Abstract
Hemoglobin-based oxygen carriers (HBOCs) have been studied for decades as red blood cell substitutes. Profound vasoconstrictor effects have limited the clinical utility of HBOCs and are attributable to avid scavenging of nitric oxide (NO). Inhaling NO can charge the body's stores of NO metabolites without producing hypotension and can prevent systemic hypertension induced when HBOCs are subsequently infused. Concurrent breathing of low NO doses can prevent pulmonary vasoconstriction after HBOC infusion without augmenting plasma methemoglobinemia.
In 2004, trauma due to automobile accidents claimed more than 43,000 lives in the US (http://www.cdc.gov/ncipc/wisqars), and many of these individuals had hemorrhagic shock when they arrived at the hospital. Although clear fluids can be used for resuscitation for brief periods, the availability of a safe and effective oxygen-carrying fluid, like a hemoglobin (Hb)-based oxygen carrier (HBOC), could provide far more effective metabolic support for the organs of the injured body during transport to a facility where type-matched blood would be available for transfusion. Our blood supply has always been subject to periodic shortages and vulnerable to infections. HIV and hepatitis virus are recent examples, and the possibility of new blood-borne infections is another reason that development of a more reliable and safe oxygen transporter is important. This elusive goal has been pursued for many years without success, most recently owning to an excess of myocardial infarction and death in patients receiving HBOCs in clinical trials (Natanson et al. 2008). We believe that the excess of morbidity and mortality associated with HBOC administration has occurred, in part, because the important NO-scavenging effects of HBOCs have been inadequately addressed.
Normally sequestered in erythrocytes, when Hb is released into the bloodstream, it triggers a sequence of reactions producing tissue injury. This tissue injury is seen in a variety of human disorders, such as malaria and sickle cell disease (O'Donnell et al. 2006, Wood et al. 2008). In the surgical patient, hemolysis is frequently produced by various hemolytic reactions (e.g. mismatched transfused blood), as well as pump oxygenator trauma to red blood cells during cardiopulmonary bypass (Kawahito et al. 2001).
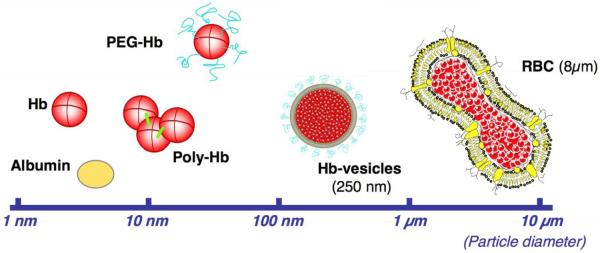
It has been known for 30 years (Savitsky et al. 1978) that infusions of tetrameric Hb produce severe vasoconstriction and hypertension in patients. Vasoconstriction appears to result from scavenging of NO by plasma ferrous (Fe2+) heme, thereby lowering the concentration of NO available to smooth muscle cells in the vascular wall. When the intracellular NO concentration falls, the activity of the NO receptor, soluble guanylate cyclase decreases; levels of its product, the intracellular second messenger cGMP, decline; and vascular smooth muscle cells contract. In the course of designing HBOCs, several strategies have been explored to reduce NO scavenging by extracellular ferrous Hb (Figure 1) and has been recently reviewed (Chang 2009). One strategy was directed at reducing delivery of tetramers to the critical abluminal region between endothelium and vascular smooth muscle (Nakai et al. 1998). One approach was to produce larger molecules of Hb by cross-linking monomers, and low molecular weight tetramers were reduced or removed. Crosslinked Hb has been infused in trauma patients when red blood cells are not available (Moore et al. 2009). In a second approach, Hb molecules were packaged in nanocells of 250 nm diameter that were coated with polyethylene glycol to reduce their transport across the endothelium (Sakai et al. 2007). In a second strategy, the structure of Hb's distal-gas binding pocket was modified by site-directed mutagenesis in E. coli to selectively reduce the rate of NO binding by 20- to 30-fold (Doherty et al. 1998, Hermann et al. 2007). A third strategy grew out of the belief that excessive oxygen delivery by HBOC with low oxygen affinity to arteriolar musculature was the cause of the vasoconstriction. This led to producing Hb molecules with reduced oxygen and NO affinity (Winslow 2003).
Figure 1.
The optimal dimension of artificial oxygen carriers. The upper limit of 10 μm in particle diameter is to prevent capillary plugging and for the sterilization by membrane filters. Small particles (<10 nm in diameter) have high rates of renal excretion and vascular wall permeabilities with side effects such as hypertension and neurological disturbances. (Figure was modified from Sakai et al, 2007; reprinted with permission from Sakai H, et al. J Intern Med 2007;263:4-15)
To further characterize the role of NO scavenging in the vasoconstrictor effects of HBOCs and to develop new strategies to prevent these vasoconstrictor effects, we have studied the hemodynamic alterations produced by infusions of various Hb preparations in mice and sheep. The majority of our studies were performed in awake animals to avoid the depressant effects of general anesthesia. We did not study hemorrhagic shock since that would greatly complicate our assessment of the hemodynamic effects of HBOCs by adding the major changes of shock, vasoconstriction, tachycardia, and reduced cardiac output. Thus, we simply intravenously infused HBOC-201 (polymerized bovine Hb produced by Biopure Corp with 3% tetrameric Hb) as a 16% of the blood volume “top load”. As a positive control for the vasoconstrictor effects of extracellular Hb, we infused a solution of Hb prepared from lysed autologous murine red blood cells. As a negative control, we transfused the same volume of autologous whole blood. In mice, systolic blood pressure (SBP) was measured with a non-invasive tail cuff blood pressure system. To perform serial hemodynamic measurements in awake lambs, catheters were placed in the carotid artery and pulmonary artery, and a tracheostomy was performed under anesthesia. The sheep were studied after recovery from the anesthesia.
We commenced our studies in mice, since selective breeding has allowed the generation of genotypes that congenitally do not express isoforms of NO synthase (NOS). To examine the vasoactivity of HBOCs, we began by studying the effects on SBP of wild-type (WT) mice by infusing either whole blood (1.44 g Hb/kg), murine tetrameric Hb (0.48 g/kg), or HBOC-201 (1.44 g/kg) over 1 min via a tail vein (see Figure 2A). Blood infusion did change SBP, while infusing either tetrameric Hb or HBOC-201 caused prolonged systemic hypertension lasting at least an hour. Subsequent studies of anesthetized WT mice at cardiac catheterization showed this hypertension to be due to profound systemic vasoconstriction.
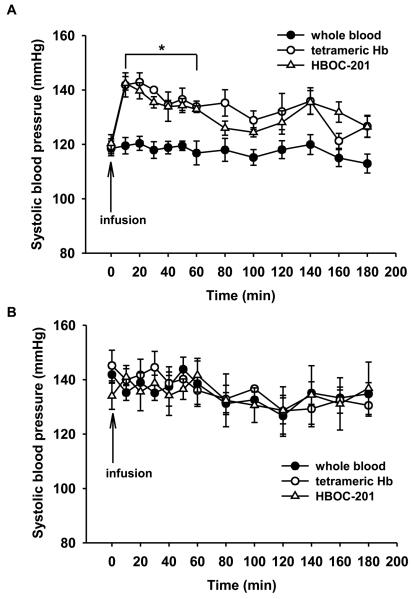
Figure 2.
(A) In WT mice, tail-cuff SBP (mmHg) was measured before and after i.v. infusion of whole blood (n=7), murine tetrameric Hb (n=5), or HBOC-201 (n=5). *P<0.001 differs versus whole blood infusion group. (B) In NOS3−/− mice, SBP was measured before and after i.v. infusion of whole blood (n=5), murine tetrameric Hb (n=5), or HBOC-201 (n=5). There was no increase of SBP after infusion of murine tetrameric Hb or HBOC-201. (Reprinted with permission from Yu B, et al. Circulation 2008;117:1982-1990)
To learn if HBOC-201-induced systemic hypertension was caused by scavenging of NO produced by endothelial NOS (NOS3, the predominant NOS expressed in endothelial cells), we studied mice that were congenitally NOS3-deficient. These mice are hypertensive, but infusion of either tetrameric Hb or HBOC-201 did not alter their blood pressure (Figure 2B). To confirm the importance of the heme component of extracellular tetrameric Hb in its vasoactive effects, we prepared tetrameric Hb in which the heme moiety was oxidized to the ferric state (Fe3+). Oxidized Hb is called methemoglobin. Infusion of methemoglobin tetramer did not produce systemic hypertension in awake WT mice. This finding further convinced us that the hypertensive reaction to cell-free Hb was due to scavenging of NO produced by NOS3 and not another type of adverse vasoconstrictor reaction (e.g excessive oxygen delivery or endothelin release) to infused Hb (Winslow 2003, Smani et al. 2007).
At first glance, it might seem useful to prevent the vasoconstriction produced by HBOC-induced NO scavenging by replacing or supplementing circulating NO metabolite levels with infused NO donors (e.g. sodium nitroprusside, nitroglycerin, nitrite, etc). Unfortunately, NO donors can produce profound systemic vasodilation and hypotension, an unwelcome effect during resuscitation from hemorrhage. As an alternative approach, we and others evaluated the ability of inhaled NO to prevent the vasoconstrictor effects of extracellular Hb and HBOCs. Inhaled NO is a selective pulmonary vasodilator that is FDA-approved to treat pulmonary hypertension and to increase systemic oxygenation in newborns (reviewed in Bloch et al. 2007). Approximately 30,000 patients are treated each year in the USA with this inhaled therapeutic agent. Inhaled NO does not produce systemic hypotension or significant vasodilation owing to its rapid intracellular combination with Hb in the red blood cell (Frostell et al. 1991). NO oxidizes the Hb, but the red blood cell contains methemoglobin reductase, an enzyme that rapidly converts the methemoglobin back to ferrous Hb.
Recent evidence suggests that inhaled NO may affect the systemic vasculature, leading to vasodilation when endogenous NO synthesis is inhibited (Cannon et al. 2001) (although this vasodilator effect is not evident in mice; Hataishi et al. 2006a). Moreover, inhaled NO can ameliorate ischemia-reperfusion injury of peripheral organs (Fox-Robichaud et al. 1998, Hataishi et al. 2006b). Inhaled NO may exert systemic effects via interaction with circulating cells as they transit the lungs. Alternatively, some NO, once inhaled, may including nitrite, RSNO, and others, that can regenerate NO in the periphery (Gaston 2006).
It was reported that the systemic vasoconstriction produced by infusion of a Hb solution into animals could be reversed by inhaling high concentration of NO gas (80 parts per million (ppm)) (Minneci et al. 2005). Exposing circulating extracellular Hb (in the absence of methemoglobin reductase) to this high concentration of inhaled NO stably converted the Hb to methemoglobin and abolished its ability to scavenge NO. Although this strategy might be useful in treating vasoconstriction due to hemolytic release of Hb, oxidation of an HBOC would render it incapable of performing its vital function of oxygen delivery as methemoglobin cannot transport oxygen.
As an alternative strategy, we sought to test the hypothesis that loading body stores of NO metabolites by breathing NO could prevent the systemic vasoconstriction induced by the subsequent infusion of an HBOC without impairing the oxygen-carrying capacity of the red blood cell substitute. Surprisingly, breathing 80 ppm NO for an hour (and then ceasing NO breathing) before injecting tetrameric Hb completely prevented the systemic vasoconstriction and hypertension in awake WT mice. We learned that shorter periods of NO inhalation (80 ppm for 15 min or 200 ppm for 7 min) also effectively blocked the systemic hypertension induced by infusing tetrameric Hb or HBOC-201. Breathing NO before HBOC-201 infusion did not increase extracellular methemoglobin levels above the 2-3.5% observed in mice receiving HBOC-201 without inhaled NO. These findings, which were confirmed by invasive hemodynamic measurements in anesthetized mice, demonstrate the pretreatment with inhaled NO can prevent HBOC-induced systemic vasoconstriction without impairing the ability of the HBOC to carry oxygen.
To obtain more complete serial hemodynamic measurements and to confirm our findings in a second species, we studied 25-kg Suffolk lambs infused a “topload” of 12 ml/kg of either autologous heparinized blood (drawn 3 days earlier in heparin) or HBOC-201. We learned that, like the mouse, lambs developed prolonged systemic hypertension to a topload of HBOC-201. Mean arterial pressure (MAP), mean pulmonary arterial pressure (PAP), systemic vascular resistance (SVR), and pulmonary vascular resistance (PVR) were all increased immediately after HBOC-201 infusion in lambs breathing at FiO2 0.3 without added NO. In contrast, infusion of autologous blood did not significantly alter hemodynamic measurements in awake lambs. Once again, we tested the strategy of pretreating lambs with inhaled NO (80 ppm for one hour) to elevate circulating NO metabolite levels before administering HBOC-201. We learned that NO pretreatment was able to prevent the HBOC-201-induced systemic hypertension. However, pretreatment with inhaled NO did not prevent the pulmonary vasoconstriction (increased PAP and PVR) or the reduced cardiac output (resulting from an increased vascular resistance) induced by HBOC-201 infusion.
Since pretreatment with inhaled NO did not prevent the acute pulmonary hypertension and systemic vasoconstriction induced in lambs by HBOC-201 infusion, we hypothesized that after “preloading” the lamb with NO metabolites, concurrent breathing of low levels of NO might block the HBOC-induced pulmonary hypertension without causing significant conversion of the circulating HBOC-201 to inactive methemoglobin. Since it was known that breathing 80 ppm NO during HBOC administration induced marked plasma methemoglobinemia (Minneci et al. 2005), we sought to identify a lower concentration of NO gas which when inhaled would dilate the pulmonary vasculature without inducing plasma methemoglobin formation. We found that continuously breathing NO (<30 ppm) after HBOC-201 administration did not markedly oxidize either plasma or intracellular Hb.
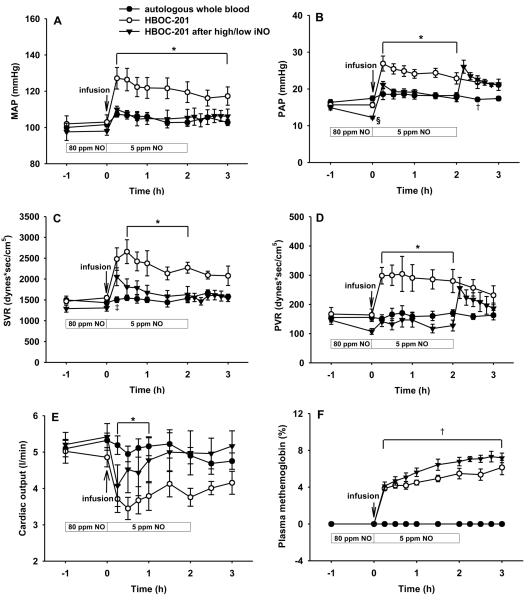
To examine whether breathing a high concentration of NO before and a lower concentration of NO after HBOC-201 infusion could block the acute pulmonary hypertension induced by HBOC administration, we pretreated lambs with 80 ppm NO breathing for 1 h, followed by infusion of HBOC-201 (12 ml/kg) while continuously breathing 5 ppm NO for 2 h. We found that this two-level combination of NO breathing prevented the increase of both systemic and pulmonary vascular resistance induced by HBOC-201 infusion (see Figure 3). Interestingly, we noted that 2 h after HBOC-201 infusion, when NO breathing was acutely discontinued, the PAP and PVR immediately increased, but there were no effects on MAP or SVR. After pretreatment with 80 ppm NO followed by continuously breathing of 5 ppm NO, the HBOC-201-induced decrease in cardiac output was markedly attenuated. After administration of HBOC-201, plasma methemoglobin levels in lambs that breathed NO at 80 ppm and then at 5 ppm did not increase beyond the levels observed in the lambs that received HBOC-201 without NO. Thus inhaling NO at a high concentration to load the animal with NO metabolites, followed by continuously breathing a low dose of NO during and after HBOC infusion reduced the vasoactive response to infusion of HBOC-201, suggesting that a simple inhalation strategy could succeed at minimizing the deleterious hemodynamic effects of infusing NO scavengers.
Figure 3.
(A) Mean arterial pressure (MAP), (B) mean pulmonary arterial pressure (PAP), (C) systemic vascular resistance (SVR), (D) pulmonary vascular resistance (PVR), (E) Cardiac output, and (F) plasma methemoglobin concentration of awake lambs after infusion of autologous whole blood (n=6), intravenous HBOC-201 (n=5), or HBOC-201 after pretreatment by breathing 80 ppm NO for 1 h followed by continuously breathing 5 ppm NO for 2 h (high/low NO, n=6). *P<0.05 HBOC-201 differs from autologous whole blood and from HBOC-201 after high/low inhaled NO, †P<0.05 autologous whole blood differs from HBOC-201 with or without high/low inhaled NO, ‡P<0.05 HBOC-201 differs from autologous whole blood, §P<0.05 differs from autologous whole blood and HBOC-201. (Reprinted with permission from Yu B, et al. Anesthesiology 2009;110:113-122)
Breathing NO leads to an accumulation of NO metabolites, including nitrate and nitrite (Hataishi et al. 2006b, Nagasaka et al. 2008). Nitrite can be converted back to NO via nitrite reductases, including deoxyhemoglobin (Lundberg et al. 2008) and xanthine oxido reductase (Casey et al. 2009). We examined whether nitrite administration could prevent the pulmonary hypertension caused by HBOC-201 infusion in lambs: sodium nitrite (1 mg/kg) was administered intravenously as an infusion before HBOC-201 was infused. The nitrite infusion transiently lowered the MAP from baseline 99±2 to 84±3 mmHg (mean±SD, P=0.008). Nitrite infusion attenuated the systemic hypertension induced by intravenous infusion of HBOC-201 but did not oxidize the HBOC. However, nitrite did not prevent the increases of PAP, PVR, or SVR or the decrease in cardiac output. In addition, nitrite infusion did not further increase plasma methemoglobin level.
Nitrate and nitrite levels were measured in plasma samples taken from lambs pretreated with inhaled NO for 1 h, from lambs treated with two levels of inhaled NO (80 ppm, 1 h before HBOC infusion and 5 ppm thereafter), and from lambs receiving a nitrite infusion. Plasma nitrate levels did not change in lambs receiving HBOC-201 alone, but dramatically increased in lambs breathing NO and in lambs given a nitrite infusion. On the other hand, plasma nitrite levels did not change in sheep breathing NO, but levels were markedly increased in the lambs given intravenous nitrite before HBOC-201 administration. After the sodium nitrite infusion, the increase in plasma nitrite levels was transient, and nitrite concentrations returned to the baseline levels by 15 min after HBOC-201 infusion. These findings suggest that increased levels of nitrite are not required for the ability of inhaled NO to prevent the vasoconstriction induced by HBOC-201. On the other hand, a bolus of nitrite followed by an infusion of a lower dose might also prevent the vasoconstriction induced by HBOCs.
The awake lamb is a commonly-used model for pulmonary circulatory studies of the human newborn, as it has a muscularized pulmonary arterial circulation. Thus, the lamb may be excessively susceptible to pulmonary vasoconstriction due to HBOC infusion. However, a muscularized pulmonary circulation is present in the human newborn as well as the pulmonary circulation of adults with chronic pulmonary hypertension or other lung vascular diseases. It is conceivable that newborns and adults with pulmonary vascular diseases may be quite susceptible to vasoconstriction from HBOC infusion (Serruys et al. 2008). High doses of inhaled NO can cause methemoglobinemia and may damage the lung and perhaps other organs (Adhikari et al. 2007). On the other hand, each year thousands of infants with hypoxic respiratory failure and pulmonary hypertension are treated with inhaled NO, usually commencing at 20-80 ppm for many hours (The Neonatal Inhaled Nitric Oxide Study Group 1997). Since most of these newborns survive without renal or respiratory failure, it is unlikely that short-term breathing of such doses of NO gas is toxic.
In conclusion, we have learned that scavenging of endothelial NO produced by NOS3 is the cause of the systemic vasoconstriction and hypertension induced by i.v. infusion of tetrameric Hb or HBOC-201 in mice. Pretreatment of mice with inhaled NO can augment body stores of NO metabolites and prevent the vasoconstriction induced by the subsequent infusion of HBOCs with impairing their ability to transport oxygen. In awake lambs, pretreatment with inhaled NO, followed by continuously breathing a low concentration of NO, can prevent the pulmonary and systemic vasoconstriction induced by HBOC-201 administration without causing any significant elevation of plasma methemoglobin levels. Although we have not yet studied the application of the combination of inhaled NO with HBOC administration for treatment of hemorrhagic shock in the laboratory, we believe that this promising combination therapy merits further evaluation as a method to enable HBOC administration without producing vasoconstriction in patients with acute traumatic anemia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. Br Med J. 2007;334:779–786. doi: 10.1136/bmj.39139.716794.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch KD, Ichinose F, Roberts JD, Jr., Zapol WM. Inhaled NO as a therapeutic agent. Cardiovasc Res. 2007;75:339–348. doi: 10.1016/j.cardiores.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon RO, 3rd, Schechter AN, Panza JA, et al. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest. 2001;108:279–287. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DB, Badejo AM, Jr, Dhaliwal JS, et al. Pulmonary vasodilator responses to sodium nitrite are mediated by an allopurino-sensitive mechanism in the rat. Am J Physiol Heart Circ Physiol. 2009;296:H524–533. doi: 10.1152/ajpheart.00543.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TMS. Nanobiotechnology for hemoglobin-based blood substitutes. Crit Care Clin. 2009;25:373–382. doi: 10.1016/j.ccc.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty DH, Doyle MP, Curry SR, et al. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat Biotechnol. 1998;16:672–676. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- Fox-Robichaud A, Payne D, Hasan SU, et al. Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. J Clin Invest. 1998;101:2497–2505. doi: 10.1172/JCI2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation. 1991;83:2038–2047. doi: 10.1161/01.cir.83.6.2038. [DOI] [PubMed] [Google Scholar]
- Gaston B. Summary: systemic effects of inhaled nitric oxide. Proc Am Thorac Soc. 2006;3:170–172. doi: 10.1513/pats.200506-049BG. [DOI] [PubMed] [Google Scholar]
- Hataishi R, Rodrigues AC, Neilan TG, et al. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006b;291:H379–H384. doi: 10.1152/ajpheart.01172.2005. [DOI] [PubMed] [Google Scholar]
- Hataishi R, Zapol WM, Bloch KD, Ichinose F. Inhaled nitric oxide does not reduce systemic vascular resistance in mice. Am J Physiol Heart Circ Physiol. 2006a;290::H1826–H1829. doi: 10.1152/ajpheart.00938.2005. [DOI] [PubMed] [Google Scholar]
- Hermann J, Corso C, Messmer KF. Resuscitation with recombinant hemoglobin rHb2.0 in a rodent model of hemorrhagic shock. 2007;107:273–280. doi: 10.1097/01.anes.0000270756.11669.64. http://www.cdc.gov/ncipc/wisqars [DOI] [PubMed]
- Kawahito S, Maeda T, Yoshikawa M, et al. Blood trauma induced by clinically accepted oxygenators. ASAIO J. 2001;47:492–495. doi: 10.1097/00002480-200109000-00019. [DOI] [PubMed] [Google Scholar]
- Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- Minneci PC, Deans KJ, Zhi H, et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EE, Moore FA, Fabian TC, et al. Human polymerized haemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA multicenter trial. J Am Coll Surg. 2009;208:1–13. doi: 10.1016/j.jamcollsurg.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Nagasaka Y, Fernandez BO, Garcia-Saura MF, et al. Brief periods of nitric oxide inhalation protect against myocardial ischemia–reperfusion injury. Anesthesiology. 2008;109:675–682. doi: 10.1097/ALN.0b013e318186316e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai K, Sakuma I, Ohta T, et al. Permeability characteristics of hemoglobin derivatives across cultured endothelial cell monolayers. J Lab Clin Med. 1998;132:313–319. doi: 10.1016/s0022-2143(98)90045-2. [DOI] [PubMed] [Google Scholar]
- Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: A meta-analysis. JAMA. 2008;299:2304–2312. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell A, Weatherall DJ, Taylor AM, Reeder JC, Allen SJ. Muscle cell injury, haemolysis and dark urine in children with falciparum malaria in Papua New Guinea. Trans R Soc Trop Med Hyg. 2006;100:817–825. doi: 10.1016/j.trstmh.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Sakai H, Sou K, Horinouchi H, Kobayashi K, Esuchida E. Haemoglobin-vesicles as artificial oxygen carriers: present situation and future visions. J Intern Med. 2007;263:4–15. doi: 10.1111/j.1365-2796.2007.01893.x. [DOI] [PubMed] [Google Scholar]
- Savitsky JP, Doczi J, Black J, Arnold JD. A clinical safety trial of stroma-free hemoglobin. Clin Pharmacol Ther. 1978;23:73–80. doi: 10.1002/cpt197823173. [DOI] [PubMed] [Google Scholar]
- Serruys PW, Vranckx P, Slagboom T, et al. Haemodynamic effects, safety, and tolerability of haemoglobin-based oxygen carrier-201 in patients undergoing PCI for CAD. Eurointervention. 2008;3:600–609. doi: 10.4244/eijv3i5a108. [DOI] [PubMed] [Google Scholar]
- Smani Y, Fifre A, Labrude P, Vigneron C, Faivre B. Pharmacological and physicochemical factors in the pressor effects of conjugated haemoglobin-based oxygen carriers in vivo. J Hypertens. 2007;25:599–608. doi: 10.1097/HJH.0b013e3280119000. [DOI] [PubMed] [Google Scholar]
- The Neonatal Inhaled Nitric Oxide Study Group Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med. 1997;336:597–604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- Winslow RM. Current status of blood substitute research: towards a new paradigm. J Intern Med. 2003;253:508–517. doi: 10.1046/j.1365-2796.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- Wood KC, Hsu LL, Gladwin MT. Sickle cell disease vasculopathy: a state of nitric oxide resistance. Free Radic Biol Med. 2008;44:1506–1528. doi: 10.1016/j.freeradbiomed.2008.01.008. [DOI] [PubMed] [Google Scholar]