Abstract
The outer leaflet of neuronal membranes is highly enriched in gangliosides. Therefore, specific neuronal roles have been attributed to this family of sialylated glycosphingolipids, e.g. in modulation of ion channels and transporters, neuronal interaction and recognition, temperature adaptation, Ca2+ homeostasis, axonal growth, (para)node of Ranvier stability and synaptic transmission. Recent developmental, ageing and injury studies on transgenic mice lacking subsets of gangliosides indicate that gangliosides are involved in maintenance rather than development of the nervous system and that ganglioside family members are able to act in a mutually compensatory manner. Besides having physiological functions, gangliosides are the likely antigenic targets of autoantibodies present in Guillain-Barré syndrome (GBS), a group of neuropathies with clinical symptoms of motor- and/or sensory peripheral nerve dysfunction. Antibody binding to peripheral nerves is thought to either interfere with ganglioside function or activate complement, causing axonal damage and thereby disturbed action potential conduction. The presynaptic motor nerve terminal at the neuromuscular junction (NMJ) may be a prominent target because it is highly enriched in gangliosides and lies outside the blood–nerve barrier, allowing antibody access. The ensuing neuromuscular synaptopathy might contribute to the muscle weakness in GBS patients. Several groups, including our own, have studied the effects of anti-ganglioside antibodies in ex vivo and in vivo experimental settings at mouse NMJs. Here, after providing a background overview on ganglioside synthesis, localization and physiology, we will review those studies, which clearly show that anti-ganglioside antibodies are capable of binding to NMJs and thereby can exert a variety of pathophysiological effects. Furthermore, we will discuss the human clinical electrophysiological and histological evidence produced so far of the existence of a neuromuscular synaptopathy contributing to muscle weakness in GBS patients.
Gangliosides are ubiquitous glycosphingolipids but are highly enriched in neurons, suggesting neuron-specific physiological functions. Furthermore, they are neuronal receptors for various paralytic microbial toxins and form antigenic targets for anti-ganglioside antibodies that are present in forms of Guillain-Barré syndrome (GBS), a neuropathy characterized by dysfunction of motor- and/or sensory peripheral nerves. Besides immune targeting of nerve trunks and roots, these anti-ganglioside antibodies may also bind to the motor nerve terminal at the neuromuscular junction (NMJ), which is especially rich in gangliosides, and thus mediate a neuromuscular synaptopathy, i.e. a structural and/or functional dysfunction of the NMJ resulting in block of synaptic transmission. Interestingly, symptoms of GBS and some known NMJ disorders overlap. We here review the animal experimental and human clinical electrophysiological evidence of a neuromuscular synaptopathy in anti-ganglioside antibody-mediated GBS, against the background of the physiological roles of gangliosides in neurons and synapses and the structure and function of the NMJ.
Gangliosides
Structure and biosynthesis
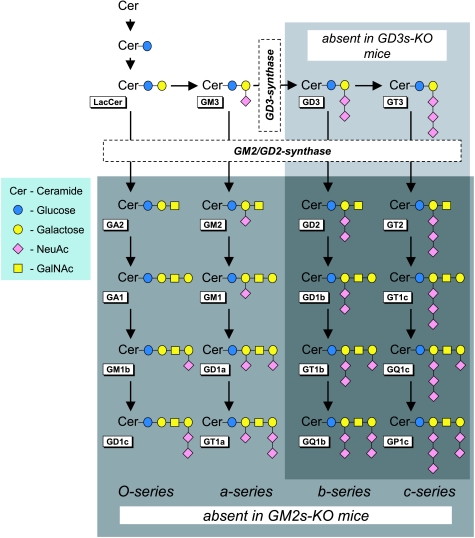
Gangliosides are amphiphilic molecules that associate with plasma- and intracellular membrane compartments. In the plasma membrane, the hydrophobic ceramide tail inserts in the membrane and the hydrophilic oligosaccharide moiety is displayed extracellularly (Figs 1 and 2C). The carbohydrate portion consists of a variable backbone chain of neutral sugars linking one or more negatively charged sialic acid residues (also called neuraminic acid, NeuAc), which defines gangliosides as a distinct glycosphingolipid group. The nomenclature is according to Svennerholm (Svennerholm, 1994), designating gangliosides as GXyz, where G indicates ganglioside, X is a letter representing the number of sialic acid molecules (M, one; D, two; T, three; Q, four), y is a number indicating the length of the neutral sugar sequence (defined as 5 minus the number of residues) and z is a letter indicating the isomeric form, reflecting the position(s) and linkage(s) of the sialic acid residues (a, b or c). Ganglioside biosynthesis takes place in the Golgi complex in parallel pathways by the addition of neutral sugar and sialic acid moieties to a glucosylceramide molecule (Fig. 1), catalysed by specific glycosyltransferases (Yu et al. 2004; Maccioni, 2007). The simple gangliosides GM3, GD3 and GT3 form the basis for complex gangliosides of the a-, b- and c-series, respectively.
Figure 1. The synthesis pathways of gangliosides and indication of the deficient ganglioside subsets in GD3s- and GM2s-KO mice.
Ganglioside nomenclature is according to Svennerholm (1994). GM2s-KO mice lack complex gangliosides (light grey rectangle); GD3s-KO mice lack b- and c-series gangliosides (dark grey rectangle). NeuAc, N-acetylneuraminic acid (= sialic acid); GalNAc, N–acetylgalactosamine.
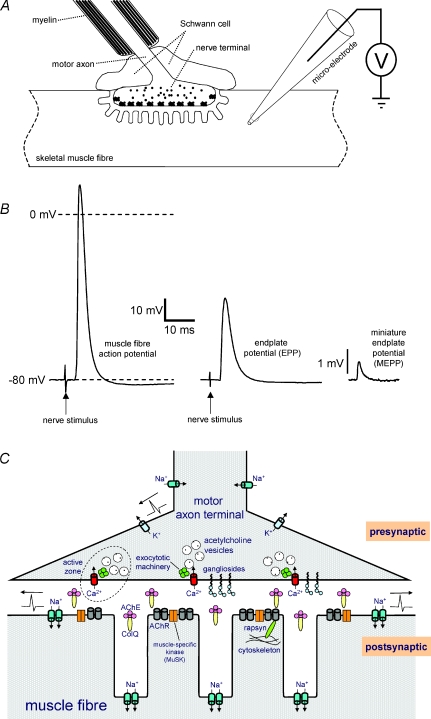
Figure 2. Structure and function of the neuromuscular junction.
A, schematic diagram depicting the neuromuscular junction (NMJ) and a perisynaptic Schwann cell. In excised muscle–nerve preparations, e.g. mouse diaphragm–phrenic nerve, the synaptic signals following from the release of ACh from the presynaptic motor nerve terminal can be measured intracellularly with a micro-electrode inserted in the muscle fibre near the NMJ. B, example intracellular recordings. Left: muscle fibre action potential triggered by successful synaptic transmission in response to nerve stimulation (arrow). Middle: a bare endplate potential (EPP), following from nerve stimulation (arrow) after blocking the muscle fibre Na+ channels with the pharmacological tool μ–conotoxin-GIIIB. Right: a miniature endplate potential, the synaptic event following from spontaneous uniquantal ACh release from the presynapse. C, schematic drawing of a NMJ, indicating the most important functional components for synaptic transmission. Gangliosides presumably co-localize with several proteins important for neurotransmitter release (e.g. Ca2+ channels) in lipid ‘rafts’ at active zones in the presynaptic membrane. AChR, ACh receptor; AChE, acetylcholinesterase; ColQ, collagen–Q.
Regional and subcellular localization
Gangliosides are particularly abundant in neurons. They compose 10–20% of the total lipid of the outer neuronal membrane layer, ten times more than in non-neuronal cells (Ledeen, 1985). Membrane gangliosides are (mainly, but not exclusively) present in small dynamic membrane ‘rafts’ characterized by high concentrations of (glyco-)sphingolipids and cholesterol (Simons & Ikonen, 1997; Kasahara et al. 2000; van der Goot & Harder, 2001; Vyas et al. 2001; Prinetti et al. 2001; Pike, 2006; Fujita et al. 2007; Hanzal-Bayer & Hancock, 2007). These rafts also contain specific proteins, e.g. GPI-anchored proteins, G-proteins and kinases, suggesting raft-associated signalling functions (van der Goot & Harder, 2001). Relatively recently it was realized that gangliosides may play an active role in the formation of lipid membrane domains, instead of only being taken up passively (Sonnino et al. 2007; Silveira e Souza et al. 2008).
Different nervous system structures can express different ganglioside patterns and levels (Schwarz & Futerman, 1996; Ogawagoto & Abe, 1998). This suggests regional-specific functions and possibly explains the specific clinical pictures amongst neuropathies associated with distinct types of anti-ganglioside antibodies (see below). For instance, human spinal cord contains a 2–fold higher ganglioside level than cauda equina, and sensory nerves contain 30% more ganglioside than motor nerves (Svennerholm, 1994). GM1 ganglioside is expressed relatively highly in ventral compared to dorsal root nerve myelin (Ogawa-Goto et al. 1992), although not confirmed by others (Svennerholm, 1994). Another example is the enrichment of GQ1b ganglioside in oculomotor nerve (Chiba et al. 1997). In addition, anti-GQ1b, -GT1a and -GD1b antibodies readily bind to NMJs in human extraocular muscles but not in axial and limb muscles (Liu et al. 2009). However, muscle spindles in the latter groups clearly bound antibodies. Immunohistochemical study of ganglioside localization is complicated by the shielding of some types of gangliosides by other glycolipids, preventing binding of certain antibodies (Schwarz & Futerman, 1996; Greenshields et al. 2009). Thus, lack of specific antibody binding does not necessarily prove absence of the ganglioside.
There are subcellular differences in ganglioside expression. For instance, several gangliosides specifically immunolocalize to either dendritic or somatic sites of cerebellar Purkinje cells and retina neurons, while being absent at axons and presynaptic nerve terminals (Schwarz & Futerman, 1996). Myelinated peripheral nerve axons have GM1 and GD1a enrichment at paranodal regions of nodes of Ranvier (Willison & O’Hanlon, 1999; Susuki et al. 2007). Cranial nerves subserving in oculomotor function have a relatively high GQ1b expression, also selectively at paranodal regions (Chiba et al. 1993, 1997). Furthermore, synaptic membrane contains a specific ganglioside content profile (Waki et al. 1994). GM1 has been shown to preferentially localize at pre- and postsynaptic membranes in cerebral cortex tissue (Hansson et al. 1977). A special class (known as Chol-1 antigens, consisting of α–isomeric forms of GM1, GD1, GT1b, GT1a and GQ1b) appears to be exclusively present at CNS cholinergic nerve terminals as well as at the NMJ (Derrington & Borroni, 1990; Schwarz & Futerman, 1996; Ando et al. 2004). At motor nerve terminals at the NMJ of humans and experimental animals, multiple types of gangliosides, including GQ1b, GM1, GD1a, GD1b, GT1a and GD3, are present although the specific profile may vary between different muscles and species (Schluep & Steck, 1988; Illa et al. 1995; Plomp et al. 1999; Goodyear et al. 1999; Goodfellow et al. 2005; Liu et al. 2009; Greenshields et al. 2009). Furthermore, perisynaptic Schwann cells at NMJs of some mouse strains express gangliosides, in particular GD3 (Halstead et al. 2005b).
Physiological functions
Functions of gangliosides postulated so far include modulation of membrane proteins, neural development, cell–cell interaction/recognition, temperature adaptation, neuronal Ca2+ homeostasis, axonal growth, (para)node of Ranvier stability and synaptic transmission (Ando, 1983; Ledeen, 1985; Thomas & Brewer, 1990; Rahmann et al. 1992; Wu & Ledeen, 1994; Lloyd & Furukawa, 1998; Ledeen & Wu, 2006; Susuki et al. 2007). While older studies assessed function by studying the effects of exogenous ganglioside application on in vitro systems, recent generation of transgenic mice lacking ganglioside-synthesizing enzymes allowed investigations into the function of endogenous gangliosides (Sheikh et al. 1999; Kawai et al. 2001; Okada et al. 2002; Inoue et al. 2002; Sugiura et al. 2005; Jennemann et al. 2005; Zitman et al. 2008, 2009). The main hypothesized functions of gangliosides will be discussed below.
Modulation of membrane protein function. Gangliosides may affect neuronal membrane protein function through (1) influencing the fluidity of the plasma membrane surrounding the protein (Kappel et al. 2000) and (2) an electrostatic influence of the negatively charged sialic acid residues, either directly or indirectly through membrane surface charge (Green & Andersen, 1991; Aubin & Prestegard, 1993; Salazar et al. 2004). Studies applying exogenous gangliosides have suggested modulatory actions on membrane-bound enzymes (Partington & Daly, 1979), ion pumps (Caputto et al. 1977; Leon et al. 1981) and ion channels (Leon et al. 1981; Carlson et al. 1994; Kappel et al. 2000; Salazar et al. 2004), hormone receptors (Bremer & Hakomori, 1982) and proteins involved in the membrane flux and intracellular homeostasis of Ca2+ (Wu et al. 1990; Wu & Ledeen, 1994; Wu et al. 2001, 2007. For reviews see Ando, 1983; Lloyd & Furukawa, 1998; Yates & Rampersaud, 1998; Ledeen & Wu, 2002). Recent studies using specific anti-ganglioside antibodies suggest a relationship between gangliosides and function of voltage-gated Ca2+ channels (Ortiz et al. 2001; Santaféet al. 2005; Buchwald et al. 2007; Nakatani et al. 2009).
Neural development. Developmental roles are suggested from the observation that ganglioside composition varies considerably in distinct nervous structures during embryogenesis, ageing or regeneration (Lloyd & Furukawa, 1998; Ngamukote et al. 2007). Furthermore, early studies showed neurotrophic and neuritogenic activity of exogenously applied gangliosides (Ledeen, 1984), as well as enhanced reinnervation and restoration of functional NMJs after peripheral nerve crush (Gorio et al. 1980). In contrast, in mice with brain-specific deletion of all glycosphingolipids, including gangliosides, embryogenesis proceeded normally; however, pups died from neurodegeneration within 3 weeks of birth (Jennemann et al. 2005). This excludes a role for gangliosides in neurodevelopment and, rather, suggests roles in maturation, maintenance or repair. This is supported by a progressive, late-onset (8–16 months) phenotype of motor coordination, gait deficits, tremor and catalepsy in mice null-mutant for GM2/GD2-synthase (GM2s-KO) (Chiavegatto et al. 2000; Sugiura et al. 2005), thereby lacking complex gangliosides (Fig. 1). Young GM2s-KO mice show no phenotype, but have histological signs of peripheral nerve degeneration (Sheikh et al. 1999), worsening upon ageing (Sugiura et al. 2005). GD3-synthase knockout (GD3s-KO) mice, lacking b- and c-series gangliosides (Fig. 1), display no overt phenotype (Kawai et al. 2001; Okada et al. 2002), but show impaired nerve regeneration (Okada et al. 2002). Compound null-mutant mice lacking both GM2/GD2-synthase and GD3-synthase only express GM3 (Fig. 1). They appear normal at birth but show sudden death with ∼50% survival at 3–6 months (Kawai et al. 2001; Inoue et al. 2002). Skin lesions develop at > 6 months of age, apparently due to sensory nerve impairment (Inoue et al. 2002). Any interpretation of observations in partially ganglioside-deficient mice is confounded by possible compensatory effects of the remaining types of gangliosides, which accumulate (Takamiya et al. 1996; Kawai et al. 2001; Okada et al. 2002; Handa et al. 2005).
Synaptic function. A role in synaptic function has been suggested from biochemical, morphological and in vitro functional studies (Thomas & Brewer, 1990; Ando et al. 1998, 2004). Firstly, ganglioside density in synaptic membranes is high (Thomas & Brewer, 1990; Ando et al. 2004). Secondly, bath-applied GM1 and GQ1b increase K+-evoked neurotransmitter release from rat brain synaptosomes, presumably via Cav2.2 Ca2+ channels (Tanaka et al. 1997). Thirdly, synapse plasticity in brain slices is affected by bath-applied gangliosides (Wieraszko & Seifert, 1985; Ramirez et al. 1990; Egorushkina et al. 1993; Tanaka et al. 1997; Furuse et al. 1998; Fujii et al. 2002). Fourthly, gangliosides co-localize in lipid rafts with key transmitter release proteins (e.g. Ca2+ channels and SNAREs) (Chamberlain et al. 2001; Lang et al. 2001; Salaun et al. 2004; Taverna et al. 2004; Davies et al. 2006). Lastly, poly-sialylated gangliosides bind Ca2+, a crucial ion in transmitter release (Rahmann et al. 1992).
Recently, we assessed directly if heterogeneity of endogenous gangliosides is essential for synaptic transmission by studying the electrophysiology of NMJs of mice lacking one or more ganglioside-synthesizing enzymes. At NMJs of GM2s-KO mice, a surprising redundancy of complex gangliosides (including GM1, GD1a and GD1b, see Fig. 1) was found for acetylcholine (ACh) release (Bullens et al. 2002). At standard measuring conditions (2 mm extracellular Ca2+, room temperature) none of the measured parameters differed from wild-type and heterozygous controls. In temperature-variation experiments, however, ∼30% reduced ACh release was found at 17°C, suggesting that complex gangliosides are involved in temperature-stabilization of synaptic transmission, as hypothesized by others (Rahmann et al. 1998). Further studies at NMJs of GD3s-KO mice as well as of compound null-mutant mice lacking both GD3s and GM2s showed a further redundancy of most gangliosides in synaptic transmission (Zitman et al. 2008). No major deficits were found in either mutant. However, compared to wild-type, ACh release at high intensity (40 Hz) nerve stimulation ran down slightly (but statistically significantly) more than at compound null-mutant NMJs. Furthermore, a temperature-specific increase of ACh release was observed at 35°C at GD3s-KO NMJs. These studies showed that synaptic transmission at the NMJ is not crucially dependent on most gangliosides and remains largely intact in the presence of GM3 only. We also investigated aged (> 9 months old) GM2s- and GD3s-KO mice, because synaptic dysfunction might develop with age and may contribute to the late-onset neurological phenotype of GM2s-KO mice (Chiavegatto et al. 2000; Sugiura et al. 2005). However, we found only subtle changes in presynaptic function (Zitman et al. 2009). ACh release at 40 Hz nerve stimulation at GM2s-KO NMJs ran down slightly more than at wild-type NMJs, and spontaneous ACh release at GD3-synthase null-mutant NMJs was somewhat higher than at wild-type, selectively at 25°C. Interestingly, we observed faster rising and decaying phases of postsynaptic responses at aged GD3s-KO NMJs, not previously seen in young GD3-synthase null-mutants or other types of (aged or young) ganglioside-deficient mice. The kinetic changes may reflect changes in postsynaptic ACh receptor (AChR) function. This effect, however, was relatively small and apparently did not endanger successful neurotransmission at the NMJ, as no muscle weakness was observed in these mice. The overall conclusion is that gangliosides may modulate temperature- and use-dependent fine-tuning of transmitter release, but are largely dispensable players in transmitter release. After all, synapses exist and function in Drosophila melanogaster, despite this organism's inability to synthesize gangliosides (Roth et al. 1992; Chen et al. 2007), showing that gangliosides are not absolutely required for synapse function. Mice lacking both GM3- and GM2-synthase show neurodegeneration and live only a few weeks (Yamashita et al. 2005). It would be interesting to study their synaptic transmission to assess (compensatory) roles of GM3.
Ganglioside-related disorders
Disorders exist with (1) disturbed ganglioside synthesis or breakdown and (2) autoantibodies against gangliosides. Examples of the first category are an infantile epilepsy syndrome (Simpson et al. 2004) and GM1- and GM2-gangliosidosis (Maegawa et al. 2006; Brunetti-Pierri & Scaglia, 2008). There is preliminary evidence of disturbed ganglioside metabolism in Huntington's disease (Desplats et al. 2007), multiple sclerosis (Marconi et al. 2006) and Alzheimer's disease (Ariga et al. 2008). With respect to the second category, GBS is the most common form of acute neuromuscular paralysis with an annual incidence of about 1–2 per 100 000 (Willison & Yuki, 2002; van Sorge et al. 2004; Ang et al. 2004; Hughes & Cornblath, 2005; Winer, 2008; van Doorn et al. 2008). Autoimmunity to gangliosides is thought to cause the neurological symptoms, although antibodies are detectable by current methods in only about half of the patients, predominantly those with axonal forms. GBS is characterized by muscle weakness, areflexia and possible sensory disturbance. Weakness peaks at ∼4 weeks from onset and, although most patients recover completely or partially, there is ∼5–10% mortality. A spectrum of clinical, serological and electrodiagnostic characteristics exist, but distinct variants can be discriminated (Willison & Yuki, 2002). The most common form in the Western world is acute inflammatory demyelinating polyneuropathy (AIDP), hallmarked by demyelination, mostly without axonal damage. In other forms, more frequent in Asia, axonal degeneration without demyelination occurs in either motor axons only (acute motor axonal neuropathy, AMAN) or both motor and sensory axons (acute motor and sensory axonal neuropathy, AMSAN). Miller Fisher syndrome (MFS), a less common variant, is characterized by ophthalmoplegia, areflexia and ataxia and, sometimes, facial and bulbar weakness with good recovery (Lo, 2007).
GBS subtypes seem to be associated with specific anti-ganglioside antibodies. AMAN and AMSAN are associated with IgG antibodies against (combinations of) either GM1, GM1b, GD1a or GalNAc-GD1a. MFS is very strongly associated (> 90% of cases) with IgG antibodies against GQ1b, often cross-reactive to GD3 and GT1a. In AIDP, anti-ganglioside antibodies have not been consistently shown to relate to particular phenotypes. Anti-GQ1b antibodies are furthermore often present in GBS forms with concomitant ophthalmoplegia. Very recently it was found that GBS patients can have antibodies that bind (at least in ELISA) to a specific combination of gangliosides and much less to the individual molecules (Kusunoki et al. 2008). About 17% of GBS sera had antibodies against a combination of two of the major gangliosides GM1, GD1a, GD1b and GT1b. Interestingly, antibodies against GD1a/GD1b and GD1b/GT1b complexes were associated with severe disease requiring ventilation (Kaida et al. 2007). In MFS, the incidence of anti-complex antibodies seems higher. Seven of 12 MFS sera (of which 10 were also positive for antibodies against GQ1b alone) were positive for antibodies against a complex of at least GQ1b or GT1a and another ganglioside such as GM1, GD1b, GD1a, GM1 or GT1b (Kaida et al. 2006).
Important early direct evidence supporting the hypothesis that anti-ganglioside antibodies cause neuropathy came from the observation that seven patients developed high titres of anti-ganglioside antibodies and a GBS-like motor neuropathy after therapeutic injection of a GM1, GD1a, GD1b and GT1b mixture (intended for pain treatment) (Illa et al. 1995).
More recently, the hypothesis was put forward that anti-ganglioside antibodies in GBS arise through a molecular mimicry mechanism (Ang et al. 2004; Yuki & Koga, 2006). About two-thirds of the patients experience a preceding airway or gastrointestinal infection, the latter being frequently caused by Campylobacter jejuni. Lipo-oligosaccharides present on isolated C. jejuni have been shown to contain ganglioside-like structures, recognized by anti-ganglioside antibodies of GBS patients. Thus, anti-ganglioside antibodies most likely arise through an immune response against C. jejuni, and subsequently cause neuropathy by cross-reacting with peripheral nerve gangliosides, probably involving complement activation (Ramaglia et al. 2008). Only about one per 3000 C. jejuni-infected people develop GBS. Therefore, either the specific C. jejuni strain involved in a particular GBS patient must be of a special nature (e.g. bear ganglioside-like epitopes on their lipo-oligosaccharides mantle), or the host must have a susceptibility factor to cause post-infectious GBS, or a combination of the two conditions must be met. Indeed, gene variants in carbohydrate-synthesizing enzyme gene clusters of C. jejuni strains isolated from GBS patients were found that enable the synthesis of ganglioside-like epitopes. Probably host susceptibility factors are immunoregulatory genes, although recent studies showed no relationship between GBS and certain HLA class II alleles or CD1 gene polymorphisms. However, single-nucleotide polymorphisms of genes encoding Fcγ-receptor-III, matrix metalloproteinase-9, tumour necrosis factor–α and mannose-binding lectin are associated with (the severity of) GBS (van Doorn et al. 2008).
The NMJ as a potential target of anti-ganglioside antibodies
Besides damaging axons, it was hypothesized 15 years ago that GBS-associated anti-ganglioside antibodies may target the NMJ. The presynaptic membrane is gangliosides-rich and, lying outside the blood–nerve barrier, readily accessible to circulating antibodies. Furthermore, GBS symptoms overlap with those of known NMJ disorders/intoxications such as botulism where Clostridium botulinum neurotoxins bind to presynaptic gangliosides at NMJs (Bullens et al. 2002), myasthenia gravis with antibodies against postsynaptic neurotransmitter receptors, and organophosphate poisoning (Dörr et al. 2006; van Doorn et al. 2008; Silverstein et al. 2008).
Structure and function of the NMJ
The NMJ transmits impulses from a motor neuron onto a skeletal muscle fibre (Ruff, 2003; Slater, 2008). Myelinated axons originating from motor neuron somata in the spinal cord anterior horn travel through a peripheral nerve into a muscle, branch off and innervate multiple fibres. Generally, each fibre has only one, single-innervated, NMJ (although during embryonic and perinatal development, multiple innervation occurs on a large scale; it remains present only at some special muscles, e.g. extraocular muscle). The distal axon ending (< 100 μm) loses myelin but, instead, is covered by 3–5 perisynaptic Schwann cells (Figs 2A and 3), involved in stabilization, regeneration and possibly also transmitter release modulation (Auld & Robitaille, 2003). Presynaptic terminals synthesize and release the neurotransmitter ACh in a tightly controlled way. A mouse motor nerve terminal contains 250 000–350 000 synaptic vesicles, each containing ∼10 000 ACh molecules (a ‘quantum’). The smaller human nerve terminals probably contain fewer vesicles (Slater, 2008). ACh is synthesized from cytosolic choline and acetylcoenzyme A and loaded into vesicles by a specific transferase. It is exocytosed at active zones by a release machinery composed of several structural and functional proteins (Sudhof, 2004) (Figs 2C and 3). A crucial functional protein is the voltage-gated Ca2+ channel (Cav2.1, at mammalian NMJs), which allows for Ca2+ influx that stimulates the neuroexocytotic machinery. Spent vesicles are recycled, probably in two different pools, depending on transmitter release rate (Perissinotti et al. 2008). A proportion of the released ACh is degraded by acetylcholinesterase in the synaptic cleft. The remainder binds and opens AChRs, clustered at high density (∼10 000–20 000 μm−2) at the tops of the typical postsynaptic membrane foldings (Figs 2 and 3). This allows the influx of positive charge, gating voltage-gated Nav1.4 channels in the folds. This causes an action potential along the muscle fibre which stimulates the contraction mechanism (Beam et al. 1989). AChR-mediated depolarizations can be measured as miniature endplate potentials (MEPPs, ∼1 mV), resulting from spontaneous release of single ACh quanta, and endplate potentials (EPPs, ∼20–40 mV), upon release of multiple quanta (‘quantal content’) by a nerve impulse (Fig. 2B and C). These events can be measured electrophysiologically with relative ease in muscle–nerve preparations from experimental animals or in biopsied human tissue (Fig. 2A and B). EPPs, not MEPPs, will normally trigger an action potential. A 3–5 × safety factor exists, i.e. the EPP is much larger than required to trigger an action potential (Wood & Slater, 2001), ensuring robustness of transmission, even at high rate when EPP rundown occurs.
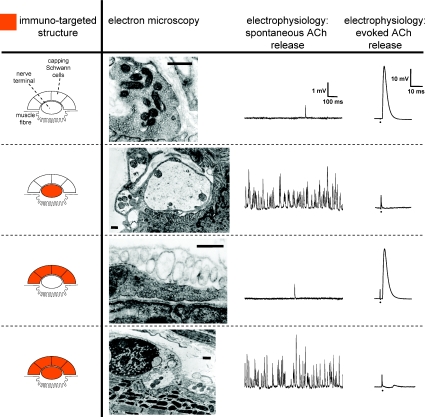
Figure 3. Overview of the ultrastructural and electrophysiological deleterious effects of anti-ganglioside antibodies when targeting either the motor nerve terminal alone, the perisynaptic Schwann cells alone, or both of these structures.
Effects of anti-ganglioside antibodies and complement in ex vivo incubation studies on NMJs of mouse muscle–nerve preparations. For details see Halstead et al. (2005b). Electron microscopy shows that immunotargeted motor axon terminals become disorganized and swollen with a reduced synaptic vesicle density and damaged mitochondria. Immunotargeted Schwann cells are ultrastructurally characterized by a swollen, electron-lucent appearance and damaged organelles. Electrophysiologically, nerve terminal damage was hallmarked by a temporary dramatic increase in spontaneous uniquantal ACh release (measured as miniature endplate potential frequency), leading to block of evoked release (measured as endplate potentials) upon nerve stimulation. The moment of stimulation in the signals of the right column is indicated by a dot and a stimulus artefact can be seen. Interestingly, acute damage of perisynaptic Schwann cells did not change the presynaptic release or the postsynaptic effect of ACh, indicating that any modulatory effect of these cells on synaptic transmission is only to occur in the longer term. Scale bars in electron micrographs, 500 nm.
Experimental evidence for synaptopathic actions of anti-ganglioside antibodies at the NMJ
Overview of our experimental mouse NMJ studies using patient sera and mouse monoclonal antibodies against gangliosides. First experimental indication that anti-ganglioside antibodies can harm NMJs came from Roberts who exposed ex vivo mouse diaphragms to three anti-GQ1b-positive MFS sera and observed transmission block (Roberts et al. 1994). Extensive studies by us on many MFS sera as well as purified IgG and human anti-GQ1b monoclonal antibody (mAb) elucidated pathophysiology in detail (Plomp et al. 1999). Firstly, immunohistochemistry showed that anti-GQ1b IgG and IgM bind to the NMJ. Secondly, electrophysiological analysis revealed a temporary (tens of minutes) but dramatic rise in MEPP frequency, peaking at a several 100-fold higher level than normal (Fig. 3), and that nerve stimulation-evoked ACh release became permanently blocked, paralysing the muscle. This effect resembled that of α–latrotoxin, from the black widow spider, which binds NMJs presynaptically and stimulates neuroexocytosis via second messengers as well as by forming pores that conduct ions, including Ca2+, causing massive ACh release and structural damage (Sudhof, 2001; Ushkaryov, 2002). Therefore, we coined the effect of anti-GQ1b antibodies as the ‘α–latrotoxin-like effect’. These effects were completely dependent on the activation of the complement cascade that is triggered by bound antibody (Walport, 2001). This was shown by loss of pathophysiological potency of sera by heating (30 min, 56°C), known to inactivate complement. Furthermore, anti-GQ1b IgG or IgM mAb only exerted pathophysiological effects when normal human serum was added as a complement source. In addition, complement deposition at NMJs was shown immunohistologically. Asynchronous twitching of muscle fibres paralleled the dramatically increased MEPP frequency. This disappeared upon AChR block by d–tubocurarine, excluding muscle fibre membrane malfunction as cause. Electrophysiological recordings showed that MEPPs frequently became superimposed due to high frequency and that summed potentials occasionally reached threshold and triggered an action potential. This twitching was used as easy readout in a bioassay where we tested synaptopathic potency of large numbers of MFS and GBS sera (Jacobs et al. 2002). Muscle strips were pre-incubated with heated serum and twitching was scored during incubation with normal human serum as complement source. In this way we found ∼80% of anti-GQ1b-positive MFS sera to induce NMJ synaptopathy, as well as 10% of the (non-MFS) GBS sera, 80% of them being anti-GQ1b-positive.
We immunized mice with lipo-oligosaccharides from GBS-associated C. jejuni and cloned mAbs that bound both lipo-oligosaccharides and gangliosides GQ1b, GT1a and GD3 (Goodyear et al. 1999). Besides showing that molecular mimicry is a likely mechanism for the generation of autoantibodies in MFS/GBS, this yielded a valuable set of anti-GQ1b/GD3/GT1a mAbs. At mouse diaphragm NMJs, these mAbs potently induced identical synaptopathic effects as produced earlier with anti-GQ1b-positive MFS and GBS sera and the human anti-GQ1b mAb. Again, antibody and complement deposits at NMJs were clearly shown (Fig. 4A and B). Subsequently, we investigated whether block of evoked ACh release (measured as EPPs) either occurred directly, or secondarily after the dramatic increase in MEPP frequency (Bullens et al. 2000). Before and after incubation of NMJs with purified anti-GQ1b-positive MFS IgG or the mouse anti-GQ1b/GD3 mAb CGM3, we measured the evoked ACh release. However, no change was found, showing that antibodies alone do not block evoked ACh release but that this occurs either as a complement-dependent effect, subsequent or parallel to the dramatic increase in MEPP frequency. It may occur either by transmitter vesicle depletion or presynaptic damage. While the first seems excluded because high frequency MEPPs stay for some time after block of evoked release (Plomp et al. 1999), the latter was clearly shown in immunofluorescence and electron microscopy (O’Hanlon et al. 2001). The electrophysiological effects coincide with loss of the cytoskeletal proteins neurofilament (heavy, 200 kDa) and type III β–tubulin (Fig. 4C and D). Ultrastructurally, disorganized terminals with a reduced synaptic vesicle density and swollen and damaged mitochondria were observed (Fig. 3), with synaptic clefts often infiltrated by Schwann cells. Immunogold labelling demonstrated a specific presynaptic binding of anti-GQ1b antibody (Halstead et al. 2004), confirming the electrophysiological absence of postsynaptic effects, e.g. MEPP amplitude reduction due to AChR block. Together, these observations suggested that anti-GQ1b antibody and complement destroy the presynaptic membrane and that ensuing Ca2+ influx activates proteases, degrading intraterminal cytoskeletal proteins.
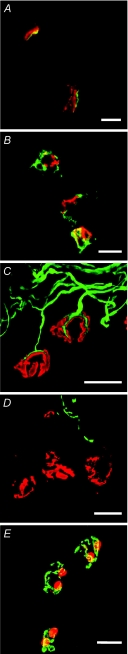
Figure 4. Immunohistochemical characterization of the deleterious effects of anti-ganglioside antibodies.
Mouse neuromuscular junctions in ex vivo muscle nerve preparations were incubated with anti-GQ1b antibody CGM3 and subsequently exposed to normal human serum as complement source. Immunohistochemical analyses showed antibody (A, green) and complement (B, green) deposition at the neuromuscular junctions, delineated by acetylcholine receptor staining using fluorescently labelled α–bungarotoxin (red). Nerve terminal damage led to loss of neurofilament staining (green) at the terminal portion of intramuscular axon branches (C, control condition; D, after treatment; acetylcholine receptor staining in red in both panels). Schwann cell death occurred, indicated by ethidium-homodimer–1 staining (E, red; acetylcholine receptors stained green). For details see O’Hanlon et al. (2001); Bullens et al. (2002); Halstead et al. (2005b). Scale bars, 20 μm.
We characterized complement in anti-GQ1b antibody-induced mouse NMJ synaptopathy and showed C1q, C3c, C4 and membrane attack complex (MAC) deposition (Halstead et al. 2004). MAC, the final complement product, is a membrane pore-forming conformation of factors C5b-9 (Walport, 2001), allowing uncontrolled ion fluxes. Three complement pathways exist which may each contribute: the classical, alternative and lectin pathway (Walport, 2001). When anti-GQ1b and complement were applied in Ca2+-free medium, no C4 or MAC deposition occurred, ruling out the alternative pathway, which is Ca2+ independent. Thus, one or both of the remaining pathways cause the effects. Proof for MAC as ultimate effector came from experiments with C6-deficient serum as complement source, which did not induce synaptopathy in C6-deficient tissue unless purified C6 was added.
Anti-GQ1b antibodies, as defined in ELISA, may in principle also exert NMJ synaptopathic effects by cross-reacting to other (sialylated) antigens, such as related glycolipids or membrane proteins. To investigate this possibility, we performed experiments at NMJs from GM2s-KO mice (see above) which lack GQ1b (and GT1a, to which most anti-GQ1b antibodies cross-react in ELISA) (Bullens et al. 2002). Monospecific anti-GQ1b IgM mAb, EM6, as well as a MFS serum induced NMJ synaptopathy in wild-type and heterozygous controls, and antibody and complement deposits were shown. However, this was not observed at NMJs of homozygous GM2s-KO mice, showing that GQ1b is the real target of ELISA-defined anti-GQ1b antibodies. We also studied monospecific mAbs against GD3, a remaining ganglioside in GM2s-KO mice. They induced little or no effect at controls, while at GM2s-KO NMJs they elicited clear synaptopathic effects. This indirectly showed that GD3 is upregulated at GM2s-KO NMJs, as in GM2s-KO brain (Takamiya et al. 1996), or becomes available for antibody binding due to the absence of steric hindering by the missing gangliosides. More importantly, they show that GD3 can substitute for GQ1b as an antigenic target in mediating synaptopathic effects at NMJs. We subsequently hypothesized that any specific anti-ganglioside mAb could induce synaptopathy, given the presence of a sufficiently high presynaptic density of the specific ganglioside. This hypothesis was tested by exposing NMJs from GD3s-KO mice (lacking b- and c-series gangliosides, and having upregulated GD1a) and GM2s-KO mice (lacking GD1a) to anti-GD1a mAb MOG35 (Goodfellow et al. 2005). This mAb readily induced the expected complement-mediated effects at GD3s-KO NMJs but failed to do so at GM2s-KO and wild-type NMJs. Anti-GD1a antibodies are often present in AMAN-GBS. GD3s-KO NMJs exposed to anti-GD1a-positive AMAN sera clearly developed synaptopathy whilst wild-type NMJs only showed moderately increased MEPP frequency without transmission block. This suggested that NMJ dysfunction may be a factor in the motor symptoms of AMAN. Recently, we also studied anti-GM1 and -GD1b mAbs and showed that they too can induce complement-dependent synaptopathy, at GD3s-KO and wild-type mouse NMJs, respectively. We made the interesting observation that for some anti-GM1 mAbs the antigenic GM1 is shielded by neighbouring gangliosides in the living neuronal membrane and only becomes available for binding under certain conditions such as created by freezing or fixation (Greenshields et al. 2009). Together, all these experimental studies show that many anti-ganglioside antibodies are capable of inducing complement-dependent neuropathogenic effects at the mouse motor nerve terminal, as long as the antigenic ganglioside is expressed at high enough density and is accessible for antibody.
Recently we generated the first paralytic in vivo mouse model for MFS (Halstead et al. 2008b). Intraperitoneal injection of anti-GQ1b/GD3 mAb CGM3 caused respiratory paralysis within hours and excised diaphragms appeared almost completely paralysed. This was due to antibody and complement causing transmission block at many (∼70%) NMJs, shown by morphological and electrophysiological analyses. This in vivo model required co-injection of human serum as complement source. Apparently, mouse complement was insufficiently activated to induce synaptopathy. The molecular explanation for this is unknown.
Overview of the experimental mouse NMJ studies of other groups. Other groups have, sometimes in different experimental settings, also investigated the effects of anti-ganglioside antibodies at mouse NMJs. Kishi and associates found increases in MEPP frequency, muscle fibre twitches and sometimes concomitant transmission block at NMJs exposed to sera from eight GBS variant patients, of which seven were anti-GQ1b-positive and one anti-GD1b-positive (Kishi et al. 2003). These effects were similar to those described by us (see above). With an extracellular recording pipette through which IgG and field stimulation was applied, Buchwald and colleagues observed complement-independent, reversible and near-complete inhibition of evoked ACh release from presynaptic motor nerve terminals by IgG from anti-GQ1b-positive or -negative MFS plasmas (Buchwald et al. 1995, 1998b). Furthermore, amplitude reduction of uniquantal responses was seen, suggesting effects on postsynaptic AChRs (Buchwald et al. 1998b). They also showed similar ACh release-inhibiting effects of IgG from several GBS sera with positivity for GM1, GQ1b or neither ganglioside, and of mouse mAbs against GM1, GD1a or GD1b (Buchwald et al. 1998a, 2007). Part of the GBS IgGs (but none of the mAbs) were reported to have a similar postsynaptic effect to the MFS IgGs, and direct competitive block of AChRs by low-affinity antibodies was hypothesized. From an ultrastructural NMJ study it was concluded that MFS IgG binds pre- and postsynaptic membranes (Wessig et al. 2001). Subsequent study of cells expressing muscle-type AChRs showed that GBS sera (either with activity against GQ1b, GM1 or neither) reduce ACh-induced currents (but only in part of the experiments) presumably through direct, competitive block of AChRs (Krampfl et al. 2003).
Santafé and colleagues studied the effects at mouse NMJs of serum and purified IgM mAb from a chronic demyelinating neuropathy patient with specificity against GM2, GalNAc-GD1a and GalNAc-GM1b, which share a terminal sugar epitope (Ortiz et al. 2001; Santaféet al. 2005, 2008). They found reversible complement-independent reduction (∼40%) of evoked ACh release and a complement-dependent increase in spontaneous release. No postsynaptic effects were noted, as judged by unchanged MEPP amplitude. Chronic mAb injection into the levator auris longus muscle of live mice greatly reduced quantal content at NMJs, as determined ex vivo, without an accompanying rise in MEPP frequency (Santaféet al. 2005, 2008). No mouse complement deposition was shown. It was hypothesized that anti-GM2 mAb inhibits presynaptic Cav2.1 Ca2+ channel function, possibly by disrupting a physiological role of GM2. A similar inhibiting action of (rabbit) anti-GalNAc-GD1a IgG was shown on Ca2+ current elicited in nerve growth factor-differentiated phaeochromocytoma cells (Nakatani et al. 2007). Anti-GM1-positive AMAN sera and additional specificity against (combinations of) GM2, GD1a, GD1b and GalNAc-GD1a, reduced Cav2.1 Ca2+ current in cerebellar Purkinje cells by 30–40%, without altering activation or inactivation kinetics, thus suggesting direct pore block (Nakatani et al. 2009). In addition, selective inhibition of intensely used Cav2.1 channels by anti-GM1 and -GD1a antibodies was suggested from studies in olfactory bulb neurons (Buchwald et al. 2007). Similarly, reversible and complement-independent block of Ca2+ channels by anti-GalNAc-GD1a antibodies was hypothesized from the observation that these antibodies blocked NMJ activity-mediated muscle fibre spikes in rat nerve–muscle co-cultures (Taguchi et al. 2004).
Together, our and other's studies indicate that NMJs may form targets of anti-ganglioside antibodies in GBS. However, a unifying mechanism is not yet established. Particularly, complement involvement in the diverse effects of anti-ganglioside antibodies is unclear and possibly complicated by the rather low complement activity in many mouse strains (Rice, 1950; Ebanks & Isenman, 1996). Neuropathy-associated anti-ganglioside antibodies are IgG–1, IgG–3 and IgM and binding of such isotypes will inevitably activate complement. Complement-independent effects, therefore, might initially take place at patient NMJs, but will probably be overwhelmed soon thereafter by complement activation, culminating in the devastating effects of MAC pore insertion.
Perisynaptic Schwann cells as target of anti-ganglioside antibodies
We first observed the binding of antiganglioside antibody to mouse perisynaptic Schwann cells when we characterized the immunolocalization of the anti-GQ1b/GD3 mouse IgM mAb CGM3 (O’Hanlon et al. 2002). Further immunofluorescence microscopy and immunogold electron microscopy studies confirmed such binding and also showed with a cell death marker (Fig. 4E) that these cells were killed by complement activation (Halstead et al. 2004). However, they may be activated first, because many cellular processes invade the synaptic cleft and enwrap the nerve terminal (O’Hanlon et al. 2001; Halstead et al. 2004). With an expanding array of different anti-ganglioside mAbs we were able to discriminate subsets that bind to either motor nerve terminals, perisynaptic Schwann cells, or both, and identify susceptibility variation between mouse strains. mAbs induced complement-mediated injury at these respective compartments as analysed with fluorescence- and electron-microscopy (Figs 3 and 4) (Halstead et al. 2005b). mAbs that selectively injured perisynaptic Schwann cells (characterized by swelling, electron-lucency and damaged organelles) had high GD3 reactivity in ELISA, suggesting high GD3 density on perisynaptic Schwann cell membranes. Others reported binding of human anti-GM2 mAb at mouse perisynaptic Schwann cells (Santaféet al. 2005). Interestingly, in view of the proposed functional roles of perisynaptic Schwann cells (Auld & Robitaille, 2003), electrophysiological analyses of NMJs of which perisynaptic Schwann cells were acutely ablated by antibody and complement in ex vivo experiments showed unchanged ACh release and postsynaptic sensitivity to ACh (Halstead et al. 2005b). This means that the proposed modulatory roles of Schwann cells, if at all applicable to the mouse NMJ, are likely to occur only in the longer term.
Proof of NMJ synaptopathy in anti-ganglioside antibody-mediated human neuropathy?
Clinical electrophysiological observations in GBS patients
The experimental studies described above strongly suggest that anti-ganglioside antibodies may induce NMJ synaptopathy in GBS. The question arises as to whether there is clinical evidence. Important early indication came from Ho and colleagues, who described an anti-GM1-positive AMAN patient with quickly improving paralysis (within weeks) after antibody removal by plasmapheresis (Ho et al. 1997). A motor-point biopsy showed severe loss of intramuscular nerve branches and many NMJs without motor nerve terminals. Sural nerve biopsy was normal, excluding proximal axonal degeneration (albeit in a sensory nerve). Importantly, electromyography showed severely reduced compound muscle action potentials (CMAPs), without reduced nerve conduction velocity, and fibrillation potentials, indicative of muscle denervation. These observations are compatible with transmission block at many NMJs due to anti-ganglioside antibody and complement-mediated destruction of motor nerve terminals. The rapid recovery indicates that only the very distal regions of motor axons were degenerated, because more proximal lesions with Wallerian degeneration would have required a longer recovery period (human axons regenerate at only < 1 mm day−1). Rapid recovery of paralysis and CMAPs has also been found in other (but not all) anti-GM1-positive AMAN-GBS patients (Kuwabara et al. 1998a,b, 2002). Another early indication of anti-ganglioside antibody-induced human NMJ synaptopathy was provided by Illa and colleagues, who showed presynaptic binding of anti-GM1 IgG from GBS patients at sectioned human NMJs (Illa et al. 1995). A first structured clinical electrophysiological study of NMJ function in acute GBS was done by Spaans, applying single-fibre electromyography (SFEMG) (Spaans et al. 2003). This technique measures variation (‘jitter’) in the delay between nerve stimulus and resulting action potential in a muscle fibre during consecutive nerve stimulation or, upon voluntary contraction, the delay time variations of pairs of action potentials recorded from two fibres of the same motor unit. Although unable to sense permanently blocked NMJs, this technique detects critically transmitting NMJs, i.e. having EPPs with amplitudes around the firing threshold. In all nine tested GBS patients, 10–32% of stimulations showed impulse blocking, not seen in healthy controls, accompanied by a normal or slightly increased jitter value. It was concluded that both axonal and NMJ dysfunction may cause paralysis in GBS.
Clinical electrophysiological observations in anti-GQ1b antibody-positive patients
Driven by the experimental findings at mouse NMJs with MFS anti-GQ1b antibodies, and the enrichment of GQ1b in oculomotor nerve (terminals) (Chiba et al. 1997; Liu et al. 2009), patients with (anti-GQ1b-positive) MFS and ophthalmoplegia variants of GBS have been electromyographically assessed for NMJ malfunction. Uncini and Lugaresi first reported NMJ dysfunction in a case of anti-GQ1b-positive MFS (with additional limb muscle weakness), serially recording CMAPs during 10 weeks after onset (Uncini & Lugaresi, 1999). Severe amplitude reduction was noted, which became maximal at around the third week and quickly normalized in the following 7 weeks, paralleling a disappearance of anti-GQ1b antibodies. Nerve conduction velocity was unchanged. Similarly, in a case of GBS with limb and bulbar paralysis, ophthalmoplegia and anti-GQ1b antibodies, markedly reduced CMAPs without nerve conduction velocity loss was observed (Wirguin et al. 2002). Remarkably, incrementing CMAPs were noted at 20 Hz nerve stimulation, suggesting NMJ abnormalities because such a phenomenon hallmarks Lambert-Eaton myasthenic syndrome, a NMJ synaptopathy with autoimmunity against presynaptic Cav2.1 Ca2+ channels, reducing ACh release (Lennon et al. 1995). However, such antibodies were not detected in this GBS patient. In one MFS and three acute ophthalmoparesis patients with anti-GQ1b-positivity, Lo and associates observed increased jitter in single-fibre electromyography at orbicularis oculi muscle which was normal again at 3 months, paralleling complete clinical recovery (Lo et al. 2004). Facial electromyography showed no reduced CMAPs or nerve conduction velocity changes. These findings suggest that part of the NMJs of these patients was critically transmitting, perhaps resulting from reduced presynaptic ACh release. Possibly, extraocular muscle was relatively severely affected, with blocked NMJs explaining the ophthalmoparesis. Similarly, increased jitter was observed in an anti-GQ1b-negative MFS case, which had disappeared 6 weeks later, upon clinical improvement (Chan et al. 2006). In another anti-GQ1b-negative MFS case, with late-onset limb weakness, reduced facial CMAPs were found, as well as increased jitter and blocked transmission in single-fibre electromyography (Sartucci et al. 2005). The patient recovered rapidly within weeks after onset and showed no electrophysiological abnormalities upon re-examination at 3 months. Similarly, increased jitter and blockings were observed in arm muscles of a MFS patient with no or borderline anti-GQ1b-positivity (Lange et al. 2006). In a larger study, the (non-weak) arm muscles of three anti-GQ1b-positive and three -negative MFS patients showed normal CMAPs (Lo et al. 2006). The positive patients showed an abnormally incrementing CMAP at 20 and 50 Hz nerve stimulation, which is puzzling because without weakness one expects the initial CMAP already to be at maximum. Similar incrementing (but initially normal) CMAP amplitudes at high-rate stimulation were reported in a non-weak arm muscle of a rare case of recurrent MFS, with the additional observation of increased jitter in single-fibre electromyography of frontalis muscle, suggesting NMJ malfunction (Tomcik et al. 2007). Intriguingly, anti-GQ1b antibodies and incremental CMAPs persisted when the MFS resolved. On the other hand, decremental (but initially normal) CMAP amplitude in MFS was also reported (Silverstein et al. 2008). Nerve stimulation of 3 Hz revealed CMAP decrement in trapezius muscle of a patient that had been first diagnosed with AChR antibody-negative myasthenia gravis, but later with MFS because weakness correlated with anti-GQ1b antibodies. However, CMAP decrement persisted when MFS resolved and anti-GQ1b antibodies disappeared. In a larger single-fibre electromyographical study of arm muscles of six MFS patients and a Bickerstaff's brain stem encephalitis patient, all anti-GQ1b-positive, findings were normal, suggesting selective effects of anti-GQ1b antibodies on extraocular, facial and bulbar muscles and relative absence of GQ1b (or less antibody access/binding) at terminal motor axons of limb muscle (Kuwabara et al. 2007). However, another anti-GQ1b-positive Bickerstaff's brain stem encephalitis patient with limb weakness showed incrementing CMAPs (Lo, 2008).
Clinical human electrophysiology versus experimental observations at mouse NMJs
NMJ synaptopathy contributing to paralysis in anti-ganglioside antibody positive (or –negative) MFS/GBS patients has not yet been unequivocally demonstrated in the clinical electrophysiological studies. It is not easy to predict the clinical electromyographical picture that should be expected on the basis of the mouse studies. If presynaptic destruction by anti-ganglioside and complement as characterized by us in mouse motor nerve terminals (Willison & Plomp, 2008) takes place in patients, CMAP amplitude will probably be reduced in affected muscles because this destructive effect will block neuromuscular transmission at (part of) the NMJs. Because of the very distal localization of the lesion, fast recovery (days to weeks) of the CMAP amplitude is to be expected when pathogenic antibodies are therapeutically removed (see below). Such a CMAP reduction with fast recovery was indeed seen in some MFS/GBS patients, but is certainly not universally observed. Possibly, it is only to be found in a minority of patients that have lesions which are restricted to distal intramuscular axons and terminals, while many patients also have concomitant proximal lesions. Fibrillation potentials following denervation can be detected with conventional needle electromyography, but will develop only within weeks of the onset of an axonal lesion. Therefore they are not yet to be expected to occur directly after block due to NMJ synaptopathy in the acute phase. Spaans and colleagues (Spaans et al. 2003) reported fibrillation potentials in only one of the nine investigated GBS patients, and only at 4 weeks after onset. SFEMG cannot directly detect permanently blocked NMJs and is therefore not informative as to whether these are present and at what scale. On the other hand, it can detect changes in ‘fibre density’ (i.e. the amount of firing fibres belonging to the same motor unit in the recording area of the SFEMG needle). However, it is unclear how this parameter would change in the case of permanently blocked/destroyed NMJs in MFS/GBS. Initially one would expect a reduction in fibre density, but in later phases an increase may appear (due to reinnervation through nerve sprouting and thus motor unit size increase, as known for other diseases hallmarked by denervation and reinnervation). Intermittently blocking NMJs without increased jitter found in a number of GBS patients (Spaans et al. 2003) suggests irregular intramuscular axonal conduction block (possibly very distally), rather than critically reduced presynaptic ACh release, which would produce increased jitter. The observed increased jitter in some MFS patients is difficult to reconcile with our synaptopathic destruction model in mice where we never observed intermediate-sized EPPs at NMJs treated with anti-GQ1b antibody and complement. EPPs either remained full size at NMJs with moderately increased MEPP frequency (mirroring low-level antibody binding and complement activation) or became blocked within minutes at NMJs with dramatically increased MEPP frequency (J. J. Plomp, unpublished observations; Plomp et al. 1999; Bullens et al. 2002). One explanation may be that the observed increased jitter in MFS patients was due to regenerating NMJs, which may already have been present at the first electromyographical examination because this takes place several days after disease onset. Regenerating NMJs are known to have sub-threshold EPPs in the early phase of recovery before increasing to full size in later phases (Lomo & Slater, 1980). Therefore, a time window must exist with around-threshold EPPs, causing increased jitter in electromyography. Alternatively, a destructive NMJ synaptopathy in patients might develop differently from that in mice, due to the undoubtedly slower rise in anti-ganglioside antibody level and, consequently, lower level of presynaptic MAC insertion. Such a dampened effect may cause a (temporary) equilibrium of damage and regeneration, in which terminals release fewer ACh quanta, producing around-threshold EPPs.
Increased jitter in MFS may also result from partial EPP inhibition by complement-independent effects of (low-affinity) anti-ganglioside antibodies, possibly involving presynaptic Cav2.1 Ca2+ channels, as suggested by some mouse NMJ studies (Taguchi et al. 2004; Santaféet al. 2005, 2008; Buchwald et al. 2007). This would resemble Lambert-Eaton myasthenic syndrome, with autoimmunity against Cav2.1 channels. Some electrophysiological features in Lambert-Eaton myasthenic syndrome, i.e. decrementing and incrementing CMAPs at low- and high-rate nerve stimulation, respectively, as well as increased jitter and blocked NMJs (Sanders, 2003; Oh et al. 2007), are indeed found in some anti-GQ1b-positive patients (albeit not in combination in one patient). However, small initial CMAPs, another Lambert-Eaton myasthenic syndrome feature, were not always found in those cases. Reversible inhibition of presynaptic ACh release by anti-ganglioside antibodies seems well compatible with a fast recovery from paralysis after antibody removal.
On the whole, the animal experimental evidence of NMJ synaptopathy in anti-ganglioside-mediated neuropathy in conjunction with the indications of NMJ dysfunction from the clinical electrophysiological studies done so far are suggestive of NMJ dysfunction in (subgroups of) MFS/GBS patients. However, in order to get a more detailed picture of the mechanisms underlying NMJ synaptopathy and its prevalence in the several clinical GBS subgroups, more patients need to be investigated electromyographically with the aim of detecting NMJ dysfunction. The monitoring of effects of experimental drugs (see below) in upcoming trials may provide excellent opportunities for such further detailed and structured clinical electrophysiological investigations (Lo, 2008).
Therapy for anti-ganglioside antibody-induced neuropathy and NMJ synaptopathy
GBS treatment with either plasmapheresis or intravenous high doses of human IgG (IVIg) is equally beneficial and the latter is standard in many centres (van Doorn et al. 2008). While the plasmapheresis mechanism is easy to understand (i.e. removal of pathogenic antibodies), that of IVIg is unclear but probably involves pleiotropic action at many levels of antibody production and binding, cytokine action, immune cellular recruitment and complement activation (Hartung, 2008). A large study demonstrated that plasmapheresis or IVIg does not affect the outcome of MFS (which is already very good in untreated cases, most fully recovering within 6 months), although IVIg slightly sped up recovery from ophthalmoplegia and ataxia (Mori et al. 2007).
These treatments will probably also improve a putative NMJ synaptopathy induced by anti-ganglioside antibodies. In the prototypical autoimmune NMJ disorder myasthenia gravis, IVIg and plasmapheresis both help to alleviate crises (Lehmann et al. 2006; Dalakas, 2008). Experimental mouse NMJ studies showed that IVIg protects from complement-independent inhibition of ACh release by GBS IgG, presumably through neutralization (Buchwald et al. 2002). In our own studies of mouse NMJ destruction by anti-ganglioside antibodies and complement, IVIg appeared highly protective by preventing anti-ganglioside antibody binding, thus precluding antibody-mediated complement activation (Jacobs et al. 2003). Furthermore, IVIg displaced anti-ganglioside antibody that had already bound antigen.
Some mouse NMJ studies indicated reversible depression of ACh release, similar to Lambert-Eaton myasthenic syndrome. If also the case in MFS/GBS patients, treatment with 3,4-diaminopyridine might be an option. This K+ channel-blocking drug is successfully used to increase ACh release at NMJs of patients with Lambert-Eaton myasthenic syndrome. However, in six studied GBS patients this drug was ineffective (Bergin et al. 1993).
New complement inhibitors may be beneficial in MFS/GBS, as in other complement-mediated disorders (Hillmen et al. 2006). Recently, we tested three inhibitors and found they effectively prevented damage by anti-GQ1b antibodies at mouse NMJs (Fig. 5) (Halstead et al. 2005a, 2008a,b). Irrespective of the existence of NMJ synaptopathy in MFS/GBS, clinical trial of such drugs will be of great interest.
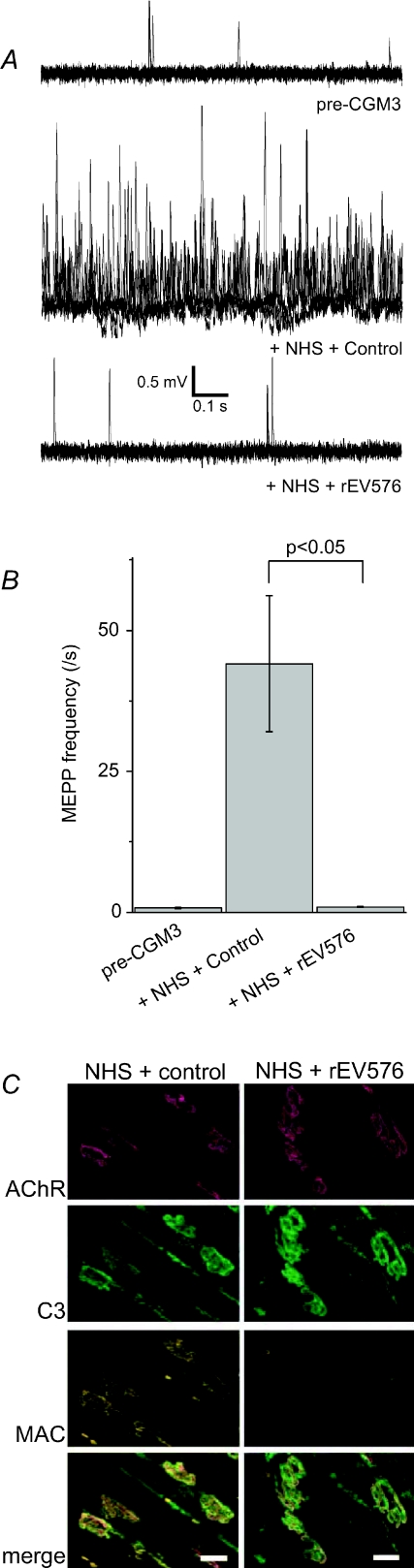
Figure 5. Complement inhibition prevents the deleterious effects of anti-ganglioside antibody at the mouse neuromuscular junction.
The complement C5 inhibitor rEV576, a recombinant form of a protein present in saliva from the soft tick Ornithodoros moubata, prevents the in vitro neuropathophysiological effects on mouse neuromuscular junctions of incubation by anti-GQ1b antibody CGM3 and subsequent normal human serum (NHS), as complement source. A and B, induction of high frequency miniature endplate potentials by NHS (1:2 diluted) is prevented by pre-adding 100 μg ml−1 rEV576. In A, five electrophysiological traces of 1 s duration are plotted overlapping for each condition. C, immunohistology showed that rEV576 prevented membrane attack complex (MAC) formation at the neuromuscular junction (identified by staining acetylcholine receptors (AChR) with fluorescently labelled α–bungarotoxin), while leaving C3 deposition intact. As control protein, bovine serum albumin was added to the NHS. For details see Halstead et al. (2008a).
Conclusions
Neuronal enrichment of gangliosides suggests a variety of neuronal functions, including at synapses. While early work suggested roles in neural development, study of recently generated transgenic mice lacking ganglioside (subsets) indicate that gangliosides are dispensable for nervous system embryogenesis but necessary for postnatal maintenance and repair. Peripheral nerve gangliosides form antigenic targets in GBS, where specific anti-ganglioside antibodies are associated with specific clinical variants, probably depending on regional expression patterns of specific gangliosides. Besides targeting peripheral nerve axons, anti-ganglioside antibodies may target distal portions of motor axons, including the terminal at the NMJ, causing a synaptopathy that contributes to muscle weakness. A large number of experimental mouse NMJ studies supports this hypothesis. Many effects were observed, ranging from complement-mediated presynaptic destruction with transmission block to more subtle, complement-independent and reversible inhibitions of ACh release. Whether or not such effects actually exist and contribute to paralysis in GBS patients is still unclear. Some clinical electrophysiological studies are in favour of NMJ malfunction (e.g. by showing small CMAPs that recover very quickly), but no consistent picture has yet appeared. Future clinical trials of new (complement-inhibiting) drugs seem a good opportunity for the structured electrophysiological study of large groups of GBS patients to resolve this matter.
Acknowledgments
Our studies are funded by the Prinses Beatrix Fonds (J.J.P.) and the Wellcome Trust (H.J.W.).
Glossary
Abbreviations
- ACh
acetylcholine
- AChR
acetylcholine receptor
- AIDP
acute demyelinating polyneuropathy
- AMAN
acute motor axonal neuropathy
- AMSAN
acute motor and sensory axonal neuropathy
- CMAP
compound muscle action potential
- EPP
endplate potential
- GBS
Guillan-Barré syndrome
- GD3s-KO
GD3-synthase knockout
- GM2s-KO
GM2/GD2-synthase knockout
- IVIg
intravenous immunogobulin
- MAC
membrane attack complex
- MEPP
miniature endplate potential
- MFS
Miller Fisher syndrome
- NMJ
neuromuscular junction
- SFEMG
single-fibre electromyography
References
- Ando S. Gangliosides in the nervous system. Neurochem Int. 1983;5:507–537. doi: 10.1016/0197-0186(83)90043-8. [DOI] [PubMed] [Google Scholar]
- Ando S, Tanaka Y, Kobayashi S, Fukui F, Iwamoto M, Waki H, Tai T, Hirabayashi Y. Synaptic function of cholinergic-specific Chol-1α ganglioside. Neurochem Res. 2004;29:857–867. doi: 10.1023/b:nere.0000018860.75734.a7. [DOI] [PubMed] [Google Scholar]
- Ando S, Tanaka Y, Waki H, Kon K, Iwamoto M, Fukui F. Gangliosides and sialylcholesterol as modulators of synaptic functions. Ann N Y Acad Sci. 1998;845:232–239. doi: 10.1111/j.1749-6632.1998.tb09676.x. [DOI] [PubMed] [Google Scholar]
- Ang CW, Jacobs BC, Laman JD. The Guillain-Barré syndrome: a true case of molecular mimicry. Trends Immunol. 2004;25:61–66. doi: 10.1016/j.it.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Ariga T, McDonald MP, Yu RK. Role of ganglioside metabolism in the pathogenesis of Alzheimer's disease – a review. J Lipid Res. 2008;49:1157–1175. doi: 10.1194/jlr.R800007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin Y, Prestegard JH. Structure and dynamics of sialic acid at the surface of a magnetically oriented membrane system. Biochemistry. 1993;32:3422–3428. doi: 10.1021/bi00064a028. [DOI] [PubMed] [Google Scholar]
- Auld DS, Robitaille R. Perisynaptic Schwann cells at the neuromuscular junction: nerve- and activity-dependent contributions to synaptic efficacy, plasticity, and reinnervation. Neuroscientist. 2003;9:144–157. doi: 10.1177/1073858403252229. [DOI] [PubMed] [Google Scholar]
- Beam KG, Tanabe T, Numa S. Structure, function, and regulation of the skeletal muscle dihydropyridine receptor. Ann N Y Acad Sci. 1989;560:127–137. doi: 10.1111/j.1749-6632.1989.tb24090.x. [DOI] [PubMed] [Google Scholar]
- Bergin PS, Miller DH, Hirsch NP, Murray NM. Failure of 3,4-diaminopyridine to reverse conduction block in inflammatory demyelinating neuropathies. Ann Neurol. 1993;34:406–409. doi: 10.1002/ana.410340318. [DOI] [PubMed] [Google Scholar]
- Bremer EG, Hakomori S. GM3 ganglioside induces hamster fibroblast growth inhibition in chemically-defined medium: ganglioside may regulate growth factor receptor function. Biochem Biophys Res Commun. 1982;106:711–718. doi: 10.1016/0006-291x(82)91769-7. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Scaglia F. GM1 gangliosidosis: review of clinical, molecular, and therapeutic aspects. Mol Genet Metab. 2008;94:391–396. doi: 10.1016/j.ymgme.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Buchwald B, Ahangari R, Weishaupt A, Toyka KV. Intravenous immunoglobulins neutralize blocking antibodies in Guillain-Barré syndrome. Ann Neurol. 2002;51:673–680. doi: 10.1002/ana.10205. [DOI] [PubMed] [Google Scholar]
- Buchwald B, Toyka KV, Zielasek J, Weishaupt A, Schweiger S, Dudel J. Neuromuscular blockade by IgG antibodies from patients with Guillain-Barré syndrome: a macro-patch-clamp study. Ann Neurol. 1998a;44:913–922. doi: 10.1002/ana.410440610. [DOI] [PubMed] [Google Scholar]
- Buchwald B, Weishaupt A, Toyka KV, Dudel J. Immunoglobulin G from a patient with Miller-Fisher syndrome rapidly and reversibly depresses evoked quantal release at the neuromuscular junction of mice. Neurosci Lett. 1995;201:163–166. doi: 10.1016/0304-3940(95)12155-2. [DOI] [PubMed] [Google Scholar]
- Buchwald B, Weishaupt A, Toyka KV, Dudel J. Pre- and postsynaptic blockade of neuromuscular transmission by Miller-Fisher syndrome IgG at mouse motor nerve terminals. Eur J Neurosci. 1998b;10:281–290. doi: 10.1046/j.1460-9568.1998.00053.x. [DOI] [PubMed] [Google Scholar]
- Buchwald B, Zhang G, Vogt-Eisele AK, Zhang W, Ahangari R, Griffin JW, Hatt H, Toyka KV, Sheikh KA. Anti-ganglioside antibodies alter presynaptic release and calcium influx. Neurobiol Dis. 2007;28:113–121. doi: 10.1016/j.nbd.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullens RW, O’Hanlon GM, Goodyear CS, Molenaar PC, Conner J, Willison HJ, Plomp JJ. Anti-GQ1b antibodies and evoked acetylcholine release at mouse motor endplates. Muscle Nerve. 2000;23:1035–1043. doi: 10.1002/1097-4598(200007)23:7<1035::aid-mus5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Bullens RW, O’Hanlon GM, Wagner E, Molenaar PC, Furukawa K, Furukawa K, Plomp JJ, Willison HJ. Complex gangliosides at the neuromuscular junction are membrane receptors for autoantibodies and botulinum neurotoxin but redundant for normal synaptic function. J Neurosci. 2002;22:6876–6884. doi: 10.1523/JNEUROSCI.22-16-06876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputto R, de Maccioni AH, Caputto BL. Studies on the functions of gangliosides in the central nervous system. Adv Exp Med Biol. 1977;83:289–295. doi: 10.1007/978-1-4684-3276-3_27. [DOI] [PubMed] [Google Scholar]
- Carlson RO, Masco D, Brooker G, Spiegel S. Endogenous ganglioside GM1 modulates L-type calcium channel activity in N18 neuroblastoma cells. J Neurosci. 1994;14:2272–2281. doi: 10.1523/JNEUROSCI.14-04-02272.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain LH, Burgoyne RD, Gould GW. SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc Natl Acad Sci U S A. 2001;98:5619–5624. doi: 10.1073/pnas.091502398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YC, Rathakrishnan R, Chan BP. Impaired neuromuscular junction transmission in anti-GQ1b antibody negative Miller Fisher variant. Clin Neurol Neurosurg. 2006;108:717–718. doi: 10.1016/j.clineuro.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Chen YW, Pedersen JW, Wandall HH, Levery SB, Pizette S, Clausen H, Cohen SM. Glycosphingolipids with extended sugar chain have specialized functions in development and behavior of Drosophila. Dev Biol. 2007;306:736–749. doi: 10.1016/j.ydbio.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Chiavegatto S, Sun J, Nelson RJ, Schnaar RL. A functional role for complex gangliosides: motor deficits in GM2/GD2 synthase knockout mice. Exp Neurol. 2000;166:227–234. doi: 10.1006/exnr.2000.7504. [DOI] [PubMed] [Google Scholar]
- Chiba A, Kusunoki S, Obata H, Machinami R, Kanazawa I. Serum anti-GQ1b IgG antibody is associated with ophthalmoplegia in Miller Fisher syndrome and Guillain-Barré syndrome: clinical and immunohistochemical studies. Neurology. 1993;43:1911–1917. doi: 10.1212/wnl.43.10.1911. [DOI] [PubMed] [Google Scholar]
- Chiba A, Kusunoki S, Obata H, Machinami R, Kanazawa I. Ganglioside composition of the human cranial nerves, with special reference to pathophysiology of Miller Fisher syndrome. Brain Res. 1997;745:32–36. doi: 10.1016/s0006-8993(96)01123-7. [DOI] [PubMed] [Google Scholar]
- Dalakas M. IVIg in other autoimmune neurological disorders: current status and future prospects. J Neurol. 2008;255(suppl. 3):12–16. doi: 10.1007/s00415-008-3004-y. [DOI] [PubMed] [Google Scholar]
- Davies A, Douglas L, Hendrich J, Wratten J, Tran VM, Foucault I, Koch D, Pratt WS, Saibil HR, Dolphin AC. The calcium channel α2δ-2 subunit partitions with CaV2.1 into lipid rafts in cerebellum: implications for localization and function. J Neurosci. 2006;26:8748–8757. doi: 10.1523/JNEUROSCI.2764-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington EA, Borroni E. The developmental expression of the cholinergic-specific antigen Chol-1 in the central and peripheral nervous system of the rat. Brain Res Dev Brain Res. 1990;52:131–140. doi: 10.1016/0165-3806(90)90228-q. [DOI] [PubMed] [Google Scholar]
- Desplats PA, Denny CA, Kass KE, Gilmartin T, Head SR, Sutcliffe JG, Seyfried TN, Thomas EA. Glycolipid and ganglioside metabolism imbalances in Huntington's disease. Neurobiol Dis. 2007;27:265–277. doi: 10.1016/j.nbd.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörr J, Dieste FJ, Klaasen van Husen D, Zipp F, Vogel HP. A case of recurrent Miller Fisher syndrome mimicking botulism. Neurol Sci. 2006;27:424–425. doi: 10.1007/s10072-006-0723-7. [DOI] [PubMed] [Google Scholar]
- Ebanks RO, Isenman DE. Mouse complement component C4 is devoid of classical pathway C5 convertase subunit activity. Mol Immunol. 1996;33:297–309. doi: 10.1016/0161-5890(95)00135-2. [DOI] [PubMed] [Google Scholar]
- Egorushkina NV, Ratushnyak AS, Egorushkin IV. The influence of exogenous gangliosides on the dynamics of the development of prolonged posttetanic potentiation. Neurosci Behav Physiol. 1993;23:435–438. doi: 10.1007/BF01183004. [DOI] [PubMed] [Google Scholar]
- Fujii S, Igarashi K, Sasaki H, Furuse H, Ito K, Kaneko K, Kato H, Inokuchi J, Waki H, Ando S. Effects of the mono- and tetrasialogangliosides GM1 and GQ1b on ATP-induced long-term potentiation in hippocampal CA1 neurons. Glycobiology. 2002;12:339–344. doi: 10.1093/glycob/12.5.339. [DOI] [PubMed] [Google Scholar]
- Fujita A, Cheng J, Hirakawa M, Furukawa K, Kusunoki S, Fujimoto T. Gangliosides GM1 and GM3 in the living cell membrane form clusters susceptible to cholesterol depletion and chilling. Mol Biol Cell. 2007;18:2112–2122. doi: 10.1091/mbc.E07-01-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse H, Waki H, Kaneko K, Fujii S, Miura M, Sasaki H, Ito KI, Kato H, Ando S. Effect of the mono- and tetra-sialogangliosides, GM1 and GQ1b, on long-term potentiation in the CA1 hippocampal neurons of the guinea pig. Exp Brain Res. 1998;123:307–314. doi: 10.1007/s002210050573. [DOI] [PubMed] [Google Scholar]
- Goodfellow JA, Bowes T, Sheikh K, Odaka M, Halstead SK, Humphreys PD, Wagner ER, Yuki N, Furukawa K, Furukawa K, Plomp JJ, Willison HJ. Overexpression of GD1a ganglioside sensitizes motor nerve terminals to anti-GD1a antibody-mediated injury in a model of acute motor axonal neuropathy. J Neurosci. 2005;25:1620–1628. doi: 10.1523/JNEUROSCI.4279-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear CS, O’Hanlon GM, Plomp JJ, Wagner ER, Morrison I, Veitch J, Cochrane L, Bullens RW, Molenaar PC, Conner J, Willison HJ. Monoclonal antibodies raised against Guillain-Barré syndrome-associated Campylobacter jejuni lipopolysaccharides react with neuronal gangliosides and paralyze muscle–nerve preparations. J Clin Invest. 1999;104:697–708. doi: 10.1172/JCI6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorio A, Carmignoto G, Facci L, Finesso M. Motor nerve sprouting induced by ganglioside treatmentPossible implications for gangliosides on neuronal growth. Brain Res. 1980;197:236–241. doi: 10.1016/0006-8993(80)90451-5. [DOI] [PubMed] [Google Scholar]
- Green WN, Andersen OS. Surface charges and ion channel function. Annu Rev Physiol. 1991;53:341–359. doi: 10.1146/annurev.ph.53.030191.002013. [DOI] [PubMed] [Google Scholar]
- Greenshields KN, Halstead SK, Zitman FM, Rinaldi S, Brennan KM, O’Leary C, Chamberlain LH, Easton A, Roxburgh J, Pediani J, Furukawa K, Furukawa K, Goodyear CS, Plomp JJ, Willison HJ. The neuropathic potential of anti-GM1 autoantibodies is regulated by the local glycolipid environment in mice. J Clin Invest. 2009;119:595–610. doi: 10.1172/JCI37338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SK, Humphreys PD, Goodfellow JA, Wagner ER, Smith RA, Willison HJ. Complement inhibition abrogates nerve terminal injury in Miller Fisher syndrome. Ann Neurol. 2005a;58:203–210. doi: 10.1002/ana.20546. [DOI] [PubMed] [Google Scholar]
- Halstead SK, Humphreys PD, Zitman FMP, Hamer J, Plomp JJ, Willison HJ. C5 inhibitor rEV576 protects against neural injury in an in vitro mouse model of Miller Fisher syndrome. J Peripher Nerv Syst. 2008a;13:228–235. doi: 10.1111/j.1529-8027.2008.00181.x. [DOI] [PubMed] [Google Scholar]
- Halstead SK, Morrison I, O’Hanlon GM, Humphreys PD, Goodfellow JA, Plomp JJ, Willison HJ. Anti-disialosyl antibodies mediate selective neuronal or Schwann cell injury at mouse neuromuscular junctions. Glia. 2005b;52:177–189. doi: 10.1002/glia.20228. [DOI] [PubMed] [Google Scholar]
- Halstead SK, O’Hanlon GM, Humphreys PD, Morrison DB, Morgan BP, Todd AJ, Plomp JJ, Willison HJ. Anti-disialoside antibodies kill perisynaptic Schwann cells and damage motor nerve terminals via membrane attack complex in a murine model of neuropathy. Brain. 2004;127:2109–2123. doi: 10.1093/brain/awh231. [DOI] [PubMed] [Google Scholar]
- Halstead SK, Zitman FM, Humphreys PD, Greenshields K, Verschuuren JJ, Jacobs BC, Rother RP, Plomp JJ, Willison HJ. Eculizumab prevents anti-ganglioside antibody-mediated neuropathy in a murine model. Brain. 2008b;131:1197–1208. doi: 10.1093/brain/awm316. [DOI] [PubMed] [Google Scholar]
- Handa Y, Ozaki N, Honda T, Furukawa K, Tomita Y, Inoue M, Furukawa K, Okada M, Sugiura Y. GD3 synthase gene knockout mice exhibit thermal hyperalgesia and mechanical allodynia but decreased response to formalininduced prolonged noxious stimulation. Pain. 2005;117:271–279. doi: 10.1016/j.pain.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Hansson HA, Holmgren J, Svennerholm L. Ultrastructural localization of cell membrane GM1 ganglioside by cholera toxin. Proc Natl Acad Sci U S A. 1977;74:3782–3786. doi: 10.1073/pnas.74.9.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzal-Bayer MF, Hancock JF. Lipid rafts and membrane traffic. FEBS Lett. 2007;581:2098–2104. doi: 10.1016/j.febslet.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Hartung HP. Advances in the understanding of the mechanism of action of IVIg. J Neurol. 2008;255(suppl. 3):3–6. doi: 10.1007/s00415-008-3002-0. [DOI] [PubMed] [Google Scholar]
- Hillmen P, Young NS, Schubert J, Brodsky RA, Socie G, Muus P, Roth A, Szer J, Elebute MO, Nakamura R, Browne P, Risitano AM, Hill A, Schrezenmeier H, Fu CL, Maciejewski J, Rollins SA, Mojcik CF, Rother RP, Luzzatto L. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355:1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- Ho TW, Hsieh ST, Nachamkin I, Willison HJ, Sheikh K, Kiehlbauch J, Flanigan K, McArthur JC, Cornblath DR, McKhann GM, Griffin JW. Motor nerve terminal degeneration provides a potential mechanism for rapid recovery in acute motor axonal neuropathy after Campylobacter infection. Neurology. 1997;48:717–724. doi: 10.1212/wnl.48.3.717. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet. 2005;366:1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- Illa I, Ortiz N, Gallard E, Juarez C, Grau JM, Dalakas MC. Acute axonal Guillain-Barré syndrome with IgG antibodies against motor axons following parenteral gangliosides. Ann Neurol. 1995;38:218–224. doi: 10.1002/ana.410380214. [DOI] [PubMed] [Google Scholar]
- Inoue M, Fujii Y, Furukawa K, Okada M, Okumura K, Hayakawa T, Furukawa K, Sugiura Y. Refractory skin injury in complex knock-out mice expressing only the GM3 ganglioside. J Biol Chem. 2002;277:29881–29888. doi: 10.1074/jbc.M201631200. [DOI] [PubMed] [Google Scholar]
- Jacobs BC, Bullens RW, O’Hanlon GM, Ang CW, Willison HJ, Plomp JJ. Detection and prevalence of α–latrotoxin-like effects of serum from patients with Guillain-Barré syndrome. Muscle Nerve. 2002;25:549–558. doi: 10.1002/mus.10060. [DOI] [PubMed] [Google Scholar]
- Jacobs BC, O’Hanlon GM, Bullens RW, Veitch J, Plomp JJ, Willison HJ. Immunoglobulins inhibit pathophysiological effects of anti-GQ1b-positive sera at motor nerve terminals through inhibition of antibody binding. Brain. 2003;126:2220–2234. doi: 10.1093/brain/awg235. [DOI] [PubMed] [Google Scholar]
- Jennemann R, Sandhoff R, Wang S, Kiss E, Gretz N, Zuliani C, Martin-Villalba A, Jager R, Schorle H, Kenzelmann M, Bonrouhi M, Wiegandt H, Grone HJ. Cell-specific deletion of glucosylceramide synthase in brain leads to severe neural defects after birth. Proc Natl Acad Sci U S A. 2005;102:12459–12464. doi: 10.1073/pnas.0500893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida K, Kanzaki M, Morita D, Kamakura K, Motoyoshi K, Hirakawa M, Kusunoki S. Anti-ganglioside complex antibodies in Miller Fisher syndrome. J Neurol Neurosurg Psychiatry. 2006;77:1043–1046. doi: 10.1136/jnnp.2006.087940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida K, Morita D, Kanzaki M, Kamakura K, Motoyoshi K, Hirakawa M, Kusunoki S. Anti-ganglioside complex antibodies associated with severe disability in GBS. J Neuroimmunol. 2007;182:212–218. doi: 10.1016/j.jneuroim.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Kappel T, Anken RH, Hanke W, Rahmann H. Gangliosides affect membrane-channel activities dependent on ambient temperature. Cell Mol Neurobiol. 2000;20:579–590. doi: 10.1023/a:1007063928449. [DOI] [PubMed] [Google Scholar]
- Kasahara K, Watanabe K, Takeuchi K, Kaneko H, Oohira A, Yamamoto T, Sanai Y. Involvement of gangliosides in glycosylphosphatidylinositol-anchored neuronal cell adhesion molecule TAG–1 signaling in lipid rafts. J Biol Chem. 2000;275:34701–34709. doi: 10.1074/jbc.M003163200. [DOI] [PubMed] [Google Scholar]
- Kawai H, Allende ML, Wada R, Kono M, Sango K, Deng C, Miyakawa T, Crawley JN, Werth N, Bierfreund U, Sandhoff K, Proia RL. Mice expressing only monosialoganglioside GM3 exhibit lethal audiogenic seizures. J Biol Chem. 2001;276:6885–6888. doi: 10.1074/jbc.C000847200. [DOI] [PubMed] [Google Scholar]
- Kishi M, Fujioka T, Miura H, Sekine A, Iguchi H, Nakazora H, Kiyozuka T, Igarashi O, Ichikawa Y, Sugimoto H, Kurihara T, Irie S, Saito T. The relation of clinical symptoms and anti-ganglioside antibodies to MEPPs frequency increase in 8 cases of variant type Guillain-Barré syndrome. J Peripher Nerv Syst. 2003;8:82–90. doi: 10.1046/j.1529-8027.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- Krampfl K, Mohammadi B, Buchwald B, Jahn K, Dengler R, Toyka KV, Bufler J. IgG from patients with Guillain-Barré syndrome interact with nicotinic acetylcholine receptor channels. Muscle Nerve. 2003;27:435–441. doi: 10.1002/mus.10349. [DOI] [PubMed] [Google Scholar]
- Kusunoki S, Kaida K, Ueda M. Antibodies against gangliosides and ganglioside complexes in Guillain-Barré syndrome: new aspects of research. Biochim Biophys Acta. 2008;1780:441–444. doi: 10.1016/j.bbagen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Kuwabara S, Asahina M, Koga M, Mori M, Yuki N, Hattori T. Two patterns of clinical recovery in Guillain-Barré syndrome with IgG anti-GM1 antibody. Neurology. 1998a;51:1656–1660. doi: 10.1212/wnl.51.6.1656. [DOI] [PubMed] [Google Scholar]
- Kuwabara S, Misawa S, Takahashi H, Sawai S, Kanai K, Nakata M, Mori M, Hattori T, Yuki N. Anti-GQ1b antibody does not affect neuromuscular transmission in human limb muscle. J Neuroimmunol. 2007;189:158–162. doi: 10.1016/j.jneuroim.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Kuwabara S, Nakata M, Sung JY, Mori M, Kato N, Hattori T, Koga M, Yuki N. Hyperreflexia in axonal Guillain-Barré syndrome subsequent to Campylobacter jejuni enteritis. J Neurol Sci. 2002;199:89–92. doi: 10.1016/s0022-510x(02)00088-6. [DOI] [PubMed] [Google Scholar]
- Kuwabara S, Yuki N, Koga M, Hattori T, Matsuura D, Miyake M, Noda M. IgG anti-GM1 antibody is associated with reversible conduction failure and axonal degeneration in Guillain-Barré syndrome. Ann Neurol. 1998b;44:202–208. doi: 10.1002/ana.410440210. [DOI] [PubMed] [Google Scholar]
- Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R. SNAREs are concentrated in cholesterol dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 2001;20:2202–2213. doi: 10.1093/emboj/20.9.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange DJ, DeAngelis T, Sivak MA. Single-fiber electromyography shows terminal axon dysfunction in Miller Fisher syndrome: a case report. Muscle Nerve. 2006;34:232–234. doi: 10.1002/mus.20544. [DOI] [PubMed] [Google Scholar]
- Ledeen RW. Biology of gangliosides: neuritogenic and neuronotrophic properties. J Neurosci Res. 1984;12:147–159. doi: 10.1002/jnr.490120204. [DOI] [PubMed] [Google Scholar]
- Ledeen RW. Gangliosides of the neuron. Trends Neurosci. 1985;8:169–174. [Google Scholar]
- Ledeen RW, Wu G. Ganglioside function in calcium homeostasis and signaling. Neurochem Res. 2002;27:637–647. doi: 10.1023/a:1020224016830. [DOI] [PubMed] [Google Scholar]
- Ledeen RW, Wu G. GM1 ganglioside: another nuclear lipid that modulates nuclear calcium. GM1 potentiates the nuclear sodium–calcium exchanger. Can J Physiol Pharmacol. 2006;84:393–402. doi: 10.1139/y05-133. [DOI] [PubMed] [Google Scholar]
- Lehmann HC, Hartung HP, Hetzel GR, Stuve O, Kieseier BC. Plasma exchange in neuroimmunological disorders: part 2. Treatment of neuromuscular disorders. Arch Neurol. 2006;63:1066–1071. doi: 10.1001/archneur.63.8.1066. [DOI] [PubMed] [Google Scholar]
- Lennon VA, Kryzer TJ, Griesmann GE, Osuilleabhain PE, Windebank AJ, Woppmann A, Miljanich GP, Lambert EH. Calcium-channel antibodies in the Lambert-Eaton syndrome and other paraneoplastic syndromes. New Engl J Med. 1995;332:1467–1474. doi: 10.1056/NEJM199506013322203. [DOI] [PubMed] [Google Scholar]
- Leon A, Facci L, Toffano G, Sonnino S, Tettamanti G. Activation of (Na+, K+)-ATPase by nanomolar concentrations of GM1 ganglioside. J Neurochem. 1981;37:350–357. doi: 10.1111/j.1471-4159.1981.tb00462.x. [DOI] [PubMed] [Google Scholar]
- Liu JX, Willison HJ, Pedrosa-Domellöf F. Immunolocalisation of GQ1b and related gangliosides in human extraocular neuromuscular junctions and muscle spindles. Invest Ophthalmol Vis Sci. 2009;50:3226–3232. doi: 10.1167/iovs.08-3333. [DOI] [PubMed] [Google Scholar]
- Lloyd KO, Furukawa K. Biosynthesis and functions of gangliosides: recent advances. Glycoconj J. 1998;15:627–636. doi: 10.1023/a:1006924128550. [DOI] [PubMed] [Google Scholar]
- Lo YL. Clinical and immunological spectrum of the Miller Fisher syndrome. Muscle Nerve. 2007;36:615–627. doi: 10.1002/mus.20835. [DOI] [PubMed] [Google Scholar]
- Lo YL. Immunotherapy for anti-GQ1b IgG antibody-mediated disorders: role of electrophysiology in human trials. Brain. 2008;132:e104. doi: 10.1093/brain/awn159. Author reply e105. [DOI] [PubMed] [Google Scholar]
- Lo YL, Chan LL, Pan A, Ratnagopal P. Acute ophthalmoparesis in the anti-GQ1b antibody syndrome: electrophysiological evidence of neuromuscular transmission defect in the orbicularis oculi. J Neurol Neurosurg Psychiatry. 2004;75:436–440. doi: 10.1136/jnnp.2003.023630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YL, Leoh TH, Dan YF, Lim LL, Seah A, Fook-Chong S, Ratnagopal P. Presynaptic neuromuscular transmission defect in the Miller Fisher syndrome. Neurology. 2006;66:148–149. doi: 10.1212/01.wnl.0000191400.77080.21. [DOI] [PubMed] [Google Scholar]
- Lomo T, Slater CR. Acetylcholine sensitivity of developing ectopic nerve–muscle junctions in adult rat soleus muscles. J Physiol. 1980;303:173–189. doi: 10.1113/jphysiol.1980.sp013279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni HJ. Glycosylation of glycolipids in the Golgi complex. J Neurochem. 2007;103(suppl. 1):81–90. doi: 10.1111/j.1471-4159.2007.04717.x. [DOI] [PubMed] [Google Scholar]
- Maegawa GH, Stockley T, Tropak M, Banwell B, Blaser S, Kok F, Giugliani R, Mahuran D, Clarke JT. The natural history of juvenile or subacute GM2 gangliosidosis: 21 new cases and literature review of 134 previously reported. Pediatrics. 2006;118:e1550–e1562. doi: 10.1542/peds.2006-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi S, Acler M, Lovato L, De Toni L, Tedeschi E, Anghileri E, Romito S, Cordioli C, Bonetti B. Anti-GD2-like IgM autoreactivity in multiple sclerosis patients. Mult Scler. 2006;12:302–308. doi: 10.1191/135248506ms1279oa. [DOI] [PubMed] [Google Scholar]
- Mori M, Kuwabara S, Fukutake T, Hattori T. Intravenous immunoglobulin therapy for Miller Fisher syndrome. Neurology. 2007;68:1144–1146. doi: 10.1212/01.wnl.0000258673.31824.61. [DOI] [PubMed] [Google Scholar]
- Nakatani Y, Hotta S, Utsunomiya I, Tanaka K, Hoshi K, Ariga T, Yu RK, Miyatake T, Taguchi K. Cav2.1 voltage dependent Ca2+ channel current is inhibited by serum from select patients with Guillain-Barré syndrome. Neurochem Res. 2009;34:149–157. doi: 10.1007/s11064-008-9735-4. [DOI] [PubMed] [Google Scholar]
- Nakatani Y, Nagaoka T, Hotta S, Utsunomiya I, Yoshino H, Miyatake T, Hoshi K, Taguchi K. IgG anti-GalNAc-GD1a antibody inhibits the voltage-dependent calcium channel currents in PC12 pheochromocytoma cells. Exp Neurol. 2007;204:380–386. doi: 10.1016/j.expneurol.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Ngamukote S, Yanagisawa M, Ariga T, Ando S, Yu RK. Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J Neurochem. 2007;103:2327–2341. doi: 10.1111/j.1471-4159.2007.04910.x. [DOI] [PubMed] [Google Scholar]
- O’Hanlon GM, Bullens RW, Plomp JJ, Willison HJ. Complex gangliosides as autoantibody targets at the neuromuscular junction in Miller Fisher syndrome: a current perspective. Neurochem Res. 2002;27:697–709. doi: 10.1023/a:1020284302718. [DOI] [PubMed] [Google Scholar]
- O’Hanlon GM, Plomp JJ, Chakrabarti M, Morrison I, Wagner ER, Goodyear CS, Yin X, Trapp BD, Conner J, Molenaar PC, Stewart S, Rowan EG, Willison HJ. Anti-GQ1b ganglioside antibodies mediate complement-dependent destruction of the motor nerve terminal. Brain. 2001;124:893–906. doi: 10.1093/brain/124.5.893. [DOI] [PubMed] [Google Scholar]
- Ogawa-Goto K, Funamoto N, Ohta Y, Abe T, Nagashima K. Myelin gangliosides of human peripheral nervous system: an enrichment of GM1 in the motor nerve myelin isolated from cauda equina. J Neurochem. 1992;59:1844–1849. doi: 10.1111/j.1471-4159.1992.tb11018.x. [DOI] [PubMed] [Google Scholar]
- Ogawagoto K, Abe T. Gangliosides and glycosphingolipids of peripheral nervous system myelins – A minireview. Neurochemical Research. 1998;23:305–310. doi: 10.1023/a:1022497114813. [DOI] [PubMed] [Google Scholar]
- Oh SJ, Hatanaka Y, Claussen GC, Sher E. Electrophysiological differences in seropositive and seronegative Lambert-Eaton myasthenic syndrome. Muscle Nerve. 2007;35:178–183. doi: 10.1002/mus.20672. [DOI] [PubMed] [Google Scholar]
- Okada M, Itoh MI, Haraguchi M, Okajima T, Inoue M, Oishi H, Matsuda Y, Iwamoto T, Kawano T, Fukumoto S, Miyazaki H, Furukawa K, Aizawa S, Furukawa K. b-series ganglioside deficiency exhibits no definite changes in the neurogenesis and the sensitivity to Fas-mediated apoptosis but impairs regeneration of the lesioned hypoglossal nerve. J Biol Chem. 2002;277:1633–1636. doi: 10.1074/jbc.C100395200. [DOI] [PubMed] [Google Scholar]
- Ortiz N, Rosa R, Gallardo E, Illa I, Tomas J, Aubry J, Sabater M, Santafe M. IgM monoclonal antibody against terminal moiety of GM2, GalNAc-GD1a and GalNAc-GM1b from a pure motor chronic demyelinating polyneuropathy patient: effects on neurotransmitter release. J Neuroimmunol. 2001;119:114–123. doi: 10.1016/s0165-5728(01)00373-3. [DOI] [PubMed] [Google Scholar]
- Partington CR, Daly JW. Effect of gangliosides on adenylate cyclase activity in rat cerebral cortical membranes. Mol Pharmacol. 1979;15:484–491. [PubMed] [Google Scholar]
- Perissinotti PP, Giugovaz TB, Uchitel OD. L–type calcium channels are involved in fast endocytosis at the mouse neuromuscular junction. Eur J Neurosci. 2008;27:1333–1344. doi: 10.1111/j.1460-9568.2008.06113.x. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- Plomp JJ, Molenaar PC, O’Hanlon GM, Jacobs BC, Veitch J, Daha MR, van Doorn PA, Van Der Meche FG, Vincent A, Morgan BP, Willison HJ. Miller Fisher anti-GQ1b antibodies: alpha-latrotoxin-like effects on motor end plates. Ann Neurol. 1999;45:189–199. doi: 10.1002/1531-8249(199902)45:2<189::aid-ana9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Prinetti A, Prioni S, Chigorno V, Karagogeos D, Tettamanti G, Sonnino S. Immunoseparation of sphingolipidenriched membrane domains enriched in Src family protein tyrosine kinases and in the neuronal adhesion molecule TAG-1 by anti-GD3 ganglioside monoclonal antibody. J Neurochem. 2001;78:1162–1167. doi: 10.1046/j.1471-4159.2001.00515.x. [DOI] [PubMed] [Google Scholar]
- Rahmann H, Jonas U, Kappel T, Hilderbrandt H. Differential involvement of gangliosides versus phospholipids in the process of temperature adaptation in vertebratesA comparative phenomenological and physicochemical study. Ann N Y Acad Sci. 1998;845:72–91. doi: 10.1111/j.1749-6632.1998.tb09663.x. [DOI] [PubMed] [Google Scholar]
- Rahmann H, Schifferer F, Beitinger H. Calcium ganglioside interactions and synaptic plasticity: effect of calcium on specific ganglioside/peptide (valinomycin, gramicidin A)-complexes in mixed mono- and bilayers. Neurochem Int. 1992;20:323–338. doi: 10.1016/0197-0186(92)90047-u. [DOI] [PubMed] [Google Scholar]
- Ramaglia V, Daha MR, Baas F. The complement system in the peripheral nerve: friend or foe? Mol Immunol. 2008;45:3865–3877. doi: 10.1016/j.molimm.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Ramirez OA, Gomez RA, Carrer HF. Gangliosides improve synaptic transmission in dentate gyrus of hippocampal rat slices. Brain Res. 1990;506:291–293. doi: 10.1016/0006-8993(90)91264-h. [DOI] [PubMed] [Google Scholar]
- Rice CE. The interchangeability of the complement components of different animal species; literature survey. Can J Comp Med Vet Sci. 1950;14:369–379. [PMC free article] [PubMed] [Google Scholar]
- Roberts M, Willison H, Vincent A, Newsom-Davis J. Serum factor in Miller-Fisher variant of Guillain-Barré syndrome and neurotransmitter release. Lancet. 1994;343:454–455. doi: 10.1016/s0140-6736(94)92694-8. [DOI] [PubMed] [Google Scholar]
- Roth J, Kempf A, Reuter G, Schauer R, Gehring WJ. Occurrence of sialic acids in Drosophila melanogaster. Science. 1992;256:673–675. doi: 10.1126/science.1585182. [DOI] [PubMed] [Google Scholar]
- Ruff RL. Neurophysiology of the neuromuscular junction: overview. Ann N Y Acad Sci. 2003;998:1–10. [PubMed] [Google Scholar]
- Salaun C, James DJ, Chamberlain LH. Lipid rafts and the regulation of exocytosis. Traffic. 2004;5:255–264. doi: 10.1111/j.1600-0854.2004.0162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar BC, Castano S, Sanchez JC, Romero M, Recio-Pinto E. Ganglioside GD1a increases the excitability of voltage-dependent sodium channels. Brain Res. 2004;1021:151–158. doi: 10.1016/j.brainres.2004.06.055. [DOI] [PubMed] [Google Scholar]
- Sanders DB. Lambert-Eaton myasthenic syndrome: diagnosis and treatment. Ann N Y Acad Sci. 2003;998:500–508. doi: 10.1196/annals.1254.065. [DOI] [PubMed] [Google Scholar]
- Santafé MM, Sabaté MM, Garcia N, Ortiz N, Lanuza MA, Tomàs J. Changes in the neuromuscular synapse induced by an antibody against gangliosides. Ann Neurol. 2005;57:396–407. doi: 10.1002/ana.20403. [DOI] [PubMed] [Google Scholar]
- Santafé MM, Sabaté MM, Garcia N, Ortiz N, Lanuza MA, Tomàs J. Anti-GM2 gangliosides IgM paraprotein induces neuromuscular block without neuromuscular damage. J Neuroimmunol. 2008;204:20–28. doi: 10.1016/j.jneuroim.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Sartucci F, Cafforio G, Borghetti D, Domenici L, Orlandi G, Murri L. Electrophysiological evidence by single fibre electromyography of neuromuscular transmission impairment in a case of Miller Fisher syndrome. Neurol Sci. 2005;26:125–128. doi: 10.1007/s10072-005-0445-2. [DOI] [PubMed] [Google Scholar]
- Schluep M, Steck AJ. Immunostaining of motor nerve terminals by IgM M protein with activity against gangliosides GM1 and GD1b from a patient with motor neuron disease. Neurology. 1988;38:1890–1892. doi: 10.1212/wnl.38.12.1890. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Futerman AH. The localization of gangliosides in neurons of the central nervous system: The use of anti-ganglioside antibodies. Biochim Biophys Acta. 1996;1286:247–267. doi: 10.1016/s0304-4157(96)00011-1. [DOI] [PubMed] [Google Scholar]
- Sheikh KA, Sun J, Liu Y, Kawai H, Crawford TO, Proia RL, Griffin JW, Schnaar RL. Mice lacking complex gangliosides develop Wallerian degeneration and myelination defects. Proc Natl Acad Sci U S A. 1999;96:7532–7537. doi: 10.1073/pnas.96.13.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira e Souza AM, Mazucato VM, de Castro RO, Matioli F, Ciancaglini P, de Paiva PT, Jamur MC, Oliver C. The α–galactosyl derivatives of ganglioside GD1b are essential for the organization of lipid rafts in RBL-2H3 mast cells. Exp Cell Res. 2008;314:2515–2528. doi: 10.1016/j.yexcr.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Silverstein MP, Zimnowodzki S, Rucker JC. Neuromuscular junction dysfunction in Miller Fisher syndrome. Semin Ophthalmol. 2008;23:211–213. doi: 10.1080/08820530802049996. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simpson MA, Cross H, Proukakis C, Priestman DA, Neville DC, Reinkensmeier G, Wang H, Wiznitzer M, Gurtz K, Verganelaki A, Pryde A, Patton MA, Dwek RA, Butters TD, Platt FM, Crosby AH. Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase. Nat Genet. 2004;36:1225–1229. doi: 10.1038/ng1460. [DOI] [PubMed] [Google Scholar]
- Slater CR. Structural factors influencing the efficacy of neuromuscular transmission. Ann N Y Acad Sci. 2008;1132:1–12. doi: 10.1196/annals.1405.003. [DOI] [PubMed] [Google Scholar]
- Sonnino S, Mauri L, Chigorno V, Prinetti A. Gangliosides as components of lipid membrane domains. Glycobiology. 2007;17:1R–13R. doi: 10.1093/glycob/cwl052. [DOI] [PubMed] [Google Scholar]
- Spaans F, Vredeveld JW, Morre HH, Jacobs BC, De Baets MH. Dysfunction at the motor end-plate and axon membrane in Guillain-Barré syndrome: a single-fiber EMG study. Muscle Nerve. 2003;27:426–434. doi: 10.1002/mus.10334. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. α–Latrotoxin and its receptors: neurexins and CIRL/latrophilins. Annu Rev Neurosci. 2001;24:933–962. doi: 10.1146/annurev.neuro.24.1.933. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Furukawa K, Tajima O, Mii S, Honda T, Furukawa K. Sensory nerve-dominant nerve degeneration and remodeling in the mutant mice lacking complex gangliosides. Neuroscience. 2005;135:1167–1178. doi: 10.1016/j.neuroscience.2005.07.035. [DOI] [PubMed] [Google Scholar]
- Susuki K, Baba H, Tohyama K, Kanai K, Kuwabara S, Hirata K, Furukawa K, Furukawa K, Rasband MN, Yuki N. Gangliosides contribute to stability of paranodal junctions and ion channel clusters in myelinated nerve fibers. Glia. 2007;55:746–757. doi: 10.1002/glia.20503. [DOI] [PubMed] [Google Scholar]
- Svennerholm L. Designation and schematic structure of gangliosides and allied glycosphingolipids. Prog Brain Res. 1994;101:XI–XIV. doi: 10.1016/S0079-6123(08)61935-4. [DOI] [PubMed] [Google Scholar]
- Taguchi K, Ren J, Utsunomiya I, Aoyagi H, Fujita N, Ariga T, Miyatake T, Yoshino H. Neurophysiological and immunohistochemical studies on Guillain-Barré syndrome with IgG anti-GalNAc-GD1a antibodies – effects on neuromuscular transmission. J Neurol Sci. 2004;225:91–98. doi: 10.1016/j.jns.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Takamiya K, Yamamoto A, Furukawa K, Yamashiro S, Shin M, Okada M, Fukumoto S, Haraguchi M, Takeda N, Fujimura K, Sakae M, Kishikawa M, Shiku H, Furukawa K, Aizawa S. Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides but exhibit only subtle defects in their nervous system. Proc Natl Acad Sci USA. 1996;93:10662–10667. doi: 10.1073/pnas.93.20.10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Waki H, Kon K, Ando S. Gangliosides enhance KCl-induced Ca2+ influx and acetylcholine release in brain synaptosomes. Neuroreport. 1997;8:2203–2207. doi: 10.1097/00001756-199707070-00023. [DOI] [PubMed] [Google Scholar]
- Taverna E, Saba E, Rowe J, Francolini M, Clementi F, Rosa P. Role of lipid microdomains in P/Q-type calcium channel (Cav2.1) clustering and function in presynaptic membranes. J Biol Chem. 2004;279:5127–5134. doi: 10.1074/jbc.M308798200. [DOI] [PubMed] [Google Scholar]
- Thomas PD, Brewer GJ. Gangliosides and synaptic transmission. Biochim Biophys Acta. 1990;1031:277–289. doi: 10.1016/0304-4157(90)90013-3. [DOI] [PubMed] [Google Scholar]
- Tomcik J, Dufek M, Hromada J, Rektor I, Bares M. Recurrent Miller Fisher syndrome with abnormal terminal axon dysfunction: a case report. Acta Neurol Belg. 2007;107:112–114. [PubMed] [Google Scholar]
- Uncini A, Lugaresi A. Fisher syndrome with tetraparesis and antibody to GQ1b: evidence for motor nerve terminal block. Muscle Nerve. 1999;22:640–644. doi: 10.1002/(sici)1097-4598(199905)22:5<640::aid-mus14>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Ushkaryov Y. α–Latrotoxin: from structure to some functions. Toxicon. 2002;40:1–5. doi: 10.1016/s0041-0101(01)00204-5. [DOI] [PubMed] [Google Scholar]
- Van Der Goot FG, Harder T. Raft membrane domains: from a liquid-ordered membrane phase to a site of pathogen attack. Semin Immunol. 2001;13:89–97. doi: 10.1006/smim.2000.0300. [DOI] [PubMed] [Google Scholar]
- van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol. 2008;7:939–950. doi: 10.1016/S1474-4422(08)70215-1. [DOI] [PubMed] [Google Scholar]
- van Sorge NM, Van Der Pol WL, Jansen MD, Van Den Berg LH. Pathogenicity of anti-ganglioside antibodies in the Guillain-Barré syndrome. Autoimmun Rev. 2004;3:61–68. doi: 10.1016/S1568-9972(03)00089-2. [DOI] [PubMed] [Google Scholar]
- Vyas KA, Patel HV, Vyas AA, Schnaar RL. Segregation of gangliosides GM1 and GD3 on cell membranes, isolated membrane rafts, and defined supported lipid monolayers. Biol Chem. 2001;382:241–250. doi: 10.1515/BC.2001.031. [DOI] [PubMed] [Google Scholar]
- Waki H, Kon K, Tanaka Y, Ando S. Facile methods for isolation and determination of gangliosides in a small scale: age-related changes of gangliosides in mouse brain synaptic plasma membranes. Anal Biochem. 1994;222:156–162. doi: 10.1006/abio.1994.1467. [DOI] [PubMed] [Google Scholar]
- Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- Wessig C, Buchwald B, Toyka KV, Martini R. Miller Fisher syndrome: immunofluorescence and immunoelectron microscopic localization of IgG at the mouse neuromuscular junction. Acta Neuropathol (Berl) 2001;101:239–244. doi: 10.1007/s004010000285. [DOI] [PubMed] [Google Scholar]
- Wieraszko A, Seifert W. The role of monosialoganglioside GM1 in the synaptic plasticity: in vitro study on rat hippocampal slices. Brain Res. 1985;345:159–164. doi: 10.1016/0006-8993(85)90847-9. [DOI] [PubMed] [Google Scholar]
- Willison HJ, O’Hanlon GM. The immunopathogenesis of Miller Fisher syndrome. J Neuroimmunol. 1999;100:3–12. doi: 10.1016/s0165-5728(99)00213-1. [DOI] [PubMed] [Google Scholar]
- Willison HJ, Plomp JJ. Anti-ganglioside antibodies and the presynaptic motor nerve terminal. Ann N Y Acad Sci. 2008;1132:114–123. doi: 10.1196/annals.1405.010. [DOI] [PubMed] [Google Scholar]
- Willison HJ, Yuki N. Peripheral neuropathies and anti-glycolipid antibodies. Brain. 2002;125:2591–2625. doi: 10.1093/brain/awf272. [DOI] [PubMed] [Google Scholar]
- Winer JB. Guillain-Barré syndrome. BMJ. 2008;337:a671. doi: 10.1136/bmj.a671. [DOI] [PubMed] [Google Scholar]
- Wirguin I, Ifergane G, Almog Y, Lieberman D, Bersudsky M, Herishanu YO. Presynaptic neuromuscular transmission block in Guillain-Barré syndrome associated with anti-GQ1b antibodies. Neuromuscul Disord. 2002;12:292–293. doi: 10.1016/s0960-8966(01)00296-6. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Slater CR. Safety factor at the neuromuscular junction. Prog Neurobiol. 2001;64:393–429. doi: 10.1016/s0301-0082(00)00055-1. [DOI] [PubMed] [Google Scholar]
- Wu G, Ledeen RW. Gangliosides as modulators of neuronal calcium. Prog Brain Res. 1994;101:101–112. doi: 10.1016/s0079-6123(08)61942-1. [DOI] [PubMed] [Google Scholar]
- Wu G, Lu ZH, Obukhov AG, Nowycky MC, Ledeen RW. Induction of calcium influx through TRPC5 channels by cross-linking of GM1 ganglioside associated with α5β1 integrin initiates neurite outgrowth. J Neurosci. 2007;27:7447–7458. doi: 10.1523/JNEUROSCI.4266-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Xie X, Lu ZH, Ledeen RW. Cerebellar neurons lacking complex gangliosides degenerate in the presence of depolarizing levels of potassium. Proc Natl Acad Sci U S A. 2001;98:307–312. doi: 10.1073/pnas.011523698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GS, Vaswani KK, Lu ZH, Ledeen RW. Gangliosides stimulate calcium flux in neuro-2A cells and require exogenous calcium for neuritogenesis. J Neurochem. 1990;55:484–491. doi: 10.1111/j.1471-4159.1990.tb04161.x. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Wu YP, Sandhoff R, Werth N, Mizukami H, Ellis JM, Dupree JL, Geyer R, Sandhoff K, Proia RL. Interruption of ganglioside synthesis produces central nervous system degeneration and altered axon-glial interactions. Proc Natl Acad Sci U S A. 2005;102:2725–2730. doi: 10.1073/pnas.0407785102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates AJ, Rampersaud A. Sphingolipids as receptor modulators. An overview. Ann N Y Acad Sci. 1998;845:57–71. doi: 10.1111/j.1749-6632.1998.tb09662.x. [DOI] [PubMed] [Google Scholar]
- Yu RK, Bieberich E, Xia T, Zeng G. Regulation of ganglioside biosynthesis in the nervous system. J Lipid Res. 2004;45:783–793. doi: 10.1194/jlr.R300020-JLR200. [DOI] [PubMed] [Google Scholar]
- Yuki N, Koga M. Bacterial infections in Guillain-Barré and Fisher syndromes. Curr Opin Neurol. 2006;19:451–457. doi: 10.1097/01.wco.0000245367.36576.e9. [DOI] [PubMed] [Google Scholar]
- Zitman FM, Todorov B, Jacobs BC, Verschuuren JJ, Furukawa K, Furukawa K, Willison HJ, Plomp JJ. Neuromuscular synaptic function in mice lacking major subsets of gangliosides. Neuroscience. 2008;156:885–897. doi: 10.1016/j.neuroscience.2008.08.034. [DOI] [PubMed] [Google Scholar]
- Zitman FM, Todorov B, Verschuuren JJ, Jacobs BC, Furukawa K, Furukawa K, Willison HJ, Plomp JJ. Neuromuscular synaptic transmission in aged ganglioside deficient mice. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.01.007. DOI 10.1016/j.neurobiolaging.2009.01.007. [DOI] [PubMed] [Google Scholar]