Abstract
The past decade has witnessed burgeoning evidence that antidepressant medications and physical exercise increase the expression of hippocampal brain-derived neurotrophic factor (BDNF). This phenomenon has gained widespread appeal because BDNF is one of the first macromolecules observed to play a central role not only in the treatment of mood disorders, but also in neuronal survival-, growth-, and plasticity-related signaling cascades. Thus, it has become critical to understand how BDNF synthesis is regulated. Much evidence exists that changes in BDNF expression result from the activation/phosphorylation of the transcription factor, cAMP-response-element binding protein (CREB) following the administration of antidepressant medications. Utilizing a mouse model genetically engineered with an inducible CREB repressor, our current study provides evidence that increases in BDNF expression and cellular survival signaling resulting from physical exercise are also dependent upon activation of this central transcription factor. The transcription and expression of hippocampal BDNF, as well as the activation of Akt, a key survival signaling molecule, were measured following acute exercise, and also following short-term treatment with the norepinephrine re-uptake inhibitor, reboxetine. We found that both interventions led to a marked increase in hippocampal BDNF mRNA, BDNF protein and Akt phosphorylation (as well as CREB phosphorylation) in wild-type mice. As expected, activation of the CREB repressor in mutant mice sharply decreased CREB phosphorylation. In addition, all measures noted above remained at baseline levels when mutant mice exercised or received reboxetine. Increases in BDNF and phospho-Akt were also prevented when mutant mice received a combination of exercise and antidepressant treatment. The results are discussed in the context of what is currently known about BDNF signaling.
Keywords: Akt, signaling, hippocampus, rat, neurotrophin, PI-3 kinase
Introduction
It is well established that physical exercise has antidepressant-like effects on mood (Labbé et al., 1988; Hill et al., 1993), and boosts overall mental health (Hill et al., 1993; Booth et al., 2002). Even in healthy animals (including humans), exercise has been shown to enhance learning (Fordyce and Wehner, 1993; van Praag et al., 2005; Winter et al., 2007) and cognition (Davey et al., 1973; Ishmail and El-Naggar, 1981; Irvine et al., 2005; Vaynman et al., 2007). At the cellular level, running exercise has been shown to boost neurogenesis (van Praag et al., 1999) and cytoarchitectural features of existing neurons (Stranahan et al., 2007). At the cellular level, such findings have been associated with a surge in the synthesis and release of monoamine neurotransmitters into the general circulation (Winter et al., 2007) and the CNS (Chaouloff, 1989; Meeusen and DeMeirleir, 1995; Pagliari and Peyrin, 1995a, 1995b; Dishman, 1997; Dishman et al., 2000). Such surges have lent support to the Monoamine Hypothesis of Depression, which suggests that a central deficiency of serotonin (5-HT) and/or norepinephrine is involved in depression (Schildkraut, 1965; Wong and Licinio, 2004). More recently, our understanding of antidepressant treatment mechanisms has been advanced by the Neurotrophin hypothesis, which invokes increased expression of brain-derived-neurotrophic factor (BDNF), resulting from increased neural activity elicited by antidepressant medications, electroconvulsive shock, or physical exercise (Duman et al., 1999; Berchtold et al., 2005; Castren, 2005; Ernst et al., 2006; Russo-Neustadt and Chen, 2005; Malberg and Blendy, 2005; Duman and Monteggia, 2006; Li et al., 2007; Ploughman et al., 2007; Tang et al., 2008). Recently, increased serum BDNF and catecholamine levels were found after intense physical exercise (Winter et al., 2007).
BDNF is now well known to play central roles in neuronal growth (Duman and Monteggia, 2006), development (Garoflos et al., 2005), plasticity (McAllister et al., 1999; Vaynman et al., 2003; Ding et al., 2006; Luikart et al., 2008) survival (Tong et al., 2001; Johnson-Farley et al., 2007), neuroprotection (Cechetti et al., 2008; White and Castellano, 2008), and repair (Duman and Monteggia, 2006). BDNF carries out these processes through a complex array of intracellular signaling pathways (Segal, 2003; Tardito et al., 2006), such as the phosphatidylinositol 3′-kinase (PI-3K)-Akt pathway (Brunet et al., 1999; 2001; Chan et al., 1999; Luikart et al., 2008) and the mitogen-activated protein kinase (MAPK) pathway (Einat et al., 2003; Vaynman et al., 2003; Hayley et al., 2005; Malberg and Blendy, 2005). Both of these pathways, as well as several others that promote neuronal survival, converge on a transcriptional regulator, cyclic-AMP responsive-element binding protein (CREB) (Shaywitz and Greenberg, 1999; Malberg and Blendy, 2005; Tardito et al., 2006; Gass and Riva, 2007).
In support of the preceding, it has been shown that BDNF transcription is dependent on CREB phosphorylation (Conti et al., 2002; Segel, 2003; Rossler et al., 2004). These 2 survival-promoting molecules been shown to be up-regulated in response to antidepressant (Duman et al., 1997; 1999; Chen et al., 2001a; Saarelainen et al., 2003; Nair and Vaidya, 2006) and/or exercise (Shen et al., 2001; Tong et al., 2001; Molteni et al., 2002; Griesbach et al., 2007; Nair et al., 2007; Vaynman et al., 2007; Muller et al., 2008), but are down-regulated following stress (Hayley et al., 2005; Duman and Monteggia, 2006; Dwivedi et al., 2006; Nair and Vaidya, 2006; Xu et al., 2006; Zheng et al., 2006). In addition, direct application of BDNF itself (Shirayama et al., 2002) or over-expression of CREB (Chen et al., 2001b) in the hippocampus has produced antidepressant-like behavioral effects.
We have gathered evidence that exercise and antidepressant treatments also share common intracellular signaling mechanisms, and that both interventions converge upon CREB activation (Chen et al., 2005). Our current study was designed to test the hypothesis that, as evidenced for antidepressant treatment (Conti et al., 2002), the activation of CREB is an essential part of the intracellular mechanism initiated by exercise and responsible for both increased transcription of hippocampal BDNF and the activation of signaling molecules necessary for maintaining neuronal survival. We employ a transgenic mouse model harboring an inducible CREB repressor, which is activated by the 4-hydroxyl metabolite of the anti-estrogenic drug, tamoxifen (Kida et al., 2002). The peak activity of this repressor is sustained for six hours (Kida et al., 2002); therefore we evaluated the involvement of CREB activation in the initial (rapid) intracellular mechanisms stimulated by exercise and a norepinephrine-selective antidepressant. We measured hippocampal BDNF mRNA, BDNF protein, P-CREB, and phospho-Akt (P-Akt) levels following 6 hours of running wheel activity, or 6 hours following a single dose of reboxetine, a norepinephrine-selective reuptake inhibitor (Sacchetti et al., 1999; Frazer, 2000; Schatzberg, 2000). Reboxetine was chosen for this study because this agent has been demonstrated to enhance hippocampal BDNF transcription more rapidly than other antidepressant agents (such as SSRIs) (Russo-Neustadt et al., 2004), and exercise-induced enhancements in BDNF and survival signaling appear to involve norepinephrine-associated mechanisms (Ivy et al., 2003; Larsen et al., 2008).
Materials and Methods
Drugs
Reboxetine was a kind gift from Pharmacia-Upjohn (Kalamazoo, MI). Four-hydroxy-tamoxifen was purchased from Sigma-Aldrich (St. Louis, MO).
Antibodies
Anti-phospho-CREB (P-CREB), anti-CREB, anti-phospho-Akt (P-Akt), and anti-Akt were purchased from Cell Signaling Technology, Inc. (Danvers, MA). Anti-BDNF was purchased from Santa Cruz Biotech (Santa Cruz, CA). Anti-GAPDH was purchased from Advanced Immunochemical, Inc. (Long Beach, CA).
Mice
All mice were housed three-four occupants of the same gender in transparent polycarbonate cages (L = 31 cm, W = 16 cm, Ht = 13 cm). Mice were fed standard rodent lab chow and water ad libitum. Every effort was made to ensure that pain, distress, and the number of mice used was kept to a minimum and were followed in strict accordance with the procedures set forth by the NIH Guide for the Care and Use of Laboratory Animals (1996).
Male breeders (BL6) harboring the LBD gene were kindly provided by Dr. Alcino Silva (UCLA), were housed singly, except when bred with 129sv females (Jackson labs, Bar Harbor, ME) to yield our experimental mice. To maintain this genetic line and generate additional experimental mice, these male breeders (LBD/BL6) were also bred with BL6 females. Males resulting from this union were then kept as future breeder males to maintain our colony, whereas the females were culled. All mouse pups were placed in separate single-sexed cages after they were weaned. All mice, except for the culled females, were genotyped at 2 months of age: Briefly, a two-mm piece was sliced from the tip of the tail of each mouse and was then cut in half (for duplicate determinations). Each half piece was then placed in a microcentrifuge tube with 100 μl tail lysis buffer (50 mM KCl, 10 mM tris HCl, pH 9.0, 0.1% triton X-100, and 0.15 mg/ml proteinase K, which was added just before use). Tail pieces were then placed in an oven overnight at 56°C. The next morning, the tubes were heated to 94°C for 15 min, placed at -80°C for 30 min, thawed on ice, centrifuged at 14,000 × g for two min, and the supernatant containing the DNA stored at -20°C till ready for PCR.
PCR
The PCR mix consisted of H2O (13 μl), 10 × polymerase buffer (2 μl), 1.25 mM dNTP mix (2.5 μl), primer mix (1 μl), tail DNA (1 μl) and Taq polymerase (0.125 μl, 5 Prime, Fisher, Tustin, CA) in a total volume of ∼20 μl. The 3 primers used were GGGCTGCAGTTGGACCTGGG, CGTTTCGGAGGTGGTTGCC, and CGTGCTCCTGAGTGCAAACGG (Operon Technologies, Inc., Alameda, CA). PCR was conducted through 30 cycles on a GeneAmp PCR System (Applied Biosystems, Foster City, CA). PCR products in duplicate were then electrophoresed on a 1.5 % agarose gel, which was then ethidium bromide-stained. CREBIR mice were distinguished from wild type (WT) mice by the presence of a 400-bp fragment (Figure 1a).
Figure 1.
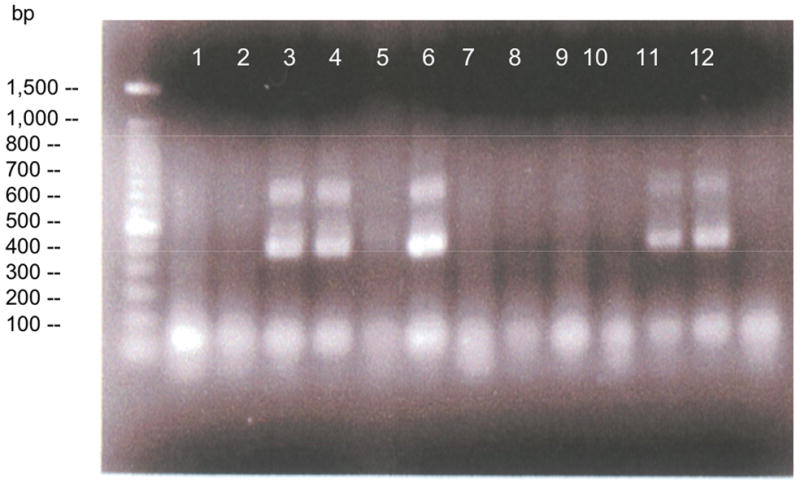
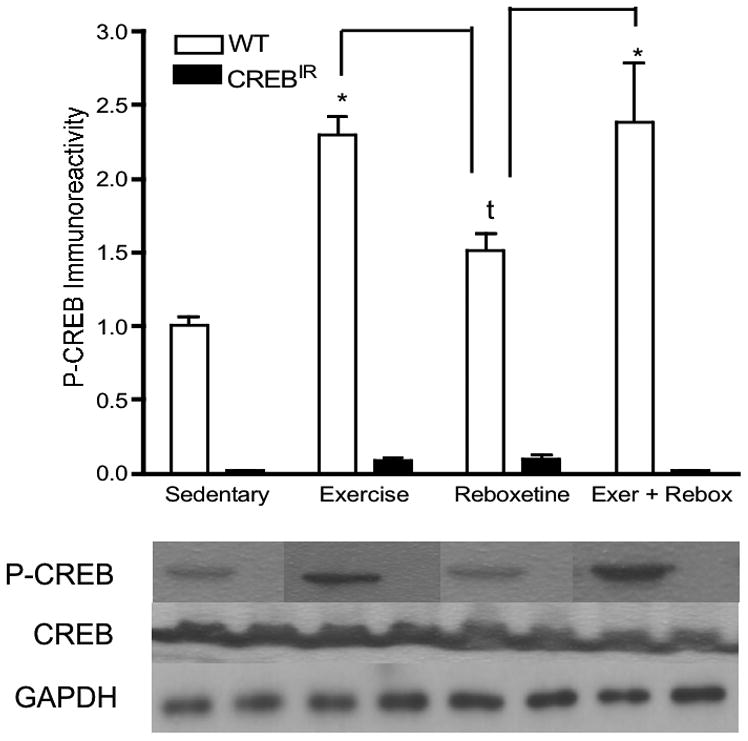
In CREBIR mice, P-CREB expression is repressed. (a) Representative 1.5 % agarose gel of PCR products of 6 mice in duplicate. Lanes 1 and 2, WT; Lanes 3 and 4, CREBIR, Lanes 5 and 6, CREBIR where the tube containing one of the duplicates (lane 5) largely evaporated during the PCR cycling process; Lanes 7 and 8, WT; Lanes 9 and 10, WT; Lanes 11 and 12, CREBIR. Note the presence of the repressor at ∼400 bp in the 3 CREBIR mice (Lanes 3, 4; 5, 6; and 11, 12). (b) WT mice express significantly more P-CREB than CREBIR mice across all treatments. Additionally, the conditionally activated mutation prevents any increase in P-CREB due to our interventions. *Among the WT mice, those who exercised (p < .0001, n = 29), received reboxetine (p = .067, n = 9), and received the combination of the two treatments (p < .0001, n = 7) expressed significantly more P-CREB than those that were sedentary (n = 12); and those that received reboxetine (n = 9) expressed significantly less P-CREB than exercising mice (p = .001, n = 29) or the combination-treated mice (p = .007, n = 7). Among the CREBIR mice, there were no significant differences among treatments in P-CREB immunoreactivity. Note the dramatic decrease in P-CREB immunoreactivity of CREBIR mice (black bars), compared to that of WT (white bars). Anti-P-CREB was very insensitive and required 60 μg protein for detection. P-CREB was undetectable in all 4 treatment groups and both genotypes, when less than 60 μg protein was applied to the gels. This large amount of protein, however, resulted in overloaded lanes for the much more sensitive anti-CREB. Because of the very small sample volumes, each mouse hippocampus was randomly assigned to a particular gel/Western film, thereby controlling for film-to-film variability. Therefore, the row of P-CREB bands and their respective CREB and GAPDH bands are a composite from several different Western blotting films. However, each P-CREB band and its respective CREB band and GAPDH band all represent the same mouse hippocampal sample (same gel lane).
Drug treatments and physical exercise
When they were approximately 2.5 months old, each experimental mouse was individually housed. All experimental mice resulting from our breeding efforts were used: males and females, WT and CREBIR. When mice were approximately 3 months old, mice randomly chosen to exercise were allowed access to a running wheel (diameter = 23 cm) for 48 hr for the purpose of pre-training to discount any effects of novelty. Following the 2 days of running access, the wheels were removed for 6 days. On the 7th day, at 18:00 hr, all mice received a single i.p. injection of 4-OH-tamoxifen (TAM, 16 mg/kg). At 24:00, mice previously chosen to exercise and/or receive reboxetine had their running wheels replaced into their cages and/or were treated with the antidepressant via i.p. injection (20 mg/kg). Mice assigned to the control condition, receiving neither of these interventions, received saline injections. Running distances were monitored using RatRun software (C. Hage Associates). At 06:00 the next morning, all mice were sacrificed by rapid decapitation, and the brains rapidly dissected: the right half of each brain was flash frozen in a dry ice/2-methylbutane bath; the left hippocampi were dissected and frozen on dry ice. Both the right hemisphere and the left hippocampi were then stored at -80°C till ready for in situ hybridization (ISH) and Western blotting, respectively.
Groups
There were a total of 123 mice used in this study and all were used for the P-CREB Westerns (see below). By gender: 62 males, 61 females. By genotype: 57 WT, 66 CREBIR. And by treatment: 21 saline-administered/sedentary (controls), 13 reboxetine-administered/sedentary, 75 saline/exercising, 14 reboxetine/exercising combination. However, for ISH, the hemispheric sections of 37 of the mice were either unavailable or deemed unusable for BDNF mRNA analyses (see below), leaving a total of 86 mice' sections suitable for ISH. By gender: 45 males, 41 females. By genotype: 46 WT, 40 CREBIR. And by treatment: 21 saline-administered/sedentary (controls), 14 reboxetine-administered/sedentary, 37 saline/exercising, 14 reboxetine/exercising combination. Finally, for BDNF and P-Akt Westerns, enough hippocampal homogenates were available from only 97 mice. By gender: 44 males, 53 females. By genotype: 53 WT, 44 CREBIR. And by treatment: 21 saline-administered/sedentary (controls), 12 reboxetine-administered/sedentary, 50 saline/exercising, 14 reboxetine/exercising combination.
In situ Hybridization
The right hemisphere of each brain was sectioned at 12 μm. In vitro transcription and ISH were carried out as previously described (Russo-Neustadt et al., 2001).
SDS-PAGE and Western Blotting
Each mouse left hippocampus was homogenized in 4 × homogenization buffer (w/v: 0.5 M Tris HCl, pH 6.8, 0.5 M EDTA, 1 % SDS) and the protein concentration determined using the method of Lowry (Lowry et al., 1951). Sixty micrograms of protein were applied to each well of a 10 % polyacrylamide gel, electrophoresed, and the proteins subsequently electrotransferred to Hybond nitrocellulose membranes (GE Healthcare, Piscataway, NJ). Western blotting for each antibody was then performed according to the respective manufacturer's specific instructions. Following Western blotting for P-CREB and P-Akt, the blots were then placed in stripping buffer (62.5 mM Tris-HCl, pH 6.7, 100 mM β-mercaptoethanol, 2 % SDS) at 55°C for 10 min with occasional stirring. Blots were then re-probed with the respective total forms of each antibody, again, in compliance with the manufacturer's specifications. Blots were then placed in stripping buffer once more and then re-probed with anti-GAPDH as loading control. Following Western blotting for BDNF, blots were placed in stripping buffer and then re-probed with anti-GAPDH, again, as loading control. Band visualization was carried out using enhanced chemiluminescence followed by apposition to hyperfilm (GE Healthcare, Piscataway, NJ).
Because of the large number of groups (2 genotypes × 2 genders × 4 treatments = 16 groups), it was not experimentally possible to assign mice to PAGE lanes according to group as presented in the bar graphs (See Results below); each gel had only 11 (if including a standard curve) or 14 lanes available (and there were 16 groups). And because of the small sample volume of each hippocampus (mean weight of hippocampi ∼ 25 mg), there was not enough of most samples to repeat on additional gels to control for gel-to-gel and therefore, film-to-film variability. All available mice hippocampi, therefore, had to be randomly assigned to a particular gel/Western blotting experiment. The assembly of the row of Western blotting bands according to the bars in the bar graph they represent in the figures (see Results), therefore, required cutting and pasting the appropriate hippocampal bands from several Western blotting films to make the composite figure. However, the usual practice of keeping each phospho form, its total form, and its loading control together remains intact. That is, each phospho form band and its respective pan form band and GAPDH band all represent the same mouse hippocampal sample (same gel lane).
Densitometry
Optical densities on the autoradiograms from both ISH and Western blotting were measured in compliance with those falling within the gray scale range on a standard curve. (MCID, St. Catherines, Ontario, Canada). For ISH, optical densities were read at the CA1, CA2, CA3, CA4/hilus, and dentate gyrus (DG) (Figure 2). For Western films, lightly exposed bands were evaluated across each film for each mouse. Optical densities of the phospho forms of CREB and Akt were corrected for by dividing them by those of the respective total forms and then again by those of GAPDH. Optical densities of BDNF bands were divided by those of GAPDH.
Figure 2.
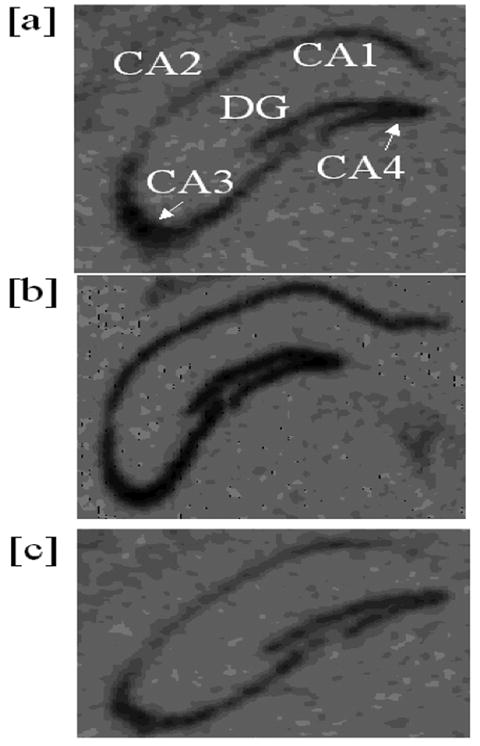
Representative autoradiograms showing hippocampal BDNF mRNA by in situ hybridization. (a) WT sedentary; (b) WT exercising; (c) CREBIR exercising.
Statistics
A three-way ANOVA (gender × genotype × treatment) was conducted to evaluate statistically significant differences in BDNF mRNA among groups for each hippocampal region (ISH) and for P-CREB, P-Akt, and BDNF (Westerns). Pair-wise comparisons between any 2 treatment groups were evaluated using post-hoc Fisher's PLSD.
Results
Running Distances
Of the mice allowed running wheels for 6 hr, there were no statistically significant differences in running distances between males (5,626 ± 354 m) and females (6,196 ± 358 m), between WT (5,648 ± 322 m) and CREBIR (6,174 ± 387 m), or between exercising (6,239 ± 392 m) and exercising-+-reboxetine- (5,747 ± 323 m) treated mice.
Phospho-CREB
In part to confirm that CREB phosphorylation was indeed repressed in TAM-induced CREBIR mice, Western blotting was performed to probe for P-CREB in the hippocampi of all mice. P-CREB was markedly decreased in all mutant mice, and significantly increased in wild-type mice receiving interventions; particularly exercise or exercise/reboxetine (Figure 1b). None of the interventions led to an increase in P-CREB beyond the low baseline in CREBIR mice. There were significant main effects for both genotype [F(1,115) = 298.13, p < .0001, n = 123] and treatment [F(3,115) = 15.40, p < .0001, n = 123] (Figure 1b). Among WT mice, sedentary mice (n = 12) expressed significantly less P-CREB than exercising mice (p < .0001, n = 29) and exercising-+-reboxetine combination mice (p < .0001, n = 7), but with a trend towards that of being less than reboxetine-treated mice (p = .067, n = 9) [F(3,53) = 15.17, p < .0001, n = 57]. Among CREBIR mice, there were no significant differences among the 4 treatment groups (F(3,62) = 1.47, p = .231, n = 66) (Figure 1b). Again, males and females were not significantly different from each other in hippocampal P-CREB [F(1,119) = .563, p = .455, n = 123].
BDNF mRNA
There were no statistically significant effects on BDNF expression with respect to gender (i.e., males and females were statistically identical) in any hippocampal regions evaluated. On the other hand, WT mice expressed significantly more BDNF mRNA than CREBIR mice resulting from the 3 exercise and/or drug treatments in all 5 hippocampal regions examined (Figure 3). That is, there were significant main effects for genotype in all 5 hippocampal regions [CA1, F(1,70) = 34.29, p < .0001, n = 86; CA2, F(1,70) = 29.93, p < .0001, n = 86; CA3, F(1,70) = 82.12, p < .0001; CA4/hilus, F(1,70) = 65.77, p < .0001, n = 86; DG, F(1,70) = 69.77, p < .0001, n = 86] and for the 3 exercise and/or drug treatments in 4 of the 5 hippocampal regions examined [CA1, F(3,70) = .688, p = .563, n = 86; CA2, F(3,70) = 2.98, p = .037, n = 86; CA3, F(3,70) = 8.57, p < .0001, n = 86; CA4/hilus, F(3,70) = 8.06, p < .0001, n = 86; DG, F(3,70) = 12.22, p < .0001, n = 86] (Figure 3). In summary, baseline BDNF mRNA levels were identical in both experimental mouse groups, but only wild-type mice showed a BDNF mRNA response to exercise and/or antidepressant. In CREBIR mice, BDNF mRNA levels remained at baseline for all treatments (Figure 3).
Figure 3.
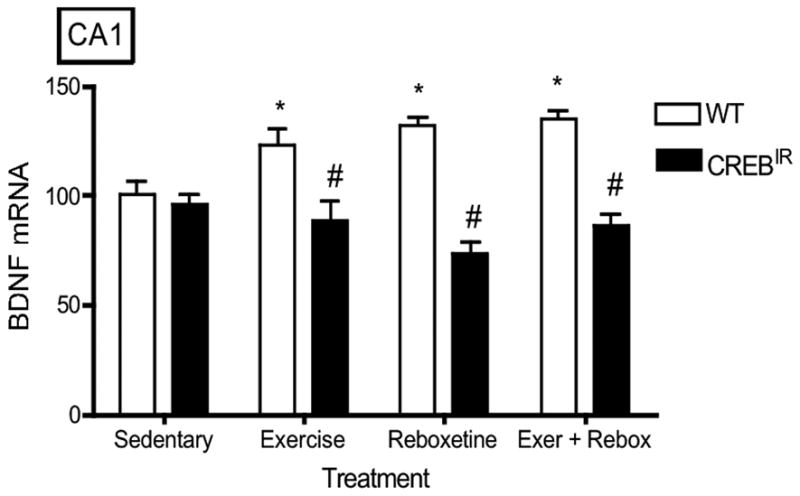
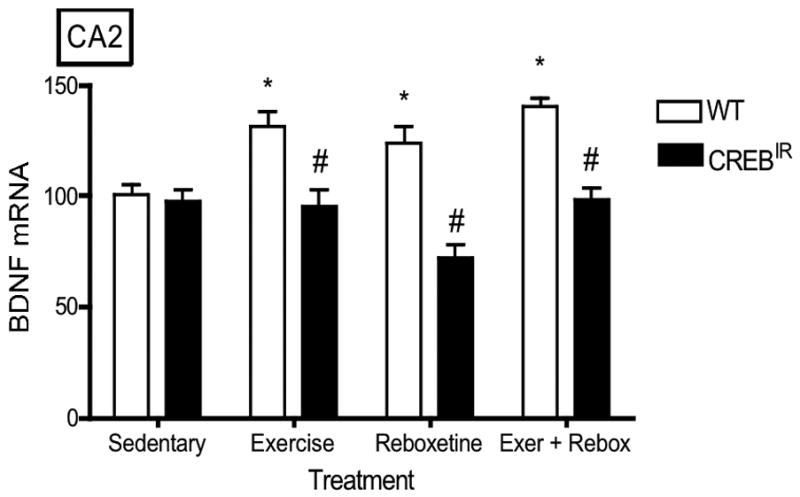
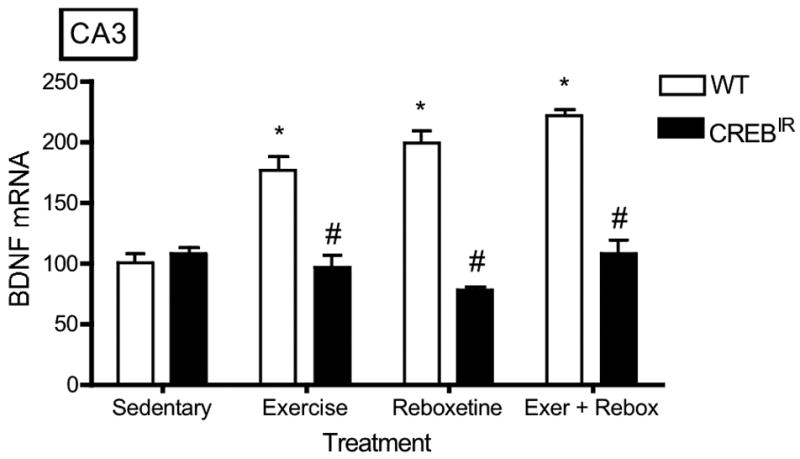
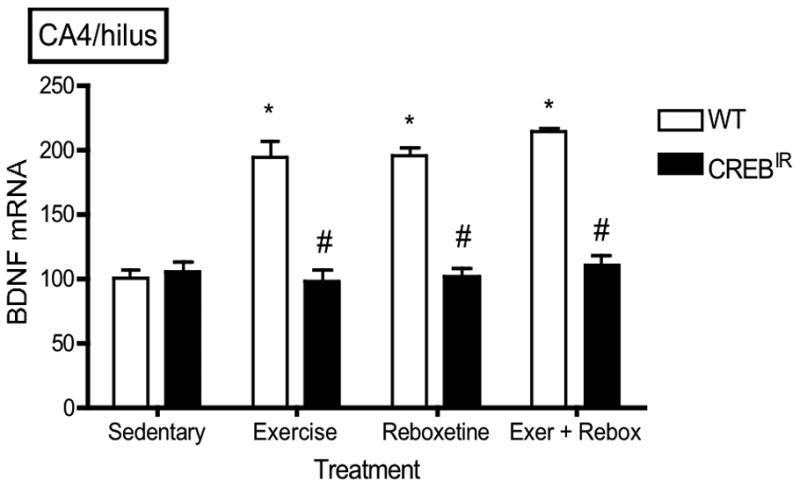
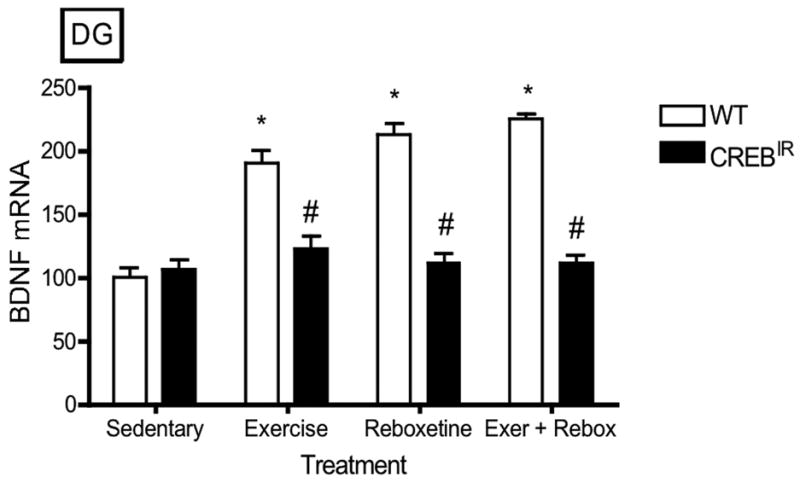
The TAM-activated CREB repressor prevented the BDNF mRNA-increasing effects of exercise, reboxetine or exercise/reboxetine in all hippocampal regions examined. (a) CA1: *Among the WT mice, those who exercised (p = .012, n = 19), received reboxetine (p = .006, n = 8), and received the combination of the two treatments (p = .004, n = 7) expressed significantly more BDNF mRNA than those that were sedentary (n = 12) [F(3,42) = 4.48, p = .008, n = 46]. # WT mice also expressed significantly more BDNF mRNA than CREBIR mice for each intervention, except for sedentary mice. Among the CREBIR mice, there were no significant differences among treatments [F(3,36) = .791, p = .507, n = 40]. (b) CA2: *Among the WT mice, those who exercised (p = .002, n = 19), received reboxetine (p = .039, n = 8), and received the combination of the two treatments (p = .001, n = 7) expressed significantly more BDNF mRNA than those that were sedentary (n = 12) [F(3,42) = 5.26, p = .004, n = 46]. # WT mice also expressed significantly more BDNF mRNA than CREBIR mice for each intervention, except for sedentary mice. Among the CREBIR mice, there were no significant differences among treatments [F(3,36) = 1.67, p = .192, n = 40]. (c) CA3: *Among the WT mice, those who exercised (p < .0001, n = 19), received reboxetine (p < .0001, n = 8), and received the combination of the two treatments (p < .0001, n = 7) expressed significantly more BDNF mRNA than those that were sedentary (n = 12) [F(3,42) = 20.81, p < .0001, n = 46]. # WT mice also expressed significantly more BDNF mRNA than CREBIR mice for each intervention, except for sedentary mice. Among the CREBIR mice, there were no significant differences among treatments [F(3,36) = 1.34, p = .276, n = 40]. (d) CA4/hilus: *Among the WT mice, those who exercised (p < .0001, n = 19), received reboxetine (p < .0001, n = 8), and received the combination of the two treatments (p < .0001, n = 7) expressed significantly more BDNF mRNA than those that were sedentary (n = 12) [F(3,42) = 18.70, p < .0001, n = 46]. # WT mice also expressed significantly more BDNF mRNA than CREBIR mice for each intervention, except for sedentary mice. Among the CREBIR mice, there were no significant differences among treatments [F(3,36) = .346, p = .792, n = 40]. (e) DG: *Among the WT mice, those who exercised (p < .0001, n = 19), received reboxetine (p < .0001, n = 8), and received the combination of the two treatments (p < .0001, n = 7) expressed significantly more BDNF mRNA than those that were sedentary (n = 12) [F(3,42) = 32.10, p < .0001, n = 46]. # WT mice also expressed significantly more BDNF mRNA than CREBIR mice for each intervention, except for sedentary mice. Among the CREBIR mice, there were no significant differences among treatments [F(3,36) = .516, p = .674, n = 40].
BDNF protein
Short-term (6 hours) exercise, a single dose of reboxetine, or the combination of these two interventions led to significant increases in hippocampal BDNF protein as measured by Western analysis. Although sedentary BDNF levels were normal, CREBIR mice showed no BDNF response to any of the interventions. There were significant main effects for genotype [F(1,81) = 23.95, p < .0001, n = 97] and treatment [F(3,81) = 2.76, p = .048, n = 97] in BDNF protein expressed (Figure 4). Among the WT mice, exercising (p = .019, n = 27) and the combination-treated mice ((p = .004, n = 7), but not the reboxetine-treated mice (p = .078, n = 7), expressed significantly more BDNF immunoreactivity than that of sedentary mice (n = 12) [F(3,49) = 3.49, p = .025, n = 53]. Among the CREBIR mice, exercising mice (n = 23) expressed significantly more BDNF immunoreactivity than those that received just reboxetine (p = .006, n = 5) and the combination treatment (p = .007, n = 7) [F(3,36) = 4.83, p = .006, n = 44]. Except for sedentary individuals (n = 12 WT, 9 CREBIR), WT mice expressed significantly more BDNF immunoreactivity than CREBIR mice as a result of exercise (p = .0003, n = 27 WT, 23 CREBIR), reboxetine (p = .0117, n = 7 WT, 5 CREBIR), or the combination treatment (p < .0001, n = 7 WT, 7 CREBIR) (Figure 4). Again, there were no significant differences between males and females [F(1,81) = 0.173, p = .679, n = 97].
Figure 4.
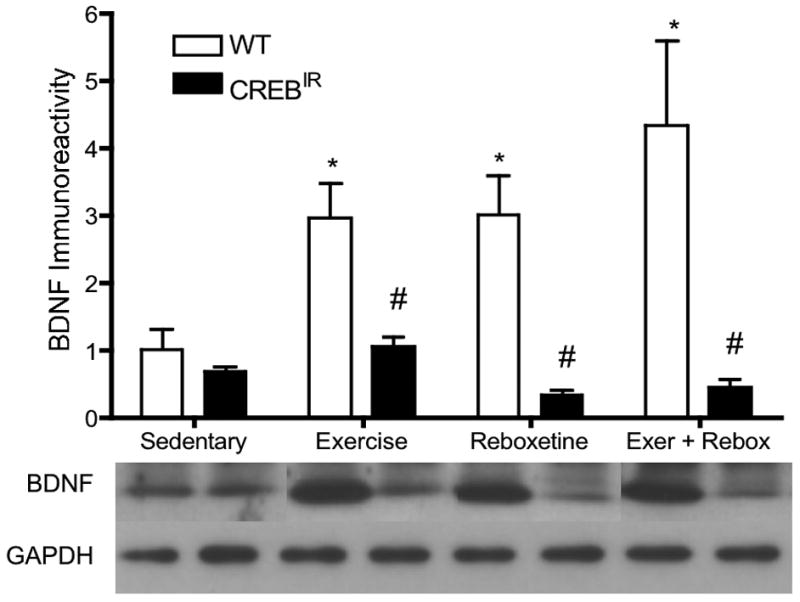
Exercise and antidepressant interventions increased hippocampal mature BDNF immunoreactivity in WT mice. No BDNF response to interventions was evident CREBIR mice. *Among the WT mice, those who exercised (p = .019, n = 27) and received the combination of the two treatments (p = .004, n = 7), but not those that received reboxetine (p = .078, n = 7), expressed significantly more mature BDNF immunoreactivity (electrophoresing as a ∼17-kDal band) than those that were sedentary (n = 12) [F(3,49) = 3.395, p = .025]. # WT mice also expressed significantly more BDNF immunoreactivity than CREBIR mice for each intervention, except for sedentary mice, who expressed the same amount between genotypes. Among the CREBIR mice, exercising mice (n = 23) expressed significantly more BDNF immunoreactivity than those that received just reboxetine (p = .006, n = 5) and the combination treatment (p = .007, n = 7). Because of the very small sample volumes, each mouse hippocampus was randomly assigned to a particular gel/Western film, thereby controlling for film-to-film variability. Therefore, the row of BDNF bands and their respective GAPDH bands are a composite from several different Western blotting films. However, each BDNF band and its respective GAPDH band all represent the same mouse hippocampal sample (same gel lane).
Phospho-Akt
In WT mice, all interventions led to a significant activation of the signaling molecule, Akt, whereas no change from baseline was evident in CREBIR mice. There were significant main effects for genotype [F(1,81) = 36.02, p < .0001, n = 97] and treatment [F(3,81) = 4.66, p = .005, n = 97] in the extent of P-Akt expressed (Figure 5). Among the WT mice, exercising (p < .0001, n = 27), reboxetine-treated (p = .027, n = 7), and the combination-treated (p < .0001, n = 7) expressed significantly more P-Akt immunoreactivity than that of sedentary mice (n = 12) [F(3,49) = 7.32, p < .0001, n = 53]. Among the CREBIR mice, there were no significant differences among the 4 treatment groups [F(3,40) = .576, p = .634, n = 44]. Except for sedentary mice (n = 12), WT individuals expressed significantly more P-Akt than CREBIR mice as a result of exercise (p < .0001, n = 27 WT, 23 CREBIR), reboxetine (p = .0097, n = 7 WT, 5 CREBIR), or the combination treatment (p < .0001, n = 7 WT, 7 CREBIR) (Figure 5). And again, there were no significant differences between males and females [F(1,81) = .017, p = .896, n = 97].
Figure 5.
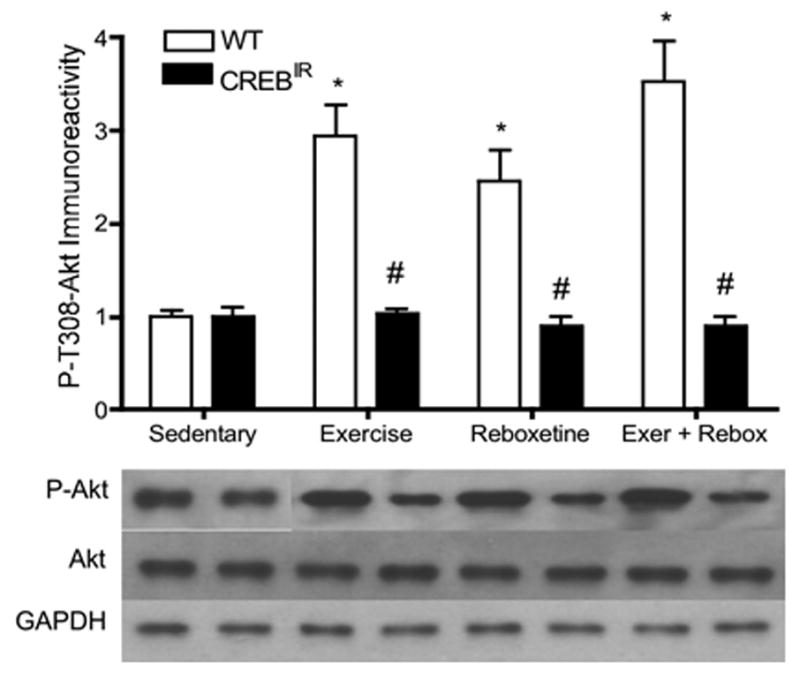
In WT mice, all interventions led to a significant activation of the signaling molecule, Akt, whereas no change from baseline was evident in CREBIR mice. *Among the WT mice, those who exercised (p < .0001, n = 27), received reboxetine (p = .027, n = 7), and received the combination of the two treatments (p < .0001, n = 7) expressed significantly more P-Akt immunoreactivity than those that were sedentary (p < .0001, n = 12). #WT mice also expressed significantly more P-Akt immunoreactivity than CREBIR mice for each intervention, except for sedentary mice, who expressed the same amount between genotypes. Among the CREBIR mice, there were no significant differences among treatments in P-Akt immunoreactivity. Because of the very small sample volumes, each mouse hippocampus was randomly assigned to a particular gel/Western film, thereby controlling for film-to-film variability. Therefore, the row of P-Akt bands and their respective Akt and GAPDH bands are a composite from several different Western blotting films. However, each P-Akt band and its respective Akt band and GAPDH band all represent the same mouse hippocampal sample (same gel lane).
Discussion
In the current study, we tested the hypothesis that the increased BDNF expression and activation of pro-survival signaling molecules resulting from an acute bout of running exercise is dependent upon the activation of CREB. Using a mouse model genetically engineered to express a repressor induced by 4-OH-tamoxifen (Kida et al., 2002), we observed that these cellular changes were indeed prevented in the absence of CREB phosphorylation. Consistent with the findings of several earlier studies (Conti et al., 2002; Segel, 2003; Rossler et al., 2004), our results also suggest that enhanced BDNF expression resulting from short-term antidepressant treatment involves CREB activation. Other investigators have suggested that the BDNF-enhancing mechanism stimulated by antidepressants depends upon the activation of this key transcription factor (Malberg and Blendy, 2005; Duman and Monteggia, 2006; Ploughman et al., 2007; Tang et al., 2008), and evidence is emerging that the pro-survival mechanisms stimulated by antidepressant treatment and running exercise may overlap (Russo-Neustadt et al., 2001) and possibly converge upon CREB (Russo-Neustadt and Chen, 2005).
With the exception of P-CREB, the baseline (sedentary, untreated) levels of the molecules we assessed were not altered when the CREB repressor was activated. Nevertheless, this mutation completely abolished a dramatic increase in BDNF mRNA expression in all regions of the hippocampi of mice participating in exercise or undergoing antidepressant treatment. These results are consistent with those of Conti et al. (2002), who showed that in CREB-deficient mice, BDNF expression was not up-regulated in response to antidepressant treatment, indicating that CREB acts upstream of BDNF in response to an antidepressant regimen. Others have found that induced over-expression of CREB in the DG, but not in the CA1 and CA3 regions, is associated with an antidepressant effect (Chen et al., 2001a, b). Our results underscore the possibility that the intracellular changes associated with not only antidepressant, but also exercise, involve CREB as a convergence point.
Recent evidence indicates that both exercise and antidepressant medications, via enhanced monoaminergic neurotransmission, increase the activation of multiple pro-survival signaling pathways, including the phosphatidylinositol-3 kinase (PI-3K) pathway and the mitogen-activated protein kinase (MAPK) pathway, and that enhanced BDNF expression may occur through a positive feed-forward mechanism (Tong et al., 2001; Chen et al., 2007; Vaynman et al., 2006), or via crosstalk between key signaling pathways (Luttrell et al., 1999; Chen et al., 2007). It has also been established that exercise-associated increases in BDNF expression are dependent on the availability of insulin-like growth factor-1 (IGF-1) (Chen and Russo-Neustadt, 2007b; Carro et al., 2000, 2001; Trejo et al., 2008), and the nitric oxide/cGMP signaling pathway has also been shown to play a vital role in the enhanced CREB expression (Riccio et al., 2006) and Akt activation (Chen and Russo-Neustadt, 2007a) associated with exercise. With so many different pathways converging on CREB, it is not surprising that any one intervention, such as exercise or antidepressants, may activate CREB several-fold above baseline (Chen and Russo-Neustadt, 2005).
Previous studies have provided ample evidence that exercise is neuroprotective against stress (Zheng et al., 2006) and other insults, such as ischemia (Gerasimov et al., 2001; Ploughman et al., 2007) by increasing the expression of BDNF (Ang et al., 2003) and that of other plasticity-related genes (Tong et al., 2001). Along the same lines, antidepressants have been shown to reverse corticosterone (stress)-induced decrease in BDNF (Dwivedi et al., 2006) suggesting that these medications are also neuroprotective. So, it is not surprising that both BDNF and CREB have antidepressant effects of elevated mood and enhanced memory and cognition ((Silva et al., 1998; Tong et al., 2001; Kida et al., 2002; Bozon et al., 2003). In addition, CREB activation has been shown to dierectly result in enhanced BDNF expression (Conti et al., 2002).
As noted above, physical exercise and antidepressant treatment both increase the availability of CNS monoamines (Dishman et al., 2000; Chaouloff et al., 1997), and norephinephrine activation in particular is essential for the BDNF-increasing effects of both interventions (Ivy et al., 2003; Garcia et al., 2003). This underscores the role that norepinephrine may have on altering plasticity-related genes (Laidenfeld et al., 2002). The antidepressant used in this study, reboxetine, is highly norephinephrine-selective (Montgomery, 1998). This agent has been shown to have rapid and robust effects on hippocampal BDNF expression in previous studies (Russo-Neustadt et al., 2004;).
Of particular clinical interest is the very acute time frame utilized in the current study. Six hours is the window for optimal activation of the CREB repressor after treatment with TAM (Kida et al., 2002), and severe inhibition of CREB activation was verified by Western analysis in this study (Figure 1b). Both exercise and reboxetine treatment led to striking elevations in hippocampal BDNF mRNA, BDNF protein and Akt phosphorylation in wild type mice during this brief time frame. Therefore, initial cellular changes occurring with both interventions are quite rapid. Exercise has been known to increase BDNF mRNA within 6 hours in rats (Oliff et al., 1998), and has recently been revealed to do so in as little as 200 min (Soya et al., 2007), but initial reports of antidepressant effects indicated that 2-3 weeks of treatment was necessary (similar to the time frame for clinical efficacy in humans) (Nibuya et al, 1995; Duman et al, 1997). On the other hand, more recent studies have examined cellular effects of very short-term antidepressant treatment, and a few agents increase BDNF expression within 4-6 hours (Sacchetti et al., 1999; Dias et al., 2003; Saarelainen et al., 2003; Vaynman et al., 2003; Rantamaki et al., 2007). Importantly, it should also be noted that our mice were allowed to run for 2 consecutive days nearly a week before their 6-hr intervention period. It is possible that this pre-training primed the molecular machinery for BDNF expression before the actual intervention (Berchtold et al., 2005). It is plausible that increased BDNF signaling is among the first in a series of steps toward enhanced neuronal functioning, such as increased expression of growth cone-associated proteins necessary for renewed dendritic arborization and synaptogenesis (Duman and Monteggia, 2006), which may require several weeks.
One possible limitation of this study, owing to the extreme shortage of hippocampal lysates from most of the mice, is the lack of P-MAPK data resulting from our interventions. Collectively, the mean weight of the left hippocampi was ∼ 25 mg. Although homogenization occurred in as small a tube as possible (0.65-ml capacity), a significant amount was lost as a natural result of the homogenization process (see Methods and Materials). This limitation, however, is tempered by earlier findings by ourselves, as well as by others, who have found P-MAPK activation at least coincident, if not dependent, on CREB phosphorylation (Waltereit and Weller, 2003; Chen et al., 2007; Shimizu et al., 2007).
In conclusion, nevertheless, it appears that not only is CREB activation an essential part of the hippocampal survival-enhancing mechanism of antidepressant treatment, but also is a key molecule in the cellular machinery activated by physical exercise. In addition, we have shown that one of the key cellular survival-promoting pathways of BDNF signaling, PI-3K-Akt, is also CREB-dependent and therefore, may reveal novel viable targets for drug discovery and amelioration of depressive symptoms.
Acknowledgments
NIMH Grant Number: MH-59776
References
- Ang ET, Wong PTH, Moochhala S, Ng YK. Neuroprotection associated with running: is it a result of increased endogenous neurotrophic factors? Neuroscience. 2003;118:335–345. doi: 10.1016/s0306-4522(02)00989-2. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Booth FW, Chakravarthy MV, Spangenburg EE. Exercise and gene expression: physiological regulation of the human genome through physical activity. Journal of Physiology. 2002;543:399–411. doi: 10.1113/jphysiol.2002.019265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozon B, Kelly A, Josselyn SA, Silva AJ, Davis S, Laroche S. MAPK, CREB, and zif268 are all required for the consolidation of recognition memory. Phil Trans R Soc Lond B. 2003;358:805–814. doi: 10.1098/rstb.2002.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and –independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Cachetti F, Fochesatto C, Scopel D, Nardin P, Goncalves CA, Netto CA, Siqueira IR. Effect of a neuroprotective exercise protocol on oxidative state and BDNF levels in the rat hippocampus. Brain Research. 2008;1188:182–188. doi: 10.1016/j.brainres.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Castren E. Is mood Chemistry? Nature Rev Neurosci. 2005;6:241–246. doi: 10.1038/nrn1629. [DOI] [PubMed] [Google Scholar]
- Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochemistry. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- Chaouloff F. Physical exercise and brain monoamines: a review. Acta Physiol Scand. 1989;137:1–13. doi: 10.1111/j.1748-1716.1989.tb08715.x. [DOI] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biological Psychiatry. 2001a;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Exercise activates the phosphatidylinositol 3′-kinase pathway. Molecular Brain Research. 2005;135:181–193. doi: 10.1016/j.molbrainres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Nitric oxide signaling participates in norepinephrine-induced activity of neuronal intracellular survival pathways. Life Sciences. 2007a;81:1280–1290. doi: 10.1016/j.lfs.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Running exercise- and antidepressant-induced increases in growth and survival-associated signaling molecules are IGF-dependent. Growth Factors. 2007b;25:118–131. doi: 10.1080/08977190701602329. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Nguyen TV, Pike CJ, Russo-Neustadt AA. Norepinephrine induces BDNF and activates the PI-3K and MAPK cascades in embryonic hippocampal neurons. Cellular Signaling. 2007;19:114–128. doi: 10.1016/j.cellsig.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Chen ACH, Shirayama Y, Shin KH, Neve RL, Duman RS. Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biological Psychiatry. 2001b;49:753–762. doi: 10.1016/s0006-3223(00)01114-8. [DOI] [PubMed] [Google Scholar]
- Conti AC, Cryan JF, Dalvi A, Lucki I, Blendy JA. cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. Journal of Neuroscience. 2002;22:3262–3268. doi: 10.1523/JNEUROSCI.22-08-03262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology. 2005;182:335–344. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- Davey CP. Physical exertion and mental performance. Ergonomics. 1973;16:595–599. doi: 10.1080/00140137308924550. [DOI] [PubMed] [Google Scholar]
- Dias BG, Banerjee SB, Duman RS, Vaidya VA. Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatment in the adult rat brain. Neuropharmacology. 2003;45:553–563. doi: 10.1016/s0028-3908(03)00198-9. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Medicine and Science in Sports and Exercise. 1997;29:63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Renner KJ, White-Welkley JE, Burke KA, Bunnell BN. Treadmill exercise training augments brain norepinephrine response to familiar and novel stress. Brain Research Bulletin. 2000;52:337–342. doi: 10.1016/s0361-9230(00)00271-9. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Archives of General Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biological Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Pandey GN. Antidepressants reverse corticosterone-mediated decrease in brain-derived neurotrophic factor expression: Differential regulation of specific exons by antidepressants and corticosterone. Neuroscience. 2006;139:1017–1029. doi: 10.1016/j.neuroscience.2005.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L, Manji HK, Chen G. The role of the extracellular signal-related kinase signaling pathway in mood modulation. Journal of Neuroscience. 2003;23:7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst C, Olson AK, Pinel JPJ, Lam RW, Christie BR. Antidepressant effects of exercise: Evidence for an adult-neurogenesis hypothesis? Journal of Psychiatry Neuroscience. 2006;31:84–92. [PMC free article] [PubMed] [Google Scholar]
- Fordyce DE, Wehner JM. Physical activity enhances spatial learning performance with an associated alteration in hippocampal protein kinase c activity in C57BL/6 and DBA/2 mice. Brain Research. 1993;619:111–119. doi: 10.1016/0006-8993(93)91602-o. [DOI] [PubMed] [Google Scholar]
- Frazer A. Norepinephrine involvement in antidepressant action. Journal of Clinical Psychiatry. 2000;61(suppl 10):25–30. [PubMed] [Google Scholar]
- Fujimaki K, Morinobu S, Duman RS. Administration of a cAMP phosphodiesterase 4 inhibitor enhances antidepressant-induction of BDNF mRNA in rat hippocampus. Neuropsychopharmacology. 2000;22:42–51. doi: 10.1016/S0893-133X(99)00084-6. [DOI] [PubMed] [Google Scholar]
- Gass P, Riva MA. CREB, neurogenesis and depression. BioEssays. 2007;29:957–961. doi: 10.1002/bies.20658. [DOI] [PubMed] [Google Scholar]
- Gerasimov VD, Artemenko DP, Krishtal OA. Preconditioning by motor activity protects rat hippocampal CA1 neurons against prolonged ischemia. Brain Research. 2001;888:326–329. doi: 10.1016/s0006-8993(00)03105-x. [DOI] [PubMed] [Google Scholar]
- Garoflos E, Stamatakis A, Mantelas A, Philippidis H, Stylianopoulou F. Cellular mechanisms underlying an effect of “early handling” on pCREB and BDNF in the neonatal rat hippocampus. Brain Research. 2005;1052:187–195. doi: 10.1016/j.brainres.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Goodman LJ, Valverde J, Lim F, Geschwind MD, Federoff HJ, Geller AI, Hefti F. Regulated release and polarized localization of brain-derived neurotrophic factor in hippocampal neurons. Molecular Cellular Neuroscience. 1996;7:222–238. doi: 10.1006/mcne.1996.0017. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Gomez-Pinilla F, Hovda DA. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. Journal of Neurotrauma. 2007;24:1161–1171. doi: 10.1089/neu.2006.0255. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Narz F, Heumann R, Lessmann V. BDNF-GFP containing secretory granules are localized in the vicinity of synaptic junctions of cultured cortical neurons. Journal of Cell Science. 1998;111:1483–1493. doi: 10.1242/jcs.111.11.1483. [DOI] [PubMed] [Google Scholar]
- Hayley S, Poulter MO, Merali Z, Anisman H. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience. 2005;135:659–678. doi: 10.1016/j.neuroscience.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Hill RD, Storandt M, Malley M. The impact of long-term exercise training on psychological function in older adults. Journal of Gerontology PSYCHOLOGICAL SCIENCES. 1993;48:P12–P17. doi: 10.1093/geronj/48.1.p12. [DOI] [PubMed] [Google Scholar]
- Hisaoka K, Maeda N, Tsuchioka M, Takebayashi M. Antidepressants induce acute CREB phosphorylation and CRE-mediated gene expression in glial cells: a possible contribution to GDNF production. Brain Research. 2008;1196:53–58. doi: 10.1016/j.brainres.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Irvine GI, Abraham WC. Enriched environment exposure alters the input-output dynamics of synaptic transmission in area CA1 of freely moving rats. Neuroscience Letters. 2005;391:32–37. doi: 10.1016/j.neulet.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Ishmail AH, El-Naggar AM. Effect of exercise on cognitive processing in adult men. Journal of Human Ergology. 1981;10:83–91. [PubMed] [Google Scholar]
- Ivy AS, Rodriguez FG, Garcia C, Chen MJ, Russo-Neustadt AA. Specific noradrenergic and serotonergic blockade inhibits the activation of hippocampal brain-derived neurotrophic factor expression resulting from physical activity and antidepressant treatment. Pharmacology, Biochemistry and Behavior. 2003;75:81–88. doi: 10.1016/s0091-3057(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Johnson-Farley NN, Patel K, Kim D, Cowen DS. Interaction of FGF-2 with IGF-1 and BDNF in stimulating Akt, ERK, and neuronal survival in hippocampal cultures. Brain Research. 2007;1154:40–49. doi: 10.1016/j.brainres.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, Pena de Ortiz S, Kogan JH, Chevere I, Masushige S, Silva AJ. CREB required for the stability of new and reactivated fear memories. Nature Neuroscience. 2002;5:348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- Labbé EE, Welsh MC, Delaney D. Effects of consistent aerobic exercise on the psychological functioning of women. Perceptual and Motor Skills. 1988;67:919–925. doi: 10.2466/pms.1988.67.3.919. [DOI] [PubMed] [Google Scholar]
- Laidenfeld D, Klein E, Ben-Shachar D. Norepinephrine alters the expression of genes involved in neuronal sprouting and differentiation: relevance for major depression and antidepressant mechanisms. Journal of Neurochemistry. 2002;83:1054–1064. doi: 10.1046/j.1471-4159.2002.01215.x. [DOI] [PubMed] [Google Scholar]
- Larsen MH, Hay-Schmidt A, Rønn LCB, Mikkelsen JD. Temporal expression of brain-derived neurotrophic factor (BDNF) mRNA in the rat hippocampus after treatment with selective and mixed monoaminergic antidepressants. European Journal of Pharmacology. 2008;578:114–122. doi: 10.1016/j.ejphar.2007.08.050. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Progress in Neurobiology. 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Li B, Suemaru K, Cui R, Araki H. Repeated electroconvulsive stimuli have long-lasting effects on hippocampal BDNF and decrease immobility time in the rat forced swim test. Life Sciences. 2007;80:1539–1543. doi: 10.1016/j.lfs.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Luikart BW, Zhang W, Wayman GA, Kwon CH, Westbrook GL, Parada LF. Neurotrophin-dependent dedritic filopodial motility: a convergence on PI3K signaling. Journal of Neuroscience. 2008;28:7006–7012. doi: 10.1523/JNEUROSCI.0195-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Blendy JA. Antidepressant action: to the nucleus and beyond. Trends in Pharmacological Sciences. 2005;26:631–638. doi: 10.1016/j.tips.2005.10.005. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annual Review of Neuroscience. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- Meeusen R, De Meirleir K. Exercise and brain neurotransmission. Sports Medicine. 1995;20:160–188. doi: 10.2165/00007256-199520030-00004. [DOI] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. European Journal of Neuroscience. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Mowla SJ, Pareek S, Farhadi HF, Petrecca K, Fawcett JP, Seidah NG, Morris SJ, Sossin WS, Murphy RA. Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. Journal of Neuroscience. 1999;19:2069–2080. doi: 10.1523/JNEUROSCI.19-06-02069.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AP, Cammarota M, de Oliveira Dietrich M, Rotta LN, Portela LV, Souza DO, Izquierdo I, Bevilaqua LR, Perry MLS. Different effect of high fat diet and physical exercise in the hippocampal signaling. Neurochem Research. 2008;33:880–885. doi: 10.1007/s11064-007-9530-7. [DOI] [PubMed] [Google Scholar]
- Nair A, Vadodaria KC, Banerjee SB, Benekareddy M, Dias BG, Duman RS, Vaidya VA. Stressor-specific regulation of distinct brain-derived neurotrophic factor transcripts and cyclic AMP response element-binding protein expression in the postnatal and adult rat hippocampus. Neuropsychopharmacology. 2007;32:1504–1519. doi: 10.1038/sj.npp.1301276. [DOI] [PubMed] [Google Scholar]
- Nair A, Vaidya VA. Cyclic AMP response element binding protein and brain-derived neurotrophic factor: Molecules that modulate our mood? Journal of Biosciences. 2006;31:423–434. doi: 10.1007/BF02704114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. Journal of Neuroscience. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliari R, Peyrin L. Norepinephrine release in the rat frontal cortex under treadmill exercise: a study with microdialysis. Journal of Applied Physiolology. 1995a;78:2121–2130. doi: 10.1152/jappl.1995.78.6.2121. [DOI] [PubMed] [Google Scholar]
- Pagliari R, Peyrin L. Physical conditioning in rats influences the central and peripheral catecholamine responses to sustained exercise. European Journal of Applied Physiolopgy and Occupational Physiology. 1995b;71:41–52. doi: 10.1007/BF00511231. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Valjent E, Corbille AG, Corvol JC, Tassin JP, Girault JA, Herve D. cAMP and extracellular signal-regulated kinase signaling in response to D-amphetamine and methylphenidate in the prefrontal cortex in vivo: role of β1-adrenoceptors. Molecular Pharmacology. 2005;68:421–429. doi: 10.1124/mol.105.011809. [DOI] [PubMed] [Google Scholar]
- Ploughman M, Granter-Button S, Chernenko G, Attwood Z, Tucker BA, Mearow KM, Corbett D. Exercise intensity influences the temporal profile of growth factors involved in neuronal plasticity following focal ischemia. Brain Research. 2007;1150:207–216. doi: 10.1016/j.brainres.2007.02.065. [DOI] [PubMed] [Google Scholar]
- Popoli M, Brunello N, Perez J, Racagni G. Second messenger-related protein kinases in the brain: Their functional role and the action of antidepressant drugs. Journal of Neurochemistry. 2000;74:21–33. doi: 10.1046/j.1471-4159.2000.0740021.x. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Alejandre H, Garcia C, Ivy AS, Chen MJ. Hippocampal brain-derived neurotrophic factor expression following treatment with reboxetine, citalopram, and physical exercise. Neuropsychopharmacology. 2004;29:2289–2199. doi: 10.1038/sj.npp.1300514. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP. Physical activity-antidepresssant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Behavioral Brain Research. 2001;120:87–95. doi: 10.1016/s0166-4328(00)00364-8. [DOI] [PubMed] [Google Scholar]
- Rantamaki T, Hendolin P, Kankaanpaa A, Mijatovic J, Piepponen P, Domenici E, Chao MV, Mannisto PT, Castren E. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cγ signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32:2152–2162. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- Riccio A, Alvania RS, Lonze BE, Ramanan N, Kim T, Huang Y, Dawson TM, Snyder SH, Ginty DD. A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. Molecular Cell. 2006;21:283–294. doi: 10.1016/j.molcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Chen MJ. Brain-derived neurotrophic factor and antidepressant activity. Current Pharmaceutical Design. 2005;11:1495–1510. doi: 10.2174/1381612053764788. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P, Castrén E. Activation of the trkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. Journal of Neuroscience. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti G, Bernini M, Bianchetti A, Parini S, Invernizzi RW, Samanin R. Studies on the acute and chronic effects of reboxetine on extracellular noradrenaline and other monoamines in the rat brain. British Journal of Pharmacology. 1999;128:1332–1338. doi: 10.1038/sj.bjp.0702926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzberg AF. Clinical efficacy of reboxetine in major depression. Journal of Clinical Psychiatry. 2000;61(suppl 10):31–38. [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: A review of supportive evidence. Americal Journal of Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Segal RA. Selectivity in neurotrophin signaling: Theme and variations. Annual Review of Neuroscience. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: A stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annual Review of Biochemistry. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Shelton RC. Cellular mechanisms in the vulnerability to depression and response to antidepressants. The Psychiatric Clinics of North America. 2000;23:713–729. doi: 10.1016/s0193-953x(05)70193-3. [DOI] [PubMed] [Google Scholar]
- Shen H, Tong L, Balazs R, Cotman CW. Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience. 2001;107:219–229. doi: 10.1016/s0306-4522(01)00315-3. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Phan T, Mansury IM, Storm DR. Proteolytic degradation of SCOP in the hippocampus contributes to activation of MAPK and memory. Cell. 2007;128:1219–1229. doi: 10.1016/j.cell.2006.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Chen ACH, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. Journal of Neuroscience. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annual Review of Neuroscience. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Soya H, Nakamura T, Deocaris CC, Kimpara A, Iimura M, Fujikawa T, Chang H, McEwen BS, Nishijima T. BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Comm. 2007;358:961–967. doi: 10.1016/j.bbrc.2007.04.173. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SW, Chu E, Hui T, Helmeste D, Law C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neuroscience Letters. 2008;431:62–65. doi: 10.1016/j.neulet.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tardito D, Perez J, Tiraboschi E, Musazzi L, Racagni G, Popoli M. Signaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: a critical overview. Pharmacological Reviews. 2006;58:115–134. doi: 10.1124/pr.58.1.7. [DOI] [PubMed] [Google Scholar]
- Thome J, Sakai N, Shin KH, Steffen C, Zhang YJ, Imprey S, Storm D, Duman RS. cAMP response element-mediated gene transcription is upregulated by chronic antidepressant treatment. Journal of Neuroscience. 2000;20:4030–4036. doi: 10.1523/JNEUROSCI.20-11-04030.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Shen H, Perreau VM, Balazs R, Cotman CW. Effects of exercise on gene expression profile in the rat hippocampus. Neurobiology of Disease. 2001;8:1046–1056. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. Journal of Neuroscience. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122:647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. The select action of hippocampal calcium calmodulin protein kinase II in mediating exercise-enhanced cognitive function. Neuroscience. 2007;114:825–833. doi: 10.1016/j.neuroscience.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltereit R, Weller M. Signaling from cAMP/PKA to MAPK and synaptic plasticity. Molecular Neurobiology. 2003;27:99–106. doi: 10.1385/MN:27:1:99. [DOI] [PubMed] [Google Scholar]
- Wong ML, Licinio J. From monoamines to genomic targets: a paradigm shift for drug discovery in depression. Nature Review Drug Discovery. 2004;3:136–151. doi: 10.1038/nrd1303. [DOI] [PubMed] [Google Scholar]
- White LJ, Castellano V. Exercise and brain health – implications for multiple sclerosis: part 1 – neuronal growth factors. Sports Medicine. 2008;38:91–100. doi: 10.2165/00007256-200838020-00001. [DOI] [PubMed] [Google Scholar]
- Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, Krueger K, Fromme A, Korsukewitz C, Floel A, Knecht S. High impact running improves learning. Neurobiology of Learning and Memory. 2007;87:597–609. doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ku B, Tie L, Yao H, Jiang W, Ma X, Li X. Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Research. 2006;1122:56–64. doi: 10.1016/j.brainres.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Zheng H, Liu Y, Li W, Yang B, Chen D, Wang X, Jiang Z, Wang H, Wang Z, Cornelisson G, Halberg F. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behavioral Brain Research. 2006;168:47–55. doi: 10.1016/j.bbr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


