Abstract
Cancer cells are known to have alterations in multiple cellular signaling pathways and because of the complexities in the communication between multiple signaling networks, the treatment and the cure for most human malignancies is still an open question. Perhaps, this is the reason why specific inhibitors that target only one pathway have been typically failed in cancer treatment. However, the in vitro and in vivo studies have demonstrated that some natural products such as isoflavones, indole-3-carbinol (I3C), 3,3′-diindolylmethane (DIM), curcumin, (−)-epigallocatechin-3-gallate (EGCG), resveratrol, lycopene, etc, have inhibitory effects on human and animal cancers through targeting multiple cellular signaling pathways and thus these “natural agents” could be classified as multi-targeted agents. This is also consistent with the epidemiological studies showing that the consumption of fruits, soybean and vegetables is associated with reduced risk of several types of cancers. By regulating multiple important cellular signaling pathways including NF-κB, Akt, MAPK, Wnt, Notch, p53, AR, ER, etc, these natural products are known to activate cell death signals and induce apoptosis in pre-cancerous or cancer cells without affecting normal cells. Therefore, non-toxic “natural agents” harvested from the bounties of nature could be useful either alone or in combination with conventional therapeutics for the prevention of tumor progression and/or treatment of human malignancies.
Keywords: cell signaling, cancer, natural products
1. Introduction
Cellular signaling is a complex signal communication network which controls basic biological activities of cells and coordinates cell actions. It has been know that signaling cascades are typically composed of three-dimensional pathways of proteins which regulate each other in the specific location of cells [1]. Because of the complex transduction of cell signaling, cancer cells always show the alterations in multiple cellular signaling pathways. Perhaps, this is the reason why the specific inhibitors that target only one pathway, most often, failed in cancer treatment. In cancer cells, the cellular signaling pathways which control cell cycle and apoptosis are almost always malfunctioning, leading to the uncontrolled cell proliferation and the formation of tumor. Several cellular signaling pathways including NF-κB, Akt, MAPK, Wnt, Notch, p53, AR, and ER, etc, have been known to control cell proliferation and apoptosis. Importantly, all of these signaling pathways have been found malfunctioning in cancer cells, resulting in cancer cell proliferation and inhibition of apoptosis [2–6]. Therefore, it is important to design a strategy that could simultaneously target multiple cellular signaling pathways so that cancer cells could get killed effectively.
In recent years, dietary compounds (natural agents) harvested from the bounties of nature have received much attention, primarily because epidemiological studies have shown that the consumption of fruits, soybean and vegetables is associated with reduced risk of several types of cancers [7–9]. The natural products including isoflavone genistein, indole-3-carbinol (I3C), 3,3′-diindolylmethane (DIM), curcumin, (−)-epigallocatechin-3-gallate (EGCG), resveratrol, lycopene, etc, have been recognized as cancer chemopreventive agents because of their anti-carcinogenic activity [10, 11]. The in vitro and in vivo studies have demonstrated that these natural products (natural agents) have inhibitory effects on various human and animal cancers [12–19]; therefore, many investigators have focused on elucidating the molecular mechanisms and identifying the targets of action of these natural products.
Soy isoflavones such as genistein, daidzein, and glycitein are mainly derived from soybean. Genistein has been found to inhibit cancer cell growth in vivo and in vitro [20–22]. I3C and its in vivo dimeric product DIM are produced from naturally occurring glucosinolates found in the family Cruciferae. I3C and DIM have shown inhibitory effects on cancer cell growth through the modulation of genes that are related to the control of cell proliferation, cell cycle, apoptosis, signal transduction, oncogenesis, and transcription regulation [14, 15]. Curcumin is a natural compound present in turmeric and has been known to possess both anti-inflammatory and antioxidant effects. However, it has also been studied as a cancer chemopreventive agent in several cancer models [17, 23]. EGCG existed in green tea has shown antioxidant and anticancer activities in several types of cancer [8, 24]. Resveratrol (3,5,4′-trihydroxystilbene) is a phytoalexin present in a wide variety of plant species including grapes, mulberries, and peanuts. Experimental studies have shown that resveratrol inhibits the growth of various cancer cells and induces apoptotic cell death [25, 26]. Lycopene rich in tomatoes has been shown to inhibit cell growth in various cancer cells with regulation of cell cycle-related genes [27, 28]. Emerging evidence from increasing number of investigations on these natural products, it is becoming clear that these natural products exert their pleiotropic effects on cancer cells through targeting multiple cellular signaling pathways including NF-κB, Akt, MAPK, Wnt, Notch, p53, AR, and ER pathways, suggesting that these natural products could be useful either alone or in combination with conventional therapeutics for the prevention of tumor progression and/or treatment of human malignancies. The roles of many of these signaling pathways are succinctly presented below in this article.
2. Malfunctioning of cellular signaling in cancer cells
In cancer cells, the altered proteins produced from the mutations or defects of genes impact the way that cell signals communicate with each other. The important cellular signaling pathways which are known to malfunction in cancer cells include NF-κB, Akt, MAPK, Wnt, Notch, p53, AR, ER, etc. among many others.
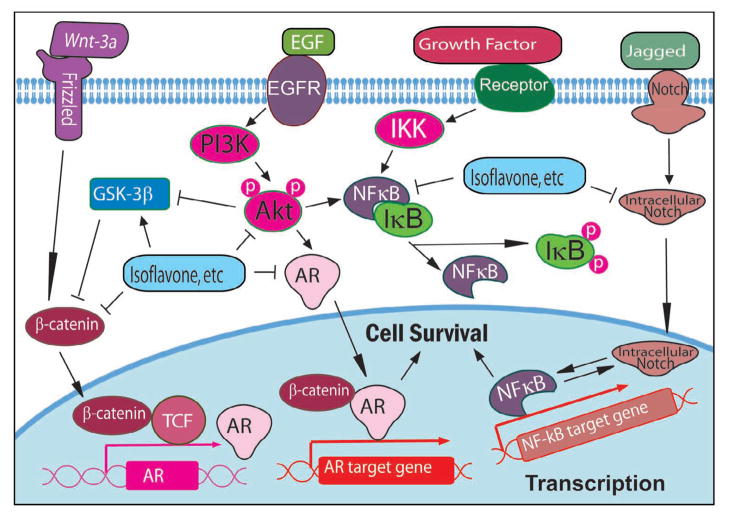
It is now well accepted that nuclear factor-κB (NF-κB) signaling pathway plays important roles in the control of cell growth, apoptosis, inflammation, stress response, and many other physiological processes [3, 29–32]. There are several important molecules such as NF-κB, IκB, IKK, within the NF-κB signaling pathway (Fig 1); however, NF-κB is the key protein in the pathway and has been described as a major culprit and a therapeutic target in cancer [33–36].
Figure 1.
Cellular signaling pathways altered by natural products.
Akt pathways plays critical roles in mammalian cell survival signaling and has been shown to be activated in various cancers [37, 38]. It has been known that Akt is activated by phospholipid binding and phosphorylation at Thr308 by PDK1 or at Ser473 by PDK2 [39]. Activated Akt functions to promote cell survival by inhibiting apoptosis through inactivation of several pro-apoptotic factors including Bad, Forkhead transcription factors, and caspase-9 [40–42]. Studies have also shown that Akt regulate the NF-κB pathway via phosphorylation and activation of molecules in the NF-κB signaling pathway (Fig 1) [43, 44] and thus Akt has also been believed to be an attractive target for cancer prevention or treatment [45].
In addition to NF-κB and Akt pathways, MAPK has also received increasing attention as a target molecule for cancer prevention and therapy. MAPK pathway consists of a three-tiered kinase core where a MAP3K activates a MAP2K that activates a MAPK (ERK, JNK, and p38), resulting in the activation of NF-κB, cell growth, and cell survival [46, 47]. It has been reported that MAPK is activated in several types of cancer and that the activation of MAPK is also linked to cancer angiogenesis, invasion, and metastasis [48].
Wnt signaling plays important roles in the embryonic developmental processes including cell proliferation, differentiation and epithelial-mesenchymal interactions. The aberrant activation of the canonical Wnt/β-catenin signaling is one of the most frequent signaling abnormalities known in human cancer. In human cancer, activated Wnt signal promotes β-catenin accumulation in the nucleus, resulting in the consequent transcriptional activation of specific target genes and the development of cancer (Fig 1). The inappropriate expression of the Wnt ligand and Wnt binding proteins and the inappropriate activation of the Wnt signaling have been found in a variety of human tumors. Therefore, inhibition of aberrant Wnt activity in cancer cell could provide an opportunity for cancer therapy [49–51].
Proper functioning of Notch signaling is required for normal development during early life. It has been found that Notch signaling plays a critical role in the regulation and maintenance of stem cells. Importantly, emerging evidence implicates dysregulation of Notch signaling in the development and progression of a number of cancers [52, 53]. Up-regulation of Notch receptors and their ligands has been observed in cervical, lung, colon, head and neck, renal, and pancreatic cancer, Hodgkin and Large-cell lymphomas [54]. In cancer cells, Notch signaling is abnormally activated, leading to the increased proliferation of cancer cells (Fig 1) and the epithelial to mesenchymal transition; therefore, Notch is now believed to be an important target in cancer therapy.
p53 is a tumor suppressor and transcription factor. p53 is critically involved in many cellular processes including cell signal transduction, cellular response to DNA-damage, genomic stability, cell cycle control, and apoptosis. For the functioning of p53 as a tumor suppressor, wild-type p53 activates the transcription of its downstream genes such as p21WAF1 and Bax to inhibit cell proliferation and induce apoptotic process in DNA damaged cells or cancer cells [55–57] where as the malfunctioning of wild-type p53 attenuates its normal function in cancer. For example, the mutations and the inactivation of p53 have been found in various cancers and the status of p53 determines the cellular response to chemotherapy [58], suggesting that normalization of the function of wild-type p53 could be viewed as novel approach for the treatment of cancer.
The deregulated hormone receptor signaling is also observed in cancer cells. It has been found that androgen receptor (AR) signaling plays important roles in the carcinogenesis and cancer progression through regulation of transcription of androgen-responsive genes (Fig 1) [59]. Prostate specific antigen (PSA), one of the AR-target genes, is a clinically important marker used to monitor diagnosis, progression, and prognosis of patients with prostate cancer. In addition, many environmental chemicals have been found to be estrogenic and have been shown to stimulate the growth of ER-positive human breast cancer cells [60, 61].
It is important to note that the cellular signaling is a complex signal network with positive or negative feedback loops and also regulated by compensatory mechanism. In cancer cells, the deregulations of multi-signaling often exist; therefore, targeting multiple signaling by natural products has opened a new avenue for cancer therapy and it appears to hold great promise. In the following sections, we will summarize the biological effects of several important “natural agents” because those agents appear to have potent biological activity against multiple cancer cell lines and tumor system.
3. Regulation of cellular signaling by natural products (natural agents)
3.1. Isoflavone
Our laboratory has investigated the effects of isoflavone genistein on multiple signaling pathways. Because NF-κB is one of the most important pathways deregulated in cancer cells, we examined NF-κB DNA-binding activity in genistein treated prostate cancer cells by electrophoresis mobility shift assay (EMSA) [62]. We found that genistein significantly inhibited the NF-κB DNA-binding activity in prostate cancer cells. Furthermore, genistein pre-treatment abrogated the activation of NF-κB stimulated by H2O2 or TNF-α. By immunochemistry and confocal microscopic analysis, we found that genistein inhibited the translocation of NF-κB to the nucleus, suggesting that genistein may reduce the NF-κB binding to its target DNA and thereby inhibit the transcription of its target genes. Other investigators have reported similar results in human lung epithelial cells and myeloid cells [63, 64]. To investigate the in vivo effects of genistein on NF-κB signaling, we conducted an in vivo study [65] where we found that when human volunteers received 50 mg of soy isoflavone supplements Novasoy™ (containing genistein, daidzein, and glycitein at a 1.3:1:0.3 ratio) twice daily for three weeks, TNF-α treatment ex-vivo failed to activate NF-κB activity in lymphocytes harvested from these volunteers, while lymphocytes from these volunteers collected prior to soy isoflavone intervention showed activation of NF-κB DNA binding activity upon TNF-α treatment ex-vivo. These results demonstrate that soy isoflavone supplementation has a protective effect against TNF-α induced NF-κB activation in humans, suggesting that soy isoflavone could exert its cancer chemopreventive activity through the regulation of NF-κB signaling. More importantly, we also found that isoflavone genistein could enhance the anti-tumor activity of chemotherapeutic agents via the down-regulation of NF-κB signaling [66], suggesting the therapeutic effects of isoflavone in cancer treatment (Fig 1).
In addition to NF-κB signaling, isoflavone genistein also regulates Akt signaling (Fig 1) [67]. By kinase activity assay and Western blot analysis, we found that genistein did not alter the level of total Akt protein; however, the phosphorylated Akt protein at Ser473 and the Akt kinase activity were decreased after genistein treatment. Genistein pre-treatment also abrogated the activation of Akt by EGF. To further explore the inhibitory mechanism of genistein on Akt and NF-κB pathways, Akt expression construct was transiently co-transfected with NF-κB-Luc reporter construct into PC-3 prostate cancer cells. Luciferase assay showed an increased luciferase activity in PC-3 cells co-transfected with the constructs. However, genistein inhibited the luciferase activity in PC-3 cells co-transfected with the constructs. These results were further confirmed by examining NF-κB DNA-binding activity in transfected cells using EMSA, suggesting that genistein exerts its inhibitory effects on NF-κB pathway through Akt pathway. We also observed similar results in MDA-MB-231 breast cancer cells [68]. Therefore, the down-regulation of NF-κB and Akt signaling pathways by genistein may be one of the molecular mechanisms by which genistein inhibits cancer cell growth and induces apoptosis.
Isoflavone genistein has also been found to inhibit the molecules in MAPK pathway. It has been reported that genistein blocked the activation of p38 MAPK by TGF-β while p38 MAPK was necessary for TGF-β-mediated induction of MMP-2 and cell invasion in prostate cancer [69]. Therefore, genistein could inhibit cancer cell invasion and metastasis by blocking the activation of p38 MAPK.
Recently, we have found that isoflavone up-regulated the expression of GSK-3β, enhanced GSK-3β binding to β-catenin, and increased the phosphorylation of β-catenin, suggesting that isoflavone could inactivate Wnt signaling to inhibit prostate cancer cell growth (Fig 1) [70]. Other investigators also reported that genistein diminished basal and Wnt-1-induced cell proliferation and attenuated Wnt-1 targets c-Myc and Cyclin D1 expression [71] and that isoflavone inhibited the expression of Wnt-5a [72], suggesting the inhibitory effects of isoflavone genistein on Wnt signaling.
The effects of isoflavone genistein on Notch signaling have been documented in several reports. We have found that genistein inhibited Notch signaling, leading to the down-regulation of NF-κB activity, the inhibition of cell proliferation, and the induction of apoptosis in pancreatic cancer cells (Fig 1) [73, 74]. Other investigators have reported that genistein could inhibit the expression of Notch-2 [72], which is consistent with our findings. These results suggest that genistein could inhibit cancer cell growth and induce apoptosis through the inhibition of Notch signaling.
To investigate the effects of genistein on p53 pathway, we measured cell growth inhibition, apoptosis, and gene expression related to apoptosis in genistein treated H460 lung cancer cells, which harbor wild type p53, and H322 lung cancer cells that possess a mutation in the p53 gene [75, 76]. Genistein was found to inhibit both H460 and H322 cell growth in a dose dependent manner and induce apoptosis in both cell lines. The expression of Bax and p21WAF1 was up-regulated in both H460 and H322 cells treated with genistein. Importantly, significantly increased p53 protein was detected in genistein-treated H460 cells, while no change in p53 expression was observed in H322 cells treated with genistein. These results suggest that genistein induces apoptosis in lung cancer cells through p53 independent pathway and, thus, may act as an anti-cancer agent regardless of the status of p53 in cancer cells.
AR signaling is also a target of genistein (Fig 1). We have previously found that genistein transcriptionally down-regulated AR, decreased nuclear AR binding to androgen responsive element (ARE) and, thereby, inhibited the transcription and protein expression of PSA in androgen-sensitive LNCaP cells [77, 78]. Other investigators also found that dietary genistein down-regulated the expression of AR in the rat prostate at concentrations comparable to those found in humans on a soy diet [79]. Recently, we have reported that isoflavone-induced inhibition of cell proliferation and induction of apoptosis are partly mediated through the regulation of the Akt/FOXO3a/GSK-3β/AR signaling network [70]. Based on these findings, we believe that the down-regulation of AR expression could be an important strategy for the prevention and/or treatment of prostate cancer, especially hormone refractory (castrate resistant) prostate cancer.
Because of the structural similarity to estrogen, isoflavones have been believed to exert their effects through ER signaling pathway. However, experimental study has found that isoflavones at different concentration may exhibit different effects [80]. Genistein at concentrations ≤1 μM may induce breast cancer cell proliferation by estrogenic agonistic properties, while genistein at 50 and 100 μM significantly arrested the growth of MCF-7 cells at G2/M phase and down-regulated mRNA expression of ERα [81], suggesting that the inhibitory action of genistein on human breast cancer cells appears to be partially mediated by the alteration of ER-dependent pathways. However, experimental studies also showed that isoflavones exert their inhibitory effects on ER-negative MDA-MB-231 breast cancer cells [82], suggesting that isoflavones may exert their effects through ER-dependent or independent pathway.
3.2. I3C and DIM
Both I3C and DIM have been found to regulate NF-κB signaling. Our laboratory have investigated whether I3C treatment could modulate NF-κB DNA binding activity in PC-3 prostate cancer cells by EMSA [14]. The results showed that I3C significantly inhibited NF-κB DNA binding activity with induction of apoptosis in PC-3 prostate cancer cells. We have also found that DIM could inhibit NF-κB DNA binding activity in PC-3, LNCaP, and C4-2B prostate cancer cells [83, 84], suggesting that inhibition of NF-κB signaling pathway may be one of the molecular mechanisms by which I3C and DIM induce apoptosis in cancer cells.
I3C and DIM also participate in the regulation of Akt signaling. We found that the phosphorylated Akt protein at Ser473 was decreased in I3C or DIM treated prostate cancer cells [84, 85]. Akt kinase assay also showed a decrease in the Akt kinase activity in I3C or DIM treated prostate cancer cells, suggesting the inactivation of Akt after I3C or DIM treatment. From the gene expression profiles of PC-3 cells exposed to I3C, we found down-regulation of PI3K expression, which is consistent with our results showing inactivation of Akt kinase by I3C [15]. These data suggest that I3C and DIM inhibited Akt signaling pathway, which may contribute to the inhibition of cell proliferation and the induction of apoptotic cell death.
We have further conducted microarray analysis to determine the alternation of gene expression profiles of PC-3 prostate cancer cells exposed to I3C or DIM [15]. From microarray data, we found that I3C and DIM treatments down-regulated the expression of MAP2K3, MAP2K4, MAP4K3, and MAPK3, suggesting the inhibitory effects of I3C and DIM on MAPK pathway. Other investigators also reported that the effects of DIM were mediated by cross talk between the protein kinase A and MAPK signaling pathways [86]. Therefore, the down-regulation of the important molecules in MAPK pathway may result in the inhibition of cancer cell survival.
It has been known that there is a crosstalk between Akt and Wnt signaling pathways through the signal communication between GSK-3β and β-catenin, two of the important molecules in Akt and Wnt pathways. Because DIM inhibits the activation of Akt, it could also inhibit Wnt activation through the crosstalk between Akt and Wnt signaling. Indeed, we found that DIM significantly increased the phosphorylation of β-catenin and inhibited β-catenin nuclear translocation [87], suggesting that DIM could also down-regulate the activation of Wnt signaling.
Several studies have focused on the potential effects of I3C and DIM on the proliferation and induction of apoptosis in human prostate cancer cell lines with different p53 status. It has been found that the induction of apoptosis by I3C was p53-independent [88]. Also, the induction of p21WAF1 expression by DIM was independent of p53 status [89].
Le et al. have reported that DIM inhibited the AR nuclear translocation, the PSA expression, and the cell proliferation induced by dihydrotestosterone (DHT) in LNCaP cells [90]. We have also found that DIM significantly inhibited Akt activation, NF-κB DNA binding activity, AR phosphorylation, AR nuclear translocation, and the expressions of AR and PSA, suggesting that DIM could interrupt the cross-talk between Akt/NF-κB and AR [83]. These results demonstrate that DIM-induced inhibition of cell proliferation and induction of apoptosis are partly mediated through the down-regulation of AR, Akt, and NF-κB signaling. In further studies focusing on the molecular effects of DIM on Akt and AR signaling, we found that DIM significantly decreased the phosphorylation of Akt and FOXO3a, inhibited FOXO3a binding to the promoter of AR, and promoted FOXO3a binding to the p27KIP1 promoter, resulting in the alteration of AR and p27KIP1 expression, the inhibition of cell proliferation, and the induction of apoptosis in both androgen-sensitive and -insensitive prostate cancer cells [87]. These results further confirm that DIM-induced inhibition of cell proliferation and induction of apoptosis are partly mediated through the regulation of Akt/FOXO3a/AR signaling.
I3C has been known to be a negative regulator of estrogen. I3C significantly inhibited the transcriptional activity of ERα, the estradiol-activated ERα signaling, and the expression of the estrogen-responsive genes [91]. When cells were treated with I3C and genistein, a synergistic effect of I3C and genistein was observed on the increase in GADD (Growth Arrest and DNA Damage) expression, the induction of apoptosis, and decrease in gene expression driven by ERα in MCF-7 breast cancer cells [92]. We and others also found that I3C and DIM could inhibit the proliferation of human breast cancer cells which are ER negative [93, 94], suggesting that anti-tumor activities of I3C and DIM could be ER independent.
3.3. Curcumin
It has been well known that curcumin is a strong inhibitor of NF-κB. Curcumin inhibited IKK, suppressed both constitutive and inducible NF-κB activation, and potentiated TNF-induced apoptosis [95]. Recent studies have shown that curcumin suppresses constitutive activation of NF-κB [96] and sensitizes human colorectal cancer xenografts in nude mice to γ-radiation by targeting NF-κB-regulated gene products [97]. It has also been reported that treatment with a liposomal formulation of curcumin resulted in a dose-dependent growth suppression of cancer cells and a decreased activation of NF-κB [98]. Expression of NF-κB target genes including cyclin D1, cyclooxygenase-2, matrix metalloproteinase-9, Bcl-2, Bcl-xL, Mcl-1L, and Mcl-1S were reduced, indicating the effect of curcumin on the NF-κB pathway. Moreover, clinical trial showed that curcumin down-regulated the expression of NF-κB and cyclooxygenase-2 in peripheral blood mononuclear cells from patients with pancreatic cancer [99]. These results clearly demonstrate that curcumin could inhibit NF-κB signaling in vitro and in vivo.
Curcumin also shows the inhibitory effect on Akt signaling. Recent studies have shown that curcumin dose- and time-dependently inhibited the phosphorylation of Akt, mTOR, and their downstream targets in prostate cancer cells [100]. Curcumin also inhibited the proliferation of cisplatin-resistant ovarian cancer cells through the inhibition of Akt activation [101]. It has also been reported that an analogue of curcumin, 4-hydroxy-3-methoxybenzoic acid methyl ester (HMBME), targeted the Akt signaling pathway, inhibited the proliferation of cancer cells and induced apoptosis [102]. Likewise, HMBME decreased the level of phosphorylated Akt, inhibited Akt kinase activity, and reduced DNA-binding activity of NF-κB [102]. Several other reports also suggest that curcumin has molecular targets within the Akt signaling pathways and that the inhibition of Akt activity may facilitate inhibition of proliferation and induction of apoptosis in cancer cells [103, 104].
The ability of curcumin to modulate MAPK signaling pathway might contribute to the inhibition of inflammation and cancer cell growth by curcumin. Curcumin has been known to inhibit the MAPK activation and the translocation of NF-κB [105]. The NF-κB targeted effects of curcumin could also be due to the inhibition of proteasome activity as reported recently by our group [106]. It has been reported that curcumin is able to attenuate experimental colitis through a reduction in the activity of p38 MAPK [107]. Gene expression profiles of curcumin treated cells showed that curcumin down-regulated the expression of MEKK4, MKK4, and JNK [108], suggesting its inhibitory effect on MAPK signaling.
Curcumin also showed its inhibitory effects on Wnt signaling. A recent study has shown that curcumin suppressed β-catenin response transcription activated by Wnt3a and inhibited the growth of various colon cancer cells [109]. It has been found that curcumin induced caspase-3-mediated degradation of β-catenin [110], leading to the decreased binding of β-catenin to TCF and the inactivation of Wnt signaling. Curcumin also down-regulated p300, which is a positive regulator of the Wnt/β-catenin pathway [109]. Gene expression profile analysis also showed that the expression of Frizzled-1 (Wnt receptor) was most strongly attenuated by curcumin [108]. Therefore, curcumin could inhibit cancer cell growth through the inhibition of Wnt signaling.
The effects of curcumin on cell growth, activation of signal transduction, and transforming activities in both androgen-dependent and independent prostate cell lines have been evaluated. It has been found that curcumin down-regulates the transactivation and expression of AR and AR-related molecules (AP-1 and NF-κB), and reduces colony formation in soft agar [111]. A number of curcumin analogues was evaluated as potential androgen receptor antagonists in the presence of AR and AR coactivator, ARA70 [112]. The results showed that some curcumin analogs possessed potent anti-androgenic activities and were superior to hydroxyflutamide, which is the currently available anti-androgen for the treatment of prostate cancer. Structure-activity relationship studies demonstrated that some moieties seem to be important factors related to the anti-androgenic activity. Therefore, these compounds could serve as a new class of anti-androgenic agents to control AR-mediated prostate cancer growth.
3.4. EGCG
It has been known that EGCG treatment could lead to a significant dose- and time-dependent inhibition of activation and translocation of NF-κB to the nucleus by suppressing the degradation of IκBα in the cytoplasm [113, 114]. EGCG could also inhibit the ATP-induced activation of NF-κB [115] and the activation of NF-κB induced by IL-1β [116]. It has been found that EGCG could stabilize p53 and negatively regulate NF-κB activity, leading to the change in the ratio of Bax/Bcl-2 in a manner that favors apoptosis [19].
EGCG has been found to inhibit PI3K/Akt activation that, in turn, resulted in the modulation of Bcl-2 family proteins, leading to the enhanced apoptosis of bladder cancer cells [117]. EGCG also inhibited VEGF-induced angiogenesis in vitro through suppression of VE-cadherin phosphorylation and inactivation of Akt molecule, suggesting inhibitory effect of EGCG on Akt signaling pathway [118]. Further studies have also shown that the cells treated with EGCG inhibited the constitutive activation of the Akt, EGFR, and Stat3 in both YCU-H891 head and neck squamous cell carcinoma and MDA-MB-231 breast carcinoma cell lines [119].
The reported effects of EGCG on MAPK pathway are controversial. EGCG showed strong inhibition of MAPK activities in transformed NIH-pATM ras fibroblasts [120]. EGCG also inhibited the phosphorylation of extracellular signal-regulated kinases 1 and 2 (ERK1/2), and suppressed p38 MAPK activity in human fibrosarcoma HT1080 cells [121]. However, EGCG has also been found to activate all three MAPKs (ERK, JNK and p38) in a dose- and time-dependent manner in human hepatoma HepG2-C8 cells [122]. It has been reported that activation of MAPK by low concentration of EGCG results in induction of ARE-mediated gene expression, whereas higher concentration of EGCG causes activation of MAPKs such as JNK leading to apoptosis [122].
Wnt signaling has been found to be inhibited by EGCG in a dose-dependent manner in breast cancer cells [123]. EGCG treatment induced transcription of HBP1 which is a suppressor of Wnt signaling. EGCG reduced both breast cancer cell tumorigenic proliferation and invasiveness through the induction of HBP1 and the subsequent inhibition of Wnt signaling [123].
It has been found that EGCG treatment resulted in a dose-dependent increase of p53 in LNCaP cells which carry wild-type p53, but not in DU145 cells carrying mutant p53 [124]. EGCG also induced stabilization of p53 and caused an up-regulation of its transcriptional activity, thereby resulting in the activation of its downstream targets such as p21WAF1 and Bax and the induction of apoptosis. In human liver cancer cells, EGCG also significantly increased the expression of p53 and p21WAF1 protein, leading to the cell cycle arrest [125].
The inhibitory effects of EGCG on AR signaling have been reported in prostate cancer in vitro and in vivo. EGCG inhibited LNCaP cell growth and the expression of AR in both mRNA and protein levels [126]. Moreover, EGCG showed a significant inhibitory effect on the androgenic inducibility of the PSA promoter and the expression of PSA. In different LNCaP sub-lines which represent different stages of prostate cancer, EGCG suppressed cell proliferation, the AR transcriptional activity, and the PSA expression at all stages [127]. Furthermore, EGCG in vivo significantly reduced cell proliferation, induced apoptosis, and decreased expression of AR, IGF-1, COX-2, and iNOS in TRAMP mice [128], suggesting the inhibitory effects of EGCG on AR signaling in vitro and in vivo.
EGCG has also been found to bind to ERα and ERβ, and elicit ER-mediated gene expression in vitro. The in vitro and in vivo studies have showed that polyphenolic catechins (EGCG and ECG) from green tea binds to ERα and ERβ, and inhibited breast cancer cell proliferation and tumor growth; but only EGCG elicited ER-mediated gene expression [129], suggesting that polyphenolic catechins may exert their chemopreventive effects through ER-dependent or independent pathway.
3.5. Resveratrol
Resveratrol have shown its inhibitory effects on the activity of NF-κB [130]. It has been found that resveratrol inhibited the TPA induced NF-κB and Cox-2 expression [131] and the ultraviolet B exposure-mediated activation of NF-κB [132]. Further studies have demonstrated that TPA treatment led to rapid induction of IKK activity, which was abolished either by resveratrol or an IKK inhibitor Bay 11-7082 [133], suggesting that resveratrol could inhibit TPA-induced expression of COX-2 and activation of NF-κB through blocking I B kinase activity.
It has been found that resveratrol inhibited skin tumorigenesis through the regulation of PI3K and Akt proteins which are implicated in cancer development and progression [134]. In LNCaP prostate cancer cells, resveratrol also dose-dependently inhibited constitutive expression of PI3K and activated Akt [135], suggesting that resveratrol inhibits cancer cell growth and induces apoptosis partly through the down-regulation of PI3K/Akt signaling.
Resveratrol has shown to significantly decrease the level of β-catenin in the nucleus of colon cancer cells. The decreased nuclear localization of β-catenin by resveratrol treatment could be due to reduced expression of lgs and pygoI which are regulators of β-catenin localization [136]. In addition to the solid tumor, resveratrol also inhibited proliferation and induced cell cycle arrest and apoptosis in Waldenstrom’s macroglobulinemia cells. These effects of resveratrol were found to be mediated via the down-regulation of Akt, MAPK, and Wnt signaling pathways [137].
It has been found that resveratrol-induced apoptosis is associated with the activation of the p53 in a dose- and a time-dependent manner [138]. Resveratrol also modulates DNA double-strand break repair pathways in p53-dependent manner [139], suggesting the regulatory effect of resveratrol on p53 signaling.
Resveratrol has been found to inhibit the expression of AR, leading to the repression of androgen up-regulated genes including PSA and AR-specific coactivator ARA70 at protein or mRNA level [140]. However, another study showed that the decreased PSA expression by resveratrol in LNCaP cells was mediated by an AR-independent mechanism [141]. Resveratrol could also down-regulate AR at the post-translational level [142], suggesting the complex mechanisms of the action of resveratrol on AR signaling.
3.6. Lycopene
Experimental studies have shown that lycopene inhibits cell growth in breast, prostate and endometrial cancer cells with regulation of cell cycle-related genes [27, 28]. It has been found that lycopene significantly inhibited the binding activity of NF-κB and the expression of NF-κB target gene MMP-9, leading to the inhibition of invasion of human hepatoma cells [143]. The inhibition of NF-κB DNA binding activity by lycopene was mediated through the down-regulation of IκB phosphorylation, NF-κB expression, and NF-κB p65 subunit translocation from cytosol to nucleus [144]. Lipopolysaccharide (LPS) stimulation has been known to activate the MAPK and NF-κB signal pathways. Another study has shown that pretreatment with 10 μM lycopene markedly inhibited the LPS-induced up-regulation of p-ERK, p-p38, p-JNK, and NF-κB [145], suggesting the inhibitory effects of lycopene on MAPK and NF-κB signaling.
In colon cancer cells, lycopene showed inhibitory effect on Akt signaling and cell proliferation [146]. Lycopene treatment suppressed Akt activation and non-phosphorylated activated β-catenin, and increased the phosphorylated form of β-catenin proteins and the expression of CDK inhibitor p27Kip1 [146]. Lycopene also induced apoptosis through down-regulation of pAkt, cyclin D1, and pBad [147], suggesting its inhibitory effect on Akt signaling. Lycopene consumption is inversely related to human prostate cancer risk. It has been reported that lycopene inhibited IGF-1 mediated Akt and AR signaling in rat prostate cancer [148]. Lycopene reduced AR and β-catenin nuclear localization and inhibited IGF-1-stimulated prostate cancer growth, perhaps by attenuating effects of IGF-1 on phosphorylation of Akt and GSK3β. Clinical trial have revealed that lycopene supplements could reduce tumor size and PSA level in localized prostate cancers [149], which is consistent with the down-regulation of AR nuclear translocation that was found during in vitro studies.
4. Summary and perspectives
The data from in vivo human and animal studies and in vitro experiments clearly indicate that natural products (natural agents) including isoflavones, I3C, DIM, curcumin, EGCG, resveratrol, and lycopene exerts their inhibitory effects on carcinogenesis and cancer progression. These effects have been believed to be mediated through the regulation of multiple cell signaling pathways including NF-κB, Akt, MAPK, Wnt, Notch, p53, AR, and ER pathways among others. Because of the complex communication between cell signaling networks, cancer cells always show alterations in multiple cellular signaling pathways. Therefore, regulation of multiple cell signaling pathways for controlling the behavior of cancer cells such as inhibition of cell growth and induction of apoptosis requires agents that could target multiple pathways, and it is now believed that many of these natural products are perfect example showing that these natural agents could target multiple pathways. Hence we believe that these non-toxic products harvested from the bounties of nature could be useful either alone or in combination with conventional therapeutics (chemotherapy and radiotherapy) for the prevention of tumor progression and/or treatment of most human malignancies. However, further in-depth mechanistic studies in vitro, and appropriate and relevant animal model studies in vivo are needed together with novel clinical trials in order to fully appreciate the value of these and other natural products in human health and diseases in the future.
Acknowledgments
The authors’ work cited in this reviews was funded by grants from the National Cancer Institute, NIH (5R01CA108535, 5R01CA083695, and 5R01CA101870 awarded to FHS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaw AS, Filbert EL. Nat Rev Immunol. 2009;9(1):47. doi: 10.1038/nri2473. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS. Cancer Cell. 2003;4(3):167. doi: 10.1016/s1535-6108(03)00216-2. [DOI] [PubMed] [Google Scholar]
- 3.Karin M, Cao Y, Greten FR, Li ZW. Nat Rev Cancer. 2002;2(4):301. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 4.Klaus A, Birchmeier W. Nat Rev Cancer. 2008;8(5):387. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 5.Sebolt-Leopold JS, Herrera R. Nat Rev Cancer. 2004;4(12):937. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 6.Stiewe T. Nat Rev Cancer. 2007;7(3):165. doi: 10.1038/nrc2072. [DOI] [PubMed] [Google Scholar]
- 7.Lee MM, Gomez SL, Chang JS, Wey M, Wang RT, Hsing AW. Cancer Epidemiol Biomarkers Prev. 2003;12(7):665. [PubMed] [Google Scholar]
- 8.Mukhtar H, Ahmad N. Toxicol Sci. 1999;52(2 Suppl):111. doi: 10.1093/toxsci/52.2.111. [DOI] [PubMed] [Google Scholar]
- 9.Smith-Warner SA, Spiegelman D, Yaun SS, Albanes D, Beeson WL, van den Brandt PA, Feskanich D, Folsom AR, Fraser GE, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Kushi LH, Miller AB, Pietinen P, Rohan TE, Speizer FE, Willett WC, Hunter DJ. Int J Cancer. 2003;107(6):1001. doi: 10.1002/ijc.11490. [DOI] [PubMed] [Google Scholar]
- 10.Surh YJ. Nat Rev Cancer. 2003;3(10):768. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 11.Khan N, Afaq F, Mukhtar H. Carcinogenesis. 2007;28(2):233. doi: 10.1093/carcin/bgl243. [DOI] [PubMed] [Google Scholar]
- 12.Lamartiniere CA, Cotroneo MS, Fritz WA, Wang J, Mentor-Marcel R, Elgavish A. J Nutr. 2002;132(3):552S. doi: 10.1093/jn/132.3.552S. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Sarkar FH. J Nutr. 2002;132(12):3623. doi: 10.1093/jn/132.12.3623. [DOI] [PubMed] [Google Scholar]
- 14.Chinni SR, Li Y, Upadhyay S, Koppolu PK, Sarkar FH. Oncogene. 2001;20(23):2927. doi: 10.1038/sj.onc.1204365. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Li X, Sarkar FH. J Nutr. 2003;133(4):1011. doi: 10.1093/jn/133.4.1011. [DOI] [PubMed] [Google Scholar]
- 16.Choudhuri T, Pal S, Agwarwal ML, Das T, Sa G. FEBS Lett. 2002;512(1–3):334. doi: 10.1016/s0014-5793(02)02292-5. [DOI] [PubMed] [Google Scholar]
- 17.Mukhopadhyay A, Bueso-Ramos C, Chatterjee D, Pantazis P, Aggarwal BB. Oncogene. 2001;20(52):7597. doi: 10.1038/sj.onc.1204997. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Hussain T, Mukhtar H. Arch Biochem Biophys. 2003;410(1):177. doi: 10.1016/s0003-9861(02)00668-9. [DOI] [PubMed] [Google Scholar]
- 19.Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Oncogene. 2003;22(31):4851. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- 20.Barnes S. Breast Cancer Res Treat. 1997;46(2–3):169. doi: 10.1023/a:1005956326155. [DOI] [PubMed] [Google Scholar]
- 21.Dixon RA, Ferreira D. Phytochemistry. 2002;60(3):205. doi: 10.1016/s0031-9422(02)00116-4. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Sarkar FH. Cancer Lett. 2002;186(2):157. doi: 10.1016/s0304-3835(02)00349-x. [DOI] [PubMed] [Google Scholar]
- 23.Shao ZM, Shen ZZ, Liu CH, Sartippour MR, Go VL, Heber D, Nguyen M. Int J Cancer. 2002;98(2):234. doi: 10.1002/ijc.10183. [DOI] [PubMed] [Google Scholar]
- 24.Katiyar SK, Afaq F, Perez A, Mukhtar H. Carcinogenesis. 2001;22(2):287. doi: 10.1093/carcin/22.2.287. [DOI] [PubMed] [Google Scholar]
- 25.Kundu JK, Surh YJ. Cancer Lett. 2008;269(2):243. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 26.Whyte L, Huang YY, Torres K, Mehta RG. Cancer Res. 2007;67(24):12007. doi: 10.1158/0008-5472.CAN-07-2464. [DOI] [PubMed] [Google Scholar]
- 27.Nahum A, Zeller L, Danilenko M, Prall OW, Watts CK, Sutherland RL, Levy J, Sharoni Y. Eur J Nutr. 2006;45(5):275. doi: 10.1007/s00394-006-0595-x. [DOI] [PubMed] [Google Scholar]
- 28.Nahum A, Hirsch K, Danilenko M, Watts CK, Prall OW, Levy J, Sharoni Y. Oncogene. 2001;20(26):3428. doi: 10.1038/sj.onc.1204452. [DOI] [PubMed] [Google Scholar]
- 29.Storz P, Toker A. Cell Cycle. 2003;2(1):9. doi: 10.4161/cc.2.1.234. [DOI] [PubMed] [Google Scholar]
- 30.Lin A, Karin M. Semin Cancer Biol. 2003;13(2):107. doi: 10.1016/s1044-579x(02)00128-1. [DOI] [PubMed] [Google Scholar]
- 31.Li Q, Verma IM. Nat Rev Immunol. 2002;2(10):725. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto Y, Gaynor RB. Curr Mol Med. 2001;1(3):287. doi: 10.2174/1566524013363816. [DOI] [PubMed] [Google Scholar]
- 33.Bharti AC, Aggarwal BB. Biochem Pharmacol. 2002;64(5–6):883. doi: 10.1016/s0006-2952(02)01154-1. [DOI] [PubMed] [Google Scholar]
- 34.Biswas DK, Dai SC, Cruz A, Weiser B, Graner E, Pardee AB. Proc Natl Acad Sci U S A. 2001;98(18):10386. doi: 10.1073/pnas.151257998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haefner B. Drug Discov Today. 2002;7(12):653. doi: 10.1016/s1359-6446(02)02309-7. [DOI] [PubMed] [Google Scholar]
- 36.Orlowski RZ, Baldwin AS. Trends Mol Med. 2002;8(8):385. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- 37.Clarke RB. Breast Cancer Res. 2003;5(3):162. doi: 10.1186/bcr596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA. Leukemia. 2003;17(3):590. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 39.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. EMBO J. 1996;15(23):6541. [PMC free article] [PubMed] [Google Scholar]
- 40.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Cell. 1999;96(6):857. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 41.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Science. 1998;282(5392):1318. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 42.Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Science. 1999;286(5445):1738. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 43.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. Nature. 1999;401(6748):82. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 44.Romashkova JA, Makarov SS. Nature. 1999;401(6748):86. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 45.Hill MM, Hemmings BA. Pharmacol Ther. 2002;93(2–3):243. doi: 10.1016/s0163-7258(02)00193-6. [DOI] [PubMed] [Google Scholar]
- 46.Sebolt-Leopold JS. Oncogene. 2000;19(56):6594. doi: 10.1038/sj.onc.1204083. [DOI] [PubMed] [Google Scholar]
- 47.Seger R, Krebs EG. FASEB J. 1995;9(9):726. [PubMed] [Google Scholar]
- 48.Fang JY, Richardson BC. Lancet Oncol. 2005;6(5):322. doi: 10.1016/S1470-2045(05)70168-6. [DOI] [PubMed] [Google Scholar]
- 49.Dihlmann S, von Knebel DM. Int J Cancer. 2005;113(4):515. doi: 10.1002/ijc.20609. [DOI] [PubMed] [Google Scholar]
- 50.Smalley MJ, Dale TC. Cancer Metastasis Rev. 1999;18(2):215. doi: 10.1023/a:1006369223282. [DOI] [PubMed] [Google Scholar]
- 51.Barker N, Clevers H. Nat Rev Drug Discov. 2006;5(12):997. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 52.Rizzo P, Osipo C, Foreman K, Golde T, Osborne B, Miele L. Oncogene. 2008;27(38):5124. doi: 10.1038/onc.2008.226. [DOI] [PubMed] [Google Scholar]
- 53.Zardawi SJ, O’Toole SA, Sutherland RL, Musgrove EA. Histol Histopathol. 2009;24(3):385. doi: 10.14670/HH-24.385. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, Li Y, Banerjee S, Sarkar FH. Cancer Lett. 2008 [Google Scholar]
- 55.el Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. Cell. 1993;75(4):817. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 56.Vogelstein B, Kinzler KW. Cell. 1992;70(4):523. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- 57.el Deiry WS. Semin Cancer Biol. 1998;8(5):345. doi: 10.1006/scbi.1998.0097. [DOI] [PubMed] [Google Scholar]
- 58.Manic S, Gatti L, Carenini N, Fumagalli G, Zunino F, Perego P. Curr Cancer Drug Targets. 2003;3(1):21. doi: 10.2174/1568009033333727. [DOI] [PubMed] [Google Scholar]
- 59.Luke MC, Coffey DS. J Androl. 1994;15(1):41. [PubMed] [Google Scholar]
- 60.Safe SH. Environ Health Perspect. 2000;108(6):487. doi: 10.1289/ehp.00108487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Environ Health Perspect. 2003;111(8):994. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis JN, Kucuk O, Sarkar FH. Nutr Cancer. 1999;35(2):167. doi: 10.1207/S15327914NC352_11. [DOI] [PubMed] [Google Scholar]
- 63.Chen CC, Sun YT, Chen JJ, Chiu KT. J Immunol. 2000;165(5):2719. doi: 10.4049/jimmunol.165.5.2719. [DOI] [PubMed] [Google Scholar]
- 64.Natarajan K, Manna SK, Chaturvedi MM, Aggarwal BB. Arch Biochem Biophys. 1998;352(1):59. doi: 10.1006/abbi.1998.0576. [DOI] [PubMed] [Google Scholar]
- 65.Davis JN, Kucuk O, Djuric Z, Sarkar FH. Free Radic Biol Med. 2001;30(11):1293. doi: 10.1016/s0891-5849(01)00535-4. [DOI] [PubMed] [Google Scholar]
- 66.Li Y, Ahmed F, Ali S, Philip PA, Kucuk O, Sarkar FH. Cancer Res. 2005;65(15):6934. doi: 10.1158/0008-5472.CAN-04-4604. [DOI] [PubMed] [Google Scholar]
- 67.Li Y, Sarkar FH. Clin Cancer Res. 2002;8(7):2369. [PubMed] [Google Scholar]
- 68.Gong L, Li Y, Nedeljkovic-Kurepa A, Sarkar FH. Oncogene. 2003;22(30):4702. doi: 10.1038/sj.onc.1206583. [DOI] [PubMed] [Google Scholar]
- 69.Huang X, Chen S, Xu L, Liu Y, Deb DK, Platanias LC, Bergan RC. Cancer Res. 2005;65(8):3470. doi: 10.1158/0008-5472.CAN-04-2807. [DOI] [PubMed] [Google Scholar]
- 70.Li Y, Wang Z, Kong D, Li R, Sarkar SH, Sarkar FH. J Biol Chem. 2008;283(41):27707. doi: 10.1074/jbc.M802759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Su Y, Simmen RC. Carcinogenesis. 2008 doi: 10.1093/carcin/bgn279. [DOI] [PubMed] [Google Scholar]
- 72.Su Y, Simmen FA, Xiao R, Simmen RC. Physiol Genomics. 2007;30(1):8. doi: 10.1152/physiolgenomics.00023.2007. [DOI] [PubMed] [Google Scholar]
- 73.Wang Z, Zhang Y, Li Y, Banerjee S, Liao J, Sarkar FH. Mol Cancer Ther. 2006;5(3):483. doi: 10.1158/1535-7163.MCT-05-0299. [DOI] [PubMed] [Google Scholar]
- 74.Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Int J Cancer. 2006;118(8):1930. doi: 10.1002/ijc.21589. [DOI] [PubMed] [Google Scholar]
- 75.Lian F, Bhuiyan M, Li YW, Wall N, Kraut M, Sarkar FH. Nutr Cancer. 1998;31(3):184. doi: 10.1080/01635589809514701. [DOI] [PubMed] [Google Scholar]
- 76.Lian F, Li Y, Bhuiyan M, Sarkar FH. Nutr Cancer. 1999;33(2):125. doi: 10.1207/S15327914NC330202. [DOI] [PubMed] [Google Scholar]
- 77.Davis JN, Muqim N, Bhuiyan M, Kucuk O, Pienta KJ, Sarkar FH. Int J Oncol. 2000;16(6):1091. doi: 10.3892/ijo.16.6.1091. [DOI] [PubMed] [Google Scholar]
- 78.Davis JN, Kucuk O, Sarkar FH. Mol Carcinog. 2002;34(2):91. doi: 10.1002/mc.10053. [DOI] [PubMed] [Google Scholar]
- 79.Fritz WA, Wang J, Eltoum IE, Lamartiniere CA. Mol Cell Endocrinol. 2002;186(1):89. doi: 10.1016/s0303-7207(01)00663-3. [DOI] [PubMed] [Google Scholar]
- 80.Martin PM, Horwitz KB, Ryan DS, McGuire WL. Endocrinology. 1978;103(5):1860. doi: 10.1210/endo-103-5-1860. [DOI] [PubMed] [Google Scholar]
- 81.Chen WF, Huang MH, Tzang CH, Yang M, Wong MS. Biochim Biophys Acta. 2003;1638(2):187. doi: 10.1016/s0925-4439(03)00082-6. [DOI] [PubMed] [Google Scholar]
- 82.Li Y, Upadhyay S, Bhuiyan M, Sarkar FH. Oncogene. 1999;18(20):3166. doi: 10.1038/sj.onc.1202650. [DOI] [PubMed] [Google Scholar]
- 83.Bhuiyan MM, Li Y, Banerjee S, Ahmed F, Wang Z, Ali S, Sarkar FH. Cancer Res. 2006;66(20):10064. doi: 10.1158/0008-5472.CAN-06-2011. [DOI] [PubMed] [Google Scholar]
- 84.Li Y, Chinni SR, Sarkar FH. Front Biosci. 2005;10:236. doi: 10.2741/1523. [DOI] [PubMed] [Google Scholar]
- 85.Chinni SR, Sarkar FH. Clin Cancer Res. 2002;8(4):1228. [PubMed] [Google Scholar]
- 86.Leong H, Riby JE, Firestone GL, Bjeldanes LF. Mol Endocrinol. 2004;18(2):291. doi: 10.1210/me.2003-0196. [DOI] [PubMed] [Google Scholar]
- 87.Li Y, Wang Z, Kong D, Murthy S, Dou QP, Sheng S, Reddy GP, Sarkar FH. J Biol Chem. 2007;282(29):21542. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- 88.Nachshon-Kedmi M, Yannai S, Haj A, Fares FA. Food Chem Toxicol. 2003;41(6):745. doi: 10.1016/s0278-6915(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 89.Hong C, Kim HA, Firestone GL, Bjeldanes LF. Carcinogenesis. 2002;23(8):1297. doi: 10.1093/carcin/23.8.1297. [DOI] [PubMed] [Google Scholar]
- 90.Le HT, Schaldach CM, Firestone GL, Bjeldanes LF. J Biol Chem. 2003;278(23):21136. doi: 10.1074/jbc.M300588200. [DOI] [PubMed] [Google Scholar]
- 91.Meng Q, Yuan F, Goldberg ID, Rosen EM, Auborn K, Fan S. J Nutr. 2000;130(12):2927. doi: 10.1093/jn/130.12.2927. [DOI] [PubMed] [Google Scholar]
- 92.Auborn KJ, Fan S, Rosen EM, Goodwin L, Chandraskaren A, Williams DE, Chen D, Carter TH. J Nutr. 2003;133(7 Suppl):2470S. doi: 10.1093/jn/133.7.2470s. [DOI] [PubMed] [Google Scholar]
- 93.Cover CM, Hsieh SJ, Tran SH, Hallden G, Kim GS, Bjeldanes LF, Firestone GL. J Biol Chem. 1998;273(7):3838. doi: 10.1074/jbc.273.7.3838. [DOI] [PubMed] [Google Scholar]
- 94.Rahman KW, Li Y, Wang Z, Sarkar SH, Sarkar FH. Cancer Res. 2006;66(9):4952. doi: 10.1158/0008-5472.CAN-05-3918. [DOI] [PubMed] [Google Scholar]
- 95.Bharti AC, Donato N, Singh S, Aggarwal BB. Blood. 2003;101(3):1053. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 96.Hussain AR, Ahmed M, Al-Jomah NA, Khan AS, Manogaran P, Sultana M, Abubaker J, Platanias LC, Al-Kuraya KS, Uddin S. Mol Cancer Ther. 2008;7(10):3318. doi: 10.1158/1535-7163.MCT-08-0541. [DOI] [PubMed] [Google Scholar]
- 97.Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, Aggarwal BB, Krishnan S. Clin Cancer Res. 2008;14(7):2128. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 98.Wang D, Veena MS, Stevenson K, Tang C, Ho B, Suh JD, Duarte VM, Faull KF, Mehta K, Srivatsan ES, Wang MB. Clin Cancer Res. 2008;14(19):6228. doi: 10.1158/1078-0432.CCR-07-5177. [DOI] [PubMed] [Google Scholar]
- 99.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Clin Cancer Res. 2008;14(14):4491. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 100.Yu S, Shen G, Khor TO, Kim JH, Kong AN. Mol Cancer Ther. 2008;7(9):2609. doi: 10.1158/1535-7163.MCT-07-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weir NM, Selvendiran K, Kutala VK, Tong L, Vishwanath S, Rajaram M, Tridandapani S, Anant S, Kuppusamy P. Cancer Biol Ther. 2007;6(2):178. doi: 10.4161/cbt.6.2.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kumar AP, Garcia GE, Ghosh R, Rajnarayanan RV, Alworth WL, Slaga TJ. Neoplasia. 2003;5(3):255. doi: 10.1016/S1476-5586(03)80057-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Squires MS, Hudson EA, Howells L, Sale S, Houghton CE, Jones JL, Fox LH, Dickens M, Prigent SA, Manson MM. Biochem Pharmacol. 2003;65(3):361. doi: 10.1016/s0006-2952(02)01517-4. [DOI] [PubMed] [Google Scholar]
- 104.Woo JH, Kim YH, Choi YJ, Kim DG, Lee KS, Bae JH, Min dS, Chang JS, Jeong YJ, Lee YH, Park JW, Kwon TK. Carcinogenesis. 2003;24(7):1199. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- 105.Kim GY, Kim KH, Lee SH, Yoon MS, Lee HJ, Moon DO, Lee CM, Ahn SC, Park YC, Park YM. J Immunol. 2005;174(12):8116. doi: 10.4049/jimmunol.174.12.8116. [DOI] [PubMed] [Google Scholar]
- 106.Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, Dou QP. Cancer Res. 2008;68(18):7283. doi: 10.1158/0008-5472.CAN-07-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salh B, Assi K, Templeman V, Parhar K, Owen D, Gomez-Munoz A, Jacobson K. Am J Physiol Gastrointest Liver Physiol. 2003;285(1):G235–G243. doi: 10.1152/ajpgi.00449.2002. [DOI] [PubMed] [Google Scholar]
- 108.Yan C, Jamaluddin MS, Aggarwal B, Myers J, Boyd DD. Mol Cancer Ther. 2005;4(2):233. [PubMed] [Google Scholar]
- 109.Ryu MJ, Cho M, Song JY, Yun YS, Choi IW, Kim DE, Park BS, Oh S. Biochem Biophys Res Commun. 2008;377(4):1304. doi: 10.1016/j.bbrc.2008.10.171. [DOI] [PubMed] [Google Scholar]
- 110.Jaiswal AS, Marlow BP, Gupta N, Narayan S. Oncogene. 2002;21(55):8414. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- 111.Nakamura K, Yasunaga Y, Segawa T, Ko D, Moul JW, Srivastava S, Rhim JS. Int J Oncol. 2002;21(4):825. [PubMed] [Google Scholar]
- 112.Ohtsu H, Xiao Z, Ishida J, Nagai M, Wang HK, Itokawa H, Su CY, Shih C, Chiang T, Chang E, Lee Y, Tsai MY, Chang C, Lee KH. J Med Chem. 2002;45(23):5037. doi: 10.1021/jm020200g. [DOI] [PubMed] [Google Scholar]
- 113.Ahmad N, Gupta S, Mukhtar H. Arch Biochem Biophys. 2000;376(2):338. doi: 10.1006/abbi.2000.1742. [DOI] [PubMed] [Google Scholar]
- 114.Afaq F, Adhami VM, Ahmad N, Mukhtar H. Oncogene. 2003;22(7):1035. doi: 10.1038/sj.onc.1206206. [DOI] [PubMed] [Google Scholar]
- 115.Guo S, Lu J, Subramanian A, Sonenshein GE. Cancer Res. 2006;66(10):5322. doi: 10.1158/0008-5472.CAN-05-4287. [DOI] [PubMed] [Google Scholar]
- 116.Kim SJ, Jeong HJ, Lee KM, Myung NY, An NH, Yang WM, Park SK, Lee HJ, Hong SH, Kim HM, Um JY. J Nutr Biochem. 2007;18(9):587. doi: 10.1016/j.jnutbio.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 117.Qin J, Xie LP, Zheng XY, Wang YB, Bai Y, Shen HF, Li LC, Dahiya R. Biochem Biophys Res Commun. 2007;354(4):852. doi: 10.1016/j.bbrc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 118.Tang FY, Nguyen N, Meydani M. Int J Cancer. 2003;106(6):871. doi: 10.1002/ijc.11325. [DOI] [PubMed] [Google Scholar]
- 119.Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. J Exp Ther Oncol. 2002;2(6):350. doi: 10.1046/j.1359-4117.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 120.Wang YC, Bachrach U. Amino Acids. 2002;22(2):131. doi: 10.1007/s007260200002. [DOI] [PubMed] [Google Scholar]
- 121.Maeda-Yamamoto M, Suzuki N, Sawai Y, Miyase T, Sano M, Hashimoto-Ohta A, Isemura M. J Agric Food Chem. 2003;51(7):1858. doi: 10.1021/jf021039l. [DOI] [PubMed] [Google Scholar]
- 122.Chen C, Yu R, Owuor ED, Kong AN. Arch Pharm Res. 2000;23(6):605. doi: 10.1007/BF02975249. [DOI] [PubMed] [Google Scholar]
- 123.Kim J, Zhang X, Rieger-Christ KM, Summerhayes IC, Wazer DE, Paulson KE, Yee AS. J Biol Chem. 2006;281(16):10865. doi: 10.1074/jbc.M513378200. [DOI] [PubMed] [Google Scholar]
- 124.Gupta S, Ahmad N, Nieminen AL, Mukhtar H. Toxicol Appl Pharmacol. 2000;164(1):82. doi: 10.1006/taap.1999.8885. [DOI] [PubMed] [Google Scholar]
- 125.Kuo PL, Lin CC. J Biomed Sci. 2003;10(2):219. doi: 10.1007/BF02256057. [DOI] [PubMed] [Google Scholar]
- 126.Ren F, Zhang S, Mitchell SH, Butler R, Young CY. Oncogene. 2000;19(15):1924. doi: 10.1038/sj.onc.1203511. [DOI] [PubMed] [Google Scholar]
- 127.Chuu CP, Chen RY, Kokontis JM, Hiipakka RA, Liao S. Cancer Lett. 2009;275(1):86. doi: 10.1016/j.canlet.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Harper CE, Patel BB, Wang J, Eltoum IA, Lamartiniere CA. Prostate. 2007;67(14):1576. doi: 10.1002/pros.20643. [DOI] [PubMed] [Google Scholar]
- 129.Goodin MG, Fertuck KC, Zacharewski TR, Rosengren RJ. Toxicol Sci. 2002;69(2):354. doi: 10.1093/toxsci/69.2.354. [DOI] [PubMed] [Google Scholar]
- 130.Estrov Z, Shishodia S, Faderl S, Harris D, Van Q, Kantarjian HM, Talpaz M, Aggarwal BB. Blood. 2003;102(3):987. doi: 10.1182/blood-2002-11-3550. [DOI] [PubMed] [Google Scholar]
- 131.Kundu JK, Shin YK, Surh YJ. Biochem Pharmacol. 2006;72(11):1506. doi: 10.1016/j.bcp.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 132.Adhami VM, Afaq F, Ahmad N. Neoplasia. 2003;5(1):74. doi: 10.1016/s1476-5586(03)80019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kundu JK, Shin YK, Kim SH, Surh YJ. Carcinogenesis. 2006;27(7):1465. doi: 10.1093/carcin/bgi349. [DOI] [PubMed] [Google Scholar]
- 134.Roy P, Kalra N, Prasad S, George J, Shukla Y. Pharm Res. 2009;26(1):211. doi: 10.1007/s11095-008-9723-z. [DOI] [PubMed] [Google Scholar]
- 135.Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N. Mol Cancer Ther. 2006;5(5):1335. doi: 10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- 136.Hope C, Planutis K, Planutiene M, Moyer MP, Johal KS, Woo J, Santoso C, Hanson JA, Holcombe RF. Mol Nutr Food Res. 2008;52(Suppl 1):S52–S61. doi: 10.1002/mnfr.200700448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roccaro AM, Leleu X, Sacco A, Moreau AS, Hatjiharissi E, Jia X, Xu L, Ciccarelli B, Patterson CJ, Ngo HT, Russo D, Vacca A, Dammacco F, Anderson KC, Ghobrial IM, Treon SP. Clin Cancer Res. 2008;14(6):1849. doi: 10.1158/1078-0432.CCR-07-1750. [DOI] [PubMed] [Google Scholar]
- 138.Alkhalaf M. Pharmacology. 2007;80(2–3):134. doi: 10.1159/000103253. [DOI] [PubMed] [Google Scholar]
- 139.Gatz SA, Keimling M, Baumann C, Dork T, Debatin KM, Fulda S, Wiesmuller L. Carcinogenesis. 2008;29(3):519. doi: 10.1093/carcin/bgm283. [DOI] [PubMed] [Google Scholar]
- 140.Mitchell SH, Zhu W, Young CY. Cancer Res. 1999;59(23):5892. [PubMed] [Google Scholar]
- 141.Hsieh TC, Wu JM. Anticancer Res. 2000;20(1A):225. [PubMed] [Google Scholar]
- 142.Harada N, Murata Y, Yamaji R, Miura T, Inui H, Nakano Y. J Nutr Sci Vitaminol (Tokyo) 2007;53(6):556. doi: 10.3177/jnsv.53.556. [DOI] [PubMed] [Google Scholar]
- 143.Huang CS, Fan YE, Lin CY, Hu ML. J Nutr Biochem. 2007;18(7):449. doi: 10.1016/j.jnutbio.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 144.Hung CF, Huang TF, Chen BH, Shieh JM, Wu PH, Wu WB. Eur J Pharmacol. 2008;586(1–3):275. doi: 10.1016/j.ejphar.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 145.Kim GY, Kim JH, Ahn SC, Lee HJ, Moon DO, Lee CM, Park YM. Immunology. 2004;113(2):203. doi: 10.1111/j.1365-2567.2004.01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tang FY, Shih CJ, Cheng LH, Ho HJ, Chen HJ. Mol Nutr Food Res. 2008;52(6):646. doi: 10.1002/mnfr.200700272. [DOI] [PubMed] [Google Scholar]
- 147.Palozza P, Sheriff A, Serini S, Boninsegna A, Maggiano N, Ranelletti FO, Calviello G, Cittadini A. Apoptosis. 2005;10(6):1445. doi: 10.1007/s10495-005-1393-2. [DOI] [PubMed] [Google Scholar]
- 148.Liu X, Allen JD, Arnold JT, Blackman MR. Carcinogenesis. 2008;29(4):816. doi: 10.1093/carcin/bgn011. [DOI] [PubMed] [Google Scholar]
- 149.Kucuk O, Sarkar FH, Djuric Z, Sakr W, Pollak MN, Khachik F, Banerjee M, Bertram JS, Wood DP. Jr Exp Biol Med (Maywood) 2002;227(10):881. doi: 10.1177/153537020222701007. [DOI] [PubMed] [Google Scholar]