Abstract
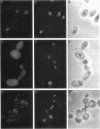
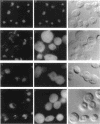
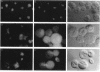
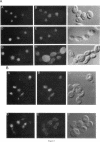
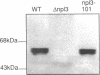
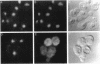
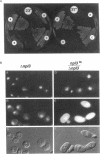
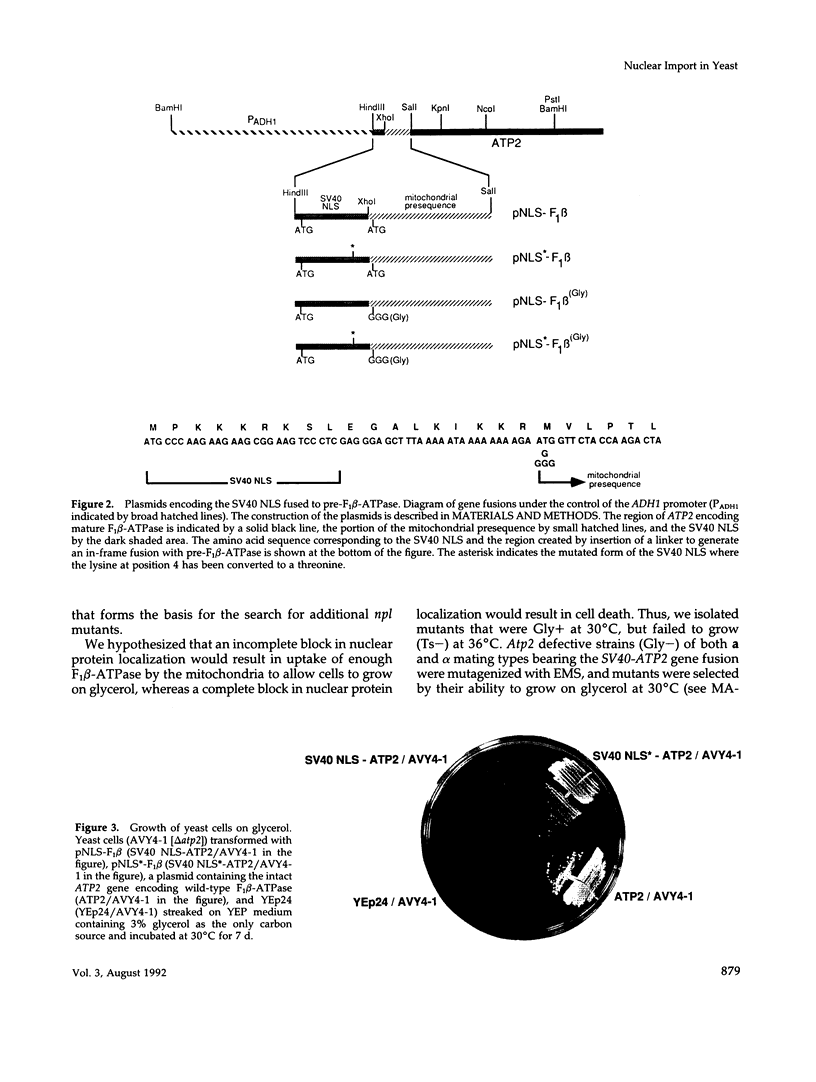
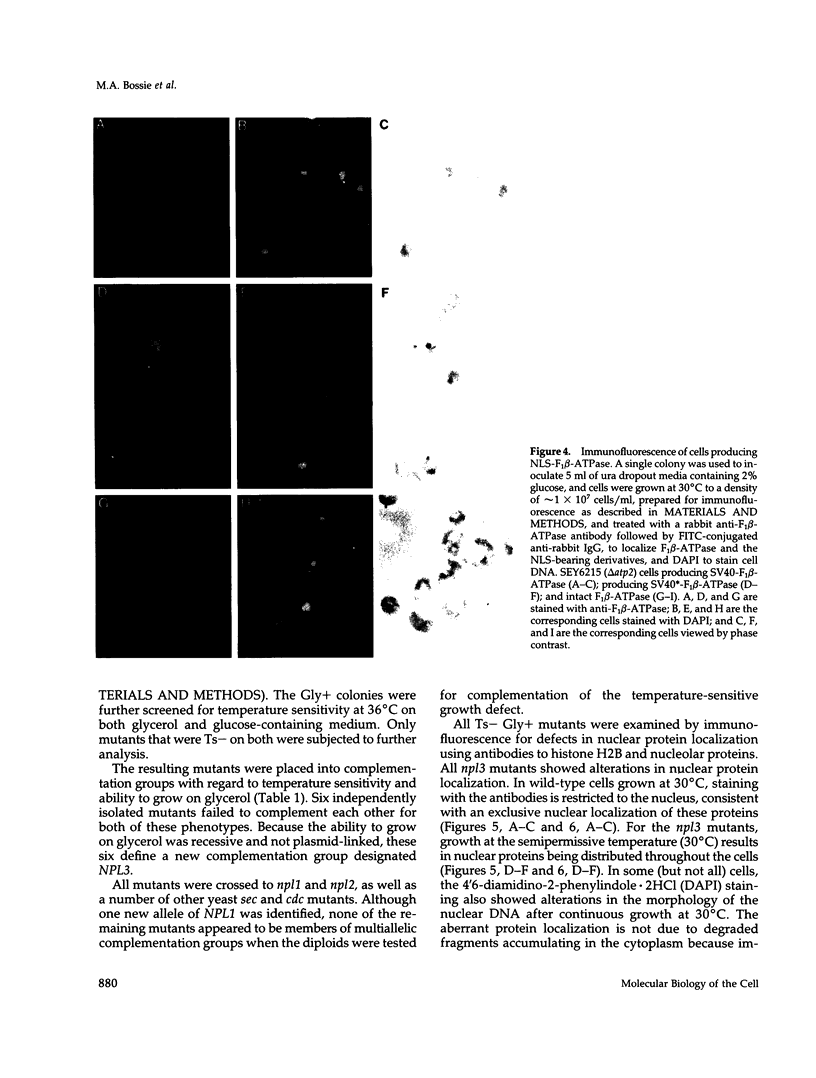
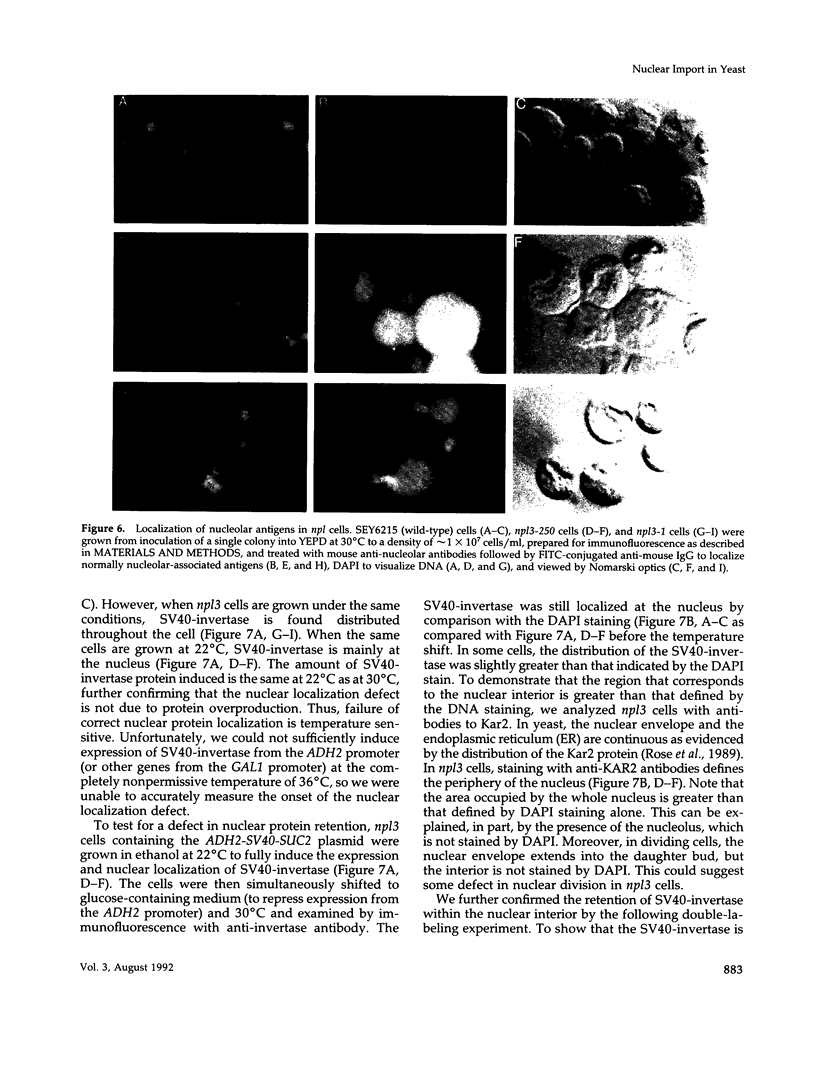
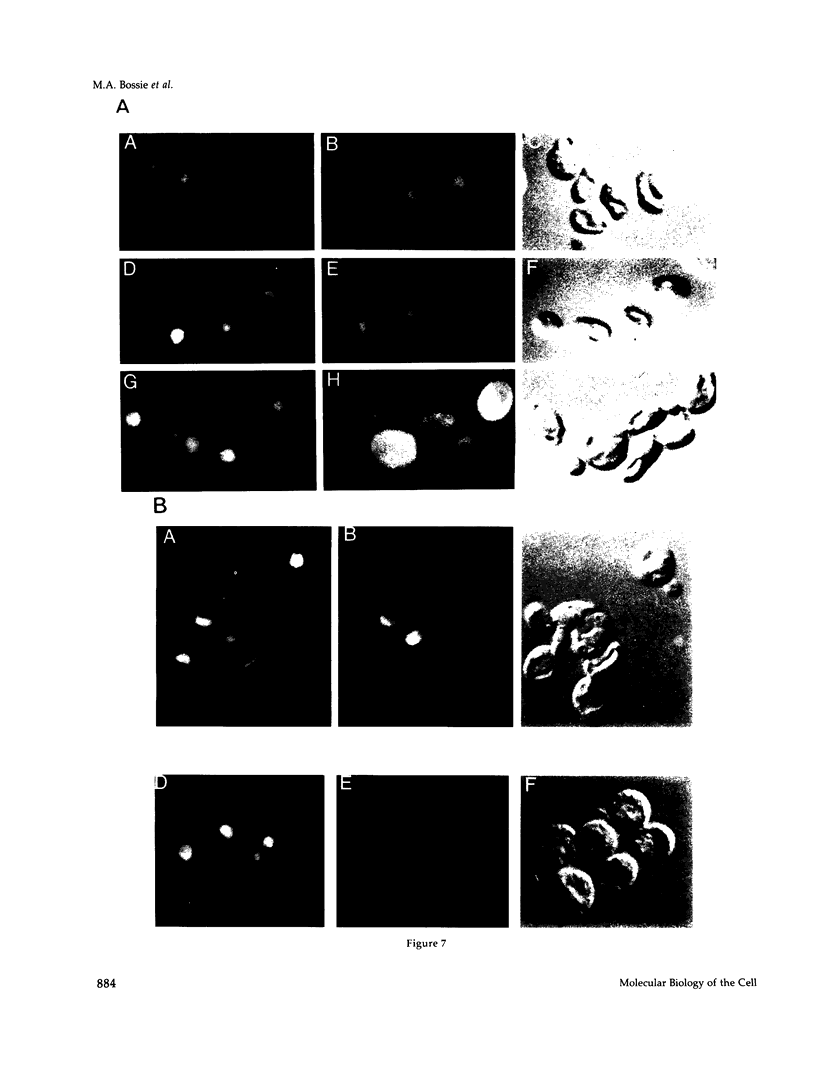
We have isolated mutants of the yeast Saccharomyces cerevisiae that are defective in localization of nuclear proteins. Chimeric proteins containing the nuclear localization sequence from SV40 large T-antigen fused to the N-terminus of the mitochondrial F1 beta-ATPase are localized to the nucleus. Npl (nuclear protein localization) mutants were isolated by their ability to grow on glycerol as a consequence of no longer exclusively targeting SV40-F1 beta-ATPase to the nucleus. All mutants with defects in localization of nucleolar proteins and histones are temperature sensitive for growth at 36 degrees C. Seven alleles of NPL3 and single alleles of several additional genes were isolated. NPL3 mutants were studied in detail. NPL3 encodes a nuclear protein with an RNA recognition motif and similarities to a family of proteins involved in RNA metabolism. Our genetic analysis indicates that NPL3 is essential for normal cell growth; cells lacking NPL3 are temperature sensitive for growth but do not exhibit a defect in localization of nuclear proteins. Taken together, these results indicate that the mutant forms of Npl3 protein isolated by this procedure are interfering with nuclear protein uptake in a general manner.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S. A., Gerace L. Cytosolic proteins that specifically bind nuclear location signals are receptors for nuclear import. Cell. 1991 Sep 6;66(5):837–847. doi: 10.1016/0092-8674(91)90431-w. [DOI] [PubMed] [Google Scholar]
- Adam S. A., Lobl T. J., Mitchell M. A., Gerace L. Identification of specific binding proteins for a nuclear location sequence. Nature. 1989 Jan 19;337(6204):276–279. doi: 10.1038/337276a0. [DOI] [PubMed] [Google Scholar]
- Adam S. A., Marr R. S., Gerace L. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J Cell Biol. 1990 Sep;111(3):807–816. doi: 10.1083/jcb.111.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedwell D. M., Strobel S. A., Yun K., Jongeward G. D., Emr S. D. Sequence and structural requirements of a mitochondrial protein import signal defined by saturation cassette mutagenesis. Mol Cell Biol. 1989 Mar;9(3):1014–1025. doi: 10.1128/mcb.9.3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt J. O., Meyer C., Fasold H., Barnard F. C., Riedel N. Interaction of a nuclear location signal with isolated nuclear envelopes and identification of signal-binding proteins by photoaffinity labeling. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9327–9331. doi: 10.1073/pnas.86.23.9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borer R. A., Lehner C. F., Eppenberger H. M., Nigg E. A. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989 Feb 10;56(3):379–390. doi: 10.1016/0092-8674(89)90241-9. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Davis L. I., Blobel G. Identification and characterization of a nuclear pore complex protein. Cell. 1986 Jun 6;45(5):699–709. doi: 10.1016/0092-8674(86)90784-1. [DOI] [PubMed] [Google Scholar]
- Davis L. I., Fink G. R. The NUP1 gene encodes an essential component of the yeast nuclear pore complex. Cell. 1990 Jun 15;61(6):965–978. doi: 10.1016/0092-8674(90)90062-j. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Nuclear targeting sequences--a consensus? Trends Biochem Sci. 1991 Dec;16(12):478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Feldherr C. M., Kallenbach E., Schultz N. Movement of a karyophilic protein through the nuclear pores of oocytes. J Cell Biol. 1984 Dec;99(6):2216–2222. doi: 10.1083/jcb.99.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay D. R., Newmeyer D. D., Price T. M., Forbes D. J. Inhibition of in vitro nuclear transport by a lectin that binds to nuclear pores. J Cell Biol. 1987 Feb;104(2):189–200. doi: 10.1083/jcb.104.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U., Darzynkiewicz E., Tahara S. M., Dathan N. A., Lührmann R., Mattaj I. W. Diversity in the signals required for nuclear accumulation of U snRNPs and variety in the pathways of nuclear transport. J Cell Biol. 1991 May;113(4):705–714. doi: 10.1083/jcb.113.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bustos J., Heitman J., Hall M. N. Nuclear protein localization. Biochim Biophys Acta. 1991 Mar 7;1071(1):83–101. doi: 10.1016/0304-4157(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Gerace L., Ottaviano Y., Kondor-Koch C. Identification of a major polypeptide of the nuclear pore complex. J Cell Biol. 1982 Dec;95(3):826–837. doi: 10.1083/jcb.95.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard J. P., Lehtonen H., Caizergues-Ferrer M., Amalric F., Tollervey D., Lapeyre B. GAR1 is an essential small nucleolar RNP protein required for pre-rRNA processing in yeast. EMBO J. 1992 Feb;11(2):673–682. doi: 10.1002/j.1460-2075.1992.tb05099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb D., Michaud N. Pathways for the nuclear transport of proteins and RNAs. Trends Cell Biol. 1991 Jul;1(1):20–24. doi: 10.1016/0962-8924(91)90065-h. [DOI] [PubMed] [Google Scholar]
- Greber U. F., Gerace L. Nuclear protein import is inhibited by an antibody to a lumenal epitope of a nuclear pore complex glycoprotein. J Cell Biol. 1992 Jan;116(1):15–30. doi: 10.1083/jcb.116.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover J. A., Cohen C. K., Willingham M. C., Park M. K. O-linked N-acetylglucosamine is attached to proteins of the nuclear pore. Evidence for cytoplasmic and nucleoplasmic glycoproteins. J Biol Chem. 1987 Jul 15;262(20):9887–9894. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Henríquez R., Blobel G., Aris J. P. Isolation and sequencing of NOP1. A yeast gene encoding a nucleolar protein homologous to a human autoimmune antigen. J Biol Chem. 1990 Feb 5;265(4):2209–2215. [PubMed] [Google Scholar]
- Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986 Sep;2(3):163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Howard E. A., Zupan J. R., Citovsky V., Zambryski P. C. The VirD2 protein of A. tumefaciens contains a C-terminal bipartite nuclear localization signal: implications for nuclear uptake of DNA in plant cells. Cell. 1992 Jan 10;68(1):109–118. doi: 10.1016/0092-8674(92)90210-4. [DOI] [PubMed] [Google Scholar]
- Imamoto-Sonobe N., Matsuoka Y., Semba T., Okada Y., Uchida T., Yoneda Y. A protein recognized by antibodies to Asp-Asp-Asp-Glu-Asp shows specific binding activity to heterogeneous nuclear transport signals. J Biol Chem. 1990 Sep 25;265(27):16504–16508. [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R. P., Hurt E. C., Kern H., Lehtonen H., Carmo-Fonseca M., Lapeyre B., Tollervey D. Evolutionary conservation of the human nucleolar protein fibrillarin and its functional expression in yeast. J Cell Biol. 1991 May;113(4):715–729. doi: 10.1083/jcb.113.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong A. Y., Clark M. W., Gilbert M., Oehm A., Campbell J. L. Saccharomyces cerevisiae SSB1 protein and its relationship to nucleolar RNA-binding proteins. Mol Cell Biol. 1987 Aug;7(8):2947–2955. doi: 10.1128/mcb.7.8.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kenan D. J., Query C. C., Keene J. D. RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci. 1991 Jun;16(6):214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- Klyce H. R., McLaughlin C. S. Characterization of temperature-sensitive mutants of yeast by a photomicrographic procedure. Exp Cell Res. 1973 Nov;82(1):47–56. doi: 10.1016/0014-4827(73)90243-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Construction and characterization of an SV40 mutant defective in nuclear transport of T antigen. Cell. 1984 Jul;37(3):801–813. doi: 10.1016/0092-8674(84)90415-x. [DOI] [PubMed] [Google Scholar]
- Lapeyre B., Bourbon H., Amalric F. Nucleolin, the major nucleolar protein of growing eukaryotic cells: an unusual protein structure revealed by the nucleotide sequence. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1472–1476. doi: 10.1073/pnas.84.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapeyre B., Mariottini P., Mathieu C., Ferrer P., Amaldi F., Amalric F., Caizergues-Ferrer M. Molecular cloning of Xenopus fibrillarin, a conserved U3 small nuclear ribonucleoprotein recognized by antisera from humans with autoimmune disease. Mol Cell Biol. 1990 Jan;10(1):430–434. doi: 10.1128/mcb.10.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. C., Mélèse T. Identification and characterization of a nuclear localization sequence-binding protein in yeast. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8808–8812. doi: 10.1073/pnas.86.22.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. C., Xue Z. X., Mélèse T. The NSR1 gene encodes a protein that specifically binds nuclear localization sequences and has two RNA recognition motifs. J Cell Biol. 1991 Apr;113(1):1–12. doi: 10.1083/jcb.113.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R. H., Thomas J. O. Identification of a human protein that interacts with nuclear localization signals. J Cell Biol. 1989 Dec;109(6 Pt 1):2623–2632. doi: 10.1083/jcb.109.6.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell R. B., Feldherr C. M. Identification of two HSP70-related Xenopus oocyte proteins that are capable of recycling across the nuclear envelope. J Cell Biol. 1990 Nov;111(5 Pt 1):1775–1783. doi: 10.1083/jcb.111.5.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier U. T., Blobel G. A nuclear localization signal binding protein in the nucleolus. J Cell Biol. 1990 Dec;111(6 Pt 1):2235–2245. doi: 10.1083/jcb.111.6.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud N., Goldfarb D. S. Multiple pathways in nuclear transport: the import of U2 snRNP occurs by a novel kinetic pathway. J Cell Biol. 1991 Jan;112(2):215–223. doi: 10.1083/jcb.112.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehrbass U., Kern H., Mutvei A., Horstmann H., Marshallsay B., Hurt E. C. NSP1: a yeast nuclear envelope protein localized at the nuclear pores exerts its essential function by its carboxy-terminal domain. Cell. 1990 Jun 15;61(6):979–989. doi: 10.1016/0092-8674(90)90063-k. [DOI] [PubMed] [Google Scholar]
- Nelson M., Silver P. Context affects nuclear protein localization in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Feb;9(2):384–389. doi: 10.1128/mcb.9.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmeyer D. D., Forbes D. J. Nuclear import can be separated into distinct steps in vitro: nuclear pore binding and translocation. Cell. 1988 Mar 11;52(5):641–653. doi: 10.1016/0092-8674(88)90402-3. [DOI] [PubMed] [Google Scholar]
- Olson M. V., Dutchik J. E., Graham M. Y., Brodeur G. M., Helms C., Frank M., MacCollin M., Scheinman R., Frank T. Random-clone strategy for genomic restriction mapping in yeast. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7826–7830. doi: 10.1073/pnas.83.20.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt R., Holzenburg A., Buhle E. L., Jr, Jarnik M., Engel A., Aebi U. Correlation between structure and mass distribution of the nuclear pore complex and of distinct pore complex components. J Cell Biol. 1990 Apr;110(4):883–894. doi: 10.1083/jcb.110.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. D., Mills A. D., Dilworth S. M., Laskey R. A., Dingwall C. Nuclear protein migration involves two steps: rapid binding at the nuclear envelope followed by slower translocation through nuclear pores. Cell. 1988 Mar 11;52(5):655–664. doi: 10.1016/0092-8674(88)90403-5. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Misra L. M., Vogel J. P. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989 Jun 30;57(7):1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Roth S., Hiromi Y., Godt D., Nüsslein-Volhard C. cactus, a maternal gene required for proper formation of the dorsoventral morphogen gradient in Drosophila embryos. Development. 1991 Jun;112(2):371–388. doi: 10.1242/dev.112.2.371. [DOI] [PubMed] [Google Scholar]
- Rothblatt J. A., Deshaies R. J., Sanders S. L., Daum G., Schekman R. Multiple genes are required for proper insertion of secretory proteins into the endoplasmic reticulum in yeast. J Cell Biol. 1989 Dec;109(6 Pt 1):2641–2652. doi: 10.1083/jcb.109.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sadler I., Chiang A., Kurihara T., Rothblatt J., Way J., Silver P. A yeast gene important for protein assembly into the endoplasmic reticulum and the nucleus has homology to DnaJ, an Escherichia coli heat shock protein. J Cell Biol. 1989 Dec;109(6 Pt 1):2665–2675. doi: 10.1083/jcb.109.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmang T., Tollervey D., Kern H., Frank R., Hurt E. C. A yeast nucleolar protein related to mammalian fibrillarin is associated with small nucleolar RNA and is essential for viability. EMBO J. 1989 Dec 20;8(13):4015–4024. doi: 10.1002/j.1460-2075.1989.tb08584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J., Schindewolf C., van Zee K., Fanning E. A mutant SV40 large T antigen interferes with nuclear localization of a heterologous protein. Cell. 1988 Jul 1;54(1):117–125. doi: 10.1016/0092-8674(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Silver P. A. How proteins enter the nucleus. Cell. 1991 Feb 8;64(3):489–497. doi: 10.1016/0092-8674(91)90233-o. [DOI] [PubMed] [Google Scholar]
- Silver P., Sadler I., Osborne M. A. Yeast proteins that recognize nuclear localization sequences. J Cell Biol. 1989 Sep;109(3):983–989. doi: 10.1083/jcb.109.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow C. M., Senior A., Gerace L. Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J Cell Biol. 1987 May;104(5):1143–1156. doi: 10.1083/jcb.104.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stochaj U., Osborne M., Kurihara T., Silver P. A yeast protein that binds nuclear localization signals: purification localization, and antibody inhibition of binding activity. J Cell Biol. 1991 Jun;113(6):1243–1254. doi: 10.1083/jcb.113.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stochaj U., Silver P. A. A conserved phosphoprotein that specifically binds nuclear localization sequences is involved in nuclear import. J Cell Biol. 1992 May;117(3):473–482. doi: 10.1083/jcb.117.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M., Vassarotti A., Douglas M. G. Nuclear genes coding the yeast mitochondrial adenosine triphosphatase complex. Primary sequence analysis of ATP2 encoding the F1-ATPase beta-subunit precursor. J Biol Chem. 1985 Dec 15;260(29):15458–15465. [PubMed] [Google Scholar]
- Tollervey D., Lehtonen H., Carmo-Fonseca M., Hurt E. C. The small nucleolar RNP protein NOP1 (fibrillarin) is required for pre-rRNA processing in yeast. EMBO J. 1991 Mar;10(3):573–583. doi: 10.1002/j.1460-2075.1991.tb07984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki L., Kanda P., Lanford R. E. Identification of four nuclear transport signal-binding proteins that interact with diverse transport signals. Mol Cell Biol. 1989 Jul;9(7):3028–3036. doi: 10.1128/mcb.9.7.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]