Abstract
Rapid, non-genomic actions of oestradiol-17β (E2) on hypothalamic neurones that may be relevant to reproductive function were described decades ago. The orphan G protein-coupled receptor GPR30, recently shown to bind oestrogens and to trigger rapid signalling in vitro, is expressed in several rat and human brain regions, including the hypothalamus. We used two complementary approaches to investigate the role of GPR30 in hypothalamic responses to E2 that are relevant to reproductive physiology. Serial blood sampling following acute administration of the selective GPR30 agonist G1 was used to assess the role of GPR30 in short latency negative feedback inhibition of luteinising hormone (LH) secretion and facilitation of prolactin (PRL) secretion in ovariectomised female rats. In vivo RNA interference (RNAi), mediated by adeno-associated virus-expressing small hairpin RNA (shRNA) infused into the mediobasal hypothalamus, was used to study the effects of GPR30 knockdown on these rapid responses to E2. Longer-term actions of E2 on female sexual behaviour (lordosis) were also examined in female rats subjected to in vivo RNAi. Administration of E2 or G1 triggered a short latency surge of PRL secretion, and animals subjected to GPR30 RNAi showed significantly less E2-dependent PRL release than animals receiving control virus. G1 did not mimic E2 negative feedback inhibition of LH secretion, and GPR30 RNAi did not interfere with E2 suppression of LH or facilitation of lordosis behaviour. These findings suggest that activation of GPR30 promotes short latency PRL secretion but does not mediate E2 negative feedback inhibition of LH secretion or E2 facilitation of female reproductive behaviour.
Five keywords: GPR30, oestrogens, prolactin, LH, lordosis
INTRODUCTION
Oestradiol-17β (E2), the major oestrogen secreted by mammalian ovaries, has widespread effects on the brain, including regulation of neural development, reproductive function, cognition, and neurone survival and repair (1–4). Oestrogens work via multiple mechanisms involving nuclear receptors and putative membrane receptors. Oestrogens have rapid actions that can be observed within seconds to minutes, strongly suggesting that receptors expressed at the plasma membrane participate in mediating cellular responses to E2 (5). Some studies demonstrate that classical oestrogen receptors (ER)-α and ER-β are expressed at the plasma membrane (6–8). However, competitive ER-α/ER-β antagonists (e.g., ICI 182,780) do not block all membrane signalling events initiated by E2, and certain responses to E2 remain in neurones lacking ER-α and ER-β (reviewed in (9)). Thus, receptors other than ER-α and ER-β likely play a key role in rapid E2 signalling. The orphan G-protein-coupled receptor GPR30 was recently reported to bind oestrogens with an affinity similar to ER-α and ER-β (10, 11), suggesting that this receptor may participate in the pleiotropic actions of oestrogens at the cell membrane (12, 13). In cell lines, E2 binding to GPR30 stimulates cAMP production, calcium mobilization and regulation of growth factor signalling pathways leading to the activation of MAPK (14–18). Despite these promising in vitro results, the physiological role of this receptor as a mediator of E2 action in the brain is unknown.
Rapid actions of E2 on hypothalamic neurones were described decades ago and may involve G-proteins (19). Several well-defined aspects of reproductive physiology and behaviour that are regulated by ovarian hormone action in the hypothalamus can be utilised to assay the role of GRP30 in E2 actions. These include the release of pituitary gonadotropins (luteinising hormone, LH) that regulate ovulation and mating behaviours (e.g., lordosis). Actions of E2 in the preoptic area (POA) and the ventromedial nucleus of the hypothalamus (VMH) ensure that female rats exhibit mating behaviour, especially lordosis, at the time of the preovulatory LH surge. E2 is also a major stimulator of prolactin (PRL) synthesis and secretion (20, 21). E2 triggers short latency PRL secretion, and this is thought to involve suppression of the inhibitory input of hypothalamic dopamine (DA) neurones (22). Whether this short latency action of E2 involves hormone interaction with a membrane receptor is still unclear.
As the newly discovered oestrogen binding protein GPR30 is expressed in several brain regions including the hypothalamus (23–25), the present study investigated the role of this receptor as a potential mediator of well-characterised neuroendocrine actions of E2 in the hypothalamus. We used in vivo administration of the selective GPR30 agonist G1 (26) and in vivo RNA interference (RNAi) of GPR30 expression in the hypothalamus to assess the role of GPR30 in mediating rapid negative feedback regulation of LH secretion and enhancement of PRL secretion. Longer-term E2 actions on lordosis behaviour were also examined in female rats subjected to GPR30 RNAi to test the hypothesis that membrane GPR30 activation is necessary for E2 facilitation of lordosis behaviour.
MATERIAL AND METHODS
Materials and Reagents
Unless otherwise noted, all cell culture and transfection reagents were purchased from InVitrogen (Carlsbad, CA, USA). E2, E2 benzoate (EB), and progesterone (P) were obtained from Steraloids, Inc. (Newport, RI, USA). The GPR30 agonist G1 was provided by ChemDiv (San Diego, CA, USA) or purchased from Calbiochem (San Diego, CA, USA). Cannulae were purchased from Plastics One (Roanoke, VA, USA). Stereotaxic injections of virus were performed using an UltraMicroPump II (World Precision Instruments, Sarasota, FL, USA). Other reagents were obtained from Sigma (St. Louis, MO, USA) or Fisher Scientific (Pittsburgh, PA, USA).
Animals
Adult female Sprague-Dawley rats (150–190 g) purchased from Taconic Farms (Germantown, NY, USA) were housed 3 per cage and maintained on a 14L:10D cycle (lights-off at 2000 h) with standard laboratory chow and tap water freely available. Animals were ovariohysterectomised (OVX) under ketamine (80mg/kg)/xylazine (4 mg/kg) anaesthesia. All procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the Albert Einstein Collegeof Medicine.
Intracerebral guide cannula implantation
Anesthetised rats were placed in a Kopf stereotaxic apparatus and secured with ear bars and a nose piece set at +5 mm. Using Bregma as a landmark and stereotaxic coordinates provided in the atlas of Pellegrino et al.(27) (A/P, +0.2 mm; M/L, +0.0 mm; D/V, −9.8 mm), a 22 ga guide cannula was implanted in the third ventricle. Cannulae were fixed with dental cement, and a dummy cannula was inserted to prevent clogging until the day of drug infusion. After surgery, animals were housed singly. Cannula placement was verified at theend of the experiments by dye injection.
Jugular vein catheterization, collection of serial blood samples, LH and PRL assay
Animals were lightly anaesthetised with ketamine/xylazine as described above, and an indwelling jugular vein catheter was placed in the right atrium. After catheter implantation, animals were housed singly and kept in a low stress environment (no loud sounds, no bedding or cage change, no room change) for the duration of the experiment. Beginning the day after surgery, catheters were kept patent with daily infusion of 0.5 ml of heparinised saline (50 IU). On the day of blood collection, after each blood sampling, an equal volume of sterile saline solution was infused to prevent dehydration. Blood samples (200 μl) were collected into 100 μl of heparinised saline (10 IU), refrigerated overnight and centrifuged at 1000 × g for 10 min.
Plasma LH was measured by RIA; the assay sensitivity was 0.2 ng/ml, and the intra- and interassay coefficients of variation were 2.3 and 5.1 %, respectively. Plasma PRL was measured by ELISA (ELISA rat PRL kit, MD Bioscience, Saint Paul, MN, USA). The assay sensitivity was 0.2 ng/ml, and the intra- and interassay coefficients of variation were 1.3 and 3.7 %, respectively. For each animal, the basal hormone level was defined as the value observed at time 0, before drug or vehicle administration.
Lordosis behaviour
After OVX, animals were housed on a reversed light:dark cycle; one week after OVX, animals were tested for lordosis behaviour in response to a single sc dose of EB (2.5 μg) followed 44 h later by P (500 μg, sc) (28). Four hours after P administration, females were placed in 20-gallon plexiglass tanks until they had received 10 mounts with pelvic thrusting from an experienced stimulus male. The lordosis quotient [LQ = (number of lordosis/10 mounts) × 100] was used to assess receptive behaviour. The intensity of lordosis was quantified on a scale of 0 to 3 according to Hardy and DeBold (29).
GPR30 in vivo RNA interference in the mediobasal hypothalamus
shRNA design and cloning into Psilencer 1.0 U6
House-designed, 19 nucleotide sequences for rat GPR30 RNAi were designed according to Reynolds et al. (30) using a downloadable Excel template written by Maurice Ho (Hong Kong University of Science and Technology, http://boz094.ust.hk/RNAi/siRNA), then matched to sequences selected by algorithms available on the web (siDirect (31), Dharmacon, Genscript siRNA Design) to minimize off target effects. Finally, a Blast search was done to minimize homology with other sequences of the rat genome. Commercially available and housed-designed interfering RNA sequences for rat GPR30 as well as sequences used for the interferon response assay and control in vivo RNAi were then cloned into P-Silencer1-U6 (ApaI, EcoRI, see Table 1 for primer sequences) as recommended by the manufacturer (Ambion, Austin, TX, USA) to be expressed as shRNA under the control of a mouse U6 polymerase III promoter. For all shRNA insertions into P-silencer, the inserted sequence was verified by direct sequencing.
Table 1.
Nucleotide sequences of short hairpin (sh)RNAs used for in vitro/in vivo RNA interference (RNAi) and interferon response assay
| ShRNA | 5′ - 3′ oligonucleotide primer sequences | |
|---|---|---|
| ShA3 | Rat GPR30 Ambion | F: CGCGAGCAGTATTACGATATCTTCAAGAGAGATATCGTAATACTGCTCGTTTTTTGGAAG |
| R: AATTCTTCCAAAAAACGAGCAGTATTACGATATCTCTCTTGAAGATATCGTAATACTGCTCGCGGGCC | ||
| ShQ4 | Rat GPR30 Qiagen | F: CGACGAGCAGTATTACGATATTCAAGAGATATCGTAATACTGCTCGTCTTTTTTGGAAG |
| R: AATTCTTCCAAAAAAGACGAGCAGTATTACGATATCTCTTGAATATCGTAATACTGCTCGTCGGGCC | ||
| ShH3 | Rat GPR30 House- designed | F: CGTCCCAGACCTGTACTTCATTTCAAGAGAATGAAGTACAGGTCTGGGATTTTTTGGAAG |
| R: AATTCTTCCAAAAAATCCCAGACCTGTACTTCATTCTCTTGAAATGAAGTACAGGTCTGGGACGGGCC | ||
| ShMora | Interferon response (Positive control) | F: CGTAAAGATTCCTGAAGAGCTTCAAGAGAGCTCTTCAGGAATCTTTACTTTTTTGGAAG |
| R: AATTCTTCCAAAAAAGTAAAGATTCCTGAAGAGCTCTCTTGAAGCTCTCAGGAATCTTTACGGGCC | ||
| ShLuc | In vivo RNA interference (shRNA control) | F: CGCCGCTGGAGAGCAACTGCATTTCAAGAGAATGCAGTTGCTCTCCAGCGGTTTTTTGGAAG |
| R: AATTCTTCCAAAAAACCGCTGGAGAGCAACTGCATTCTCTTGAAATGCAGTTGCTCTCCAGCGGCGGGCC | ||
Oligonucleotide primers (F: Forward, R: reverse) were designed to express RNAi sequences as shRNA when inserted (ApaI, EcoRI) into Psilencer 1.0 U6 (Ambion, Austin, Tx, USA). For each pair of primers, the specific RNAi sequence is underlined. The same loop (in bold, TTCAAGAGA, proposed by Brummelkamp (63) and shown to produce efficient RNAi in rat brain cells (37) was used to express all RNAi sequences as shRNA.
When the RNAi sequence does not start with a G, a G was added at the 5′ end of the RNAi sequence in the forward primer to start pol III transcription. To enhance silencing, a GGAA sequence was added at the 3′ end of the forward primer following the poly T (terminator).
ShA3 and shQ4 sequences were derived from commercially available double strand RNA sequences designed for rat GPR30 RNAi (Ambion and Qiagen (Valencia, CA, USA) respectively). Among house-designed RNAi sequences for rat GPR30, shA3 was selected to be expressed by AAV for in vivo GPR30 RNAi. ShMora sequence was published as a positive control to measure in vitro induction of interferon response (32). ShLuc was already published as a suitable control sequence for in vivo RNAi in mice and rat hypothalamus (39, 64).
Rat GPR30 in vitro RNAi
HEK293 cells expressing rat GPR30 (V5 tagged in C-terminus; see supplemental material for rat GPR30 PCR cloning and stable expression in HEK293 cells) were transfected (Lipofectamine Plus) in 24 well plates with P-Silencer1-U6 (Ambion) expressing selected shRNA for GPR30 (see Table 1 for sequences) or the empty vector (PS). Forty-eight h later, cells were lysed in 200 μl of Laemmli buffer, the samples sonicated and heated at 70°C for 10 min. Fifteen μl of cell lysate were loaded on a 4–15% acrylamide gel, subjected to electrophoresis and transferred onto nitrocellulose membrane for immunoblotting with an anti-V5 antibody (1/5000, InVitrogen) according to the manufacturer’s recommendation. After incubation with anti-rabbit HRP conjugated antibody (1/5000, Amersham Life Science, Pittsburgh, PA, USA), membranes were treated with enhanced chemiluminescence reagents (Amersham Life Science) and apposed to Biomax film (Eastman Kodak Co., Rochester, NY, USA) for signal detection. Membranes were then stripped (Re-blot Plus Strong, Chemicon, Temecula, CA, USA) and reprobed for GAPDH (mouse monoclonal GAPDH antibody 1/5000, Ambion) as a loading and cell viability control under the same conditions as for GPR30-V5 detection.
Interferon response assay
Commercially available and house-designed shRNAs for rat GPR30 RNAi (Table 1) were tested for their ability to induce an interferon response using a reporter assay where luciferase is expressed under the control of a promoter activated by interferon (mx2) (32). Briefly, HEK293 cells in 24 well plates were co-transfected with three vectors: (1) CMV GFP (control), empty P-silencer or P-silencer expressing shRNA, (2) reporter vector (mx2::firefly luciferase), and (3) normalising vector (CMV::Renilla luciferase) at 0.45, 1.35, and 0.15 μg per well, respectively. Twenty-four h later, cells were lysed, and luciferase activity was measuredusing the Dual-Luciferase Reporter system (Promega, Madison, WI, USA). Activation of the interferon response was measured as the ratio of firefly luciferase/renilla luciferase and expressed as % of reporter expression with the control (GFP). Activation of the reporter with shRNA sequences selected for rat GPR30 RNAi was compared to that observed with shMora (sequence targeting MORF4L1, a protein involved in chromatin regulation, see sequence in Table 1), a positive control known to induce interferon and promote off target effects (32).
Production of adeno-associated virus (AAV) particles expressing control shRNA or specific rat GPR30 shRNA
The Psilencer 1.0 U6 expression cassette containing a selected shRNA sequence for rat GPR30 RNAi (shH3) and control RNAi (shLuc) (see Table 1) were transferred from P-Silencer 1-U6 by restriction enzyme digestion (BamHI) into a proprietary plasmid (Genedetect, Auckland, New Zealand) to produce chimaeric AAV1/2 particles (33) expressing either the specific or the control shRNA under the control of a mouse U6 promotor (34) and eGFP under the control of an independent promoter. The final recombinant AAV1/2 expression cassette, ITR-U6-shRNA-terminator-CAG-eGFP-WPRE-BGH-polyA-ITR (ITR, inverted terminal repeat, U6: mouse U6 RNA polymerase III promoter, CAG: chicken β-actin promoter modified with CMV early enhancer (35), WPRE: cis-acting woodchuck postregulatory regulatory element (36), BGH-polyA: Bovine growth hormone polyadenylation signal) was already shown to successfully knock down Ca2+/calmodulin-dependent protein kinase II in rat brain cells (37). The stock of recombinant virus particles for control and specific GPR30 vivo RNA interference in the hypothalamus were produced by Genedetect (Auckland, New Zealand), aliquoted and frozen at −80 until use.
Virus infusion
Female rats anesthetised with ketamine/xylazine as described above were placed in a Kopf stereotaxic apparatus and secured with ear bars and a nose piece set at −3 mm. Using Bregma as a landmark and stereotaxic coordinates (A/P, −3 mm; M/L, −0.5 mm; D/V, −8.8 mm with respect to Bregma) provided in the atlas of Paxinos and Watson (38), animals received bilateral infusions of virus expressing the control shRNA (shLuc) or GPR30 shRNA (shH3) into the mediobasal hypothalamus. Virus injections were performed using a Hamilton microsyringe controlled by a programmable infusion pump mounted on the stereotaxic apparatus. Four μl of AAV1/2 particles (1.1 × 1012 particles/ml) were bilaterally infused at a rate of 200 nl/min. The injector remained in place for an additional 10 min following infusion.
Virus transduction
Three to six weeks after virus infusion animals were perfused with 4% paraformaldehyde. Forty μm brain cryo-sections were mounted on Superfrost slides using Prolong anti-fade reagent (InVitrogen). Direct fluorescence of eGFP expression was visualised using a fluorescent microscope on sections collected throughout the entire hypothalamus.
Quantification of in vivo GPR30 knockdown by semiquantitative real time PCR
Two independent cohorts of 12 animals were injected with the control virus (n=6/cohort) or the virus expressing the specific shRNA for GPR30 (n=6/cohort) as described above. Animals were OVX four weeks after virus infection. One week later, they were decapitated under deep anaesthesia, and the hypothalamus was dissected. The specimens were then immediately frozen on dry ice and kept at −80°C for determination of GPR30 mRNA expression by semiquantitative, real time PCR using GAPDH as housekeeping gene.
DNA-free total RNA was purified using the RNeasy Lipid Mini kit from Qiagen (Valencia, CA, USA) including a DNase step. Reverse transcription (RT) was performed using the High Capacity cDNA RT Kit with RNase inhibitor (Applied Biosystems, Foster city, CA) using 500 ng of RNA per 20 μl of RT reaction. Change in gene expression was measured by real time PCR using TaqMan Gene Expression Assays and Master Mix from Applied Biosystems according to manufacturer’s instructions. The final reaction mix contained the proprietary TaqMan probes and primers for the normalizer (rat GAPDH endogenous control, primer limited, VIC®/MGB probe, part number 4374966, context sequence NM_017008.3) and the specific target (ratGPR30, primer limited, Fam probe, assay ID Rn00592091, context sequence NM_133573.1). Real time PCR was performed using an ABI PRISM 7900HT (Applied Biosystems) in multiplex condition using 50 ng of cDNA per 20 μl of total reaction mix. Amplified transcripts for GPR30 were quantified using the comparative threshold cycle method using GAPDH as a normalizer. The fold change in GPR30 expression was then calculated as 2−ΔΔCT where CT = threshold cycle, ΔCT = CT (GPR30) − CT (GAPDH), ΔΔCT = ΔCT (shGPR30)−ΔCT(shControl).
Effects of GPR30 agonist G1 on PRL and LH secretion
Females rats were OVX and implanted with a cannula aimed at the third ventricle. One week later, animals were implanted with an indwelling jugular vein catheter for serial blood sampling. Each animal (n=10) was subjected to two experimental days of drug injection and blood sampling to individually measure their response to both the vehicle and the specific drug. On experimental day 1 at 1000 h, a blood sample was taken to establish basal LH and PRL secretion. Animals were then immediately infused icv with 3 μl of vehicle (100% DMSO, n=5), E2 (3 μg, n=2) or G1 (30 μg, n=3). Blood samples were taken 1, 3, 4, 5 and 7 h after drug or vehicle infusion. The next day, animals were not subjected to blood sampling, and catheters were flushed with heparinised saline. Twenty-four h later, on experimental day 2, animals injected on experimental day 1 with vehicle were then injected at 1000 h with either E2 (n=3) or G1 (n=2), and animals that received E2 or G1 on experimental day 1 were injected with vehicle (n=5). Blood samples were collected as described for experimental day 1.
Effects of GPR30 in vivo RNA interference in the hypothalamus on E2 regulation of PRL and LH secretion and on lordosis behaviour
Four weeks after virus injection into the mediobasal hypothalamus, animals subjected to control (n=6) or GPR30 specific (n=6) in vivo RNAi and control animals (n=6, not injected with virus) were OVX and transferred to reversed dark/light cycle housing condition. One week later, they were tested for lordosis behaviour as described above. Immediately after behaviour testing, animals were returned to standard housing conditions, and one week later, an indwelling jugular vein catheter was placed in the right atrium. Two days after catheter placement, at 1000 h, a blood sample was taken to establish basal LH and PRL secretion. Immediately after, animals received a single intravenous injection of E2 (10 μg), and blood samples were taken 1, 3, 5 and 7 h after E2 injection. A last sample was taken 24 h after E2 injection.
Animal weights and vaginal smears were monitored starting one week before virus injection and continuing until OVX (vaginal smears) or the end of the experiment (body weight). At the end of the experiment, virus transduction in the hypothalamus was verified for each animal by visualising GFP expression.
Statistical analysis
Data were expressed as mean ± standard error (SEM). One-way ANOVA was used to evaluate GPR30 RNAi efficiency and induction of interferon response in vitro. T-test was used to evaluate GPR30 in vivo knockdown. Two-way ANOVA with repeated measures on time was used to determine differences in LH and PRL secretion over the time in response to drug treatment or RNAi. A P value of ≤0.05 was accepted as statistically significant. Post-hoc tests used were Newman-Keuls for one-way ANOVA and Bonferonni for two-way ANOVA with repeated measures
RESULTS
The GPR30 agonist G1 promotes PRL secretion but does not inhibit LH secretion
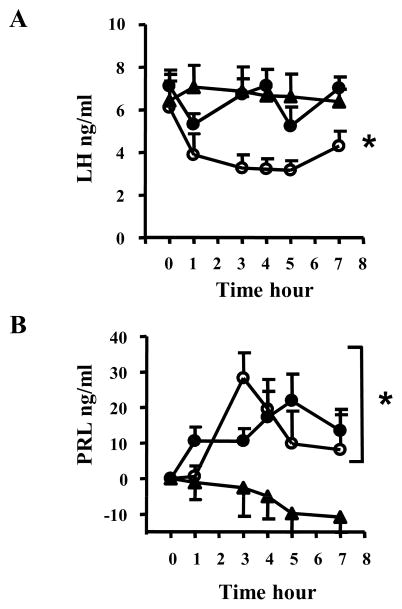
To assess whether GPR30 mediates rapid neuroendocrine effects of E2 in the hypothalamus, we determined whether the selective GPR30 agonist G1 mimics E2 negative feedback inhibition of LH secretion or short latency facilitation of PRL secretion. Because the permeability of G1 through the blood-brain-barrier is unknown, OVX animals outfitted with a jugular vein cannula were injected icv with vehicle (100% DMSO), E2, (3 μg) or G1 (30 μg). As shown in Figure 1, when OVX rats are infused with E2, LH levels decline rapidly and significantly (p < 0.05) relative to vehicle controls. In contrast, G1 does not modify LH secretion (Figure 1A).
Figure 1. Icv injection of G1 triggers a surge of PRL but does not mediate negative feedback on LH secretion.
Plasma LH (A) and PRL (B) were measured in OVX animals in response to icv injection of E2 (open circle, n=5), G1 (filled circle, n=5) or the vehicle (triangle, n=10). In (B), PRL results are expressed after subtracting the basal value for each animal. Plasma PRL and LH were measured by ELISA and RIA, respectively.
In A: Two way ANOVA, main effect F(df) = 4.06 (2,85), P<0.05.
In B: Two way ANOVA, main effect F(df) = 5.14 (2,90), P< 0.05
*P<0.05 vs. vehicle
In preliminary studies (data not shown), we observed that animals given icv infusions show significantly higher and more variable basal PRL (17 ± 14.5 ng/ml, n=21) than animals given intravenous injections through the catheter used for blood sampling (11.8 ± 7 ng/ml, n=28; t-test, p < 0.01). Because PRL secretion is highly sensitive to stressors, several experimental factors related to icv injection (surgical stress, physical restraint) might have stressed these animals. Therefore, values of PRL over time after icv drug infusion are expressed for each animal after subtracting basal PRL at time 0 (before drug or vehicle infusion). There was a significant main effect of both E2 and G1 on PRL (F(2,90)= 5.14, p <0.05 ). As expected, infusion of E2 significantly elevates PRL relative to vehicle infusion (p <0.05) with a peak at 3 hr post-infusion (Figure 1B). Interestingly, there was a significant increase of PRL secretion in response to icv administration of G1 (p < 0.05 versus vehicle). The onset of the response to G1 started earlier (1 hr vs 3 hr for E2), and the peak was delayed to 5 hr post-infusion of G1.
GPR30 in vivo RNA interference in the mediobasal hypothalamus
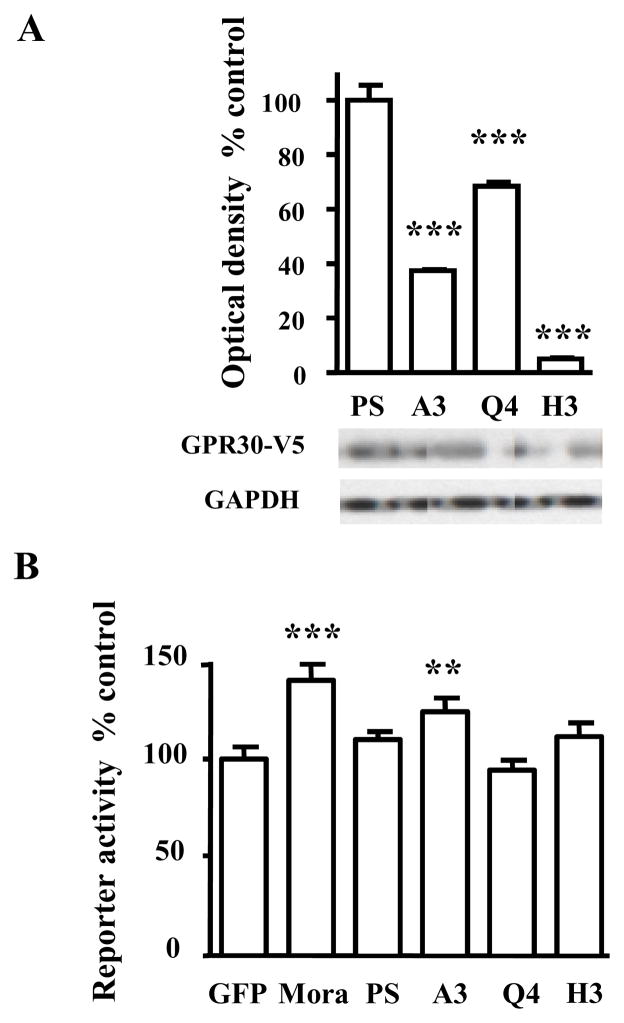
Because no selective GPR30 antagonists are available, we used GPR30 RNAi in the mediobasal hypothalamus to verify that GPR30 can mediate short latency E2 effects on PRL secretion. For this purpose, HEK293 clones expressing rat GPR30 (V5-tagged) (see supplemental data) were used to test shRNA sequences designed to specifically knock down rat GPR30 expression (see Figure 2A and Table 1).
Figure 2. In vitro rat GPR30 knockdown by RNAi and interferon response.
A) Lysates of HEK293 cells expressing rat GPR30-V5 and transfected for 48 hr with empty P-silencer (PS) or PS expressing 3 different shRNA sequences for rat GPR30 RNA interference (A3, Q4, H3; see sequences in Table 1) were probed by immunoblotting for the expression of rat GPR30 protein using an anti-V5 monoclonal antibody. Expression of GAPDH was followed to confirm equal protein loading and to verify that the conditions of interference did not differentially affect cell viability. Results are expressed as percent of the ratio of intensity GAPDH/GPR30 for empty PS and are representative of 2 independent experiments performed in triplicate. (ANOVA, ***: p<0.001 vs. control (PS))
B) Relative luciferase activity was measured 24 h after HEK293 cells were co-transfected with the interferon-inducible reporter (Mx2, luciferase) and EGFP (control), empty P-Silencer (PS), P-silencer expressing shMora (positive control) or P-Silencer expressing shRNA for rat GPR30 RNAi (ShA3, ShQ4, ShH3; see sequences in Table 1). Results are expressed as % of relative luciferase activity observed for the control and are representative of 3 experiments performed in triplicate. (ANOVA ***:p<0.001 vs control (GFP), **: p<0.01 vs control).
We wished to achieve knockdown of GPR30 in the hypothalamus by RNAi with minimal off-target effects. One of the most serious off-target effects in brain RNAi is the induction of an interferon response. The interferon response can be monitored in vitro using a reporter assay where luciferase is expressed under the control of a promoter activated by interferon (32). Commercially available and house-designed sequences (Table 1) were cloned into P-silencer to express the corresponding shRNA and then tested for their ability to reduce GPR30 protein expression (Figure 2A) and to induce an interferon response (Figure 2B) in HEK293 cells. Among the sequences tested, shA3 produced effective knockdown of GPR30 but triggered a significant interferon response. shQ4 did not induce an interferon response but was a poor interfering agent. shH3 efficiently knocked down GPR30 protein and did not induce an interferon response that was significantly different from the negative control or empty P-silencer. This sequence was therefore selected for in vivo GPR30 RNAi. While a scrambled sequence of the shRNA is typically used as a control for RNAi in vitro, scrambled RNAi sequences can trigger undesired off-target effects in vivo. Therefore, we picked a published (39) control shRNA sequence (shLuc) that did not promote any dysfunction in hypothalamic neurones when employed in a study using in vivo RNAi to knock down ERα in the VMH in mice and rats (see Table 1).
Several classes of viral vectors that deliver transgenes to the CNS have been used for expressing shRNA in vivo. We selected the chimaeric AAV1/2 for its ability to transduce neurones from different brain regions with high efficiency and without toxicity (37, 40–43). We transferred shH3 and the control sequence shLuc by restriction enzyme digestion into shuttle plasmids for production of AAV1/2 particles expressing shRNA to interfere with rat GPR30 mRNA production (shGPR30) or AAV1/2 virus particles expressing the control shRNA (shControl). As shown in Figure 3, 3–6 weeks after injection, virus particles carrying either control or specific shRNA sequence spread throughout the entire hypothalamus from rostral regions such as the POA to more caudal areas such as the arcuate nucleus and VMH.
Figure 3. In vivo GPR30 RNAi in the mediobasal hypothalamus.
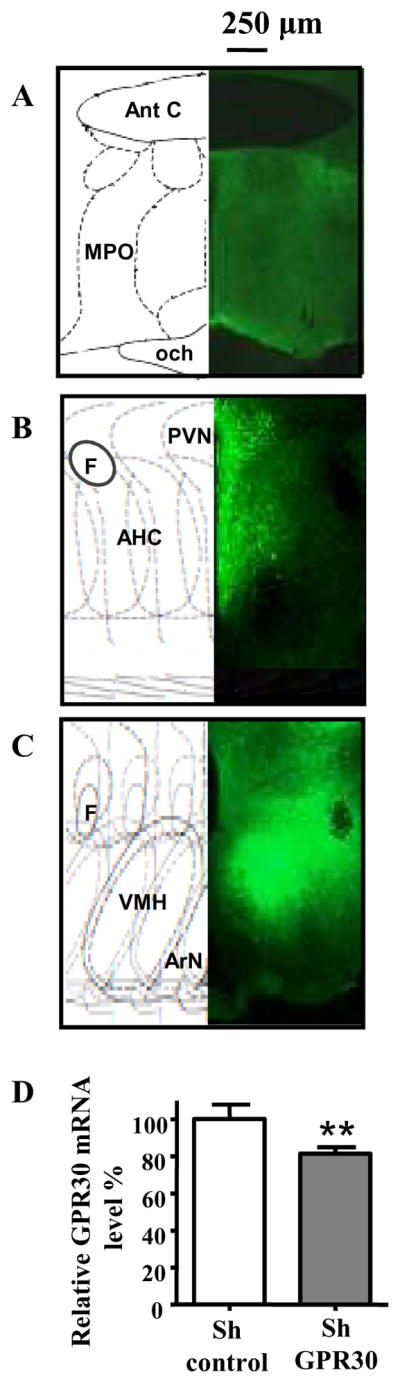
A to C: Typical spreading of virus expressing control or specific GPR30 shRNA, 3 to 6 weeks after bilateral injection of AAV1/2 into the mediobasal hypothalamus. Spreading of virus expressing eGFP was visible under the microscope by direct fluorescence visualisation. Fluorescence was seen at the level of the POA (A), paraventricular nucleus (B), and in the arcuate nucleus, median eminence and VMH (C).
D: Quantification of GPR30 in vivo knockdown. Animals were bilaterally injected with the control virus (shControl, n=12) or the virus expressing the specific shRNA for GPR30 (shGPR30, n=12) in the mediobasal hypothalamus. Five weeks later, GPR30 mRNA expression was measured by real time PCR using GAPDH as normalizer. The values in animals injected with the virus expressing shGPR30 are expressed relative to the values in animals receiving the control virus. **: P<0.005, T-test Ant C: Anterior commissure, MPO: medial preoptic area, och: optic chiasma, PVN:paraventricular nucleus, F: Fornix, AHC: Anterior Hyp. central area, VMH: Ventromedial hypothalamic nucleus, ArN: Arcuate nucleus.
Reduction of GPR30 mRNA by in vivo RNAi was measured by semiquantitative, real time RT-PCR on two groups of animals injected with control virus or virus expressing shGPR30 as described above. These two independent experiments showed a reproducible and significant (P<0.005) but modest reduction (20%) in total hypothalamic GPR30 mRNA expression in the animals subjected to GPR30 RNAi compared to those receiving control virus (Figure 3D).
GPR30 in vivo RNA interference in the hypothalamus reduces E2 facilitation of PRL secretion but not LH secretion or lordosis behaviour
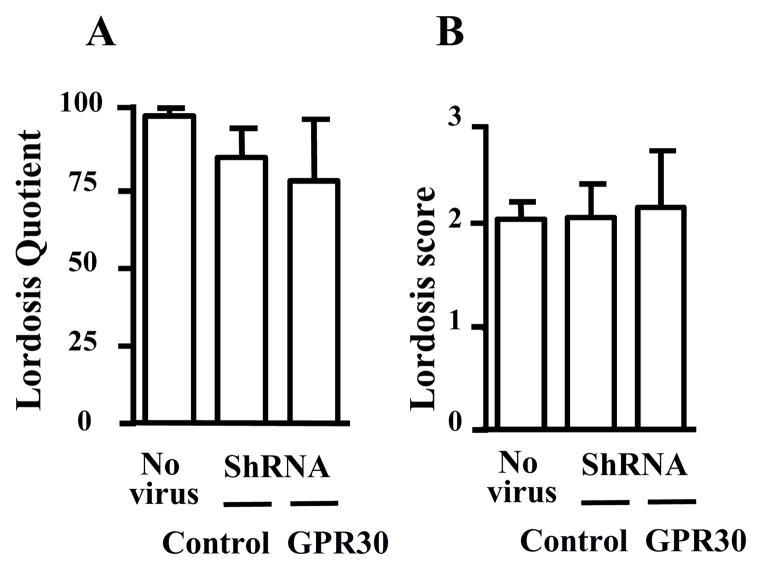
All virus-injected animals resumed normal estrous cycles within 7 days after bilateral injection of AAV1/2 into the hypothalamus and had comparable weight gain over a survival period of 6 weeks (data not shown). Four weeks after control or specific GPR30 interfering virus infusion, animals were OVX and tested 1 week later for lordosis behaviour in response to a hormonal treatment known to induce female sexual receptivity. As seen in Figure 4, animals injected with either the control or the specific virus for GPR30 RNAi exhibited lordosis quotients and lordosis scores that were not significantly different from the values observed for naive animals not injected with virus.
Figure 4. GPR30 RNAi in the mediobasal hypothalamus does not interfere with lordosis behaviour.
OVX female rats subjected to control (shControl, n=6) or specific GPR30 RNA interference (shGPR30, n=6) in the mediobasal hypothalamus and control animals (OVX female rats not subjected to virus injection; n= 6) were tested for lordosis behavior in response to a single injection of EB (2.5μg) followed 44 h later by P (500 μg). Behavioral testing was performed 4 h after P injection. No significant difference in either lordosis quotient or lordosis score was seen between groups (ANOVA )
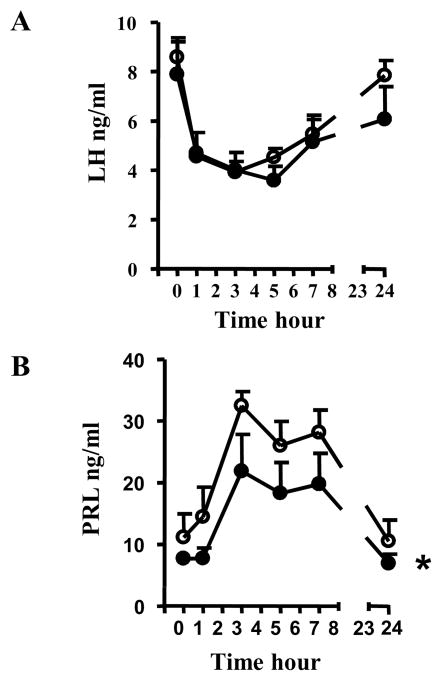
One week later, the same animals were implanted with a catheter for serial blood sampling after intravenous injection of E2. As observed in our previous experiment with icv injection, intravenous E2 (10 μg) significantly (p < 0.05) reduced LH secretion within 1 hr in animals subjected to either control or specific GPR30 RNAi (Figure 5A). As shown in Figure 5B, there was a significant main effect of virus administration on E2-induced PRL secretion. Animals injected with both control and specific GPR30 shRNA show an increase of PRL in response to systemic E2, with a peak at 3 h post-injection. However, animals injected with the specific GPR30 shRNA show significantly lower PRL elevations in response to E2 compared to animals subjected to control RNAi (P<0.05).
Figure 5. GPR30 RNAi in the mediobasal hypothalamus does not affect E2 negative feedback on LH secretion but reduces E2-induced PRL secretion.
OVX female rats subjected to control (shcontrol, open circle n=6) or specific GPR30 (shGPR30, filled circle, n=6) RNA interference in the mediobasal hypothalamus were injected iv with 10 μg of E2. Serial blood sample collection started 5 min before drug injection (T=0). Plasma LH (A) and PRL (B) were measured by RIA and ELISA, respectively.
In A: Two way ANOVA, no main effect of virus.
In B: Two way ANOVA, main effect of virus, F(df)= 5.33 (1,45), P<0.05
* P<0.05 versus shcontrol
DISCUSSION
Physiological relevance of GPR30
The present findings suggest that activation of GPR30 promotes short latency PRL secretion but does not mediate E2 negative feedback inhibition of LH secretion or E2 facilitation of female reproductive behaviour. Administration of the GPR30 agonist G1 into the third ventricle triggers a PRL surge similar in amplitude to the one observed in response to E2. Moreover, administration of shRNA for GPR30 RNAi bilaterally into the hypothalamus significantly reduces the PRL surge observed in response to intravenous E2. In contrast, G1 failed to reduce LH secretion in OVX females. Likewise, rats subjected to GPR30 RNAi showed normal negative feedback suppression of LH release and facilitation of lordosis behaviour in response to E2. These observations agree with a recent report that female GPR30 deficient mice were as fertile as their wild type littermates (44), suggesting that hypothalamic GPR30 is not necessary for normal function of the hypothalamic-pituitary-gonadal axis. The observation that ICI-182,780, an antagonist for the classical ER-α and ER-β, failed to block E2-stimulated PRL secretion in vitro and in vivo (45, 46) already suggested that another receptor might be involved in this regulation.
The most plausible mechanism for a rapid increase in PRL release is that E2 reduces the tonic inhibitory effect of DA, thus freeing the pituitary gland to express its inherent capacity to secret PRL. The rapid secretion of PRL in response to E2 is abolished by inhibition of the DA transporter (DAT), suggesting that short latency effects of oestrogens on PRL secretion may involve regulation of the DAT (47). Watson and colleagues recently examined the roles of ER-α, ER-β and GPR30 in E2 modulation of DA efflux from PC12 cells. Their findings might provide an explanation of how GPR30 action on hypothalamic DA neurones could block DA release, thereby increasing PRL secretion. GPR30 RNAi increased E2-mediated DA efflux via the DAT, and application of the GPR30 agonist G1 reduced the basal efflux of DA through the DAT. These observations, taken together, strongly suggest that GPR30 modulation of DAT activity might reduce synaptic availability of DA (48). Whether GPR30 is expressed in hypothalamic DA neurones, and whether GPR30 mediates E2 regulation of PRL release via actions on hypothalamic DA neurones, remains to be investigated.
Another potential mechanism of GPR30 regulation of PRL secretion is suggested by recent observations that oxytocin neurones of the paraventricular and supraoptic nuclei express GPR30 (25). Several studies suggest that E2 promotes rapid release of PRL via activation of a PRL releasing factor, and that oxytocin may be such a factor (20). For example, an oxytocin antagonist prevents the proestrous surge of PRL (49), and passive immunization with oxytocin antisera delays and reduces PRL surges induced by E2 (50). Thus, GPR30 activation in oxytocin neurones might stimulate oxytocin secretion, which in turn could promote PRL secretion.
The discrepancy between the robust PRL response to G1 (PRL surge amplitude similar to that produced by E2) and the modest inhibition of E2-induced PRL secretion observed in animals subjected to GPR30 RNAi in the mediobasal hypothalamus may indicate that GPR30 expressed in the pituitary (23, 51) also participates in the short latency facilitation of PRL secretion by E2. G1 and E2 injected into the third ventricle could have activated GPR30 in both the hypothalamus and the pituitary, leading to similar PRL surge amplitudes. Knockdown of GPR30 only in the hypothalamus might partially reduce E2-induced PRL secretion due to the remaining effect of GPR30 in the pituitary, where RNAi was not achieved. Another explanation is that our in vivo RNAi procedure did not achieve complete GPR30 knockdown in the arcuate nucleus, locus of the major hypothalamic DA neurones that regulates PRL secretion.. If GPR30 expressed in arcuate DA neurones is the major mediator of the short latency PRL responses to E2, our RNAi protocol may not have effected complete knockdown of GPR30 in this particular hypothalamic nucleus.
E2 action in the VMH is both necessary and sufficient for the facilitation of lordosis behaviour, and this behavioural response requires ER-α (39). However, the requirement for ER-α does not preclude a role for membrane actions in E2 facilitation of reproductive behaviour. Use of a two-pulse E2 treatment delivered directly to the VMH, in which one pulse was membrane-limited, provided evidence that E2 actions at the cell membrane can potentiate the genomic actions mediated by ER-α (52). Furthermore, activators of cAMP-dependent protein kinase, one of the signalling pathways activated by GPR30, effectively substituted for the membrane-limited pulse of E2. Thus, the molecular mediator of E2 action at the cell membrane may be a receptor able to directly activate Gαs, leading to cAMP production and protein kinase A activation. These observations prompted us to ask whether GPR30 transduces the membrane-mediated potentiation of E2’s genomic action on lordosis behaviour. However, expression of virus carrying GPR30 shRNA in the mediobasal hypothalamus (including the VMH) does not interfere with hormonal facilitation of lordosis behaviour.
Rapid, non-genomic actions of E2 were implicated in negative feedback regulation of LH secretion decades ago. For example, it takes only about 16 min to detect significant changes in the firing rate of POA neurones following intravenous injection of E2. GnRH neurones themselves are a target of the rapid actions of E2 (53), and recent evidence suggests that GPR30 rapidly modulates GnRH release in embryonic primate GnRH neurones (54, 55). However, our findings suggest that GPR30 is not involved in E2 negative feedback inhibition of LH release in adult female rats. Icv administration of the agonist G1 did not mimic the short latency inhibition of LH secretion observed in response to E2 Even though G1 significantly increased PRL secretion in the same animals. Likewise, GPR30 RNAi in the hypothalamus had no effect on E2 inhibition of LH secretion. The discrepancy between our results and the findings of Noel et al. may reflect our use of adult animals infused with drugs or RNAi in vivo rather than cultured embryonic neurones or species differences (rats versus primates). One might also question whether we achieved sufficient in vivo knockdown of GPR30 in the rostral hypothalamus, where GnRH neurones and critical afferents that project to GnRH neurones are located (see Figure 3). However, if GPR30 plays a key role in negative feedback inhibition of LH secretion, we would have expected to see suppression of LH when G1 was infused icv. This was clearly not the case.
Although we interpret our findings as indicative of GPR30-mediated actions of E2 at the cell membrane, our in vivo data do not speak to the controversy regarding the subcellular localization of GPR30 (10, 18, 56). Therefore, we cannot verify that the observed actions of G1 involve GPR30 expressed at the plasma membrane and/or in the endoplasmic reticulum. Likewise, we have no direct evidence that E2 or G1 binds to GPR30, but the observed effects on PRL secretion imply that this is probable.
Technical considerations in use of in vivo RNAi
Animals injected with control or specific virus for GPR30 RNAi exhibited normal oestrous cycles for 3 weeks before OVX. This observation, coupled with the fact that infection of the entire hypothalamus with virus expressing shRNA did not interfere with lordosis behaviour, suggests that our experimental conditions did not produce neuronal toxicity. The modest effect of GPR30 RNAi on PRL secretion raises the question whether our in vivo RNAi procedures produced sufficient knockdown of GPR30 protein. In situ hybridization and immunohistochemistry demonstrate almost complete mRNA or protein knockdown in the brain when the target protein is expressed at high levels (34, 37, 57). However, quantifying knockdown of low expression genes, such as G-protein coupled receptors, is more challenging using these techniques. We could not get reliable signals from western blots on brain samples using available antibodies developed for the human GPR30, and to date, no antibody specific for rat GPR30 is available. Therefore, we measured the reduction of GPR30 mRNA expression in the entire hypothalamus by semiquantitative, real time RT-PCR. Two independent experiments showed a reproducible but modest reduction (20%) in total hypothalamic GPR30 mRNA expression in the animals subjected to GPR30 RNAi compared to those receiving control virus. Virus-mediated in vivo RNAi in the brain rarely leads to more than a 50% reduction of mRNA levels in dissected brain samples. Nonetheless, physiological effects of virus-mediated modulation of gene expression in the hypothalamus are reported when the observed level of mRNA knockdown (30–40%, (58–60)) or mRNA overexpression (20%, (58)) is quite modest. Thus, we consider it likely that the 20% reduction of GPR30 mRNA observed in animals injected with virus expressing the specific GPR30 shRNA is responsible for the modest but significant reduction in E2-dependent PRL secretion.
The recent report that GPR30 is highly expressed in the brain vasculature (61), and that such tissue would be included in our dissection but not infected by AAV, might explain why we detected such a modest reduction of hypothalamic GPR30 mRNA. Indeed, it is well known that AAVs are poor transducers of epithelial cells, including blood vessels (62). The chimaeric serotype virus used for our study (AAV1/2) was designed to infect neurones with high efficiency; however, we do not know if this particular serotype can infect blood vessels. Thus, the true extent of RNAi-mediated reduction of GPR30 mRNA in hypothalamic nuclei that regulate PRL secretion could have been masked by the remaining expression of GPR30 in blood vessels that were not targeted by the virus.
In conclusion, we report the novel finding that the putative membrane oestrogen receptor GPR30 participates in short latency enhancement of PRL secretion by E2. We also document the safety of in vivo administration of AAV1/2-expressing shRNA and the widespread expression of virus throughout the hypothalamus. In the absence of pharmacological inhibitors of GPR30, this method may be a reasonable strategy for studying GPR30 actions in vivo. If GPR30 regulates PRL secretion by modulating DA neurotransmission in the mediobasal hypothalamus, it might be interesting to examine the role of this oestrogen receptor in other DA systems where GPR30 is expressed (23).
Supplementary Material
Acknowledgments
We thank Dr. Jun Shu and Mr. Zewei Jiang for technical support and Brigitte Mann (Northwestern University Radioimmunoassay Core, Evanston, IL) for conducting serum LH and PRL measurements. This work was supported by R37 MH41414 and the D.P. Purpura Department of Neuroscience, Albert Einstein College of Medicine.
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose.
References
- 1.Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4(1):43–58. doi: 10.1177/1534582305277152. [DOI] [PubMed] [Google Scholar]
- 2.Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138(3):929–38. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: Basic mechanisms and clinical implications. Steroids. 2007;72 (5):381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kipp M, Karakaya S, Pawlak J, Araujo-Wright G, Arnold S, Beyer C. Estrogen and the development and protection of nigrostriatal dopaminergic neurons: concerted action of a multitude of signals, protective molecules, and growth factors. Frontiers in neuroendocrinology. 2006;27(4):376–90. doi: 10.1016/j.yfrne.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Raz L, Khan MM, Mahesh VB, Vadlamudi RK, Brann DW. Rapid estrogen signaling in the brain. Neurosignals. 2008;16(2–3):140–53. doi: 10.1159/000111559. [DOI] [PubMed] [Google Scholar]
- 6.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Molecular endocrinology (Baltimore, Md) 2006;20(9):1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 7.Sheldahl LC, Shapiro RA, Bryant DN, Koerner IP, Dorsa DM. Estrogen induces rapid translocation of estrogen receptor beta, but not estrogen receptor alpha, to the neuronal plasma membrane. Neuroscience. 2008;153(3):751–61. doi: 10.1016/j.neuroscience.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Frontiers in neuroendocrinology. 2009 doi: 10.1016/j.yfrne.2009.04.011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronnekleiv OK, Malyala A, Kelly MJ. Membrane-initiated signaling of estrogen in the brain. Semin Reprod Med. 2007;25(3):165–77. doi: 10.1055/s-2007-973429. [DOI] [PubMed] [Google Scholar]
- 10.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A Transmembrane Intracellular Estrogen Receptor Mediates Rapid Cell Signaling. Science (New York, NY) 2005;307(5715):1625–30. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 11.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an Estrogen Membrane Receptor Coupled to a G Protein in Human Breast Cancer Cells. Endocrinology. 2005;146(2):624–32. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 12.Prossnitz ER, Arterburn JB, Sklar LA. GPR30: A G protein-coupled receptor for estrogen. Molecular and cellular endocrinology. 2007;265–266:138–42. doi: 10.1016/j.mce.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB. The ins and outs of GPR30: a transmembrane estrogen receptor. The Journal of steroid biochemistry and molecular biology. 2008;109(3–5):350–3. doi: 10.1016/j.jsbmb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625–30. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 15.Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16(1):70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 16.Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16(8):362–7. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146(2):624–32. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 18.Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148(7):3236–45. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- 19.Kelly MJ, Ronnekleiv OK. Membrane-initiated estrogen signaling in hypothalamic neurons. Molecular and cellular endocrinology. 2008;290(1–2):14–23. doi: 10.1016/j.mce.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80(4):1523–631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 21.Grattan DR, Kokay IC. Prolactin: a pleiotropic neuroendocrine hormone. Journal of neuroendocrinology. 2008;20(6):752–63. doi: 10.1111/j.1365-2826.2008.01736.x. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocrine reviews. 2001;22(6):724–63. doi: 10.1210/edrv.22.6.0451. [DOI] [PubMed] [Google Scholar]
- 23.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. Journal of endocrinology. 2007;193(2):311–21. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 24.Xu H, Qin S, Carrasco GA, Dai Y, Filardo EJ, Prossnitz ER, Battaglia G, Doncarlos LL, Muma NA. Extra-nuclear estrogen receptor GPR30 regulates serotonin function in rat hypothalamus. Neuroscience. 2009;158(4):1599–607. doi: 10.1016/j.neuroscience.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakamoto H, Matsuda K, Hosokawa K, Nishi M, Morris JF, Prossnitz ER, Kawata M. Expression of G protein-coupled receptor-30, a G protein-coupled membrane estrogen receptor, in oxytocin neurons of the rat paraventricular and supraoptic nuclei. Endocrinology. 2007;148(12):5842–50. doi: 10.1210/en.2007-0436. [DOI] [PubMed] [Google Scholar]
- 26.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2(4):207–12. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 27.Pellegrino L, Pellegrino A, Cushman A. A Stereotaxic Atlas of the Rat Brain. New York: Plenum Press; 1979. [Google Scholar]
- 28.Clark AS, Roy EJ. Effective intervals for the administration of estradiol pulses and the induction of sexual behavior in female rats. Physiology & behavior. 1987;39(5):665–7. doi: 10.1016/0031-9384(87)90171-5. [DOI] [PubMed] [Google Scholar]
- 29.Hardy DF, DeBold JF. Effects of coital stimulation upon behavior of the female rat. J Comp Physiol Psychol. 1972;78(3):400–8. doi: 10.1037/h0032536. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A. Rational siRNA design for RNA interference. Nat Biotechnol. 2004;22(3):326–30. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- 31.Naito Y, Yamada T, Ui-Tei K, Morishita S, Saigo K. siDirect: highly effective, target-specific siRNA design software for mammalian RNA interference. Nucleic Acids Res. 2004;32(Web Server issue):W124–9. doi: 10.1093/nar/gkh442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez VA, Ridenour DA, Sabatini BL. Retraction of synapses and dendritic spines induced by off-target effects of RNA interference. J Neurosci. 2006;26(30):7820–5. doi: 10.1523/JNEUROSCI.1957-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10(2):302–17. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 34.Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med. 2003;9(12):1539–44. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- 35.Xu L, Daly T, Gao C, Flotte TR, Song S, Byrne BJ, Sands MS, Parker Ponder K. CMV-beta-actin promoter directs higher expression from an adeno-associated viral vector in the liver than the cytomegalovirus or elongation factor 1 alpha promoter and results in therapeutic levels of human factor X in mice. Hum Gene Ther. 2001;12(5):563–73. doi: 10.1089/104303401300042500. [DOI] [PubMed] [Google Scholar]
- 36.Paterna JC, Moccetti T, Mura A, Feldon J, Bueler H. Influence of promoter and WHV post-transcriptional regulatory element on AAV-mediated transgene expression in the rat brain. Gene Ther. 2000;7(15):1304–11. doi: 10.1038/sj.gt.3301221. [DOI] [PubMed] [Google Scholar]
- 37.Babcock AM, Standing D, Bullshields K, Schwartz E, Paden CM, Poulsen DJ. In vivo inhibition of hippocampal Ca2+/calmodulin-dependent protein kinase II by RNA interference. Mol Ther. 2005;11(6):899–905. doi: 10.1016/j.ymthe.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic Press; 1982. [Google Scholar]
- 39.Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(27):10456–60. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grimm D, Pandey K, Kay MA. Adeno-associated virus vectors for short hairpin RNA expression. Methods in enzymology. 2005:392381–405. doi: 10.1016/S0076-6879(04)92023-X. [DOI] [PubMed] [Google Scholar]
- 41.Peel AL, Klein RL. Adeno-associated virus vectors: activity and applications in the CNS. Journal of neuroscience methods. 2000;98(2):95–104. doi: 10.1016/s0165-0270(00)00183-7. [DOI] [PubMed] [Google Scholar]
- 42.Choi VW, McCarty DM, Samulski RJ. AAV hybrid serotypes: improved vectors for gene delivery. Current gene therapy. 2005;5(3):299–310. doi: 10.2174/1566523054064968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taymans JM, Vandenberghe LH, Haute CV, Thiry I, Deroose CM, Mortelmans L, Wilson JM, Debyser Z, Baekelandt V. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Human gene therapy. 2007;18(3):195–206. doi: 10.1089/hum.2006.178. [DOI] [PubMed] [Google Scholar]
- 44.Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biology of reproduction. 2009;80(1):34–41. doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- 45.Steyn FJ, Anderson GM, Grattan DR. Differential effects of centrally-administered oestrogen antagonist ICI-182,780 on oestrogen-sensitive functions in the hypothalamus. Journal of neuroendocrinology. 2007;19(1):26–33. doi: 10.1111/j.1365-2826.2006.01499.x. [DOI] [PubMed] [Google Scholar]
- 46.Kansra S, Yamagata S, Sneade L, Foster L, Ben-Jonathan N. Differential effects of estrogen receptor antagonists on pituitary lactotroph proliferation and prolactin release. Molecular and cellular endocrinology. 2005;239(1–2):27–36. doi: 10.1016/j.mce.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Demaria JE, Nagy GM, Lerant AA, Fekete MI, Levenson CW, Freeman ME. Dopamine transporters participate in the physiological regulation of prolactin. Endocrinology. 2000;141(1):366–74. doi: 10.1210/endo.141.1.7281. [DOI] [PubMed] [Google Scholar]
- 48.Alyea RA, Laurence SE, Kim SH, Katzenellenbogen BS, Katzenellenbogen JA, Watson CS. The roles of membrane estrogen receptor subtypes in modulating dopamine transporters in PC-12 cells. Journal of neurochemistry. 2008;106(4):1525–33. doi: 10.1111/j.1471-4159.2008.05491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnston CA, Negro-Vilar A. Role of oxytocin on prolactin secretion during proestrus and in different physiological or pharmacological paradigms. Endocrinology. 1988;122(1):341–50. doi: 10.1210/endo-122-1-341. [DOI] [PubMed] [Google Scholar]
- 50.Samson WK, Lumpkin MD, McCann SM. Evidence for a physiological role for oxytocin in the control of prolactin secretion. Endocrinology. 1986;119(2):554–60. doi: 10.1210/endo-119-2-554. [DOI] [PubMed] [Google Scholar]
- 51.Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150(4):1722–30. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- 52.Kow L-M, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci U S A. 2004;101(33):12354–7. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly MJ, Ronnekleiv OK, Eskay RL. Identification of estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Brain Res Bull. 1984;12(4):399–407. doi: 10.1016/0361-9230(84)90112-6. [DOI] [PubMed] [Google Scholar]
- 54.Terasawa E, Noel SD, Keen KL. Rapid action of oestrogen in luteinising hormone-releasing hormone neurones: the role of GPR30. Journal of neuroendocrinology. 2009;21(4):316–21. doi: 10.1111/j.1365-2826.2009.01839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Molecular endocrinology. 2009;23(3):349–59. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149(10):4846–56. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- 57.Scammell TE, Arrigoni E, Thompson MA, Ronan PJ, Saper CB, Greene RW. Focal deletion of the adenosine A1 receptor in adult mice using an adeno-associated viral vector. J Neurosci. 2003;23 (13):5762–70. doi: 10.1523/JNEUROSCI.23-13-05762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang C, Gagnon D, Vachon P, Tremblay A, Levy E, Massie B, Michaud JL. Adenoviral-mediated modulation of Sim1 expression in the paraventricular nucleus affects food intake. J Neurosci. 2006;26(26):7116–20. doi: 10.1523/JNEUROSCI.0672-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, Bi S. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. J Neurosci. 2009;29(1):179–90. doi: 10.1523/JNEUROSCI.4379-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCrimmon RJ, Shaw M, Fan X, Cheng H, Ding Y, Vella MC, Zhou L, McNay EC, Sherwin RS. Key role for AMP-activated protein kinase in the ventromedial hypothalamus in regulating counterregulatory hormone responses to acute hypoglycemia. Diabetes. 2008;57(2):444–50. doi: 10.2337/db07-0837. [DOI] [PubMed] [Google Scholar]
- 61.Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. Expression Pattern of Gpr30 in LacZ Reporter Mice. Endocrinology. 2008 doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- 62.Jazwa A, Jozkowicz A, Dulak J. New vectors and strategies for cardiovascular gene therapy. Curr Gene Ther. 2007;7(1):7–23. doi: 10.2174/156652307779940243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296(5567):550–3. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 64.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104(7):2501–6. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.