Abstract
Elevated maternal plasma levels of homocysteine (Hcy) are associated with pregnancy complications and adverse neonatal outcomes, suggesting placental transport of Hcy may impact on fetal development. However, such transport mechanisms have not been defined. In this study we characterise Hcy transport mechanisms across the microvillous plasma membrane (MVM) of the syncytiotrophoblast, the transporting epithelium of human placenta. Three candidate transport systems, systems L, A and y+L, were examined utilising competitive inhibition to investigate the effects of Hcy on the uptake of well-characterised radiolabelled substrates for each system into isolated MVM vesicles, and that of model substrates on 10 μm[35S]l–Hcy uptake. System L activity was inhibited by both l-Hcy and dl–Hcy, comparable to model substrates including 2–aminobicyclo[2.2.1]heptane-2-carboxylic acid (BCH). System L constituted the major transport mechanism, with significant BCH inhibition (∼69%) of [35S]l–Hcy uptake. System A activity was also inhibited by l–Hcy and dl-Hcy with a smaller contribution (∼21%) to [35S]l–Hcy uptake. Inhibition by l–Hcy and dl–Hcy of system y+L activity was Na+ sensitive with a significant inhibition constant (Ki) shift observed following K+ replacement; l–arginine reduced [35S]l–Hcy uptake by ∼19%. Kinetic modelling of [35S]l–Hcy uptake resolved two, Na+-independent, transport components (Km 72 μm and 9.7 mm). This study provides evidence for the involvement of systems L, A and y+L in placental Hcy transport. Such transport, by competing with endogenous amino acids for transporter activity, could have major implications for syncytiotrophoblast metabolism and function as well as fetal development.
Homocysteine (Hcy) is a thiol group-containing amino acid metabolite formed in the methionine (Met) cycle (Selhub, 1999). As a branch point metabolite, Hcy can be remethylated back to Met or converted to cysteine through the transsulphuration pathway (Selhub, 1999). Perturbation of these metabolic pathways leads to hyperhomocysteinaemia (HHcy), a condition in which the plasma concentration of total Hcy (tHcy), comprising reduced and oxidized forms of Hcy, is elevated. Plasma tHcy is regulated by several factors including nutritional deficiencies in the vitamins that act as co-factors or co-substrates in Hcy metabolism (folate, vitamins B12 and B6), or genetic defects in the enzymes responsible for Hcy metabolism (Refsum et al. 1998; Selhub, 1999).
Evidence from several epidemiological studies has revealed that HHcy is associated with a diverse array of health complications of considerable public health significance, reflecting the pleiotropic nature of the effects elicited by Hcy. Indeed, such studies have identified Hcy as an independent risk factor for cardiovascular disease, stroke, cognitive dysfunction and osteoporosis (Refsum et al. 2006; Selhub, 2006). Hcy is also an independent risk for obstetric vascular disease. Raised maternal plasma levels of tHcy are associated with various vascular-related complications of pregnancy including pre-eclampsia (Ray & Laskin, 1999; Vollset et al. 2000; de la Calle et al. 2003), placental abruption (Goddijn-Wessel et al. 1996; Ray & Laskin, 1999; Vollset et al. 2000; de la Calle et al. 2003), recurrent pregnancy loss (Ray & Laskin, 1999; Quéréet al. 2001; de la Calle et al. 2003), fetal growth restriction (Vollset et al. 2000; de la Calle et al. 2003) and stillbirth (Vollset et al. 2000), as well as adverse outcomes for the baby such as neural tube defects and congenital malformations (Vollset et al. 2000; de la Calle et al. 2003). Supplementation with folic acid and B group vitamins has proved beneficial in normalising maternal tHcy and improving pregnancy outcome (Quéréet al. 2001; de la Calle et al. 2003) signifying that some of the risks are modifiable.
Maternal plasma tHcy concentration is significantly lowered during pregnancy (Murphy et al. 2004) but this is not accounted for by haemodilution, a decrease in albumin levels to which 70–80% of tHcy is bound, or folic acid supplementation (Murphy et al. 2002). Indeed it has been proposed that this is a physiological adaptation to pregnancy (Murphy et al. 2002). Maternal plasma tHcy concentration is higher than fetal plasma tHcy concentration and is a major determinant of the latter, with a significant, positive correlation between these two variables (Malinow et al. 1998; Molloy et al. 2002; Murphy et al. 2004). These observations are compatible with the concept that Hcy is transported by the placenta, supported further by the evidence of a decreasing concentration gradient of plasma tHcy from maternal vein to umbilical vein to umbilical artery (Malinow et al. 1998). However, Hcy transport mechanisms in the placenta have not previously been explored.
We reasoned that maternofetal transfer of Hcy across the transporting epithelium of the human placenta, the syncytiotrophoblast, would involve amino acid transporters that are intrinsic to both the maternal-facing microvillous plasma membrane (MVM) and the fetal-facing basal plasma membrane of the syncytiotrophoblast which transport neutral amino acids (Jansson, 2001; Cleal & Lewis, 2008). These comprise Na+-dependent and Na+-independent mechanisms with some transport mechanisms common to both syncytiotrophoblast plasma membranes, including systems A, L and y+L (Jansson, 2001; Cleal & Lewis, 2008).
System A activity is mediated by three highly homologous isoforms of the sodium-coupled neutral amino acid transporter (SNAT) family, namely SNAT1 (SLC38A1), SNAT2 (SLC38A2) and SNAT4 (SLC38A4), all of which are expressed in the syncytiotrophoblast and localised to MVM (Desforges et al. 2009). System y+L comprises a heterodimeric complex with the CD98 heavy chain linked to either y+LAT1 (SLC7A7) or y+LAT2 (SLC7A6) light chain (Torrents et al. 1998; Pfeiffer et al. 1999) which confer functional activity and substrate specificity. Both y+LAT light chains are expressed in human placenta at the mRNA level (Dye et al. 2004). System L is also a heterodimeric transport system in which CD98 associates with LAT1 (SLC7A5) and LAT2 (SLC7A8) light chains, both of which are expressed in human placenta at the mRNA level (Kudo & Boyd, 2000). LAT1 is particularly enriched in MVM compared to basal plasma membrane (Okamoto et al. 2002) whilst the distribution of LAT2 remains unknown. Functional evidence suggests LAT2 may be present on both MVM and basal plasma membrane (Kudo & Boyd, 2001; Lewis et al. 2007). It has also been proposed that LAT4 (SLC43A2), a monomeric amino acid transporter mediating system L–type transport, and which is highly expressed in placenta (Bodoy et al. 2005), might also mediate placental transport of system L amino acid substrates across the basal plasma membrane (Cleal & Lewis, 2008).
Each of these three transport mechanisms, systems A, L and y+L, have the common characteristic of being able to utilise Met as a substrate. Based on the biochemical similarity of Met to Hcy, we hypothesised that these transporters would also support Hcy as a substrate. To test this hypothesis, we investigated whether these candidate transport systems are involved in Hcy transport across the MVM of human placenta, the first plasma membrane barrier to maternofetal amino acid transfer. Isolated MVM plasma membrane vesicles were employed in a dual strategy of examining the effect of (i) unlabelled Hcy on the uptake of well-characterised radiolabelled substrates for each transport system and (ii) unlabelled model substrates for each transport system on radiolabelled Hcy uptake.
Methods
Materials
All chemicals were purchased from either Sigma-Aldrich Co. Ltd (Poole, UK) or VWR International (Lutterworth, UK) unless otherwise stated. l–Hcy was custom-synthesized from l–Met (Department of Chemistry, University of Manchester) by the method of Shiraiwa et al. (2000) with >94% purity confirmed by HPLC analysis and no evidence of oxidized product homocystine. [14C]α-(methylamino)isobutyric acid (MeAIB; 58.8 mCi mmol−1), [35S]l–Met (>1000 Ci mmol−1) and [3H]l–arginine (Arg; 50 Ci mmol−1) were obtained from Perkin Elmer (Beaconsfield, UK), GE Healthcare (Little Chalfont, UK) and MP Biomedicals (London, UK), respectively.
Tissue acquisition
Placentas were obtained with written informed consent in accordance with ethical approval (Central Manchester Local Research Ethics Committee). Placentas were collected from normal, uncomplicated, singleton pregnancies at term (38–41 weeks) within 30 min of vaginal delivery or Caesarean section. Gestational age was calculated from the first day of the last menstrual period and confirmed by ultrasound scan.
Isolation of MVM vesicles
MVM vesicles were prepared from human placenta as described previously (Method 3 in Glazier et al. 1988). Protein concentration was measured by the method of Lowry et al. (1951). Membrane purity was assessed by measuring the enrichment of alkaline phosphatase (activity in vesicles/initial homogenate), as a marker of MVM (Glazier et al. 1988). Vesicles were suspended in intravesicular buffer (of the following composition: 290 mm sucrose, 5 mm Tris, 5 mm Hepes, pH 7.4) unless indicated otherwise. MVM vesicles were stored at 4°C prior to transport assay which were performed within 48 h of isolation. Alkaline phosphatase activity was enriched 20 ± 1-fold in MVM vesicles (n= 50), of comparable purity to our previous studies (Mahendran et al. 1993).
Transport assays
Strategy for the characterisation of l–Hcy transport mechanisms in MVM of human placenta
To characterise the transport mechanisms for l–Hcy across human placental MVM, we firstly undertook competitive inhibition studies, examining whether l–Hcy inhibited the uptake of radiolabelled model substrates for systems A, L and y+L, the three candidate transport systems under investigation. The inhibitory effect of l–Hcy was compared to that of well-characterised amino acid substrates for each transport mechanism; a similar pattern of inhibition was taken to indicate mediation by a common transport pathway. Additionally, the effect of dl–Hcy was included for reference, this being the commercial source of Hcy used previously by others to characterise transport mechanisms (Naggar et al. 2003; Hultberg, 2004). Secondly, in a more direct approach, uptake of radiolabelled [35S]l–Hcy was measured and the effect of unlabelled model substrates for each candidate transport system determined.
Effect of Hcy on system A activity in MVM vesicles
System A activity in MVM vesicles was measured as the Na+-dependent uptake of [14C]MeAIB, a non-metabolisable substrate transported by system A (Mackenzie & Erickson, 2004), as described previously (Mahendran et al. 1993). Briefly, uptakes at initial rate (30 s) were performed by adding 20 μl MVM vesicles to 20 μl extravesicular buffer (EVB) with Na+ (EVB-Na+; 145 mm NaCl, 5 mm Tris, 5 mm Hepes, pH 7.4) or without Na+ (EVB-K+; 145 mm KCl, 5 mm Tris, 5 mm Hepes, pH 7.4) containing 0.33 mm[14C]MeAIB. Competitive inhibition of system A activity by 20 mm l–Hcy and dl–Hcy was compared to that of l–alanine (Ala), l–serine (Ser), l–Met, and MeAIB as paradigm substrates for this transport mechanism. 2-Aminobicyclo[2.2.1]heptane-2-carboxylic acid (BCH), which is not transported by system A (Johnson & Smith, 1988), was included as a negative control. In order to explore the concentration dependency of inhibition, the effect of l–Hcy on Na+-dependent [14C]MeAIB uptake into MVM was examined over a broad concentration range (50 μm–20 mm), and the concentration dependency of inhibition compared to that of l–Met and MeAIB.
Effect of Hcy on system L activity in MVM vesicles
System L activity in MVM vesicles was measured as the Na+-independent uptake of [35S]l–Met, a model substrate for system L (Ganapathy et al. 1986; Johnson & Smith, 1988). [35S]l–Met uptakes were performed initially over 15–60 s to establish the initial rate by adding 20 μl MVM vesicles to 20 μl EVB-K+ containing 0.4 μm[35S]l–Met. Competitive inhibition by Hcy of system L activity in MVM vesicles was examined at apparent initial rate (30 s; found in the time course experiments to be on the linear part of the uptake) by the addition of unlabelled l–Hcy and dl–Hcy (0.1, 1, 10 and 20 mm), with comparison to the effects of l–leucine (Leu), BCH and l–Met as model substrates for this transport mechanism (Ganapathy et al. 1986; Johnson & Smith, 1988). The effect of dl–Hcy was also examined for reference. MeAIB, an amino acid analogue not transported by system L (Ganapathy et al. 1986), was included as a negative control. To investigate the concentration dependency of inhibition, the effect of unlabelled l–Hcy was examined over an expansive concentration range (0.5 μm–20 mm) and compared to the effect by l–Met.
Effect of Hcy on system y+L activity in MVM vesicles
System y+L activity was measured as the uptake of 0.2 μm[3H]l–Arg following pre-incubation of MVM vesicles with 4 μm valinomycin for 1 h as described previously (Ayuk et al. 2000). MVM vesicles were suspended in intravesicular buffer (50 mm KCl, 50 mm choline chloride, 100 mm mannitol, 20 mm Hepes-Tris, pH 7.4) and uptakes performed at initial rate (30 s) by adding 20 μl MVM vesicles to 100 μl EVB (50 mm, NaCl, 50 mm KCl, 100 mm mannitol, 20 mm Hepes-Tris, pH 7.4) containing 0.24 μm[3H]l–arginine (Ayuk et al. 2000). Competitive inhibition of system y+L activity by Hcy was examined by comparing the effect of unlabelled dl–Hcy to that of cationic (l–Arg and l–lysine (Lys)) and neutral (l–Leu, l–glutamine (Gln) and l–Met) amino acid substrates for this transporter (Devés & Boyd, 1998).
The Na+ dependency of inhibition by l–Hcy and dl–Hcy of system y+L activity was investigated and compared to that of l–Met as a model neutral amino acid substrate. Uptake of [3H]l–Arg was measured in the presence of competing amino acid (50 μm–10 mm) with and without Na+ (with K+ replacement) to allow determination of the inhibition constant (Ki) under both conditions. Ki values were determined using a statistical program (Graphpad Prism 4, San Diego, CA, USA) from the equation of Cheng & Prusoff (1973) where Km= 7.6 μm for system y+L was applied, as determined previously in MVM (Ayuk et al. 2000). An increase in Ki when Na+ was replaced by K+ was taken to indicate transport by system y+L (Devés & Boyd, 1998; Ayuk et al. 2000).
Measurement of [35S]l–Hcy uptake into MVM vesicles
[35S]l–Hcy was prepared from [35S]l–Hcy thiolactone (5 mm; 357 μCi μmol−1) by alkaline hydrolysis (Duerre & Miller, 1966) using either 5 m NaOH or KOH (allowing for transport measurements to be performed in the presence or absence of Na+, respectively), as described previously (Büdy et al. 2006), but with modifications as described below. Following alkaline hydrolysis for 2 min at 37°C, the mixture was immediately neutralised with 0.1 m TES buffer (pH 7.4) containing 50 mm Tris(2-carboxyethyl)phosphine hydrochloride (TCEP.HCl) as reductant to preserve the thiol group (Cline et al. 2004) and an appropriate volume of 5 m HCl added to achieve a pH of 7.4. The concentration of [35S]l–Hcy was measured by quantification of thiol concentration using a commercial kit according to the manufacturer's instructions (Measure-iT Thiol Assay Kit; Invitrogen, Paisley, UK). Converted [35S]l–Hcy was stored at −20°C between transport assays.
Uptake of [35S]l–Hcy into MVM vesicles was performed as described previously for system A (Mahendran et al. 1993). Uptake was initiated by adding 20 μl MVM vesicles (∼250–300 μg protein) to 20 μl EVB-Na+ or EVB-K+ containing 20 μm[35S]l–Hcy. Non-specific binding of tracer to MVM plasma membrane was quantified following disruption of vesicle integrity with 0.2% Triton.
To characterise the kinetic properties of [35S]l–Hcy uptake into MVM vesicles, uptakes were performed at 30 s (taken to be initial rate; see Results) in the presence of increasing concentrations of unlabelled l–Hcy (5 μm–10 mm). To examine the involvement of candidate transport mechanisms systems A, L and y+L in [35S]l–Hcy transport across human placental MVM, competitive inhibition of [35S]l–Hcy uptake into MVM vesicles was performed in the presence of model substrates for each transport system; MeAIB, BCH and l–Arg for systems A, L and y+L, respectively.
Statistical analysis
Data are presented as the mean ±s.e.m., where n is the number of placentas. Competitive inhibition of amino acid uptake into MVM was analysed by Kruskal–Wallis with Dunn's multiple comparison test, two-way ANOVA with Bonferroni post test or Friedman with Dunn's multiple comparison test as appropriate. For each transport mechanism, the half-maximal inhibitory concentration (IC50) was derived by non-linear regression to a one-site transport system model. All statistical analyses were performed using a statistical program (Graphpad Prism 4, San Diego, CA, USA).
Kinetic modelling of l–Hcy competitive inhibition curves was achieved by comparison to Michaelis–Menten equations with up to two saturable components (SIMFIT version 6.0.18; W.G. Bardsley, University of Manchester; http://www.simfit.man.ac.uk). The SIMFIT computer package supports curve fitting of data with discrimination of kinetically distinct transporters by determination of the best-fit to the Michaelis–Menten equation. The data were fitted using a program within SIMFIT which assumes the kinetic transformation process is the same whether the substrate is labelled or not, so if the radiolabelled substrate is fixed ([hot]), the initial rate of uptake (y) will be proportional to the concentration of unlabelled substrate ([cold]) added, allowing isotope displacement kinetics to be modelled to the following equation:
where [hot] is the concentration of radiolabelled substrate, [cold] is the concentration of unlabelled substrate, Km is the Michaelis constant and Vmax is the maximal velocity. The best fit to the data was determined by the F test, comparing the closeness of fit (weighted sum of squares of the variance) against the number of parameters in the model.
Results
Inhibition of system A activity by Hcy
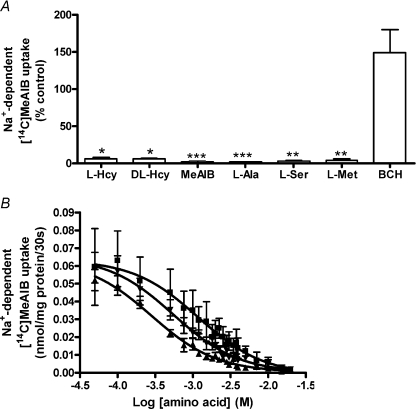
Both l–Hcy and dl–Hcy caused a profound inhibition of system A activity in MVM vesicles, similar to that observed with the model amino acid substrates for this transport mechanism (Fig. 1A). BCH, which is not transported by system A, was not inhibitory, as expected (Fig. 1A). Inhibition of system A activity by l–Hcy was concentration dependent, also observed for the positive controls l–Met and MeAIB (Fig. 1B). The IC50 for l–Hcy (0.53 ± 0.04 mm) was not significantly different from that for l–Met (0.27 ± 0.02 mm) or MeAIB (1.13 ± 0.12 mm; n= 6 for all). However, IC50 for l–Met was significantly lower (P < 0.001) than that for MeAIB, suggesting a higher preference for the former substrate.
Figure 1. Hcy inhibition of system A activity in MVM vesicles.
A, effect of l–Hcy and dl–Hcy on system A activity at initial rate (30 s) compared to the model system A substrates MeAIB, l–Ala, l–Ser and l–Met. BCH served as negative control (20 mm for all). All data are expressed as a percentage of control uptake (no amino acid; 100%). Data are shown as mean ±s.e.m. (n= 9) except for BCH (n= 4). *P < 0.05, **P < 0.01, ***P < 0.001 vs control (Kruskal–Wallis with Dunn's multiple comparison test). B, effect of varied concentrations (50 μm–20 mm) of l–Hcy (▾), MeAIB (▪) and l–Met (▴) on the Na+-dependent uptake of [14C]MeAIB (0.165 mm) at initial rate. Data are mean ±s.e.m. (n= 6). Data from individual experiments were fitted by non-linear regression to a sigmoidal dose–response model.
Inhibition of system L activity by Hcy
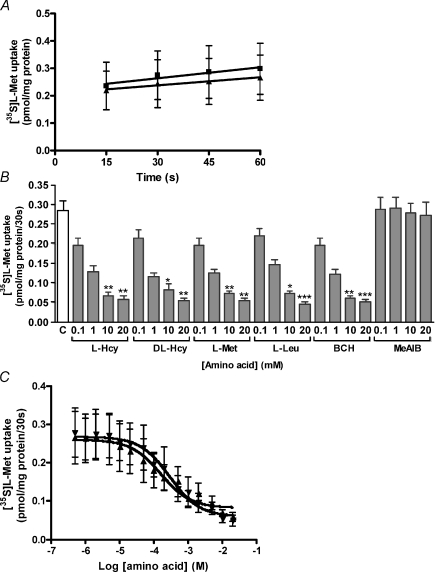
In preliminary studies we explored the possibility of using [3H]l–Leu as a paradigm substrate for system L, but observed that uptake equilibrium was achieved extremely rapidly, within 5 s, the earliest time point that could be reproducibly measured (data not shown). We therefore employed [35S]l–Met as a system L substrate (Fig. 2) and initially examined the linearity and Na+ independence of [35S]l–Met uptake. Uptake of [35S]l–Met was linear over 15–60 s and unaltered by an inwardly directed Na+ gradient (Fig. 2A). Therefore, system L activity in MVM vesicles was measured as the Na+-independent uptake of [35S]l–Met with 30 s taken to indicate apparent initial rate of uptake. We use the term ‘apparent initial rate’ here as extrapolation of the regression line to time zero in Fig. 2A yielded an uptake value of ∼0.2 pmol (mg protein)−1, greater than the residual component of uptake following BCH inhibition (∼0.05 pmol (mg protein)−1; Fig. 2B), suggesting there is an extremely rapid initial uptake of [35S]l–Met by a BCH-inhibitable component. The dose-dependent inhibition by both l–Hcy and dl–Hcy of Na+-independent [35S]l–Met uptake into MVM was of comparable magnitude to that exhibited by all system L substrates (l–Met, l–Leu, BCH) examined (Fig. 2B), suggesting mediation by a common transport mechanism. As expected, uptake was unaltered in the presence of MeAIB, a specific system A substrate not transported by system L, included as a negative control. The concentration dependency of inhibition was further explored, comparing the effect of l–Hcy to l–Met as reference (Fig. 2C). The observed similarity in the inhibition profiles between these two amino acids, as illustrated in Fig. 2C, lends further support to the concept that these two amino acids are transported by a common transport mechanism. Indeed, the IC50 was not significantly different between l–Hcy (0.29 ± 0.05 mm) and l–Met (0.17 ± 0.07 mm; n= 6), suggesting l–Hcy has a relatively high affinity for system L.
Figure 2. Hcy inhibition of system L activity in MVM vesicles.
A, time-dependent uptake of 0.2 μm[35S]l–Met into MVM vesicles over 15–60 s in the presence (▪) and absence (▴) of an inwardly directed Na+ gradient. Data are mean +s.e.m. (n= 8) with linear regression plot shown (r2 > 0.9, P < 0.05 for both conditions). There was no significant difference between the slopes of the lines. B, effect of varied concentrations (0.1, 1, 10 and 20 mm) of l–Hcy, dl–Hcy, l–Met, l– l–Leu, BCH and MeAIB on uptake of 0.2 μm[35S]l–Met into MVM vesicles. Data are mean +s.e.m. (n= 6, except at 20 mm where n= 5). *P < 0.05, **P < 0.01, ***P < 0.001 vs control (C) (Kruskal–Wallis with Dunn's multiple comparison test). C, effect of varied concentrations (0.5 μm–20 mm) of l–Hcy (▾) or l–Met (▴) on uptake of 0.2 μm[35S]l–Met into MVM vesicles. Data are mean ±s.e.m. (n= 3). Data from individual experiments were fitted by non-linear regression to a sigmoidal dose–response model.
Inhibition of system y+L activity by Hcy
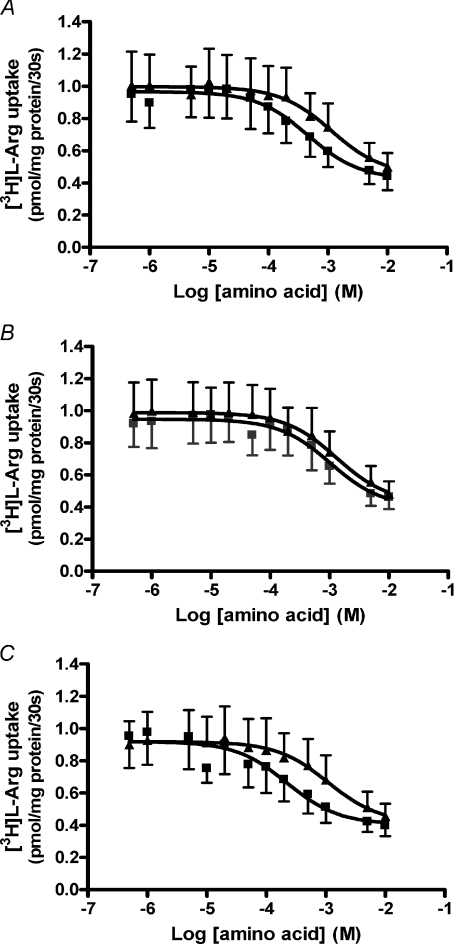
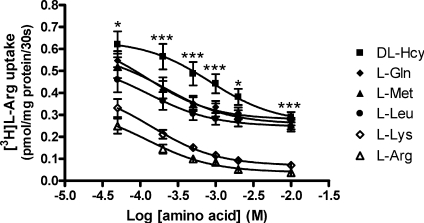
As shown in Fig. 3, l–Hcy (Fig. 3A), dl–Hcy (Fig. 3B) and l–Met included as model substrate for system y+L (Fig. 3C), all demonstrated concentration-dependent inhibition of 3[H]l–Arg uptake into MVM under both Na+-containing and Na+-free (by K+ replacement) conditions. Abolition of the inwardly directed Na+ gradient across the MVM vesicular membrane resulted in an upward shift in the inhibition curve for l–Hcy (Fig. 3A), dl–Hcy (Fig. 3B) and l–Met (Fig. 3C). This shift was most notably divergent for l–Hcy (Fig. 3A) and l–Met (Fig. 3C). The Na+ dependency of inhibition was lost at higher substrate concentrations, a pattern characteristic of system y+L (Devés & Boyd, 1998). Table 1 provides confirmation of the Na+ dependency of inhibition for all three amino acids as shown by the significantly lower Ki values found in the presence of Na+. This implies a higher affinity of neutral amino acid binding in the presence of Na+ providing positive identification of system y+L activity (Devés & Boyd, 1998; Ayuk et al. 2000). As might be predicted from the preference of system y+L for the l–stereoisomer form of neutral amino acids (Devés & Boyd, 1998), the Ki shift for dl–Hcy was less pronounced than for l–Hcy. However, further experiments examining the effect of dl–Hcy revealed a similar inhibitory capacity at high concentration (10 mm) compared to other system y+L neutral amino acid substrates (l–Gln, l–Met, l–Leu), although at lower concentrations dl–Hcy was less effective (Fig. 4). Figure 4 also serves to demonstrate that there was a component of [3H]l–Arg uptake that was resistant to neutral amino acid inhibition but which was inhibitable by the cationic amino acid substrates l–Arg and l–Lys; this can be attributed to system y+ activity (Ayuk et al. 2000).
Figure 3. Na+ dependency of Hcy inhibition of [3H]l–Arg uptake into MVM vesicles.
Effect of l–Hcy (A), dl–Hcy (B) and l–Met (C) as positive control on 0.2 μm[3H]l–Arg uptake by MVM vesicles in the presence (▪) or absence (▴) of an inwardly directed Na+ gradient at initial rate (30 s). Data are mean ±s.e.m. (n= 6) and were fitted by non-linear regression to a one-site binding model.
Table 1.
Ki values for amino acid inhibition of 0.2 μm[3H]l–arginine uptake into MVM vesicles
|
Ki (μm) |
|||
|---|---|---|---|
| Amino acid | Na+ | K+ | Ki shift§ |
| l–Hcy | 535 ± 184 | 1419 ± 435* | 2.6 |
| dl–Hcy | 826 ± 100 | 1354 ± 216* | 1.6 |
| l–Met | 177 ± 20 | 1021 ± 320* | 5.8 |
Data are mean ±s.e.m., (n= 6). *P < 0.05 vs Na+ (paired t test). §Ki shift was calculated as ratio (K+/Na+) of mean Ki value.
Figure 4. Hcy inhibition of [3H]l–Arg uptake into MVM vesicles.
Effect of unlabelled (0.05–10 mm) neutral (dl–Hcy, l–Gln, l–Met, l–Leu) and cationic (l–Lys, l–Arg) amino acids on 0.2 μm[3H]l–Arg uptake into MVM vesicles at initial rate (30 s). Data are mean ±s.e.m., except where this falls within size of symbol (n= 6). *P < 0.05, ***P < 0.001 dl–Hcy vs all other amino acids, except at 10 mm, l–Lys and l–Arg only (two-way ANOVA with Bonferroni's post test).
Uptake of [35S]l–Hcy by human placental MVM vesicles
Time course and effect of inwardly directed Na+ gradient
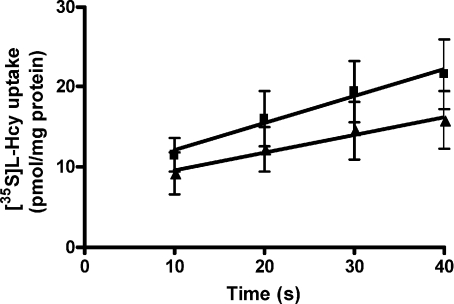
Uptake of 10 μm[35S]l–Hcy into MVM was time dependent and linear over 10–40 s both in the presence and absence of an inwardly directed Na+ gradient (Fig. 5). The rate of uptake was not significantly different between these two conditions, suggesting that Na+-independent transport mechanisms of [35S]l–Hcy uptake prevail under the conditions of assay. The magnitude of uptake was, however, significantly higher in the presence of Na+ (P < 0.05, paired t test). Although these observations would be consistent with there being unidirectional uptake of [35S]l–Hcy across the MVM plasma membrane into an intravesicular space under both conditions, disruption of vesicular integrity by 0.2% Triton indicated there was considerable binding of [35S]l–Hcy to the MVM plasma membrane. The degree of binding measured at 40 s was similar in the presence or absence of Na+ (9.6 ± 1.1 and 10.8 ± 0.9 pmol (mg protein)−1, respectively, n= 5). Addition of unlabelled l–Hcy demonstrated this component was largely exchangeable with ‘cold’l–Hcy, as shown by marked inhibitory capacity (Fig. 6). We therefore reasoned that this did not reflect irreversible binding of [35S]l–Hcy to the MVM plasma membrane and took the displacement by exogenous l–Hcy to infer that this component represented binding of [35S]l–Hcy to transport moieties prior to translocation across MVM. All uptake data are therefore shown uncorrected for binding. For all subsequent measurements, 30 s was taken to indicate initial rate of uptake.
Figure 5. Linearity of [35S]l–Hcy uptake into MVM vesicles.
Uptake of 10 μm[35S]l–Hcy into MVM vesicles in the presence (▪) or absence (▴; with K+ replacement) of an inwardly directed Na+ gradient over 10–40 s. Linear regression plot is shown (r2 > 0.97, P < 0.05 for both conditions). Data are presented as mean ±s.e.m. (n= 6).
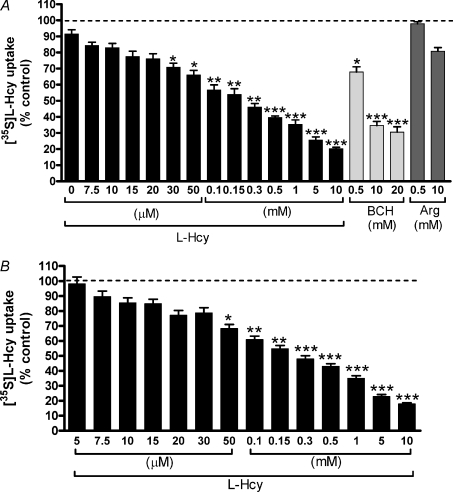
Figure 6. Inhibition by unlabelled amino acids or analogues of [35S]l–Hcy uptake into MVM vesicles.
Effect of unlabelled amino acids or analogues on uptake of 10 μm[35S]l–Hcy into MVM vesicles at initial rate (30 s) in the absence (A) or the presence (B) of an inwardly directed Na+ gradient shown as percentage of control uptake represented by the dashed line. Concentration-dependent inhibition by l–Hcy (black bars) was observed under both conditions. The system L analogue BCH (pale grey bars) significantly inhibited uptake at all concentrations, whereas the system y+L substrate l– Arg (dark grey bars) failed to evoke significant inhibition. Data are presented as mean ±s.e.m. (n= 5–7). *P < 0.05, **P < 0.01, ***P < 0.001 vs control (Kruskal–Wallis with Dunn's multiple comparison test).
Effect of amino acids or analogues on [35S]l–Hcy uptake
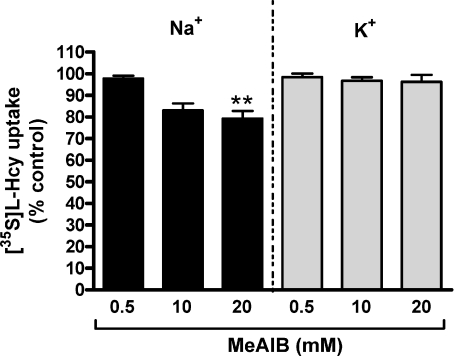
The effect of exogenously added l–Hcy on [35S]l–Hcy uptake as well as that for substrates of the three candidate systems, BCH (system L), l–Arg (system y+L) and MeAIB (system A), are shown in Figs 6 and 7. Under both Na+-free (Fig. 6A) and Na+-containing (Fig. 6B) conditions, [35S]l–Hcy uptake was significantly inhibited by l–Hcy in a concentration-dependent manner. At 10 mm l–Hcy, ∼80% inhibition of control uptake was observed under both conditions. The inhibitory potential of BCH and l–Arg was only examined under Na+-free conditions (Fig. 6A) as these substrates are transported in a Na+-independent manner by systems L and y+L, respectively. BCH significantly inhibited [35S]l–Hcy uptake at all three concentrations tested (0.5, 10 and 20 mm). In contrast, no significant inhibition of [35S]l–Hcy uptake was observed with 0.5 or 10 mm l–Arg, although a mean reduction of ∼19% was apparent at the higher concentration; this is likely to be accounted for by mechanisms other than system y+L, that is system y+, mediating l–Arg transport across MVM (Ayuk et al. 2000). Figure 7 shows that 20 mm MeAIB significantly inhibited uptake of [35S]l–Hcy and that this inhibition was dependent of the presence of Na+.
Figure 7. MeAIB inhibition of [35S]l–Hcy uptake into MVM vesicles.
Effect of unlabelled MeAIB on uptake of 10 μm[35S]l–Hcy into MVM vesicles at initial rate (30 s) in the presence (black bars) or the absence (K+ replacement; grey bars) of an inwardly directed Na+ gradient shown as percentage of control uptake. 20 mm MeAIB significantly inhibited uptake and this inhibition was dependent on the presence of Na+. Data are presented as mean +s.e.m. (n= 5). **P < 0.01 vs control (Friedman with Dunn's multiple comparison test).
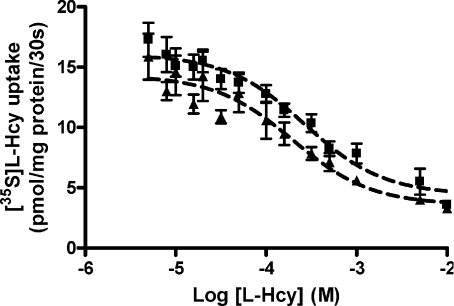
Kinetic analysis of [35S]l–Hcy uptake
Based on these data suggesting the primary involvement of system L amino acid transporter in [35S]l–Hcy transport across MVM, we examined whether kinetic modelling of the individual l–Hcy inhibition curves (Fig. 8) would reveal discrimination of two kinetic components reflecting mediation by both LAT light chains. In all four MVM vesicle isolates examined, and for both assay conditions, the best fit (F test, P < 0.01) was to a two saturable transporter model. Derived estimates of Km and Vmax for the two transport mechanisms were not significantly different between Na+-containing and Na+-free conditions and have therefore been pooled. The kinetic characteristics for each transport system were Km1= 72 ± 13 μm, Vmax1= 750 ± 161 pmol (mg protein)−1 (30 s)−1 and Km2= 9748 ± 894 μm, Vmax2= 60341 ± 4910 pmol (mg protein)−1 (30 s)−1 (n= 8).
Figure 8. Kinetic modelling of [35S]l–Hcy uptake into MVM vesicles.
Effect of unlabelled l–Hcy (5 μm–10 mm) on uptake of 10 μm[35S]l–Hcy into MVM vesicles at initial rate (30 s) in the presence (▪) or absence (▴; with K+ replacement) of an inwardly directed Na+ gradient. Kinetic modelling using non-linear regression was performed for each individual experiment under both conditions, and this revealed that a two-site transporter model (dashed lines) was preferred over a one-site model in all cases. There was a significant improvement in the correlation coefficient for a two-site model (r2 > 0.98 for all) compared to a one-site model (r2 < 0.96 for all). Data are mean ±s.e.m. (n= 4).
Discussion
This study characterises three potential influx transport mechanisms for l–Hcy across MVM of human term placenta, the first plasma membrane barrier to maternofetal nutrient transfer. The three transport systems we elected to study here, systems A, L and y+L, were considered prime candidates based on (i) the extensive characterisation of these transport mechanisms in MVM of human placenta (Jansson, 2001; Cleal & Lewis, 2008) and (ii) the ability of all three transport mechanisms to utilise l–Met, the methylated derivative of l–Hcy, as substrate (Devés & Boyd, 1998; Kanai et al. 1998; Meier et al. 2002; Mackenzie & Erickson, 2004; Cleal & Lewis, 2008). Our evidence supports the involvement of each of these neutral amino acid transport mechanisms in mediating l–Hcy transport across MVM of human placenta. Considering the magnitude of maximal inhibition of [35S]l–Hcy uptake into MVM vesicles by substrates of systems A (∼21%), L (∼69%) and y+L (∼19%) observed here, it would appear that involvement by other transport systems, such as system X−AG which is localised to the MVM and imports l–Hcy in other cell types (Büdy et al. 2006; Jiang et al. 2007), is minimal. Involvement of system ASC (Büdy et al. 2006; Jiang et al. 2007) can be discounted as its activity is confined to basal plasma membrane in human placenta (Cleal & Lewis, 2008).
We infer that system A transports l–Hcy across MVM based on our observations of (i) concentration-dependent inhibition by l–Hcy of [14C]MeAIB uptake, a paradigm substrate for system A, (ii) the comparable inhibition of [14C]MeAIB uptake by Hcy to that of model system A substrates at high substrate concentration, and (iii) the ability of MeAIB to inhibit [35S]l–Hcy uptake. These data demonstrating a system A-mediated component of l–Hcy transport are consistent with the observations of others in some (Naggar et al. 2003; Hultberg, 2004; Büdy et al. 2006) but not all (Ewadh et al. 1990; Jiang et al. 2007) cell types.
SNAT1, 2 and 4 isoforms of system A are all localised to MVM (Desforges et al. 2009), allowing for the possibility that more than one SNAT isoform mediates Hcy transport across MVM. Based on the comparability of IC50 values observed for l–Hcy and l–Met inhibition of [14C]MeAIB uptake, and the relatively high preference of SNAT1 and SNAT2 for l–Met as substrate (Hatanaka et al. 2000; Wang et al. 2000), we speculate that l–Hcy transport across MVM may involve either, or both, of these two SNAT isoforms. Our observation that the racemic mixture dl–Hcy was equally effective as l–Hcy in inhibiting [14C]MeAIB uptake into MVM is consistent with the demonstration that both enantiomers of Hcy can be transported (Jiang et al. 2007) and that d–enantiomers of amino acids can be taken up into cells by various transport mechanisms, including systems A and L, although stereoselectivity usually favours the l–enantiomer (Shikano et al. 2007).
Our evidence of (i) concentration-dependent inhibition by l–Hcy and dl–Hcy of Na+-independent [35S]l–Met uptake, a preferred substrate for both LAT1 and LAT2 (Kanai et al. 1998; Pineda et al. 1999; Meier et al. 2002), (ii) Hcy inhibition of [35S]l–Met uptake with similar capacity to that of model system L substrates, and (iii) marked inhibition of [35S]l–Hcy uptake by BCH, collectively supports the concept that system L transports Hcy across MVM. Further, our data suggest that system L predominates as the transport mechanism for Hcy across MVM as shown by BCH inhibiting ∼69%[35S]l–Hcy uptake. This agrees well with previous observations in a variety of cell types that system L is a major contributor to cellular Hcy uptake (Ewadh et al. 1990; Limpach et al. 2000; Naggar et al. 2003; Büdy et al. 2006; Jiang et al. 2007).
The notion that system L activity predominates would also be consistent with our kinetic analyses of [35S]l–Hcy uptake into MVM vesicles, which discriminated both a high-, and low-affinity, Na+-independent, transport component with a Km value of ∼72 μm and 9.7 mm, respectively. These Km values fall within the range of values (12 μm to >10 mm) previously cited for substrate affinities for LAT1 and LAT2, which is governed by LAT isoform substrate preference; this can vary by orders of magnitude between LAT1 and LAT2 for the same amino acid (Rossier et al. 1999). Whilst the CD98/LAT1 variant of system L transports neutral amino acids with large side chains and generally exhibits higher substrate affinity (Rossier et al. 1999), CD98/LAT2 has broader substrate selectivity and accepts smaller neutral amino acids (Pineda et al. 1999). We therefore speculate that the two kinetically distinguishable components of [35S]l–Hcy uptake might represent transport by CD98/LAT1 and CD98/LAT2 heterodimers, respectively. The ability of dl–Hcy to inhibit [35S]l–Met uptake into MVM vesicles with the same efficacy as l–Hcy, and the profoundly greater ability of d-amino acids to inhibit LAT1 over LAT2 (Rajan et al. 2000) strongly implicates the involvement of LAT1. The notion that LAT2 is involved in Hcy transport across MVM would have been greatly bolstered by the positive identification of LAT2 in MVM, but such investigations have been hampered by poor antibody specificity (Lewis et al. 2007).
Our demonstration of a Hcy-sensitive Arg transporter that exhibited a significant reduction in its affinity for Hcy when Na+ was replaced by K+, and which showed a similar inhibitory capacity to other neutral amino acids at high concentration, led to the designation of this transport mechanism as y+L (Eleno et al. 1994; Devés et al. 1998; Devés & Boyd, 1998). The magnitude of the Ki shift for l–Met found here in MVM (5.8-fold), compares favourably to that of other studies with l–Gln (5-fold; Eleno et al. 1994) providing confidence in this attribution. System y+L activity demonstrated substrate stereoselectivity, preferring l–Hcy over d-Hcy, as judged by the smaller magnitude of the observed Ki shift for dl–Hcy and the lower inhibitory capacity of dl–Hcy compared to other l–amino acids substrates of system y+L over a broad concentration range, entirely consistent with system y+L activity (Devés et al. 1998; Devés & Boyd, 1998). Whilst l–Hcy was found to be an effective inhibitor of system y+L activity, measured here as neutral amino acid-sensitive [3H]l–Arg uptake into MVM vesicles, the reciprocal experiment revealed that 20 mm l–Arg was only able to inhibit ∼19% of [35S]l–Hcy transport. We attribute this to the overlying action of system y+ activity which makes a major contribution to l–Arg transport across MVM, as shown here by the neutral amino acid-resistant component (Fig. 4) and characterised previously (Ayuk et al. 2000). Our demonstration that system y+L mediates Hcy transport across MVM agrees with the observation of others in platelets demonstrating Hcy inhibition of a Leu-sensitive, Arg-transporting component, taken to be system y+L activity (Brunini et al. 2003; Leoncini et al. 2003). The co-expression of y+LAT1 and y+LAT2 mRNA in human placenta (Dye et al. 2004) allows for the possibility that either of these light chain variants form a heterodimeric complex with CD98 to mediate system y+L activity and transport l–Hcy. Both y+LAT1 and y+LAT2 exhibit a strong preference for l–Met, l–Gln and l–Leu (Pfeiffer et al. 1999; Bröer et al. 2000), precluding their functional delineation using these amino acid substrates.
The collective evidence presented here reveals that, as for other amino acids, l–Hcy is transported by multiple transport mechanisms across the MVM of human placenta. It is of particular interest that systems L, A and y+L all support l–Hcy transport in MVM, as the latter two transport mechanisms may work in parallel to provide system L with amino acid substrates for exchange (Verrey, 2003). However, of these three mechanisms, system L-mediated l–Hcy influx is likely to be of prime importance at maternal physiological concentrations of l–Hcy. This notion is proposed based on our evidence that this transport mechanism makes the major contribution to MVM uptake of 10 μm l–Hcy with the kinetic discrimination a high-affinity (∼72 μm) transport component ascribed to system L. The predominance of a particular transport mechanism for l–Hcy appears to be governed by cell type (Naggar et al. 2003; Jiang et al. 2007), as does the apparent Km for the same transport mechanism. Hence, the Km for system l–mediated Hcy transport in human aortic endothelial cells is 19 μm (Büdy et al. 2006) whilst that in human umbilical vein endothelial cell (HUVEC) is 160 μm (Ewadh et al. 1990). It is noteworthy that the Km value for the high-affinity component observed here falls within this range.
There are important pathophysiological implications of these data. The scenario that Hcy is transported by, or accumulates within, the syncytiotrophoblast raises the possibility that Hcy could impact on syncytiotrophoblast function in several ways: reduction in the provision of amino acids to the developing fetus by competing with other substrates of systems L, A and y+L; perturbation of placental metabolism; altered vascular function; and induction of apoptosis. Indeed, the concept that Hcy is transported across the MVM into the syncytiotrophoblast is supported by the observation that Hcy inhibits [14C]MeAIB uptake into placental villous fragments (Tsitsiou et al. 2008).
System L activity in MVM would be particularly susceptible to perturbations in maternal Hcy status based on the evidence presented here. As system L provides essential amino acids for both fetal development and placental metabolism, with placental supply of essential amino acids only just matching fetal demand for protein synthesis (Chien et al. 1993), such an interaction is likely to impact on fetal growth. This concept is compatible with the pre-disposition to fetal growth restriction and a lower birth weight in mothers with HHcy (Vollset et al. 2000). Furthermore, as systems L and A activities in MVM are reduced in association with fetal growth restriction (Jansson & Powell, 2007), maternal HHcy would serve to further exacerbate the situation by restricting amino acid supply.
In summary, this study has shown that systems L, A and y+L each support Hcy as a substrate to mediate transport of this amino acid across MVM of human syncytiotrophoblast. System L activity predominated with kinetic discrimination of a high-affinity and low-affinity transport component that was independent of an inwardly directed Na+ gradient across MVM. The involvement of these three amino acid transport mechanisms suggests that in pregnancies compromised by maternal HHcy, Hcy competes with endogenous amino acid substrates for transporter activity, thereby diminishing placental amino acid influx and provision of amino acids to the developing fetus.
Acknowledgments
This work was supported by the Medical Research Council (MRC) (G0500647; J.D.G., S.W.D.'S., C.P.S.) and a MRC Doctoral Training Studentship (E.T.). This work was also supported by Grant HL52234 from the National Heart, Lung and Blood Institute of the National Institutes of Health (D.W.J.). The Maternal and Fetal Health Research Group is supported by the Manchester NIHR Biomedical Research Centre. We extend our grateful thanks to Professor Jonathan Clayden and Mr Lee Mullen (Department of Chemistry, University of Manchester) for the synthesis of l–Hcy. We would also like to thank Professor Mike Taggart and Dr Austin Elliot for their academic input and Ms Alicia Requena Jimenez for technical support. We are also very grateful to Dr W Bardsley for his advice concerning the kinetic modelling of the data. We thank the midwives and medical staff at St Mary's Hospital, Manchester, for their help in obtaining placentas. The authors declare no conflict of interest.
Glossary
Abbreviations
- Ala
l–alanine
- Arg
l–arginine
- BCH
2-aminobicyclo[2.2.1]heptane-2-carboxylic acid
- EVB
extravesicular buffer
- Gln
l–glutamine
- Hcy
homocysteine
- HHcy
hyperhomocysteinaemia
- Leu
l–leucine
- Lys
l–lysine
- MeAIB
α-(methylamino)isobutyric acid
- Met
l–methionine
- MVM
microvillous plasma membrane
- Ser
l–serine
- SNAT
sodium-coupled neutral amino acid transporter
- tHcy
total Hcy
Author contributions
E.T., C.P.S., S.W.D'S., D.W.J., J.D.G. designed research. E.T., O.C., J.D.G. performed research. E.T., C.P.S., J.D.G. analysed data. E.T., C.P.S., S.W.D'S., D.W.J., J.G. wrote paper.
References
- Ayuk PT, Sibley CP, Donnai P, D'Souza S, Glazier JD. Development and polarization of cationic amino acid transporters and regulators in the human placenta. Am J Physiol Cell Physiol. 2000;278:C1162–C1171. doi: 10.1152/ajpcell.2000.278.6.C1162. [DOI] [PubMed] [Google Scholar]
- Bodoy S, Martin L, Zorzano A, Palacin M, Estevez R, Bertran J. Identification of LAT4, a novel amino acid transporter with system L activity. J Biol Chem. 2005;280:12002–12011. doi: 10.1074/jbc.M408638200. [DOI] [PubMed] [Google Scholar]
- Bröer A, Wagner CA, Lang F, Bröer S. The heterodimeric amino acid transporter 4F2hc/y+LAT2 mediates arginine efflux in exchange with glutamine. Biochem J. 2000;349:787–795. doi: 10.1042/bj3490787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunini TM, Yaqoob MM, Novaes Malagris LE, Ellory JC, Mann GE, Mendes Ribeiro AC. Increased nitric oxide synthesis in uraemic platelets is dependent on l–arginine transport via system y+L. Pflugers Arch. 2003;445:547–550. doi: 10.1007/s00424-002-0978-7. [DOI] [PubMed] [Google Scholar]
- Büdy B, O’Neill R, DiBello PM, Sengupta S, Jacobsen DW. Homocysteine transport by human aortic endothelial cells: identification and properties of import systems. Arch Biochem Biophys. 2006;446:119–130. doi: 10.1016/j.abb.2005.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Chien PF, Smith K, Watt PW, Scrimgeour CM, Taylor DJ, Rennie MJ. Protein turnover in the human fetus studied at term using stable isotope tracer amino acids. Am J Physiol Endocrinol Metab. 1993;265:E31–E35. doi: 10.1152/ajpendo.1993.265.1.E31. [DOI] [PubMed] [Google Scholar]
- Cleal JK, Lewis RM. The mechanisms and regulation of placental amino acid transport to the human foetus. J Neuroendocrinol. 2008;20:419–426. doi: 10.1111/j.1365-2826.2008.01662.x. [DOI] [PubMed] [Google Scholar]
- Cline DJ, Redding SE, Brohawn SG, Psathas JN, Schneider JP, Thorpe C. New water-soluble phosphines as reductants of peptide and protein disulfide bonds: reactivity and membrane permeability. Biochemistry. 2004;43:15195–15203. doi: 10.1021/bi048329a. [DOI] [PubMed] [Google Scholar]
- de la Calle M, Usandizaga R, Sancha M, Magdaleno F, Herranz A, Cabrillo E. Homocysteine, folic acid and B-group vitamins in obstetrics and gynaecology. Eur J Obstet Gynecol Reprod Biol. 2003;107:125–134. doi: 10.1016/s0301-2115(02)00305-6. [DOI] [PubMed] [Google Scholar]
- Desforges M, Mynett KJ, Jones RL, Greenwood SL, Westwood M, Sibley CP, Glazier JD. The SNAT4 isoform of the system A amino acid transporter is functional in human placental microvillous plasma membrane. J Physiol. 2009;587:61–72. doi: 10.1113/jphysiol.2008.161331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devés R, Angelo S, Rojas AM. System y+L: the broad scope and cation modulated amino acid transporter. Exp Physiol. 1998;83:211–220. doi: 10.1113/expphysiol.1998.sp004105. [DOI] [PubMed] [Google Scholar]
- Devés R, Boyd CA. Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol Rev. 1998;78:487–545. doi: 10.1152/physrev.1998.78.2.487. [DOI] [PubMed] [Google Scholar]
- Duerre JA, Miller CH. Preparation of L–homocysteine from L–homocysteine thiolactone. Anal Biochem. 1966;17:310–315. doi: 10.1016/0003-2697(66)90209-0. [DOI] [PubMed] [Google Scholar]
- Dye JF, Vause S, Johnston T, Clark P, Firth JA, D'Souza SW, Sibley CP, Glazier JD. Characterization of cationic amino acid transporters and expression of endothelial nitric oxide synthase in human placental microvascular endothelial cells. FASEB J. 2004;18:125–127. doi: 10.1096/fj.02-0916fje. [DOI] [PubMed] [Google Scholar]
- Eleno N, Deves R, Boyd CA. Membrane potential dependence of the kinetics of cationic amino acid transport systems in human placenta. J Physiol. 1994;479:291–300. doi: 10.1113/jphysiol.1994.sp020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewadh MJ, Tudball N, Rose FA. Homocysteine uptake by human umbilical vein endothelial cells in culture. Biochim Biophys Acta. 1990;1054:263–266. doi: 10.1016/0167-4889(90)90097-w. [DOI] [PubMed] [Google Scholar]
- Ganapathy ME, Leibach FH, Mahesh VB, Howard JC, Devoe LD, Ganapathy V. Characterization of tryptophan transport in human placental brush-border membrane vesicles. Biochem J. 1986;238:201–208. doi: 10.1042/bj2380201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier JD, Jones CJ, Sibley CP. Purification and Na+ uptake by human placental microvillus membrane vesicles prepared by three different methods. Biochim Biophys Acta. 1988;945:127–134. doi: 10.1016/0005-2736(88)90475-0. [DOI] [PubMed] [Google Scholar]
- Goddijn-Wessel TA, Wouters MG, van de Molen EF, Spuijbroek MD, Steegers-Theunissen RP, Blom HJ, Boers GH, Eskes TK. Hyperhomocysteinemia: a risk factor for placental abruption or infarction. Eur J Obstet Gynecol Reprod Biol. 1996;66:23–29. doi: 10.1016/0301-2115(96)02383-4. [DOI] [PubMed] [Google Scholar]
- Hatanaka T, Huang W, Wang H, Sugawara M, Prasad PD, Leibach FH, Ganapathy V. Primary structure, functional characteristics and tissue expression pattern of human ATA2, a subtype of amino acid transport system A. Biochim Biophys Acta. 2000;1467:1–6. doi: 10.1016/s0005-2736(00)00252-2. [DOI] [PubMed] [Google Scholar]
- Hultberg B. Extracellular concentration of homocysteine in human cell lines is influenced by specific inhibitors of cyst(e)ine transport. Clin Chem Lab Med. 2004;42:378–383. doi: 10.1515/CCLM.2004.067. [DOI] [PubMed] [Google Scholar]
- Jansson T. Amino acid transporters in the human placenta. Pediatr Res. 2001;49:141–147. doi: 10.1203/00006450-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond) 2007;113:1–13. doi: 10.1042/CS20060339. [DOI] [PubMed] [Google Scholar]
- Jiang X, Yang F, Brailoiu E, Jakubowski H, Dun NJ, Schafer AI, Yang X, Durante W, Wang H. Differential regulation of homocysteine transport in vascular endothelial and smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1976–1983. doi: 10.1161/ATVBAHA.107.148544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LW, Smith CH. Neutral amino acid transport systems of microvillous membrane of human placenta. Am J Physiol Cell Physiol. 1988;254:C773–C780. doi: 10.1152/ajpcell.1988.254.6.C773. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CA. Heterodimeric amino acid transporters: expression of heavy but not light chains of CD98 correlates with induction of amino acid transport systems in human placental trophoblast. J Physiol. 2000;523:13–18. doi: 10.1111/j.1469-7793.2000.t01-1-00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y, Boyd CA. Characterisation of l–tryptophan transporters in human placenta: a comparison of brush border and basal membrane vesicles. J Physiol. 2001;531:405–416. doi: 10.1111/j.1469-7793.2001.0405i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoncini G, Pascale R, Signorello MG. Effects of homocysteine on l-arginine transport and nitric oxide formation in human platelets. Eur J Clin Invest. 2003;33:713–719. doi: 10.1046/j.1365-2362.2003.01203.x. [DOI] [PubMed] [Google Scholar]
- Lewis RM, Glazier J, Greenwood SL, Bennett EJ, Godfrey KM, Jackson AA, Sibley CP, Cameron IT, Hanson MA. L–serine uptake by human placental microvillous membrane vesicles. Placenta. 2007;28:445–452. doi: 10.1016/j.placenta.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Limpach A, Dalton M, Miles R, Gadson P. Homocysteine inhibits retinoic acid synthesis: a mechanism for homocysteine-induced congenital defects. Exp Cell Res. 2000;260:166–174. doi: 10.1006/excr.2000.5000. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch. 2004;447:784–795. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- Mahendran D, Donnai P, Glazier JD, D'Souza SW, Boyd RD, Sibley CP. Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res. 1993;34:661–665. doi: 10.1203/00006450-199311000-00019. [DOI] [PubMed] [Google Scholar]
- Malinow MR, Rajkovic A, Duell PB, Hess DL, Upson BM. The relationship between maternal and neonatal umbilical cord plasma homocyst(e)ine suggests a potential role for maternal homocyst(e)ine in fetal metabolism. Am J Obstet Gynecol. 1998;178:228–233. doi: 10.1016/s0002-9378(98)80005-7. [DOI] [PubMed] [Google Scholar]
- Meier C, Ristic Z, Klauser S, Verrey F. Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J. 2002;21:580–589. doi: 10.1093/emboj/21.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy AM, Mills JL, McPartlin J, Kirke PN, Scott JM, Daly S. Maternal and fetal plasma homocysteine concentrations at birth: the influence of folate, vitamin B12, and the 5,10-methylenetetrahydrofolate reductase 677C→T variant. Am J Obstet Gynecol. 2002;186:499–503. doi: 10.1067/mob.2002.121105. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Scott JM, Arija V, Molloy AM, Fernandez-Ballart JD. Maternal homocysteine before conception and throughout pregnancy predicts fetal homocysteine and birth weight. Clin Chem. 2004;50:1406–1412. doi: 10.1373/clinchem.2004.032904. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Scott JM, McPartlin JM, Fernandez-Ballart JD. The pregnancy-related decrease in fasting plasma homocysteine is not explained by folic acid supplementation, hemodilution, or a decrease in albumin in a longitudinal study. Am J Clin Nutr. 2002;76:614–619. doi: 10.1093/ajcn/76.3.614. [DOI] [PubMed] [Google Scholar]
- Naggar H, Fei YJ, Ganapathy V, Smith SB. Regulation of reduced-folate transporter-1 (RFT-1) by homocysteine and identity of transport systems for homocysteine uptake in retinal pigment epithelial (RPE) cells. Exp Eye Res. 2003;77:687–697. doi: 10.1016/j.exer.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Sakata M, Ogura K, Yamamoto T, Yamaguchi M, Tasaka K, Kurachi H, Tsurudome M, Murata Y. Expression and regulation of 4F2hc and hLAT1 in human trophoblasts. Am J Physiol Cell Physiol. 2002;282:C196–C204. doi: 10.1152/ajpcell.2002.282.1.C196. [DOI] [PubMed] [Google Scholar]
- Pfeiffer R, Rossier G, Spindler B, Meier C, Kuhn L, Verrey F. Amino acid transport of y+l–type by heterodimers of 4F2hc/CD98 and members of the glycoprotein-associated amino acid transporter family. EMBO J. 1999;18:49–57. doi: 10.1093/emboj/18.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda M, Fernandez E, Torrents D, Estevez R, Lopez C, Camps M, Lloberas J, Zorzano A, Palacin M. Identification of a membrane protein, LAT-2, that co-expresses with 4F2 heavy chain, an L–type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J Biol Chem. 1999;274:19738–19744. doi: 10.1074/jbc.274.28.19738. [DOI] [PubMed] [Google Scholar]
- Quéré I, Mercier E, Bellet H, Janbon C, Marès P, Gris JC. Vitamin supplementation and pregnancy outcome in women with recurrent early pregnancy loss and hyperhomocysteinemia. Fertil Steril. 2001;75:823–825. doi: 10.1016/s0015-0282(01)01678-8. [DOI] [PubMed] [Google Scholar]
- Rajan DP, Kekuda R, Huang W, Devoe LD, Leibach FH, Prasad PD, Ganapathy V. Cloning and functional characterization of a Na+-independent, broad-specific neutral amino acid transporter from mammalian intestine. Biochim Biophys Acta. 2000;1463:6–14. doi: 10.1016/s0005-2736(99)00224-2. [DOI] [PubMed] [Google Scholar]
- Ray JG, Laskin CA. Folic acid and homocyst(e)ine metabolic defects and the risk of placental abruption, pre-eclampsia and spontaneous pregnancy loss: A systematic review. Placenta. 1999;20:519–529. doi: 10.1053/plac.1999.0417. [DOI] [PubMed] [Google Scholar]
- Refsum H, Nurk E, Smith AD, Ueland PM, Gjesdal CG, Bjelland I, Tverdal A, Tell GS, Nygard O, Vollset SE. The Hordaland Homocysteine Study: a community-based study of homocysteine, its determinants, and associations with disease. J Nutr. 2006;136:1731S–1740S. doi: 10.1093/jn/136.6.1731S. [DOI] [PubMed] [Google Scholar]
- Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- Rossier G, Meier C, Bauch C, Summa V, Sordat B, Verrey F, Kuhn LC. LAT2, a new basolateral 4F2hc/CD98-associated amino acid transporter of kidney and intestine. J Biol Chem. 1999;274:34948–34954. doi: 10.1074/jbc.274.49.34948. [DOI] [PubMed] [Google Scholar]
- Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- Selhub J. The many facets of hyperhomocysteinemia: studies from the Framingham cohorts. J Nutr. 2006;136:1726S–1730S. doi: 10.1093/jn/136.6.1726S. [DOI] [PubMed] [Google Scholar]
- Shikano N, Nakajima S, Kotani T, Ogura M, Sagara J, Iwamura Y, Yoshimoto M, Kubota N, Ishikawa N, Kawai K. Transport of D-[1-14C]-amino acids into Chinese hamster ovary (CHO-K1) cells: implications for use of labeled d-amino acids as molecular imaging agents. Nucl Med Biol. 2007;34:659–665. doi: 10.1016/j.nucmedbio.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Shiraiwa T, Nakagawa K, Kanemoto N. Facile synthesis of optically active homocysteine from methionine. Chem Lett. 2000:468–469. doi: 10.1248/cpb.50.1081. [DOI] [PubMed] [Google Scholar]
- Torrents D, Estévez R, Pineda M, Fernández E, Lloberas J, Shi YB, Zorzano A, Palacín M. Identification and characterization of a membrane protein (y+L amino acid transporter-1) that associates with 4F2hc to encode the amino acid transport activity y+L. A candidate gene for lysinuric protein intolerance. J Biol Chem. 1998;273:32437–32445. doi: 10.1074/jbc.273.49.32437. [DOI] [PubMed] [Google Scholar]
- Tsitsiou E, Greenwood SL, Sibley CP, D'Souza SW, Glazier JD. Homocysteine inhibition of system A amino acid transporter activity in human placenta. Reprod Sci. 2008;15:91A. [Google Scholar]
- Verrey F. System L: heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch. 2003;445:529–533. doi: 10.1007/s00424-002-0973-z. [DOI] [PubMed] [Google Scholar]
- Vollset SE, Refsum H, Irgens LM, Emblem BM, Tverdal A, Gjessing HK, Monsen AL, Ueland PM. Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine study. Am J Clin Nutr. 2000;71:962–968. doi: 10.1093/ajcn/71.4.962. [DOI] [PubMed] [Google Scholar]
- Wang H, Huang W, Sugawara M, Devoe LD, Leibach FH, Prasad PD, Ganapathy V. Cloning and functional expression of ATA1, a subtype of amino acid transporter A, from human placenta. Biochem Biophys Res Commun. 2000;273:1175–1179. doi: 10.1006/bbrc.2000.3061. [DOI] [PubMed] [Google Scholar]