Abstract
Matrix metalloproteinase-2 (MMP-2), a ubiquitously expressed zinc-dependent endopeptidase, and poly(ADP-ribosyl) polymerase (PARP), a nuclear enzyme regulating DNA repair, are activated by nitroxidative stress associated with various pathologies. As MMP-2 plays a detrimental role in heart injuries resulting from enhanced nitroxidative stress, where PARP and MMP inhibitors are beneficial, we hypothesized that PARP inhibitors may affect MMP-2 activity. Using substrate degradation assays to determine MMP-2 activity we found that four PARP inhibitors (3-AB, PJ-34, 5-AIQ, and EB-47) inhibited 64 kDa MMP-2 in a concentration-dependent manner. The IC50 values of PJ-34 and 5-AIQ were in the high micromolar range and comparable to those of known MMP-2 inhibitors doxycycline, minocycline or o-phenanthroline, whereas those for 3-AB and EB-47 were in the millimolar range. Co-incubation of PARP inhibitors with doxycycline showed an additive inhibition of MMP-2 that was significant for 3-AB alone. These data demonstrate that the protective effects of some PARP inhibitors may include inhibition of MMP-2 activity.
Keywords: Matrix metalloproteinase-2, Oxidative stress, Peroxynitrite, Poly(ADP-ribosyl)polymerase
Introduction
Matrix metalloproteinases (MMPs), a family of 28 structurally related, multidomain Zn-dependent endopeptidases, are key enzymes in the development and remodeling of tissues and organs, including embryogenesis and angiogenesis [1,2]. MMPs are first expressed intracellularly and maintained in a latent form by an interaction between a conserved cysteine (Cys) residue in the autoinhibitory propeptide domain and a Zn2+ in the catalytic site [3]. The disruption of this interaction by proteolytic removal of the autoinhibitory propeptide domain or by sulfhydryl-reactive compounds has been suggested to trigger enzyme activation [4].
Increasing evidence suggests that the activity of MMPs is enhanced in several cardiovascular diseases, including ischemic heart disease, heart failure, stroke, and atherosclerosis [1,5–7]. MMP-2 is expressed in the heart at substantial levels and its activity is increased in myocardial ischemia–reperfusion injury [5–8]. An important mediator of acute cardiac contractile failure is nitroxidative stress, which occurs during heart exposure to ischemia–reperfusion [9] or pro-inflammatory cytokines [10], and involves an overproduction of nitroxidative species, particularly peroxynitrite [8–11]. We recently reported that MMP-2 can undergo activation by peroxynitrite that involves the covalent modification of the critical Cys residue in the propeptide domain [12].
Poly(ADP-ribose)polymerases (PARPs) are a family of 17 Zn-containing enzymes, present in the nuclei of all eukaryotes, that participate in the regulation of DNA repair, gene transcription, genomic stability, and cell death [13,14]. The activity of PARP-1 (the major contributor to PARP activity in higher eukaryotes) is increased about 500-fold in response to DNA strand breaks [13]. PARPs mediate the post-translational modification of nuclear proteins, particularly histones, to allow them to unpack the chromatin at the site of the DNA strand break, and expose the damage site to nuclear repair proteins. As a consequence of their important cellular functions, PARPs have received attention as major targets for drug design for the treatment of cancer, diabetes, inflammation, retroviral infection, and cardiovascular diseases [14].
PARPs and MMPs can both be activated by nitroxidative stress associated with cardiac diseases [1,8]. PARP inhibitors substantially improve the outcome of myocardial damage following ischemia–reperfusion, hypoxia-reoxygenation, cardiopulmonary bypass, cardiac transplantation, or doxorubicin-induced injury [8,14–16] by reducing PARP over-activation and cell death [17,18]. Pharmacological inhibition of MMP-2 has beneficial effects in injury induced by ischemia–reperfusion [5], pro-inflammatory cytokines [19], direct infusion of peroxynitrite [20], or myocardial infarct [21,22].
Since there is evidence suggesting a cross-talk between PARP and MMP-2, we hypothesized that the beneficial effects of PARP inhibitors may also involve the direct inhibition of MMP-2. Thus, we investigated the effect of four PARP inhibitors (3-AB, 5-AIQ, PJ-34, and EB-47) on the activity of MMP-2 and compared the inhibitory potencies of these compounds with those of known MMP inhibitors, including doxycycline, minocycline, o-phenanthroline and GM 6001.
Materials and methods
All reagents were of analytical grade and unless otherwise specified were purchased from Sigma–Aldrich (Oakville, ON). Human recombinant 64 kDa MMP-2 was obtained from Calbiochem (San Diego, CA); OmniMMP® fluorogenic peptide substrate from Biomol (Plymouth Meeting, PA); and Coomassie Blue R-250 from Bio-Rad (Hercules, CA). 3-Aminobenzamide (3-AB), 2-(dimethylamino)-N-(5,6-dihydro-6-oxophenanthridin-2-yl)acetamide (PJ-34), 5-aminoisoquinolinone (5-AIQ) and 1-piperazineacetamide-4-[1-(6-amino-9H-purin-9-yl)-1-deoxy-d-ribofuranuron]-N-(2,3-dihydro-1H-isoindol-4-yl)-1-one (EB-47) were purchased from Sigma–Aldrich, Calbiochem or Inotek Pharmaceuticals (Beverly, MA) and fresh stock solutions of these compounds were prepared in 1% DMSO or distilled water.
Kinetic analysis of MMP-2 activity in the presence of PARP and MMP inhibitors
In the OmniMMP kinetic assay the enzyme is present in a homogenous, aqueous buffer that is optimal for enzyme activity and, importantly, the enzyme is not subjected to a denaturation process. The hydrolysis of the OmniMMP® fluorogenic substrate (15 µM, prepared in 1.8% v/v DMSO) by 64 kDa MMP-2 (0.2 nM in 50 mM Tris, pH 7.6, 0.5 or 10 mM CaCl2, 0.05% Brij-35, 1 nM or 10µM ZnSO4) was measured at 37 °C in a plate reader-based protocol [23] in the presence of PARP (PJ-34, 5-AIQ, 3-AB, and EB-47) or MMP (doxycycline, minocycline, GM 6001, and o-phenanthroline) inhibitors. Assays were made in a total volume of 120 µl in black polystyrene half-area plates (Corning, NY), and contained MMP-2 (60 µl in 2 × reaction buffer) and substrate (60 µl) or DMSO vehicle. Fluorescence associated with (7-meth-oxycoumarin-4-yl)acetyl-tagged cleavage product was measured every 30 s for 1 h (λex 328 nm, λem 393 nm) in a SPECTRAmax Gemini XPS (Molecular Devices, Sunnyvale, CA) fluorescence plate reader. PJ-34 concentrations greater than 100 µM interfered with the fluorescence readings (data not shown) and the effects of the inhibitor were not evaluated at these concentrations. The rate of product formation in each well was determined through linear regression of the experimental data (r2 > 0.98) using SOFTmax Pro 4.8 software (Molecular Devices, Sunnyvale, CA). Appropriate lag times, to preclude data prior to equilibration at 37 °C, and end times, to preclude data following a loss of linearity, were entered manually prior to linear regression of data to obtain slopes. The experimental rates in the presence of inhibitors were normalized to the rates of the vehicle controls. All experiments were performed in three to five replicates and the inhibitor concentrations required to produce 50% enzyme inhibition (IC50) were determined from the non-linear curve fit of data using GraphPad Prism version 4.03 (GraphPad Software, San Diego, CA).
Estimation of MMP-2 activity in the presence of PARP and MMP inhibitors
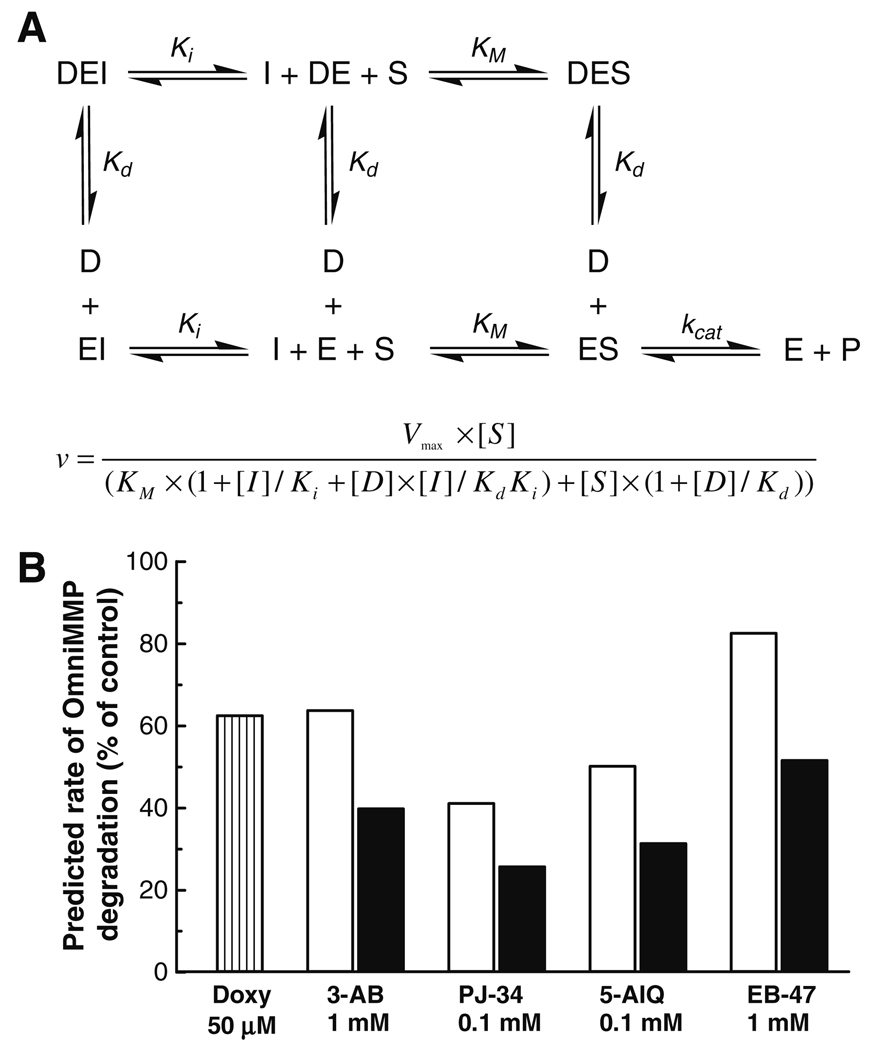
MMP-2 inhibition data were generated with an equation (Fig. 4A) which includes the following assumptions: (i) the effects of PARP inhibitors are competitive with the substrate; (ii) doxycycline binds to a site distinct from the catalytic site that results in complete loss of enzyme activity [24]; and (iii) binding of doxycycline does not affect the affinity of either the substrate or of other inhibitors, and vice versa. KM was fixed at 33.3 µM [23], the enzyme activity at 15 µM substrate is considered here as 100%, the concentration of doxycycline and PARP inhibitors are the same as the ones in the co-incubation experiment (Fig. 3), and the Ki values were calculated from the Cheng–Prusoff equation using the IC50 values.
Fig. 4.
(A) Model and equation of MMP-2 inhibition by simultaneous exposure of MMP-2 to PARP inhibitors and doxycycline; I, PARP inhibitor; D, doxycycline; S, substrate; P, products; Kd, inhibitor constant for doxycycline; Ki, inhibitor constant for each PARP inhibitor; KM, Michaelis constant. (B) Theoretical rates of MMP-2 inhibition by PARP inhibitors co-incubated with 50 µM doxycycline calculated based on the equation in (A).
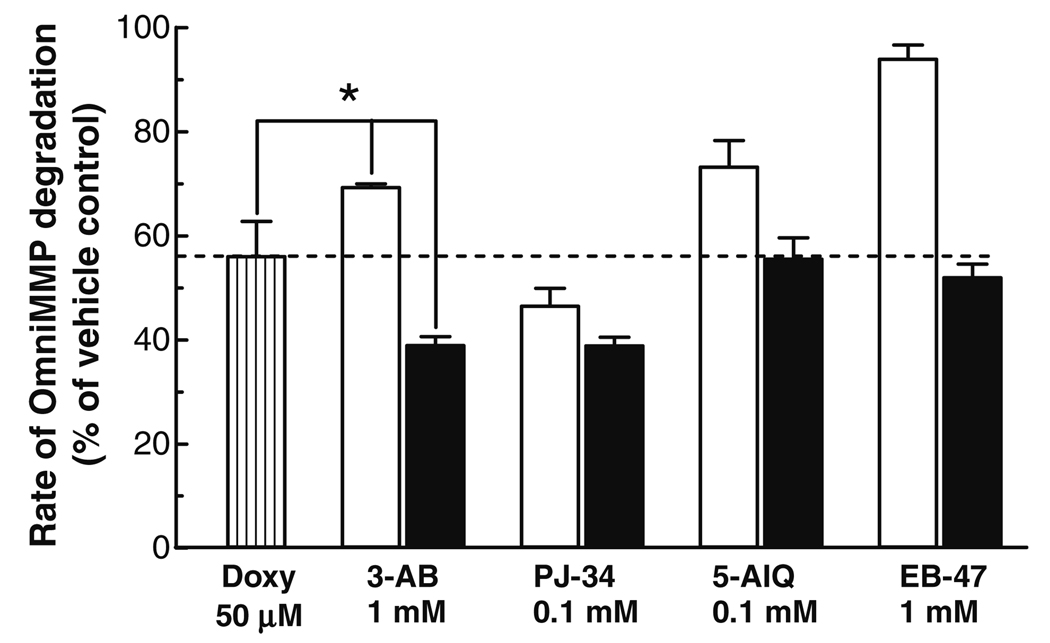
Fig. 3.
Effect of PARP and doxycycline co-incubation on MMP-2 activity. PARP inhibitors were incubated at 37 °C alone (open bars) or together with 50 µM doxycycline (Doxy) (closed bars) in the presence of 0.2 nM MMP-2, 15 µM OmniMMP fluorogenic substrate and 50 mM Tris buffer, pH 7.6, containing 10 mM CaCl2 and 10 µM ZnSO4. Data represent means ± SD of three to five replicate determinations. *Statistically significant differences compared to doxycycline or 3-AB treatment; P < 0.05, one-way ANOVA with Newman–Keuls post hoc test.
Gelatinolytic activity of MMP-2 in the presence of PARP and MMP inhibitors
Gelatinolytic activity of 64 kDa MMP-2 by zymography was performed as previously described [5] with some modifications. Briefly, 10 ng of 64 kDa MMP-2 were electrophoresed on an 8% polyacrylamide gel containing 2 mg/ml gelatin for 60–90 min (150 V, ambient temperature). After washing with Triton X-100 (2.5% v/v, 3 × 20 min), the gels were cut and one electrophoresed protein per strip was separately incubated overnight at 37 °C in 20 ml zymography buffer in the absence or presence of PJ-37, EB-47, 5-AIQ (10, 30, and 100 µM), and 3-AB (30, 100, and 1000 µM). For comparison, gel strips were incubated in the absence or presence of MMP inhibitors, i.e. o-phenanthroline (3, 10, and 30 µM), doxycycline (10, 30, and 100 µM) and GM 6001 (0.3, 1, and 3 µM). Gels were stained with 0.05% Coomassie blue and subsequently destained. Gelatinolytic activities were detected as transparent bands against the blue-stained background.
Statistical analysis
All data are presented as mean ± standard deviation (SD). Comparisons between multiple groups were performed using one-way ANOVA followed by Newman–Keuls post hoc test. Two-tailed P values <0.05 were considered statistically significant.
Results
Effect of Zn2+ on MMP-2 activity measured by OmniMMP assay
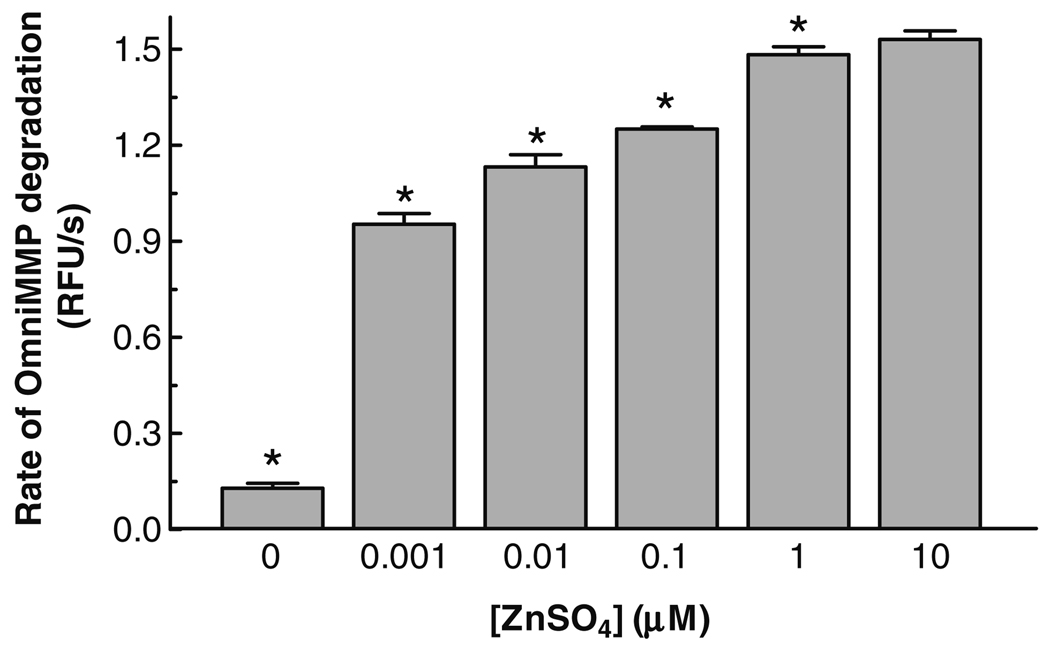
MMP-2 is a Zn2+-dependent endopeptidase [25] and some MMP inhibitors can complex divalent cations. Thus, we investigated the effect of different Zn2+ concentrations on the activity of MMP-2 measured by its proteolysis of a small fluorogenic peptide substrate, OmniMMP. We observed that when the assay buffer did not contain ZnSO4, the enzyme activity was minimal, but when 1 nM ZnSO4 was present in the assay buffer there was an approximately 10-fold increase in enzyme activity. Increasing concentrations of ZnSO4 resulted in a non-linear concentration-dependent increase of MMP-2 activity, which reached a maximal plateau at 1–10 µM ZnSO4 (Fig. 1). Consequently, the effects of PARP or MMP inhibitors were determined at ZnSO4 concentrations of 1 nM, a concentration that should minimally affect the free concentration of any chelating compound, and at 10 µM, a concentration required for maximal enzyme activity.
Fig. 1.
Effect of different ZnSO4 concentration on MMP-2 activity measured in the OmniMMP® fluorogenic substrate kinetic assay. ZnSO4 was incubated with 0.2 nM 64 kDa MMP-2 and 15 µM OmniMMP fluorogenic substrate at 37 °C in 50 mM Tris, pH 7.6, containing 10 mM CaCl2. Data represent means ± SD of triplicate determinations. *Statistically significant difference compared to the 10 µM ZnSO4 group, P < 0.05, one-way ANOVA with Newman–Keuls post hoc test.
Effect of PARP and MMP inhibitors on MMP-2 activity measured by OmniMMP assay
Initially we tested the inhibitory effects of PARP inhibitors, in comparison with MMP inhibitors, using gelatin zymography. This was inconclusive (Fig. 1S) and since zymography is not suitable for kinetic studies we tested the effects of PARP and MMP-2 inhibitors using a kinetic assay in aqueous solution.
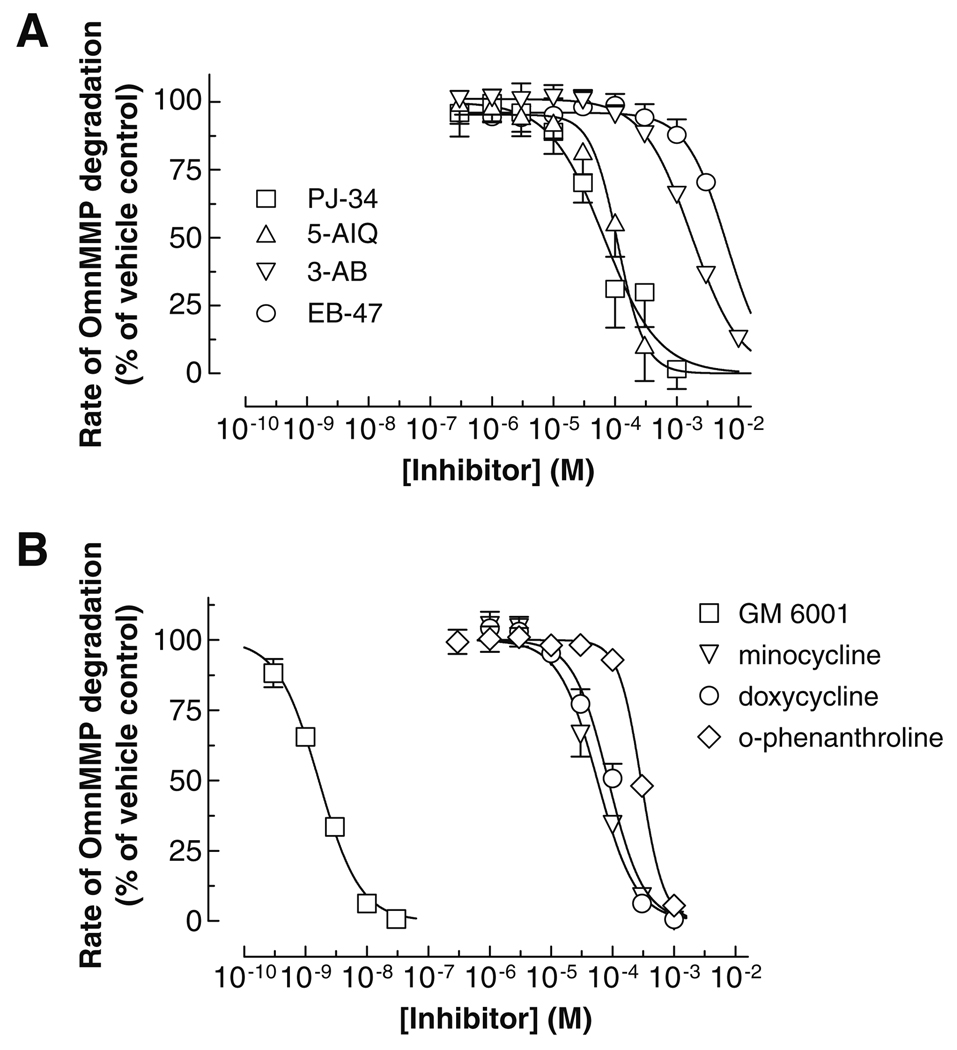
Using 1 nM and 10 µM ZnSO4 the OmniMMP fluorescent kinetic assay was used to characterize the inhibitory potencies (IC50s) of PARP inhibitors on MMP-2. In the presence of 10 µM Zn2+, the PARP inhibitors blocked MMP-2 activity in a concentration-dependent manner, with the following rank order of IC50 values: PJ-34 < 5-AIQ << 3-AB < EB-47 (Fig. 2A), comparable to the effects of MMP inhibitors minocycline and doxycycline (Fig. 2B). PJ-34 and 5-AIQ were comparable in their IC50s, which were significantly lower than those of 3-AB and EB-47 (Table 1).
Fig. 2.
Concentration-dependent effect of PARP inhibitors (A) and MMP inhibitors (B) on MMP-2 activity using the OmniMMP® fluorogenic substrate kinetic assay. PARP and MMP inhibitors were incubated with 0.2 nM 64 kDa MMP-2 and 15 µM OmniMMP fluorogenic substrate at 37 °C in 50 mM Tris buffer, pH 7.6, containing 10 mM CaCl2 and 10 µM ZnSO4. Data represent means ± SD of three to five replicate determinations and were fitted to sigmoidal (variable slope) curves.
Table 1.
Potencies of tested compounds at inhibiting MMP-2.
| Inhibitor | IC50 ± SD (µM) | |
|---|---|---|
| 10 µM ZnSO4 | 1 nM ZnSO4 | |
| PJ-34 | 56 ± 14 | 27 ± 10 |
| 5-AIQ | 102 ± 34 | n.d. |
| 3-AB | 1760 ± 220* | 1000 ± 200 |
| EB-47 | >3000 | n.d. |
| o-Phenanthroline | 293 ± 14 | 116 ± 9 |
| Minocycline | 57 ± 6 | n.d. |
| Doxycycline | 86 ± 13 | 93 ± 12 |
| GM 6001 | 0.0018 ± 0.0002* | 0.0063 ± 0.0006 |
Statistically significant difference compared to any other inhibitor, P < 0.05, one-way ANOVA with Newman–Keuls post hoc test. Reaction conditions: 50 mM Tris, pH 7.6, containing 10 mM CaCl2, 0.05% Brij-35, 10 µM ZnSO4; 37°C. n.d., not determined.
Since MMP-2 is a Zn2+-dependent enzyme [25], it is possible that MMP-2 activity and potency of MMP inhibitors, which are also metal ion chelators, may be affected by the Zn2+ concentration in the assay buffer. At 10 µM ZnSO4 the potencies of PJ-34 and 3-AB were not significantly different than those at 1 nM ZnSO4 (Table 1). Interestingly, there was a significant 2.5-fold decrease in the potency of o-phenanthroline at 10 µM ZnSO4 compared to o-phenanthroline’s potency at 1 nM ZnSO4, suggesting that, at least in part, some o-phenanthroline was unavailable for inhibiting MMP-2 due to the formation of a Zn2+ complex. The IC50 values of MMP inhibitors in the presence of 10 µM Zn2+ increased in the following rank order: GM 6001 <<< minocycline < doxycycline < o-phenanthroline (Table 1).
In order to investigate whether there is an additive effect of PARP inhibitors in the presence of a MMP inhibitor, PARP inhibitors were incubated with doxycycline at concentrations that would produce significant but not more than 50% inhibition of MMP-2 when incubated alone. Co-incubation of 50 µM doxycycline with PARP inhibitors revealed that there is a significant (P < 0.05, one-way ANOVA with Newman–Keuls post hoc test) potentiation (≈45% increase) of the MMP-2 inhibitory effect only for 3-AB compared to doxycycline alone (Fig. 3).
Our theoretical assumptions (Fig. 4) for a possible mode of how PARP inhibitors and doxycycline inhibit MMP-2 revealed a good concordance with the experimental data for PARP inhibitors alone, or for the co-incubation of PARP inhibitors with doxycycline (Fig. 3 and Fig 4), except for 5-AIQ.
Discussion
The present study demonstrates, using a fluorescence kinetic assay, that two PARP inhibitors PJ-34 and 5-AIQ inhibit MMP-2 at comparable levels with pan-specific MMP inhibitors (e.g. doxycycline and o-phenanthroline), and that the PARP inhibitor 3-AB demonstrates a significant additive effect in inhibiting MMP-2 when co-administered with doxycycline.
The affinity of most known MMP inhibitors relies on: (i) the specific chelation of the catalytic Zn2+ (e.g. hydroxamate groups), and (ii) the nature of the specificity loop in the large and hydrophobic S1′ pocket which surrounds the catalytic Zn2+ [26–28]. Recently designed MMP inhibitors do not interact with the catalytic Zn2+, but bind in the S1′ pocket and interact with amino acid residues (e.g. Tyr, Thr, Phe, and Met) in the specificity loop, thereby interfering with the enzyme’s catalytic activity [29]. The tested PARP inhibitors contain planar electron-rich aromatic rings that establish strong electronic interactions with residues (e.g. Tyr) within PARP’s active site [30]. The observed MMP-2 inhibitory effect may be the result of such interactions between PARP inhibitors and domains on the enzyme that control the conformation at the catalytic site. This assumption is supported by the curve fittings to an equation derived from the theoretical model we used (Fig. 4). The differences between the theoretical and experimental data could reflect that binding of doxycycline affects the enzyme’s affinity for substrates and for PARP inhibitors, and vice versa. However, attempts to prove this kinetically would be futile due to the large number of associated variables.
Zn2+ is implicated in various important cellular functions and in the heart affects cardiac muscle differentiation and regeneration, cardiac conductance, acute stress responses, and recovery of transplanted heart mechanical function [31]. Low serum levels of Zn2+ are associated with an increased incidence of cardiovascular disease [32,33]. Furthermore, supplementation with zinc ionophores in animal models improved the recovery of hearts subjected to ischemia–reperfusion injury [34,35]. Considering our observation that the activity of MMP-2 is increased by nanomolar concentrations of Zn2+ (Fig. 1) it may be possible that the activity of MMP-2 in the injured heart is modulated by intracellular Zn2+. Notably, the MMP-2 inhibitory potency of PJ-34 and 3-AB was not influenced by Zn2+ concentration (Fig. 2) suggesting that these compounds may interact with enzyme domains other than the catalytic site.
The apparent discrepancy in the behavior of PARP inhibitors in the zymography (Fig. 1S) versus the kinetic assay (Fig. 2) can be explained considering the substrate concentration and the inhibition mechanism. The IC50 of doxycycline, a non-competitive inhibitor of MMP-2 [24], is not influenced by substrate concentration. In contrast, PARP inhibitors compete with the MMP-2 substrate by possibly binding to a site which overlaps the substrate binding site, with the consequence that the degree of inhibition is proportional to the substrate concentration. Considering the substrate concentration in gelatin zymography (20 µM, assuming a MW of 100 kDa) and the KM for gelatin (89.3 nM [36]), IC50s in the millimolar range for PARP inhibitors (e.g. 9.2 mM for PJ-34) would be necessary.
The effectiveness of some PARP inhibitors is different in vitro and in vivo. For example, 3-AB displays a potency for PARP inhibition in the micromolar range in vitro and in the millimolar range in animal models [30,37]. PJ-34 and 3-AB were also found neuroprotective in vivo at micromolar and millimolar concentrations [38], similar to those used in the present study. It is possible that part of the observed in vivo effectiveness of PARP inhibitors may involve the attenuation of intracellular MMP-2 activity.
The potencies demonstrated by some second generation PARP inhibitors for inhibiting MMP-2 may represent an alternative approach for designing specific MMP-2 inhibitors, or for combined MMP-2/PARP inhibitor therapeutic strategies with potential benefits in cardiac diseases or inflammation.
Supplementary Material
Acknowledgments
This project was supported by Canadian Institute of Health Research operating grants MOP-77526 to R.S. and MOP-77529 to A.H., and by the Intramural Program of NIH/NIAAA to P.P. R.S. is a Senior Scientist of the Alberta Heritage Foundation for Medical Research. A.C.N. is a recipient of Alberta Heritage Foundation for Medical Research and Heart and Stroke Foundation of Canada Fellowships. We thank Owen Degenhardt for technical assistance.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbrc.2009.07.080.
References
- 1.Schulz R. Intracellular targets of matrix metalloproteinase-2 in cardiac disease: rationale and therapeutic approaches. Annu. Rev. Pharmacol. Toxicol. 2007;47:211–242. doi: 10.1146/annurev.pharmtox.47.120505.105230. [DOI] [PubMed] [Google Scholar]
- 2.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 3.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. USA. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgunova E, Tuuttila A, Bergmann U, Tryggvason K. Structural insight into the complex formation of latent matrix metalloproteinase 2 with tissue inhibitor of metalloproteinase 2. Proc. Natl. Acad. Sci. USA. 2002;99:7414–7419. doi: 10.1073/pnas.102185399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung PY, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R. Matrix metalloproteinase-2 contributes to ischemia–reperfusion injury in the heart. Circulation. 2000;101:1833–1839. doi: 10.1161/01.cir.101.15.1833. [DOI] [PubMed] [Google Scholar]
- 6.Lalu MM, Pasini E, Schulze CJ, Ferrari-Vivaldi M, Ferrari-Vivaldi G, Bachetti T, Schulz R. Ischaemia–reperfusion injury activates matrix metalloproteinases in the human heart. Eur. Heart J. 2005;26:27–35. doi: 10.1093/eurheartj/ehi007. [DOI] [PubMed] [Google Scholar]
- 7.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol. Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 8.Pacher P, Schulz R, Liaudet L, Szabo C. Nitrosative stress and pharmacological modulation of heart failure. Trends Pharmacol. Sci. 2005;26:302–310. doi: 10.1016/j.tips.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasmin W, Strynadka KD, Schulz R. Generation of peroxynitrite contributes to ischemia–reperfusion injury in isolated rat hearts. Cardiovasc. Res. 1997;33:422–432. doi: 10.1016/s0008-6363(96)00254-4. [DOI] [PubMed] [Google Scholar]
- 10.Ferdinandy P, Danial H, Ambrus I, Rothery RA, Schulz R. Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circ. Res. 2000;87:241–247. doi: 10.1161/01.res.87.3.241. [DOI] [PubMed] [Google Scholar]
- 11.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viappiani S, Nicolescu AC, Holt A, Sawicki G, Crawford BD, Leon H, VanMulligen T, Schulz R. Activation and modulation of 72 kDa matrix metlloproteinase-2 by peroxynitrite and glutathione. Biochem. Pharmacol. 2009;77:826–834. doi: 10.1016/j.bcp.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat. Rev. Mol. Cell. Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 14.Pacher P, Szabo C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc. Drug Rev. 2007;25:235–260. doi: 10.1111/j.1527-3466.2007.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pacher P, Liaudet L, Mabley J, Komjati K, Szabo C. Pharmacologic inhibition of poly(adenosine diphosphate-ribose) polymerase may represent a novel therapeutic approach in chronic heart failure. J. Am. Coll. Cardiol. 2002;40:1006–1016. doi: 10.1016/s0735-1097(02)02062-4. [DOI] [PubMed] [Google Scholar]
- 16.Pacher P, Szabo C. Role of the peroxynitrite–poly(ADP-ribose) polymerase pathway in human disease. Am. J. Pathol. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pacher P, Liaudet L, Bai P, Virag L, Mabley JG, Hasko G, Szabo C. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J. Pharmacol. Exp. Ther. 2002;300:862–867. doi: 10.1124/jpet.300.3.862. [DOI] [PubMed] [Google Scholar]
- 18.Pacher P, Liaudet L, Mabley JG, Cziraki A, Hasko G, Szabo C. Beneficial effects of a novel ultrapotent poly(ADP-ribose) polymerase inhibitor in murine models of heart failure. Int. J. Mol. Med. 2006;17:369–375. [PMC free article] [PubMed] [Google Scholar]
- 19.Gao CQ, Sawicki G, Suarez-Pinzon WL, Csont T, Wozniak M, Ferdinandy P, Schulz R. Matrix metalloproteinase-2 mediates cytokine-induced myocardial contractile dysfunction. Cardiovasc. Res. 2003;57:426–433. doi: 10.1016/s0008-6363(02)00719-8. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Sawicki G, Schulz R. Peroxynitrite-induced myocardial injury is mediated through matrix metalloproteinase-2. Cardiovasc. Res. 2002;53:165–174. doi: 10.1016/s0008-6363(01)00445-x. [DOI] [PubMed] [Google Scholar]
- 21.Villarreal FJ, Griffin M, Omens J, Dillmann W, Nguyen J, Covell J. Early short-term treatment with doxycycline modulates postinfarction left ventricular remodeling. Circulation. 2003;108:1487–1492. doi: 10.1161/01.CIR.0000089090.05757.34. [DOI] [PubMed] [Google Scholar]
- 22.Matsumura S, Iwanaga S, Mochizuki S, Okamoto H, Ogawa S, Okada Y. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J. Clin. Invest. 2005;115:599–609. doi: 10.1172/JCI22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sariahmetoglu M, Crawford BD, Leon H, Sawicka J, Li L, Ballermann BJ, Holmes C, Berthiaume LG, Holt A, Sawicki G, Schulz R. Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J. 2007;21:2486–2495. doi: 10.1096/fj.06-7938com. [DOI] [PubMed] [Google Scholar]
- 24.Garcia RA, Pantazatos DP, Gessner CR, Go KV, Woods VL, Jr, Villarreal FJ. Molecular interactions between matrilysin and the matrix metalloproteinase inhibitor doxycycline investigated by deuterium exchange mass spectrometry. Mol. Pharmacol. 2005;67:1128–1136. doi: 10.1124/mol.104.006346. [DOI] [PubMed] [Google Scholar]
- 25.Morgunova E, Tuuttila A, Bergmann U, Isupov M, Lindqvist Y, Schneider G, Tryggvason K. Structure of human pro-matrix metalloproteinase-2: activation mechanism revealed. Science. 1999;284:1667–1670. doi: 10.1126/science.284.5420.1667. [DOI] [PubMed] [Google Scholar]
- 26.Engel CK, Pirard B, Schimanski S, Kirsch R, Habermann J, Klingler O, Schlotte V, Weithmann KU, Wendt KU. Structural basis for the highly selective inhibition of MMP-13. Chem. Biol. 2005;12:181–189. doi: 10.1016/j.chembiol.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Skiles JW, Gonnella NC, Jeng AY. The design, structure, and therapeutic application of matrix metalloproteinase inhibitors. Curr. Med. Chem. 2001;8:425–474. doi: 10.2174/0929867013373417. [DOI] [PubMed] [Google Scholar]
- 28.Feng Y, Likos JJ, Zhu L, Woodward H, Munie G, McDonald JJ, Stevens AM, Howard CP, De Crescenzo GA, Welsch D, Shieh HS, Stallings WC. Solution structure and backbone dynamics of the catalytic domain of matrix metalloproteinase-2 complexed with a hydroxamic acid inhibitor. Biochim. Biophys. Acta. 2002;1598:10–23. doi: 10.1016/s0167-4838(02)00307-2. [DOI] [PubMed] [Google Scholar]
- 29.Johnson AR, Pavlovsky AG, Ortwine DF, Prior F, Man CF, Bornemeier DA, Banotai CA, Mueller WT, McConnell P, Yan C, Baragi V, Lesch C, Roark WH, Wilson M, Datta K, Guzman R, Han HK, Dyer RD. Discovery and characterization of a novel inhibitor of matrix metalloprotease-13 that reduces cartilage damage in vivo without joint fibroplasia side effects. J. Biol. Chem. 2007;282:27781–27791. doi: 10.1074/jbc.M703286200. [DOI] [PubMed] [Google Scholar]
- 30.Woon EC, Threadgill MD. Poly(ADP-ribose)polymerase inhibition—where now? Curr Med. Chem. 2005;12:2373–2392. doi: 10.2174/0929867054864778. [DOI] [PubMed] [Google Scholar]
- 31.Korichneva I. Zinc dynamics in the myocardial redox signaling network. Antioxid. Redox Signal. 2006;8:1707–1721. doi: 10.1089/ars.2006.8.1707. [DOI] [PubMed] [Google Scholar]
- 32.Lee DH, Folsom AR, Jacobs DR., Jr Iron, zinc, and alcohol consumption and mortality from cardiovascular diseases: the Iowa Women’s Health Study. Am. J. Clin. Nutr. 2005;81:787–791. doi: 10.1093/ajcn/81.4.787. [DOI] [PubMed] [Google Scholar]
- 33.Soinio M, Marniemi J, Laakso M, Pyorala K, Lehto S, Ronnemaa T. Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care. 2007;30:523–528. doi: 10.2337/dc06-1682. [DOI] [PubMed] [Google Scholar]
- 34.Karagulova G, Yue Y, Moreyra A, Boutjdir M, Korichneva I. Protective role of intracellular zinc in myocardial ischemia/reperfusion is associated with preservation of protein kinase C isoforms. J. Pharmacol. Exp. Ther. 2007;321:517–525. doi: 10.1124/jpet.107.119644. [DOI] [PubMed] [Google Scholar]
- 35.Powell SR, Hall D, Aiuto L, Wapnir RA, Teichberg S, Tortolani AJ. Zinc improves postischemic recovery of isolated rat hearts through inhibition of oxidative stress. Am. J. Physiol. 1994;266:H2497–H2507. doi: 10.1152/ajpheart.1994.266.6.H2497. [DOI] [PubMed] [Google Scholar]
- 36.Young TN, Pizzo SV, Stack MS. A plasma membrane-associated component of ovarian adenocarcinoma cells enhances the catalytic efficiency of matrix metalloproteinase-2. J. Biol. Chem. 1995;270:999–1002. doi: 10.1074/jbc.270.3.999. [DOI] [PubMed] [Google Scholar]
- 37.Curtin NJ. PARP inhibitors for cancer therapy. Expert Rev. Mol. Med. 2005;7:1–20. doi: 10.1017/S146239940500904X. [DOI] [PubMed] [Google Scholar]
- 38.Goebel DJ, Winkler BS. Blockade of PARP activity attenuates poly(ADP-ribosyl)ation but offers only partial neuroprotection against NMDA-induced cell death in the rat retina. J. Neurochem. 2006;98:1732–1745. doi: 10.1111/j.1471-4159.2006.04065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.