ2- and CSQ1-associated RyR2 channels increased with increasing luminal Ca2+ concentration. When 0.25, 0.5, or 1 mM luminal Ca2+ was present, the RyR2 Po was significantly (p < 0.05) elevated compared to when no CSQ was present. When CSQ2 was exchanged for CSQ1, the RyR2 Po was not significantly different. For example, the Po at 1 mM luminal Ca2+ was 0.36 ± 0.07 with CSQ2 and 0.28 ± 0.08 with CSQ1. The Po at 0.25 mM luminal Ca2+ was 0.07 ± 0.03 with CSQ2 and 0.09 ± 0.07 with CSQ1 present.
Fig. 3 C shows results from this kind of study on RyR1 channels. The mean Po of RyR1 channels with no CSQ, or with CSQ1 or CSQ2 present, was always low, regardless of the luminal Ca2+ concentration. Although the Po values of CSQ2-associated RyR1 channels appear to be elevated at the 0.5 and 1 mM luminal Ca2+ marks, these values were not statistically different from the Po values of CSQ1-associated RyR1 channels (0.089 ± 0.045 vs. 0.009 ± 0.017 and 0.085 ± 0.035 vs. 0.007 ± 0.029, respectively; n = 6). We note, however, that a statistical significant difference might be achieved if a much larger data set could be evaluated, and thus, this particular result (lack of CSQ2 action on RyR1) may not be entirely definitive. To address the possibility that the Po is too low here to “see” an inhibitory action of luminal Ca2+ when CSQ is present, another set of studies evaluating CSQ1 action on preactivated RyR1 channels was done (Fig. 3 C, inset). In these very different experimental conditions (see the Fig. 3 C legend), the Po with no CSQ present was high (∼0.4). Addition of 5 or 10 μg/ml CSQ1 did not change the Po over the ∼40 min of recording. Further, a subsequent reduction in luminal Ca2+ with CSQ1 present in these conditions also did not change the Po (see the Supporting Material). Thus, no CSQ1-dependent luminal Ca2+ regulation of RyR1 was detected, and this is likely not due to degraded CSQ1 samples, because the very same protein generated positive results when applied to RyR2 channels.
Discussion
Our results indicate that the mechanism of CSQ-dependent luminal Ca2+ regulation of the RyR2 protein complex may involve cardiac TD (not JC) and does not distinguish between CSQ2 and CSQ1. These results also show that this mechanism mediates a large decrease of RyR2 Po (0.4–∼0.02) upon a reduction of luminal free Ca2+ from 1 to 0.05 mM. In contrast, no significant CSQ-dependent luminal Ca2+ regulation of single RyR1 channels was detected.
It is believed by some that cytosolic ATP (or caffeine) is a necessary cofactor for RyR luminal Ca2+ regulation. We show here that cytosolic ATP is not required for luminal RyR2 Ca2+ regulation. The cofactor idea arises in part from the many studies done using cytosolic ATP with no cytosolic Mg2+ present. Such studies work with channels that have potentiated cytosolic Ca2+ sensitivity (see Fig. 1 B). Caffeine is also known to potentiate cytosolic Ca2+ sensitivity (2). The point is that this makes channels abnormally susceptible to Ca2+ “feed through”, where Ca2+ passing through the channel acts on cytosolic Ca2+ sites. This makes it difficult to distinguish true luminal Ca2+ regulation from feed-through Ca2+ effects. Here, this situation is minimized because the cytosolic Ca2+ sensitivity of our channels is closer to that present in cells. Also, our lumen-to-cytosol Ca2+ flux is relatively small (∼0.2 pA; see Methods). This is less than the smallest lumen-to-cytosol Ca2+ flux (0.25 pA) reported to activate single caffeine-activated RyR2 channels (41) and about half the Ca2+ flux amplitude thought to occur normally in cells (49). Further, we observed substantial changes in RyR2 channel function (with or without CSQ present) when the same Ca2+ flux was present. Thus, we do not believe that Ca2+ feed through substantially contributes to the luminal regulatory effects reported here.
In cardiac muscle cells, a single action potential (AP) triggers RyR2 opening that may liberate up to ∼50% of the releasable intra-SR Ca2+ (46,48). This will result in a substantial fall in the intra-SR free Ca2+ concentration and represents a reasonably large signal that can drive an RyR2 luminal Ca2+ regulatory mechanism (14,26,50,51). However, the situation is different in skeletal muscle. In skeletal muscle, a single AP is thought to liberate a much smaller fraction (∼10%) of the releasable intra-SR Ca2+ (47,52). This will result in a relatively small change in the intra-SR free Ca2+ concentration and thus a smaller intra-SR Ca2+ signal to drive an RyR1 luminal Ca2+ regulatory mechanism (after an AP). The implication is that CSQ-dependent luminal RyR regulation is likely to be more important in cardiac than skeletal muscle. Our results support this possibility. A similar interpretation has also been put forward in a recent review article (53). Nevertheless, there is evidence that luminal Ca2+ does regulate the RyR1 channel (15,17,54,55), and this is discussed below. First, however, we discuss the role of CSQ and luminal Ca2+ in the regulation of RyR2 channels.
CSQ-dependent RyR2 regulation
Our results contribute to a growing consensus that CSQ2-dependent luminal Ca2+ RyR2 regulation exists and operates in cells (13,14,25,31,36,50). Our results are consistent with those of most published works. For example, Gyorke and Gyorke (18) reported luminal Ca2+ regulation (KD = 2.2 mM) of native canine RyR2 channels that were activated by cytosolic ATP (no Mg2+, 1 μM Ca2+). We demonstrate a similar luminal Ca2+ regulation (KD = 0.687 mM) of native rat RyR2 channels that were activated only by 1 μM of cytosolic Ca2+ (14). The different KD values are likely a consequence of the different species and/or cytosolic activation methods used. Gyorke et al. (36) showed that a luminal Ca2+ change from 20 μM to 5 mM elevated the Po from ∼0.08 to ∼0.40. They used native RyR2 channels that were activated by 6 μM cytosolic Ca2+ with cytosolic MgATP present. They also showed that removal of RyR2-associated proteins (e.g., TD and JC) disrupted the regulation and that replacing these proteins restored it. Comparing the Gyorke et al. (36) Po points (described above) with ours plotted in Fig. 3 B reveals a remarkable agreement. One apparent disagreement between our results and those already published revolves around how the acute addition of CSQ2 to the luminal bath affects the single RyR2 Po. Gyorke et al. (36) reported that CSQ2 addition reduced the Po of RyR2 channels (∼0.31 to ∼0.03 with 20 μM luminal Ca2+ present). In Fig. 3 B, we show that there is little (if any) change in Po upon CSQ2 addition at a similarly low luminal Ca2+ concentration (50 μM). However, our channels were activated by just 0.75 μM cytosolic Ca2+, and the Po was already quite low when we added CSQ2. We believe that the discrepancy stems from the initial activation status of the CSQ-free channel. If the channel is activated to an extent greater than that dictated by the applicable CSQ-dependent luminal Ca2+-Po relationship, then adding CSQ will inhibit, as observed by Gyorke et al. (36). If the channel is activated to a degree below the relationship, then adding CSQ will activate, as observed in this study.
Although the mechanistic details are still being debated, there is evidence that TD and JC proteins are somehow involved in CSQ2-dependent luminal Ca2+ RyR2 regulation (36,50). These proteins are integral SR proteins and are therefore likely associated with the RyR2 channels we examined here. Indeed, Chaps solubilization of RyR channels (which likely removes TD and JC) is known to make channels insensitive to CSQ-dependent luminal Ca2+ regulation (36,54). Our affinity binding results indicate that CSQ2 binds to cardiac TD (but not cardiac JC), consistent with the results of Terentyev et al. (50). Our binding studies also show that CSQ1 does not interact with cardiac TD (Fig. 1 A), whereas our single-channel results show that both CSQ isoforms regulate RyR2 channels (Fig. 3 B). A possible explanation is that the sensitivity of our binding assay was not adequate to detect an interaction between CSQ1 and cardiac TD. Another possibility is that CSQ1 regulation of RyR2 is TD-independent, as proposed previously for CSQ1 regulation of RyR1 (56,57). Indeed, more recent works have presented evidence that TD is not essential for normal EC coupling (58–60). Knollmann (59) proposes that TD acts to maintain the structural, and thus functional, integrity of the RyR2 release unit and helps anchor CSQ in the junctional SR (jSR). Yet some CSQ is retained in the jSR of TD knockout mice. This could be because CSQ binds to the residual JC present in these mice (61), directly to RyR2 (60), or to some other jSR protein. Our results suggest that one of the latter possibilities is the case. Note that our results indicate that both CSQ isoforms regulate RyR2 in a similar way (Table 2), suggesting that they act through a common mechanism.
Perhaps the most comprehensive study (thus far) on the interactions of CSQ2 with TD and JC is that of Zhang et al. (31). That study showed that canine cardiac JC and CSQ2 interact when 1 mM Ca2+ is present. As mentioned above, this interaction was not observed in our study. This could be due to the different species used, the different SR preparation methods used, and/or the different quantities of protein used in the CSQ-affinity chromatography. Recently, it was shown that animals deficient in cardiac CSQ, TD, or JC are viable and have no gross abnormalities in their normal (unstressed) cardiac function (25,62–64). This implies either that cardiac CSQ, TD, and JC are not vital to normal RyR2 function, and/or that the animals in the studies have compensated well for the deficiencies. In any event, this and the discrepancies described above, highlight our unsettled and poor understanding of the RyR-CSQ-TD-JC functional interaction.
CSQ-dependent RyR1 regulation
A key conclusion from our study is that CSQ-dependent luminal Ca2+ regulation of single RyR2 channels is very different from that of RyR1 channels. Indeed, we observed no significant CSQ-dependent luminal Ca2+ regulation of RyR1 channels in experimental conditions where RyR2 channels were substantially regulated. This result is not likely due to some failure of the CSQ removal process, since the effectiveness of the process is well established (14,17,28,36). Even if endogenous CSQ1 remained associated with the RyR1 channel (i.e., the removal process failed), our primary observation that there is no CSQ1-dependent luminal Ca2+ RyR1 regulation is still valid, because either way (endogenously retained or exogenously replaced), CSQ1 is present and the channel was insensitive to the luminal Ca2+. If there is CSQ1-dependent RyR1 regulation and we were simply unable to detect it, then that mechanism must be substantially different from the mechanism in cardiac muscle. We believe that the primary role of CSQ1 in skeletal muscle is as an intra-SR Ca2+ buffer (65) and not as an RyR1 regulator.
CSQ1-dependent regulation of single RyR1 channels has been reported in bilayers, elsewhere (17,37,54). Szegedi et al. (37) showed that adding ∼15 μM dephosphorylated CSQ1 to single purified RyR1 channels increased their Po, but that the same amount of phosphorylated CSQ1 did not do so. Beard et al. (17) reported that addition of ∼0.4 μM CSQ1 to RyR1 channels decreased the Po. Here, CSQ1 addition (0.1 μM) evoked no detectable change in RyR1 Po. One possible explanation for our result is that our CSQ1 concentration was too low. We do not believe this is the case, because 1), the CSQ we applied was sufficient to substantially alter single RyR2 function, 2), doubling CSQ concentration generated no additional effect, and, 3) even the 0.1 μM CSQ1 concentration applied here should provide ample protein for the high-affinity CSQ1-RyR1 interaction (56). We believe that the most likely explanation for the disparate results lies in the different methods of RyR1 channel activation used in the different studies. For example, RyR1 channels activated by ATP (no Mg2+), as in the Beard et al. (17) study, and those activated by just a low cytosolic Ca2+ level, as done in this study, might be expected to respond to regulatory challenges differently. The likely reason for the discrepancy between our study and the Szegedi et al. (37) results is that we used native RyR1 channels, which are likely still associated with TD and JC, whereas they used CHAPS-purified channels, which likely lack these potentially important proteins. Wang et al. (54) also showed that CSQ1 enhances RyR channel activity. They examined CSQ1-dependent Ca2+ regulation of RyR1/RyR3 channels isolated from C2C12 myotubes. The apparent disparity between our results and theirs could be due to the presence of RyR3 channels in their preparation, since in our study only RyR1 channels were examined.
Fast-twitch skeletal muscle contains primarily CSQ1, whereas slow-twitch muscle contains both CSQ1 and CSQ2 (15). Slow-twitch muscle also contains other cardiac isoforms (e.g., SR ATPase, troponin C, etc.) (66). Here, we show that there was no significant difference in single RyR1 channel luminal Ca2+ regulation when CSQ1 or CSQ2 were present. Thus, the CSQ2 is not likely to make RyR1 channels in slow-twitch muscles operate differently than they do in fast-twitch muscles. The CSQ2 could, however, change the intra-SR Ca2+ buffer properties or it could be interacting with the RyR3 channels that are present (67).
Finally, an analogous CSQ isoform swap study on ATP-activated channels was recently published (60). The authors report that the RyR2 Po is increased by both CSQ isoforms, that CSQ2 dissociation reduces RyR2 Po, and that RyR1 Po is modestly elevated by CSQ2. These findings are consistent with our results. Wei et al. (60) also report that CSQ1 inhibits RyR1 and a similar CSQ2-associated RyR2 Po at 100 nM and 1 mM luminal Ca2+ concentration. This is contrary to our results as well as to other published reports (e.g., (14,18,22)). We detected no CSQ1 action on RyR1 channels, although previous studies have detected such action (e.g., (17,19,37)). This could be due to differences in species, membrane potentials, and/or—of most importance—how the channels are activated. Since several studies have reported that CSQ action depends on the experimental conditions used (e.g., (18,68,73)), we believe that this is the primary cause of the apparent discrepancies outlined above.
Supporting Material
Methods and a figure are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(09)01293-4.
Supporting Material
Acknowledgments
This work was supported by National Institutes of Health grants HL57832 and AR54098 (to M.F.) and HL71741 (to J.R.F), and by a Telethon grant (GGP04066) to P.V.
References
- 1.Bers D. Kluwer Academic,Dordrecht; The Netherlands: 2001. Excitation-Contraction Coupling and Cardiac Contractile Force. [Google Scholar]
- 2.Fill M., Copello J. Ryanodine receptor calcium release channels. Physiol. Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 3.Jorgensen A.O., Shen A.C., Campbell K.P., MacLennan D.H. Ultrastructural localization of calsequestrin in rat skeletal muscle by immunoferritin labeling of ultrathin frozen sections. J. Cell Biol. 1983;97:1573–1581. doi: 10.1083/jcb.97.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franzini-Armstrong C., Kenney L.J., Varriano-Marston E. The structure of calsequestrin in triads of vertebrate skeletal muscle: a deep-etch study. J. Cell Biol. 1987;105:49–56. doi: 10.1083/jcb.105.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLennan D.H., Wong P.T. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 1971;68:1231–1235. doi: 10.1073/pnas.68.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fliegel L., Ohnishi M., Carpenter M.R., Khanna V.K., Reithmeier R.A. Amino acid sequence of rabbit fast-twitch skeletal muscle calsequestrin deduced from cDNA and peptide sequencing. Proc. Natl. Acad. Sci. USA. 1987;84:1167–1171. doi: 10.1073/pnas.84.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho J.H., Oh Y.S., Park K.W., Yu J., Choi K.Y. Calsequestrin, a calcium sequestering protein localized at the sarcoplasmic reticulum, is not essential for body-wall muscle function in Caenorhabditis elegans. J. Cell Sci. 2000;113:3947–3958. doi: 10.1242/jcs.113.22.3947. [DOI] [PubMed] [Google Scholar]
- 8.Arai M., Otsu K., MacLennan D.H., Alpert N.R., Periasamy M. Effect of thyroid hormone on the expression of mRNA encoding sarcoplasmic reticulum proteins. Circ. Res. 1991;69:266–276. doi: 10.1161/01.res.69.2.266. [DOI] [PubMed] [Google Scholar]
- 9.Fujii J., Willard H.F., MacLennan D.H. Characterization and localization to human chromosome 1 of human fast-twitch skeletal muscle calsequestrin gene. Somat. Cell Mol. Genet. 1990;16:185–189. doi: 10.1007/BF01233048. [DOI] [PubMed] [Google Scholar]
- 10.Scott B., Simmerman H., Collins J., Nadal-Ginard B., Jones L. Complete amino acid sequence of canine cardiac calsequestrin deduced by cDNA cloning. J. Biol. Chem. 1988;263:8958–8964. [PubMed] [Google Scholar]
- 11.Divet A., Paesante S., Grasso C., Cavagna D., Tiveron C. Increased Ca2+ storage capacity of the skeletal muscle sarcoplasmic reticulum of transgenic mice over-expressing membrane bound calcium binding protein junctate. J. Cell. Physiol. 2007;213:464–474. doi: 10.1002/jcp.21121. [DOI] [PubMed] [Google Scholar]
- 12.di Barletta M.R., Viatchenko-Karpinski S., Nori A., Memmi M., Terentyev D. Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2006;114:1012–1019. doi: 10.1161/CIRCULATIONAHA.106.623793. [DOI] [PubMed] [Google Scholar]
- 13.Lahat H., Pras E., Olender T., Avidan N., Ben-Asher E. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am. J. Hum. Genet. 2001;69:1378–1384. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin J., Valle G., Nani A., Nori A., Rizzi N. Luminal Ca2+ regulation of single cardiac ryanodine receptors: insights provided by calsequestrin and its mutants. J. Gen. Physiol. 2008;131:325–334. doi: 10.1085/jgp.200709907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paolini C., Quarta M., Nori A., Boncompagni S., Canato M. Reorganized stores and impaired calcium handling in skeletal muscle of mice lacking calsequestrin-1. J. Physiol. 2007;583:767–784. doi: 10.1113/jphysiol.2007.138024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yano K., Zarain-Herzberg A. Sarcoplasmic reticulum calsequestrins: structural and functional properties. Mol. Cell. Biochem. 1994;135:61–70. doi: 10.1007/BF00925961. [DOI] [PubMed] [Google Scholar]
- 17.Beard N., Sakowska M., Dulhunty A., Laver D. Calsequestrin is an inhibitor of skeletal muscle ryanodine receptor calcium release channels. Biophys. J. 2002;82:310–320. doi: 10.1016/S0006-3495(02)75396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gyorke I., Gyorke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys. J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikemoto N., Ronjat M., Mészáros L.G., Koshita M. Postulated role of calsequestrin in the regulation of calcium release from sarcoplasmic reticulum. Biochemistry. 1989;28:6764–6771. doi: 10.1021/bi00442a033. [DOI] [PubMed] [Google Scholar]
- 20.Laver D.R., O'Neill E.R., Lamb G.D. Luminal Ca2+-regulated Mg2+ inhibition of skeletal RyRs reconstituted as isolated channels or coupled clusters. J. Gen. Physiol. 2004;124:741–758. doi: 10.1085/jgp.200409092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sitsapesan R., Williams A. The gating of the sheep skeletal sarcoplasmic reticulum Ca2+-release channel is regulated by luminal Ca2+ J. Membr. Biol. 1995;146:133–144. doi: 10.1007/BF00238004. [DOI] [PubMed] [Google Scholar]
- 22.Terentyev D., Viatchenko-Karpinski S., Valdivia H., Escobar A., Gyorke S. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ. Res. 2002;91:414–420. doi: 10.1161/01.res.0000032490.04207.bd. [DOI] [PubMed] [Google Scholar]
- 23.Tripathy A., Meissner G. Sarcoplasmic reticulum lumenal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophys. J. 1996;70:2600–2615. doi: 10.1016/S0006-3495(96)79831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu L., Meissner G. Regulation of cardiac muscle Ca2+ release channel by sarcoplasmic reticulum lumenal Ca2+ Biophys. J. 1998;75:2302–2312. doi: 10.1016/S0006-3495(98)77674-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knollmann B.C., Chopra N., Hlaing T., Akin B., Yang T. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J. Clin. Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terentyev D., Nori A., Santoro M., Viatchenko-Karpinski S., Kubalova Z. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ. Res. 2006;98:1151–1158. doi: 10.1161/01.RES.0000220647.93982.08. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi Y., Alseikhan B., Jones L. Localization and characterization of the calsequestrin-binding domain of triadin 1. Evidence for a charged β-strand in mediating the protein-protein interaction. J. Biol. Chem. 2000;275:17639–17646. doi: 10.1074/jbc.M002091200. [DOI] [PubMed] [Google Scholar]
- 28.Beard N., Casarotto M., Wei L., Varsanyi M., Laver D. Regulation of ryanodine receptors by calsequestrin: effect of high luminal Ca2+ and phosphorylation. Biophys. J. 2005;88:3444–3454. doi: 10.1529/biophysj.104.051441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowery O.H., Rosenbrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 30.Saito A., Seiler S., Chu A., Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J. Cell Biol. 1984;99:875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L., Kelley J., Schmeisser G., Kobayashi Y., Jones L. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J. Biol. Chem. 1997;272:23389–23397. doi: 10.1074/jbc.272.37.23389. [DOI] [PubMed] [Google Scholar]
- 32.Shin D.W., Ma J., Kim D.H. The Asp-rich region at the carboxyl-terminus of calsequestrin binds to Ca(2+) and interacts with triadin. FEBS Lett. 2000;486:178–182. doi: 10.1016/s0014-5793(00)02246-8. [DOI] [PubMed] [Google Scholar]
- 33.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Sumida M., Wang T., Mandel F., Froehlich J.P., Schwartz A. Transient kinetics of Ca2+ transport of sarcoplasmic reticulum. A comparison of cardiac and skeletal muscle. J. Biol. Chem. 1978;253:8772–8777. [PubMed] [Google Scholar]
- 35.Tu Q., Velez P., Cortes-Gutierrez M., Fill M. Surface charge potentiates conduction through the cardiac ryanodine receptor channel. J. Gen. Physiol. 1994;103:853–867. doi: 10.1085/jgp.103.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gyorke I., Hester N., Jones L., Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys. J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szegedi C., Sarkozi S., Herzog A., Jona I., Varsanyi M. Calsequestrin: more than “only” a luminal Ca2+ buffer inside the sarcoplasmic reticulum. Biochem. J. 1999;337:19–22. [PMC free article] [PubMed] [Google Scholar]
- 38.Gillespie D., Xu L., Wang Y., Meissner G. (De)constructing the ryanodine receptor: modeling ion permeation and selectivity of the calcium release channel. J. Phys. Chem. B. 2005;109:15598–15610. doi: 10.1021/jp052471j. [DOI] [PubMed] [Google Scholar]
- 39.Reference deleted in proof.
- 40.Copello J.A., Barg S., Sonnleitner A., Porta M., Diaz-Sylvester P. Differential activation by Ca2+, ATP and caffeine of cardiac and skeletal muscle ryanodine receptors after block by Mg2+ J. Membr. Biol. 2002;187:51–64. doi: 10.1007/s00232-001-0150-x. [DOI] [PubMed] [Google Scholar]
- 41.Xu L., Mann G., Meissner G. Regulation of cardiac Ca2+ release channel (ryanodine receptor) by Ca2+, H+, Mg2+, and adenine nucleotides under normal and simulated ischemic conditions. Circ. Res. 1996;79:1100–1109. doi: 10.1161/01.res.79.6.1100. [DOI] [PubMed] [Google Scholar]
- 42.Sonnleitner A., Fleischer S., Schindler H. Gating of the skeletal calcium release channel by ATP is inhibited by protein phosphatase 1 but not by Mg2+ Cell Calcium. 1997;21:283–290. doi: 10.1016/s0143-4160(97)90116-0. [DOI] [PubMed] [Google Scholar]
- 43.Chopra N., Laver D., Davies S.S., Knollmann B.C. Amitriptyline activates cardiac ryanodine channels and causes spontaneous sarcoplasmic reticulum calcium release. Mol. Pharmacol. 2009;75:183–195. doi: 10.1124/mol.108.051490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosales R.A., Fill M., Escobar A.L. Calcium regulation of single ryanodine receptor channel gating analyzed using HMM/MCMC statistical methods. J. Gen. Physiol. 2004;123:533–553. doi: 10.1085/jgp.200308868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saftenku E., Williams A.J., Sitsapesan R. Markovian models of low and high activity levels of cardiac ryanodine receptors. Biophys. J. 2001;80:2727–2741. doi: 10.1016/S0006-3495(01)76241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bers D.M. Macromolecular complexes regulating cardiac ryanodine receptor function. J. Mol. Cell. Cardiol. 2004;37:417–429. doi: 10.1016/j.yjmcc.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 47.Launikonis B.S., Zhou J., Royer L., Shannon T.R., Brum G. Depletion “skraps” and dynamic buffering inside the cellular calcium store. Proc. Natl. Acad. Sci. USA. 2006;103:2982–2987. doi: 10.1073/pnas.0511252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shannon T., Ginsburg K., Bers D. Potentiation of fractional sarcoplasmic reticulum calcium release by total and free intra-sarcoplasmic reticulum calcium concentration. Biophys. J. 2000;78:334–343. doi: 10.1016/S0006-3495(00)76596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kettlun C., Gonzalez A., Rios E., Fill M. Unitary Ca2+ current through mammalian cardiac and amphibian skeletal muscle ryanodine receptor channels under near-physiological ionic conditions. J. Gen. Physiol. 2003;122:407–417. doi: 10.1085/jgp.200308843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terentyev D., Viatchenko-Karpinski S., Vedamoorthyrao S., Oduru S., Györke I. Protein protein interactions between triadin and calsequestrin are involved in modulation of sarcoplasmic reticulum calcium release in cardiac myocytes. J. Physiol. 2007;583:71–80. doi: 10.1113/jphysiol.2007.136879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chopra N., Kannankeril P.J., Yang T., Hlaing T., Holinstat I. Modest reductions of cardiac calsequestrin increase sarcoplasmic reticulum Ca2+ leak independent of luminal Ca2+ and trigger ventricular arrhythmias in mice. Circ. Res. 2007;101:617–626. doi: 10.1161/CIRCRESAHA.107.157552. [DOI] [PubMed] [Google Scholar]
- 52.Launikonis B.S., Zhou J., Royer L., Shannon T.R., Brum G. Confocal imaging of [Ca2+] in cellular organelles by SEER, shifted excitation and emission ratioing of fluorescence. J. Physiol. 2005;567:523–543. doi: 10.1113/jphysiol.2005.087973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ríos E., Launikonis B.S., Royer L., Brum G., Zhou J. The elusive role of store depletion in the control of intracellular calcium release. J. Muscle Res. Cell Motil. 2006;27:337–350. doi: 10.1007/s10974-006-9082-5. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y., Xu L., Duan H., Pasek D.A., Eu J.P. Knocking down type 2 but not type 1 calsequestrin reduces calcium sequestration and release in C2C12 skeletal muscle myotubes. J. Biol. Chem. 2006;281:15572–15581. doi: 10.1074/jbc.M600090200. [DOI] [PubMed] [Google Scholar]
- 55.Wei L., Varsányi M., Dulhunty A.F., Beard N.A. The conformation of calsequestrin determines its ability to regulate skeletal ryanodine receptors. Biophys. J. 2006;91:1288–1301. doi: 10.1529/biophysj.106.082610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herzog A., Szegedi C., Jona I., Herberg F., Varsanyi M. Surface plasmon resonance studies prove the interaction of skeletal muscle sarcoplasmic reticular Ca(2+) release channel/ryanodine receptor with calsequestrin. FEBS Lett. 2000;472:73–77. doi: 10.1016/s0014-5793(00)01431-9. [DOI] [PubMed] [Google Scholar]
- 57.Murray B.E., Ohlendieck K. Complex formation between calsequestrin and the ryanodine receptor in fast- and slow-twitch rabbit skeletal muscle. FEBS Lett. 1998;429:317–322. doi: 10.1016/s0014-5793(98)00621-8. [DOI] [PubMed] [Google Scholar]
- 58.Allen P.D. Triadin, not essential, but useful. J. Physiol. 2009;587:3123–3124. doi: 10.1113/jphysiol.2009.172015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knollmann B.C. New roles of calsequestrin and triadin in cardiac muscle. J. Physiol. 2009;587:3081–3087. doi: 10.1113/jphysiol.2009.172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei L., Hanna A.D., Beard N.A., Dulhunty A.F. Unique isoform-specific properties of calsequestrin in the heart and skeletal muscle. Cell Calcium. 2009;45:474–484. doi: 10.1016/j.ceca.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 61.Chopra N., Yang T., Asghari P., Moore E.D., Huke S. Ablation of triadin causes loss of cardiac Ca2+ release units, impaired excitation-contraction coupling, and cardiac arrhythmias. Proc. Natl. Acad. Sci. USA. 2009;106:7636–7641. doi: 10.1073/pnas.0902919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong C., Kwon S., Kim D.H. Multiple functions of junctin and junctate, two distinct isoforms of aspartyl β-hydroxylase. Biochem. Biophys. Res. Commun. 2007;362:1–4. doi: 10.1016/j.bbrc.2007.07.166. [DOI] [PubMed] [Google Scholar]
- 63.Shen X., Franzini-Armstrong C., Lopez J.R., Jones L.R., Kobayashi Y.M. Triadins modulate intracellular Ca(2+) homeostasis but are not essential for excitation-contraction coupling in skeletal muscle. J. Biol. Chem. 2007;282:37864–37874. doi: 10.1074/jbc.M705702200. [DOI] [PubMed] [Google Scholar]
- 64.Yuan Q., Fan G., Dong M., Altschafl B., Diwan A. Sarcoplasmic reticulum calcium overloading in junctin deficiency enhances cardiac contractility but increases ventricular automaticity. Circulation. 2007;115:300–309. doi: 10.1161/CIRCULATIONAHA.106.654699. [DOI] [PubMed] [Google Scholar]
- 65.Pape P.C., Fénelon K., Lamboley C.R.H., Stachura D. Role of calsequestrin evaluated from changes in free and total calcium concentrations in the sarcoplasmic reticulum of frog cut skeletal muscle fibres. J. Physiol. 2007;581:319–367. doi: 10.1113/jphysiol.2006.126474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sukovich D.A., Shabbeer J., Periasamy M. Analysis of the rabbit cardiac/slow twitch muscle sarcoplasmic reticulum calcium ATPase (SERCA2) gene promoter. Nucleic Acids Res. 1993;21:2723–2728. doi: 10.1093/nar/21.11.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Protasi F., Takekura H., Wang Y., Chen S.R., Meissner G. RYR1 and RYR3 have different roles in the assembly of calcium release units of skeletal muscle. Biophys. J. 2000;79:2494–2508. doi: 10.1016/S0006-3495(00)76491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laver D.R. Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. Clin. Exp. Pharmacol. Physiol. 2007;34:889–896. doi: 10.1111/j.1440-1681.2007.04708.x. [DOI] [PubMed] [Google Scholar]
- 69.Sitsapesan R., Williams A.J. Regulation of the gating of the sheep cardiac sarcoplasmic reticulum Ca(2+)-release channel by luminal Ca2+ J. Membr. Biol. 1994;137:215–226. doi: 10.1007/BF00232590. [DOI] [PubMed] [Google Scholar]
- 70.Zoghbi M.E., Copello J.A., Villalba-Galea C.A., Vélez P., Diaz Sylvester P.L. Differential Ca2+ and Sr2+ regulation of intracellular divalent cations release in ventricular myocytes. Cell Calcium. 2004;36:119–134. doi: 10.1016/j.ceca.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 71.Meissner G. Regulation of mammalian ryanodine receptors. Front. Biosci. 2002;7:d2072–d2080. doi: 10.2741/A899. [DOI] [PubMed] [Google Scholar]
- 72.Sárközi S., Szegedi C., Szentesi P., Csernoch L., Kovács L. Regulation of the rat sarcoplasmic reticulum calcium release channel by calcium. J. Muscle Res. Cell Motil. 2000;21:131–138. doi: 10.1023/a:1005630321863. [DOI] [PubMed] [Google Scholar]
- 73.Laver, D. 2009. Luminal Ca(2+) activation of cardiac ryanodine receptors by luminal and cytoplasmic domains. Eur. Biophys. J. http://www.ncbi.nlm.nih.gov/pubmed/19255753. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


