Abstract
Objectives:
To examine the ultrastructure of microvessels in normal and atherosclerotic coronary arteries and its association with plaque phenotype.
Background:
Microvessels in atherosclerotic plaques are an entry point for inflammatory and red blood cells. Yet there is limited data on the ultrastructural integrity of microvessels in human atherosclerosis.
Methods:
Microvessel density (MVD) and ultrastructural morphology were determined in the adventitia, intima-media border, and atherosclerotic plaque of 28 coronary arteries using immunohistochemistry for endothelial cells (Ulex europeaus, CD31/CD34), basement membrane (laminin, collagen IV), and mural cells (desmin, alpha-smooth muscle (SM) actin, smoothelin, SM1, SM2, Smemb). Ultrastructural characterization of microvessel morphology was performed by electron microscopy (EM).
Results:
MVD was increased in advanced plaques compared to early plaques, which correlated with lesion morphology. Adventitial MVD was higher than intraplaque MVD in normal arteries and early plaques, but adventitial and intraplaque MVD were similar in advanced plaques. Although microvessel basement membranes were intact, the percentage of thin-walled microvessels was similarly low in normal and atherosclerotic adventitia, in the adventitia and the plaque, and in all plaque types. Intraplaque microvascular endothelial cells (EC) were abnormal, with membrane blebs, intracytoplasmic vacuoles, open EC-EC junctions, and basement membrane detachment. Leukocyte infiltration was frequently observed by EM, and confirmed by CD45RO and CD68 immunohistochemistry.
Conclusions:
MVD was associated with coronary plaque progression and morphology. Microvessels were thin-walled in normal and atherosclerotic arteries, and the compromised structural integrity of microvascular endothelium may explain the microvascular leakage responsible for intraplaque hemorrhage in advanced human coronary atherosclerosis.
Keywords: Coronary atherosclerosis, angiogenesis, microvascular leakage, junctions, ultrastructure
Introduction
Atherosclerotic plaque rupture and symptomatic coronary disease are closely related to the presence of intraplaque and adventitial angiogenesis (1,2). Intraplaque microvessels are surrounded by macrophages and red blood cells, and hemorrhage, secondary cholesterol accumulation and inflammation in atherosclerotic plaques are associated with plaque rupture (3-6).
Macrophages and red blood cells are thought to extravasate from the intraplaque microvessel lumen into the plaque tissue (7). Microvascular structural integrity determines microvessel leakage, but a comprehensive analysis of intraplaque microvessel structure is lacking. Physiological angiogenesis starts with increased microvessel permeability and matrix degradation, followed by endothelial cell (EC) migration and/or proliferation. The resulting thin-walled, permeable sprouts are matured by basement membrane formation and mural cell recruitment. Research on pathological angiogenesis in tumors has established features of microvascular leakage (8). The majority of tumor microvessels are endothelial-lined tubes without coverage by basement membrane or mural cells, and these thin-walled structures are prone to leakage (8-10). The possible fragility of plaque microvessels corroborates the suggested leakage of macrophages and red blood cells.
Microvascular leakage is also characterized by a disruption of endothelial integrity, which is normally maintained by inter-cellular junctions (11). Microvessels with aberrant endothelial junctions demonstrate microvascular leakage (9). Based on the findings in physiological and pathological angiogenesis, we hypothesize that the structural integrity of plaque microvessels in human atherosclerosis is incomplete, possibly explaining the inflammatory infiltration and red blood cell extravasation.
Therefore, microvessel density and ultrastructure were studied in human atherosclerotic plaques of varying morphology and in normal coronary arteries using quantitative analysis of microvessel density, endothelial morphology and integrity, and mural cell and basement membrane presence by light and electron microscopy.
Materials and methods
Tissue collection
Atherosclerotic coronary arteries (n=28) were obtained at autopsy from 28 sudden death donors (Table 1). Collection, storage, and use of tissue and patient data were performed in agreement with institutional ethical guidelines. Samples were processed and classified, based on plaque morphology, as intimal thickening (IT, normal), pathological intimal thickening (PIT), a thick fibrous cap atheroma with an early (E-FA) or a late necrotic core (L-FA), a thin fibrous cap atheroma (TCFA) or a ruptured plaque, as described previously (12). Samples were included in the study without any pre-selection based on angiogenesis.
Table 1.
Demographics and clinical characteristics
| Frequency % (n)* | |
|---|---|
| Age (years)† | 55.5 (2.8) |
| Male | 89 (25) |
| Ethnicity Caucasian | 71 (21) |
| African-American | 25 (7) |
| Hypertension | 26 (6) |
| Hypercholesterolemia | 9 (2) |
| Smoking | 30 (7) |
| Alcohol use | 22 (5) |
| Statin therapy | 0 (0) |
| Type 2 diabetes mellitus | 9 (2) |
| History of cardiovascular disease | 17 (4) |
unless stated otherwise;
mean (SEM).
Immunohistochemistry
Immunohistochemistry was performed on paraffin-embedded sections with IT (n=5), PIT (n=5), E-FA (n=4), L-FA (n=5), TCFA (n=5) or ruptured plaques (n=4). Serial sections were stained with primary antibodies (Table 2) against macrophages (CD68), endothelial cells (Ulex aeropaeus lectin-1, CD31/CD34 cocktail), mural cells (alpha smooth muscle actin (αSMA), desmin), smooth muscle differentiation markers (smoothelin, smooth muscle (SM) myosin heavy chain SM1, SM2, and non muscle-type MHC SMemb), basement membrane (laminin, collagen IV), leukocytes (CD45RO), and mast cells (mast cell tryptase, MCT). Staining was visualized using 3,3′-diaminobenzidine tetrachloride (DAKO, Glostrup, Denmark) tinted with 0.04% nickel chloride (Sigma-Aldrich, St. Louis, MO). Evaluation of the staining pattern in control tissue, in which marker expression had previously been described, and omission of a primary antibody served as specificity controls.
Table 2.
Immunohistochemical methods
| Antibody | Company, product code | Expression | Dilution | Incubation | Antigen retrieval | Blocking | Detection |
|---|---|---|---|---|---|---|---|
| CD68 | Dako,M0814 | Macrophage | 1:300 | O/N | EDTA pH = 8 | Pre-AB Block | Powervision-HRP |
|
Ulex europeaus lectin-1 |
Vector Laboratories, L-1060 |
Endothelial cell | 1:400 | 1hr, RT | None | 10% Horse serum | LSAB-HRP |
| CD31/CD34 | Dako, M0823/Monosan, MON1164 |
Endothelial cell | 1:50- 1:200 |
1hr, RT | EDTA pH = 8 | Pre-AB block | Powervision-HRP |
| Desmin | Sigma,#1033 | Mural cell | 1:50 | O/N | EDTA pH = 8 | Pre-AB block | Powervision-HRP |
| αSMA | DAKO, M0851 | Mural cell | 1:400 | 1hr, RT | None | None | Powervision-HRP ABC-AP (double*) |
| Smoothelin | Monosan, MON 2094 | SM differentiation | 1:10 | 1hr, RT | cryosection | Pre-AB | Powervision-HRP |
| SM1 | Seigaku #7599 | SM differentiation | 1:800 | 1hr, RT | cryosection | Pre-AB | Powervision-HRP |
| SM2 | Seigaku, #7601 | SM differentiation | 1:800 | 1hr, RT | cryosection | Pre-AB | Powervision-HRP |
| SMemb | Seigaku, #7602 | SM differentiation | 1:800 | 1hr, RT | cryosection | Pre-AB | Powervision-HRP |
| Collagen IV | Eurodiagnostica, PCO2233 | Basement membrane | 1:4000 | 1hr, RT | Pepsin | Pre-AB block | Powervision-HRP |
| Laminin | Neomarkers, 86103 | Basement membrane | 1:100 | 1hr, RT | Protease 8 | Pre-AB block | Powervision-HRP |
| CD45 | DAKO, M0701 | Leukocyte | 1:500 | 1hr, RT | None | None | Powervision-HRP |
| Mast cell tryptase | DAKO M7052 | Mast cells | 1:1000 | 1hr, RT | None | None | Powervision-HRP |
Abbreviations: Pepsin, 0.1% pepsin in 0.1N HCl; Protease 8, 0.05% protease 8 (Sigma-Aldrich, St. Louis, MO) in 0.05M Tris/0.01M CaCl2, pH=7.6; Pre-AB Block, Powervision pre-antibody blocking reagent (Immunologic, Duiven, the Netherlands); LSAB-HRP, LSAB Strep-Avidin horseradish peroxidase conjugated (DAKO, Glostrup, Denmark); ABC-AP, alkaline phospatase conjugated avidin biotin complex (Vector laboratories, Burlingame, CA); Powervision-HRP, horseradish-peroxidase conjugated Powervision goat anti-mouse/rabbit/rat antibody (Immunologic);
double, double staining with U. europeaus. Other abbreviations are explained in text.
Quantitative morphometry
Histological and morphometric analysis of atherosclerosis was performed as described elsewhere.(13) Maximal intimal thickness was taken as the maximal distance perpendicular from the plaque surface to the internal elastic lamina (IEL). The maximal percentage of artery stenosis was calculated as (IEL perimeter − lumen perimeter)/(IEL perimeter)×100.
Microvessels were quantified in hotspots (≥ 3 microvessels) using ×200 fields of three regions: the adventitia, intima-media (IM) border, and intraplaque region (close to the necrotic core and away from the media) using image-processing software (IVision, Scanalytics, Rockville, MD). Adventitial microvessels were quantified using ULEX+ microvessels in one hotspot per quadrant, whereas CD31+CD34+ microvessels in the IM border and intraplaque regions were counted in ≤ 3 hotspots. The intima-media border was limited to twice the thickness of the normal media. Microvessel density (MVD) was computed as: the total number of vessels/total area, and mural cell coverage as: αSMA+ microvessel/total microvessel. When mural cells were present, mural cell layers were differentiated as single or multiple layers. The average across hotspots of each measurement was computed per region.
Electron microscopy
Coronary artery sections with normal intima (n=1) or advanced plaques (FA n=2; ruptured n=4) were obtained from 7 additional donors (60 ± 6 years, n=6 males, n=5 Caucasian, n=2 African-American). Tissue fragments of ∼1 mm3 were fixed overnight in 2.5% glutaraldehyde (Ted Pella, Redding, CA), post-fixed in 1% osmium tetroxide solution, dehydrated and embedded in epoxy resin. Semi-thin (1 μm) serial sections were stained with toluidine blue to localize microvessels. Ultra-thin sections (70-90 nm) were mounted on Formvar (1595 E, Merck)-coated 75 mesh copper grids, and counterstained with uranyl acetate and lead citrate before analysis on a Philips CM100 transmission electron microscope.
Statistical analysis
The overall difference between plaque types in terms of plaque morphology, MVD, and mural cell coverage was tested using a non-parametric Kruskall-Wallis test, followed by a Mann-Whitney test to determine which plaque types were different. A two-tailed Fisher exact test was used to test the frequency distribution of concentric/eccentric morphology between plaque types. Spearmann's non-parametric correlation coefficient ρ was calculated for plaque morphology, MVD, and mural cell coverage (SPSS 12.0 Chicago, IL). Results were considered statistically different when p<0.05.
Results
Plaque morphology changed with plaque progression
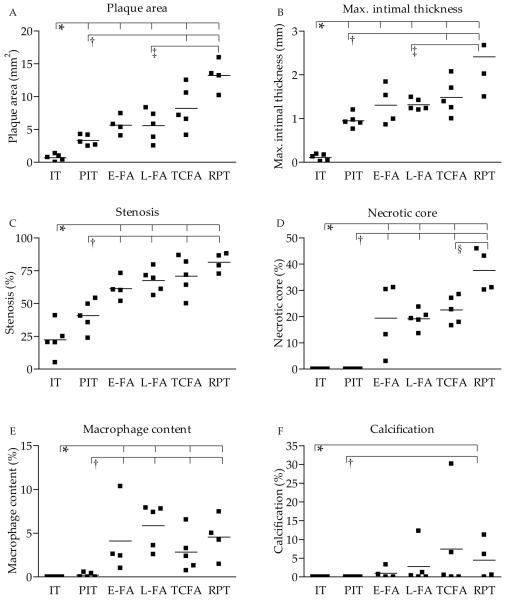
Plaque area, maximal intimal thickness, stenosis, necrotic core, macrophage, and calcification, significantly increased with progression of atherosclerosis (Figure 1A-F). Ruptured plaques were more often concentric than eccentric as compared to PIT, early, and late FA plaque stages (Table 3). The relation between plaque composition and plaque stage were as reported in the literature, indicating that a representative coronary plaque selection had been collected.
Figure 1. Atherosclerotic plaque morphology in normal and atherosclerotic coronary arteries.
A. Plaque area, B. maximal intimal thickness, C. stenosis, D. necrotic core content, E. macrophage content, and F. calcification content in normal coronary artery and various coronary plaque types. * p-value<0.05 vs. IT; † p<0.05 vs. PIT; ‡ p<0.05 vs. L-FA; § p<0.05 vs. TCFA. Abbreviations: IT, normal intimal thickening; PIT, pathological intimal thickening; FA, thick fibrous cap atheroma with an early or (E-FA) late necrotic core (L-FA); TCFA, thin fibrous cap atheroma; RPT, ruptured plaque
Table 3.
Frequencies of concentric vessel morphology in different plaque types
| Plaque type | Concentric % (n) |
|---|---|
| PIT* | 20 (1) |
| E-FA | 40 (2) |
| L-FA | 40 (2) |
| TCFA | 80 (4) |
| Rupture | 100 (5) |
indicates p-value <0.05 vs. rupture. Abbreviations: PIT, pathological intimal thickening; FA, thick fibrous cap atheroma with an early or (E-FA) late necrotic core (L-FA); TCFA, thin fibrous cap atheroma; RPT, ruptured plaque
Microvessel density increased with early plaque progression and plaque morphology
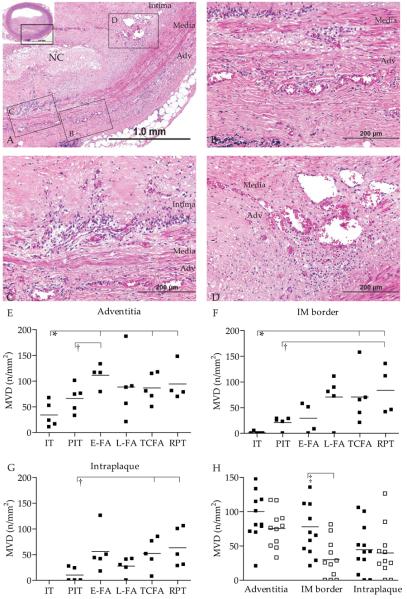
Angiogenesis in atherosclerotic plaques was present in three regions, viz. the adventitia, IM border, and intraplaque region (Figure 2A-D). Microvessel density (MVD) in the adventitia (p=0.03), IM border (p=0.01), and intraplaque region (p=0.025) (Figure 2E-G) significantly increased with plaque progression. A significant difference in MVD was observed between normal arteries or early plaque stages (PIT) and advanced plaques (FA and ruptured) in all arterial wall regions. However, MVD in late core fibroatheromas did not differ from that in ruptured plaques (Figure 2E-G) Likewise, plaque morphometry was generally similar between late core fibroatheromas, TCFAs and ruptured plaques (Figure 1). MVD was significantly higher in concentric plaques, presumably as advanced plaques, with concomitant higher MVD, were more often concentric (Figure 2H).
Figure 2. Microvessel density increases with atherosclerotic progression.
A. Detail of hematoxylin and eosin stained coronary artery with thick cap fibroatheroma (NC, necrotic core), inset shows entire coronary artery. Boxed regions B-D illustrate the regions of interest and correspond to photographs 2B showing microvessels in the adventitia, 2C intima-media border, and D. in the intraplaque region. E. Mean microvessel density (MVD) was quantified in adventitia, F. in the IM border, and G. in the intraplaque region, and increased with progression. H. Microvessel density was higher in concentric (black squares) than in eccentric (open squares) plaques. * p-value<0.05 vs. IT; † p<0.05 vs. PIT; ‡ p<0.05 eccentric vs. concentric; Abbreviations: IT, normal intimal thickening; PIT, pathological intimal thickening; FA, thick fibrous cap atheroma with an early or (E-FA) late necrotic core (L-FA); TCFA, thin fibrous cap atheroma; RPT, ruptured plaque
In advanced plaques, the MVD was similar in all vessel wall regions, while in early plaques, adventitial MVD was significantly higher than IM border and intraplaque MVD (Figure 2E-G). In addition, the trend of increasing adventitial MVD with plaque stage did not correlate with the intraplaque and IM border MVD trends (ρ=0.30, p=0.140 and ρ=0.35, p=0.056 respectively). MVD magnitude and trends were similar in IM border and intraplaque regions (ρ=0.44, p=0.028). Microvessels were observed invading from the adventitia, through the medial elastic lamina breaches into the plaque, in line with previous studies showing the adventitia as the origin of microvessel sprouting (Figure 3A-B) (2,14). Together, these results suggest that the onset of adventitial microvessels in atherogenesis preceded that in the IM border and intraplaque regions.
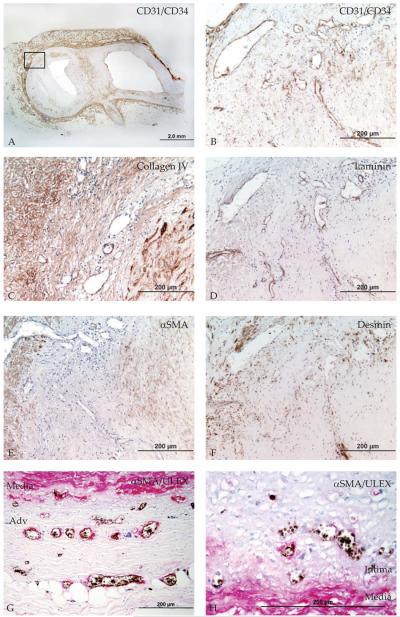
Figure 3. Basement membrane and mural cell coverage in normal and atherosclerotic coronary arteries.
A. Coronary fibroatheroma stained with CD31/CD34 cocktail. Boxed region indicates origin of B. CD31+CD34+ microvessels run from the adventitia through the media into the plaque. C Adjacent section stained with laminin or D. collagen IV, showing intact microvessel basement membrane in the adventitia, intima-media (IM) border and plaque. E. Adjacent section stained with alpha smooth muscle actin (aSMA), showing mostly αSMA− microvessels in the adventitia, IM border and plaque. F. Adjacent section stained with desmin shows some desmin+ microvessels in the adventitia, IM border and intraplaque region. G Adjacent section double-stained with lectin-1 (black) and αSMA (red) shows infrequent mural cell coverage in the adventitia and H. in the IM border.
MVD in all three regions correlated significantly with plaque morphology (Table 4). However, this effect was no longer observed after stratification for plaque stage. No association was observed between MVD and plaque calcification (Table 4).
Table 4.
Spearmann's ρ correlation coefficients of microvessel density and plaque morphology
| Microvessel density | |||
|---|---|---|---|
| Adventitia | IM border | Intraplaque | |
| Plaque area | 0.53† | 0.785† | 0.63† |
| Max. intimal thickness | 0.54† | 0.71† | 0.68† |
| Stenosis | 0.60† | 0.70† | 0.47* |
| Necrotic core content | 0.57† | 0.52† | 0.51† |
| Macrophage content | 0.49† | 0.58† | 0.57† |
| Calcification content | 0.35 | 0.65† | 0.37 |
p-values <0.05,
<0.01
Basement membrane is intact, but mural cell coverage is low in normal and atherosclerotic coronary arteries
Immunohistochemistry of basement membrane (laminin, collagen IV) and mural cells (αSMA, desmin) was performed to establish microvessel structure in normal and atherosclerotic coronary arteries. Microvessels in all regions were generally surrounded by laminin, and some also by collagen IV (Figure 3C-D), indicating that the microvessel basement membrane was present and intact. In contrast, mural cells were infrequently present (Figure 3E-H).
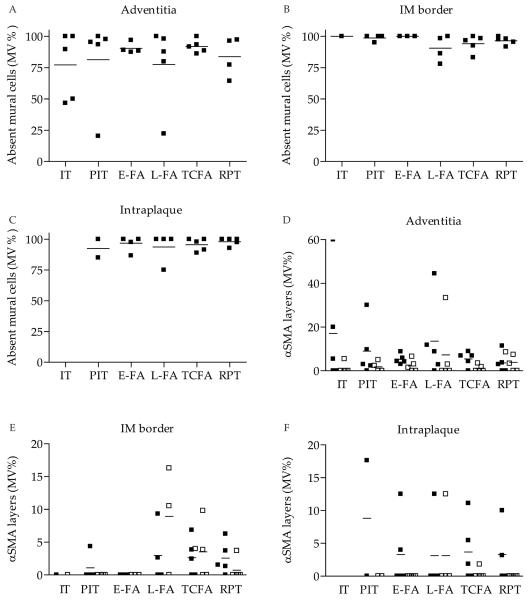
Mural cells surrounding microvessels may be pericytes or smooth muscle cells, a distinction mostly based on whether they share a basement membrane with the ECs or not. However, morphology and marker expression may vary with the tissue and/or pathology, hindering the identification of pericytes or smooth muscle cells (15). We therefore did not distinguish between mural cell types. On histology, the single or multi-layered mural cells most likely represented vascular smooth muscle cells, although EM also showed single-layered pericytes sharing basement membrane with EC. Thus, αSMA and desmin expressing cells were encountered surrounding arterial wall microvessels, but at low percentages (Figure 3E-H). Moreover, well-established markers of SMC differentiation SM1 and SM2 were also infrequently expressed, while microvessels remained mostly negative for SMemb or the late differentiation marker, smoothelin (data not shown). Unexpectedly, the percentage of thin-walled microvessels was not different between normal and atherosclerotic arteries, or between the different plaque types (Figure 4A-C). If present, mural cell layers (single or multiple) did not differ between plaque types either (Figure 4D-F). Also, no difference in mural cell coverage was observed between different vessel wall regions, nor was mural cell coverage correlated with plaque morphology.
Figure 4. Quantification of mural cell coverage in various plaque types.
A. Mural cell coverage was low in the adventitia, B. intima-media (IM) border, and C. intraplaque region of both normal and atherosclerotic coronary arteries. D. Percentage of microvessels with single (black squares) and multiple (open squares) mural cell layers in adventitia, E. IM border and F. intraplaque region. All p-values>0.05. Abbreviations: IT, normal intimal thickening; PIT, pathological intimal thickening; FA, thick fibrous cap atheroma with an early or (E-FA) late necrotic core (L-FA); TCFA, thin fibrous cap atheroma; RPT, ruptured plaque
Plaque microvessels show abnormal EC morphology, aberrant EC junctions, and leukocyte adherence
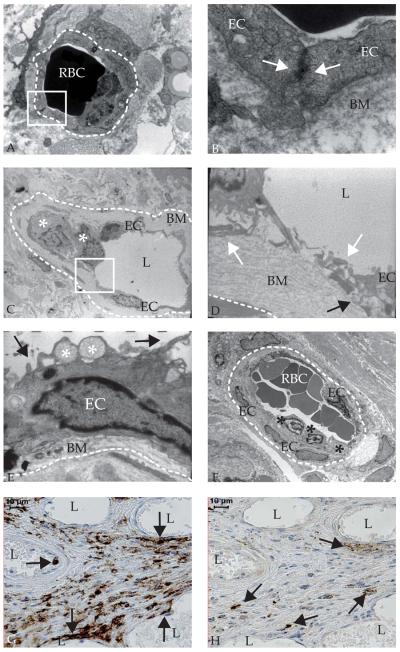
As microvascular leakage is also characterized by a disruption of endothelial integrity, inter-endothelial junctions were studied using electron microscopy. Inter-endothelial junctions were intact in adventitial microvessels in a non-diseased coronary artery (Figure 5A-B). Junctions of intraplaque microvessels were quantitatively analyzed in atherosclerotic coronary arteries with advanced fibroatheromas and ruptured plaques. Although an intact basement membrane was generally observed (Figure 5C-F), inter-endothelial contact was incomplete or completely absent in 76% of analyzed microvessels and endothelial junctions (Figure 5D, Table 5). Thus, endothelial integrity was severely compromised. In addition, EC morphology frequently represented an activated, dysfunctional status, characterized by blebbing and spike-like protrusions of the cell membrane. Also, numerous intracytoplasmic vacuoles, a sign of increased secretory capacity, were observed (Figure 5E). Moreover, membrane detachment was frequently observed (Figure 5E, Table 5). In addition, cells were adhering to the microvessel lumen and infiltrating into the plaque in 50% (n=13) of microvessels (Figure 5C,F, Table 5). These were identified as monocytes, based on nuclear morphology, and CD45RO (Figure 5G) and CD68 (not shown) immunoreactivity. Mature mast cells were frequently present in the plaque in the proximity of microvessels (Figure 5H). As mast cell tryptase is only expressed by tissue-resident, matured mast cells, it cannot fully be excluded that immature mast cells adhere to the luminal side of plaque microvessels. Nevertheless, the nuclear morphology and CD68 expression indicates that adhering cells are monocytes (Figure 5C,F). Thus, abnormal EC morphology and junctions were associated with monocyte/macrophage infiltration.
Figure 5. Intraplaque microvessels show abnormal endothelial cell (EC) morphology, aberrant junctions, and leukocyte infiltration as demonstrated by electron microscopy.
A. Ultrastructure of an adventitial microvessel (dashed line indicates circumference) in a non-diseased coronary artery with luminal red blood cells (RBC) (electron microscopy (EM, 8000x) B. Magnification of the boxed region in A of microvessel with intact basement membrane (BM) and inter-endothelial junction indicated by close contact (white arrows) between endothelial cells (20,000x) C. Ultrastructure of intraplaque microvessel with leukocytes (white asterisks) (650x) D. Magnification of the boxed region in C, showing aberrant inter-EC junction (white arrows) and basement membrane detachment (black arrow) (4600x) E. Dysfunctional EC ultrastructure in an intraplaque microvessel (6300x): membrane blebs (black arrow) and intracytoplasmic vacuoles (white asterisk) F. Leukocytes (asterisks) adhering to intraplaque microvessel endothelium (460x) G. Immunohistochemistry shows CD45+ cells in and near microvessels (arrows). H. Immunohistochemistry showing mast cell tryptase positive cells at larger distance from microvessels (arrows). L, lumen.
Table 5.
Electron microscopy analysis of endothelial cell (EC) morphology, junctions, and leukocyte infiltration in intraplaque microvessels
| Occurrence (N) | |
|---|---|
| Microvessels with all junctions intact | 24% (8) |
| Microvessels with >1 open junction | 76% (19) |
| Microvessels with all junctions open | 33% (8) |
| Total open junctions | 59% (34) |
| Basement membrane detachment | 42% (11) |
| Leukocyte infiltration | 50% (13) |
Discussion
The association of microvessel density and microvessel ultrastructure with coronary atherosclerosis plaque type and morphology was determined using structural parameters related to microvascular leakage: basement membrane and mural cell coverage, and endothelial junction integrity. Microvessel density was increased in fibroatheromas and ruptured plaques compared to normal and pathological intimal thickening, and was associated with plaque morphology. Microvessels were observed meandering from the adventitia through the media into the atherosclerotic plaque. The adventitia is the origin of >95% of plaque microvessels, as comprehensively demonstrated by 3D angiography and serial sectioning (2,14). Other reports confirmed that the onset of adventitial angiogenesis preceded intraplaque angiogenesis (16) and coincided with the onset of atherosclerosis (17).
Thus, angiogenesis and atherogenesis develop in close conjunction. Intraplaque microvessels are rare in small animal models of atherosclerosis (18), whereas they are abundant in human plaques (1,2,16,19). This suggests that microvessels need not be abundantly present to allow initiation and development of atherosclerosis. The growth of intraplaque microvessels may thus simply be a physiological reaction. Regardless of whether microvessel plaque content is merely a reaction to plaque growth, growing evidence suggests that the abnormal microvessel structure may be involved in atherosclerotic plaque destabilization.
Microvessels in our human samples with coronary atherosclerosis commonly showed incomplete mural cell coverage (Figure 3E-F), and a compromised structural integrity accompanied by extensive macrophage infiltration (Figure 5C-G). Contrary to our expectations, mural cell coverage was infrequent even in normal coronary arteries, and was similar in all plaque types and vessel wall regions. Hence, incomplete mural cell coverage cannot explain microvascular and subsequent intraplaque leakage. Recent reports also described intraplaque angiogenesis lacking mural cell coverage in atherosclerotic femoral (20) and carotid arteries (21,22). Plaques of symptomatic patients demonstrated significantly fewer microvessels covered with smooth muscle cells than those of asymptomatic patients (21), in contrast to our study of sudden death victims. Unfortunately, these studies did not examine mural cell coverage in normal carotid or femoral arteries, hindering the extrapolation of our results to other arteries. Nonetheless, the phenomenon of thin-walled microvessels does not seem to be restricted to coronary arteries.
As the normal adventitia represents a physiological arterial wall, it is unclear why the morphology of arterial wall angiogenesis differs from the accepted morphology of physiological angiogenesis. Microvessel morphology is generally adapted to local tissue demand, suggesting that mural cells are dispensable in arterial wall angiogenesis. Smooth muscle cell coverage provides vessel stabilization, possibly preventing microvessel compression in situations of high tissue pressure. A recent review described the mathematical theory of arterial wall and microvessel stress in relation to angiogenic perfusion and compression (23). High arterial wall stress, determined by arterial lumen pressure, and low microvessel lumen pressure may lead to the transient (cardiac cycle-dependent) compression of microvessels surrounded by loose structures. Arterial wall stress drops sharply close to the adventitia according to Lamé's law (23). This relatively low adventitial stress might reduce compression, suggesting that the presence of smooth muscle cells in the arterial adventitia is dispensable.
This still does not explain the absence of mural cell in intraplaque angiogenesis. Intraplaque microvessel stress is determined by low microvessel flow and a pressure drop from the adventitia. As microvessel diameters are similar in the adventitia and plaque (Sluimer, unpublished observations), however, the pressure drop from the adventitia to the plaque may be limited (24). Thus, unchanged intraplaque microvessel stress and higher plaque stress suggest that mural cells are necessary to withstand compression, and yet they are absent. As plaque microvessel stress and flow are currently unknown, their association with mural cell coverage remains to be established.
Infrequent mural cell coverage may also be explained by ongoing or inadequate mural cell recruitment. Ongoing recruitment seems unlikely, as pericytes were recruited within 2 weeks in a corneal angiogenesis model (25), whereas angiogenesis associated with atherosclerotic progression is likely a matter of decades. In addition, ECs in microvessels are not (or no longer) proliferating nor apoptotic (19,26). Although pericytes (20) and their recruitment signals have previously been demonstrated in human and mouse atherosclerosis (27,28), inadequate mural cell recruitment to intraplaque angiogenesis has not yet been investigated in vivo.
Perhaps the origin of microvascular leakage is not mural cell coverage but the compromised EC morphology and integrity demonstrated by electron microscopy. The findings in this study are not likely to be caused by artifacts of electron microscopy analysis, as electron microscopy is generally able to detect intact microvessel junctions in several species, tissues, and biopsy sources (29,30). Moreover, the morphology of cells surrounding the microvessels appeared healthy, suggestive of successful tissue collection. Importantly, a previous report showed that reduced expression of the adherens junction marker VE-Cadherin in plaque microvessels coincided with open junctions, substantiating the validity of our morphological EM results (31).
The strikingly deviant EC morphology has also been observed for large artery endothelium at the initiation of atherosclerosis and in hyperglycemic conditions, while inter-endothelial junctions between arterial ECs appeared intact (32). The increased malfunction of intraplaque microvascular compared to arterial endothelium is likely explained by the amplified inflammatory and angiogenic response in advanced compared to early plaques, which may stimulate microvascular permeability.
An important factor determining microvascular permeability is vascular endothelial growth factor (VEGF) (33). VEGF and its key stimulus hypoxia initiate angiogenesis by disrupting endothelial junctions (34), and both hypoxia and VEGF are present throughout atherogenesis (19). A chronic VEGF stimulus might be involved in the aberrant integrity of microvascular ECs and microvascular leakage. ECs in microvessels require mural cells to exert an adequate barrier function. The abnormal EC morphology and missing EC junctions may also be caused by the absence of pericytes, as shown in the platelet-derived growth factor B (PDGFB−/−) and the PDGF-receptor β−/− mouse with failed pericyte recruitment and EC blebbing (35). Actually, these mice also showed increased VEGF expression, and decreased capillary perfusion as a result of ECs protruding into and possibly blocking the lumen (36). This suggests compromised microvessel blood flow and increased plaque hypoxia.
In addition, enlarged gaps between ECs and EC blebbing are associated with inflammation and reactive oxygen species, produced by macrophages in atherosclerotic plaques (37,38). Previous studies (39) as well as our electron microscopy images show macrophage-microvessel adhesion and transmigration. In addition, macrophage accumulation in the proximity of intraplaque microvessels and increased adhesion molecule expression on microvascular endothelium (40) are suggestive of macrophage infiltration from permeable microvessels. Also, junctional adhesion molecules (JAMs) enhance macrophage transmigration and JAM expression in turn is stimulated by inflammatory infiltration (41). Altogether, we propose that macrophages may not only originate from intraplaque microvessels, but after extravasation may further aggravate microvascular leakage.
In conclusion, microvessel density is associated with plaque progression and morphology. Microvessel mural cell coverage is incomplete in normal and atherosclerotic human coronary arteries, and is thus unlikely to account for microvascular leakage. However, microvascular leakage may be explained by the compromised structural integrity of intraplaque angiogenesis, and this leaky morphology may explain the extensive leukocyte infiltration, intraplaque hemorrhage and plaque instability.
Acknowledgments
We thank Lila Adams, Hedwig Avalone and Michael Cooper for excellent technical assistance and Rob Reneman and Arnold Hoeks for stimulating discussions.
Financial disclosure: Research was partly supported by a travel grant from the Netherlands Organization for Scientific research (J.C.S) and a grant from the National Institutes of Health (RO1HL071148-05, R.V.). M.J.A.P.D and V.W.M.v.H. participate in the European Vascular Genomics Network (http://www.evgn.org), a Network of Excellence supported by the European Community's Sixth Framework Program for Research Priority 1 (contract LSHMCT-2003-503254).
Selected abbreviations
- IT
intimal thickening, normal
- PIT
pathological intimal thickening
- E-FA
thick fibrous cap atheroma with an early core
- L-FA
thick fibrous cap atheroma with late necrotic core
- TCFA
thin fibrous cap atheroma
- MVD
microvessel density
- αSMA
alpha smooth muscle actin
- IM border
intima-media border
- EC
endothelial cell
- EM
electron microscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: none
References
- 1.Moreno PR, Purushothaman KR, Fuster V, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110:2032–8. doi: 10.1161/01.CIR.0000143233.87854.23. [DOI] [PubMed] [Google Scholar]
- 2.Virmani R, Kolodgie FD, Burke AP, et al. Atherosclerotic Plaque Progression and Vulnerability to Rupture Angiogenesis as a Source of Intraplaque Hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–61. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 3.Bot I, de Jager SC, Zernecke A, et al. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation. 2007;115:2516–25. doi: 10.1161/CIRCULATIONAHA.106.660472. [DOI] [PubMed] [Google Scholar]
- 4.Kockx MM, Cromheeke KM, Knaapen MW, et al. Phagocytosis and macrophage activation associated with hemorrhagic microvessels in human atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:440–6. doi: 10.1161/01.ATV.0000057807.28754.7F. [DOI] [PubMed] [Google Scholar]
- 5.Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–25. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 6.de Nooijer R, Verkleij CJ, von der Thusen JH, et al. Lesional overexpression of matrix metalloproteinase-9 promotes intraplaque hemorrhage in advanced lesions but not at earlier stages of atherogenesis. Arterioscler Thromb Vasc Biol. 2006;26:340–6. doi: 10.1161/01.ATV.0000197795.56960.64. [DOI] [PubMed] [Google Scholar]
- 7.Virmani R, Narula J, Farb A. When neoangiogenesis ricochets. Am Heart J. 1998;136:937–9. doi: 10.1016/s0002-8703(98)70144-9. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 9.Hashizume H, Baluk P, Morikawa S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156:1363–80. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 11.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–70. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 12.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–75. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 13.Kolodgie FD, Burke AP, Skorija KS, et al. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2523–9. doi: 10.1161/01.ATV.0000244681.72738.bc. [DOI] [PubMed] [Google Scholar]
- 14.Kumamoto M, Nakashima Y, Sueishi K. Intimal neovascularization in human coronary atherosclerosis: its origin and pathophysiological significance. Hum Pathol. 1995;26:450–6. doi: 10.1016/0046-8177(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 15.Abramsson A, Berlin O, Papayan H, Paulin D, Shani M, Betsholtz C. Analysis of mural cell recruitment to tumor vessels. Circulation. 2002;105:112–7. doi: 10.1161/hc0102.101437. [DOI] [PubMed] [Google Scholar]
- 16.Fleiner M, Kummer M, Mirlacher M, et al. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation. 2004;110:2843–50. doi: 10.1161/01.CIR.0000146787.16297.E8. [DOI] [PubMed] [Google Scholar]
- 17.Lappalainen H, Laine P, Pentikainen MO, Sajantila A, Kovanen PT. Mast cells in neovascularized human coronary plaques store and secrete basic fibroblast growth factor, a potent angiogenic mediator. Arterioscler Thromb Vasc Biol. 2004;24:1880–5. doi: 10.1161/01.ATV.0000140820.51174.8d. [DOI] [PubMed] [Google Scholar]
- 18.Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation. 2005;112:1813–24. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- 19.Sluimer JC, Gasc JM, van Wanroij JL, et al. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol. 2008;51:1258–65. doi: 10.1016/j.jacc.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Wilkinson FL, Kirton JP, et al. Hepatocyte growth factor and c-Met expression in pericytes: implications for atherosclerotic plaque development. J Pathol. 2007;212:12–9. doi: 10.1002/path.2155. [DOI] [PubMed] [Google Scholar]
- 21.Dunmore BJ, McCarthy MJ, Naylor AR, Brindle NP. Carotid plaque instability and ischemic symptoms are linked to immaturity of microvessels within plaques. J Vasc Surg. 2007;45:155–9. doi: 10.1016/j.jvs.2006.08.072. [DOI] [PubMed] [Google Scholar]
- 22.Mazzone A, Epistolato MC, Gianetti J, et al. Biological features (inflammation and neoangiogenesis) and atherosclerotic risk factors in carotid plaques and calcified aortic valve stenosis: two different sites of the same disease? Am J Clin Pathol. 2006;126:494–502. doi: 10.1309/W75NTE5QBC9DXE03. [DOI] [PubMed] [Google Scholar]
- 23.Ritman EL, Lerman A. The dynamic vasa vasorum. Cardiovasc Res. 2007;75:649–58. doi: 10.1016/j.cardiores.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoeks AP, Reesink KD, Hermeling E, Reneman RS. Local blood pressure rather than shear stress should be blamed for plaque rupture. J Am Coll Cardiol. 2008;52:1107–8. doi: 10.1016/j.jacc.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 25.Cursiefen C, Hofmann-Rummelt C, Kuchle M, Schlotzer-Schrehardt U. Pericyte recruitment in human corneal angiogenesis: an ultrastructural study with clinicopathological correlation. Br J Ophthalmol. 2003;87:101–6. doi: 10.1136/bjo.87.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tricot O, Mallat Z, Heymes C, Belmin J, Leseche G, Tedgui A. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation. 2000;101:2450–3. doi: 10.1161/01.cir.101.21.2450. [DOI] [PubMed] [Google Scholar]
- 27.Pilarczyk K, Sattler KJ, Galili O, et al. Placenta growth factor expression in human atherosclerotic carotid plaques is related to plaque destabilization. Atherosclerosis. 2006 doi: 10.1016/j.atherosclerosis.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 28.Khurana R, Moons L, Shafi S, et al. Placental growth factor promotes atherosclerotic intimal thickening and macrophage accumulation. Circulation. 2005;111:2828–36. doi: 10.1161/CIRCULATIONAHA.104.495887. [DOI] [PubMed] [Google Scholar]
- 29.Luksha L, Poston L, Gustafsson JA, Hultenby K, Kublickiene K. The oestrogen receptor beta contributes to sex related differences in endothelial function of murine small arteries via EDHF. J Physiol. 2006;577:945–55. doi: 10.1113/jphysiol.2006.121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baloyannis SJ. Pathological alterations of the climbing fibres of the cerebellum in vascular dementia: a Golgi and electron microscope study. J Neurol Sci. 2007;257:56–61. doi: 10.1016/j.jns.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Bobryshev YV, Cherian SM, Inder SJ, Lord RS. Neovascular expression of VEcadherin in human atherosclerotic arteries and its relation to intimal inflammation. Cardiovasc Res. 1999;43:1003–17. doi: 10.1016/s0008-6363(99)00125-x. [DOI] [PubMed] [Google Scholar]
- 32.Simionescu M, Popov D, Sima A, et al. Pathobiochemistry of combined diabetes and atherosclerosis studied on a novel animal model. The hyperlipemic-hyperglycemic hamster. Am J Pathol. 1996;148:997–1014. [PMC free article] [PubMed] [Google Scholar]
- 33.van Hinsbergh VW, van Nieuw Amerongen GP. Intracellular signalling involved in modulating human endothelial barrier function. J Anat. 2002;200:549–60. doi: 10.1046/j.1469-7580.2002.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suarez S, Ballmer-Hofer K. VEGF transiently disrupts gap junctional communication in endothelial cells. J Cell Sci. 2001;114:1229–35. doi: 10.1242/jcs.114.6.1229. [DOI] [PubMed] [Google Scholar]
- 35.Hellstrom M, Gerhardt H, Kalen M, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–53. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 37.Hazell LJ, Arnold L, Flowers D, Waeg G, Malle E, Stocker R. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest. 1996;97:1535–44. doi: 10.1172/JCI118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonald DM, Thurston G, Baluk P. Endothelial gaps as sites for plasma leakage in inflammation. Microcirculation. 1999;6:7–22. [PubMed] [Google Scholar]
- 39.Balakrishnan KR, Kuruvilla S. Images in cardiovascular medicine. Role of inflammation in atherosclerosis: immunohistochemical and electron microscopic images of a coronary endarterectomy specimen. Circulation. 2006;113:e41–3. doi: 10.1161/CIRCULATIONAHA.105.537464. [DOI] [PubMed] [Google Scholar]
- 40.O'Brien KD, McDonald TO, Chait A, Allen MD, Alpers CE. Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation. 1996;93:672–82. doi: 10.1161/01.cir.93.4.672. [DOI] [PubMed] [Google Scholar]
- 41.Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7:467–77. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]