Abstract
Skeletal muscle capillarisation responds to physiological and pathological conditions with a remarkable plasticity. Angiomotin was recently identified as a new pro-angiogenic molecule. Angiomotin is expressed as two protein isoforms, p80 and p130. Whereas p80 stimulates endothelial cell migration and angiogenesis, p130 is rather characteristic of stabilized and matured vessels. To date, how angiomotin expression is physiologically regulated in vivo remains largely unknown. We thus investigated (1) whether angiomotin was physiologically expressed in skeletal muscle; (2) whether exercise training, known to stimulate muscle angiogenesis, affected angiomotin expression; and (3) whether such regulation was altered in obesity, a pathological situation often associated with an impaired angiogenic activity and some capillary rarefaction in skeletal muscle. Two models of obesity were used: a high fat diet regime and Zucker Diabetic Fatty rats (ZDF). Our results provide evidence that angiomotin was expressed both in capillaries and myofibres. In non-obese rats, the p80 isoform was increased in plantaris muscle in response to endurance training whereas p130 was unaffected. In obese animals, no change was observed for p80 whereas training significantly decreased p130 expression. Exercise training induced angiogenesis in plantaris from both obese and non-obese rats, possibly through the modulation of angiomotin level and its consequences on RhoA–ROCK signalling. In conclusion, any increase in p80 or decrease in p130, as respectively observed in non-obese and obese animals, led to an increased ratio between p80 and p130 isoforms. This increased angiomotin p80/p130 ratio might then directly reflect the enhanced angiogenic ability of skeletal muscle in response to exercise training.
Angiogenesis, the de novo formation of capillaries, is a complex and multi-step biological process requiring proliferation, migration and assembly of endothelial cells to form new vessels. Such events are tightly regulated by angiogenic and angiostatic factors (Risau, 1997; Carmeliet, 2003, 2005). Angiomotin was recently identified in various endothelial cell lines as a transmembrane receptor for the angiostatic factor angiostatin (Troyanovsky et al. 2001; Bratt et al. 2005). In vivo, angiomotin was detected at the surface of blood vessels of both healthy and pathological tissues such as placenta, retina, Kaposi's sarcoma and breast tumours (Troyanovsky et al. 2001; Levchenko et al. 2004, 2008; Holmgren et al. 2006; Jiang et al. 2006; Aase et al. 2007). These studies have also highlighted its important role in both promoting angiogenesis and maintaining established vessels.
Alternative splicing of angiomotin mRNA results in two protein isoforms of 80 and 130 kDa that exert very distinct roles during angiogenesis (Bratt et al. 2002; Ernkvist et al. 2006, 2008). The p80 angiomotin isoform strongly stimulates in vitro and in vivo the migration of endothelial cells, a key event of the angiogenic process (Ernkvist et al. 2008). Interestingly, angiostatin binding to angiomotin extracellular domain strongly inhibits such an effect (Bratt et al. 2005; Ernkvist et al. 2008). In contrast, p130 angiomotin isoform only exerts a very weak stimulatory effect on endothelial cell migration (Ernkvist et al. 2008). The p130 protein was identified as tightly associated to cytoskeleton actin filaments and highly involved in vessel stabilization and maturation (Ernkvist et al. 2006, 2008).
Inhibition of angiomotin function by gene knockout, siRNA silencing, or antibody treatment strongly impairs endothelial cells migration in vitro and angiogenesis in vivo (Holmgren et al. 2006; Aase et al. 2007; Levchenko et al. 2008). Angiomotin-deficient endothelial cells lack the in vitro chemotactic response to the key angiogenic factor vascular endothelial growth factor (VEGF) (Levchenko et al. 2003; Ernkvist et al. 2008). In vivo inhibition of angiomotin has shown promising results for the prevention of pathological angiogenesis in murine models of choroidal neovascularization and breast cancer (Holmgren et al. 2006; Aase et al. 2007; Levchenko et al. 2008). Angiomotin has also been described as an important actor in vasculogenesis in mouse and zebrafish embryos (Shimono & Behringer, 2003; Aase et al. 2007). This, in addition to its important role during angiogenesis, vessel maturation and stabilization, undoubtedly raises the question of whether any therapeutic approaches to systemically inhibit angiomotin's function might also exert harmful side-effects in healthy tissues.
Interestingly, skeletal muscles represent the most abundant tissue of the body and they express high levels of angiomotin (Troyanovsky et al. 2001: Bratt et al. 2002). Moreover, microcirculation is a critical component of muscle function since capillaries provide myofibres with oxygen and nutriments, and remove carbon dioxide and metabolic waste. As myofibres respond to physiological or pathological conditions with a remarkable plasticity, it is crucial that the microcirculation remains well matched with the myofibres’ needs in order to preserve muscle function (Hudlicka et al. 1992; Birot & Bigard, 2003). Depending on conditions, such muscle angio-adaptation can involve either angiogenesis or some vascular regression. Given its role not only during angiogenesis but also for vessel stabilization and maturation, angiomotin might thus represent an important actor in muscle angio-adaptation. To date, angiomotin expression in skeletal muscle has never been investigated in response to physiological or pathological conditionings. Our objective was, then, to study how p80 and p130 angiomotin isoforms were expressed in skeletal muscle in response to endurance training, a well-described and powerful physiological stimulus for muscle angiogenesis (Hudlicka, 1991; Birot et al. 2003; Prior et al. 2004). We also investigated whether obesity or type-2 diabetes could possibly alter angiomotin response to exercise training. Previous studies have indeed reported some capillary regression as well as an impaired angiogenic activity in skeletal muscles from obese or diabetic rodents (Rivard et al. 1999; Frisbee, 2002; Stapleton et al. 2008). This is of particular interest since exercise training has recently been proposed as an efficient physiological alternative to blunt capillary rarefaction in the metabolic syndrome (Frisbee et al. 2006).
Methods
Animal experiments
All animal experiments were approved by Montreal University Institutional Animal Care Committee and were conducted accordingly to the directives of the Canadian Council on Animal Care.
First animal model
Female Sprague–Dawley rats were purchased from Charles River (Saint-Constant, QC, Canada) and housed on a 12 : 12 h light–dark cycle, with water and food access ad libitum. After 1 week of acclimatization, animals were subjected to different dietary regimes for 8 weeks. Rats were fed with either standard (SD, 11% lipids) or high fat (HFD, 42% lipids) diets. During the 8 weeks of dietary regimes, SD and HFD groups were each divided into sedentary (SED) or trained (TR) subgroups (6 rats per group). Endurance training consisted in a running exercise on a rodent treadmill (Quinton Instruments, Seattle, WA, USA) 5 times per week (60 min per day, 25 m min−1, 4% slope).
Second animal model
Five-week-old male Lean (n= 9) and Zucker Diabetic Fatty (ZDF, n= 16) rats were purchased from Charles River Laboratories (Canada) and housed as described above with free access to water and food (Purina 5008). After acclimatization, ZDF rats were assigned to either sedentary (SED-ZDF, n= 8) or trained (TR-ZDF, n= 8) groups. Lean rats were kept sedentary. All animals were individually placed for 7 weeks into wheel-running cages. Sedentary Lean and ZDF rats had a locked wheel whereas the trained ZDF group had a free wheel allowing spontaneous and voluntary activity.
Killing and tissue processing
Rats were weighed, and anaesthetized by ketamine (80 mg kg−1)–xylazine (10 mg kg−1) intraperitoneal injection. Soleus, plantaris and extensor digitorum longus muscles were harvested, weighed and fast-frozen in liquid nitrogen. Killing was performed by exsanguination and heart removal.
The onset of obesity was estimated in the sedentary high-fat diet (SED-HFD) Sprague–Dawley group by measuring the mean body weight and the mean visceral fat pad mass. Development of obesity and type-2 diabetes in sedentary Zucker Diabetic Fatty rats (SED-ZDF) was evidenced by comparing plasma glucose, epididymal white adipose tissue (EWAT) weight and mean body weight with Lean values as previously described (Bergeron et al. 2006).
Western blotting
Proteins were extracted from 20–40 mg of frozen muscles using a RIPA lysis buffer containing 1 mg ml−1 phenylmethylsulfonyl fluoride (PMSF), 1 mmol l−1 Na3VO4, 1 mm NaF (Sigma-Aldrich, Montreal, Canada), 1× protease inhibitors cocktail (Roche Diagnostics, Laval, Canada). Muscle lyses were performed in a Retsch MM301 tissue lyser (Retsch GmbH, Haan, Germany). Denaturizing samples were separated on SDS-PAGE and blotted onto a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA, USA). After blocking with 5% fat-free milk, membranes were probed for angiomotin, cytochrome c oxidase subunit IV (COX-IV), phospho-myosin phosphatase target subunit (MYPT), phospho-insulin receptor substrate-1 (IRS-1), anti-β-tubulin and β-actin protein detection using appropriate antibodies: anti-angiomotin (kindly provided by Dr Tony Pawson, Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, Canada), anti-COX-IV antibody (A21348, Invitrogen), anti-phospho-MYPT (Thr853, no. 4563, Cell Signaling, Pickering, ON, Canada), anti-phospho-IRS-1 (Ser636/639, no. 2388, Cell Signaling), anti-β-tubulin (no. 2148, Cell Signaling), anti-β-actin (sc-47778, Santa Cruz Biotechnology, Santa Cruz, CA, USA), horseradish peroxidase (HRP)-conjugated anti-mouse (NA-931, GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA) and HRP-conjugated anti-rabbit (p0217, Dako North America Inc., Carpinteria, CA, USA). Platelet endothelial cell adhesion molecule-1 (PECAM/CD31) detection in skeletal muscle tissue using commercially available antibodies often requires a signal amplification. PECAM was detected using a mouse anti-rat CD31 (clone TLD-3A12, BD Pharmingen, Mississauga, Canada) and successive incubations with a biotinylated rabbit anti-mouse rat-adsorbed (BA-2001, Vector Laboratories, Burlingame, CA, USA) and HRP-conjugated streptavidin (no. 550946, BD Pharmingen, Mississauga, Canada). Incubation without CD31 primary antibody was performed to ensure signal specificity at 130 kDa (Fig. 1B). Proteins were visualized using an enhanced chemiluminescence procedure (sc-2048, Santa Cruz Biotechnology). Quantification was carried out using NIH Image 1.62 software.
Figure 1. Angiomotin expression in rat skeletal muscle.
A, immunofluorescence staining on plantaris muscle cryosections revealed that angiomotin protein is expressed in both capillaries (stained for isolectin-B4) and skeletal myofibres (unstained) as respectively illustrated by the arrowheads and arrows. Scale bar, 50 μm. B, PECAM/CD31 protein was detected in skeletal muscle by Western blotting. Probing of the membrane without any primary antibody ensured the specificity of PECAM detection. C, Western blot illustrating angiomotin and PECAM protein expression in extensor digitorum longus, plantaris and soleus muscles. β-Actin protein detection was used as a loading control. D, densitometric analysis of PECAM expression. Data are presented as means ±s.e.m. (n= 5/muscle type). Significantly different from soleus muscle: *P≤ 0.05; **P≤ 0.01; ***P≤ 0.001. E, linear regression between angiomotin and PECAM protein expression in all muscles analysed (SOL, EDL, PLA). Abreviations: Amot, angiomotin; PECAM, platelet endothelial cell adhesion molecule-1; EDL, extensor digitorum longus; PLA, plantaris; SOL, soleus.
Immunohistochemistry
Angiomotin was detected on 8 μm muscle frozen sections after incubation with anti-angiomotin antibody (same as for Western blotting) and a fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit antibody (111-095-144, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). Capillaries were visualized as previously described (Rivilis et al. 2002; Holmgren et al. 2006), either after staining for alkaline phosphatase activity using FAST BCIP/NBT tablets (Sigma-Aldrich) or after incubation with biotinylated isolectin-B4 (L2140, Sigma-Aldrich) and TRITC-conjugated streptavidin (016-020-084, Jackson ImmunoResearch Laboratories). Pictures were acquired using an Axio-Imager (Zeiss, Jena, Germany) equipped with an AxioCam camera (Zeiss) and analysed with Axio-Vision 4.5 software (Zeiss).
Statistical analyses
Statistical analyses were performed with Prism5 for Mac OSX (v. 5.0). Data presented are mean ±s.e.m. One way or two way ANOVA using the Newman–Keuls post hoc test was applied and results were considered to be statistically significant when P≤ 0.05.
Results
Angiomotin expression in skeletal muscle
Staining of plantaris frozen sections showed that angiomotin protein was expressed both in capillaries (colocalization with isolectin-B4, arrowhead on Fig. 1A) and skeletal myofibres (arrow on Fig. 1A). Fig. 1C and D indicates that oxidative and slow-twitch soleus muscle (SOL), respectively, expressed 40% and 55% more angiomotin protein than the extensor digitorum longus (EDL) and the plantaris muscles (PLA): 1.11 ± 0.11 in SOL vs. 0.66 ± 0.14 in EDL (P≤ 0.05) and vs. 0.50 ± 0.09 in PLA (P≤ 0.01). Figure 1B illustrates the specific detection of the endothelial marker PECAM/CD31 protein in skeletal muscle by Western blotting. Figure 1C and D indicates that SOL respectively expressed 34% and 52% more PECAM protein than EDL and PLA: 1.33 ± 0.09 in SOL vs. 0.88 ± 0.04 in EDL (P≤ 0.01) and vs. 0.64 ± 0.09 in PLA (P≤ 0.001). Figure 1E shows that the expressions of angiomotin and PECAM proteins were significantly correlated in soleus, plantaris and extensor digitorum longus muscles (r2= 0.85, P≤ 0.0001).
Obesity and type-2 diabetes in sedentary animals
Table 1 evidences that a high fat diet (HFD) regime led to a significant increased mean body weight in sedentary rats (SED-HFD) when compared with animals fed with a standard diet (SED-SD): 352 ± 9 vs. 271 ± 4 g, P≤ 0.05). The HFD regime also led to a significantly higher visceral fat mass (mg (100 g)−1) in sedentary and trained animals (respectively SED-HFD and TR-HFD) when respectively compared to sedentary and trained animals fed with a standard diet: 9.6 ± 0.7 in SED-HFD vs. 5.9 ± 0.5 in SED-SD and 8.3 ± 0.5 in TR-HFD vs. 6.7 ± 1.2 in TR-SD, P≤ 0.05.
Table 1.
Effect of high fat diet and exercise training on body weight and visceral fat tissue
| Sedentary |
Trained |
|||
|---|---|---|---|---|
| Regime | SD | HFD | SD | HFD |
| Body weight (g) | 271 ± 4 | 352 ± 9* | *342 ± 8* | 333 ± 3* |
| Visceral fat pads (g (100 g)−1) | 5.9 ± 0.5 | 9.6 ± 0.7† | 6.7 ± 1.2 | 8.3 ± 0.5† |
High fat diet regime led to a significant higher mean body weight in sedentary animals (*P≤ 0.05). The consequence of HFD regime was not observable when combined with exercise training. HFD regime led to an increased visceral fat mass in both sedentary and trained HFD rats when respectively compared to sedentary and trained SD animals (†P≤ 0.05). Data are means ±s.e.m., n= 6 animals/group.
Table 2 shows that both sedentary and trained Zucker Diabetic Fatty rats (SED-ZDF and TR-ZDF) presented a higher mean body weight (382 ± 14 and 381 ± 6 g, respectively) when compared to Lean animals (303 ± 6 g, P≤ 0.005) as well as a significantly increased epididymal white adipose tissue (EWAT) mass (respectively 9.7 ± 0.4 and 9.0 ± 0.4 vs. 3.9 ± 0.8 mg g−1, P≤ 0.001). Glycaemia (mg dl−1) was significantly higher in sedentary ZDF rats (296 ± 42 mg dl−1) when compared to Lean and trained ZDF animals (respectively 133 ± 3 and 149 ± 19 mg dl−1, P≤ 0.05). Although trained TR-ZDF became obese, exercise training prevented any significant difference in glycaemia between these TR-ZDF rats and Lean animals.
Table 2.
Running distance, glycaemia and morphometric values in ZDF rats
| Lean | Sedentary ZDF | Trained ZDF | |
|---|---|---|---|
| Body weight (g) | 303 ± 6 | 382 ± 14*** | 381 ± 6*** |
| Plantaris (mg (g body weight)−1) | 0.95 ± 0.03 | 0.58 ± 0.03*** | 0.65 ± 0.03*** |
| EWAT (mg (g body weight)−1) | 3.9 ± 0.8 | 9.7 ± 0.4*** | 9.0 ± 0.4*** |
| Glycaemia (mg dl−1) | 133 ± 3 | 296 ± 42*** | 149 ± 19*** |
| Mean running distance after 42 days (km) | n/a | n/a | 4.7 ± 0.4*** |
Zucker Diabetic Fatty rats (ZDF) presented a higher mean body weight than Lean animals (**P≤ 0.005). Plantaris muscle was atrophied in sedentary (n= 8) and trained (n= 8) ZDF rats when compared to Lean (n= 9) animals (***P≤ 0.001). Epididymal white adipose tissue (EWAT) was significantly increased in sedentary and trained ZDF rats when compared to Lean animals (***P≤ 0.001). Glycaemia (mg dl−1) was significantly increased in sedentary ZDF rats when compared to Lean animals and trained ZDF (*P≤ 0.05). Exercise training prevented any significant increase in glycaemia in trained ZDF rats when compared to Lean animals. n/a: non applicable. Data are means ±s.e.m.
Exercise training and muscle oxidative capacity
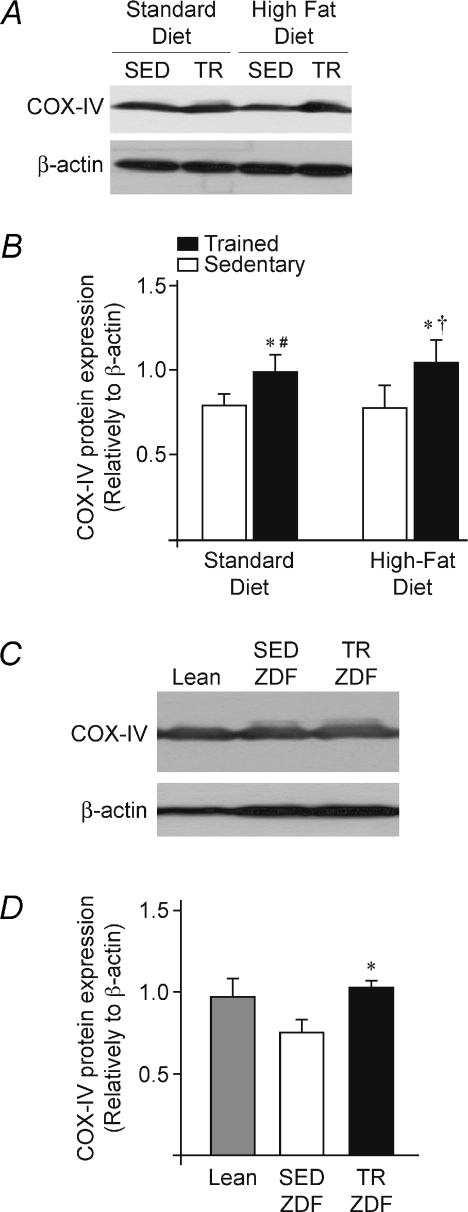
COX-IV protein expression was determined in all muscle samples as an indicator of oxidative capacity improvement in response to exercise training (Sheehan et al. 2004). Figure 2 shows that neither HFD-induced obesity nor ZDF-induced obesity/diabetes affected COX-IV protein expression: SED-SD (0.79 ± 0.03) vs. SED-HFD (0.79 ± 0.06), TR-SD (0.99 ± 0.05) vs. TR-HFD (1.04 ± 0.07), and Lean (0.98 ± 0.10) vs. SED-ZDF (0.76 ± 0.07). In contrast, exercise training significantly increased COX-IV protein expression in both models: SED-SD vs. TR-SD (0.79 ± 0.03 vs. 0.99 ± 0.05, P≤ 0.05), SED-HFD vs. TR-HFD (0.79 ± 0.06 vs. 1.04 ± 0.07, P≤ 0.05), SED-ZDF vs. TR-ZDF (0.76 ± 0.07 vs. 1.01 ± 0.05, P≤ 0.05).
Figure 2. Exercise training and muscle oxidative capacity.
A, representative Western blots for COX-IV protein expression in plantaris muscles from sedentary (SED) and trained (TR) Sprague–Dawley rats fed with either standard (SD) or high-fat (HFD) diets. β-Actin protein detection was used as a loading control. B, densitometric analysis of COX-IV expression in the four experimental groups (SED-SD, SED-HFD, TR-SD and TR-HFD). Data are presented as means ±s.e.m. (n= 6 rats/group). Significant effect of exercise training: *P≤ 0.05. Significant differences: TR-HFD vs. SED-SD (†P≤ 0.05); TR-SD vs. SED-HFD (#P≤ 0.05). C, representative Western blots for COX-IV protein expression in plantaris muscles from sedentary (SED) and trained (TR) Zucker Diabetic Fatty rats (ZDF), and sedentary Lean. β-Actin protein detection was used as a loading control. D, densitometric analysis of COX-IV expression in the three experimental groups (n= 9 Lean, n= 8 SED-ZDF, n= 8 TR-ZDF). Data are presented as means ±s.e.m. Significant effect of exercise training: *P≤ 0.05.
Exercise training and skeletal muscle angiogenesis
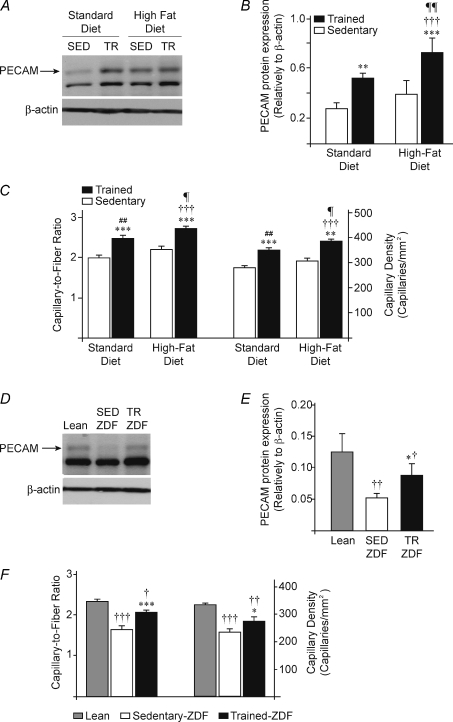
Skeletal muscle capillarisation was estimated by determining three parameters: PECAM protein expression, the capillary density (CD) and the capillary-to-fibre ratio (C/F) in all samples (Fig. 3). HFD-induced obesity did not affected basal muscle capillarisation and PECAM protein expression. In both SD and HFD groups, programmed training significantly increased PECAM expression respectively by 88% (P≤ 0.01) and 85% (P≤ 0.001): SED-SD (0.28 ± 0.03) vs. TR-SD (0.52 ± 0.02), SED-HFD (0.39 ± 0.06) vs. TR-HFD (0.72 ± 0.09) (Fig. 3A and B). Capillary density was also significantly increased in SD and HFD groups by respectively 24% (P≤ 0.001) and 25% (P≤ 0.01): SED-SD (280 ± 8) vs. TR-SD (349 ± 10,), SED-HFD (308 ± 11) vs. TR-HFD (384 ± 10) (Fig. 3C). Similar increased were observed for the capillary-to-fibre ratio: SED-SD vs. TR-SD (+24%, 2.01 ± 0.06 vs. 2.49 ± 0.07, P≤ 0.001), SED-HFD vs. TR-HFD (+24%, 2.20 ± 0.08 vs. 2.73 ± 0.07, P≤ 0.001) (Fig. 3C).
Figure 3. Exercise training and skeletal muscle angiogenesis.
A, representative blots for PECAM protein expression in sedentary (SED) and trained (TR) rats fed with standard (SD) or high-fat (HFD) diets. B, densitometric analysis of PECAM expression. Data are presented as means ±s.e.m. (n= 6 rats/group). Significant effect of exercise training: **P≤ 0.01; ***P≤ 0.001. Significant differences: versus SED-SD (†††P≤ 0.001); versus TR-SED (¶¶P≤ 0.01). C, quantitative measurement of capillary-to-fibre ratio and capillary density in plantaris muscles from all above groups. Data are expressed as means ±s.e.m. (n= 6 rats/group). Significant effect of exercise training: **P≤ 0.01; ***P≤ 0.001. Significant differences: versus SED-SD (†††P≤ 0.001); versus TR-SD (¶P≤ 0.05); versus SED-HFD (##P≤ 0.01). D, representative Western blots for PECAM protein expression in plantaris muscles from Lean, sedentary (SED) or trained (TR) ZDF rats. β-Actin protein detection was used as a loading control. E, densitometric analysis of PECAM expression. Data are presented as means ±s.e.m. (n= 9 Lean, n= 8 SED-ZDF, n= 8 TR-ZDF). Significant effect of exercise training: *P≤ 0.05. Significantly different from Lean: †P≤ 0.05; ††P≤ 0.01. F, quantitative measurement of capillary-to-fibre ratio and capillary density in plantaris muscles from Lean, SED-ZDF and TR-ZDF. Data are expressed as means ±s.e.m. (n= 9 Lean, n= 8 SED-ZDF, n= 8 TR-ZDF). Significant effect of exercise training: *P≤ 0.05; ***P≤ 0.001. Significantly different from Lean: †P≤ 0.05; ††P≤ 0.01; †††P≤ 0.001.
In the ZDF model, obese and diabetic SED-ZDF rats presented a lower PECAM protein expression than in Lean rats (−60%, 0.051 ± 0.004 vs. 0.125 ± 0.015, P≤ 0.01, Fig. 3D and E) as well as a reduced basal capillarisation (CD: −30%, 233 ± 12 vs. 329 ± 7, P≤ 0.001; C/F: −29%, 1.67 ± 0.07 vs. 2.33 ± 0.05, P≤ 0.001, Fig. 3F). Voluntary training significantly increased PECAM protein expression in TR-ZDF rats compared to SED-ZDF (+70%, 0.087 ± 0.009 vs. 0.051 ± 0.004, P≤ 0.05, Fig. 3D and E) as well as muscle capillarisation (CD: +17%, 273 ± 13 vs. 233 ± 12, P≤ 0.05; C/F: +24%, 2.08 ± 0.05 vs. 1.67 ± 0.07, P≤ 0.001, Fig. 3F). However, both PECAM protein expression and capillarisation remained lower in TR-ZDF compared to Lean animals (PECAM: −31%, 0.087 ± 0.009 vs. 0.125 ± 0.015, P≤ 0.05 (Fig. 3D and E); CD: −17%, 273 ± 13 vs. 329 ± 7, P≤ 0.01; C/F: −11%, 2.08 ± 0.05 vs. 2.33 ± 0.05, P≤ 0.05, Fig. 3F).
Angiomotin expression in obese and non-obese animals
All plantaris muscles from sedentary (SED) and trained (TR) Sprague–Dawley rats fed with either a standard (SD) or a high fat diet (HFD) expressed both p80 and p130 angiomotin isoforms (Fig. 4A).
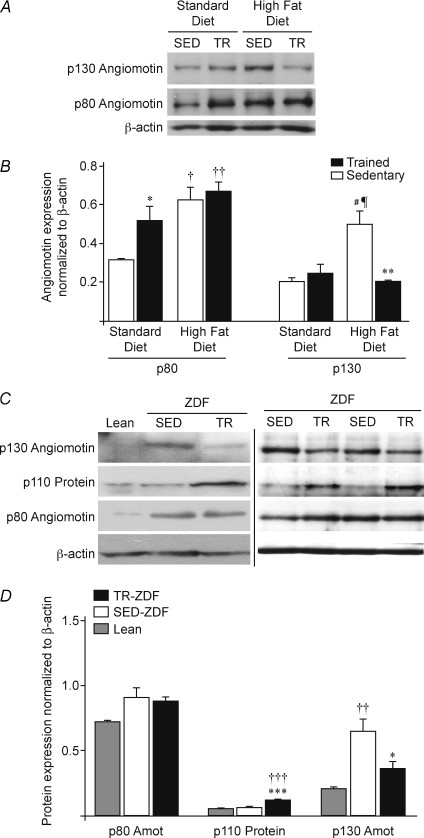
Figure 4. Expression of angiomotin isoforms in response to exercise training.
A, representative Western blots for p80 and p130 angiomotin (Amot) protein expression in plantaris muscles from sedentary (SED) and trained (TR) Sprague–Dawley rats fed with either standard (SD) or high-fat (HFD) diets. β-Actin protein detection was used as a loading control. B, densitometric analysis of p80 and p130 expression in the four experimental groups (SED-SD, SED-FHD, TR-SD and TR-HFD, n= 6 rats/group). Data are presented as means ±s.e.m. Significant effect of exercise training: *P≤ 0.05; **P≤ 0.01. Significant differences: versus SED-SD (†P≤ 0.05; ††P≤ 0.01); versus SED-SD (#P≤ 0.05); versus TR-SD (¶P≤ 0.05). C, representative Western blots for p80 and p130 angiomotin (Amot), as well as p110 protein expression in plantaris muscles from Lean, sedentary (SED) or trained (TR) ZDF rats. β-Actin protein detection was used as a loading control. D, densitometric analysis of p80, p110 and p130 proteins in Lean, SED-ZDF and TR-ZDF. Data are presented as means ±s.e.m. (n= 9 Lean, n= 8 SED-ZDF, n= 8 TR-ZDF). Significant effect of exercise training: *P≤ 0.05; ***P≤ 0.001. Significantly different from Lean: ††P≤ 0.01; †††P≤ 0.001.
Sedentary HFD rats significantly expressed more p80 (+97%) and p130 (+145%) than sedentary SD animals (respectively 0.63 ± 0.06 vs. 0.32 ± 0.01, P≤ 0.05; and 0.50 ± 0.07 vs. 0.20 ± 0.02, P≤ 0.05, Fig. 4B). Expressions of p80 and p130 angiomotin were differently affected by exercise training in SD and HFD groups (Fig. 4A and B). In SD rats, no change was detected for p130 angiomotin (0.20 ± 0.02 in SED-SD vs. 0.25 ± 0.05 in TR-SD) whereas p80 expression was significantly increased by 62% in trained animals (0.32 ± 0.01 in SED-SD vs. 0.52 ± 0.08 in TR-SD, P≤ 0.05). In contrast, exercise training did not affect p80 expression in HFD rats (0.63 ± 0.06 in SED-HFD vs. 0.67 ± 0.05 in TR-HFD), whereas it decreased p130 level by 59% (0.50 ± 0.07 in SED-HFD vs. 0.21 ± 0.01 in TR-HFD, P≤ 0.05).
Angiomotin response to voluntary exercise in Zucker Diabetic Fatty rats
Expression of p80 and p130 angiomotin proteins was analysed in plantaris muscles from sedentary Lean animals as well as in sedentary (SED-ZDF) and trained (TR-ZDF) ZDF rats (Fig. 4C and D). No significant variation was observed for p80 angiomotin between Lean and SED-ZDF (0.71 ± 0.01 vs. 0.90 ± 0.07). In contrast, p130 angiomotin expression was 224% higher in SED-ZDF compared to Lean animals (0.64 ± 0.10 vs. 0.20 ± 0.01, P≤ 0.01). As previously observed in TR-HFD rats, p80 angiomotin was not affected by exercise training in TR-ZDF compared to SED-ZDF (0.86 ± 0.05 vs. 0.90 ± 0.07) whereas the p130 isoform was significantly decreased by 44% (0.36 ± 0.06 vs. 0.64 ± 0.10, P≤ 0.05). Despite this significant decrease, the p130 level remained 82% higher in TR-ZDF than in Lean animals, although the difference was not significant. The p110 protein was strongly and significantly increased in TR-ZDF muscles by respectively 91% (P≤ 0.001) and 155% (P≤ 0.001) when compared to SED-ZDF and Lean samples (0.118 ± 0.004 in TR-ZDF, 0.062 ± 0.006 in SED-ZDF, and 0.046 ± 0.007 in Lean).
Angiomotin p80/p130 ratio in response to exercise training
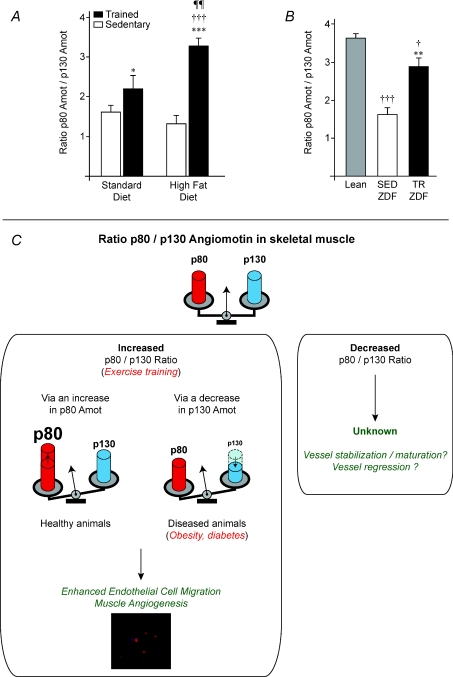
The ratio between p80 and p130 angiomotin isoforms was significantly increased in response to exercise in all conditions (SD, HFD and ZDF; Fig. 5A and B). The p80/p130 ratio was increased in response to programmed exercise by 36% in SD (1.62 ± 0.15 vs. 2.20 ± 0.34, P≤ 0.05) and by 148% in the HFD group (3.27 ± 0.21 vs. 1.32 ± 0.21, P≤ 0.001, Fig. 5A). In our ZDF model, obese and diabetic SED-ZDF rats expressed a 56% lower p80/p130 ratio than Lean animals (1.61 ± 0.19 vs. 3.62 ± 0.12, P≤ 0.001, Fig. 5B). Voluntary exercise significantly increased this ratio by 78% between TR-ZDF and SED-ZDF (2.85 ± 0.25 vs. 1.61 ± 0.19, P≤ 0.01). Despite such exercise-induced increase, the ratio remained 21% lower in obese but non-diabetic TR-ZDF compared to Lean animals (P≤ 0.05).
Figure 5. Change in angiomotin p80/p130 ratio in response to exercise training.
A, angiomotin isoforms ratio in sedentary (SED) and trained (TR) Sprague–Dawley rats fed with standard (SD) or high fat (HFD) diets. Significant effect of exercise training: *P≤ 0.05; ***P≤ 0.001. Significant differences: TR-HFD vs. SED-SD (†††P≤ 0.001); TR-HFD vs. TR-SD (¶¶P≤ 0.01). Data are presented as means ±s.e.m. (n= 6 rats/group). B, p80/p130 ratio in sedentary Lean, sedentary (SED-ZDF) and voluntary trained (TR-ZDF) Zucker Diabetic Fatty rats. Data are presented as means ±s.e.m. (n= 9 Lean, n= 8 SED-ZDF, n= 8 TR-ZDF). Significant effect of exercise training: **P≤ 0.01. Significantly different from Lean: †P≤ 0.05; †††P≤ 0.001. C, schematic illustration of angiomotin p80/p130 ratio as a new indicator of muscle angio-adaptation.
MYPT and IRS-1 phosphorylation and total angiomotin level
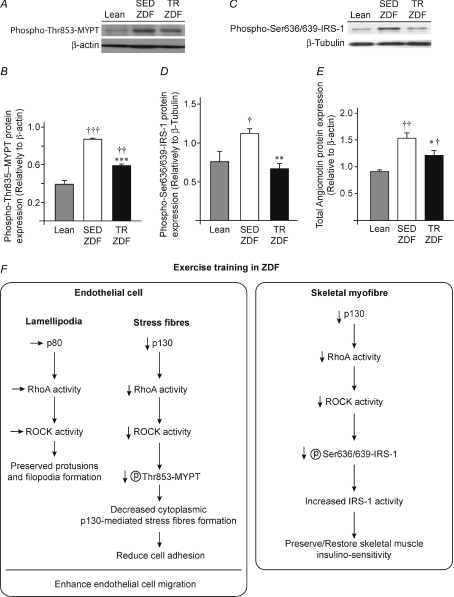
Phosphorylated protein myosin phosphatase target subunit-1 (MYPT) on residue threonine 853 and insulin receptor substrate-1 (IRS-1) on serine residues 636/639 were measured by Western blotting (Fig. 6A–D). Phospho-MYPT was increased by 124% in sedentary ZDF (P≤ 0.001) and by 50% in trained ZDF (P≤ 0.01) compared to Lean animals (respectively 0.86 ± 0.02 and 0.58 ± 0.03 vs. 0.39 ± 0.05, Fig. 6A and B). Exercise training decreased phospho-MYPT by 33% in TR-ZDF compared to SED-ZDF (P≤ 0.001). Phospho-IRS-1 protein expression was higher in SED-ZDF than in Lean animals (+48%, 1.11 ± 0.07 vs. 0.75 ± 0.15, P≤ 0.05, Fig. 6C and D). Exercise training decreased phospho-IRS-1 protein expression by 40% (P≤ 0.01) in TR-ZDF (0.67 ± 0.06) and no significant difference was observed between TR-ZDF and Lean animals. Total angiomotin protein expression (sum of p80 + p130 isoforms) was increased by 69% in SED-ZDF (1.54 ± 0.10, P≤ 0.01) and by 34% in TR-ZDF rats (1.22 ± 0.10, P≤ 0.05) compared to Lean animals (0.91 ± 0.02) (Fig. 6G). Exercise training decreased by 32% total angiomotin expression in TR-ZDF (1.22 ± 0.10 vs. 1.54 ± 0.10, P≤ 0.05). However, total angiomotin in TR-ZDF remained 34% higher than in Lean animals (P≤ 0.05). Interestingly in ZDF animals, phospho-IRS-1, phospho-MYPT and total angiomotin protein levels were similarly reduced by exercise training (−40%, −33% and −32%, respectively).
Figure 6. MYPT and IRS-1 activation.
A, representative Western blots for phospho-MYPT on serine 853 in plantaris muscles from Lean, sedentary (SED) or trained (TR) ZDF rats. β-Actin protein detection was used as a loading control. B, densitometric analysis of phospho-MYPT protein expression. C, representative Western blots for phospho-IRS-1 on serine 636/639 in plantaris muscles from Lean, sedentary (SED) or trained (TR) ZDF rats. αβ-Tubulin protein detection was used as a loading control. D, densitometric analysis of phospho-IRS-1 serine 636/639 protein expression. E, total angiomotin expression (p80+p130) in Lean, SED-ZDF and TR-ZDF. Data obtained from densitometric analysis represented in Fig. 4D. Data in all panels are presented as means ±s.e.m. (n= 9 Lean, n= 8 SED-ZDF, n= 8 TR-ZDF). Significant effect of exercise training: *P≤ 0.05; **P≤ 0.01; ***P≤ 0.001. Significantly different from Lean: †P≤ 0.05; ††P≤ 0.01; †††P≤ 0.001. F, potential mechanism by which exercise training might affect angiomotin/RhoA/ROCK signalling pathway.
Discussion
The remarkable plasticity of the vascular network in skeletal muscle must be tightly regulated in response to physiological or pathological conditions to prevent either excessive or insufficient capillarisation and to maintain an optimal muscle function (Hudlicka et al. 1992; Birot & Bigard, 2003). Angiomotin appears as an important actor for in vivo angiogenesis as well as for the maintenance of established vessels (Holmgren et al. 2006; Aase et al. 2007; Levchenko et al. 2008). Although initially described in endothelial cells from tumours, angiomotin was also identified in other cell types such as epithelial cells, and in various healthy tissues such as skeletal muscle and retina (Troyanovsky et al. 2001; Bratt et al. 2002; Shimono & Behringer, 2003; Wells et al. 2006). To our knowledge, the present study demonstrates for the first time how angiomotin expression is regulated in skeletal muscle in response to physiological and pathological conditions. We confirmed angiomotin protein expression in skeletal muscle and clearly identified its presence not only in capillaries but also in myofibres. The platelet endothelial cell adhesion molecule-1 (PECAM) is a commonly used marker for endothelial cells and capillaries. The strong and significant correlation between angiomotin and PECAM protein expression in soleus, plantaris and extensor digitorum longus skeletal muscles suggests that although not restricted to blood vessels, angiomotin expression seems to represent a good indicator of skeletal muscle capillarisation.
We investigated then whether angiomotin expression was affected in plantaris muscle in response to endurance training, a well described physiological stimulus for skeletal muscle angiogenesis (Hudlicka et al. 1992). We found that exercise training significantly stimulated p80 angiomotin expression by 62% in healthy female Sprague–Dawley rats. Previous studies have shown that p80 angiomotin strongly stimulated in vitro and in vivo the migration of endothelial cells and thus appeared as the main angiogenic isoform (Levchenko et al. 2003; Holmgren et al. 2006; Aase et al. 2007; Ernkvist et al. 2008). Together with our present data, these findings supported the idea that p80 angiomotin could play an important role during exercise-induced muscle angiogenesis. In accordance with this, the efficiency of our exercise training protocol in enhancing muscle capillarisation was determined by measuring three capillary parameters: PECAM protein expression, capillary density and capillary-to-fibre ratio. In healthy Sprague–Dawley rats, exercise training significantly increased all these parameters. In addition, training also significantly increased COX-IV protein expression in plantaris. Such protein measurement has previously been shown to strongly reflect COX activity and mitochondrial content (Sheehan et al. 2004).
Obesity and type-2 diabetes are pathological conditions often associated with both vascular regression and impairment for inducing skeletal muscle angiogenesis (Rivard et al. 1999; Frisbee 2002; Frisbee et al. 2007). Endurance training was recently shown to efficiently blunt capillary rarefaction in skeletal muscle from obese rats (Frisbee et al. 2006). We thus investigated whether angiomotin response to exercise training still persisted in obese rats. Female Sprague–Dawley rats developed obesity when fed with a high-fat diet (HFD) as evidenced in our study by the significant increase in visceral fat tissue and body weight. Zucker Diabetic Fatty (ZDF) rats progressively developed obesity and type-2 diabetes (Bergeron et al. 2006). Whereas our sedentary ZDF rats became indeed obese and diabetic, voluntary exercise training efficiently blunted diabetes development. Our trained ZDF rats thus became obese but never diabetic.
In HFD and ZDF animals, exercise training surprisingly induced an opposite effect on angiomotin expression compared to healthy SD animals. p80 angiomotin was unaffected by exercise training in obese HFD and ZDF rats, whereas p130 angiomotin expression was respectively decreased by 59% and 44%. Such an effect was not due to the sex of the animals as we observed the same response in male ZDF and female Sprague–Dawley HFD rats. This was also independent of the mode of training as forced treadmill training and spontaneous voluntary training both led to similar angiomotin regulation. p130 angiomotin was recently shown to promote vessel stabilization and maturation (Aase et al. 2007; Ernkvist et al. 2008). Thus, we could speculate that any decrease in p130 angiomotin expression could enhance vessel destabilization, an indispensable key event of the angiogenic process. Obese animals, unable to increase p80 expression in response to exercise, might instead decrease their p130 level as an alternative mechanism to maintain angiogenic ability in skeletal muscle.
Our hypothesis was supported by measuring plantaris muscle capillarisation. PECAM expression, capillary density and capillary-to-fibre ratio were not statistically different between sedentary HFD and sedentary SD Sprague–Dawley rats. Interestingly, exercise training significantly and similarly increased these parameters in both SD and HFD groups.
In contrast, we observed some capillary regression in sedentary ZDF compared to sedentary Lean, as evidenced by significantly lower PECAM expression, capillary density and capillary-to-fibre ratio. In this model, we decided then to investigate whether voluntary exercise training could preserve muscle capillarisation in ZDF animals. Interestingly, exercise training significantly improved all three capillary parameters in trained ZDF rats. However, PECAM expression, capillary density and capillary-to-fibre ratio remained significantly lower in trained ZDF compared to sedentary Lean animals. Interestingly, this was perfectly associated with the changes in p130 angiomotin expression. Sedentary ZDF expressed more p130 than Lean rats. Despite a decrease in response to exercise training, the p130 level remained higher in trained ZDF compared to sedentary Lean animals.
Altogether, our data suggest that exercise-induced angiogenesis might require an increase of the ratio between p80 and p130 isoforms. In healthy non-obese rats, this was achieved through the increased expression of p80. Such increase was impaired by obesity. However, the exercise-induced decrease in p130 in obese rats seems sufficient to increase the p80/p130 ratio and to preserve exercise-induced angiogenic activity in muscle tissue. Such a ratio might then represent a new indicator to estimate in vivo skeletal muscle angio-adaptation. In contrast to what we observed in response to exercise, it would be very interesting to investigate whether any decrease in the p80/p130 ratio would enhance vessel stabilization or even lead to some vascular regression. The potential implication of angiomotin p80/p130 ratio in determining skeletal muscle angio-adaptation is illustrated in Fig. 5C.
Modulation of the p80/p130 ratio during the angiogenic process has already been described in mouse retina (Ernkvist et al. 2008). Newborn mice present an avascular retina and the capillary network immediately starts to develop after birth (Fruttiger, 2002; Gerhardt et al. 2003). Capillaries spread from the centre of the retina toward the periphery within the first week post-birth. The angiogenic activity is very intense as endothelial cells migrate to form vessels and only p80 angiomotin was detected during this period. At day 7 post-birth, the newly formed capillaries at the surface of the retina stabilize and mature. At this time, p80 angiomotin expression had decreased and p130 angiomotin was now detectable. In adult retina, only p130 angiomotin was expressed (Ernkvist et al. 2008). In addition to its stabilization role, this supports the hypothesis that low levels of p130 are required to obtain a maximal angiogenic activity.
Moreover in the study from Ernkvist and co-workers, a specific band at 110 kDa was strongly detected at day 7 and then progressively decreased whereas p130 angiomotin expression increased. The authors did not comment on this p110 protein since it was unfortunately not cloned and was unidentified. We also observed this p110 protein in our ZDF rats. As we used a different anti-angiomotin antibody (Wells et al. 2006) than Ernkvist and co-workers (Ernkvist et al. 2008), we could speculate that p110 might represent a transition isoform between p80 and p130 angiomotin. Interestingly, p110 was significantly increased in response to exercise training. The question of whether p110 protein could represent a transition angiomotin isoform between the decreasing p130 and the increasing p80 remains to be answered, and furthers investigations will be needed to address the issue.
Angiomotin p80 and p130 isoforms are both expressed in tight junctions. However, the p80 isoform is specifically expressed in lamellipodia while p130 is associated to stress fibres (Bratt et al. 2005; Ernkvist et al. 2006, 2009). This could explain each isoform specific function. Interestingly, both p80 and p130 functions might require modulation of RhoA and Rho-associated kinase (ROCK) signalling (Ernkvist et al. 2009). By increasing RhoA activity in lamellipodia, p80 enhances cell migration, whereas p130 controls cell shape and cell static adhesion by modulating stress fibre formation also via a ROCK-dependent mechanism (Bratt et al. 2005; Ernkvist et al. 2006). Altogether these data suggest that both p80 and p130 could modulate the RhoA–ROCK signalling pathway. Phosphorylation of myosin phosphatase target subunit-1 (MYPT) is a good indicator of ROCK activity (Kanda et al. 2006). Interestingly, MYPT is involved in cell migration ability (Garcia et al. 1998; Somlyo et al. 2003). ROCK inactivates MYPT by phosphorylation on Thr853, thus leading to activation of myosin light chain MLC, favouring in turn stress fibre formation (Matsumura et al. 2001). Any decrease in angiomotin–RhoA–ROCK signalling would then contribute to higher activated MYPT, lower activated MLC, and stress fibre disorganisation. Total angiomotin and phospho-MYPT were both higher in sedentary ZDF than in Lean animals. Interestingly, exercise training significantly lowered them both. The observed decrease in total angiomotin expression was achieved by decreasing p130 expression without altering the p80 level. This would result in preserving p80 RhoA activity in the leading front of migrating cells (Ernkvist et al. 2008). p130-mediated ROCK activity would be decreased, thus disorganising stress fibres. Altogether, such events would enhance endothelial cell migration ability.
One surprising finding in our study was the preservation of normoglycaemia in spontaneously trained ZDF rats. It was then very tempting to imagine that angiomotin might also play a role in such an exercise training effect via the RhoA–ROCK pathway. Phosphorylation of insulin receptor substrate-1 (IRS-1) on serine residues inhibits its function and could induce insulin resistance (White 2002). Interestingly, ROCK has been identified as the kinase responsible for IRS-1 phosphorylation on serine residues 636/639 in humans, equivalent to serine 632/635 in mouse (Furukawa et al. 2005). Such phosphorylations were previously observed in skeletal muscle cells from patients with type-2 diabetes, and shown to reduce the insulin-signalling pathway (Bouzakri et al. 2003). Sedentary ZDF rats expressed more phospho-(Ser636/639)-IRS-1 than Lean animals. Interestingly, exercise training counteracted IRS-1 phosphorylation in ZDF rats. As angiomotin was also detected in myofibres, any decrease in its expression level could prevent such IRS-1 inhibitory phosphorylation, thus maintaining IRS-1 function and glycaemia control.
We could then hypothesise that exercise training, by reducing angiomotin expression in obese animals, could inhibit the angiomotin–RhoA–ROCK pathways, thus contributing to enhance endothelial cells’ migratory ability and to preserve muscle insulino-sensitivity (Fig. 6F illustrated such a potential mechanism).
In conclusion our study shows that angiomotin isoforms were differently affected by exercise training between skeletal muscles from obese and non-obese animals. As a new and original physiological concept, we propose that the angiomotin p80/p130 ratio could reflect skeletal muscle angio-adaptation. Such findings are of high interest since they provide the first evidence that angiomotin is tightly regulated in both healthy and diseased tissues in response to a physiological stimulus. As several anti-angiogenic therapeutic strategies are currently under development to inhibit systemically or locally angiomotin function, our results strongly reiterate the necessity to better characterize angiomotin expression and function in healthy tissues in order to prevent any harmful side-effects arising from these therapeutic approaches.
Acknowledgments
We thank Dr Tony Pawson (Samuel Lunenfeld Research Institute, Toronto) for kindly providing the anti-Amot antibody, M. Christian Charbonneau (IRIC, University of Montreal) for his help in microscopy, Dr David A. Hood (MHRC, York University, Toronto) for kindly providing COX-IV antibody, and Dr Tara L. Haas for sharing laboratory space. Catherina Le Bel was supported by a summer NSERC award (NSERC-USRA). This study was supported by funding from the Natural Sciences and Engineering Research Council of Canada (NSERC Individual Discovery Grant no. 341258).
Glossary
Abbreviations
- COX-IV
cytochrome c oxidase subunit IV
- EWAT
epididymal white adipose tissue
- HFD
high-fat diet
- IRS-1
insulin receptor substrate-1
- MYPT
myosin phosphatase target subunit
- SD
standard diet
- SED
sedentary
- TR
trained
- VEGF
vascular endothelial growth factor
- ZDF
Zucker Diabetic Fatty
Author contributions
E.R. and O.B. contributed to the design of the study, performed experiments, analysed and interpreted data, and wrote the manuscript. N.C., S.D., C.G., and C.LeB. performed experiments and analysed data. R.B. and J.-M.L. contributed to the design of animal models and to the interpretation of the data. All authors contributed to the drafting and revision of the manuscript content and gave their final approval of the version to be published. Experiments were carried out at the University of Montreal (Department of Kinesiology) and at York University, Toronto (Muscle Health Research Center).
References
- Aase K, Ernkvist M, Ebarasi L, Jakobsson L, Majumdar A, Yi C, Birot O, Ming Y, Kvanta A, Edholm D, Aspenstrom P, Kissil J, Claesson-Welsh L, Shimono A, Holmgren L. Angiomotin regulates endothelial cell migration during embryonic angiogenesis. Genes Dev. 2007;21:2055–2068. doi: 10.1101/gad.432007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron R, Yao J, Woods JW, Zycband EI, Liu C, Li Z, Adams A, Berger JP, Zhang BB, Moller DE, Doebber TW. Peroxisome proliferators-activated receptor (PPAR)-α agonism prevents the onset of type 2 diabetes in Zucker diabetic fatty rats: A comparison with PPARγ agonism. Endocrinology. 2006;142:4252–4262. doi: 10.1210/en.2005-1535. [DOI] [PubMed] [Google Scholar]
- Birot O, Bigard AX. Responses of the capillary bed in trained skeletal muscle. Sci Sports. 2003;18:1–10. [Google Scholar]
- Birot OJ, Koulmann N, Peinnequin A, Bigard XA. Exercise-induced expression of vascular endothelial growth factor mRNA in rat skeletal muscle is dependent on fibre type. J Physiol. 2003;552:213–221. doi: 10.1113/jphysiol.2003.043026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzakri K, Roques M, Gual P, Espinosa S, Guebre-Egziabher F, Riou JP, Laville M, Le Marchand-Brustel Y, Tanti JF, Vidal H. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes. 2003;52:1319–1325. doi: 10.2337/diabetes.52.6.1319. [DOI] [PubMed] [Google Scholar]
- Bratt A, Wilson WJ, Troyanovsky B, Aase K, Kessler R, Meir EGW, Holmgren L. Angiomotin belongs to a novel protein family with conserved coiled-coil and PDZ binding domains. Gene. 2002;298:69–77. doi: 10.1016/s0378-1119(02)00928-9. [DOI] [PubMed] [Google Scholar]
- Bratt A, Birot O, Sinha I, Veitonmaki N, Aase K, Ernkvist M, Holmgren L. Angiomotin regulates endothelial cell-cell junctions and cell motility. J Biol Chem. 2005;280:34859–34869. doi: 10.1074/jbc.M503915200. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Ernkvist M, Aase K, Ukomadu C, Wohlschlegel J, Blackman R, Veitonmaki N, Bratt A, Dutta A, Holmgren L. p130-Angiomotin associates to actin and controls endothelial cell shape. FEBS J. 2006;273:2000–2011. doi: 10.1111/j.1742-4658.2006.05216.x. [DOI] [PubMed] [Google Scholar]
- Ernkvist M, Birot O, Sinha I, Veitonmaki N, Nyström S, Aase K, Holmgren L. Differential roles of p80- and p130-angiomotin in the switch between migration and stabilization of endothelial cells. Biochim Biophys Acta. 2008;1783:429–437. doi: 10.1016/j.bbamcr.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Ernkvist M, Persson NL, Audebert S, Lecine P, Sinha I, Liu M, Schlueter M, Horowitz A, Aase K, Weide T, Borg JP, Majumdar A, Holmgren L. The Amot/Patj/Syx signaling complex spatially controls RhoA GTPase activity in migrating endothelial cells. Blood. 2009;113:244–253. doi: 10.1182/blood-2008-04-153874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee JC. Obesity, insulin resistance, and microvessel density. Microcirculation. 2002;298:69–77. doi: 10.1080/10739680701282945. [DOI] [PubMed] [Google Scholar]
- Frisbee JC, Samora JB, Peterson J, Bryner R. Exercise training blunts microvascular rarefaction in the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2006;291:H2483–H2492. doi: 10.1152/ajpheart.00566.2006. [DOI] [PubMed] [Google Scholar]
- Frisbee JC, Samora JB, Basile DP. Angiostatin does not contribute to skeletal muscle microvascular rarefaction with low nitric oxide bioavailability. Microcirculation. 2007;14:145–153. doi: 10.1080/10739680601131242. [DOI] [PubMed] [Google Scholar]
- Fruttiger M. Development of the mouse retinal vasculature: angiogenesis versus vasculogenesis. Invest Ophtalmol Vis Sci. 2002;43:522–527. [PubMed] [Google Scholar]
- Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ, Lee YH, Kaibuchi K, Kahn BB, Masuzaki H, Kim JK, Lee SW, Kim YB. Cell Metab. 2005;2:119–129. doi: 10.1016/j.cmet.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Verin AD, Herenyiova M, English D. Adherent neutrophils activate endothelial myosin light chain kinase: role in transendothelial migration. J Appl Physiol. 1998;84:1817–1821. doi: 10.1152/jappl.1998.84.5.1817. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Physiol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren L, Ambrosino E, Birot O, Tullus C, Veitonmaki N, Levchenko T, Carlson L-M, Musiani P, Lezzi M, Curcio C, Forni G, Cavallo F, Kiessling R. A DNA vaccine targeting angiomotin inhibits angiogenesis and suppresses tumor growth. Proc Natl Acad Sci U S A. 2006;103:9208–9213. doi: 10.1073/pnas.0603110103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudlicka O. What makes blood vessels grow? J Physiol. 1991;444:1–24. doi: 10.1113/jphysiol.1991.sp018863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev. 1992;72:369–417. doi: 10.1152/physrev.1992.72.2.369. [DOI] [PubMed] [Google Scholar]
- Jiang WG, Watkins G, Douglas-Jones A, Holmgren L, Mansel RE. Angiomotin and angiomotin like proteins, their expression and correlation with angiogenesis and clinical outcome in human breast cancer. BMC Cancer. 2006;23:6–16. doi: 10.1186/1471-2407-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T, Wakino S, Homma K, Yoshioka K, Tatematsu S, Hasegawa K, Takamatsu I, Sugano N, Hayashi K, Saruta T. Rho-kinase as a molecular target for insulin resistance and hypertension. FASEB J. 2006;20:169–171. doi: 10.1096/fj.05-4197fje. [DOI] [PubMed] [Google Scholar]
- Levchenko T, Aase K, Troyanovsky B, Bratt A, Holmgren L. Loss of responsiveness to chemotactic factors by deletion of the C-terminal protein interaction site of angiomotin. J Cell Sci. 2003;116:3803–3810. doi: 10.1242/jcs.00694. [DOI] [PubMed] [Google Scholar]
- Levchenko T, Bratt A, Arbiser JL, Holmgren L. Angiomotin expression promotes hemangioendothelioma invasion. Oncogene. 2004;23:1469–1473. doi: 10.1038/sj.onc.1207264. [DOI] [PubMed] [Google Scholar]
- Levchenko T, Veitonmaki N, Lundkvist A, Gerhardt H, Ming Y, Berggren K, Kvanta A, Carlsson R, Holmgren L. Therapeutic antibodies targeting angiomotin inhibits angiogenesis in vivo. FASEB J. 2008;22:880–889. doi: 10.1096/fj.07-9509com. [DOI] [PubMed] [Google Scholar]
- Matsumura F, Totsukawa G, Yamakita Y, Yamashiro S. Role of myosin light chain phosphorylation in the regulation of cytokinesis. Cell Struct Funct. 2001;26:639–644. doi: 10.1247/csf.26.639. [DOI] [PubMed] [Google Scholar]
- Prior BM, Yang HT, Terjung RL. What makes vessels grow with exercise training? J Appl Physiol. 2004;97:1119–1128. doi: 10.1152/japplphysiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, Peters K, Isner JM. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999;154:355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivilis I, Milkiewicz M, Boyd P, Goldstein J, Brown MD, Egginton S, Hansen FM, Hudlicka O, Haas TL. Differential involvement of MMP-2 and VEGF during muscle stretch- versus shear stress-induced angiogenesis. Am J Physiol Heart Circ Physiol. 2002;283:H1430–H1438. doi: 10.1152/ajpheart.00082.2002. [DOI] [PubMed] [Google Scholar]
- Sheehan TE, Kumar PA, Hood DA. Tissue-specific regulation of cytochrome c oxidase subunit expression by thyrid hormone. Am J Physiol. 2004;286:968–974. doi: 10.1152/ajpendo.00478.2003. [DOI] [PubMed] [Google Scholar]
- Shimono A, Behringer RR. Angiomotin regulates visceral endoderm movements during mouse embryogenesis. Curr Biol. 2003;13:613–617. doi: 10.1016/s0960-9822(03)00204-5. [DOI] [PubMed] [Google Scholar]
- Somlyo AV, Phelps C, Dipierro C, Eto M, Read P, Barrett M, Gibson JJ, Burnitz MC, Myers C, Somlyo AP. Rho kinase and matrix metalloproteinase inhibitors cooperate to inhibit angiogenesis and growth of human prostate cancer xenotransplants. FASEB J. 2003;17:223–234. doi: 10.1096/fj.02-0655com. [DOI] [PubMed] [Google Scholar]
- Stapleton PA, James ME, Goodwill AG, Frisbee JC. Obesity and vascular dysfunction. Pathophysiology. 2008;15:79–89. doi: 10.1016/j.pathophys.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyanovsky B, Levchenko T, Mansson G, Matvijenko O, Holmgren L. Angiomotin: An angiostatin binding protein that regulates endothelial cell migration and tube formation. J Cell Biol. 2001;152:1247–1254. doi: 10.1083/jcb.152.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells CD, Fawcett JP, Traweger A, Yamanaka Y, Goudreault M, Elder K, Kulkarni S, Gish G, Virag C, Lim C, Coldwill K, Starostine A, Metalnikov P, Pawson T. A rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell. 2006;125:535–548. doi: 10.1016/j.cell.2006.02.045. [DOI] [PubMed] [Google Scholar]
- White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002;283:E413–422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]