Abstract
Large-conductance Ca2+-activated K+ (BK) channels can regulate cellular excitability in complex ways because they are able to respond independently to two distinct cellular signals, cytosolic Ca2+ and membrane potential. In rat chromaffin cells (RCC), inactivating BKi and noninactivating (BKs) channels differentially contribute to RCC action potential (AP) firing behavior. However, the basis for these differential effects has not been fully established. Here, we have simulated RCC action potential behavior, using Markovian models of BKi and BKs current and other RCC currents. The analysis shows that BK current influences both fast hyperpolarization and afterhyperpolarization of single APs and that, consistent with experimental observations, BKi current facilitates repetitive firing of APs, whereas BKs current does not. However, the key functional difference between BKi and BKs current that accounts for the differential firing is not inactivation but the more negatively shifted activation range for BKi current at a given [Ca2+].
Introduction
Ca2+- and voltage-dependent K+ (BK) channels are widely expressed ion channels, but our understanding of the precise role that BK channels play in regulating cellular excitability remains incomplete. The specific role that BK channels play in sculpting cellular electrical activity often depends not only on the specific properties of the BK channels, but also on aspects of Ca2+ regulation and other ion channels. Among different cells, BK channels exhibit substantial functional differences, arising from factors that include alternative splice variants of the α subunit (1–3), tissue-specific expression of auxiliary β subunits (4), splice-variant-specific covalent modifications (5), and perhaps cell-specific association with other proteins (6). As a consequence, BK channels can differ in apparent Ca2+ dependence and kinetic properties, such that cells may differ with regard to the rates of BK-channel-mediated repolarization, rapidity of BK channel activation, and durations and magnitude of Ca2+-mediated afterhyperpolarizations. It has been shown that among different cell types, BK channels promote the occurrence of plateau potentials (7), participate in action potential broadening (8), promote repetitive firing (9,10), and contribute to action potential repolarization and brief afterhyperpolarizations (8,9). Yet how specific biophysical properties of BK channels in a given cell may account for specific effects on excitability remains a topic of investigation. For example, dependent on the Ca2+ sensitivity of BK channels in a given cell, the range of voltages and submembrane Ca2+ found in the cell, and other voltage-dependent K+ conductances, the physiological consequences of BK activation might differ in substantive ways. Thus, the functional role of BK channels in any given cell will depend not only on BK channel properties but also on the interplay of BK currents with other conductances that together shape the time course and extent of voltage trajectories and Ca2+ elevations.
Rat adrenal medullary chromaffin cells (RCCs) provide an interesting system in which to examine the potential physiological roles of different BK channel variants. The repetitive firing of action potentials (APs) in RCCs leads to the elevation of intracellular Ca2+, which triggers secretion of catecholamines (11,12). Furthermore, the specific firing patterns of APs may influence secretory responses (13) and may be physiologically regulated (10,14). Previous work has shown that, dependent on the presence of two specific BK channel variants, an inactivating variant (termed BKi) and a noninactivating variant (termed BKs), RCCs exhibit differential firing behaviors in response to current injection (9,15). For RCCs, an essentially full set of ionic conductances has been identified that includes a TASK-mediated leak K+ conductance (16), voltage-dependent Na+ current (17), voltage-dependent Ca2+ current (18), voltage-dependent K+ current (19–21), small-conductance Ca2+-dependent SK-type K+ current (22,23), and the two distinct types of BK current (9,24). Although complete kinetic descriptions of all currents are not available, each current has been sufficiently well described that simplified kinetic models can be defined that provide reasonable recapitulations of the native currents. Thus, RCCs provide a system in which the specific contributions of different currents to electrical behavior can be examined in a computational fashion.
Here, we provide empirical descriptions of the known components of RCC membrane currents and utilize them in simulations of the voltage behavior of an idealized RCC under current-clamp. We compare two cell models, one with BKi current and one with BKs current. The simulated voltage behaviors demonstrate that, consistent with the behavior of native RCCs, model cells with BKi currents are better able to fire repetitively during depolarizing constant-current injection, whereas model cells with BKs currents fire in a phasic fashion. Furthermore, this analysis establishes that BKi current supports repetitive firing not because of inactivation, but because of a more negatively shifted range of activation of BKi channels compared to BKs channels.
Materials and Methods
Chromaffin cell preparation
Rat chromaffin cells were isolated as described previously (22). Briefly, chromaffin cells were dispersed from adrenal medullas isolated from two to three rats aged ∼2–3 months. Isolated cells were cultured with Dulbecco's modified Eagle's medium (DMEM) in a standard CO2 incubator at 37°C and 5% CO2 and used for experiments in 2–6 days.
Expression in Xenopus oocytes
Xenopus laevis oocytes (stage IV) were harvested for injection as described in previous work (25). The mSlo1 α and β2 auxiliary subunits were identical to those described in previous work (25,26). A total of 0.1–0.5 ng cRNA was injected into stage IV Xenopus oocytes with a ratio of α/β2 = 1:2 by weight, ensuring a large molar excess of β subunit RNA. Oocytes were used 3–5 days after injection of cRNA.
Electrophysiology
Whole-cell recordings from RCCs utilized the perforated-patch method (27). Series resistance was in the range 8–15 MΩ, of which 80–90% was electronically compensated. For BK channel recordings in oocytes, inside-out patches were bathed with constantly flowing solutions of defined Ca2+ from a multibarrel local application system. All currents were typically digitalized at 20 kHz and filtered at 5 kHz (Bessel low-pass filter; −3 dB) during digitization. For action potential experiments, all recordings were performed using the current-clamp capabilities of the Axopatch amplifier. All experiments were performed at room temperature (22–25°C).
Solutions
Electrophysiological recordings of BK channels were performed after 2–7 days incubation of oocytes in ND-96 (containing (in mM) 96 NaCl, 2.0 KCl, 1.8 CaCl2, 1.0 MgCl2, and 5.0 HEPES, pH 7.5) supplemented with sodium pyruvate (2.5 mM), penicillin (100 U/ml), streptomycin (100 mg/ml), and gentamicin (50 mg/ml). For inside-out patch recordings, the pipette extracellular solution contained (in mM) 140 potassium methanesulphonate, 20 KOH, 10 HEPES, and 2 MgCl2, pH 7.0. The cytosolic solution contained (in mM) 140 potassium methanesulphonate, 20 KOH, and 10 HEPES, pH 7.0, and one of the following (in mM): 5 EGTA (for nominally 0 Ca2+, and 0.5-, 1-, and 4-μM Ca2+ solutions), 5 HEDTA (for 10 μM Ca2+ solution), 10 HEDTA (for 60 μM Ca2+ solution) or no added Ca2+ buffer (for 100 μM and higher Ca2+), as defined by the EGTAETC program (E. McCleskey, Vollum Institute, Portland, OR).
The normal bath saline for perforated patch-clamp experiments contained (in mM) 150 NaCl, 5.4 KCl, 10 HEPES, 1.8 CaCl2, and 2.0 MgCl2, pH 7.4, adjusted with N-methylglucamine. For the 0 Ca2+ normal saline, 2 mM Mg2+ was substituted for 1.8 mM Ca2+ into normal saline. For perforated patch-clamp recordings, the pipette solution contained (in mM), as described previously (28), 120 K-aspartate, 30 KCl, 10 HEPES (H+), and 2 MgCl2 titrated to pH 7.4 with N-methylglucamine, with amphotericin and pluronic acid included for permeabilization (27). For all RCC recordings, 200 nM apamin was added to extracellular solutions to block small-conductance Ca2+-activated K+ (SK) currents. In a similar way, 200 nM tetrodotoxin was used to reduce voltage-dependent Na+ current. All salts and chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Data analysis
Data were analyzed with IGOR (Wavemetrics, Lake Oswego, OR), Clampfit (Molecular Devices, Sunnyvale, CA), SigmaPlot (SPSS Science, Chicago, IL). The fractional value of activation or steady-state inactivation was generated from the peak value of inactivating current or the steady-state value for noninactivating current and fit with a single Boltzmann function of the form
| (1) |
where V50 is the voltage at which the f(V) = 0.5, and κ is a factor reflecting the steepness of the activation curve or the steady-state inactivation curve. Fractional recovery curves of inactivating currents were fitted to an exponential function as follows:
| (2) |
Here τr is the recovery time constant. Unless stated otherwise, all data are presented as the mean ± SD.
Mathematical modeling and simulations
The differential equations for the kinetic modeling were solved numerically, using a fifth-order Runge-Kutta integration method. The integrating routines were written and executed with software CeL (Huazhong University of Science and Technology, Wuhan, Hubei, China), compiled with the C++ compiler to run under Windows XP.
Results
We begin with empirical definitions of the kinetic properties of several of the main current components of rat chromaffin cells, specifically including INa, ICa, IKv, BKi, and BKs currents. We omit Ca2+-dependent SK current, since experiments comparing the impact of BK current on RCC firing behavior were done in the presence of apamin (9). We begin with definitions of a model and a set of gating parameters for each current component, with the aim of providing a reasonable approximation of the native current in RCCs. We then simulate current clamp behavior during brief or more sustained current injection to determine whether the properties of the two kinds of BK current account for differences in firing behavior.
Definition of Na current
Voltage-dependent Na+ current is the predominant inward current in RCCs (11), likely arising from NaV1.7 and β1/β3 subunits (29). For RCC Na+ current, we utilized a model containing 12 states (30), the parameters of which are given in Fig. S1 of the Supporting Material. Parameters were determined based on properties of channels arising from NaV1.7 and β1/β3 subunits (see Table S1). Activation and inactivation behaviors of the simulated Na+ current are summarized in Fig. S1 B. The normalized fractional activation curve gives a half-maximal activation voltage (V50) of −17.5 mV with slope κ = 5.2 mV; for fractional availability, V50 is −39.8 mV with slope κ = 3.4 mV (Fig. S1 C). The simulated Na+ current inactivation time constant (τi) is 1.5 ms at −10 mV (Fig. S1 C). The recovery time constants at various membrane potentials (Fig. S1 D) are also consistent with previous results for Na+ currents in RCCs (11).
Definition of voltage-gated K current
The molecular identities of voltage-dependent K+ in RCCs remain incompletely defined, although multiple types of Kv channels have been described in bovine chromaffin cells (19). Some evidence suggests the involvement of two distinct subunits, Kv1.2 and Kv1.5, which may participate in mediating oxygen sensitivity of RCC K+ currents as either homomultimers or heteromultimers (20,21,31,32). Both subunits result in channels of generally similar properties (33,34), although the activation properties of each can be subject to regulation (33,35). Here, we assume a single component of Kv current with properties similar to those of Kv1.2, with a half-maximal activation voltage of V50 = −4.3 mV and an activation time constant (τa) of 6 ms at 20 mV (34,36). For the simulation of Kv current, we utilized a linear five-state activation model with parameters given in Fig. S2 A. The simulated currents yielded V50 = −0.8 mV for activation, which is close to the V50 of Kv1.2 current (Fig. S2).
Definition of Ca2+ current and cytosolic Ca2+
A variety of high-voltage-activated (HVA) calcium channels have been identified in adult RCCs (18,37). We ignore a low-voltage-activated (LVA) calcium current identified in embryonic chromaffin cells (38). To avoid iterative calculation of each HVA component, we utilize a single HVA Ca2+ current (39) whose gating model and parameters (Table S2) are defined in Fig. S3 A. Based on earlier work (18), the Vh for HVA calcium current in RCC was −3 mV (κ = 9 mV), with an activation time constant (τa) of ∼1 ms at +10 mV (18). Simulated currents (see examples in Fig. S3) yielded a V50 = 2.3 mV (κ = 6 mV).
In modeling cellular electrical properties, it is important to include a procedure for relating Ca2+ influx to cellular Ca2+ concentrations. BKi channels colocalize to some extent with HVA channels both in RCCs and elsewhere (6,18,37,40–43). Yet all BK channels in a cell may not be identically coupled to Ca2+ channels, and distinct BK channel variants may be coupled to different sets of HVA Ca2+ channels. For RCCs, the available evidence suggests that most BK channels are sufficiently close to Ca2+ channels to sense local Ca2+ increases during brief depolarizations (37). For the purposes of this study, we have assumed that activation of all BK channels by Ca2+ depends strictly on net influx and local diffusion of Ca2+ near individual Ca2+ channels. As such, we have employed a formulation first utilized by Beeler and Reuter (44) to convert Ca2+ influx to a local Ca2+ concentration:
| (3) |
where etrans is an arbitrary transfer coefficient that scales calcium influx to the calcium concentration, and ediff is the calcium diffusion coefficient.
Kinetic properties of BKs channels
Completely inactivating BKi channels and the noninactivating BKs channels largely segregate among different RCCs, although some cells of mixed phenotype can be found (9,24). The BKs current is thought to arise from channels containing only four Slo1 α subunits. BKi channels arise from the coassembly of four Slo1 α subunits and up to four auxiliary β2 subunits (26,45). We characterize BKi cells as those that express almost exclusively BKi current, whereas BKs cells are those that express almost exclusively BKs current.
The kinetic and steady-state properties of nonactivating BK current have been well studied in several laboratories (46,47). Rather than employ a full BK gating scheme (46,47), we have utilized the 10-state Monod-Wyman-Changeux kinetic model (47) shown in Fig. S4 A. This model includes four Ca2+-binding steps, with all the voltage dependence assigned to the transitions between closed and open states, i.e., the C-O equilibrium. Simulations with this model result in predicted currents (Fig. S4) that closely reproduce the features of BKs current in RCCs.
Kinetic properties of BKi channels
Although a BKi channel can have up to four β2 subunits, the average number of β2 subunits/channel in RCCs is ∼3 (24). Here, we assume that each BKi channel has four β2 subunits. This results in only a small difference in inactivation rate and level of steady-state inactivation compared to a population of native BKi channels, each containing an average of three β2 subunits (24,45). Although many properties of α + β2 currents have been defined previously (26,48), to obtain a more complete description of α + β2 currents, we recorded from excised patches from oocytes to define activation (Fig. S5 A1), steady-state inactivation (Fig. S5 A2), and deactivation (Fig. S5 A3) of these currents. The overall dependence of BKi current activation on Ca2+ and the shift in steady-state inactivation as a function of Ca2+ was defined over the range 0–60 μM Ca2+ (Fig. S5, B1 and B2) (18). The instantaneous current-voltage properties determined (Fig. S5 B3) showed that net current at +100 mV is around two times that at −100 mV (49). Inactivation (Fig. S5 C1), activation, and deactivation (Fig. S5 C2) time constants were also defined, and the properties of recovery from inactivation at different voltages were determined (Fig. S5 D).
Inactivation of BK channels mediated by β2 subunits involves a two-step process in which a preinactivated open state precedes the fully inactivated state, C-O-O∗-I, where C, O, O∗, and I are the closed, open, preinactivated-open, and inactivated states, respectively (50). Here, for simplicity, we use a one-step inactivation model (C-O-I; Fig. 1 A). An unusual aspect of BKi current inactivation is that inactivated channels can recover from inactivation without reopening (51,52). This aspect of BKi behavior is not incorporated in the model described here and might impact on the net BKi current flux during tail currents. Table 1 lists the parameters used for simulation of BKi currents. Constants for BKi activation and deactivation differ from those used for BKs simulations, because at any given [Ca2+] above ∼1 μM, activation of channels containing β2 subunits is shifted to more negative voltages than for those not containing β2 subunits (26,53).
Figure 1.
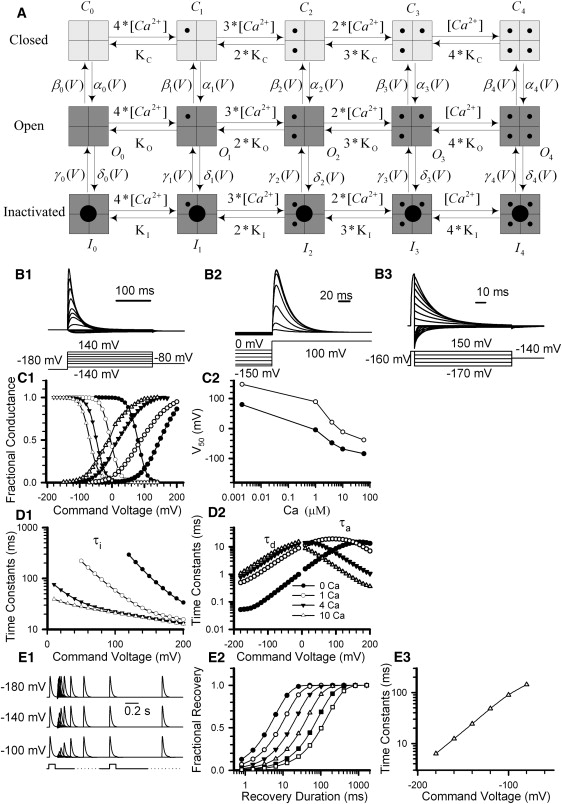
Properties of simulated BKi currents. (A) The scheme summarizes a 10-state activation model for BKi gating composed of four Ca2+ binding steps with voltage dependence assigned to the closed-to-open conformational step. The scheme includes a single-step inactivation process from the open states. Transition rates are defined in Table 1. (B) Standard voltage protocols were used to generate simulated current traces, allowing definition of activation behavior (B1), steady-state inactivation properties (B2), and deactivation behavior (B3) of BKi currents. Simulation conditions assumed symmetrical 160 mM K+ solution, and 10 μM internal Ca2+. Currents in B were scaled by the instantaneous I/V generated from native α + β2 currents (Fig. S5). (C1) Fractional conductance and fractional availability are plotted as a function of membrane potential, based on measurements of BKi currents. Solid lines are fits to the Boltzmann equation (Eq. 1). Curves correspond to Ca2+ concentrations of ∼2 nM (solid circles), 1 μM (open circles), 4 μM (inverted triangles) and 10 μM (triangles). (C2) The V50 of activation (open circles) and steady-state inactivation (solid circles) are plotted as a function of [Ca2+] based on the simulated BKi currents. The V50 values for activation and steady-state inactivation are 147.4 and 79.9 mV in 2 nM Ca2+, 89.4 and −4.5 mV in 1 μM Ca2+, 20.7 and −48.0 mV in 4 μM Ca2+, −11.9 and −68.3 mV in 10 μM Ca2+, and −38.2 and −84.0 mV in 60 μM Ca2+. (D1) Inactivation time constants (τi) of simulated BKi current are plotted as a function of voltage for 2 nM (solid circles), 1 μM (open circles), 4 μM (inverted triangles), and 10 μM (triangles). (D2) Deactivation time constants (τd) and activation time constants (τa) are plotted as a function of potential for 2 nM (solid circles), 1 μM (open circles), 4 μM (inverted triangles), and 10 μM (triangles). (E1) Traces show the time course of recovery from inactivation of simulated BKi channels elicited by a paired-pulse protocol (activation steps to 100 mV) separated by steps of different duration to −180, −140, and −100 mV. (E2) The fractional recovery of BKi channels is plotted as a function of recovery duration for −180, −160, −140, −120, −100, and −80 mV in 10 μM Ca2+. Solid lines were fitted to Eq. 2. Recovery time constants (τr) are 6.4, 12.4, 24.4, 48.4, 89.4, and 143.9 ms at −180, −160, −140, −120, −100, and −80 mV, respectively. (E3) Recovery time constants are plotted as a function of recovery voltage at 10 μM internal Ca2+.
Table 1.
Parameters used for current simulations in BKi cells
| s−1 | s−1 | ||
|---|---|---|---|
| A0 | 1 | B0 | 810 |
| A1 | 2 | B1 | 135 |
| A2 | 12 | B2 | 67.5 |
| A3 | 120 | B3 | 56.25 |
| A4 | 288 | B4 | 11.25 |
| zCO | 0.513 | zOC | 0.5745 |
| s−1 | s−1 | ||
| C0 | 15 | D0 | 7.000 |
| C1 | 18 | D1 | 3.360 |
| C2 | 21 | D2 | 1.568 |
| C3 | 25 | D3 | 0.7468 |
| C4 | 30 | D4 | 0.3584 |
| zOI | 0.1293 | zIO | 0.8617 |
| KC | 7.2 | KO | 0.6 |
| KI | 0.24 | ||
| Ca2+ on-rates per site | 109 M−1 s−1 | ||
| Ca2+ off-rates from Cn per binding site | 109KC (6,600 s−1) | ||
| Ca2+ off-rates from On per binding site | 109KO (550 s−1) | ||
| Ca2+ off-rates from In per binding site | 109KI (220 s−1) | ||
Simulated BKi currents (Fig. 1 B) were generated with voltage protocols identical to those used to define gating properties of expressed α + β2 currents (Fig. S5). In these simulated BKi currents, the voltage dependence of conductance, the shift in steady-state inactivation with voltage, and the general behavior of the V50 both for activation and steady-state inactivation (Fig. 1 C) closely mirrored those of the native BKi currents (Fig. S5 B). Furthermore, τa and τd for the simulated BKi currents (Fig. 1 D) provided reasonable approximations of the behavior of experimentally measured BKi currents (Fig. S5 C). The simulated BKi currents also exhibited a voltage dependence of recovery from inactivation (Fig. 1 E) similar to that observed in the native BKi currents (Fig. S5 D). Thus, these gating behaviors of both the simulated BKi and BKs currents seem sufficiently close to native currents to allow their impact on current clamp behavior to be assessed.
Characterization of the AP firing behavior in an RCC first requires identification of the principle BK current component in that cell (9). To accomplish this, a direct step to +90 mV in an RCC typically activates very little, if any, BK current, since net Ca2+ influx at that potential is negligible. Therefore, comparison of currents at +90 mV, either with or without a conditioning step, to a potential that results in robust Ca2+ elevation was used in native RCCs to provide clear visualization of whether the BK current exhibits inactivation (BKi) or very slow decay (BKs), reflecting Ca2+ clearance (9).
Patterns of AP firing in response to constant-current injection in native and model RCCs
The two distinct patterns of repetitive AP firing during 2-s current injections in BKs and BKi cells (9) suggest that BK current properties determine the differences between the two cell types. We therefore utilized model cells, with parameters defined in Table 2, to test whether the type of BK current might account for the current clamp behavior. For both BKi and BKs model cell simulations, all parameters except BK channel gating properties were identical. Model cell simulations of AP behavior were calculated from Eqs. A1 and A2 (see the Appendix in the Supporting Material) and Eq. 3. Values of conductance for each channel type were selected to yield conductances in the current-clamp simulations that compared with those evoked by AP clamp voltage commands in experiments on native cells. The instantaneous rectification of BKi current was ignored in these simulations, since the instantaneous I/V relationship is largely linear from −60 to +60 mV.
Table 2.
Model cell parameters
| Channel | Gmax (nS) | Reversal potential (mV) |
|---|---|---|
| Na | 54 | 55 |
| Kv | 1.5 | −70 |
| BKS/BKi | 90 | −60 |
| Ca | 1 | 60 |
| Leak | 0.45 | −55 |
| Ca2+ transition coefficient | 0.006 μM pA−1 ms−1 | |
| Ca2+ diffusion coefficient | 0.004 ms−1 | |
| Global intracellular [Ca2+]i | 10 nM | |
| VRest | −55 mV | |
Model cell was 15 μm in diameter, with a membrane capacity of Cm = 10 pF.
In response to different levels of current injection (Fig. 2 A), the BKi model cell exhibited a somewhat accommodating repetitive firing, whereas the BKs model cell exhibited a phasic response with only one or a few APs. The number of APs over 0 mV increased with the amount of injected current in the BKi model cell, whereas the number in the BKs model cell initially increased and then decreased (Fig. 2 B). This behavior approximates that observed in native RCCs (9). In the model cell with BKi current, the afterhyperpolarizations between APs are better able to return the potential to near-resting potentials, which presumably has a major impact on the availability of voltage-dependent Na+ current for subsequent APs. This probably results from the fact that BKi current is more effectively activated at a given [Ca2+] and voltage, resulting in more prolonged tail current at a given voltage and [Ca2+]. The persistence of the membrane potential of the BKs model cell at more depolarized levels during current injection is also consistent with previously reported results (9).
Figure 2.
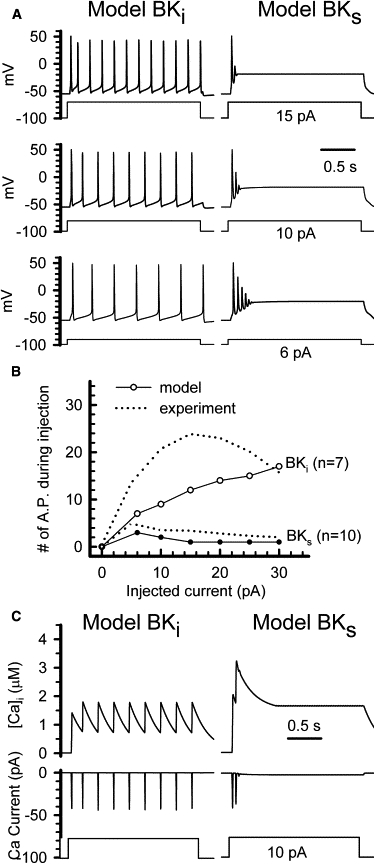
BKi and BKs currents differentially contribute to AP firing in model cells. (A) Model cells containing either BKi (left) or BKs (right) current were used to simulate action potentials in response to constant-current injection of different amplitude (lower, 6 pA; middle, 10 pA; upper, 20 pA). The resting potential was −55 mV. (B) The number of action potentials elicited during a 2-s period of injected current are plotted as a function of the amplitude of injected current for model BKi (open circles) or model BKs (solid circles) cells. The dotted lines correspond to experimental results obtained previously (9) in native RCCs. (C) Left: The upper trace shows the simulated time course of the local calcium concentration in a model BKi cell; the middle traces shows calcium currents activated by an injected current of 10 pA in the model BKi cell (lower trace). Right: The upper trace shows the time course of the local calcium concentration in a BKs model cell, whereas the middle trace shows calcium currents activated in the BKs model cell by an injected current of 10 pA (lower trace).
We compared the impact of these current-clamp behaviors on cytosolic Ca2+ using Eq. 3. It should be kept in mind that Eq. 3 does not provide a physically realistic estimate of [Ca2+]i, but rather gives a relative value that allows Ca2+ influx to be scaled to BK current activation, based on the assumption that all BK channels are sampling this same concentration of Ca2+. However, as long as BKi and BKs channels are organized similarly with regard to Ca2+ channels, Eq. 3 provides a meaningful comparison of relative increases in Ca2+ in the two types of model cells (Fig. 2 C). In both model cells, the Ca2+ concentration rapidly increases to 1–3 μM after a few APs, which is consistent with observations by others (54). In the BKi model cell, the simulation reveals a distinct rise and fall between individual APs, with little diminution in peak Ca2+ current influx. In contrast, in the BKs model cell, Ca2+ is maintained at a higher level due to a small sustained calcium influx through HVA Ca2+ channels that appears at ∼−25 mV. This sustained Ca2+ level is maintained despite the reduction of peak Ca2+ current per action potential that occurs as peak action potential is diminished.
The Ca2+ dependence of AP firing in native RCCs and simulated model cells
The Ca2+ dependence of repetitive AP firing in native BKi or BKs RCCs was examined through application of 0-Ca2+ saline (Fig. 3 A) in the presence of 200 nM apamin to block SK currents. In both cell types, removal of external Ca2+ reduced the response to injected current to one or two APs, indicating that the ability to fire repetitive APs in both BKi and BKs cells is Ca2+-dependent. In model BKi and BKs cells, when both SK and BK current are excluded from the simulations, the number of APs evoked by 10 pA constant-current injection was similarly reduced to one or two APs (Fig. 3 B). The ability to fire repetitively will also potentially be influenced by Kv current density (see Fig. 5). In our model cells, Kv current density is small relative to the maximal BK current (see Table 2) because of the small amplitude of Kv current relative to BK current observed in voltage-clamp experiments in both BKi and BKs cells (9). The inability of either native BKi or BKs cells to fire repetitively in 0 Ca2+ is also indicative that the Kv current density is low.
Figure 3.
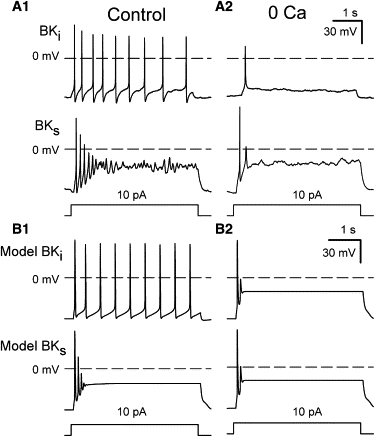
Repetitive AP firing in RCCs is abolished by 0 Ca2+. (A1) Action potentials were elicited by constant-current injection in identified BKi (upper) and BKs (lower) RCCs using perforated-patch recording with normal extracellular (1.8 mM Ca2+) saline. (A2) Traces were recorded in 0 Ca2+ normal saline (with 2 mM Mg2+). In all cases, the extracellular saline contained 200 nM apamin. (B1) Action potentials activated by constant-current injection were simulated in BKi (upper) and BKs (lower) model cells. SK current was inactive. (B2) The traces were obtained from model simulations in which only Ca2+ current was removed from the simulation.
Figure 5.
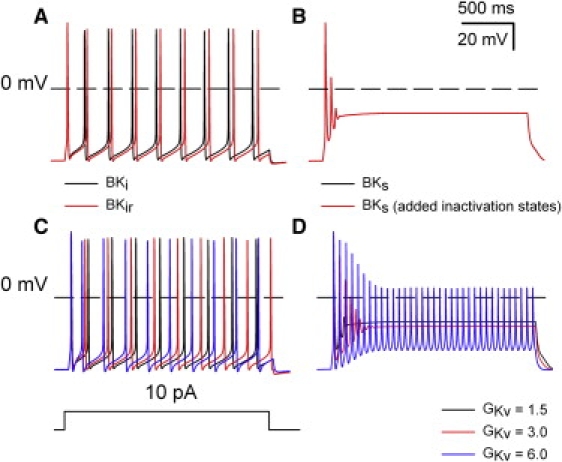
Ability of BK current in RCCs to support repetitive firing arises not from inactivation but from the shifted range of activation compared to BKs current. (A) APs were elicited by a 2-s 10-pA current injection in the model BKi cell either with inactivation intact (black line) or with transitions to inactivated states removed (red line). (B) APs were elicited by a 2-s 10-pA current injection either in the model BKs cell (black line) or the model BKs cell with inactivated states implemented (red line). (C) The density of Kv channels in the BKi cell was increased from 1.5 to 3.0 and then 6.0, producing a slight increase in the AP firing rate. (D) An increase of Kv current density in a BKs cell supports repetitive firing with reduced AP amplitude.
Contributions of BK current to AHPs in native and model BKi and BKs cells
We next examined the Ca2+ dependence of afterhyperpolarizations (AHPs) after single APs in either native or model cells. In native RCCs, single APs were elicited by a brief 100-pA depolarizing current pulse and examined with either 1.8 or 0 mM extracellular Ca2+ saline (with 200 nM apamin to block SK current). In BKi cells, removal of external Ca2+ resulted in a pronounced slowing of repolarization, particularly in the second half of the repolarization. Furthermore, a small AHP after the AP was abolished in 0 Ca2+ (Fig. 4 A, left). In contrast, in BKs cells, there was little clear change in the AP waveform (Fig. 4 A, right), suggesting that there is little BKs current activation with a single AP.
Figure 4.
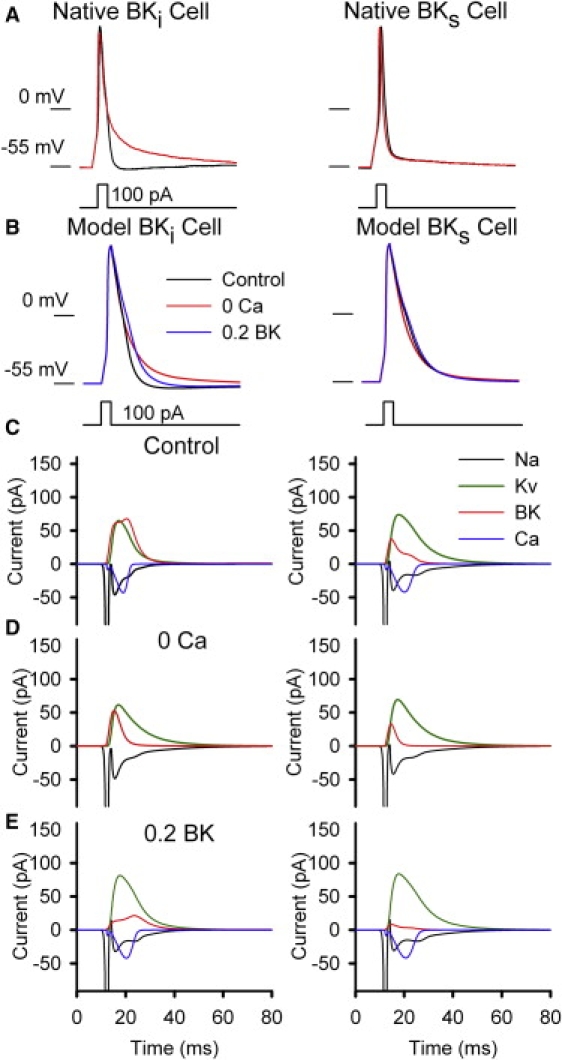
BK current differentially contributes to AP repolarization in BKi and BKs cells. (A) APs elicited by a 100-pA depolarizing current pulse are shown for identified BKi (left) and BKs (right) RCCs in both control and 0-Ca2+ saline, as indicated. The extracellular saline contained 200 nM apamin. (B) The simulated waveforms of APs elicited by 100-pA depolarizing current pulses are shown for a model BKi (left) cell and a model BKs (right) cell in control and 0 Ca2+, as indicated. SK currents were removed in all cases. (C–E) Individual current components (Na, Kv, BK, and Ca) appearing in a single AP are shown for a model BKi (left) cell and a model BKs (right) cell, for control saline (C), 0 Ca2+ saline (D), and with nominal 100 nM CTX (0.2 BK; E).
We also evoked single APs in BKi and BKs model cells (also with no SK current) and tested the consequences of Ca2+ removal. For the BKi model cell, removal of Ca2+ current resulted in slowing of repolarization similar to that seen in the native RCCs, and the AHP after the AP was also abolished (Fig. 4 B, left). In contrast, for the BKs model cell, with Ca2+ current removed, the AP repolarization actually became slightly faster (Fig. 4 B, right), presumably because of the removal of inward Ca2+ current during the later stages of the AP. The effect of ∼100 nM charybdotoxin (CTX) (EC50 (half-maximal effective concentration) ∼ 25 nM) (9) was also simulated by reducing the available BK current by 80%. For the BKi model cell, the simulated AP exhibited a pronounced prolongation with only a minor effect in the BKs model cell (Fig. 4 B). Both the 0-Ca2+ and simulated CTX effects closely mirror those seen in native RCCs (9). Specifically, CTX (0.2 BK) mediates a delay in repolarization immediately after the AP peak, whereas 0 Ca2+ only impacts on AP repolarization at late times in the decay process.
We next examined the contributions of each voltage-dependent current that is activated during the evoked APs in both the BKi (Fig. 4 C, left) and BKs (Fig. 4 C, right) model cells. The net BK current flux was substantially larger in the BKi model cell, whereas the duration of Ca2+ current was clearly more prolonged in the BKs model cell. In the BKs model cell, the primary repolarizing current was mediated by Kv channel. When Ca2+ current was omitted (Fig. 4 D), BK current in both cell types was diminished but still substantial. The relatively small reduction of BK current elicited by a single AP presumably reflects the ability of voltage alone to activate BK channels (46), particularly at early times during an AP. A contributing factor to the slow repolarization in BKi cells with 0 Ca2+ appears to be the prominent secondary persistence of Na+ current (Fig. 4 D, left). The BKs model cell in normal saline also shows more persistence of Na+ current (Fig. 4 C, right), consistent with the slower overall repolarization in the BKs model cell. The ability of the simulated CTX effect to delay repolarization, in comparison to the effect of 0 Ca2+, appears to arise because of the ability of CTX to block the early activation of BK current that occurs during the initial upswing of an AP.
Overall, the contribution of BK current to repolarization in BKi and BKs model cells closely mimics the contribution of these currents to native RCCs. The primary difference between native and model cells is that the model cells exhibit more prolonged AP durations, particularly in the BKs model cell. The reasons for this difference will be examined in future iterations of this modeling effort, but contributing factors might include the density of BK channels, the density of Na+ and Ca2+ channels, or the approximation made for coupling of Ca2+ influx to BK activation. It will be noted (Fig. 4, C and D) that Kv current contributes somewhat more prominently to net outward current in model BKs cells than in BKi cells, although both model cells contain the same number of BK channels. This presumably arises because the weaker BK activation in BKs cells slightly delays repolarization, allowing for the enhanced activation of Kv current.
Inactivation is not responsible for the repetitive firing associated with BKi channels
One proposed explanation for the difference in repetitive firing behavior between BKi and BKs cells is that the range of activation for BKi current is more negatively shifted (15), allowing it to play a more prominent role in AHPs between APs. To test this idea, we compared the ability of BKi current with inactivation intact to its ability with inactivated states removed. In response to depolarizing current injection, a simulation in which BKi channels do not inactivate exhibits a slight increase in firing rate in comparison to the normal BKi model cell (Fig. 5 A). This small increase in firing rate probably arises because of slightly stronger total BK activation in the absence of some very weak cumulative inactivation. We then added inactivated states to the model for BKs current without changing any of the transitions between closed and open states. In response to constant-current injection, a model cell with inactivating BKs channels exhibited a phasic firing behavior essentially identical to that of the standard BKs model cell (Fig. 5 B). This directly confirms the idea that the key aspect of BK channels that supports repetitive firing in RCCs is not inactivation itself but the more negatively shifted range of activation produced by the β2 subunit. Since Kv channels are also expected to influence repetitive firing behavior, we also examined the ability of increases in Kv current density to influence repetitive firing in the BKi and BKs model cells (Fig. 5, C and D). Not unexpectedly, an increase in Kv current density, although it has little effect in the BKi model cell, is able to support repetitive firing in the BKs model cell. The absence of appreciable repetitive firing in native BKs cells is therefore consistent with the modest amounts of Kv current densities used in our simulations.
Discussion
We have examined whether the properties of inactivating and noninactivating BK channels predict the previously observed differential AP firing behavior among RCCs that contain either predominantly BKi or BKs current (9,15). The overall conclusion is that the presence of BKi current contributes to the ability of a model BKi cell to fire repetitively during constant current injection, whereas in model cells with exclusively BKs current, constant current injection results in only one or very few APs. These differences arise, not because of the inactivation behavior of BKi current, but from the more negatively shifted range of activation of BKi channels at a given [Ca2+]i in comparison to BKs current. Specifically, the voltage activation curve for activation of BK channels arising from α + β2 subunits (26,53) is shifted to more negative values at a given Ca2+. Because of this shift in gating, BKi channels better contribute to a robust afterhyperpolarization among APs, which presumably allows Na+ channels to recover from inactivation and participate in repetitive firing. The ability of a shift in activation range of a K+ channel to influence repetitive firing is not surprising, and others have observed that shifting the gating range of a Kv channel by even as little as 20 mV can impact substantially on the ability of a model cell to exhibit repetitive firing (10,55). The gratifying aspect of the simulations presented here is that model cells, based on plausible differences in BK channel properties, were able to reproduce the differential current-clamp properties of native BKi and BKs RCCs. Although it has been previously proposed that this might arise from the difference in activation properties of the two BK currents (9,15), the simulations presented here provide stronger support for this idea. A shift in BK gating properties, probably arising from a change in expression of Slo1 α-subunit splice variants, has also been shown to underlie changes in RCC firing properties after hypophysectomy (10).
The differences in range of activation of BKi and BKs current also contribute to other properties of native BKi and BKs cells. In both native and model cells with BKi channels, after single evoked APs, BK channels contribute clearly to AP repolarization and afterhyperpolarizations, whereas in cells with BKs current, very little BK current appears to be activated during single APs. This is consistent with differences in the effect of 0 Ca2+ or CTX application on afterhyperpolarizations in native BKi and BKs cells (9). Although both 0 Ca2+ and CTX have been observed to produce a slowing of repolarization in native BKi cells, the effects are quite distinct (9). Whereas 0 Ca2+ results in a slowing of repolarization at later times in the AP decay time course, essentially abolishing the afterhyperpolarizations, CTX results in a delay in repolarization immediately after the peak of the AP. This behavior was nicely mimicked by the model BKi cell. A powerful aspect of the simulation approach is that it provides a potential explanation for these differences by allowing examination of each individual current component during a simulation. This approach revealed that the differences between 0 Ca2+ and CTX arise because CTX more effectively blocks BK current during the initial upswing of the AP. Furthermore, in 0 Ca2+, there is a secondary persistence of inward Na+ current that contributes to the slow AP repolarization. We feel that neither the greater effect of CTX on early BK current nor the increased late Na+ current in 0 Ca2+ would have seemed obvious factors contributing to the difference between effects of 0 Ca2+ and CTX. This highlights the potential power of realistic models of simulation guided by experiment.
Left unanswered by the analysis presented here is the physiological role of BKi inactivation in RCCs. One potential role of inactivating BK channels has been proposed in regard to use-dependent spike prolongation both in hippocampal pyramidal cells (56) and also in neurons of the rat lateral amygdala (8). In both cases, AP broadening is fully developed within 3–5 action potentials and is favored by firing frequencies >20 Hz, indicative that any inactivating channel mediating these effects must exhibit a more rapid and complete inactivation than is typically observed for β2-mediated inactivation of BK channels in RCCs. The analysis presented here sheds no light on this question.
An important future improvement over the models used here will be the replacement of the 10-state gating model for BKi and BKs current activation with a more complete model of BK activation. Although this will be more computationally intensive, a complete allosteric model incorporating independent Ca2+ and voltage-dependent transitions (46) should allow a better approximation of the Ca2+-dependence of activation and deactivation behavior for both BKi and BKs current. BK activation and deactivation time constants vary with [Ca2+] in complex ways, particularly at Ca2+ <1 μM contingent upon activation of either of two distinct types of Ca2+ binding sites (57). This behavior cannot be readily approximated solely by the 10-state model. Furthermore, activation and deactivation are markedly slowed by the β2 subunit (4) in ways that should be better described by a more complete gating model. Although the models used here capture the essential differences in behavior between these two types of BK current, it will be interesting to know to what extent the observed effects on AP firing may arise not only from the equilibrium differences in Vh, but also specific differences in the Ca2+-dependence of channel gating behavior.
A primary goal of this work was to build a simulation approach based on Markovian models of channel gating, rather the often used Hodgkin-Huxley formalisms. We have utilized a number of simplifying assumptions. First, detailed kinetic models and quantitative estimates of current densities for many of the underlying currents in RCCs are not yet available. When such information becomes available, more physically plausible models with appropriate kinetic rates can be easily implemented into the current approach. Second, we have treated different Ca2+ current components as a single entity driving elevations in local Ca2+. Given the overlap in the ranges of voltage-dependence of activation and kinetic features of different HVA currents, this assumption seems acceptable. A circumstance in which specific definition of separate Ca2+ current components would be useful would be in cases where there is differential modulation of specific Ca2+ currents, perhaps with specific coupling to BK channels. Third, we have used a simple procedure for relating Ca2+ influx to effective [Ca2+]i (44). Explicit calculation of [Ca2+] in different submembrane compartments that takes into account cytosolic Ca2+ buffering and extrusion would allow a more realistic examination of how BK activation tracks cytosolic [Ca2+]. However, in regards to the evaluation of the differential contribution of BKi and BKs current to AP firing, the assumptions used here seem acceptable. With further refinement of this model and the inclusion of additional data-based estimates of the properties of various current, this approach promises to provide a useful evaluation of how different conductances impact on cellular excitability.
Supporting Material
Five figures, three tables, an appendix, and references are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(09)01218-1.
Supporting Material
Acknowledgments
This work was supported by grants from the National Science Foundation of China (30770522), the National Institutes of Health (GM081748 to C.L.), and the Program of Introducing Talents of Discipline to Universities (B08029).
Footnotes
Liang Sun, Yu Xiong, and Xuhui Zeng contributed equally to this work.
Contributor Information
Anlian Qu, Email: alqu@mail.hust.edu.cn.
Jiuping Ding, Email: jpding@mail.hust.edu.cn.
References
- 1.Saito M., Nelson C., Salkoff L., Lingle C.J. A cysteine-rich domain defined by a novel exon in a slo variant in rat adrenal chromaffin cells and PC12 cells. J. Biol. Chem. 1997;272:11710–11717. doi: 10.1074/jbc.272.18.11710. [DOI] [PubMed] [Google Scholar]
- 2.Dworetzky S.I., Trojnacki J.T., Gribkoff V.K. Cloning and expression of a human large-conductance calcium-activated potassium channel. Brain Res. Mol. Brain Res. 1994;27:189–193. doi: 10.1016/0169-328x(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 3.Tseng-Crank J., Yao J.A., Berman M.F., Tseng G.N. Functional role of the NH2-terminal cytoplasmic domain of a mammalian A-type K channel. J. Gen. Physiol. 1993;102:1057–1083. doi: 10.1085/jgp.102.6.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orio P., Rojas P., Ferreira G., Latorre R. New disguises for an old channel: MaxiK channel β-subunits. News Physiol. Sci. 2002;17:156–161. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- 5.Salkoff L., Butler A., Ferreira G., Santi C., Wei A. High-conductance potassium channels of the SLO family. Nat. Rev. Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 6.Berkefeld H., Sailer C.A., Bildl W., Rohde V., Thumfart J.O. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science. 2006;314:615–620. doi: 10.1126/science.1132915. [DOI] [PubMed] [Google Scholar]
- 7.Van Goor F., Li Y.X., Stojilkovic S.S. Paradoxical role of large-conductance calcium-activated K+ (BK) channels in controlling action potential-driven Ca2+ entry in anterior pituitary cells. J. Neurosci. 2001;21:5902–5915. doi: 10.1523/JNEUROSCI.21-16-05902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faber E.S., Sah P. Ca2+-activated K+ (BK) channel inactivation contributes to spike broadening during repetitive firing in the rat lateral amygdala. J. Physiol. 2003;552:483–497. doi: 10.1113/jphysiol.2003.050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solaro C.R., Prakriya M., Ding J.P., Lingle C.J. Inactivating and noninactivating Ca2+- and voltage-dependent K+ current in rat adrenal chromaffin cells. J. Neurosci. 1995;15:6110–6123. doi: 10.1523/JNEUROSCI.15-09-06110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovell P.V., McCobb D.P. Pituitary control of BK potassium channel function and intrinsic firing properties of adrenal chromaffin cells. J. Neurosci. 2001;21:3429–3442. doi: 10.1523/JNEUROSCI.21-10-03429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lou X.L., Yu X., Chen X.K., Duan K.L., He L.M. Na+ channel inactivation: a comparative study between pancreatic islet β-cells and adrenal chromaffin cells in rat. J. Physiol. 2003;548:191–202. doi: 10.1113/jphysiol.2002.034405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Z., Misler S. Action potential-induced quantal secretion of catecholamines from rat adrenal chromaffin cells. J. Biol. Chem. 1995;270:3498–3505. [PubMed] [Google Scholar]
- 13.Duan K., Yu X., Zhang C., Zhou Z. Control of secretion by temporal patterns of action potentials in adrenal chromaffin cells. J. Neurosci. 2003;23:11235–11243. doi: 10.1523/JNEUROSCI.23-35-11235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovell P.V., King J.T., McCobb D.P. Acute modulation of adrenal chromaffin cell BK channel gating and cell excitability by glucocorticoids. J. Neurophysiol. 2004;91:561–570. doi: 10.1152/jn.01101.2002. [DOI] [PubMed] [Google Scholar]
- 15.Lingle C.J., Solaro C.R., Prakriya M., Ding J.P. Calcium-activated potassium channels in adrenal chromaffin cells. Ion Channels. 1996;4:261–301. doi: 10.1007/978-1-4899-1775-1_7. [DOI] [PubMed] [Google Scholar]
- 16.Inoue M., Harada K., Matsuoka H., Sata T., Warashina A. Inhibition of TASK1-like channels by muscarinic receptor stimulation in rat adrenal medullary cells. J. Neurochem. 2008;106:1804–1814. doi: 10.1111/j.1471-4159.2008.05521.x. [DOI] [PubMed] [Google Scholar]
- 17.Fenwick E.M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J. Physiol. 1982;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prakriya M., Lingle C.J. BK channel activation by brief depolarizations requires Ca2+ influx through L- and Q-type Ca2+ channels in rat chromaffin cells. J. Neurophysiol. 1999;81:2267–2278. doi: 10.1152/jn.1999.81.5.2267. [DOI] [PubMed] [Google Scholar]
- 19.Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J. Physiol. 1985;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conforti L., Millhorn D.E. Selective inhibition of a slow-inactivating voltage-dependent K+ channel in rat PC12 cells by hypoxia. J. Physiol. 1997;502:293–305. doi: 10.1111/j.1469-7793.1997.293bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conforti L., Bodi I., Nisbet J.W., Millhorn D.E. O2-sensitive K+ channels: role of the Kv1.2-subunit in mediating the hypoxic response. J. Physiol. 2000;524:783–793. doi: 10.1111/j.1469-7793.2000.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neely A., Lingle C.J. Two components of calcium-activated potassium current in rat adrenal chromaffin cells. J. Physiol. 1992;453:97–131. doi: 10.1113/jphysiol.1992.sp019220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park Y.B. Ion selectivity and gating of small conductance Ca2+-activated K+ channels in cultured rat adrenal chromaffin cells. J. Physiol. 1994;481:555–570. doi: 10.1113/jphysiol.1994.sp020463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding J.P., Li Z.W., Lingle C.J. Inactivating BK channels in rat chromaffin cells may arise from heteromultimeric assembly of distinct inactivation-competent and noninactivating subunits. Biophys. J. 1998;74:268–289. doi: 10.1016/S0006-3495(98)77785-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei A., Solaro C., Lingle C., Salkoff L. Calcium sensitivity of BK-type KCa channels determined by a separable domain. Neuron. 1994;13:671–681. doi: 10.1016/0896-6273(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 26.Xia X.M., Ding J.P., Lingle C.J. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J. Neurosci. 1999;19:5255–5264. doi: 10.1523/JNEUROSCI.19-13-05255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrington J., Solaro C.R., Neely A., Lingle C.J. The suppression of Ca2+- and voltage-dependent outward K+ current during mAChR activation in rat adrenal chromaffin cells. J. Physiol. 1995;485:297–318. doi: 10.1113/jphysiol.1995.sp020731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prakriya M., Solaro C.R., Lingle C.J. [Ca2+]i elevations detected by BK channels during Ca2+ influx and muscarine-mediated release of Ca2+ from intracellular stores in rat chromaffin cells. J. Neurosci. 1996;16:4344–4359. doi: 10.1523/JNEUROSCI.16-14-04344.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan K., Stevens E.B., Shah B., Cox P.J., Dixon A.K. β3: an additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proc. Natl. Acad. Sci. USA. 2000;97:2308–2313. doi: 10.1073/pnas.030362197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuo C.C., Bean B.P. Na+ channels must deactivate to recover from inactivation. Neuron. 1994;12:819–829. doi: 10.1016/0896-6273(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 31.Thompson R.J., Nurse C.A. Anoxia differentially modulates multiple K+ currents and depolarizes neonatal rat adrenal chromaffin cells. J. Physiol. 1998;512:421–434. doi: 10.1111/j.1469-7793.1998.421be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fearon I.M., Thompson R.J., Samjoo I., Vollmer C., Doering L.C. O2-sensitive K+ channels in immortalised rat chromaffin-cell-derived MAH cells. J. Physiol. 2002;545:807–818. doi: 10.1113/jphysiol.2002.028415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steidl J.V., Yool A.J. Differential sensitivity of voltage-gated potassium channels Kv1.5 and Kv1.2 to acidic pH and molecular identification of pH sensor. Mol. Pharmacol. 1999;55:812–820. [PubMed] [Google Scholar]
- 34.Grissmer S., Nguyen A.N., Aiyar J., Hanson D.C., Mather R.J. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol. Pharmacol. 1994;45:1227–1234. [PubMed] [Google Scholar]
- 35.Rezazadeh S., Kurata H.T., Claydon T.W., Kehl S.J., Fedida D. An activation gating switch in Kv1.2 is localized to a threonine residue in the S2–S3 linker. Biophys. J. 2007;93:4173–4186. doi: 10.1529/biophysj.107.116160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neshatian L., Leung Y.M., Kang Y., Gao X., Xie H. Distinct modulation of Kv1.2 channel gating by wild type, but not open form, of syntaxin-1A. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G1233–G1242. doi: 10.1152/ajpgi.00473.2006. [DOI] [PubMed] [Google Scholar]
- 37.Prakriya M., Lingle C.J. Activation of BK channels in rat chromaffin cells requires summation of Ca2+ influx from multiple Ca2+ channels. J. Neurophysiol. 2000;84:1123–1135. doi: 10.1152/jn.2000.84.3.1123. [DOI] [PubMed] [Google Scholar]
- 38.Bournaud R., Hidalgo J., Yu H., Jaimovich E., Shimahara T. Low threshold T-type calcium current in rat embryonic chromaffin cells. J. Physiol. 2001;537:35–44. doi: 10.1111/j.1469-7793.2001.0035k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L., Bischofberger J., Jonas P. Differential gating and recruitment of P/Q-, N-, and R-type Ca2+ channels in hippocampal mossy fiber boutons. J. Neurosci. 2007;27:13420–13429. doi: 10.1523/JNEUROSCI.1709-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loane D.J., Lima P.A., Marrion N.V. Co-assembly of N-type Ca2+ and BK channels underlies functional coupling in rat brain. J. Cell Sci. 2007;120:985–995. doi: 10.1242/jcs.03399. [DOI] [PubMed] [Google Scholar]
- 41.Marrion N.V., Tavalin S.J. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- 42.Berkefeld H., Fakler B. Repolarizing responses of BKCa-Cav complexes are distinctly shaped by their Cav subunits. J. Neurosci. 2008;28:8238–8245. doi: 10.1523/JNEUROSCI.2274-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcantoni A., Baldelli P., Hernandez-Guijo J.M., Comunanza V., Carabelli V. L-type calcium channels in adrenal chromaffin cells: role in pace-making and secretion. Cell Calcium. 2007;42:397–408. doi: 10.1016/j.ceca.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 44.Beeler G.W., Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J. Physiol. 1977;268:177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y.-W., Ding J.P., Xia X.-M., Lingle C.J. Consequences of the stoichiometry of Slo1 α and auxiliary β subunits on functional properties of BK-type Ca2+-activated K+ channels. J. Neurosci. 2002;22:1550–1561. doi: 10.1523/JNEUROSCI.22-05-01550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horrigan F., Aldrich R. Coupling between voltage-sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J. Gen. Physiol. 2002;120:267–305. doi: 10.1085/jgp.20028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox D.H., Cui J., Aldrich R.W. Allosteric gating of a large conductance Ca-activated K+ channel. J. Gen. Physiol. 1997;110:257–281. doi: 10.1085/jgp.110.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding J., Lingle C. Steady-state and closed-state inactivation properties of inactivating BK channels. Biophys. J. 2002;82:2448–2465. doi: 10.1016/S0006-3495(02)75588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng X.-H., Xia X.-M., Lingle C.J. Redox-sensitive extracellular gates formed by auxiliary β subunits of calcium-activated potassium channels. Nat. Struct. Biol. 2003;10:448–454. doi: 10.1038/nsb932. [DOI] [PubMed] [Google Scholar]
- 50.Lingle C.J., Zeng X.-H., Ding J.-P., Xia X.-M. Inactivation of BK channels mediated by the N-terminus of the β3b auxiliary subunit involves a two-step mechanism: possible separation of binding and blockade. J. Gen. Physiol. 2001;117:583–605. doi: 10.1085/jgp.117.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solaro C.R., Ding J.P., Li Z.W., Lingle C.J. The cytosolic inactivation domains of BKi channels in rat chromaffin cells do not behave like simple, open-channel blockers. Biophys. J. 1997;73:819–830. doi: 10.1016/S0006-3495(97)78114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benzinger G.R., Xia X.M., Lingle C.J. Direct observation of a preinactivated, open state in BK channels with β2 subunits. J. Gen. Physiol. 2006;127:119–131. doi: 10.1085/jgp.200509425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallner M., Meera P., Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane β-subunit homolog. Proc. Natl. Acad. Sci. USA. 1999;96:4137–4142. doi: 10.1073/pnas.96.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meinrenken C.J., Borst J.G., Sakmann B. Local routes revisited: the space and time dependence of the Ca2+ signal for phasic transmitter release at the rat calyx of Held. J. Physiol. 2003;547:665–689. doi: 10.1113/jphysiol.2002.032714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu Y.C., Fettiplace R. A development model for generating frequency maps in the reptilian and avian cochleas. Biophys. J. 1996;70:2557–2570. doi: 10.1016/S0006-3495(96)79827-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shao L.R., Halvorsrud R., Borg-Graham L., Storm J.F. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J. Physiol. 1999;521:135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng X.H., Xia X.M., Lingle C.J. Divalent cation sensitivity of BK channel activation supports the existence of three distinct binding sites. J. Gen. Physiol. 2005;125:273–286. doi: 10.1085/jgp.200409239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


