Abstract
The Tec-family protein tyrosine kinase IL-2–inducible T cell kinase (ITK) mediates T cell activation, as does the adaptor protein SLP-76 (SH2-domain–containing leukocyte protein of 76 kD), which forms a complex with ITK and other intracellular signaling enzymes. One of these enzymes is phospholipase C–γ1 (PLC-γ1), which mediates T cell receptor (TCR)–stimulated intracellular calcium mobilization leading to the activation of transcription factors such as nuclear factor of activated T cells. The Src-family tyrosine kinase Lck and the Syk-family tyrosine kinase ζ chain–associated protein kinase of 70 kD (ZAP-70), together with ITK, are necessary for the phosphorylation of PLC-γ1 in response to TCR stimulation. ITK is thought to phosphorylate a specific tyrosine residue of PLC-γ1 that is required for its activation. The mechanism of activation of ITK appears to involve the interaction between SLP-76 and ITK, which not only initiates ITK activity but is also important to maintain the kinase activity of ITK. This suggests that SLP-76 acts as more than a neutral adaptor in mediating T cell activation; SLP-76 also directly influences the kinase activity of ITK, allowing ITK to phosphorylate PLC-γ1.
Signals from the T cell receptor (TCR) are required for T cell activation and the generation of an immune response. Critical for TCR stimulation is the resulting increase in intracellular calcium concentration ([Ca2+]i), which is important for the activation of transcription factors that regulate gene expression. The Tec-family protein tyrosine kinase IL-2–inducible T cell kinase (ITK) plays an important role in this pathway, stimulating the secretion of interleukin-2 (IL-2), as well as the T helper 2 (TH2) cytokines IL-4, -5, and -13 (1). ITK mediates TCR-stimulated increases in [Ca2+]i in part by phosphorylating and activating phospholipase C–γ1 (PLC-γ1) (2–5). Activated PLC-γ1 generates the second messengers inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) from the hydrolysis of phosphatidylinositol-4,5-bisphosphate (PIP2) (Fig. 1). Whereas IP3 triggers increases in [Ca2+]i, which leads to the activation of nuclear factor of activated T cells (NFAT) and nuclear factor κB (NF-κB) signaling pathways, DAG activates Ras-dependent signals, such as the extracellular signal–regulated kinase (ERK) pathway, which are important for the induction of cytokines such as IL-2 (6–8). SH2-domain–containing leukocyte protein of 76 kD (SLP-76), an adaptor protein that is critical for T cell development and function, is also required for PLC-γ1 activation (9). ITK and SLP-76 form a complex during T cell activation, and work by Bogin et al. sheds new light on the functional consequences of this interaction. This study has implications for our understanding of the mechanism of activation of ITK, as well as the role of the SLP-76 signaling complex in TCR signaling (10).
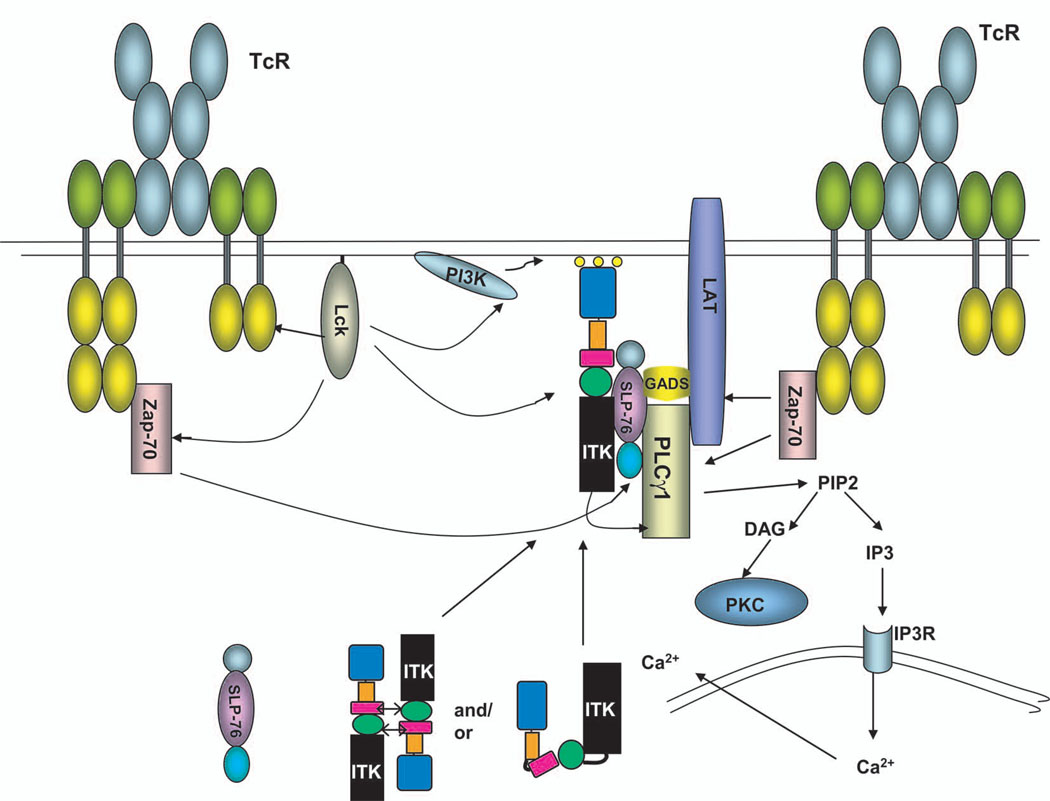
Fig. 1.
TCR signaling pathways leading to ITK and SLP-76. ITK may exist as a folded monomer or a dimer in its inactive state. Upon stimulation of the TCR, ITK is recruited to the plasma membrane, where it interacts with SLP-76 and becomes activated.
ITK contains an N-terminal pleckstrin homology (PH) domain; a Tec homology (TH) domain, which contains a Zn2+-binding BH [Bruton's tyrosine kinase (Btk) homology] motif; a proline-rich region (PRR), a Src homology 3 (SH3) domain; an SH2 domain; and a C-terminal kinase domain (Fig. 2A) (11). Stimulation of the TCR leads to the activation of the Src-family tyrosine kinases Lck and Fyn, which phosphorylate and activate phosphoinositide 3-kinase (PI3K) and the Syk-family tyrosine kinase member ζ chain–associated protein kinase of 70 kD (ZAP-70). Activated PI3K generates membrane phosphatidylinositol-3,4,5-trisphosphate (PIP3). ITK is recruited to the cell membrane through the interaction of its PH domain with PIP3, where it is phosphorylated by Lck (12). Although ZAP-70 is required for the activation of ITK, the precise role it plays has been unclear (13).
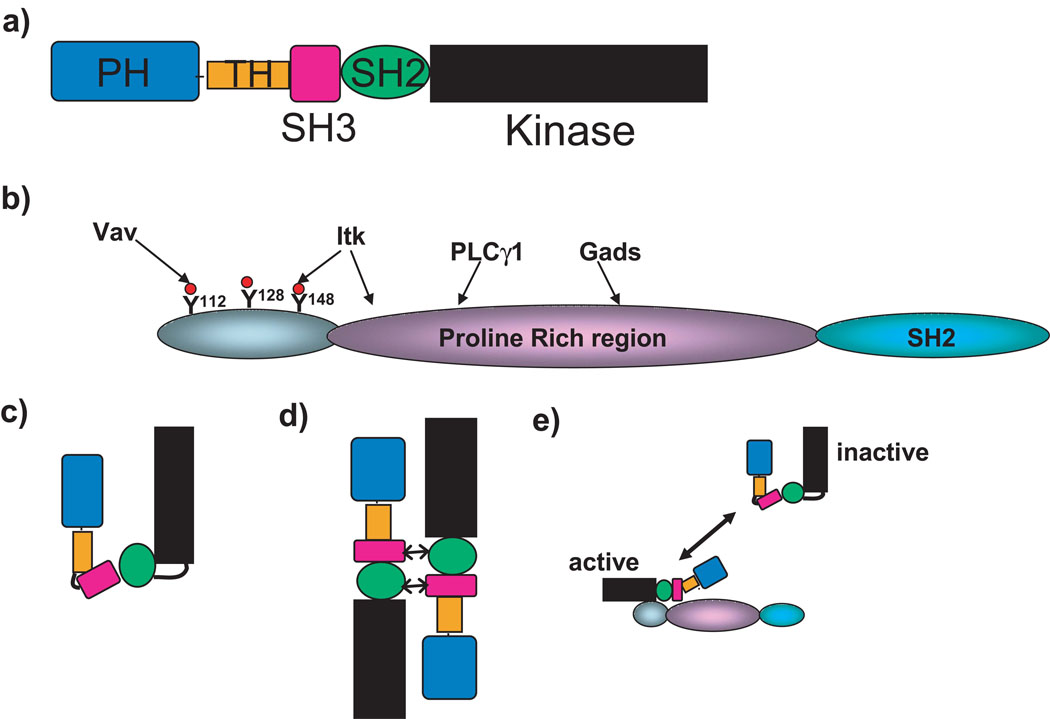
Fig. 2.
Proposed structure of ITK. (A) Structural domains of ITK. (B) Structural domains of SLP-76, including those sites implicated in interactions with Vav, ITK, PLC-γ1, and Gads. (C) Intramolecular folded conformation of inactive ITK. (D) Alternative head-to-tail dimer of inactive ITK. (E) Relationship between SLP-76 and ITK and the resulting effect on the kinase activity of ITK.
SLP-76 has an N-terminal acidic domain containing three tyrosine phosphorylation sites, a central PRR, and a C-terminal SH2 domain (Fig. 2B) (14, 15). The SH3 domain of PLC-γ1 interacts with the PRR of SLP-76, and this interaction is important for PLC-γ1 activation (16–18). The SH2 domain of ITK binds directly to Tyr145 within the N-terminus of SLP-76, and the SH3 domain of ITK binds the PRR of SLP-76 (19, 20). TCR-stimulated tyrosine phosphorylation and activation of PLC-γ1 are substantially reduced in the absence of either ITK or SLP-76, resulting in dramatically decreased Ca2+ mobilization (5, 9). The SH2 and SH3 domains of ITK and amino acids 157–223 (denoted the P-I region in the central PRR) of SLP-76 are important in mediating PLC-γ1 activation, indicating that multiple protein-protein interactions play a role in this process (2, 19, 21). It was thought that the functional consequence of the interaction between ITK and SLP-76 was the proper localization of ITK with PLC-γ1, because early work indicated that SLP-76 was not required for the activation of ITK (9).
The ITK, SLP-76, and PLC-γ1 complex also interacts with the integral membrane adaptor protein LAT (linker for activation of T cells) through the growth factor receptor–bound protein 2 (Grb2)–related adaptor protein Gads, which bridges SLP-76 and LAT. LAT, similar to ZAP-70, is also required for the activation of ITK; however, its role in this process remains unclear (13). Whereas the interaction between LAT and PLC-γ1 is essential for the membrane localization of PLC-γ1, it is the interaction between SLP-76 and ITK that activates PLC-γ1 (22). Bogin et al. show that the SLP-76:PLC-γ1:ITK complex is critical for the tyrosine phosphorylation of PLC-γ1 on Tyr783 (10), a site that is important for its activation (23). Of greater interest, the authors also suggest that the interaction with SLP-76 is critical for maintaining the catalytic activity of ITK. In the absence of SLP-76, TCR-stimulated activation of ITK was greatly reduced in both magnitude and duration. In vitro experiments showed that the majority of the active ITK in activated T cells was associated with SLP-76, because the removal of ITK from the immunoprecipitated SLP-76 complex by high salt elution resulted in a substantial loss of the catalytic activity of ITK (10). However, when the eluted ITK was desalted and added back to SLP-76, the kinase activity of ITK was recovered and SLP-76 was phosphorylated, although the site of phosphorylation is not known. Bogin et al. suggest a model similar to that for Src kinases, whereby ITK interacts with SLP-76 through its SH2 or SH3 domains, or both, thus allowing the maintenance of ITK in an active conformation. These findings have implications for our understanding of the structure of ITK and perhaps those of other Tec kinases.
Although the structure of full-length ITK is unknown, Src-family tyrosine kinases, which are similar in structure to Tec kinases (with the exception of the TH and PH domains), are maintained in an inactive conformation due to intramolecular interactions between their SH1, SH2, and SH3 domains and their kinase domains (24, 25). These interactions allosterically prevent the activation of the kinase domain. However, the kinase activity of Src kinases is increased because of either ligand binding to the inhibitory domains or tyrosine phosphorylation of the kinase domain, which release the inhibitory interactions from the kinase domain (24, 25). Unlike Src-family kinases, the conformation of ITK may be determined by both intramolecular and intermolecular interactions (Fig. 2, C and D) (26, 27). To date, two kinds of interactions have been shown. The first, unique to Tec-family kinases, is an interaction between the SH3 and PRR domains of ITK, which maintains ITK in a folded conformation (26). The second interaction occurs between the SH2 domain of one ITK molecule and the SH3 domain of a second ITK, which causes their dimerization in a head-to-tail configuration (27, 28). Both interactions may inhibit the catalytic activity of ITK by blocking the kinase domain of ITK and precluding its function. In addition, these ITK-ITK interactions may block the association of ITK with other signaling partners, such as SLP-76. However, ITK may form dimers only in the vicinity of receptors at the membrane that activate ITK [such as the inducible costimulator (ICOS)]; thus, it is unclear whether dimers of ITK are inactive (29). Nevertheless, when the TCR is stimulated, any intramolecular and intermolecular interactions within the cytoplasmic pool of ITK are disrupted because of competitive binding of ITK domains with other proteins in signaling complexes (19), presumably increasing the proportion of ITK in the active conformation. Through the interaction of SLP-76 with both the SH2 and SH3 domains of ITK, SLP-76 may determine the conformation and active state of ITK. The study by Bogin et al. suggests that the TCR-stimulated association between SLP-76 and ITK not only activated ITK but was also required to maintain the activity of ITK (Fig. 2E). Because ITK interacts with Tyr145 of SLP-76, the data also suggest that the phosphorylation of this residue or the interaction between the SH3 domain of ITK and SLP-76, or both, may play critical roles in the activation of ITK. In addition, the requirement for both LAT and ZAP-70 for the activation of ITK may be to allow the formation of a SLP-76–LAT complex and the phosphorylation of Tyr145 of SLP-76 by ZAP-70, which promotes the subsequent assembly of the SLP-76–ITK complex (30).
Because a large fraction of active ITK is associated with SLP-76, most of the substrates of ITK might be found within the SLP-76–containing complex. This includes not only PLC-γ1 but also Vav (a guanine nucleotide exchange factor for the Rho-family small guanosine triphosphatases), because Vav interacts with SLP-76. ITK phosphorylates Vav, and in the absence of Vav tyrosine phosphorylation of ITK is drastically reduced, suggesting that Vav is required for the activation of ITK (31–33). Whether SLP-76 is required for the interaction between Vav and ITK is not known; however, because both SLP-76 and Vav are required for the full activation of ITK and because they form a multiprotein complex, these findings emphasize the importance of the proper assembly of the SLP-76–containing signaling complex for the stimulation and maintenance of ITK activity. Fluorescence microscopy experiments in which T cells were stimulated on coverslips coated with antibody to TCR showed that a complex containing SLP-76 first clusters near an activated TCR and then moves to the center of the contact interface between the TCR and the coverslip containing the antibody to TCR (34, 35). It may be inferred that ITK accompanies SLP-76 in this movement, but it would be interesting to determine whether the maintenance of the activity of ITK through its interaction with SLP-76 is required or involved in this movement. These new findings suggest that the partnership between SLP-76 and ITK is critical to keep the music going for ITK and its substrates!
References
- 1.Berg LJ, Finkelstein LD, Lucas JA, Schwartzberg PA. Tec family kinases in T lymphocyte development and function. Annu. Rev. Immunol. 2005;23:549–600. doi: 10.1146/annurev.immunol.22.012703.104743. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Villar JJ, Kanner SB. Regulated association between the tyrosine kinase Emt/Itk/Tsk and phospholipase-C gamma 1 in human T lymphocytes. J. Immunol. 1999;163:6435–6441. [PubMed] [Google Scholar]
- 3.Liao XC, Littman DR, Weiss A. Itk and Fyn make independent contributions to T cell activation. J. Exp. Med. 1997;186:2069–2073. doi: 10.1084/jem.186.12.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao XC, Littman DR. Altered T cell receptor signaling and disrupted T cell development in mice lacking Itk. Immunity. 1995;3:757–769. doi: 10.1016/1074-7613(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 5.Liu KQ, Bunnell SC, Gurniak CB, Berg LJ. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J. Exp. Med. 1998;187:1721–1727. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roose J, Mollenauer M, Gupta VA, Stone J, Weiss A. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol. Cell. Biol. 2005;25:4426–4441. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebinu J, Stang SL, Teixeira C, Bottorff DA, Hooton J, Blumberg PM, Barry M, Bleakley RC, Ostergaard HL, Stone JC. RasGRP links T-cell receptor signaling to Ras. Blood. 2000;95:3199–3203. [PubMed] [Google Scholar]
- 8.Tsukamoto H, Irie A, Nishimura Y. B-Raf contributes to sustained extracellular signal-regulated kinase activation associated with interleukin-2 production stimulated through the T cell receptor. J. Biol. Chem. 2004;279:48457–48465. doi: 10.1074/jbc.M403087200. [DOI] [PubMed] [Google Scholar]
- 9.Yablonski D, Kuhne MR, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 10.Bogin Y, Ainey C, Beach D, Yablonski D. SLP-76 mediates and maintains activation of the Tec family kinase ITK via the T cell antigen receptor-induced association between SLP-76 and ITK. Proc. Natl. Acad. Sci. U.S.A. 2007;104:6638–6643. doi: 10.1073/pnas.0609771104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith CI, Islam TC, Mattsson PT, Mohamed AJ, Nore BF, Vihinen M. The Tec family of cytoplasmic tyrosine kinases: Mammalian Btk, Bmx, Itk, Tec, Txk and homologs in other species. Bioessays. 2001;23:436–446. doi: 10.1002/bies.1062. [DOI] [PubMed] [Google Scholar]
- 12.August A, Sadra A, Dupont B, Hanafusa H. Src-induced cell kinase (ITK) requires phosphatidylinositol 3-kinase activity and the Pleckstrin homology domain of inducible T cell kinase. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11227–11232. doi: 10.1073/pnas.94.21.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shan X, Wange RL. Itk/Emt/Tsk activation in response to CD3 cross-linking in Jurkat T cells requires ZAP-70 and Lat and is independent of membrane recruitment. J. Biol. Chem. 1999;274:29323–29330. doi: 10.1074/jbc.274.41.29323. [DOI] [PubMed] [Google Scholar]
- 14.Jackman JK, Motto DG, Sun Q, Tanemoto M, Turck CW, Peltz GA, Koretzky GA, Findell PR. Molecular cloning of SLP-76, a 76-kDa tyrosine phosphoprotein associated with Grb2 in T cells. J. Biol. Chem. 1995;270:7029–7032. doi: 10.1074/jbc.270.13.7029. [DOI] [PubMed] [Google Scholar]
- 15.Fang N, Motto DG, Ross SE, Koretzky GA. Tyrosines 113, 128, and 145 of SLP-76 are required for optimal augmentation of NFAT promoter activity. J. Immunol. 1996;157:3769–3773. [PubMed] [Google Scholar]
- 16.Singer AL, Bunnell SC, Obstfeld AE, Jordan MS, Wu JN, Myung PS, Samelson LE, Koretzky GA. Roles of the proline-rich domain in SLP-76 subcellular localization and T cell function. J. Biol. Chem. 2004;279:15481–15490. doi: 10.1074/jbc.M313339200. [DOI] [PubMed] [Google Scholar]
- 17.Deng L, Velikovsky CA, Swaminathan CP, Cho S, Mariuzza RA. Structural basis for recognition of the T cell adaptor protein SLP-76 by the SH3 domain of phospholipase C gamma1. J. Mol. Biol. 2005;352:1–10. doi: 10.1016/j.jmb.2005.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonen R, Beach D, Ainey C, Yablonski D. T cell receptor-induced activation of phospho lipase C-gamma1 depends on a sequence independent region of SLP-76. J. Biol. Chem. 2005;280:8364–8370. doi: 10.1074/jbc.M409437200. [DOI] [PubMed] [Google Scholar]
- 19.Bunnell SC, Diehn M, Yaffe MB, Findell PR, Cantley LC, Berg LJ. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J. Biol. Chem. 2000;275:2219–2230. doi: 10.1074/jbc.275.3.2219. [DOI] [PubMed] [Google Scholar]
- 20.Su YW, Zhang Y, Schweikert J, Koretzky GA, Reth M, Wienands J. Interaction of SLP adaptors with the SH2 domain of Tec family kinases. Eur. J. Immunol. 1999;29:3702–3711. doi: 10.1002/(SICI)1521-4141(199911)29:11<3702::AID-IMMU3702>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 21.Yablonski D, Kadlecek T, Weiss A. Identification of a phospholipase C-gamma1 (PLC-gamma1) SH3 domain-binding site in SLP-76 required for T-cell receptor-mediated activation of PLC-gamma1and NFAT. Mol. Cell. Biol. 2001;21:4208–4218. doi: 10.1128/MCB.21.13.4208-4218.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beach D, Gonen R, Bogin Y, Reischl IG, Yablonski D. Dual role of SLP-76 in mediating T cell receptor-induced activation of phospholipase C-gamma1. J. Biol. Chem. 2007;282:2937–2946. doi: 10.1074/jbc.M606697200. [DOI] [PubMed] [Google Scholar]
- 23.Serrano CJ, Graham L, DeBell K, Rawat R, Veri MC, Bonvini E, Rellahan BL, Reischl IG. A new tyrosine phosphorylation site in PLC gamma 1: The role of tyrosine 775 in immune receptor signaling. J. Immunol. 2005;174:6233–6237. doi: 10.4049/jimmunol.174.10.6233. [DOI] [PubMed] [Google Scholar]
- 24.Sicheri F, Moarefi I, Kuriyan J. Crystal structure of the Src family tyrosine kinase Hck. Nature. 1997;385:602–609. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- 25.Gonfloni S, Weijland A, Kretzschmar J, Superti-Furga G. Crosstalk between the catalytic and regulatory domains allows bidirectional regulation of Src. Nat. Struct. Biol. 2000;7:281–286. doi: 10.1038/74041. [DOI] [PubMed] [Google Scholar]
- 26.Andreotti AH, Bunnell SC, Feng S, Berg LJ, Schreiber SL. Regulatory intramolecular association in a tyrosine kinase of the Tec family. Nature. 1997;385:93–97. doi: 10.1038/385093a0. [DOI] [PubMed] [Google Scholar]
- 27.Brazin KN, Fulton DB, Andreotti AH. A specific intermolecular association between the regulatory domains of a Tec family kinase. J. Mol. Biol. 2000;302:607–623. doi: 10.1006/jmbi.2000.4091. [DOI] [PubMed] [Google Scholar]
- 28.Colgan J, Asmal M, Neagu M, Yu B, Schneidkraut J, Lee Y, Sokolskaja E, Andreotti A, Luban J. Cyclophilin A regulates TCR signal strength in CD4+ T cells via a proline-directed conformational switch in Itk. Immunity. 2004;21:189–201. doi: 10.1016/j.immuni.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Qi Q, Sahu N, August A. Tec kinase Itk forms membrane clusters specifically in the vicinity of recruiting receptors. J. Biol. Chem. 2006;281:38529–38534. doi: 10.1074/jbc.M609180200. [DOI] [PubMed] [Google Scholar]
- 30.Jordan MS, Sadler J, Austin JE, Finkelstein LD, Singer AL, Schwartzberg PL, Koretzky GA. Functional hierarchy of the N-terminal tyrosines of SLP-76. J. Immunol. 2006;176:2430–2438. doi: 10.4049/jimmunol.176.4.2430. [DOI] [PubMed] [Google Scholar]
- 31.Dombroski D, Houghtling RA, Labno CM, Precht P, Takesono A, Caplen NJ, Billadeau DD, Wange RL, Burkhardt JK, Schwartzberg PL. Kinase-independent functions for Itk in TCR-induced regulation of Vav and the actin cytoskeleton. J. Immunol. 2005;174:1385–1392. doi: 10.4049/jimmunol.174.3.1385. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Villar JJ, Whitney GS, Sitnick MT, Dunn RJ, Venkatesan S, O'Day K, Schieven GL, Lin TA, Kanner SB. Phosphorylation of the linker for activation of T-cells by Itk promotes recruitment of Vav. Biochemistry. 2002;41:10732–10740. doi: 10.1021/bi025554o. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds LF, Smyth LALF, Norton T, Freshney N, Downward J, Kioussis D, Tybulewicz VL. Vav1 transduces T cell receptor signals to the activation of phospholipase C-gamma1 via phosphoinositide 3-kinase-dependent and - independent pathways. J Exp Med. 2002;195:1103–1114. doi: 10.1084/jem.20011663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bunnell SC, Hong DI, Kardon JR, Yamazaki T, McGlade CJ, Barr VA, Samelson LE. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J. Cell Biol. 2002;158:1263–1275. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bunnell SC, Singer AL, Hong DI, Jacque BH, Jordan MS, Seminario MC, Barr VA, Koretzky GA, Samelson LE. Persistence of cooperatively stabilized signaling clusters drives T-cell activation. Mol. Cell. Biol. 2006;26:7155–7166. doi: 10.1128/MCB.00507-06. [DOI] [PMC free article] [PubMed] [Google Scholar]