Abstract
Purpose of review
Several studies have shown that the pattern electroretinogram, a direct, objective method of measuring retinal ganglion cell function, is altered early in ocular hypertension and glaucoma. Renewed interest in the pattern electroretinogram for early detection of pre-perimetric glaucoma has been sparked by noninvasive and reproducible methods of recording using skin electrodes.
Recent findings
With the noninvasive pattern electroretinogram, response abnormalities have been detected in up to 50% of glaucoma suspects with normal standard perimetry. In early glaucoma (with either normal or high intraocular pressure), a reduction of intraocular pressure has sometimes yielded improvement in pattern electroretinogram amplitude. A prolonged steady-state stimulus presentation reduces the pattern electroretinogram amplitude and increases optic nerve blood flow in normal subjects, suggesting that sustained activity of retinal ganglion cells is physiologically associated with autoregulatory changes of the neural-vascular system. It is unknown whether this autoregulation is altered in glaucoma. The multifocal pattern electroretinogram does not seem to have an advantage over the pattern electroretinogram in the early detection of glaucoma. The photopic negative response of the diffuse flash electroretinogram has shown changes in glaucoma, but may not be able to detect retinal dysfunction in normal tension glaucoma.
Summary
The pattern electroretinogram is a noninvasive, direct, objective method that may be useful to clinicians in detecting early retinal ganglion cell dysfunction in glaucoma suspects. The pattern electroretinogram may also optimize treatment strategies based on improvement of retinal ganglion cell function.
Keywords: glaucoma, neuroprotection, pattern electroretinogram, retinal ganglion cell
Introduction
The pattern electroretinogram (PERG) is a retinal bio-potential that is evoked when a high-contrast patterned stimulus (alternating stripes or checkerboards) is viewed on a television monitor [1]. When the alternation rate is slow (typically 2–4 reversals per second), a transient PERG is generated with a waveform characterized by a positive wave peaking about 50 ms after the contrast reversal (P50), followed by a negative wave peaking about 95 ms after contrast reversal (N95). For faster reversal rates (typically 16 reversals per second), a steady-state, sinusoidal-like waveform is generated, whose period corresponds to the reversal frequency [2]. While the traditional flash ERG is unaltered in conditions that impair retinal ganglion cell (RGC) function, the PERG closely depends on normal RGC activity, and therefore represents an objective and direct measure of RGC function [3–5]. The PERG has been shown by many investigators to be altered in ocular hypertension [6,7], for example, and glaucoma (see [8, 9] for a review). Recent developments of the technique allow easy recording in the clinical setting with good reproducibility [10••]. This article will review the PERG and the resurgence of interest in this complementary technique to aid in the earlier diagnosis and monitoring of pre-perimetric glaucoma. Recent publications comparing PERG with the photopic negative response and the multifocal PERG will also be reviewed.
Pattern electroretinogram in normal subjects
Several previous reports (for example, [11,12]) have demonstrated that the PERG amplitude and phase decrease (latency increases) with increasing age in normal controls. These results were confirmed by Porciatti and Ventura [10••] using a new paradigm called PER-GLA (PERG for glaucoma), where skin electrodes are taped on the lower eyelids, and steady-state responses are automatically processed. The setup for non-invasive PERG recording, and examples of normal and abnormal responses, are displayed in Figs. 1 and 2, respectively. Physiological age-related PERG changes may be due to neural losses as well as reduction of retinal illuminance resulting from senile miosis, and reduction in image contrast secondary to cataract or other opacities of the optical media. When the response is normalized for senile miosis [11,12], however, and senile lenses are substituted with pseudophakic implants [12], the PERG of aged subjects is still substantially reduced and delayed as compared with the PERG of younger subjects. This indicates, at least in part, an age-dependent loss of neural activity that can be detected by PERG.
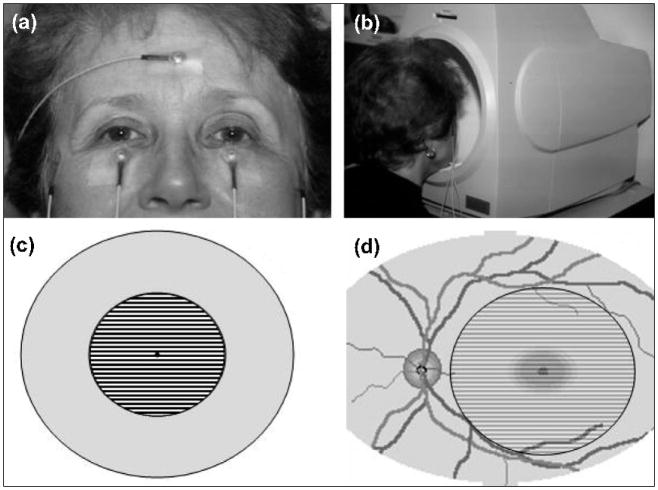
Figure 1. Setup for the non-invasive PERG.
(a) Standard skin electrodes are taped on the lower eyelids (active), temples (reference), and forehead (common ground). (b) The subject looks for 3 minutes at the center of the stimulus placed inside a ganzfeld bowl. (c) Pattern stimulus consisting of black and white stripes alternating 16.6 times per second. (d) The stimulus covers a circular retinal area with a 12.5 degree radius centered on the fovea.
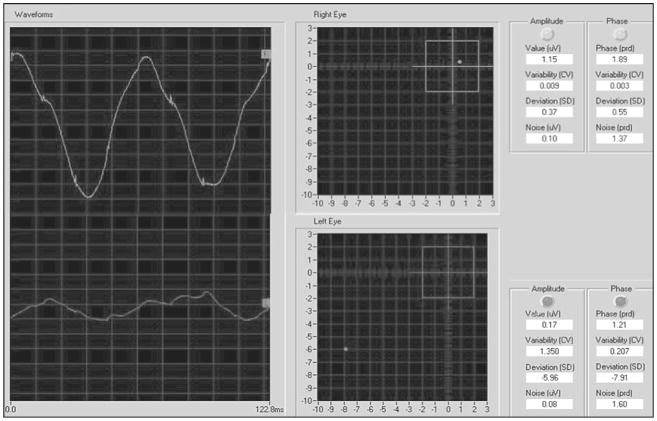
Figure 2. Example of normal PERG (right eye, upper left) and abnormal PERG (left eye, bottom left).
PERGs have an approximately sinusoidal waveform, whose frequency corresponds to the reversal rate. The analyzed period (122.8 ms) exactly corresponds to two contrast reversals. PERG waveforms are automatically analyzed by Digital Fourier Transform to isolate the PERG sinusoidal component (16.6 Hz) from the background noise, and measure its amplitude in microvolts and phase in radians (latency) (right-hand tables). Amplitude and phase are also expressed in standard deviations (SDs) from age-corrected normal averages (right-hand tables) and plotted one against the other in a polar diagram (center panels). The box in the polar diagram represents the 95% (±2 SD) tolerance interval for amplitude (vertical) and phase (horizontal). In the example, the right eye has an amplitude and phase close to the age-corrected normal average. The left eye has a reduced amplitude (about −6 SDs from normal) and a delayed phase (about −8 SDs from normal). The program also calculates an index of intrinsic variability (coefficient of variation (CV) between the amplitude and phase recorded during the first 1.5 minutes and the second 1.5 minutes of the acquisition) and an index of a noise response (amplitude and phase of a difference waveform between the first 1.5 minutes and the second 1.5 minutes of the acquisition).
Pattern electroretinogram in early glaucoma
Ventura et al. [13••] recorded the steady-state PERG with the PERGLA paradigm in 200 glaucoma suspects (classified on the basis of optic disc abnormalities and normal visual fields), and in 42 patients with early manifest glaucoma (EMG). The PERG was abnormal in amplitude, phase, or interocular asymmetry in amplitude and phase in 52% of glaucoma suspect patients and 69% of EMG patients [13••]. These results confirm previous data showing a high sensitivity of PERG for glaucoma detection (for example, [9]). The high frequency of PERG abnormalities in glaucoma suspect patients suggests that many of these patients do not have so-called ‘physiological cupping’, but rather have a dysfunction of the RGC themselves and/or the circuitry of the inner retina impinging on RGCs. PERG amplitude losses increased with increasing perimetric defects, increasing optic nerve head cupping, and increasing age. Age-related PERG abnormalities were relatively larger in patients with more damaged visual fields and larger cups. Compared to white glaucoma suspects, a lower PERG amplitude was found in black glaucoma suspects and EMG patients, but not in black normal controls [13••]. Based on the association between PERG abnormalities and known risk factors for glaucoma, Ventura et al. [13••] conclude that PERG may have a predictive potential for the development or progression of the disease, or both.
Pattern electroretinogram and perimetry
PERG and perimetry test different aspects of visual function; therefore, PERG and visual field results often dissociate, particularly in the early stages of the disease. The PERG is a direct and objective measure of the electrical activity of the RGC population of the central retina (more than 40% of the total RGC population) in response to a suprathreshold stimulus. PERG is a mass response. Perimetry is a subjective response to focal threshold stimuli covering central and more peripheral retinal regions, which depends on RGC function as well as on other postretinal factors. Postretinal factors may exacerbate the reduction of sensitivity due to RGC loss or even mask it. Exacerbating factors include a geniculate/cortical component resulting from glaucomatous deafferentation [14], as well as an unspecific cortical component that may further reduce the response owing to a variety of conditions affecting the aging brain. Masking factors include compensatory mechanisms of cortical plasticity that tend to fill in gaps in the visual field caused by glaucomatous deafferentation [15].
As mentioned above, the PERG is often abnormal when there are no perimetric defects in the central field in ocular hypertension [6], suspicion of glaucoma [13••], or early manifest glaucoma [9,16•]. Here abnormality of PERG signifies a generalized RGC dysfunction, RGC drop out, or a combination of both. The complementary nature of PERG and perimetry was noted in an elegant study by Bayer and collaborators [17], who concluded that the PERG improved the possibility of short-wavelength automated perimetry (SWAP) to detect glaucomatous optic neuropathy in eyes with primary open angle glaucoma (POAG) but with still normal standard visual fields. An abnormal PERG may also represent a useful parameter to predict future losses of the visual field [18–20].
What about when a peripheral defect in the field occurs in the face of a normal PERG? Perimetry may detect an area of peripheral focal field loss in patients when the majority of central RGCs are functioning normally; thus the PERG is expected to be normal. Hood et al. [16•] cautioned that the transient PERG may miss glaucomatous damage in about 30% of patients with visual field loss confirmed by multifocal visual evoked potentials. Graham et al. [21] and Ventura et al. [13••] also reported comparable percentages of false negatives with 95% specificity in early manifest glaucoma. One explanation is that in normal subjects there is a wide range in the numbers of RGCs from 770 000 to 1.7 million [22]. Consequently, a wide range of PERG amplitudes among control subjects is expected [9,11,12]. Hood et al. [16•] suggest that, for subjects with high baseline RGC number and PERG amplitude, RGC losses due to glaucoma must be substantial to reduce the PERG amplitude below the lower tolerance interval.
Another appealing explanation for a normal PERG in patients with reproducible visual field loss may be offered by Caprioli et al. [23], who suggest that distinct types of field loss, diffuse compared with focal, may be caused by different mechanisms of glaucomatous optic nerve damage. A more diffuse loss of visual field sensitivity is hypothesized by the authors to be pressure–dependent, and is thought to be secondary to diffuse axonal dysfunction leading to progressive concentric enlargement of the optic nerve cup, and an evenly distributed thinning of the disk rim. Clinicians all too often see glaucoma patients with this type of progressively even cupping in the face of normal visual fields, and we have shown that PERG is often abnormal in such cases, reflecting diffuse RGC dysfunction [13••]. In contrast, a localized type of visual field loss may be less pressure–dependent [23], and such focal losses are believed to be due to an accelerated ganglion cell death, perhaps owing to vascular or other focal neurotoxic factors, leading to dense scotomata. Where focal scotomata exist in the visual field, the PERG may still be normal if the majority of RGCs are still unaffected. An abnormal PERG is helpful in further raising our suspicion when the visual field is normal, that is, in about 50% of our patients. On the other hand, when the visual field is abnormal, our definition of glaucoma does not require PERG confirmation to declare the disease. A normal PERG in the face of an abnormal field is a reassuring finding to the patient and clinician. A normal PERG means that there exists a relatively large population of functioning RGCs, and herein lies another possible parameter to monitor the patient for progression.
False positive pattern electroretinogram abnormality
Diabetic retinopathy [24], Parkinson’s disease [25], and macular degeneration [26] depress the PERG. Central cortical or posterior subcapsular cataracts that impair vision may also significantly reduce the PERG amplitude [27]. This represents a limitation for the clinical application of PERG in glaucoma as well as in other diseases of the anterior visual pathway.
Multifocal pattern electroretinogram
In order to establish a more precise relationship between local retinal dysfunction and local field defects, there has been a flurry of activity surrounding the multifocal ERG (MERG) [28]. The MERG stimulus consists of an array of small patches (typically 103 that cover a central area of 48 degrees) that flicker ON and OFF according to a pseudorandom sequence, and elicit spatially localized ERGs. The MERGs are not substantially different in nature from the conventional photopic ERGs, and applications of MERGs in glaucoma have been disappointing. Hood et al. [29] conclude that damage to ganglion cell axons is not a sufficient condition to produce changes in the MERG. In patients with clear abnormalities in MERG waveforms, these changes are poorly correlated with local reductions in field sensitivity.
In order to increase the specificity of the multifocal technique to detect localized retinal dysfunction of RGCs in glaucoma, the stimulus was modified to generate a multifocal PERG (MPERG). Stiefelmeyer et al. [30•] recently carried out a study to further determine if the MPERGs are altered in a characteristic manner in glaucoma patients to permit the diagnosis of an abnormality. In agreement with previous studies [31, 32], Stiefelmeyer et al. [30•] reported that no topographic correlation of focally abnormal amplitudes and corresponding localized visual field defects could be shown even for advanced absolute scotomata. In addition, in patients with moderate to advanced glaucoma, the MPERG changes were quite small, and one would expect even smaller changes in pre-perimetric glaucoma. The MPERG, in its current form, does not seem to have a useful clinical application in glaucoma patients.
Metabolically challenging pattern electroretinogram stimulus
In a recent study, Porciatti et al. [33••] showed that the PERG in response to a prolonged presentation of a stimulus that maximizes PERG amplitude [12] (a fine grating pattern of high contrast, reversing 16 times per second) induces autoregulatory changes in RGC activity in normal subjects. Stimuli of comparable characteristics also induce an increase in optic nerve head blood flow [34,35]. With a continuous presentation of this stimulus, the PERG displays a slow decline of amplitude at constant phase, until reaching a dynamic equilibrium after approximately 2 minutes at a level approximately 30% lower than the initial amplitude. Neural activity has a high metabolic cost [36], reflected by a local increase in blood flow and in oxygen and glucose consumption [37]. Porciatti et al. [33••] suggest that in RGCs continually exposed to optimal stimuli, the metabolic demand may be larger than the available supply; therefore, RGCs must reduce their activity (reflected in a reduction in PERG amplitude) to maintain a dynamic equilibrium compatible with the energy budget [38].
The challenge of a prolonged steady-state stimulus might help us to identify those individuals whose PERG fatigues prematurely or excessively. Future studies may indicate whether these individuals are more likely to progress. This may help identify those 10% of patients who progress more rapidly and go on to develop bilateral blindness [39].
Metabolic challenge may also explain why the steady-state PERG (16 reversals per second) yields a substantially greater reduction in amplitude in patients with glaucoma compared to the standard transient PERG (2 reversals per second) (see Fig. 4 in [9]). The difference between steady state and transient PERG in glaucoma may be understood assuming that there is a higher energy demand under steady-state conditions that is not met in metabolically compromised glaucomatous retinas.
Retinal ganglion cell activity may be also shown in the diffuse flash electroretinogram
While RGCs do not contribute to the conventional flash ERG, under specific recording conditions it is possible to record signals consistent with RGC activity. Viswanathan and collaborators [40] first identified the ‘photopic negative response’ or PhNR, which is a slow (time-to-peak longer than 100 ms) negative potential following the positive b-wave of the ERG in response to a red flash superimposed on a blue background. They suggested that PhNR was caused by RGC activity because it was greatly reduced by pharmacologically suppressing spiking activity with tetrodotoxin (TTX), and it was markedly reduced in monkeys with experimental glaucoma due to IOP elevation. The same group [41] later demonstrated that PhNR and PERG were similarly affected by TTX and experimental glaucoma in monkeys. Colotto et al. [42] demonstrated that the PhNR of the focal ERG and the transient PERG may be similarly affected in human glaucoma.
In patients with ocular hypertension, POAG and normal tension glaucoma (NTG), Aldebasi et al. [43•] recently compared the negative component of transient PERG (N95) to the negative component (PhNR) of the diffuse-flash ERG in response to stimuli selective for either the S(blue) cones or the L(red)+M(green) cones. To elicit the S-cone ERG, they used a blue–green heterochromatic flicker superimposed on a red background. To elicit the (L+M)-cone ERG, they used a red flash on a blue background. Aldebasi et al. [43•] found that in ocular hypertension (OHT) and POAG, but not NTG, a reduction in amplitude was noted in the late negative waves of all three types of ERG compared to controls, and this was thought to reflect a reduction in RGC activity. This study shows that in OHT and glaucoma not only is the central PERG abnormal, but also the ERG from the retina as a whole (involving signals from many types of neurons) may be dysfunctional (see also [44]).
The percentage of amplitude reduction was notably greater for the PERG than for the S-cone or (L+M)-cone ERG. Unlike the PERG, which showed an alteration in NTG, the S-cone ERG was not significantly reduced in the NTG group. The authors surmise that the S-cone pathway may be more pressure–sensitive than the (L+M)-cone pathway (see also [45,46]). An alternative explanation may be that paracentral defects tend to occur more noticeably in NTG [47]. Since PERG is recorded from the posterior pole, PERG may be more sensitive in detecting paracentral RGC pathology. Aldebasi et al. [43•] conclude that selected types of flash ERG could serve as a useful alternative to the PERG in the assessment of clinical glaucomatous neuropathy. This may be particularly helpful in the aging population as the flash-ERG is less affected by cataracts than the PERG. The PhNR technique is a promising but still evolving technology. Future studies are needed to establish its clinical utility in the management of glaucoma.
Impaired retinal ganglion cell activity may improve after intraocular pressure reduction
The PERG amplitude reflects the total amount of electrophysiological activity of RGCs. Reduction of PERG amplitude in glaucoma may be due to lack of activity of dead/missing RGCs, reduced activity of viable RGCs, or a combination of both. Recent results of Ventura and Porciatti [48••] indicate that the abnormal PERG recorded in eyes with early stages of glaucoma may often improve after IOP reduction. This indicates that initial PERG abnormalities reflect, at least in part, RGC dysfunction which may be restored after IOP reduction. Results do not exclude, however, that the PERG effects might also be due to changes occurring in the inner retina that do not directly involve RGCs. Of interest was the fact that PERG improvement after IOP lowering occurred not only in OHT and POAG eyes, but also in NTG eyes. PERG improvements in NTG, compared to POAG eyes, were associated with smaller IOP reductions. These results might be explained by assuming that RGCs of NTG have a lower threshold for IOP insult compared to POAG eyes. Consequently, a pressure-dependent RGC dysfunction occurs at lower IOP levels in NTG compared to POAG, and PERG improvement occurs with smaller IOP reductions.
In addition to IOP lowering, PERG improvement appears to depend on other factors. First, in order to have a substantial improvement, the PERG must be abnormal. No significant changes occur in treated normal subjects [48••]. In addition, initial PERG abnormality in glaucoma patients had to be associated with normal or early altered visual fields. Conversely, little PERG improvement occurred in eyes with severe visual field defects [48••]. A possible explanation for the interaction between PERG improvement and the extent of visual field loss would be that in eyes with a ‘more’ preserved visual field there exists a larger population of RGCs with reversible dysfunction compared with advanced disease states. That PERG abnormality is reversible implies that in the early stages of glaucoma RGC dysfunction may precede RGC death.
This conclusion of RGC dysfunction before death is an apparent contradiction to the current opinion that structural changes of the optic nerve fiber layer precede the appearance of visual dysfunction, as measured by standard automated perimetry [49,50]. It is possible that viable RGCs may display subtle anatomical changes that are not reflected in the number of RGC axons, but may manifest themselves in an altered electrical responsiveness. In primate models of glaucoma, it has been shown that somata and dendritic arbors of RGCs may undergo significant shrinkage [51,52]. Shrunken RGCs are less responsive, particularly to patterned stimuli as well as to stimuli presented at increased temporal frequencies [53••].
Conclusion
In summary, PERG is a useful clinical tool. When abnormal, PERG further raises suspicion, and may predict the development of future visual field defects [18]. Abnormal PERG may also predict progression in patients with early visual field loss [20], and even provide unique information on the efficacy of treatment based on reversal of dysfunction [48••]. Earlier treatment may spare neurons from premature RGC death, and older patients from greater degrees of visual loss. The more advanced the glaucoma, the lesser the chances for reversal of dysfunction.
When the PERG is normal, glaucoma is not ruled out, and the clinician should maintain the level of suspicion engendered by all of the risk factors at hand. The PERG should not serve as a substitute for perimetry in screening for glaucoma, but should be used as a complement when fields are normal, yet an increased suspicion is unsettling. A normal PERG is useful in reassuring clinicians and patients that a major RGC population is still alive and functional, and may be followed for deterioration as a marker for progression.
Acknowledgments
Sponsorship: This study was supported by NIH-NEI RO1 EY014957, The Glaucoma Foundation, NIH center grant P30-EY014801, and by an unrestricted grant to the University of Miami from Research to Prevent Blindness.
Abbreviations
- IOP
intraocular pressure
- MERG
multifocal electroretinogram
- NTG
normal tension glaucoma
- PERG
pattern electroretinogram
- RGC
retinal ganglion cell
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 213).
- 1.Riggs LA, Johnson EP, Schick AML. Electrical responses of the human eye to moving stimulus pattern. Science. 1964;144:567–568. doi: 10.1126/science.144.3618.567. [DOI] [PubMed] [Google Scholar]
- 2.Bach M, Hawlina M, Holder GE, et al. for the International Society for Clinical Electrophysiology of Vision. Standard for pattern electroretinography. Doc Ophthalmol. 2000;101:11–18. doi: 10.1023/a:1002732114721. [DOI] [PubMed] [Google Scholar]
- 3.Maffei L, Fiorentini A. Electroretinographic responses to alternating gratings before and after section of the optic nerve. Science. 1981;211:953–955. doi: 10.1126/science.7466369. [DOI] [PubMed] [Google Scholar]
- 4.Fiorentini A, Maffei L, Pirchio M, et al. The ERG in response to alternating gratings in patients with diseases of the peripheral visual pathway. Invest Ophthalmol Vis Sci. 1981;21:490–493. [PubMed] [Google Scholar]
- 5.Zrenner E. The physiological basis of the pattern electroretinogram. In: Osborne N, Chader G, editors. Progress in Retinal Research. Vol. 9 Oxford: Pergamon Press; 1990. [Google Scholar]
- 6.Porciatti V, Falsini B, Brunori S, et al. Pattern electroretinogram as a function of spatial frequency in ocular hypertension and early glaucoma. Doc Ophthalmol. 1987;65:349–355. doi: 10.1007/BF00149941. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein GW, Arden GB, Hitchings RA, et al. The pattern electroretinogram (PERG) in ocular hypertension and glaucoma. Arch Ophthalmol. 1988;106:923–928. doi: 10.1001/archopht.1988.01060140069027. [DOI] [PubMed] [Google Scholar]
- 8.Berninger TA, Arden GB. The pattern electroretinogram. Eye. 1988;2 (Suppl):S257–S283. doi: 10.1038/eye.1988.149. [DOI] [PubMed] [Google Scholar]
- 9.Bach M. Electrophysiological approaches for early detection of glaucoma. Eur J Ophthalmol. 2001;11 (Suppl 2):S41–S49. doi: 10.1177/112067210101102s05. [DOI] [PubMed] [Google Scholar]
- 10 ••.Porciatti V, Ventura LM. Normative data for a user-friendly paradigm for pattern electroretinogram recording. Ophthalmology. 2004;111:161–168. doi: 10.1016/j.ophtha.2003.04.007. A description of the noninvasive PERG technique together with age-specific norms and repeatability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trick GL, Nesher R, Cooper DG, et al. The human pattern ERG: alteration of response properties with aging. Optom Vis Sci. 1992;69:122–128. doi: 10.1097/00006324-199202000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Porciatti V, Burr DC, Morrone MC, Fiorentini A. The effects of aging on the pattern electroretinogram and visual evoked potential in humans. Vision Res. 1992;32:1199–1209. doi: 10.1016/0042-6989(92)90214-4. [DOI] [PubMed] [Google Scholar]
- 13 ••.Ventura LM, Porciatti V, Ishida K, et al. Pattern electroretinogram abnormality and glaucoma. Ophthalmology. 2005;112:10–19. doi: 10.1016/j.ophtha.2004.07.018. Application of the PERGLA paradigm in a large population of glaucoma suspects and early manifest glaucoma, and a study of the associations between PERG abnormalities and risk factors for glaucoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yucel YH, Zhang Q, Weinreb RN, et al. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res. 2003;22:465–481. doi: 10.1016/s1350-9462(03)00026-0. [DOI] [PubMed] [Google Scholar]
- 15.Lam DY, Kaufman PL, Gabelt BT, et al. Neurochemical correlates of cortical plasticity after unilateral elevated intraocular pressure in a primate model of glaucoma. Invest Ophthalmol Vis Sci. 2003;44:2573–2581. doi: 10.1167/iovs.02-0779. [DOI] [PubMed] [Google Scholar]
- 16 •.Hood DC, Xu L, Thienprasiddhi P, et al. The pattern electroretinogram in glaucoma patients with confirmed visual field deficits. Invest Ophthalmol Vis Sci. 2005;46:2411–2418. doi: 10.1167/iovs.05-0238. A working model for explaining false negative transient PERGs in perimetric glaucoma. [DOI] [PubMed] [Google Scholar]
- 17.Bayer AU, Maag KP, Erb C. Detection of optic neuropathy in glaucomatous eyes with normal standard visual fields using a test battery of short-wavelength automated perimetry and pattern electroretinography. Ophthalmology. 2002;109:1350–1361. doi: 10.1016/s0161-6420(02)01100-4. [DOI] [PubMed] [Google Scholar]
- 18.Pfeiffer N, Tillmon B, Bach M. Predictive value of the pattern electroretinogram in high-risk ocular hypertension. Invest Ophthalmol Vis Sci. 1993;34:1710–1715. [PubMed] [Google Scholar]
- 19.Arai M, Yoshimura N, Sakaue H, et al. A 3-year follow-up study of ocular hypertension by pattern electroretinogram. Ophthalmologica. 1993;207:187–195. doi: 10.1159/000310431. [DOI] [PubMed] [Google Scholar]
- 20.Bayer AU, Erb C. Short wavelength automated perimetry, frequency doubling technology perimetry, and pattern electroretinography for prediction of progressive glaucomatous standard visual field defects. Ophthalmology. 2002;109:1009–1017. doi: 10.1016/s0161-6420(02)01015-1. [DOI] [PubMed] [Google Scholar]
- 21.Graham SL, Drance SM, Chauhan BC, et al. Comparison of psychophysical and electrophysiological testing in early glaucoma. Invest Ophthalmol Vis Sci. 1996;37:2651–2662. [PubMed] [Google Scholar]
- 22.Jonas JB, Schmidt AM, Muller-Bergh JA, et al. Human optic nerve fiber count and optic disc size. Invest Ophthalmol Vis Sci. 1992;33:2012–2018. [PubMed] [Google Scholar]
- 23.Caprioli J, Sears M, Miller JM. Patterns of early visual field loss in open-angle glaucoma. Am J Ophthalmol. 1987;104:98. doi: 10.1016/0002-9394(87)90314-x. [DOI] [PubMed] [Google Scholar]
- 24.Falsini B, Porciatti V, Scalia G, et al. Steady-state pattern electroretinogram in insulin-dependent diabetics with no or minimal retinopathy. Doc Ophthalmol. 1989;73:193–200. doi: 10.1007/BF00155037. [DOI] [PubMed] [Google Scholar]
- 25.Sartucci F, Orlandi G, Lucetti C, et al. Changes in pattern electroretinograms to equiluminant red-green and blue-yellow gratings in patients with early Parkinson’s disease. J Clin Neurophysiol. 2003;20:375–381. doi: 10.1097/00004691-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Holder GE. Pattern electroretinography (PERG) and an integrated approach to visual pathway diagnosis. Prog Retin Eye Res. 2001;20:531–561. doi: 10.1016/s1350-9462(00)00030-6. [DOI] [PubMed] [Google Scholar]
- 27.Bach M, Mathieu M. Different effect of dioptric defocus vs. light scatter on the pattern electroretinogram (PERG) Doc Ophthalmol. 2004;108:99–106. doi: 10.1023/b:doop.0000018415.00285.56. [DOI] [PubMed] [Google Scholar]
- 28.Sutter EE, Tran D. The field topography of ERG components in man–I. The photopic luminance response. Vision Res. 1992;32:433–446. doi: 10.1016/0042-6989(92)90235-b. [DOI] [PubMed] [Google Scholar]
- 29.Hood DC, Greenstein VC, Holopigian K, et al. An attempt to detect glaucomatous damage to the inner retina with the multifocal ERG. Invest Ophthalmol Vis Sci. 2000;41:1570–1579. [PubMed] [Google Scholar]
- 30 •.Stiefelmeyer S, Neubauer AS, Berninger T, et al. The multifocal pattern elec troretinogram in glaucoma. Vision Res. 2004;44:103–112. doi: 10.1016/j.visres.2003.08.012. A confirmation that the multifocal PERG does not show local alterations consistent with perimetric defects. [DOI] [PubMed] [Google Scholar]
- 31.Klistorner AI, Graham SL, Martins A. Multifocal pattern electroretinogram does not demonstrate localised field defects in glaucoma. Doc Ophthalmol. 2000;100:155–165. doi: 10.1023/a:1002772520539. [DOI] [PubMed] [Google Scholar]
- 32.Lindenberg T, Horn FK, Korth M. Multifocal steady-state pattern-reversal electroretinography in glaucoma patients. Ophthalmologe. 2003;100:453–458. doi: 10.1007/s00347-002-0759-x. [DOI] [PubMed] [Google Scholar]
- 33 ••.Porciatti V, Sorokac N, Buchser W. Habituation of retinal ganglion cell activity in response to steady state pattern visual stimuli in normal subjects. Invest Ophthalmol Vis Sci. 2005;46:1296–1302. doi: 10.1167/iovs.04-1242. Steady-state presentation of an optimal PERG stimulus reduces the PERG amplitude, suggesting that RGCs autoregulate their function in response to an increased metabolic demand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Logean E, Falsini B, Riva CE. Optic Nerve Head Blood Flow Responses Elicited Using Pattern Contrast Reversal Checkerboard Stimuli. ARVO. 2002 abstract # 3314. [Google Scholar]
- 35.Riva CE, Falsini B, Logean E. Flicker-evoked responses of human optic nerve head blood flow: luminance versus chromatic modulation. Invest Ophthalmol Vis Sci. 2001;42:756–762. [PubMed] [Google Scholar]
- 36.Ames A., 3rd CNS energy metabolism as related to function. Brain Res Rev. 2000;34:42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- 37.Raichle ME. Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci USA. 1998;95:765–772. doi: 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raichle ME, Gusnard DA. Appraising the brain’s energy budget. Proc Natl Acad Sci USA. 2002;99:10237–10239. doi: 10.1073/pnas.172399499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quigley HA. Glaucoma: macrocosm to microcosm the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2005;46:2662–2670. doi: 10.1167/iovs.04-1070. [DOI] [PubMed] [Google Scholar]
- 40.Viswanathan S, Frishman LJ, Robson JG, et al. The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40:1124–1136. [PubMed] [Google Scholar]
- 41.Viswanathan S, Frishman LJ, Robson JG. The uniform field and pattern ERG in macaques with experimental glaucoma: removal of spiking activity. Invest Ophthalmol Vis Sci. 2000;41:2797–2810. [PubMed] [Google Scholar]
- 42.Colotto A, Falsini B, Salgarello T, et al. Photopic negative response of the human ERG: losses associated with glaucomatous damage. Invest Ophthalmol Vis Sci. 2000;41:2205–2211. [PubMed] [Google Scholar]
- 43 •.Aldebasi YH, Drasdo N, Morgan JE, North RV, S-cone L. + M-cone, and pattern, electroretinograms in ocular hypertension and glaucoma. Vision Res. 2004;44:2749–2756. doi: 10.1016/j.visres.2004.06.015. A comparison between transient PERG and PhNR in response to stimuli selective for the S-cone pathway and the (L+M)-cone pathway in glaucoma. [DOI] [PubMed] [Google Scholar]
- 44.Porciatti V, Di Bartolo E, Nardi N, Fiorentini A. Responses to chromatic and luminance contrast in glaucoma: a psychophysical and electrophysiological study. Vision Res. 1997;37:1975–1987. doi: 10.1016/s0042-6989(97)00018-7. [DOI] [PubMed] [Google Scholar]
- 45.Greenstein VC, Hood DC, Ritch R, et al. S (blue) cone pathway vulnerability in retinitis pigmentosa, diabetes and glaucoma. Invest Ophthalmol Vis Sci. 1989;30:1732–1737. [PubMed] [Google Scholar]
- 46.Trick GL. Visual dysfunction in normotensive glaucoma. Doc Ophthalmol. 1993;85:125–133. doi: 10.1007/BF01371128. [DOI] [PubMed] [Google Scholar]
- 47.Chauhan BC, Drance SM, Douglas GR, et al. Visual field damage in normal-tension and high-tension glaucoma. Am J Ophthalmol. 1989;108:636–642. doi: 10.1016/0002-9394(89)90854-4. [DOI] [PubMed] [Google Scholar]
- 48 ••.Ventura LM, Porciatti V. Restoration of retinal ganglion cell function in early glaucoma after intraocular pressure reduction: a pilot study. Ophthalmology. 2005;112:20–27. doi: 10.1016/j.ophtha.2004.09.002. PERG abnormality may be reversed after IOP lowering in early stages of both OAG and NTG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109:77–83. doi: 10.1001/archopht.1991.01080010079037. [DOI] [PubMed] [Google Scholar]
- 50.Tuulonen A, Lehtola J, Airaksinen PJ. Nerve fiber layer defects with normal visual fields. Do normal optic disc and normal visual field indicate absence of glaucomatous abnormality? Ophthalmology. 1993;100:587–597. doi: 10.1016/s0161-6420(93)31598-8. (discussion 597–8) [DOI] [PubMed] [Google Scholar]
- 51.Morgan JE. Retinal ganglion cell shrinkage in glaucoma. J Glaucoma. 2002;11:365–370. doi: 10.1097/00061198-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 52.Weber AJ, Kaufman PL, Hubbard WC. Morphology of single ganglion cells in the glaucomatous primate retina. Invest Ophthalmol Vis Sci. 1998;39:2304–2320. [PubMed] [Google Scholar]
- 53 ••.Weber AJ, Harman CD. Structure-function relations of parasol cells in the normal and glaucomatous primate retina. Invest Ophthalmol Vis Sci. 2005;46:3197–3207. doi: 10.1167/iovs.04-0834. In monkeys with hypertensive glaucoma, living RGCs are shrunken and are less responsive. This may provide a neurophysiological rationale for PERG abnormality in early glaucoma. [DOI] [PMC free article] [PubMed] [Google Scholar]