Abstract
Taste cells use multiple signalling mechanisms to generate unique calcium responses to distinct taste stimuli. Some taste stimuli activate G-protein coupled receptors (GPCRs) that cause calcium release from intracellular stores while other stimuli depolarize taste cells to cause calcium influx through voltage-gated calcium channels (VGCCs). We recently demonstrated that a constitutive calcium influx exists in taste cells that is regulated by mitochondrial calcium transport and that the magnitude of this calcium influx correlates with the signalling mechanisms used by the taste cells. In this study, we used calcium imaging to determine that sodium–calcium exchangers (NCXs) also routinely contribute to the regulation of basal cytosolic calcium and that their relative role correlates with the signalling mechanisms used by the taste cells. RT-PCR analysis revealed that multiple NCXs and sodium–calcium–potassium exchangers (NCKXs) are expressed in taste cells. Thus, a dynamic relationship exists between calcium leak channels and calcium regulatory mechanisms in taste cells that functions to keep cytosolic calcium levels in the appropriate range for cell function.
Activation of taste receptors on the apical end of taste cells in the oral cavity is the first step in the detection of gustatory stimuli. Ionic taste stimuli (sour and salty) directly activate ligand-gated receptors to cause cell depolarization and calcium influx through voltage-gated calcium channels (VGCCs). The more chemically complex taste stimuli (bitter, sweet and umami) activate a G-protein coupled receptor (GPCR) pathway that depends on calcium release from internal stores. Taste cells that use the GPCR signalling pathway do not express VGCCs and lack conventional, chemical synapses (Medler et al. 2003; Clapp et al. 2006; DeFazio et al. 2006). Instead, these taste cells rely on the opening of hemichannels to directly release neurotransmitter onto afferent gustatory neurons (Huang et al. 2007; Romanov et al. 2007, 2008).
Strong evidence indicates that taste cells expressing VGCCs have conventional chemical synapses and can therefore be identified as type III or presynaptic taste cells (Kinnamon et al. 1985, 1988; Finger & Simon, 2000; Yang et al. 2000a; Yee et al. 2001; Medler et al. 2003; DeFazio et al. 2006; Tomchik et al. 2007). Taste cells that use GPCR signalling and rely on calcium release from internal stores have also been labelled as type II or receptor cells (Finger & Simon, 2000; Yang et al. 2000b; Medler et al. 2003; Clapp et al. 2004, 2006; DeFazio et al. 2006; Tomchik et al. 2007). Within the context of this study, we grouped taste cells based on their reliance of calcium influx through VGCCs or calcium release from internal calcium stores to generate an output signal. These calcium signalling mechanisms appear to approximately correlate with other cell classification systems that currently exist within the taste field.
While these two taste cell populations use different signalling pathways and generate significantly different calcium responses (DeFazio et al. 2006; Hacker et al. 2008), they both must increase cytosolic calcium to produce normal synaptic signals (Medler et al. 2003; Richter et al. 2003; DeFazio et al. 2006; Huang et al. 2007; Romanov et al. 2007). As a result, all transducing taste cells must tightly regulate intracellular calcium levels; however, our current understanding of how cytosolic calcium is regulated in taste cells is lacking.
We recently reported that mitochondria make significant contributions to the regulation of cytosolic calcium in taste cells even in the absence of other stimuli (Hacker & Medler, 2008). Unlike most cells, taste cells have a constitutive calcium influx across the plasma membrane which is in part regulated by the calcium transport capabilities of the mitochondria. However, it is unlikely that mitochondria are solely responsible for the regulation of this calcium influx since mitochondria primarily function as temporary calcium stores. Ultimately, the calcium must be extruded out of the taste cell to maintain physiologically low calcium levels inside the cell. We hypothesized that calcium extrusion mechanisms, specifically sodium–calcium exchangers, likely contribute to the regulation of cytosolic calcium in taste cells.
We isolated mouse taste receptor cells and recorded cytosolic calcium changes with fura 2-AM. We replaced external sodium with lithium to inhibit sodium–calcium exchanger (NCX) activity (Blaustein & Santiago, 1977) and found that in un-stimulated taste cells, inhibiting the NCX activity caused an increase in cytosolic calcium levels. RT-PCR analysis of mRNA from isolated taste buds revealed the presence of multiple NCXs, including potassium-dependent sodium–calcium exchangers (NCKXs). This study provides the first evidence that NCXs contribute to the regulation of cytosolic calcium levels in taste cells and provides a new understanding of how taste cells routinely regulate and maintain cytosolic calcium at physiologically low levels.
Methods
Taste cell isolation
Taste buds were harvested from circumvallate (CV) and foliate (Fol) papillae of C57BL/6 mice (n= 49). Mice were killed with CO2 and cervical dislocation. Mice were cared for in compliance with the University at Buffalo Animal Care and Use Committee procedures. Tongues were removed and then injected under the lingual epithelium with 100 μl of an enzymatic solution containing 0.5 mg of collagenase B (Roche, Indianapolis, IN, USA), 3 mg of dispase II (Roche), and 1 mg of trypsin inhibitor (Sigma, St Louis, MO, USA) per millilitre of Tyrode solution (140 mm NaCl, 5 mm KCl, 1 mm MgCl2, 3 mm CaCl2, 10 mm Hepes, 10 mm glucose and 1 mm pyruvic acid, pH 7.4). Tongues were incubated with oxygenated Tyrode solution for 20 min before the epithelial layer was peeled off and incubated in Ca2+-free Tyrode solution (140 mm NaCl, 5 mm KCl, 10 mm Hepes, 2 mm BAPTA, 10 mm glucose and 1 mm pyruvic acid, adjusted to pH 7.4) for 30 min. Foliate and circumvallate taste cells were removed with a capillary pipette using gentle suction and were identified based on their characteristic morphology (Hacker et al. 2008).
Calcium imaging
Isolated taste cells from the CV and Fol papillae were plated onto coverslips pre-coated with Cell Tak (BD Bioscience, San Jose, CA, USA). Cells were loaded with 2 μm fura 2-AM (Molecular Probes, Invitrogen) containing the non-ionic dispersing agent Pluronic F-127 (Molecular Probes, Invitrogen). Loaded cells were visualized using an Olympus IX71 microscope with 40× oil immersion lens and images were captured using a SensiCam QE camera (Cooke Corporation, Romulus, MI, USA). Excitation wavelengths of 340 and 380 nm were used with an emission wavelength of 510 nm. Images were collected every 4 s using Imaging Workbench 5.2 (Indec Biosystems, Santa Clara, CA, USA). Since our shuttering rate was relatively slow, a faster sampling (0.5 s) was done on a subset of cells to determine if we were accurately measuring the peak fluorescence increases. There was no difference in the peak amplitude of the response to stimulus in taste cells when images were captured every 4 s and when the images were captured every 0.5 s (n= 18, P= 0.12, online Supplemental Fig. S1), indicating that peak fluorescence responses could accurately be measured at the slower sampling rate. Faster capture rates sometimes damaged the taste cells and impaired the cell's ability to recover from a stimulus (see Supplemental Fig. S1), so we used the slower sampling rate for these experiments to maximize the available time frame of the experiments. During experiments, cells were kept under constant perfusion with Tyrode's solution followed by alternating changes in solutions. All solutions were bath applied using a gravity flow perfusion system (Automate Scientific, San Francisco, CA, USA) and laminar flow perfusion chambers (RC-25F, Warner Scientific, Hamden, CT, USA). Control experiments determined that there is approximately a 3 s delay between the onset of stimulus application and the stimulus contact with the target cell in our experimental set-up. Stimulus application time is reported in the figures with no compensation for the delay due to stimulus delivery. An evoked response was defined as an increase in fluorescence that was greater than two standard deviations above baseline. Data were plotted in graphs without any filtering and statistically analysed using OriginPro 7.5 software. The amplitude of the cytosolic calcium elevations were calculated as [(peak − baseline)/baseline]× 100 and were reported as percent increases over baseline. In most experiments, we compared the percent increases over baseline in order to not confound the data due to differences in baseline calcium values. Statistical comparisons were made using a one-way ANOVA with a Bonferroni's post hoc analysis or Student's t test, either independent or paired as appropriate. The limit of significance was set at P < 0.05.
Data were collected as a ratio of fluorescence intensities. All fluorescence values were calibrated using the fura 2-AM calcium imaging calibration kit (Invitrogen). The effective dissociation constant, Kd, was calculated to be 253 nm and calcium concentrations were determined using the formula outlined by Grynkiewicz et al. (1985). Most taste cells had baseline calcium values ranging from 50 nm to 150 nm. As this is a primary preparation and taste cells are enzymatically dissociated from each other, the cells can sometimes become damaged in this process. In order to reduce the potential of analysing damaged cells that could confound our results, taste cells with baseline values higher than 200 nm were deemed to be unhealthy and were not included in the analysis. There were a total of 18 cells out of 164 tested cells (approximately 11%) that were excluded from our study because of high resting baseline calcium levels. While this cut-off value was somewhat arbitrary, 200 nm was approximately twice the average baseline calcium value which we felt was a reasonable, conservative cut-off value for our analysis. It is possible that taste cells with higher baseline calcium values are a specific subset of taste cells that have distinct properties from other taste cells; however, for this study these data points were not included in the analyses.
Solutions
All chemicals were purchased from Sigma unless otherwise noted. We used each of the following solutions: bitter (10 mm denatonium benzoate in Tyrode's solution), high-K+ (Tyrode's solution replacing 50 mm NaCl with 50 mm KCl), lithium Tyrode's (Tyrode's solution with LiCl2 substituted for NaCl2), NMG Tyrode's (Tyrode's solution with N-methyl-d-glucamine substituted for Na+). Additional solutions were made by dilution with Tyrode's or lithium Tyrode's: 5 μm or 100 μm KB-R7943 (Tocris, Ellisville, MO, USA), 50 μm SN-6 (Tocris), 1 μm FCCP (Tocris) and 10 μm SB-366791 (Tocris).
RT-PCR analysis
Isolated taste buds were manually collected by gentle suction as described above and placed in lysis buffer from the RNeasy Mini Kit from Qiagen (Valencia, CA, USA). This procedure minimizes the potential contamination of the sample with non-taste cells. RNA was purified according to the manufacturer's instructions. Total RNA isolated from brain or retinal tissue was used as a positive control. PCR analysis for GAPDH was performed in a 50 μl reaction using 3 μl cDNA from each sample to determine sample quality and to check for genomic contamination. If any genomic DNA was present, the sample was discarded and a new sample was collected. cDNA from isolated taste buds and the appropriate control tissue were tested for the expression of NCX and NCKX isoforms. Previously published primers were used to check for the presence of NCKX1 (Poon et al. 2000), NCKX2 (Wang & Seed, 2003) and NCKX6 (Cai & Lytton, 2004). NCX, NCKX3 and NCKX4 primers were designed with the Oligo software (version 6.71) to check for the presence of NCX1 (5′GGGGATGATCATTGAACAT and 3′CCCATCTAAGAAATTGTCAAC), NCX2 (5′ATGGTGCCAGTCGCATCTT and 3′GTCCTCGGTACGGTAGTCCAC), NCX3 (5′TCATGTCAGTGAAAGTATTGG and 3′GGCTGTCCCTTCTACTGTC), NCKX3 (5′ATCTCATTGGGGAAGATAGAA and 3′AATGGCCAGCGCATAG) and NCKX4 (5′AGCCCTTTACCCTTTACCCTA and 3′GATTCACAACATTAGCCGAATAAC). The identities of all PCR products were confirmed by DNA sequencing.
Results
Responses to inhibiting sodium–calcium exchangers were variable
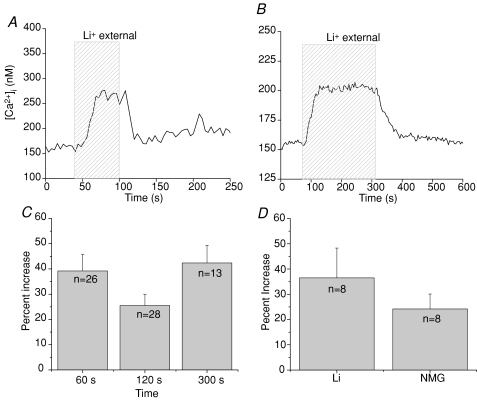
Isolated taste receptor cells were loaded with fura 2-AM and changes in cytosolic calcium were measured when external sodium was replaced with lithium. The loss of external sodium inhibits the extrusion of cytosolic calcium by NCXs on the plasma membrane because lithium cannot substitute for sodium in these transporters. However, the lithium replacement of sodium in the external solution does not affect the activity of sodium channels because lithium can substitute for sodium in these channels (Blaustein & Santiago, 1977). Therefore, replacing sodium with lithium allows for the selective inhibition of NCX activity. In taste cells, removing external sodium but not stimulating the cell in any other way, caused an increase in cytosolic calcium in approximately 92% of taste cells tested (n= 146, Fig. 1A). The amplitude of the calcium increases that occurred in response to removing external sodium varied from just 3.9% over baseline calcium levels up to a 406% increase over basal calcium (max. peak value = 482 nm). The average percent increase in cytosolic calcium when external sodium was removed was 69% over baseline values (average peak value = 163 nm, sderr = 6.8 nm, n= 135).
Figure 1. Removing external sodium affects cytosolic calcium in most taste cells.
A, replacing external sodium with lithium (hatched area, 60 s) caused a reversible increase in cytosolic calcium levels in 92% of taste cells tested (n= 146). B, the prolonged removal of external sodium (4 min, hatched area) caused a sustained increase in cytosolic calcium that was maintained until sodium was replaced in the external solution and the exchangers were again functional. C, the amplitude of the lithium-dependent calcium elevation was not dependent on the length of time that sodium was removed. Comparisons between removing external sodium for 60 s, 120 s or 300 s revealed no significant differences using one-way ANOVA in the amplitude of the measured calcium response (P= 0.11). Data were plotted as average percent increase (with standard error) over baseline calcium values. D, substituting NMG for sodium in the external solution exerted similar effects compared to replacing external sodium with lithium in the same cells. Analysis using a paired Student's t test found no significant differences between the effects of these two substitutes for external sodium on the resulting elevation in cytosolic calcium levels (P= 0.296, n= 8).
Cytosolic calcium remained elevated as long as external sodium was absent and exchanger function was inhibited. In Fig. 1B, external sodium was replaced with lithium for 240 s (4 min) during which time the cytosolic calcium increased from a baseline value of 150 nm to a peak value of 200 nm in this cell. Cytosolic calcium levels remained elevated until sodium was returned to the external solution and the NCXs were again functional. We determined that the amplitudes of the cytosolic calcium elevations were not dependent on the length of time that external sodium was absent from the cells. A one-way ANOVA and post hoc Bonferroni's test were performed to detect any differences in the size of the calcium responses from taste cells with NCXs inhibited for three different time periods (Fig. 1C) but no significant differences were detected (P= 0.11). Therefore, these cytosolic calcium elevations do not tend to increase indefinitely as long as the exchangers are inhibited, but rather appear to have a discrete calcium response that plateaus until the NCXs are again functional and the cytosolic calcium can decrease back to baseline levels.
We also compared the effects of replacing external sodium with either the impermeant cation N-methyl-d-glucamine (NMG) or lithium on the same taste cells using a Student's paired t test. Both conditions caused comparable changes in cytosolic calcium levels (Fig. 1D, P= 0.296, n= 8). This indicates that the elevated cytosolic calcium that occurred in the lithium external solution was due to the removal of external sodium which inhibits exchanger activity, rather than some non-specific effect of lithium.
In Supplementary Fig. S2, we used linear regression to determine if there were any correlative relationships that could explain the variability in the amplitudes of the responses. We found that peak calcium values positively correlated with baseline calcium values with a R2 value of 0.7 (P < 0.001) while the percent change in cytosolic calcium over baseline negatively correlated with increasing baseline calcium values (R2=−0.26, P < 0.001). When resting calcium values are higher, the amplitude of the calcium response due to NCX inhibition is smaller even though the absolute value of the peak concentration increases. This suggests that the cell uses alternate mechanisms when NCXs are inhibited to prevent cytosolic calcium from increasing indefinitely and thus avoid the non-specific activation of calcium-dependent processes.
The amplitude of the lithium-dependent calcium increase correlates with calcium signalling mechanisms
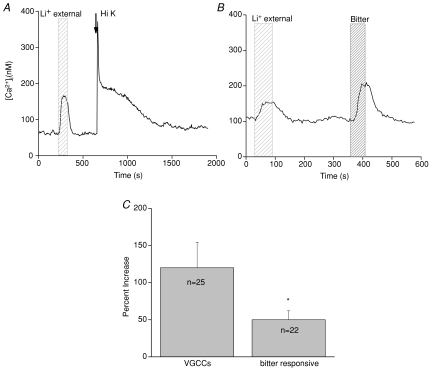
We next determined if the variability in the amplitude of the calcium elevations due to NCX inhibition correlated with the specific calcium signalling mechanisms employed by the taste cells (either calcium influx through VGCCs or stimulus-induced calcium release from internal stores). We recently demonstrated that activating different calcium signalling mechanisms in taste cells generates significantly different calcium responses and that these calcium signalling mechanisms were selectively expressed in separate taste cell populations (Hacker et al. 2008). For the current experiments, we identified the presence of a particular calcium signalling mechanism in the taste cell and measured the amplitude of the calcium elevations when NCXs were inhibited within these two populations of taste cells. The presence of VGCCs was determined by the taste cell's responsiveness to 50 mm KCl (Fig. 2A), while taste cells that depend on calcium release from internal stores were identified by their sensitivity to denatonium benzoate, a bitter compound (Fig. 2B). Since we did not test cells for their responsiveness to all tastants that depend on calcium release from internal stores (Zhang et al. 2003), our values may not reflect the calcium elevations due to NCX inhibition in all types of taste cells that depend on calcium release from internal stores. However, the goal of these experiments was only to determine if there were any differences in the amplitude of the cytosolic calcium responses when NCXs were inhibited that correlated with the calcium signalling mechanisms expressed in those taste cells.
Figure 2. Amplitudes of the lithium-induced calcium elevations varied significantly across different populations of taste cells.
The amplitude of the calcium elevation when external sodium was replaced with lithium was compared in different taste cell populations. A, a trace showing the calcium response when external sodium was replaced with lithium (wide-hatched area) in a taste cell that expresses VGCCs (arrow, Hi K). B, a trace showing the calcium response when external sodium was replaced with lithium (wide-hatched area) in a taste cell that is bitter sensitive (narrow-hatched area). C, taste cells that release calcium from internal stores in response to bitter taste stimuli but lack VGCCs had significantly smaller lithium-dependent calcium elevations compared to taste cells with VGCCs (Student's t test, *P < 0.05).
We found that taste cells which depend on calcium release from internal stores and which lack VGCCs had significantly smaller elevations in cytosolic calcium when NCXs were inhibited compared to taste cells that express VGCCs (Fig. 2C, P < 0.05). There were no significant differences between the baseline calcium levels for these two cell groups (average baseline for bitter responsive cells = 100 nm, average baseline for high K+-responsive cells = 97 nm, P= 0.83), indicating that these amplitude differences were not due to differences in baseline calcium levels. Instead, it is more likely that there are differences in the properties of the calcium leak channels or the calcium regulatory mechanisms that are contributing to the measured differences. Future studies are needed to address this question.
Lithium-induced cytosolic calcium increases are not due to reverse-mode NCX activity
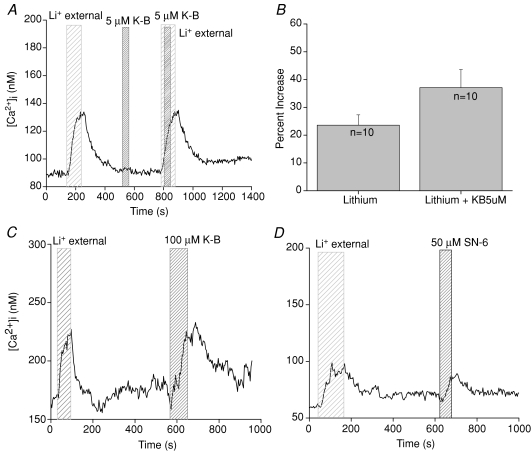
As removing external sodium was sufficient to cause an increase in intracellular calcium, it was possible that this elevated cytosolic calcium was due to the sodium–calcium exchangers functioning in reverse mode due to the change in the sodium gradient across the plasma membrane. Since exchanger function depends on the ionic gradients across the membrane, a sudden shift in those gradients can cause the exchangers to reverse their action and remove sodium from the cytosol while adding calcium into the cytosol. This is primarily a concern for sodium–calcium exchangers and not potassium-dependent sodium–calcium exchangers because the potassium gradient has not reversed to allow those transporters to reverse their function (Blaustein & Lederer, 1999). To determine if we were measuring a calcium influx through the exchangers in reverse mode, we replaced external sodium with lithium in the presence of the reverse mode inhibitor KB-R7943 (5 μm) (Kimura et al. 1999; Seki et al. 2002; Watanabe et al. 2006). Low concentrations of KB-R7943 preferentially inhibit the reverse mode activity of sodium–calcium exchangers (Kimura et al. 1999; Watanabe et al. 2006). If the calcium elevations were due to the reverse mode activity of the NCXs, then 5 μm KB-R7943 should abolish the lithium-induced calcium elevations. There was no significant difference in the amplitude of the calcium elevation when NCXs were inhibited in the presence of 5 μm KB-R7943 compared to the amplitude of the calcium response when only NCXs were inhibited (Fig. 3A and B, n= 10, P= 0.11). In these experiments, 5 μm KB-R7943 sometimes increased the amplitude of the cytosolic calcium elevation when external sodium was removed, but this difference was not significantly different from control. Therefore, the lithium-dependent cytosolic calcium elevations were not due to the reverse mode activity of the NCXs.
Figure 3. Lithium-induced cytosolic calcium increases are not due to reverse mode NCX activity.
A, external sodium was replaced with lithium (wide-hatched area) in the presence of 5 μm KB-R7943 (narrow-hatched area labelled K-B), a reverse mode inhibitor for sodium–calcium exchangers. Substituting external sodium with lithium caused an elevation in cytosolic calcium while 5 μm KB-R7943 alone had no effect. When 5 μm KB-R7943 was applied in the absence of external sodium, it did not significantly affect the amplitude of the calcium response (B, paired Student's t test, n= 10, P= 0.11). C, application of 100 μm KB-R7943 in normal external solution (narrow-hatched area) evoked similar cytosolic calcium increases compared to the calcium elevations that occurred when external sodium was removed (n= 6, P= 0.09, wide-hatched area). D, 50 μm SN-6 (narrow-hatched area) generated comparable cytosolic calcium elevations compared to the calcium responses due to only inhibiting NCXs (n= 9, P= 0.49, wide-hatched area).
Higher concentrations of reverse mode NCX blockers, including KB-R7943, inhibit NCXs in the forward direction (Watanabe et al. 2006). There are conflicting reports as to the selectivity of KB-R7943 for other channels; some studies report that KB-R7943 can inhibit voltage-gated sodium and calcium channels as well as inward rectifying potassium channels in cardiac ventricular cells (Watano et al. 1996), while studies in cardiomyocyte cultures found that KB-R7943 was much more specific in its inhibitory actions for NCXs (Iwamoto et al. 1996). In taste cells, application of 100 μm KB-R7943 in normal external solution evoked similar cytosolic calcium increases compared to the lithium-induced calcium elevations in the same cells (Fig. 3C, n= 6, P= 0.09). As a precaution against measuring non-specific KB-R7943 effects, we also used the NCX blocker SN-6, which at high concentrations also inhibits the forward mode activity of NCXs (Niu et al. 2007b) and has been reported to be more specific in its actions than KB-R7943 (Watanabe et al. 2006; Niu et al. 2007a,b;). In taste cells, 50 μm SN-6 generated comparable cytosolic calcium elevations compared to the calcium responses due to removal of extracellular sodium (paired Student's t test, n= 9, P= 0.49, Fig. 3D). These data support the conclusion that inhibiting NCX forward-mode activity causes an increase in cytosolic calcium, even in the absence of cell stimulation.
NCXs contribute to the regulation of the constitutive calcium influx to maintain basal calcium levels
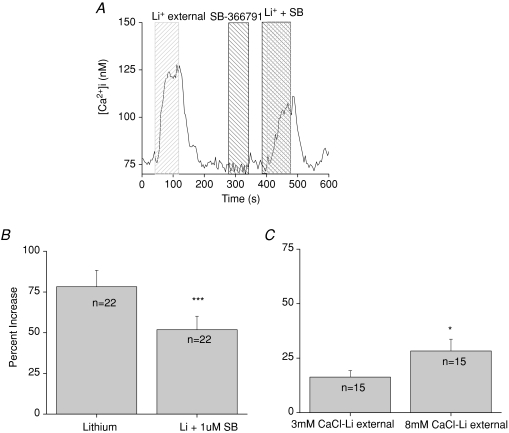
Mouse taste cells have a constitutive calcium influx across the plasma membrane that can be regulated by mitochondrial calcium uptake but ultimately must be extruded at the plasma membrane to maintain physiological low calcium levels inside the cell (Hacker & Medler, 2008). While multiple channels appear to contribute to this calcium influx, we have identified TRPV1, or a taste variant of TRPV1, as one of the channels involved in this constitutive influx (Lyall et al. 2004; Hacker & Medler, 2008). Removing external sodium in the presence of the TRPV1 antagonist SB-366791 caused a significant reduction in the amplitude of the calcium elevation (Student's paired t test, n= 22, P < 0.0001, Fig. 4A and B), which suggests that TRPV1 is constitutively passing calcium into the taste cell that is subsequently extruded by the exchangers. When the channel's activity was blocked, the magnitude of the lithium-induced cytosolic calcium elevation significantly decreased. Removing SB-366791 restored the cytosolic calcium elevations back to original levels when the NCXs were subsequently inhibited.
Figure 4. TRPV1 antagonist significantly reduces lithium-induced cytosolic calcium elevations.
A, application of 1 μm SB-366791, a specific TRPV1 antagonist, significantly reduced the amplitude of the calcium response due to inhibition of NCX activity (B, paired Student's t test, n= 22, ***P < 0.0001) while having no effect on basal calcium levels when applied alone. C, increasing calcium concentrations in the external solution to 8 mm caused a significant increase in the amplitude of the cytosolic calcium response to inhibiting NCX activity (paired Student's t test, n= 15, *P < 0.05).
Varying external calcium concentrations while inhibiting the exchangers caused a significantly larger increase in cytosolic calcium compared to the calcium elevation that occurred when exchangers were inhibited in normal external calcium (Fig. 4C). This agrees with our earlier study (Hacker & Medler, 2008) in which we reported that changing external calcium concentrations alone affected cytosolic calcium levels in taste cells and significantly affected the cytosolic calcium response that occurred when the mitochondria were not functioning. These data suggest a dynamic relationship between constitutively active channels at the plasma membrane and calcium regulatory mechanisms in the taste cells that serves to control cytosolic calcium levels in these cells.
NCXs and mitochondria work together to regulate cytosolic calcium
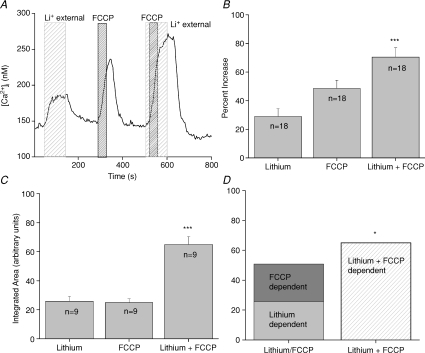
Inhibiting mitochondrial calcium transport using the protonophore FCCP caused an elevation in cytosolic calcium that was maintained as long as the mitochondria were not able to sequester calcium. There is a constitutive calcium influx through channels at the plasma membrane that can be taken up by the mitochondria (Hacker & Medler, 2008) but how this calcium is removed from the cell has not previously been addressed. We hypothesized that NCXs are important in the routine extrusion of this constitutive calcium influx and determined that both NCXs and mitochondria contribute to the regulation of the calcium influx to keep internal cytosolic calcium levels low. Analysis of the FCCP-dependent, lithium-dependent and the lithium + FCCP-induced cytosolic calcium increases using a one-way ANOVA determined that the peak amplitudes of the lithium + FCCP responses were significantly larger than the peak amplitudes of the calcium responses to either FCCP or lithium alone (Fig. 5A and B, n= 18, P < 0.0001). Inhibiting mitochondrial function with FCCP was reversible and subsequent inhibition of the NCXs generated calcium elevations that were comparable to the initial responses (data not shown). When we added the amplitude of the cytosolic calcium elevation due to inhibiting mitochondrial calcium uptake to the amplitude of the cytosolic calcium response when NCXs were inhibited, there was no difference in the amplitude of these combined responses compared to the amplitude of the response when we co-applied FCCP and lithium (Student's paired t test, n= 18, P= 0.18). These data suggested that the increase in response amplitude seen in Fig. 5A and B may have been due to the additive effects of two separate events that both affect cytosolic calcium (inhibiting NCX activity and inhibiting mitochondrial calcium uptake).
Figure 5. Exchangers and mitochondrial calcium transport work together to regulate cytosolic calcium levels in taste cells.
A, replacement of external sodium with lithium (wide-hatched area) for 100 s caused an elevation in cytosolic calcium. Applying FCCP for 40 s (1 μm, narrow-hatched area) to inhibit mitochondrial calcium uptake also elevated cytosolic calcium. Adding FCCP when NCXs were inhibited caused a much larger increase in cytosolic calcium compared to FCCP or lithium alone. B, analysis using one-way ANOVA of the average peak increases for FCCP, external lithium and FCCP + external lithium revealed that FCCP + lithium was significantly larger than the amplitude of the responses to lithium or FCCP alone (n= 18, ***P < 0.0001). C, the area under the curve for each condition was integrated and compared using the one-way ANOVA. Lithium + FCCP was significantly larger than either FCCP or lithium alone (n= 9, ***P < 0.0001). D, the integrated area under the lithium + FCCP curve was significantly larger than the combination of the integrated areas reported in C for the calcium elevations that occurred when FCCP and lithium were applied individually (n= 9, *P < 0.05). Adding the area of the FCCP-dependent calcium response to the integrated area of the calcium response when NCXs were inhibited was only 78% of the integrated area of the calcium response that was generated when FCCP was applied while NCXs were inhibited.
To further address this question, we integrated the area for the calcium responses and found that the area under the curve for lithium + FCCP was also significantly higher than the area under the curves when each mechanism was individually inhibited (Fig. 5C, n= 9, P < 0.0001). The area under the curve was measured beginning at the onset of the calcium elevation until the calcium levels returned to baseline values. FCCP was always applied for 40s while sodium was always removed for 100 s so both mechanisms were disabled for the same amount of time whether they were individually inhibited or co-inhibited. When we added the average integrated area for the lithium-induced calcium elevation to the area of the FCCP-induced calcium elevation, the total area value was only 78% of the average calculated area of the calcium response when FCCP and lithium were co-applied. Therefore, adding the two individual areas generated a significantly smaller response than the area under the curve when both mechanisms were inhibited (Fig. 5D, n= 9, P < 0.05). These data indicate that the calcium response that occurred when mitochondrial calcium uptake and exchanger activity were both inhibited were not simply additive (i.e. the last calcium elevation seen in Fig. 5A).
Multiple isoforms of both NCXs and NCKXs are expressed in taste cells
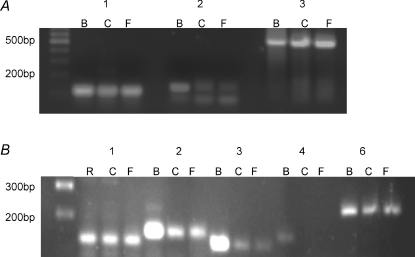
As our physiological data strongly indicated that NCXs are important in regulating cytosolic calcium in taste cells, we performed RT-PCR analysis to identify which NCX isoforms are expressed in taste cells. All three identified NCX isoforms (NCX1-3) were expressed in circumvallate and foliate taste cells (Fig. 6A). We also tested for the expression of sodium–calcium–potassium exchangers (NCKXs) which exchange sodium ions for calcium ions in a potassium-dependent manner (Cervetto et al. 1989; Dong et al. 2001; Prinsen et al. 2002; Szerencsei et al. 2002). There are six identified NCKX isoforms (NCKX1–6) (Prinsen et al. 2002; Szerencsei et al. 2002; Cai & Lytton, 2004) of which we were able to amplify PCR products for NCKX1–3 and NCKX6 (Fig. 6B) in taste cells. We excluded NCKX5 from our analysis because its physiological function has not been well established but appears to have a role in pigmentation in zebrafish and humans (Schnetkamp, 2004; Lamason et al. 2005). The identities of all PCR products were confirmed with sequence analysis. While these initial studies did not quantify the relative expression of each of the exchanger isoforms, this RT-PCR analysis supports our conclusion that exchangers are expressed in taste cells and indicate that multiple isoforms may be contributing to the regulation of cytosolic calcium in taste cells.
Figure 6. RT-PCR analysis reveals the presence of multiple exchangers in taste cells.
A, isolated mRNA from taste buds located in the circumvallate (C) and foliate (F) papillae was subjected to RT-PCR analysis to identify the sodium–calcium exchangers expressed in taste buds. mRNA isolated from brain tissue (B) was run as a positive control. Predicted PCR sizes for each primer set were: NCX1 (1) 100 bp, NCX2 (2) 150 bp and NCX3 (3) 540 bp. Molecular weight markers are in the far left lane. PCR product identities were confirmed for each of the PCR products using sequence analysis. NCX2 primers generated primer dimers in the C and F NCX2 samples. B, isolated mRNA from taste buds located in the circumvallate (C) and foliate (F) papillae was subjected to RT-PCR analysis to identify the sodium–calcium–potassium exchangers expressed in taste buds. Molecular weight markers are in the far left lane. mRNA isolated from brain tissue (B) or retinal tissue (R) was run as a positive control. Predicted PCR sizes for each primer set were: NCKX1 (1) 130 bp, NCKX2 (2) 150 bp, NCKX3 (3) 120 bp, NCKX4 (4) 146 bp and NCKX6 (6) 237 bp band. PCR product identities were confirmed for each of the products using sequence analysis. All samples were tested to ensure they contained no genomic contamination (see Methods).
Discussion
Taste cells have many unique features compared to neurons and other excitable cells. Like neurons, taste cells are excitable, can fire action potentials, have presynaptic specializations and release neurotransmitters. However, taste cells are derived from surrounding epithelial cells (Stone et al. 1995) and are continually replaced every 10 to 14 days (Farbman, 1980; Finger & Simon, 2000). Taste cells are heterogeneous and express distinct signalling mechanisms that correlate with the kind of taste stimulus they detect. Some taste cells form conventional chemical synapses with afferent gustatory neurons and rely on the opening of VGCCs to generate a sufficient increase in cytosolic calcium to cause vesicular release of neurotransmitter (Medler et al. 2003; Clapp et al. 2006; DeFazio et al. 2006). Other taste cells use a completely unique synaptic mechanism that relies on cell depolarization and calcium release from internal stores to open a hemichannel to release neurotransmitter (Huang et al. 2007; Romanov et al. 2007). These taste cells do not have conventional chemical synapses and lack VGCCs (Medler et al. 2003; Clapp et al. 2006; DeFazio et al. 2006). While it is well established that these two groups of taste cells rely on different mechanisms to increase cytosolic calcium in response to stimulation, very little study has focused on understanding how cytosolic calcium is regulated in these cells, which is key to their ability to form an appropriate cellular response to taste stimuli.
This study is the first to describe the presence of sodium–calcium exchangers in taste cells and reports on their unique function in the regulation of cytosolic calcium in these cells. While many cells rely on NCXs to recover from stimulus-evoked calcium increases (Blaustein & Lederer, 1999; Prinsen et al. 2002; Szerencsei et al. 2002; Schnetkamp, 2004; Iwamoto et al. 2007), in taste cells NCX activity is important for the regulation of cytosolic calcium even in the absence of cell stimulation. This is due to the presence of a constitutive calcium influx in taste cells that is also regulated in part by mitochondrial calcium transport (Hacker & Medler, 2008). In an earlier study, we found that maintaining appropriate resting calcium levels in taste cells depended on mitochondrial calcium transport, external calcium concentrations and a continual calcium influx through multiple channels, one of which is TRPV1 (Hacker & Medler, 2008), or a taste variant of TRPV1 (Lyall et al. 2004).
Unlike most cells, inhibiting mitochondrial calcium uptake elevated cytosolic calcium levels even in taste cells that had never been stimulated (Hacker & Medler, 2008). In our current study, we found that inhibiting NCX activity also increased cytosolic calcium even if the taste cells had not been stimulated. When external calcium concentrations were increased and NCXs were inhibited, the calcium elevations became significantly larger (Fig. 4C). These data identify a relationship between external calcium concentrations, plasma membrane calcium leak channels and calcium regulatory mechanisms that is critical to the basal regulation of cytosolic calcium in taste cells. Like most cells, it is important for cytosolic calcium in taste cells to be maintained at physiological low concentrations to prevent the non-specific activation of calcium-dependent processes and to allow the cells to respond appropriately to external stimuli. Unlike most cells, the apical ends of taste cells are in physical contact with the external environment in order to detect any taste stimuli that are present in the oral cavity. In general, this environment will have very low ionic content but changes as nutrients are consumed. Being subjected to variable environmental conditions may have created a need for taste cells to have additional mechanisms in place to ensure that cytosolic calcium levels are appropriately maintained at all times.
Inhibiting NCXs caused elevations in cytosolic calcium that were variable in amplitude; some responses were quite large while in other cells, inhibiting NCXs generated very small increases in cytosolic calcium. Analysis of these data revealed that this variability correlated with the expression of specific calcium signalling mechanisms expressed by the taste cell (Fig. 2). Taste cells that express VGCCs and can be classified as type III/presynaptic taste cells had significantly larger calcium elevations when NCXs were inhibited compared to taste cells that release calcium from internal stores and do not have VGCCs (type II/receptor taste cells). The baseline calcium values for these two groups of cells were virtually identical, so the variable responses were not due to differences in resting calcium levels between these cells. These findings parallel the results from our earlier study which showed inhibiting mitochondrial calcium uptake caused variable increases in cytosolic calcium that were similarly correlated with the expression of these two calcium signalling mechanisms (calcium influx via VGCCs or calcium release from internal stores) (Hacker & Medler, 2008). Type III taste cells with VGCCs have large calcium influxes that generate a significantly larger calcium load on the cell compared to the calcium loads in type II taste cells that rely on calcium release from internal stores (Hacker et al. 2008). Taken together, the data from the current study and our earlier studies (Hacker et al. 2008; Hacker & Medler, 2008) suggest that the overall regulation of cytosolic calcium in taste cells is related to the calcium signalling mechanisms used by those cells.
Currently, we do not know if these differences are due to unique characteristics of the channel(s) responsible for calcium influx at the plasma membrane or due to different properties of the calcium regulatory mechanisms used by these taste cells. Our RT-PCR analysis revealed the expression of multiple sodium–calcium exchangers and sodium–potassium–calcium exchangers in taste cells. There may be differential expression of these protein isoforms that correlates with specific calcium signalling mechanisms in taste cells. It has been reported in mouse that multiple NCX and NCKX isoforms are differentially expressed throughout the olfactory and vomeronasal organs and that NCXs have unique expression profiles within different areas of the olfactory neurons (Pyrski et al. 2007). These data suggest that exchangers have distinct functions within the olfactory neurons which may be a common feature of the peripheral sensory systems. We have not yet characterized the expression patterns of the different exchanger isoforms in taste cells because the available antibodies did not produce labelling that was localized at the plasma membrane but labelled epitopes throughout the cytosol (data not shown). Future studies are needed to address these questions.
We know that mitochondrial calcium uptake contributes to the regulation of basal calcium levels (Hacker & Medler, 2008) and we have now shown that NCXs also contribute to maintaining appropriate calcium concentrations in these cells. One possibility is that these are two separate mechanisms that work independently in taste cells. The second possibility is that these mechanisms work together to regulate cytosolic calcium. Our data indicate that mitochondrial calcium transport and sodium–calcium exchangers work together to maintain cytosolic calcium at appropriately low levels, even when taste cells have not been stimulated (Fig. 5). The calcium load was significantly larger when both mechanisms were inhibited compared to inhibiting each mechanism individually (Fig. 5D). The average increase in cytosolic calcium when both mechanisms were inhibited was 128% of the sum of the calcium increases when these mechanisms were individually blocked, so the effect of inhibiting both mechanisms had more than an additive effect on the cytosolic calcium levels. If these mechanisms were independent of each other, the calcium response when they were both inhibited should have been approximately equal to the combination of their individual responses. Our data reveal that when NCXs are inhibited, the mitochondria can compensate for some of the increased calcium load to keep cytosolic calcium levels, at least within the time frame of our experiments. Conversely, when the mitochondria are inhibited but NCXs are still functional, some of the excess calcium load is being extruded out of the cell by the NCXs to reduce the increases in cytosolic calcium. However, when both mechanisms are inhibited, the cell is subjected to a significantly larger calcium load. These data suggest an interdependent relationship between the mitochondria and NCXs at the plasma membrane of taste cells that is reminiscent of the relationship in many cell types between mitochondrial calcium transport and the activity of SERCA pumps on internal calcium stores which jointly regulate any calcium leak from internal stores in order to keep cytosolic calcium levels low (Landolfi et al. 1998; Csordas et al. 1999; Hajnoczky et al. 1999, 2000a,b; Pacher et al. 2000; Szalai et al. 2000; Arnaudeau et al. 2001; Csordas & Hajnoczky, 2001; Csordas et al. 2001; Johnson et al. 2002; Jackson & Thayer, 2006; Kopach et al. 2008).
Acknowledgments
The authors wish to thank Margaret Starostik for her technical assistance as well as Dr Evanna Gleason and Dr Scott Medler for their insightful comments. This work was supported by NIH Grant DC006358 to K.M.
Glossary
Abbreviations
- CV
circumvallate papillae
- Fol
foliate papillae
- GPCR
G-protein coupled receptor
- NCX
sodium–calcium exchanger
- NCKX
potassium-dependent sodium–calcium exchanger
- VGCC
voltage-gated calcium channel
Author contributions
K.M. conceived the study. A.L. and K.M. performed experiments, analysed data and wrote the manuscript.
Supplemental material
References
- Arnaudeau S, Kelley WL, Walsh JV, Jr, Demaurex N. Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J Biol Chem. 2001;276:29430–29439. doi: 10.1074/jbc.M103274200. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Santiago EM. Effects of internal and external cations and of ATP on sodium-calcium and calcium-calcium exchange in squid axons. Biophys J. 1977;20:79–111. doi: 10.1016/S0006-3495(77)85538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Lytton J. Molecular cloning of a sixth member of the K+-dependent Na+/Ca2+ exchanger gene family, NCKX6. J Biol Chem. 2004;279:5867–5876. doi: 10.1074/jbc.M310908200. [DOI] [PubMed] [Google Scholar]
- Cervetto L, Lagnado L, Perry RJ, Robinson DW, McNaughton PA. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature. 1989;337:740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signalling pathway. J Comp Neurol. 2004;468:311–321. doi: 10.1002/cne.10963. [DOI] [PubMed] [Google Scholar]
- Csordas G, Hajnoczky G. Sorting of calcium signals at the junctions of endoplasmic reticulum and mitochondria. Cell Calcium. 2001;29:249–262. doi: 10.1054/ceca.2000.0191. [DOI] [PubMed] [Google Scholar]
- Csordas G, Thomas AP, Hajnoczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Thomas AP, Hajnoczky G. Calcium signal transmission between ryanodine receptors and mitochondria in cardiac muscle. Trends Cardiovasc Med. 2001;11:269–275. doi: 10.1016/s1050-1738(01)00123-2. [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Light PE, French RJ, Lytton J. Electrophysiological characterization and ionic stoichiometry of the rat brain K+-dependent Na+/Ca2+ exchanger, NCKX2. J Biol Chem. 2001;276:25919–25928. doi: 10.1074/jbc.M103401200. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Renewal of taste bud cells in rat circumvallate papillae. Cell Tissue Kinet. 1980;13:349–357. doi: 10.1111/j.1365-2184.1980.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Finger TE, Simon SA. Cell biology of taste epithelium. In: Finger TE, Silver WL, Restrepo D, editors. The Neurobiology of Taste and Smell. New York: Wiley-Liss; 2000. pp. 287–314. [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hacker K, Laskowski A, Feng L, Restrepo D, Medler K. Evidence for two populations of bitter responsive taste cells in mice. J Neurophysiol. 2008;99:1503–1514. doi: 10.1152/jn.00892.2007. [DOI] [PubMed] [Google Scholar]
- Hacker K, Medler KF. Mitochondrial calcium buffering contributes to the maintenance of basal calcium levels in mouse taste cells. J Neurophysiol. 2008;100:2177–2191. doi: 10.1152/jn.90534.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnoczky G, Csordas G, Krishnamurthy R, Szalai G. Mitochondrial calcium signalling driven by the IP3 receptor. J Bioenerg Biomembr. 2000a;32:15–25. doi: 10.1023/a:1005504210587. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Csordas G, Madesh M, Pacher P. The machinery of local Ca2+ signalling between sarcoendoplasmic reticulum and mitochondria. J Physiol. 2000b;529:69–81. doi: 10.1111/j.1469-7793.2000.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnoczky G, Hager R, Thomas AP. Mitochondria suppress local feedback activation of inositol 1,4,5-trisphosphate receptors by Ca2+ J Biol Chem. 1999;274:14157–14162. doi: 10.1074/jbc.274.20.14157. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T, Watanabe Y, Kita S, Blaustein MP. Na+/Ca2+ exchange inhibitors: a new class of calcium regulators. Cardiovasc Hematol Disord Drug Targets. 2007;7:188–198. doi: 10.2174/187152907781745288. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Watano T, Shigekawa M. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J Biol Chem. 1996;271:22391–22397. doi: 10.1074/jbc.271.37.22391. [DOI] [PubMed] [Google Scholar]
- Jackson JG, Thayer SA. Mitochondrial modulation of Ca2+-induced Ca2+ release in rat sensory neurons. J Neurophysiol. 2006;96:1093–1104. doi: 10.1152/jn.00283.2006. [DOI] [PubMed] [Google Scholar]
- Johnson PR, Tepikin AV, Erdemli G. Role of mitochondria in Ca2+ homeostasis of mouse pancreatic acinar cells. Cell Calcium. 2002;32:59–69. doi: 10.1016/s0143-4160(02)00091-x. [DOI] [PubMed] [Google Scholar]
- Kimura J, Watano T, Kawahara M, Sakai E, Yatabe J. Direction-independent block of bi-directional Na+/Ca2+ exchange current by KB-R7943 in guinea-pig cardiac myocytes. Br J Pharmacol. 1999;128:969–974. doi: 10.1038/sj.bjp.0702869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnamon JC, Sherman TA, Roper SD. Ultrastructure of mouse vallate taste buds: III. Patterns of synaptic connectivity. J Comp Neurol. 1988;270:1–10. 56–57. doi: 10.1002/cne.902700102. [DOI] [PubMed] [Google Scholar]
- Kinnamon JC, Taylor BJ, Delay RJ, Roper SD. Ultrastructure of mouse vallate taste buds. I. Taste cells and their associated synapses. J Comp Neurol. 1985;235:48–60. doi: 10.1002/cne.902350105. [DOI] [PubMed] [Google Scholar]
- Kopach O, Kruglikov I, Pivneva T, Voitenko N, Fedirko N. Functional coupling between ryanodine receptors, mitochondria and Ca2+ ATPases in rat submandibular acinar cells. Cell Calcium. 2008;43:469–481. doi: 10.1016/j.ceca.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Lamason RL, Mohideen MA, Mest JR, Wong AC, Norton HL, Aros MC. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310:1782–1786. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- Landolfi B, Curci S, Debellis L, Pozzan T, Hofer AM. Ca2+ homeostasis in the agonist-sensitive internal store: functional interactions between mitochondria and the ER measured in situ in intact cells. J Cell Biol. 1998;142:1235–1243. doi: 10.1083/jcb.142.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Alam RI, Russell OF, Malik SA, Bigbee JW, DeSimone JA. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol. 2004;558:147–159. doi: 10.1113/jphysiol.2004.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medler KF, Margolskee RF, Kinnamon SC. Electrophysiological characterization of voltage-gated currents in defined taste cell types of mice. J Neurosci. 2003;23:2608–2617. doi: 10.1523/JNEUROSCI.23-07-02608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu CF, Watanabe Y, Iwamoto T, Yamashita K, Satoh H, Urushida T, Hayashi H, Kimura J. Electrophysiological effects of SN-6, a novel Na+/Ca2+ exchange inhibitor on membrane currents in guinea pig ventricular myocytes. Ann N Y Acad Sci. 2007a;1099:534–539. doi: 10.1196/annals.1387.037. [DOI] [PubMed] [Google Scholar]
- Niu CF, Watanabe Y, Ono K, Iwamoto T, Yamashita K, Satoh H, Urushida T, Hayashi H, Kimura J. Characterization of SN-6, a novel Na+/Ca2+ exchange inhibitor in guinea pig cardiac ventricular myocytes. Eur J Pharmacol. 2007b;573:161–169. doi: 10.1016/j.ejphar.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Pacher P, Csordas P, Schneider T, Hajnoczky G. Quantification of calcium signal transmission from sarco-endoplasmic reticulum to the mitochondria. J Physiol. 2000;529:553–564. doi: 10.1111/j.1469-7793.2000.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon S, Leach S, Li XF, Tucker JE, Schnetkamp PP, Lytton J. Alternatively spliced isoforms of the rat eye sodium/calcium+potassium exchanger NCKX1. Am J Physiol Cell Physiol. 2000;278:C651–C660. doi: 10.1152/ajpcell.2000.278.4.C651. [DOI] [PubMed] [Google Scholar]
- Prinsen CF, Cooper CB, Szerencsei RT, Murthy SK, Demetrick DJ, Schnetkamp PP. The retinal rod and cone Na+/Ca2+-K+ exchangers. Adv Exp Med Biol. 2002;514:237–251. [PubMed] [Google Scholar]
- Pyrski M, Koo JH, Polumuri SK, Ruknudin AM, Margolis JW, Schulze DH, Margolis FL. Sodium/calcium exchanger expression in the mouse and rat olfactory systems. J Comp Neurol. 2007;501:944–958. doi: 10.1002/cne.21290. [DOI] [PubMed] [Google Scholar]
- Richter TA, Caicedo A, Roper SD. Sour taste stimuli evoke Ca2+ and pH responses in mouse taste cells. J Physiol. 2003;547:475–483. doi: 10.1113/jphysiol.2002.033811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Khokhlov AA, Kolesnikov SS. Voltage dependence of ATP secretion in mammalian taste cells. J Gen Physiol. 2008;132:731–744. doi: 10.1085/jgp.200810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetkamp PP. The SLC24 Na+/Ca2+-K+ exchanger family: vision and beyond. Pflugers Arch. 2004;447:683–688. doi: 10.1007/s00424-003-1069-0. [DOI] [PubMed] [Google Scholar]
- Seki S, Taniguchi M, Takeda H, Nagai M, Taniguchi I, Mochizuki S. Inhibition by KB-r7943 of the reverse mode of the Na+/Ca2+ exchanger reduces Ca2+ overload in ischemic-reperfused rat hearts. Circ J. 2002;66:390–396. doi: 10.1253/circj.66.390. [DOI] [PubMed] [Google Scholar]
- Stone LM, Finger TE, Tam PP, Tan SS. Taste receptor cells arise from local epithelium, not neurogenic ectoderm. Proc Natl Acad Sci U S A. 1995;92:1916–1920. doi: 10.1073/pnas.92.6.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalai G, Csordas G, Hantash BM, Thomas AP, Hajnoczky G. Calcium signal transmission between ryanodine receptors and mitochondria. J Biol Chem. 2000;275:15305–15313. doi: 10.1074/jbc.275.20.15305. [DOI] [PubMed] [Google Scholar]
- Szerencsei RT, Winkfein RJ, Cooper CB, Prinsen C, Kinjo TG, Kang K, Schnetkamp PP. The Na/Ca-K exchanger gene family. Ann N Y Acad Sci. 2002;976:41–52. doi: 10.1111/j.1749-6632.2002.tb04712.x. [DOI] [PubMed] [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;27:10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003;31:e154. doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Koide Y, Kimura J. Topics on the Na+/Ca2+ exchanger: pharmacological characterization of Na+/Ca2+ exchanger inhibitors. J Pharmacol Sci. 2006;102:7–16. doi: 10.1254/jphs.fmj06002x2. [DOI] [PubMed] [Google Scholar]
- Watano T, Kimura J, Morita T, Nakanishi H. A novel antagonist, No. 7943, of the Na+/Ca2+ exchange current in guinea-pig cardiac ventricular cells. Br J Pharmacol. 1996;119:555–563. doi: 10.1111/j.1476-5381.1996.tb15708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Crowley HH, Rock ME, Kinnamon JC. Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. J Comp Neurol. 2000a;424:205–215. doi: 10.1002/1096-9861(20000821)424:2<205::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Yang R, Tabata S, Crowley HH, Margolskee RF, Kinnamon JC. Ultrastructural localization of gustducin immunoreactivity in microvilli of type II taste cells in the rat. J Comp Neurol. 2000b;425:139–151. doi: 10.1002/1096-9861(20000911)425:1<139::aid-cne12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC. ‘Type III’ cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol. 2001;440:97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signalling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.