Abstract
Literature is reviewed indicating that greater tendency to manage anger via direct verbal or physical expression (trait anger-out) is associated with increased acute and chronic pain responsiveness. Neuroimaging data are overviewed supporting overlapping neural circuits underlying regulation of both pain and anger, consisting of brain regions including the rostral anterior cingulate cortex, orbitofrontal cortex, anterior insula, amygdala, and periaqueductal gray. These circuits provide a potential neural basis for observed positive associations between anger-out and pain responsiveness. The role of endogenous opioids in modulating activity in these interlinked brain regions is explored, and implications for understanding pain-related effects of anger-out are described. An opioid dysfunction hypothesis is presented in which inadequate endogenous opioid inhibitory activity in these brain regions contributes to links between trait anger-out and pain. A series of studies is presented that supports the opioid dysfunction hypothesis, further suggesting that gender and genetic factors may moderate these effects. Finally, possible implications of interactions between trait anger-out and state behavioral anger expression on endogenous opioid analgesic activity are described.
Keywords: anger expression, anger-out, chronic pain, acute pain, mechanisms, opioids, genetic, neuroimaging, fMRI
Introduction
For more than 60 years, the experience of pain has been reported to be associated with various negative emotional states, including depression, anxiety, fear, and anger (Chapman et al., 1946; Hemphill et al., 1952; Ramzy & Wallerstein, 1958; Schachter, 1957; Webb & Lascelles, 1962). Studies have generally found that higher levels of negative emotions are associated with greater acute and chronic pain intensity (e.g., Bruehl et al., 2002; Janssen, 2002; Linton, 2000; Staud, 2004), and possibly with increased risk of developing chronic pain (Linton, 2005). Although these positive associations between pain and negative emotional states undoubtedly exist, underlying mechanisms remain only incompletely understood.
From an evolutionary perspective, an association between emotions and pain responses might be expected, given that pain in times past was often the result of situations threatening survival of the organism (e.g., attack). Anger and fear would be the two emotions most likely to be elicited under such threatening circumstances, as they reflect motivational states underlying the fight and flight responses, respectively (Averill, 1983; Fendt & Fanselow, 1999; Sewards & Sewards, 2002). Consistent with the idea of anger as a primary emotional reaction to physical pain, human experimental studies confirm that acute physical discomfort triggers significantly increased anger and anger-related thoughts, even more so than fearful reactions (Berkowitz, 1990). In addition to pain triggering emotional reactions, acute emotional states can also affect pain, and in some cases are even associated with analgesia. For example, there is a significant animal literature indicating that acute fear reactions to threat are associated with subsequent reductions in pain sensitivity, in part via endogenous opioid mechanisms deriving from the periaqueductal gray (De Oca et al., 1998; Lichtman & Fanselow, 1990; Rau et al., 2005). Similarly, interconnections between neural systems underlying anger and pain might be expected, as will be described below, although the nature of these interactions may be complex.
It has been increasingly recognized that while every individual experiences emotions, there is great variability in how individuals regulate these emotions. For example, in response to being unfairly criticized, one individual might experience anger but become quiet and withdrawn whereas another may lash out verbally and pound the table with her fist. Although both of these individuals may experience similar levels of subjective anger, the manner in which it is displayed and regulated differs dramatically. The study of emotional regulation is a relatively new field (Gross, 1998). Emotional regulation refers to a “broad constellation of processes that serve to either amplify, attenuate, or maintain the strength of emotional reactions” (Davidson, 1998, p. 308). These regulatory processes may be either voluntary or automatic (Davidson, 1998; Gross, 1998), and have been identified as potentially important contributors to physical and mental health (Gross, 1998).
Two basic emotional regulatory strategies that have received a great deal of empirical research attention are suppression and direct expression (Gross, 1998). In the context of the emotion of anger, these two regulatory strategies are usually referred to as “anger-in” and “anger-out” respectively (Spielberger et al., 1985). Trait anger-out, the focus of this review, is defined as the dispositional tendency to regulate anger through direct verbal or physical expression, which in more extreme instances may include verbal aggression, sarcasm, arguing, physical expressions such as striking out or slamming doors, and generally “losing one's temper” (Spielberger et al., 1985). Anger-out impacts not only on the amplification or attenuation of angry feelings, but more importantly, appears related to an individual's sensitivity to acute and chronic pain (Bruehl et al., 2006a).
This review will focus on the pain-related effects of anger-out and will overview literature addressing neurohumoral mechanisms that may contribute to these effects. After detailing several key conceptual issues, we will summarize the literature regarding associations between trait anger-out and acute and chronic pain responsiveness. Next, we will examine relevant neuroimaging literature that suggests significant overlap in the brain structures underlying the experience and regulation of both anger and pain. We will then explore the role of endogenous opioids as modulators of activity in these overlapping brain circuits. Finally, we will provide an overview of a series of studies suggesting that individual differences in endogenous opioid activity in these brain regions may contribute to the pain-related effects of anger-out. It is hoped that this review will highlight the value of moving beyond research simply demonstrating that cognitive and emotional processes can influence pain responsiveness, to examining and documenting how these processes affect pain. Such research may ultimately prove useful for improving the management of acute and chronic pain.
Conceptual Issues
Given the focus of this review on regulation of emotions, the question of what emotions are must first be addressed. Emotions have been described as reactions that are typically of short duration involving alterations in subjective experience, behavioral response tendencies, and central, autonomic, and endocrine response systems (Lang, 1995). There is as yet no universally-accepted conceptual model of emotion, and while it is beyond the scope of this review, a brief overview of several models of emotion may be useful. One dominant early model proposed a relatively unitary “limbic system” underlying all emotions (MacLean 1952). More recently, alternative unitary models have been proposed, such as the idea that strong unpleasant emotions are processed exclusively in the right hemisphere (Adolphs et al., 1999). Dimensional models have also been described that categorize all emotions based on valence (positive/negative) and action tendency (approach/avoidance; Davidson, 1998), with different brain regions contributing to these dimensions. Finally, multisystem models have been described that propose a small set of neurally-determined affect programs that underlie a limited number of discrete fundamental emotions (e.g., fear, anger, disgust). A meta-analytic evaluation of brain imaging evidence for these various models of emotion provided the strongest support for the affect program model, with results suggesting that brain regions underlying fear, anger, and disgust are at least partially distinct (Murphy et al., 2003). While the following review is not dependent on the conceptual model of emotion that is assumed to be correct, the affect program model is not inconsistent with the model of anger-pain associations to be described below.
Another conceptual issue that must be addressed is the distinction between anger expression and aggression. Many behaviors such as yelling or striking out that are associated with the anger-out construct that is the focus of this paper can be considered verbally or physically aggressive. Although anger-out clearly shares some behavioral similarities with aggression, it is not the same construct (Martin et al., 2000), as the latter may be motivated by heterogeneous factors, such as impulsiveness or dominance, rather than anger (e.g., Gray et al., 1991; Lesch & Merschdorf, 2000; Martin et al., 2000). Thus, it is not clear to what extent the literature regarding the neurobiology of aggression (e.g., 5-HT mechanisms; Davidson et al. 2000) is relevant to understanding mechanisms of anger-out and vice versa. Discussion in this review is intended to address the topic of anger-out rather than the related but conceptually distinct construct of aggression.
Some researchers have argued against examining broad categorizations of anger regulation such as anger-in and anger-out (Linden et al., 2003). However, the literature regarding anger regulation and pain considers these two broad categories almost exclusively, and it is therefore not practical at this time to examine the issue otherwise. Evidence for the validity of the construct of trait anger-out is provided by research indicating that when individuals were asked to recall a recent anger-provoking episode and describe what they actually did to manage their anger, common strategies were verbal aggression (49% of episodes), talking negatively to others about the instigator (34%), displaced aggression against an object (26%) or person (25%), and physical aggression against the instigator (10%; Averill, 1983). Thus, while the category of anger-out may be broad, there does appear to be a set of characteristic outwardly-directed strategies by which anger is managed by some individuals, which may be best summarized under the rubric of anger-out. Validation for the most commonly used measure of trait anger-out, the Spielberger Anger Expression Inventory (SAEI; Spielberger et al., 1985), is provided by research employing diary methodology that revealed significant associations between elevated trait anger-out on the SAEI and more frequent use of expressive anger regulation strategies in vivo (Porter et al., 1999). Further supporting the validity of the anger-out subscale, Faber and Burns (1996) found that scores on this scale predicted the degree of verbally expressed anger during provocation. Except where otherwise indicated, the measure of anger-out used in all studies reviewed below is the SAEI.
Although not the focus of this review, it should be noted that other dimensions of anger and anger regulation may be pain-relevant. These include state anger (intensity of current anger), trait anger (frequency with which anger is typically experienced), anger-in (regulation of anger by suppression), and anger control (calm and controlled modulation of anger; Spielberger et al., 1999). State and trait anger both reflect the experience rather than the regulation of anger, but there is evidence that both may be associated positively with pain intensity (e.g., Bruehl et al., 2002). Anger-in has been reported to be associated positively with pain responses in numerous studies, although unlike for anger-out, this effect appears to be due in large part to shared variance with general negative affectivity (i.e., neuroticism; Burns et al., 2008). Anger control, like anger-out, is an active anger regulation strategy, but differs from anger-out in its emphasis on managing anger through nonaggressive behaviors (Deffenbacher et al., 1996). Not surprisingly, anger-out and anger control display a relatively large inverse association (r = −0.58), with anger control negatively correlated with pain outcomes (Lombardo et al, 2005). While limited work suggests that the pain-related effects of anger-out are not due to overlap with trait anger (Burns et al., 1998; Martin et al., 1999) or anger-in (Bruehl et al., 2007b), studies have not specifically addressed the role, if any, that state anger or anger control might play in determining the pain-related effects of anger-out.
One final conceptual distinction also needs to be addressed, and will be discussed in greater detail below in the context of specific study outcomes. It is important to distinguish between the pain-related effects of expressive anger regulation when considered from the trait perspective versus the state perspective. That is, the effects of a self-reported tendency to manage angry emotions through direct expression (trait anger-out) may need to be examined separately from the effects of actual behavioral anger expression in a given anger-provoking context (state anger-out). As will be detailed below, interactions between state and trait anger expression may be a crucial determinant of the pain-related effects of anger-out.
Associations Between Trait Anger-Out and Pain-Related Variables
A thorough review of the published literature using Medline and PsychInfo revealed 24 published studies regarding relationships between trait anger-out and acute or chronic pain-related variables. Results of these studies are summarized in Table 1 (in the order in which they are described below). One published study was not included in this review due to reporting inconsistencies that leave data interpretation unclear (Ham et al., 1994). Of the 24 studies reviewed, 20 (83.3%) reported results providing at least some support for a significant positive relationship between anger-out and acute or chronic pain severity/dysfunction. The relative consistency of this finding that emerges across a variety of pain samples suggests that the relationship between trait anger-out and indices of pain is reliable, although the minority of nonsignificant studies suggest that moderating factors or methodological issues may reduce or eliminate this effect in some cases. Studies testing associations between trait anger-out and acute pain are reviewed briefly below, followed by studies regarding associations with chronic pain and dysfunction.
Table 1.
Studies addressing associations between trait anger-out and pain-related outcomes.
| Reference |
Sample |
Acute Pain Stimulus or Clinical Pain Outcome |
Significant Results |
|---|---|---|---|
|
Gelkopf (1997) |
Healthy (n=21) |
Cold Pressor Task |
↑ Anger-Out = ↓ Pain Tolerance |
|
Janssen et al. (2001) |
Healthy (n=56) |
Cold Pressor Task with and without harassment |
↓ Control of Anger Expression (↑ Anger-Out?) = ↓ Pain Threshold and Tolerance regardless of harassment condition |
|
Burns et al. (2004) |
Healthy (n=53) |
Cold Pressor Task with and without harassment |
↑ Anger-Out = ↓ Pain Tolerance but only after harassment |
|
Burns et al. (2007) |
Healthy (n=187) |
Cold Pressor Task with and without harassment/with and without suppression of anger-related thoughts |
↑ Anger-Out = ↑ Pain Intensity but only after harassment with anger suppression |
| Bruehl et al. (2002) | Healthy (n=45) | Finger Pressure Pain Task | ↑ Anger-Out = ↑ Pain Intensity |
| |
CLBP (n=43) |
Ischemic Pain Task |
|
| Bruehl et al. (2007) | Healthy (n=14) | Finger Pressure Pain Task | ↑ Anger-Out = ↑ Pain Intensity only in the CLBP group |
| |
CLBP (n=13) |
Ischemic Pain Task |
|
| Bruehl et al. (2008) | Healthy (n=53) | Finger Pressure Pain Task | No significant main effects of Anger-Out |
| CLBP (n=34) | Ischemic Pain Task | ||
| |
|
Thermal Pain Task |
|
|
Voulgari et al (1991) |
Post-Surgical Patients (n = 162) |
Post-Surgical Pain Intensity Ratings |
↑ Extrovert Hostility (↑ Anger-Out ?) = ↑ Pain Intensity |
| Bruehl et al. (2006) |
Post-Surgical Patients (n=48) |
Post-Surgical Pain Intensity Ratings |
↑ Anger-Out = ↑ Sensory Pain Intensity |
| Martin et al. (1999) | Community Sample (n=65) | Weekly somatic symptoms including pain | ↑ Anger-Out = ↑ Somatic Symptoms |
| Study 1 |
|
|
|
| Martin et al. (1999) | Healthy (n=180) | Weekly somatic symptoms including pain | ↑ Anger-Out = ↑ Somatic Symptoms |
| Study 2 |
|
|
|
| Materazzo et al. (2000) | Migraine (n=28) | Two-week daily headache severity diary | ↑ Baseline Anger-Out = ↑ Mean Daily Headache Severity (prospective) only in migraine sample |
| |
Tension-Type Headache (n=14) |
|
|
|
Venable et al. (2001) |
Mixed Headache Diagnoses (n=65) |
Retrospective ratings of typical headache intensity |
No significant Anger-Out effects |
| Sayar et al. (2004) | Fibromyalgia (n=50) | Chronic pain intensity ratings | ↑ Anger-Out = ↑ Pain Intensity in Fibromyalgia sample. Directionally positive but nonsignificant in arthritis sample. |
| |
Rheumatoid Arthritis (n=20) |
|
|
|
Gaskin et al. (1992) |
Mixed chronic pain diagnoses (n=60) |
Chronic pain intensity ratings |
Multiple regression indicated no significant effects of anger-out when controlling for state and trait depression and anxiety. Zero-order correlations not reported. |
|
Kerns et al. (1994) |
Mixed chronic pain diagnoses (n= 142) |
Chronic pain intensity ratings |
↑ Anger-Out = ↑ Pain Intensity |
|
Lombardo et al. (2005) |
Mixed chronic pain diagnoses (n= 564) |
Chronic pain intensity ratings |
↑ Anger-Out = ↑ Pain Intensity |
|
Bruehl et al. (2002) |
CLBP (n=43) |
7-day chronic pain intensity diary |
↑ Anger-Out = ↑ Mean Weekly Sensory Pain Intensity |
|
Bruehl et al. (2003a) |
CLBP (n=71) |
7-day chronic pain intensity diary |
↑ Anger-Out = ↑ Mean Weekly Sensory Pain Intensity |
|
Carson et al. (2005) |
CLBP (n=61) |
Chronic pain intensity ratings |
Effects of anger-out directionally positive but nonsignificant |
| Bruehl et al. (2003b) | CRPS (n=34) | Chronic pain intensity ratings | ↑ Anger-Out = ↑ Pain Intensity in CRPS group but not in the Non-CRPS group |
| |
Non-CRPS Limb Pain (n=50) |
|
|
|
Burns & Bruehl (2005) |
Mixed chronic pain diagnoses (n= 136) |
Chronic pain intensity ratings |
↑ Anger-Out = ↑ Pain Intensity only in patients not taking opioid analgesics |
|
Duckro et al. (1995) |
Posttraumatic headache (n = 84) |
Headache-related disability |
↑ Anger-Out = ↑ Headache-Related Disability |
|
Burns et al. (1998) |
Mixed chronic pain diagnoses (n=101) |
Improvements in lifting capacity following multidisciplinary pain treatment |
↑ Anger-Out = ↓ Improvements in Lifting Capacity in Male Patients only |
| Burns et al. (1996) | Mixed chronic pain diagnoses (n=135) | Chronic pain intensity ratings | ↑ Anger-Out (+ High Hostility) = ↑ Pain Intensity in Female patients but ↓ Pain Intensity in Male patients |
Acute Pain Responsiveness
Several published studies have examined the effects of trait anger-out on acute experimental pain responses in healthy samples. Higher anger-out scores on the Multidimensional Anger Inventory (Siegel, 1986) were associated with significantly shorter cold pressor tolerance times in one study (Gelkopf, 1997). In another, subjects not inclined to control expression of anger (a characteristic correlated with elevated anger-out) displayed lower cold pressor pain threshold and tolerance (Janssen et al., 2001). Other studies have replicated these findings, but further suggest a role for key moderating factors. Burns et al. (2004) reported that individuals high in trait anger-out displayed lower cold pressor pain tolerance, but only for subjects acutely angered (by a harassment manipulation) prior to undergoing the pain task. Moderating effects of emotional suppression/nonsuppression on acute pain responses have also been reported (Burns et al., 2007). Greater anger-out was associated with significantly higher ratings of cold pressor pain intensity, but only for subjects who underwent anger-induction while instructed to suppress anger-related thoughts. Findings of these latter two studies suggest that associations between greater anger-out and elevated acute pain responsiveness may be strongest in the context of acute anger arousal, particularly when individuals characterized by high trait anger-out are not able to express their anger.
Acute pain responses also appear to be related to trait anger-out in individuals with chronic pain. Higher anger-out scores were associated with significantly greater pain intensity ratings in response to finger pressure pain and ischemic forearm pain tasks similarly in healthy individuals and those with chronic low back pain (CLBP; Bruehl et al., 2002). However, in a separate smaller sample (14 healthy and 13 CLBP patients), greater anger-out was associated with significantly higher acute pain ratings only in CLBP subjects (Bruehl et al., 2007a). The absence of pain-related anger-out effects among healthy controls in this latter study may in part be a reflection of the substantially lower statistical power available in this study compared to the Bruehl et al. (2002) study, which had a sample more than three times as large. However, statistical power issues would not appear to explain findings of a more recent study in a relatively large sample (n=87) that failed to reveal significant main effects relationships between anger-out and acute experimental pain ratings in either healthy controls or CLBP patients (Bruehl et al., 2008) The only pain-related effects of anger-out in this latter study were found to be moderated by genetic status (detailed below).
A limited number of studies have examined trait anger-out measures as they relate to acute clinical pain responses. Using the Foulds’ Hostility Questionnaire (Foulds, 1965), high levels of “extroverted hostility” (conceptually similar to anger-out) predicted greater post-surgical pain intensity in patients undergoing elective surgery (Voulgari et al., 1991). More recently, anger-out was found to correlate significantly and positively with post-surgical sensory pain intensity ratings in patients undergoing elective coronary artery bypass graft surgery (Bruehl et al., 2006b). Finally, significant positive associations between anger-out and self-reported weekly somatic symptoms, including pain, have also been reported in both community and college student samples (Martin et al., 1999).
Chronic Pain
A larger literature has examined relationships between trait anger-out and chronic pain severity and dysfunction. In a prospective headache diary study, Materazzo et al. (2000) found that higher anger-out at baseline predicted greater subsequent mean daily headache severity. These effects were significant in a sample of migraine patients, but not in a smaller sample of patients with chronic tension-type headache, most likely due to inadequate statistical power in the latter sample. Another study failed to find significant relationships between anger-out and retrospective ratings of typical headache pain severity in patients with tension-type or mixed headache types (Venable et al., 2001). These negative findings may have been influenced by differences in pain assessment methodology compared to the Materazzo et al. (2000) study, that is, daily diary headache pain ratings versus retrospective ratings of typical headache pain.
Pain-related effects of anger-out have been more extensively explored in non-headache chronic pain patients. Elevated anger-out was associated with significantly higher chronic pain intensity in fibromyalgia patients, although similar positive correlations failed to reach statistical significance in a smaller sample of rheumatoid arthritis patients due to statistical power issues (Sayar et al., 2004). Gaskin et al. (1992) examined anger-out/chronic pain intensity relationships in a chronic pain sample with various diagnoses, and reported no significant effects of anger-out in stepwise regression analyses that controlled simultaneously for state and trait depression and anxiety. Unfortunately, zero-order correlations between anger-out and pain intensity were not reported. Some evidence suggests that the nature of the chronic pain diagnosis could affect the strength of anger-out/pain relationships. Bruehl et al. (2003b) reported that anger-out showed significant positive correlations with chronic pain intensity in patients with Complex Regional Pain Syndrome (CRPS), but not in non-CRPS limb pain patients. One interpretation of these results is that the anger-out/pain intensity relationship may be stronger in pain conditions reflecting catecholamine-sensitive pain mechanisms as in CRPS, although this findings remains to be replicated.
Associations between trait anger-out and chronic back pain intensity have been reported in several studies. For example, significant positive correlations between anger-out and chronic pain intensity ratings were reported in two separate samples of male veterans experiencing primarily CLBP (Kerns et al., 1994; Lombardo et al., 2005). Significant positive correlations between baseline anger-out and subsequent mean 7-day diary ratings of chronic back pain intensity have also been described in two studies (Bruehl et al., 2002; 2003a). In a different sample of chronic pain patients experiencing primarily CLBP, elevated anger-out was related to significantly greater chronic pain severity, but only in patients not regularly taking opioid analgesics (Burns & Bruehl, 2005). It may be notable in this regard that all subjects in the Bruehl et al. (2002; 2003a) studies above were also not taking daily opioid analgesics. In contrast to the significant findings above, others have reported positive but nonsignificant associations between anger-out and CLBP intensity (Carson et al., 2005). Although reasons for the negative findings in this latter study are not known, it may be relevant that the studies with positive findings above used the original SAEI (Spielberger et al., 1985), whereas the Carson et al. (2005) study used a revised version embedded in a larger anger-focused questionnaire (the State-Trait Anger Expression Inventory II; Spielberger, 1999).
Two studies have addressed the effects of anger-out on chronic pain-related functioning rather than intensity of chronic pain per se. Duckro et al. (1995) used path analysis in a sample of posttraumatic headache patients, and found that greater anger-out was associated with greater headache-related disability. High levels of anger-out have also been shown to be associated with low levels of improvement in lifting capacity among male but not female chronic pain patients undergoing multidisciplinary pain treatment (Burns et al,. 1998).
Possible Gender Differences
As suggested by the findings of Burns et al. (1998), gender differences may be an important but often overlooked source of variability in the relationship observed between trait anger-out and pain. Burns et al. (1996) reported no overall significant relationship between anger-out and pain severity ratings among 135 married patients with chronic pain (predominately CLBP). However, high anger-out in combination with elevated hostility was found to predict elevated pain severity among female patients. Thus, a psychosocial profile combining increased hostile and suspicious attitudes with a tendency to regulate anger through direct expression was a risk factor for elevated pain severity specifically among women. In contrast, among male patients, low anger-out scores combined with elevated hostility predicted more severe pain (Burns et al., 1996). These results suggest that gender may in some cases moderate associations between anger-out and chronic pain variables (Burns et al., 1996; 1998).
Summary
Although some null findings have been reported, more than 80% of published studies indicate that elevated trait anger-out is associated with increased experimental and clinical acute pain responsiveness, greater chronic pain intensity, and greater chronic pain-related dysfunction (Table 1). Analyses conducted in several studies indicate that these effects are specific to trait anger-out rather than being attributable to statistical overlap with general negative affect (e.g., depression, anxiety, trait anger; Bruehl et al., 2002; 2003b; Burns et al., 1996; 1998; Burns & Bruehl, 2005; Martin et al., 1999). The possibility that anger-out effects on pain could be due to overlap with general emotional expressivity (i.e., tendency towards expressive regulation of all emotions) has also been addressed in one study (Burns et al., 2007). This study found that anger-out was associated with greater pain particularly under conditions of emotional suppression. Anger-out scores were uncorrelated with a measure a general emotional expressivity (the Emotion Expressivity Scale; Kring et al., 1994), with the latter showing a different pattern of pain-related effects than anger-out. This latter finding is consistent with other work indicating no association between anger-out and general emotional expressivity (Kring et al., 1994). Overall, these results suggest that the pain-related effects of anger-out are not due to simple overlap with a tendency to regulate emotions in general via expressive strategies.
Of note in the review above are findings indicating that a number of factors such as gender, acute anger arousal and suppression, and use of opioid analgesics may in some cases moderate the pain-related effects of trait anger-out. Still, the fact that anger-out appears related not only to clinical pain intensity in chronic pain populations but also to acute pain sensitivity in healthy individuals suggests the possibility that the effects of anger-out may be mediated by differences in central pain regulatory pathways that impact on both chronic and acute pain responsiveness.
Possible Neural Interconnections
Given that elevated trait anger-out appears to be associated with greater pain responsiveness, is there evidence for plausible neural substrates that could account for these associations? As noted by others, existing literature suggests that there are common opioid-mediated neural pathways that contribute to the regulation of both emotional states and physical states including pain (Rhudy et al. 2005; 2006; Ribeiro et al., 2005). Brain regions which neuroimaging studies suggest are involved in regulation of both anger and pain are summarized in Table 2. Neuroimaging data addressing the neural circuitry underlying pain responsiveness and pain modulation will next be reviewed.
Table 2.
Brain regions exhibiting endogenous opioid activity that are linked to responsiveness to pain and anger stimuli in neuroimaging studies.
Neural Circuitry of Pain
A prior review of the pain neuroimaging literature concluded that responses to acute pain are consistently associated with activity in several brain regions, including the dorsolateral prefrontal cortex, SI, SII, anterior insula, and anterior cingulate cortex (ACC; Peyron et al., 2000). The ACC is subdivided into “affective” and “cognitive” divisions (Devinsky et al., 1995), with acute pain activating primarily the affective division (the rostral ACC; Peyron et al., 2000). Within the rostral ACC, specific areas frequently showing pain-related activation include Brodmann's areas 24, 25, and 32 (Peyron et al., 2000). Not surprisingly, experimental studies suggest that the ACC may be more involved in modulating the affective component of pain rather than its sensory component (Price, 2000).
Although many findings indicate that acute pain is associated with increased activity in the ACC, other results suggest that this may not always be the case. For example, one study employing co-registered PET and fMRI scanning showed decreased activity in ACC region 32 in response to acute thermal pain stimuli (Vogt et al., 1996). Apparently discrepant findings such as these highlight not only the importance of considering subregions within functionally diverse structures such as the ACC, but also raise one potential interpretive issue regarding pain neuroimaging studies. That is, it may in some cases be difficult to distinguish between pain-related brain activations per se as opposed to activations of pain inhibitory circuits (Wagner et al., 2007).
The methodology of several more recent neuroimaging studies allows at least tentative conclusions to be drawn regarding structures that may contribute to endogenous pain inhibition rather than pain sensation itself. In an fMRI study (Valet et al., 2004), a distraction manipulation reduced acute pain responsiveness, and greater efficacy of this distraction manipulation was found to be associated with increased activation in the ACC, orbitofrontal cortex (OFC), and periaqueductal gray (PAG). MRI-based diffusion tensor imaging indicates that brain regions including the prefrontal cortex (which includes the OFC) and amygdala, both of which participate in descending pain modulation, impact on pain via connections to the PAG (Hadjipavlou et al., 2006). Another fMRI study also suggested that a distraction manipulation reduced acute pain responsiveness through activation of the PAG (Tracey et al., 2002).
Results of some studies permit tentative conclusions to be drawn regarding the directional nature of interactions between the components of this pain modulation circuitry. Functional interactions between pain-related brain regions observed on fMRI during distraction were consistent with the ACC exerting top-down influences on the PAG (Valet et al., 2004). Connectivity analyses reported in PET scan studies support the idea of the ACC exerting top-down influences on pain through descending activation of the PAG as well (Peyron et al., 2007). This view is also consistent with results of a retrograde labeling study in monkeys which found that the prefrontal cortex, ACC, and insula all project to the PAG (Devinsky et al., 1995; Hardy & Leichnetz, 1981). It is notable that the PAG is a primary source of endogenous opioid analgesia in the neuraxis (Devinsky et al., 1995). In sum, available studies suggest that pain-related activity in the ACC, OFC, and insula all could elicit opioid-mediated analgesia through descending influences on the PAG (Wagner et al., 2007). Studies specifically addressing a role for opioids in the brain regions above that underlie pain sensation and modulation are now summarized.
Studies of Exogenous Opioid Activation
Results from neuroimaging studies indicate that administration of exogenous opioids alters activity in several pain-related brain regions, thereby revealing presence of opioid receptors in these regions. Two PET studies indicate that in the absence of painful stimulation, administration of exogenous opioids increases activation in the ACC (Adler et al., 1997; Firestone et al., 1996). Work using SPECT scan methods similarly found that in the absence of painful stimuli, exogenous opioid administration produced activations in the ACC as well as the amygdala (Schlaepfer et al., 1998). Findings using PET methodology indicate that exogenous opioids administered in the context of acute pain stimulation increase activity in both the rostral ACC (Petrovic et al., 2002) and the PAG (Petrovic et al., 2002; Wagner et al., 2007). Degree of opioid-induced activation of the rostral ACC correlated with PAG activation (Petrovic et al., 2002), consistent with the proposed top-down influence of the ACC on PAG activity described previously.
Dose-response relationships have also been described. In one study, the greater the exogenous opioid dose given during acute heat pain stimulation, the greater the activity increases observed in the PAG and ACC, although interestingly, decreases were observed in the insula and prefrontal cortex (Wager et al., 2007). These findings that an exogenous opioid agonist simultaneously increases ACC activity while decreasing insula activity stand in contrast to studies showing that acute pain stimuli often activate both regions simultaneously (Peyron et al., 2000). As noted by Wagner et al. (2007), such apparent discrepancies may arise in part from differences between brain activation patterns related to processing acute pain sensation and activation patterns associated with specific activation of pain inhibitory circuitry. Such findings highlight the complexity of interpreting activation patterns in neuroimaging studies. While activation by exogenous opioids indicates the presence of opioid receptors in the brain regions above, it does not necessarily imply that these circuits are involved in naturally-elicited endogenous opioid analgesic responses. This issue will now be examined.
Studies of Endogenous Opioid Activation
Several neuroimaging studies have addressed the role of endogenous opioids in modulating acute pain responsiveness. These studies have generally used PET scan methodology. In such studies, reduced binding potential of exogenous mu opioid radiotracers is inferred to reflect increased opioid receptor occupancy by endogenous ligands, and presumably greater endogenous opioid activity. For example, a PET study examining responses to brief thermal pain stimuli found that endogenous opioid analgesia elicited by the pain stimulus took place through activation of the rostral ACC and insula (Sprenger et al., 2006). Using a more sustained acute pain stimulus (controlled infiltration of hypertonic saline), Zubieta et al. (2001) found that acute pain triggered significant endogenous opioid analgesia deriving from activity not only in the ACC and insula, but also in the prefrontal cortex and amygdala. Ratings of sensory pain intensity correlated negatively with degree of endogenous opioid activity in the amygdala and PAG, whereas ratings of affective pain intensity were correlated negatively with opioid activation in the ACC. This latter finding is consistent with other evidence that the ACC is more involved in determining the affective component of pain (Price, 2000). The investigators further concluded that the amygdala is involved in endogenous opioid analgesia via direct connections with the PAG, consistent with results of Hadjipavlou et al. (2006).
Several PET studies have examined the role of endogenous opioids in placebo analgesia. In one such study, degree of placebo analgesia to acute pain was found to be correlated with increased activation of the ACC and OFC (Petrovic et al., 2002). Three other studies similarly found that rostral ACC and OFC activation contributed to placebo analgesia by enhancing pain-induced opioid release (Bingel et al., 2006; Scott et al., 2008; Wager et al., 2007), with results also supporting a role for the PAG, anterior insula, and amygdala in these effects. Consistent with findings in PET studies using exogenous opioids, connectivity analyses revealed that activation of placebo analgesia occurred through endogenous opioid-mediated connections between the rostral ACC and the PAG (Bingel et al., 2006; Wager et al., 2007).
In summary, neuroimaging evidence suggests that a network of brain regions including the ACC, insula, OFC, amygdala, and PAG all contribute to processing and modulation of nociceptive input. Moreover, these studies indicate that endogenous analgesia elicited by acute pain involves endogenous opioid mechanisms arising from interactions between these brain regions. While PET studies indicate the presence of opioid receptors in the ACC, insula, OFC, and amygdala, it is not known to what extent endogenous analgesia derives directly from opioid inhibitory activity in these areas. However, connectivity analyses and neuroanatomic studies clearly suggest that top-down influences of the ACC, and possibly other brain regions, on opioid release from the PAG contribute to the observed endogenous analgesia. As will now be detailed, there appears to be a great deal of overlap between the network of pain processing regions described above and those involved in regulation of emotions in general, and anger in particular.
Neural Circuitry of Anger Regulation
Before examining the literature pertaining to the neural substrates of anger regulation, several issues affecting interpretation of these studies must be addressed. Gross (1998) notes that the neural circuits underlying emotion regulation likely differ as a function of the specific emotion studied, in line with the affective program model of emotion described previously. As a consequence, an understanding of the brain circuitry underlying anger regulation specifically must necessarily focus on neuroimaging studies that have involved the actual arousal of anger. A few studies have used methods such as eliciting anger via autobiographical recall of anger-provoking incidents or other anger-provoking imagery (e.g., Damasio et al., 2000; Dougherty et al., 1999; Pietrini et al., 2000). However, most neuroimaging studies addressing anger-related factors have studied reactions to viewing angry faces or hearing angry speech, methods that do not address anger arousal itself (Davidson & Irwin, 1999). In addition, most studies on this topic simply address differences in brain activation patterns between acute anger arousal and an emotionally neutral state. Studies specifically addressing the effects of state anger expression versus nonexpression on brain activity are quite limited, but will be highlighted in this review. Finally, with the exception of a small unpublished pilot study conducted recently in our lab (described below), there are no studies that have specifically addressed trait anger-out as it relates to brain activations in response to pain or emotional stimuli, so the review below must necessarily make inferences from related studies.
Similar to the network of brain structures involved in pain regulation as summarized previously, emotional responses, affective style, and emotion regulation appear to be determined by a network of functionally interconnected structures within the brain that has been termed by some the rostral limbic system (Davidson et al., 2000; Devinsky et al., 1995). These structures include the amygdala, septum, OFC, anterior insula, ACC, ventral striatum including the nucleus accumbens, and several brainstem nuclei including the PAG (Davidson et al., 2000; Devinsky et al., 1995). As implied by the neuroimaging studies reviewed previously, there are a variety of interconnections among the components of this network. For example, the insula and OFC share reciprocal projections and have links to the amygdala as well. The rostral ACC has reciprocal projections to and from the amygdala, and also receives input from the OFC (Zald & Kim, 1996a). This interlinked rostral limbic system is believed to be responsible for controlling executive, social, affective, and motivational behavior, and several of these structures (e.g., rostral ACC, OFC, amygdala) are considered key neural substrates of emotional processing and regulation (Bush et al., 2000; Devinsky et al., 1995; Dougherty et al., 1999; Hariri et al., 2000; Phan et al., 2005; Posner et al., 2007; Thayer & Lane, 2000).
With regard to the focus of this review, it is notable that both anger and impulsive aggression, which is behaviorally similar to anger-out, appear to be regulated by this circuit (Davidson et al., 2000). Davidson et al. (2000) proposed that activation of the OFC and ACC when anger is aroused is part of an automatic regulatory response that modulates the intensity of anger expression. They further hypothesized that reduced ACC and OFC activation in the context of anger would be associated with greater aggressive behavior. Thus, to the extent that anger-out may reflect aggressive responding to angry emotions, one might expect individuals more extreme in trait anger-out to display lower ACC and OFC activation.
Studies of behavior associated with dysfunction or lesions in these areas lend mixed support to this hypothesis. OFC lesions appear to be associated with diminished aggression but also a lack of social restraint (Zald & Kim, 1996b), with the latter finding supporting the hypothesis above. Seizures in the ACC can cause irritability and emotional lability, and ACC lesions or tumors can cause disinhibition, impulsivity, aggressive behavior with little provocation, lack of social restraint, and impaired social judgment (Devinsky et al., 1995). While dysfunctional activity in the ACC (e.g., due to lesions) appears associated with anger-out like behavior, total absence of ACC activity (due to cingulotomy) paradoxically leads to improvements in both pathological aggression and pain (e.g., Cohen et al., 2001). Given that the ACC is part of an interconnected functional network (the rostral limbic system), one speculation is that these opposing patterns of results might be due to differing impacts of dysfunctional ACC input into an intact system as opposed to absent ACC input that may lead to compensatory changes elsewhere in the network. While the direction of ACC-mediated effects on anger-related aggression is not entirely clear, the findings above suggest that dysfunctional ACC activity could contribute both to anger-out behaviors and enhanced pain responsiveness. Other research suggests a possible role for the ACC in verbal aggression like that associated with anger-out. The ACC has been shown to contribute to emotionally-charged vocalization in animals (Devinsky et al., 1995), although comparable human studies are not available. In sum, the studies above suggest that both the ACC and the OFC could play a role in regulating anger-related aggressive behavior, which appears similar in many ways to the behavioral characteristics of more extreme anger-out.
Neuroimaging studies specifically regarding anger and its regulation will now be reviewed. A meta-analysis of location activations in neuroimaging studies of emotion (fMRI or PET) supported the concept of a distinct activation pattern characteristic of anger (Murphy et al., 2003). The most common activation regions in response to anger-related stimuli are the OFC, the rostral ACC, and the dorsomedial prefrontal cortex, with these activations tending to be symmetric (Murphy et al., 2003). Conclusions from this meta-analysis must be tempered by the fact that it reflected a predominance of studies examining responses to angry faces or voices rather than arousal of anger itself.
Results of studies that involve eliciting personally-relevant anger through autobiographical recall of an anger-provoking incident consistently implicate several brain regions associated with anger arousal. Dougherty et al. (1999) used PET imaging to compare brain activity between neutral imagery and autobiographical anger recall conditions. Anger-provoking imagery significantly increased self-reported anger, and was associated with significant activations including the right rostral ACC (areas 24 and 32) and the left OFC. In a similar PET study, autobiographical anger recall also increased self-reported anger, as well as skin conductance and heart rate, compared to a neutral condition (Damasio et al., 2000). Anger provocation was associated with increased ACC activity in the same areas reported above (areas 24 and 32; Damasio et al., 2000). However, in contrast to the findings of Dougherty et al. (1999), anger was associated with decreased activity in the OFC, consistent with hypotheses of Davidson et al., 2000, as well as increased activity in the anterior insula. Sample characteristics may help account for the differing patterns of findings, with a roughly equal gender distribution in the Damasio et al. (2000) sample but an entirely male sample in Dougherty et al. (1999). This possibility is supported by findings of significant gender effects on insula activation in the former study. Differences in sample size may also have contributed, as the Damasio et al. (2000) study had a much larger sample (n=41) than the Dougherty et al. (1999) study (n=8), with the former study more likely to produce stable findings. Use of a similar PET study design in a sample of cocaine-dependent men found that autobiographical anger recall was associated with significantly reduced activity in the frontal cortex and insula, but increased activity in the ACC like that noted above (Drexler et al., 2000). The unique nature of this latter sample makes comparisons with findings in healthy samples difficult.
The few neuroimaging studies of acute anger arousal (autobiographical recall) have not addressed the possible moderating role of individual trait differences in the pattern of activations observed. Such individual difference variables could be important for understanding brain activation patterns associated with anger regulation, and may help explain some of the differing patterns of results in the studies summarized previously. For example, although not examining anger arousal or pain responses specifically, one published study has examined the impact of trait anger-out on resting brain activity. Higher trait anger-out scores were associated with greater mid-frontal and anterior frontal cortical activity on EEG, with significant left sided asymmetry (Hewig et al., 2004). Other studies suggest that personality traits (e.g., extraversion), dispositional affectivity (including trait anger), and family stress history may alter the pattern of brain activation to emotional stimuli (Canli et al., 2004; Hamann & Canli, 2004; Harmon-Jones, 2007; Taylor et al., 2006).
Trait anger-out is by definition an individual difference variable, reflecting the degree to which individuals differ in their tendency to manage anger through overt expressive strategies. Therefore, the neural substrates of anger-out would best be elucidated not by designs simply examining anger arousal, but by designs explicitly examining the impact of trait anger-out or manipulations of state anger expression on brain activation patterns. Regarding the former, only one published study (using EEG as described above) has examined anger-out directly as it relates to brain activity (Hewig et al., 2004). Regarding the latter, the impact of anger expression manipulations on anger-related brain activation has not been well-studied, although results of limited research manipulating regulation of other negative emotional states may have some relevance.
Several studies have explored the impact of manipulations designed to up- or downregulate emotional responses to pictures with negative emotional valence. Eippert et al. (2007) examined fMRI activation patterns in subjects exposed to threat-related pictures, and reported that conscious upregulation of subjects’ emotional reactions (imagine the pictures are real and happening to them) was associated with increased amygdala activity, whereas conscious emotional downregulation (attend to the pictures but detach and distance themselves emotionally) was associated with increased activation of the ACC, OFC, and dorsolateral prefrontal cortex. Work using PET imaging also implicates increased OFC activity in voluntary suppression of emotions elicited by viewing negative pictures, further finding that greater attending to emotions increased amygdala activation (Ohira et al., 2006). Results of these two studies are not inconsistent with suggestions of Davidson et al. (2000) of an association between greater OFC and ACC activity and reduced aggressive behavior.
Other fMRI work suggests that cognitive reappraisal of visual stimuli with negative valence (attend to pictures but interpret them so that negative emotions are no longer felt) successfully reduced negative emotions via increased activation of the medial and lateral prefrontal regions, but decreased activation of the amygdala and OFC (Ochsner et al., 2002). Another fMRI study examined responses to negative emotion-inducing films, and found that conscious reduction of negative emotional reactions through reappraisal (look at pictures objectively to reduce negative emotions) decreased amygdala and insula activity, whereas voluntary suppression of negative emotions increased activity in these areas (Goldin et al., 2008). The finding by Ochsner et al. (2002) of an association between diminished OFC activity and decreased negative emotions seems to differ from the pattern noted above by Ohira et al. (2006) and Eippert et al. (2007). Differences between the Ohira et al. (2006) and Ochsner et al. (2002) studies might be attributed to methodological differences (i.e., differing effects of active suppression versus reappraisal, respectively). However, differences between the Eippert et al. (2007) findings and Ochsner et al. (2002) cannot be explained easily by differences in the experimental conditions, as both involved subjects distancing themselves from the emotional stimulus. All of the studies above suffered from a similar methodological weakness in that the emotions were induced with negative visual stimuli, and thus their relevance to in vivo emotional responses is not known. Moreover, for the purposes of the present review, none of these studies examined the emotion of anger specifically. Regardless, the studies above do suggest that manipulations that sustain or increase emotional arousal would most likely be associated with increased amygdala activity, and possibly decreased OFC activity.
One study using imaginal anger induction suggests specifically that behavioral anger expression versus nonexpression (i.e., state expression rather than trait expressiveness) may affect patterns of brain activation during anger arousal. Pietrini et al. (2000) used PET imaging to examine regional cerebral blood flow changes in response to a neutral imaginal stimulus (riding in an elevator with the subject's mother and two men) versus an anger-provoking imaginal stimulus. The latter manipulation had three conditions varying in degree of anger expression allowed. That is, subjects were asked to imagine riding in an elevator with two men who assault the subject's mother and the subject could: 1) not act and must restrain himself or herself cognitively, 2) could aggress but was physically restrained, or 3) could engage in unrestrained anger-related aggression. The two conditions in which subjects could aggress (#2 and #3) were associated with significantly decreased activity in the OFC. Significantly larger decreases in OFC activity were observed in the unrestrained aggression condition. In both conditions in which aggression was restrained (#1 and #2), decreases in left ACC activity were also observed (ACC area 32). Results of this study are consistent with hypotheses proposed by Davidson et al. (2000) in that aggression and particularly unrestrained aggression were associated with reduced OFC activity, although expected associations between unrestrained aggression and reduced ACC activity were not observed.
Although the findings of this imaginal anger expression study are intriguing, other work using a direct behavioral anger expression manipulation reported a different pattern of results. Harmon-Jones & Sigelman (2001) induced anger experimentally in subjects by providing them with unfair negative written personal evaluations from a study confederate regarding the subjects’ performance in an experimental task. Subjects were subsequently given the opportunity to express their anger (without their knowledge) by assigning the confederate to taste unpleasant versus benign-tasting substances. Greater behavioral anger expression in this manipulation (i.e., assigning unpleasant tasting substances) was associated with increased left prefrontal activation. Thus, while results of Pietrini et al. (2000) suggest that imaginal anger-related aggression was associated with decreased activity in the OFC, results of Harmon-Jones and Sigelman (2001) suggest that anger expressive behavior was associated with increased activity in the prefrontal region, an area that includes the OFC. Because this latter study used EEG rather than fMRI, the resolution was insufficient to localize the brain activations further, so it cannot be determined whether activity in the OFC specifically was increased or decreased during behavioral anger expression. If these two studies indeed reflect opposing findings regarding OFC activation during anger expression, the possibility must be considered that entirely imaginal anger arousal and expression as in Pietrini et al. (2000) may be associated with a different brain activation pattern than a more naturalistic interpersonal anger stimulus with actual behavioral anger expression (Harmon-Jones & Sigelman, 2001). If true, this might raise questions about the generalizability of the fMRI literature on anger, which is highly dependent on manipulations such as imagery-based anger inductions and presentation of anger-related auditory or visual stimuli due to methodological constraints while subjects are in the fMRI magnet (cf. Davidson & Irwin, 1999).
In summary, studies evoking arousal of anger and manipulating expression of that anger suggest that the ACC, OFC, and insula are likely involved in anger regulation. The pattern of observed activations and deactivations in these areas sometimes differs across studies depending on the design employed. However, findings of an association between acute anger arousal and increased ACC activity are fairly consistent, supporting the contention by Davidson et al. (2000) that such activations represent an automatic anger regulatory response to modulate the intensity of anger expression. The ACC, OFC, and insula are all involved in nociceptive processing as well, in part via top-down influences on the PAG, the primary source of endogenous opioid analgesia in the neuroaxis. Evidence for a role of opioid-mediated pathways in these brain structures as they relate to emotional regulation will next be examined.
Role of Endogenous Opioids in Emotional Regulation
The literature reviewed previously in the context of pain indicates that the brain regions comprising the rostral limbic system (e.g., ACC, OFC, insula, amygdala, PAG) all possess opioid receptors, with the PAG clearly exhibiting significant opioid analgesic activity. Given the presence of opioid receptors in these brain regions, it is likely that opioid activity would impact on emotional regulation subserved by these areas as well. Experimental studies do suggest a possible role for endogenous opioids in emotional regulation processes. For example, dysphoric clinical states such as major depression and premenstrual phase dysphoric disorder appear often to be associated with impaired endogenous opioid inhibitory activity (Burnett et al., 1999; Facchinetti et al., 1994; Young et al., 2000), although some findings regarding depression do appear to contradict this (Kennedy et al., 2006). In non-pathological states, higher levels of circulating endogenous opioids have been shown to be associated with greater emotional stability (Zorrilla et al., 1995) and greater positive mood after exercise (Wildmann et al., 1986). Furthermore, several reports indicate that pharmacological opioid blockade results in increased anger (e.g., Martin del Campo et al., 1994; Pickar et al., 1982). Regarding style of anger regulation, it is notable clinically that early recovery from heroin abuse, which is associated with acutely deficient endogenous opioid activity, is associated with increases specifically in outwardly expressed anger (Powell & Taylor, 1992).
The studies above provide evidence, albeit indirect, that endogenous opioids may be involved in regulation of emotional states, including anger. Other work more directly supports the idea of opioid-mediated emotional regulation. Zubieta et al. (2003) used a PET scan design with a mu opioid selective radiotracer in 14 healthy women. Greater increases in negative affect resulting from autobiographical recall of a sad memory were associated with reduced endogenous opioid activity in the rostral ACC and amygdala. Moreover, the magnitude of increased negative affect resulting from this sadness manipulation correlated with the degree of endogenous opioid deactivation in the rostral ACC and insula. Although anger was not specifically evaluated in this study, results indicate a role for endogenous opioids in regulation of negative emotional states, and moreover, suggest that this regulation involves opioids in the rostral ACC, insula, and amygdala. It should be noted that results of a similar PET study (Kennedy et al., 2006) indicated that patients with major depressive disorder exhibited increased endogenous opioid activity in response to sadness induction, in contrast to the decreases observed in healthy individuals. These latter findings are supported by recent work indicating that patients with major depressive disorder exhibited greater opioid analgesia (reflected in opioid blockade effects) in response to induced negative affect than did healthy controls (Frew & Drummond, 2008). Thus, the nature of opioid modulation of acute negative emotional states may be influenced by concurrent mood disorders and other individual difference factors which as yet have been little explored.
More recently, neuroimaging work suggests that endogenous opioid activity in the rostral limbic system may also contribute to regulation of positive emotional states (Boecker et al., 2008). Ten athletes were tested before and immediately after endurance training using PET imaging with a mu opioid radiotracer. Results indicated that the degree of euphoria resulting from endurance training was correlated significantly and positively with the degree of endogenous opioid receptor binding in brain regions including the OFC, ACC, and insula. Taken together with results of Zubieta et al. (2003), endogenous opioids in key regions of the rostral limbic system appear likely to play a role in regulation of both negative and positive emotional states.
With regard to the issue of regulation of anger in particular, results of one animal study may also be worth noting. Miller et al. (2004) found that in rhesus monkeys, presence of a single nucleotide polymorphism of the mu opioid receptor gene (C77G) was associated with increased aggressive threat communication (teeth baring, ear-flapping, and other facial displays), behavior that may have some parallels to aggressive verbal communication often characteristic of elevated anger-out. While this C77G polymorphism was associated with increased beta-endorphin binding, it also was associated with evidence suggesting decreased production of the precursor molecule of beta-endorphin, a key endogenous mu opioid receptor agonist (Miller et al., 2004). These results would be consistent with an association between greater aggressive communication in primates and reduced beta-endorphin availability.
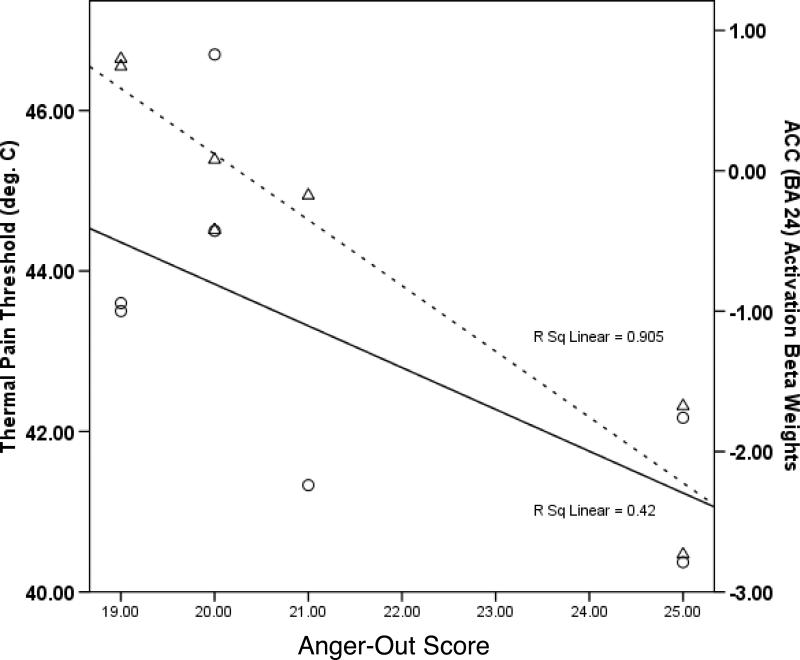
Although no published studies have examined trait anger-out as it relates to pain-related brain activations in the opioid-rich brain structures that appear to underlie both pain and anger regulation, recent fMRI pilot work in our lab does bear on this issue. Seven healthy subjects completed a psychometric measure of trait anger-out and then were scanned while undergoing thermal forearm pain stimulation using an event-related design. Pain-specific activations were derived (pain tolerance minus warmth threshold), and the brain regions in Table 2 were all examined in a priori region of interest analyses as they related to anger-out. Consistent with the EEG findings of Hewig et al. (2004) described above, higher anger-out scores were associated with significantly (p<.05) greater left OFC and dorsolateral prefrontal cortex activation in response to pain. In addition, higher anger-out was associated significantly (p<.05) with decreased activation of the insula and PAG, and both increased and decreased ACC activity (areas 32 and 24 respectively). Interestingly, the association between greater anger-out and increased ACC area 32 activity appears consistent with findings of Pietrini et al. (2000), who reported that imagery of unrestrained aggression was associated with greater activity in ACC area 32. Figure 1 displays thermal pain threshold (circles) and activation beta weights for ACC area 24 (triangles) as a function of anger-out. The correlations between pain threshold and anger-out related activity in ACC areas 24 and 32 were r = 0.61, p<.15 and r = −0.73, p<.06, respectively. Although not reaching statistical significance due to sample size limitations, this pattern suggests the possibility that greater anger-out may have been be associated with decreased pain threshold (i.e., increased pain sensitivity) to an extent similar to that which ACC activation in area 24 was diminished and activation in area 32 was increased. Given the role described previously of ACC/PAG links as a contributor to endogenous opioid antinociception (e.g., Petrovic et al., 2002; Wager et al., 2007), it is notable that activity in the PAG showed a strong positive relationship with activity in ACC area 24 (r = 0.81, p<.05) and a strong negative relationship with activity in ACC area 32 (r = −0.91, p<.005), and that PAG activation also displayed a relatively large but marginally significant positive correlation with pain threshold (r = 0.69, p<.10). This pattern is not inconsistent with elevated anger-out contributing to elevated acute pain sensitivity through an association with diminished endogenous opioid analgesia deriving from the PAG, although this possibility was not directly tested. While the findings above are consistent with activation networks reported by others, these results should be interpreted cautiously given the limited sample size of this pilot work.
Figure 1.
Scatterplot of thermal pain threshold (circles) and activation beta weights for ACC area 24 (triangles) as a function of anger-out score. Solid and broken best fit lines are for pain threshold and ACC activation, respectively.
The studies above support the plausibility of a connection between trait anger-out and endogenous opioid function. The opioid dysfunction hypothesis of anger-out will now be described, followed by a summary of studies testing this hypothesis.
Associations Between Anger-Out and Endogenous Opioids
In 2002, we proposed an opioid dysfunction hypothesis to account for the observed associations between greater trait anger-out and increased acute and chronic pain responsiveness (Bruehl et al., 2002). This model was based on literature exploring the overlapping neural circuits likely to contribute to regulation of both anger and pain, as well as structural and functional data suggesting a role for endogenous opioids in this regulation. Endogenous opioids have much the same effects as exogenous opioids; that is, analgesia, diminished negative affect, and reduced physiological reactivity (McCubbin et al., 1988; Martin del Campo et al., 1994; Millan, 2002; Pickar et al., 1982). Thus, we hypothesized that inadequate endogenous opioid inhibitory activity in the rostral limbic system might simultaneously lead to elevated pain sensitivity and reduced ability to modulate anger when it is experienced, thereby leading to a tendency towards more overt expressions of anger (high trait anger-out). Within this absolute opioid dysfunction model (i.e., absolute inability to recruit opioid analgesia regardless of circumstances), the previously reported positive relationships between trait anger-out and pain sensitivity were proposed to be mediated by impairments in overlapping opioid inhibitory systems modulating both pain and anger regulation.
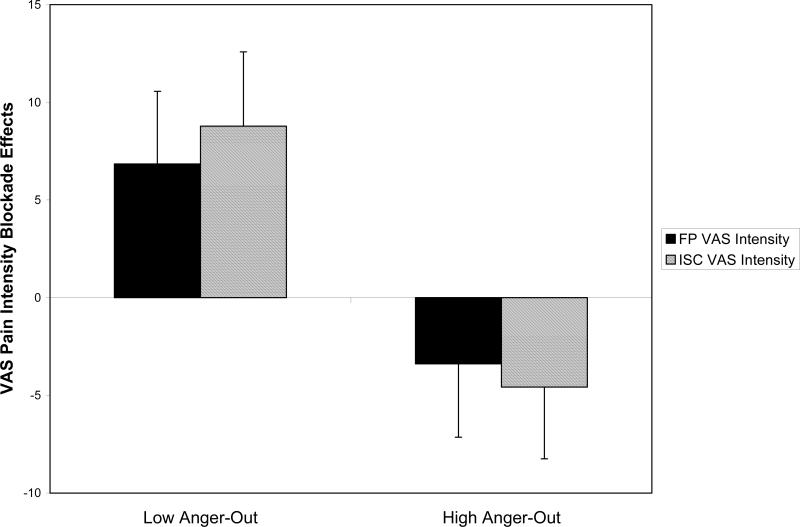
We have conducted a series of studies to test this absolute opioid dysfunction hypothesis. Using a within-subject, placebo-controlled opioid blockade (naloxone) methodology, Bruehl et al. (2002) tested for associations between anger-out and opioid analgesic system function. The sample included 43 patients with CLBP and 45 pain-free healthy controls. Opioid analgesic function was indexed by deriving blockade effects representing changes in acute pain responses induced by opioid blockade (naloxone condition pain ratings minus placebo condition pain ratings). Larger positive blockade effect values indicated greater increases in pain intensity following opioid blockade, and thereby provided evidence for effective endogenous opioid analgesia in the placebo condition. Blockade effect results for finger pressure and ischemic acute pain tasks are summarized in Figure 2. Low scorers on the trait anger-out measure exhibited effective endogenous opioid analgesia, as reflected in positive blockade effects (i.e., increased pain ratings from placebo to opioid blockade conditions). In contrast, subjects scoring high on anger-out did not display any increases in pain ratings from placebo to opioid blockade conditions, but rather, appeared to have some degree of paradoxical analgesia in response to opioid blockade. Similar findings were observed across multiple pain measures on both acute pain stimuli. Thus, while subjects lower in anger-out exhibited what might be considered “normal” opioid analgesia to acute pain, those higher in anger-out did not, consistent with an association between elevated anger-out and endogenous opioid dysfunction. Similar effects were observed in CLBP patients and healthy controls alike, suggesting that this apparent anger-related opioid dysfunction was not a consequence of having chronic pain.
Figure 2.
Effects of trait anger-out on opioid blockade effects derived from acute finger pressure (FP) and forearm ischemic task (ISC) visual analog scale (VAS) pain intensity ratings. Blockade effects (y-axis) represent naloxone condition pain ratings minus placebo condition pain ratings. Larger positive blockade effects indicate greater opioid analgesia. Raw VAS ratings used to derive blockade effects potentially ranged from 0−100mm.
An association between elevated anger-out and opioid analgesic system dysfunction was also suggested by work using an alternative index of opioid function: pain-induced changes in plasma endogenous opioids (Bruehl et al., 2007a). Beta-endorphin (BE) is an endogenous mu opioid receptor agonist which has clinically meaningful analgesic properties (Millan, 2002). Plasma BE was assessed at rest and again following exposure to three laboratory acute pain tasks (finger pressure, ischemic, and thermal) in a sample of 14 healthy controls and 13 CLBP subjects. It was assumed that acute pain would trigger increased BE release in individuals with well-functioning opioid systems. As expected, acute pain ratings correlated positively with anger-out scores in the CLBP group. Greater pain-induced release of BE was associated with significantly lower pain ratings in both groups, indicating that the BE differences examined in the study contributed to observed differences in acute pain responsiveness. Hierarchical multiple regression indicated that greater anger-out predicted significantly lower pain-induced release of BE (see Figure 3), consistent with the opioid dysfunction hypothesis. In addition, statistical control of general negative affect did not eliminate the association between anger-out and BE. Finally, mediation analyses (conducted as per Baron & Kenny, 1986) revealed that differences in BE release associated with anger-out partially mediated the hyperalgesic effects of anger-out on pain unpleasantness. These findings further supported unique associations between anger-out and impaired endogenous opioid analgesic systems, and suggested that this opioid dysfunction was in part due to differences in opioid release.
Figure 3.
Scatterplot of anger-out scores and residualized changes in plasma beta-endorphin (BE, in ng/ml) in response to acute pain stimulation. Positive BE change values indicate greater release of BE in response to acute pain stimuli. BE changes are residualized for pre-pain baseline (i.e., baseline corrected change) and differences across assay plates.
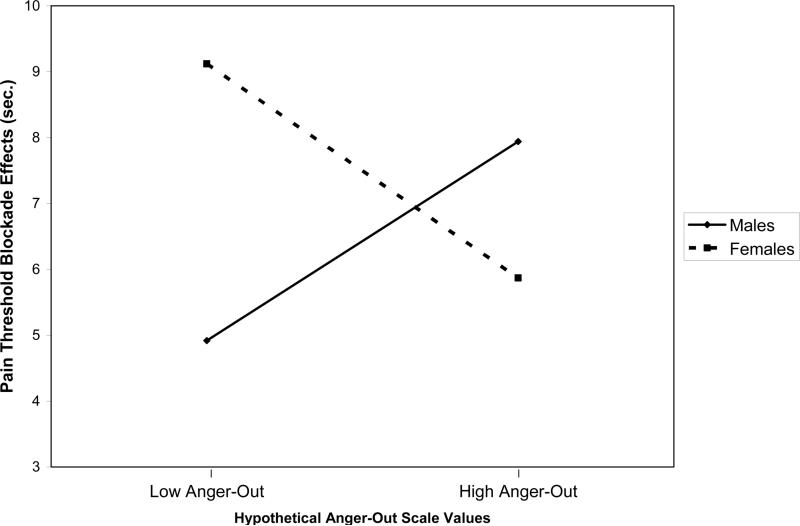
Another attempt to replicate findings of an association between trait anger-out and opioid dysfunction was conducted using a different acute pain stimulus and a different opioid blockade methodology (Bruehl et al., 2007b). Results of this study suggested that gender might be an important variable to consider (Bruehl et al., 2007b). One hundred forty-five healthy subjects underwent acute electrocutaneous pain stimulation on two occasions, once after receiving placebo and once after receiving oral opioid blockade with naltrexone. As above, opioid analgesic function was indexed by deriving blockade effects reflecting changes in pain responses induced by opioid blockade. Hierarchical regressions revealed that in females, elevated anger-out was associated with a significantly lower degree of opioid analgesia, as was noted in the other studies described above (see Figure 4). Male subjects, however, exhibited a significant anger-out/opioid relationship in the opposite direction. Gender X anger-out interactions on opioid blockade effects were significant for electrocutaneous pain threshold, with similar marginally significant interactions (p's<.10) noted for nociceptive flexion reflex threshold, pain tolerance, and pain ratings. Overlap with negative affect again did not account for the observed opioid-related effects. These findings provided additional evidence of an association between trait anger-out and endogenous opioid analgesia, but further suggested that gender may moderate these effects. Unfortunately, due to sample size issues, the other studies described above were unable to test with adequate statistical power for interactions between gender and anger-out on endogenous opioid function.
Figure 4.
Effects of anger-out on pain threshold opioid blockade effects in male and female participants. Anger-out values plotted are hypothetical values representing one standard deviation (SD) below and above the sample mean. Pain threshold blockade effects (y-axis) reflect placebo condition pain threshold values minus naloxone condition pain threshold values. Larger blockade effects indicate greater opioid analgesia.
That gender might affect associations between anger-out and endogenous opioids is not surprising in light of prior work suggesting possible influences of gender on opioid system function. For example, gender-related response differences to exogenous opioid analgesics have previously been described (Aubrun et al., 2005; Cepeda & Carr, 2003). Several studies also suggest gender differences in endogenous opioid function. Frew and Drummond (2007) reported that in a healthy sample, experimentally-induced discouragement triggered significant opioid-mediated analgesia in females but not males. In contrast, previous brain imaging work suggested that pain-induced endogenous opioid analgesia was significantly greater in males than in females (Zubieta et al., 2002). One possible source for such gender differences in opioid function is the known influence of female sex hormones (estradiol) on endogenous opioid antinociception, which may differ across the menstrual cycle (Smith et al., 2006). At present, knowledge of gender influences on endogenous opioid function is insufficient to develop clear hypotheses to explain findings such as those of Bruehl et al. (2007b), particularly given that this study did not control for menstrual phase influences.
Regardless of their source, it is interesting to note the similarity between the gender effects on anger-out/opioid associations in Bruehl et al. (2007b) to those of Burns et al. (1996) regarding effects of anger-out on chronic pain intensity. Among subjects with elevated hostility, high anger-out was found to be associated with greater chronic pain intensity in female patients but lower chronic pain intensity in male patients (Burns et al., 1996). These findings parallel results of Bruehl et al. (2007b) which suggested relative opioid hypofunction in high anger-out female subjects and relative opioid hyperfunction in high anger-out male subjects. Possible gender moderation effects on anger-out/opioid associations remain to be replicated.
The issue of whether opioid dysfunction related to elevated anger-out impacts not only on acute pain responses, but also on clinical chronic pain intensity has also been investigated. Applying the methodology of Baron and Kenny (1986) in a sample of 71 CLBP patients, we tested whether positive associations between trait anger-out and chronic pain intensity were mediated by endogenous opioid dysfunction (Bruehl et al., 2003a). Results of sequential hierarchical regressions suggested that opioid dysfunction measured in the laboratory partially mediated the positive association observed between trait anger-out and mean daily chronic pain intensity (based on a 7-day pain diary measure). Specifically, while anger-out was a significant predictor of chronic pain intensity when entered alone (p<.05), entry of an index of endogenous opioid dysfunction (i.e., opioid blockade effects on acute pain) in a hierarchical regression prior to entry of anger-out resulted in anger-out no longer being a significant predictor of chronic pain, with a corresponding decrease from 7% to 3% in chronic pain variance accounted for by anger-out (Bruehl et al., 2003a). This 57% reduction in variance accounted for by anger-out in chronic pain intensity when a measure of endogenous opioid system dysfunction was statistically removed provided additional support for the opioid dysfunction hypothesis. According to the Baron and Kenny (1986) methodology for testing mediation, the observed pattern of findings indicated that the positive association between trait anger-out and chronic pain intensity was partially mediated by opioid antinociceptive system dysfunction.
Individual differences in pain responsiveness reflect not only differences in sensory pain intensity per se, but also differences in the affective component of pain, often termed pain-related suffering. The impact of trait anger-out on pain-related suffering and the role of endogenous opioid system differences in these effects have recently been explored (Bruehl et al., 2008a). Seventy-nine CLBP patients and 46 healthy controls received opioid blockade (8mg naloxone i.v.) and placebo in randomized, counterbalanced order in separate sessions. During each, subjects experienced finger pressure and ischemic forearm pain tasks, with emotional state assessed at baseline and post-pain (changes therefore reflected emotional reactivity to pain). To index opioid modulation of this emotional reactivity, blockade effects were derived by subtracting placebo from naloxone condition emotional reactivity. Significant interactions revealed that in CLBP subjects but not controls, greater anger-out was associated with deficient opioid modulation of anxiety, anger, and fear reactivity to acute pain (see Figure 5 for anger reactivity blockade effects). These results suggested that opioid dysfunction associated with trait anger-out may affect not only pain intensity, but also pain-related suffering. However, unlike the other studies described previously, these findings of an anger-out/opioid association were observed in subjects with CLBP but not in healthy controls. One likely explanation for this relates to the fact that the CLBP group reported significantly greater emotional reactivity to pain during the placebo condition than did controls. Healthy controls may simply not have experienced a sufficient level of emotional reactivity to optimally elicit opioid modulatory mechanisms, whereas the CLBP subjects did, thereby permitting more efficient detection of differences in opioid function attributable to anger-out in the latter group.
Figure 5.
Blockade effects on emotional reactivity (anger) to acute pain stimulation by anger-out score. Anger-out values plotted are hypothetical values representing one standard deviation (SD) below and above the sample mean. Anger blockade effects (y-axis) reflect naloxone condition changes in anger from baseline to post-pain minus the comparable placebo condition anger reactivity values. Larger positive blockade effects indicate greater endogenous opioid modulation of anger reactivity. CLBP = Chronic Low Back Pain.
Other studies (Burns et al., 2003; 2004) provide indirect support for the opioid dysfunction hypothesis. Relationships between anger-out and acute pain sensitivity observed following experimental anger induction were found to overlap statistically with the increased blood pressure reactivity elicited during anger (Burns et al., 2004). Given the known role of central endogenous opioid activity in modulating not only acute pain responses (Millan, 2002) but also blood pressure stress reactivity (McCubbin et al., 1988), these results are consistent with the presence of a common central mechanism contributing to both findings, possibly opioid-mediated. Burns and Bruehl (2005) also reported indirect evidence suggesting dysfunctional opioid analgesic systems in high anger-outs. Elevated anger-out was associated with significantly greater chronic pain severity, but only in subjects not regularly taking opioid analgesics. These results are consistent with the notion that exogenous opioids in high anger-outs may have served to supplement deficient endogenous opioid systems and eliminate the negative effects of trait anger-out on chronic pain severity.
Although the studies above provide both direct and indirect support for the hypothesis that elevated anger-out is associated with endogenous opioid system dysfunction, replication of these findings is required. In addition, future studies must address the situational determinants that may influence how the anger-out/opioid link is expressed (see below).
Genetic Factors
To the extent that effects of anger-out are opioid–mediated, and further, that endogenous opioid analgesic function is influenced by genetic differences in opioid receptors (e.g., beta-endorphin receptor binding; Bond et al., 1998), genotype X phenotype interactions relevant to the opioid dysfunction hypothesis might be expected to occur. One reasonable genetic target is the A118G single nucleotide polymorphism (SNP) of the mu opioid receptor gene. This SNP appears to affect experimental acute pain sensitivity and responsiveness to both exogenous and endogenous opioid agonists. The variant G allele has been associated with lower acute pain responsiveness (Fillingim et al., 2005; Lotsch et al., 2006), greater beta-endorphin receptor binding (Bond et al., 1998), and greater Ca2+ inhibition on opioid receptor activation (Margas et al., 2007). Other work indicates that although the variant G allele does not influence exogenous opioid receptor binding (Bond et al., 1998), it is associated with reduced receptor expression (Kroslak et al., 2007; Zhang et al., 2005) and increased exogenous opioid analgesic requirements (Chou et al., 2006; Klepstad et al., 2004; Lotsch et al., 2002; Skarke et al., 2003). Two studies have explored possible genetic mediation or moderation of the pain-related effects of anger-out.
Forty-eight patients undergoing elective coronary artery bypass graft surgery completed a measure of trait anger-out, DNA samples were obtained, and post-surgical opioid analgesic demands were recorded (Bruehl et al., 2006b). The correlation between A118G SNP status and trait anger-out was quite small (point-biserial r = 0.06), suggesting that this SNP was unlikely to be a mediator of anger-out's pain-related effects. However, significant moderation by the A118G SNP was observed for the effects of anger-out on postoperative opioid analgesic demands (see Figure 6). Regression analyses indicated that in subjects with the wild-type mu opioid receptor gene (homozygous A allele), the relationship between anger-out scores and analgesic use was weakly positive (r = 0.07), whereas it was significantly larger in subjects with the variant G allele (r = 0.53). Greater trait anger-out appeared to be associated with larger exogenous opioid requirements primarily in subjects with the A118G variant.
Figure 6.
Effects of anger-out on analgesic use (mg of morphine equivalents) in subjects with wild-type versus A118G variant allele of the mu opioid receptor gene.
Possible mediation or moderation of the effects of trait anger-out on experimental acute pain responsiveness by the A118G SNP has also been examined (Bruehl et al., 2008b). Genetic samples and a measure of trait anger-out were obtained in 87 subjects who participated in controlled laboratory acute pain tasks (ischemic, finger pressure, thermal). McGill Pain Questionnaire (MPQ) Sensory and Affective ratings for each pain task were standardized and combined for analyses. As in Bruehl et al. (2006b), A118G status was uncorrelated with anger-out scores, ruling out genetic mediation of anger-out effects on acute pain. However, significant anger-out X A118G interactions on both McGill Pain Questionnaire (MPQ) Sensory and Affective pain ratings were observed. Simple effects tests for both pain measures revealed that whereas anger-out was nonsignificantly hyperalgesic in subjects homozygous for the wild-type allele (similar in direction to prior work), anger-out was significantly hypoalgesic in the smaller subset of subjects with the variant G allele (p's<.05). For the MPQ-Affective measure, this interaction arose both from low pain sensitivity in high anger-out subjects with the G allele and heightened pain sensitivity in low anger-out subjects with the G allele relative to responses in homozygous wild-type subjects. Anger-out X A118G interactions were not accounted for statistically by overlap with general negative affect, suggesting the presence of unique effects of expressive anger regulation. Results further supported opioid-related genotype X phenotype interactions involving trait anger-out.
Results of these studies can be viewed in context of prior work indicating that the A118G SNP is associated simultaneously with reduced acute pain responsiveness (Fillingim et al., 2005; Lotsch et al., 2006) and increased exogenous opioid analgesic requirements (e.g., Klepstad et al., 2004; Lotsch et al., 2002). Results of Bruehl et al. (2008b) suggest the former effect is more pronounced in those highest in anger-out, whereas those of Bruehl et al. (2006b) suggest the latter effect is also more pronounced in those higher in anger-out. Thus, both studies indicate that previously reported pain-related effects of the A118G SNP are greatest in individuals highest in trait anger-out. Mechanisms accounting for these effects remain to be determined. Regardless, results of these two studies further highlight a likely role for opioid factors in determining the pain-related effects of anger-out.
Trait X State Interactions
Bushman et al. (2001) reported that people high in trait anger-out, unlike those low in anger-out, engage in behavioral anger expression because they believe it will make them feel better. The idea that catharsis is beneficial among individuals high in anger-out may be intuitively appealing when viewed in terms of psychodynamic conceptualizations of emotion regulation. Yet at first glance, it seems difficult to reconcile the supposed positive effects of catharsis with consistent findings that trait anger-out is associated with greater pain responsiveness. However, this apparent paradox may be explained by methodological issues in existing studies. Most published studies of anger regulation have focused on the pain-related effects of anger-out assessed solely as a trait (using questionnaires), rather than examining the immediate pain-related effects of behavioral (state) anger expression in anger-provoking contexts. Indeed, previous work clearly highlights the potential value of examining trait X state interactions involving trait anger-out and actual anger regulation on pain outcomes (Bushman et al., 2001; Engebretson et al., 1989).
In brief, this trait X state “matching hypothesis” (Engebretson et al., 1989) posits that among individuals with a strong disposition to regulate anger via direct expression (high anger-out), the opportunity to behaviorally express anger when it is experienced helps reduce anger arousal as well as the negative health effects of such arousal. This notion makes sense from the perspective that one function of negative affect regulation processes (such as direct expression) may be to attenuate the experienced emotion (Gross, 1999), and in essence restore “emotional homeostasis.” Thus, it would be surprising if direct anger expression did not have a functional regulatory benefit under some circumstances and among certain people.
Behavioral Anger Expression and Pain
Harassment manipulations that evoke acute situational anger but do not allow this anger to be directly expressed appear to exacerbate the negative effects of trait anger-out on pain responses (Burns et al., 2004). For example, trait anger-out was associated with increased pain sensitivity most strongly under a condition of anger-induction (harassment) in which there was no opportunity to behaviorally express anger (Burns et al., 2004). This finding may be taken as evidence of a trait X situation mismatch for individuals high in anger-out, that is, they are angered but not permitted to engage in their preferred anger regulation strategy (overt behavioral anger expression). The importance of this issue is further highlighted by recent work in our lab indicating that deliberate attempts to suppress anger by subjects with elevated anger-out scores were associated with even greater pain responsiveness (Burns et al., 2007).
Review of the literature suggests that the issue of behavioral anger expression versus non-expression could be one key to fully understanding the health impacts of anger-out. Verbalization of anger by certain individuals reduces the intensity of those angry emotions and improves cardiovascular post-stress recovery, consistent with the concept of behavioral anger expression being an adaptive form of emotional regulation for some (Bushman et al., 2001; Engebretson et al., 1989; Faber & Burns, 1996; Lai & Linden, 1992; Suchday & Larkin, 2001). Results of one study suggest that behavioral anger expression could have similar beneficial effects with regard to pain as well (Burns et al., 2003), although definitive conclusions in this regard are not yet possible. Subjects higher in anger-out displayed diminished acute pain responsiveness relative to low anger-outs after undergoing an anger recall interview in which anger was elicited and verbally expressed (Burns et al., 2003). Similar significant effects were not found following recall and expression of sadness or joy, suggesting that there may be emotion-specific beneficial effects of verbal anger expression on acute pain responses among subjects higher in anger-out.
Another recent study also raises the possibility of analgesic effects of behavioral anger expression, in this case among individuals with chronic pain (Graham et al., 2008). In a sample of 102 chronic pain patients, 51 were randomly assigned to recall a recent anger-provoking incident and then express their anger directly in written form for 20 minutes on two occasions (2 ½ weeks apart), while 51 other patients were assigned to simply write about their plans for the upcoming day. Over a 9-week period, patients in the anger expression condition reported significantly greater improvements in control over pain and marginally significant improvements in clinical pain intensity relative to subjects not expressing anger. Although this study did not examine possible moderating effects of trait anger-out on changes in these pain-related outcomes, these results are consistent with possible beneficial effects of behavioral anger expression in high anger-out patients.
Although providing only indirect evidence, work examining EEG brain asymmetry supports the possibility that behavioral anger expression versus nonexpression could be a crucial determinant of the effects of anger-out. Experimental arousal of anger in the absence of any opportunity to behaviorally express anger was not associated significantly with frontal EEG asymmetry (Harmon-Jones, 2007), whereas anger provocation with an opportunity to behaviorally express anger was associated with left-sided asymmetry in prefrontal EEG activity (Harmon-Jones & Sigelman, 2001). Combined with other work indicating that left frontal EEG asymmetry is more prominent in high anger-out individuals (Hewig et al., 2004), these studies support a possibly unique brain activation pattern in individuals higher in anger-out that may be dependent on behavioral expression of anger. The brain circuitry described previously portrays one possible way in which such activation patterns could be associated with differences in pain responsiveness, possibly via opioid mechanisms.
The Opioid Triggering Hypothesis
The findings reviewed above suggest that elevated trait anger-out in the absence of anger arousal and/or expression is associated with increased pain sensitivity, and these associations appear to be mediated in part by impaired endogenous opioid analgesic function in individuals high in anger-out (Bruehl et al., 2002; 2003a; 2007a; 2007b). Other results indicate that for individuals with high trait anger-out, behavioral expression of anger when it is aroused elicits diminished pain and improves cardiovascular post-stress recovery (Burns et al., 2003). Given the important inhibitory role of endogenous opioids in modulation of both pain and cardiovascular stress responding (McCubbin et al., 1988; Millan, 2002), these findings are consistent with the hypothesis that for high anger-out individuals but not those low in anger-out, behavioral anger expression triggers endogenous opioid inhibitory systems that otherwise remain relatively inactive even when exposed to pain and psychosocial stress. Thus, for individuals with a predominately expressive anger regulation style, behavioral anger expression may be functional and adaptive. Individuals low in anger-out, in contrast, appear to elicit adequate endogenous opioid analgesia even in the absence of anger arousal and expression (Bruehl et al., 2002; 2003a). That is, for those high in trait anger-out, behavioral anger expression might activate otherwise quiescent opioid inhibitory mechanisms and thereby eliminate response differences relative to individuals low in anger-out. If true, individuals with high trait anger-out might best be conceptualized not as having an absolute inability to activate opioid inhibitory systems, but rather as having an increased threshold for activating those systems, with behavioral anger expression being the trigger. Opioid analgesia triggered by anger expression, if it occurs, might be viewed as similar to the opioid analgesia triggered by fear that has been noted in animal studies (Lichtman & Fanselow, 1990).
If the opioid triggering hypothesis is valid, then the possibility that operant reinforcement mechanisms could contribute to development of trait anger-out might need to be considered, given evidence that physiological processes can be operantly conditioned (Ax, 1990; Koers et al., 1997; Poon & Siniaia, 2000) and that endogenous opioids can serve as behavioral reinforcers (e.g., Hayward et al., 2002; Lett et al., 2001). The opioid triggering hypothesis is still in the process of being evaluated experimentally in our lab.
Conclusions
Elevated trait anger-out is associated with increased responding to both acute and chronic pain stimuli. The pain-related effects of anger-out do not appear to be a result of shared variance with general negative affect, but rather, seem to be unique to an expressive anger regulation style. Evidence from neuroimaging studies suggests that a network of interconnected brain regions including the OFC, rostral ACC, anterior insula, amygdala, and PAG underlies regulation of both pain and anger. Endogenous opioids appear to modulate activity in this brain network. Results of a series of studies support the hypothesis that the hyperalgesic effects of elevated trait anger-out derive in part from deficient endogenous opioid activity, most likely resulting from altered activity or connectivity in these brain regions. Factors such as gender, opioid analgesic use, genetic status, and state behavioral anger expression may moderate the opioid-related effects of trait anger-out. Brain imaging evidence suggests that trait anger-out and actual behavioral anger expression are both likely subserved by similar opioid-rich brain circuits. While existing studies support an association between elevated trait anger-out and reduced opioid analgesic function, the possibility that behavioral anger expression in high anger-out individuals triggers increased opioid analgesia has not be subjected to direct empirical evaluation. Until such studies have been conducted, pathways by which trait anger-out and behavioral anger expression might interact to determine expression of endogenous opioid analgesia remain an open question. This review highlights the potential value of exploring neurohumoral mechanisms that may contribute to the health-related effects of processes often considered to be purely psychological in character. Findings from studies like those reviewed may reveal new directions for addressing the pain and dysfunction associated with chronic pain conditions.
Acknowledgements
This work was supported in part by National Institute of Health grants R01-NS038145, R01-NS050578, R01-NS046694, and R01-MH071260, General Clinical Research Center Grant RR-00095, and NICHD Grant P30 HD15052 (Vanderbilt Kennedy Center for Research on Human Development). We wish to acknowledge the support and assistance of Elizabeth Stringer, John Gore, Ph.D., Malcolm Avison, Ph.D., Limin Chen, M.D., and the Vanderbilt Imaging Institute in obtaining the fMRI pilot data regarding anger-out. The authors also wish to acknowledge the contributions of the Vanderbilt University Program in Human Genetics DNA Resources Core for assistance in the genetic work described in this review. The first author would like to express his gratitude for comments provided by Ken Leiser and Jayne Cornwell during preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler LJ, Gyulai FE, Diehl DJ, Mintun MA, Winter PM, Firestone LL. Regional brain activity changes associated with fentanyl analgesia elucidated by positron emission tomography. Anesth. Analg. 1997;84:120–126. doi: 10.1097/00000539-199701000-00023. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Russell JA, Tranel D. A role for the human amygdala in recognizing emotional arousal from unpleasant stimuli. Psychol. Sci. 1999;10:167–171. [Google Scholar]
- Aubrun F, Salvi N, Coriat P, Riou B. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology. 2005;103:156–160. doi: 10.1097/00000542-200507000-00023. [DOI] [PubMed] [Google Scholar]
- Averill JR. Studies on anger and aggression: implications for theories of emotion. Am. Psychol. 1983;38:1145–1160. doi: 10.1037//0003-066x.38.11.1145. [DOI] [PubMed] [Google Scholar]
- Ax AF. Individual differences in autonomic learning: a quarter century of reflection. Int. J. Psychophys. 1990;10:1–9. doi: 10.1016/0167-8760(90)90039-g. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psych. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Boecker H, Sprenger T, Spilker ME, Henriksen G, Koppenhoefer M, Wagner KJ, Valet M, Berthele A, Tolle TR. The runner's high: opioidergic mechanisms in the human brain. Cereb. Cortex. 2008 2008 Feb 21; doi: 10.1093/cercor/bhn013. [Epub ahead of print], PMID: 18296435. [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc. Natl. Acad. Sci. USA. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehl S, al'Absi M, France CR, France J, Harju A, Burns JW, Chung OY. Anger management style and endogenous opioid function: is gender a moderator? J. Behav. Med. 2007b;30:209–219. doi: 10.1007/s10865-007-9099-2. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Burns JW, Chung OY, Quartana P. Anger management style and emotional reactivity to noxious stimuli among chronic pain patients and healthy controls: the role of endogenous opioids. Health Psychol. 2008a;27:204–214. doi: 10.1037/0278-6133.27.2.204. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Burns JW, Chung OY, Ward P, Johnson B. Anger and pain sensitivity in chronic low back pain patients and pain-free controls: The role of endogenous opioids. Pain. 2002;99:223–233. doi: 10.1016/s0304-3959(02)00104-5. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Chung OY, Burns JW. Differential effects of expressive anger regulation on chronic pain in CRPS and non-CRPS limb pain patients. Pain. 2003b;104:647–654. doi: 10.1016/S0304-3959(03)00135-0. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Chung OY, Burns JW. Anger expression and pain: an overview of findings and possible mechanisms. J. Behav. Med. 2006a;29:593–606. doi: 10.1007/s10865-006-9060-9. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Chung OY, Burns JW. The mu opioid receptor A118G gene polymorphism moderates effects of trait anger-out on acute pain sensitivity. Pain. 2008b doi: 10.1016/j.pain.2008.05.014. doi:10.1016/j.pain.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehl S, Chung OY, Burns JW, Biridepalli S. The association between anger expression and chronic pain intensity: evidence for partial mediation by endogenous opioid dysfunction. Pain. 2003a;106:317–324. doi: 10.1016/S0304-3959(03)00319-1. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Chung OY, Burns JW, Diedrich L. Trait anger expressiveness and pain-induced beta-endorphin release: support for the opioid dysfunction hypothesis. Pain. 2007a;130:208–215. doi: 10.1016/j.pain.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehl S, Chung OY, Donahue BS, Burns JW. Anger regulation style, postoperative pain, and relationship to the A118G mu opioid receptor gene polymorphism: a preliminary study. J. Behav. Med. 2006b;29:161–169. doi: 10.1007/s10865-005-9030-7. [DOI] [PubMed] [Google Scholar]
- Burnett FE, Scott LV, Weaver MG, Medbak SH, Dinan TG. The effect of naloxone on adrenocorticotropin and cortisol release: evidence for a reduced response in depression. J. Affect. Disorders. 1999;53:263–268. doi: 10.1016/s0165-0327(98)00127-x. [DOI] [PubMed] [Google Scholar]
- Burns JW, Bruehl S. Anger management style, opioid analgesic use, and chronic pain severity: a test of the opioid-deficit hypothesis. J. Behav. Med. 2005;28:555–563. doi: 10.1007/s10865-005-9020-9. [DOI] [PubMed] [Google Scholar]
- Burns JW, Bruehl S, Caceres C. Anger management style, blood pressure reactivity and acute pain sensitivity: Evidence for a Trait x Situation model. Ann. Behav. Med. 2004;27:195–204. doi: 10.1207/s15324796abm2703_7. [DOI] [PubMed] [Google Scholar]
- Burns JW, Johnson BJ, Devine J, Mahoney N, Pawl R. Anger manger management style and prediction of treatment outcome among male and female chronic pain patients. Behav. Res. Ther. 1998;36:1051–1062. doi: 10.1016/s0005-7967(98)00080-1. [DOI] [PubMed] [Google Scholar]
- Burns JW, Johnson BJ, Mahoney N, Devine J, Pawl R. Anger management style, hostility and spouse responses: gender differences in predictors of adjustment among chronic pain patients. Pain. 1996;64:445–453. doi: 10.1016/0304-3959(95)00169-7. [DOI] [PubMed] [Google Scholar]
- Burns JW, Kubilus A, Bruehl S. Emotion induction moderates effects of anger management style on acute pain sensitivity. Pain. 2003;106:109–118. doi: 10.1016/s0304-3959(03)00298-7. [DOI] [PubMed] [Google Scholar]
- Burns JW, Quartana P, Bruehl S. Anger management style moderates effects of emotion suppression during initial stress on pain and cardiovascular responses during subsequent pain-induction. Ann. Behav. Med. 2007;34:154–165. doi: 10.1007/BF02872670. [DOI] [PubMed] [Google Scholar]
- Burns JW, Quartana P, Bruehl S. Anger inhibition and pain: conceptualizations, evidence and new directions. J. Behav. Med. 2008;31:259–279. doi: 10.1007/s10865-008-9154-7. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bushman BJ, Baumeister RF, Phillips CM. Do people aggress to improve their mood? Catharsis beliefs, affect regulation opportunity, and aggressive responding. J. Pers. Soc. Psych. 2001;81:17–32. [PubMed] [Google Scholar]
- Canli T, Amin Z, Haas B, Omura K, Constable RT. A double dissociation between mood states and personality traits in the anterior cingulate. Behav. Neurosci. 2004;118:897–904. doi: 10.1037/0735-7044.118.5.897. [DOI] [PubMed] [Google Scholar]
- Carson JW, Keefe FJ, Goli V, Fras AM, Lynch TR, Thorp SR, Buechler JL. Forgiveness and chronic low back pain: a preliminary study examining the relationship of forgiveness to pain, anger, and psychological distress. J. Pain. 2005;6:84–91. doi: 10.1016/j.jpain.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Cepeda MS, Carr DB. Women experience more pain and require more morphine than men to achieve a similar degree of analgesia. Anesth. Analg. 2003;97:1464–1468. doi: 10.1213/01.ANE.0000080153.36643.83. [DOI] [PubMed] [Google Scholar]
- Chapman WP, Cohen ME, Cobb S. Measurements related to pain in neurocirculatory asthenia, anxiety neurosis, or effort syndrome: levels of heat stimulus perceived as painful and producing wince and withdrawal reactions. J. Clin. Invest. 1946;25:890–896. doi: 10.1172/JCI101777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WY, Wang CH, Liu PH, Liu CC, Tseng CC, Jawan B. Human opioid receptor A118G polymorphism affects intravenous patient-controlled analgesia morphine consumption after total abdominal hysterectomy. Anesthesiology. 2006;105:334–337. doi: 10.1097/00000542-200608000-00016. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Paul R, Zawacki TM, Moser DJ, Sweet L, Wilkinson H. Emotional and personality changes following cingulotomy. Emotion. 2001;1:38–50. doi: 10.1037/1528-3542.1.1.38. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LLB, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: perspectives from affective neuroscience. Cognition Emot. 1998;12:307–330. [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn. Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation – a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Deffenbacher JL, Oetting ER, Lynch RS, Morris CD. The expression of anger and its consequences. Behav. Res. Ther. 1996;34:575–90. doi: 10.1016/0005-7967(96)00018-6. [DOI] [PubMed] [Google Scholar]
- De Oca BM, DeCola JP, Maren S, Fanselow MS. Distinct regions of the periaqueductal gray are involved in the acquisition and expression of defensive responses. J Neurosci. 1998;18:3426–3432. doi: 10.1523/JNEUROSCI.18-09-03426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Shin LM, Alpert NM, Pitman RK, Orr SP, Laska M, Macklin ML, Fischman AJ, Rach SL. Anger in healthy men: a PET study using script-driven imagery. Biol. Psychiatry. 1999;46:466–472. doi: 10.1016/s0006-3223(99)00063-3. [DOI] [PubMed] [Google Scholar]
- Drexler K, Schweitzer JB, Quinn CK, Gross R, Ely TD, Muhammad F, Kilts CD. Neural activity related to anger in cocaine-dependent men: a possible link to violence and relapse. Am. J. Addict. 2000;9:331–339. doi: 10.1080/105504900750047382. [DOI] [PubMed] [Google Scholar]
- Duckro PN, Chibnall JT, Tomazic TJ. Anger, depression, and disability: a path analysis of relationships in a sample of chronic posttraumatic headache patients. Headache. 1995;35:7–9. doi: 10.1111/j.1526-4610.1995.hed3501007.x. [DOI] [PubMed] [Google Scholar]
- Eippert F, Veit R, Wesikopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapp. 2007;28:409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebretson TO, Matthews KA, Scheier MF. Relations between anger expression and cardiovascular reactivity: Reconciling inconsistent findings through a matching hypothesis. J. Pers. Soc. Psych. 1989;57:513–521. doi: 10.1037//0022-3514.57.3.513. [DOI] [PubMed] [Google Scholar]
- Faber SD, Burns JW. Anger management style, degree of expressed anger, and gender influence cardiovascular recovery from interpersonal harassment. J. Behav. Med. 1996;19:31–53. doi: 10.1007/BF01858173. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Fiorni L, Martignoni, Sances G, Costa A, Genazzani AR. Changes of opioid modulation of the hypothalamo-pituitary-adreanal axis in patients with severe premenstrual syndrome. Psychosom. Med. 1994;56:418–422. doi: 10.1097/00006842-199409000-00006. [DOI] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Kaplan L, Staud R, Ness TJ, Glover TL, Campbell CM, Mogil JS, Wallace MR. The A118G single nucleotide polymorphism of the mu-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. J. Pain. 2005;6:159–167. doi: 10.1016/j.jpain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Adler LJ, Gyulai FE, Diehl DJ, Mintun MA, Winter PM, Firestone LL. Regional brain activity changes associated with fentanyl analgesia elucidated by positron emission tomography. Anesth. Analg. 1997;84:120–126. doi: 10.1097/00000539-199701000-00023. [DOI] [PubMed] [Google Scholar]
- Foulds GA. Personality and personal illness. Tavistock; London: 1965. [Google Scholar]
- Frew AK, Drummond PD. Negative affect, pain and sex: the role of endogenous opioids. Pain. 2007;132(Suppl 1):S77–85. doi: 10.1016/j.pain.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Frew AK, Drummond PD. Stress-evoked opioid release inhibits pain in major depressive disorder. Pain. 2008 doi: 10.1016/j.pain.2008.04.022. doi:10.1016/j.pain.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Gaskin ME, Greene AF, Robinson ME, Geisser ME. Negative affect and the experience of chronic pain. J. Psychsom. Res. 1992;36:707–713. doi: 10.1016/0022-3999(92)90128-o. [DOI] [PubMed] [Google Scholar]
- Gelkopf M. Laboratory pain and styles of coping with anger. J. Psychol. 1997;131:121–123. doi: 10.1080/00223989709603510. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol. Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JE, Lobel M, Glass P, Lokshina I. Effects of written anger expression in chronic pain patients: making meaning from pain. J. Behav. Med. 2008;31:201–212. doi: 10.1007/s10865-008-9149-4. [DOI] [PubMed] [Google Scholar]
- Gray A, Jackson N, McKinlay JB. The relation between dominance, anger, and hormones in normally aging men: results from the Massachusetts Male Aging Study. Psychosom. Med. 1991;53:375–385. doi: 10.1097/00006842-199107000-00003. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: an integrative review. Rev. Gen. Psychol. 1998;2:271–299. [Google Scholar]
- Gross JJ. Emotion regulation: past, present, future. Cognit. Emot. 1999;13:551–573. [Google Scholar]
- Hadjipavlou G, Dunckley P, Behrens TE, Tracey I. Determining anatomical connectivities between cortical and brainstem pain processing regions in humans: a diffusion tensor imaging study in healthy controls. Pain. 2006;123:169–178. doi: 10.1016/j.pain.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Ham LP, Andrasik F, Packard RC, Bundrick CM. Psychopathology in individuals with post-traumatic headaches and other pain types. Cephalalgia. 1994;14:118–126. doi: 10.1046/j.1468-2982.1994.1402118.x. [DOI] [PubMed] [Google Scholar]
- Hamann S, Canli T. Individual differences in emotion processing. Curr. Opin. Neurobiol. 2004;14:233–238. doi: 10.1016/j.conb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Hardy SG, Leichnetz GR. Cortical projections to the periaqueductal gray in the monkey: a retrograde and orthograde horseradish peroxidase study. Neurosci. Lett. 1981;22:97–101. doi: 10.1016/0304-3940(81)90070-7. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. Trait anger predicts relative left frontal cortical activation to anger-inducing stimuli. Int. J. Psychophysiol. 2007;66:154–60. doi: 10.1016/j.ijpsycho.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Sigelman J. State anger and prefrontal brain activity: evidence that insult-related relative left-prefrontal activation is associated with experienced anger and aggression. J. Pers. Soc. Psychol. 2001;80:797–803. [PubMed] [Google Scholar]
- Hayward MD, Pintar JE, Low MJ. Selective reward deficit in mice lacking beta-endorphin and enkephalin. J. Neuroscience. 2002;22:8251–8258. doi: 10.1523/JNEUROSCI.22-18-08251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemphill RE, Hall KR, Crookes TG. A preliminary report on fatigue and pain tolerance in depressive and psychoneurotic patients. J. Ment. Sci. 1952;98:433–440. doi: 10.1192/bjp.98.412.433. [DOI] [PubMed] [Google Scholar]
- Hewig J, Hagemann D, Seifert J, Naumann E, Bartussek D. On the selective relation of frontal cortical asymmetry and anger-out versus anger-control. J. Pers. Soc. Psychol. 2004;87:926–939. doi: 10.1037/0022-3514.87.6.926. [DOI] [PubMed] [Google Scholar]
- Janssen SA. Negative affect and sensitization to pain. Scand. J. Psychol. 2002;43:131–137. doi: 10.1111/1467-9450.00278. [DOI] [PubMed] [Google Scholar]
- Janssen SA, Spinhoven P, Brosschot JF. Experimentally induced anger, cardiovascular reactivity, and pain sensitivity. J. Psychosom. Res. 2001;51:479–485. doi: 10.1016/s0022-3999(01)00222-7. [DOI] [PubMed] [Google Scholar]
- Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch. Gen. Psychiatry. 2006;63:1199–1208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- Kerns RD, Rosenberg R, Jacob MC. Anger expression and chronic pain. J. Behav. Med. 1994;17:57–67. doi: 10.1007/BF01856882. [DOI] [PubMed] [Google Scholar]
- Kring AM, Smith DA, Neale JM. Individual differences in dispositional expressiveness: development and validation of the Emotional Expressivity Scale. J. Pers. Soc. Psychol. 1994;66:934–949. doi: 10.1037//0022-3514.66.5.934. [DOI] [PubMed] [Google Scholar]
- Klepstad P, Rakvag TT, Kaasa S, Holthe M, Dale O, Borchgrevink PC, Baar C, Vikan T, Krokan HE, Skorpen F. The 118 A>G polymorphism in the human muopioid receptor gene may increase morphine requirements in patients with pain caused by malignant disease. Acta Anaesthesiol. Scand. 2004;48:1232–1239. doi: 10.1111/j.1399-6576.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- Koers G, Gaillard AW, Mulder G. Evoked heart rate and blood pressure in an S1-S2 paradigm. Biol. Psychol. 1997;46:247–74. doi: 10.1016/s0301-0511(97)00015-x. [DOI] [PubMed] [Google Scholar]
- Kroslak T, LaForge S, Gianotti RJ, Ho A, Nielsen DA, Kreek MJ. The single nucleotide polymorphism A118G alters functional properties of the human mu opioid receptor. J. Neurochem. 2007;103:77–87. doi: 10.1111/j.1471-4159.2007.04738.x. [DOI] [PubMed] [Google Scholar]
- Lai JY, Linden W. Gender, anger expression style, and opportunity for anger release determine cardiovascular reaction to and recovery from anger provocation. Psychosom. Med. 1992;54:297–310. doi: 10.1097/00006842-199205000-00006. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: studies of motivation and attention. Am. Psychol. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Merschdorf U. Impulsivity, aggression, and serotonin: a molecular psychobiological perspective. Behav. Sci. Law. 2000;18:581–604. doi: 10.1002/1099-0798(200010)18:5<581::aid-bsl411>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Koh MT. Naloxone attenuates the conditioned place preference induced by wheel running in rats. Physiol. Behav. 2001;72:355–358. doi: 10.1016/s0031-9384(00)00427-3. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Fanselow MS. Opioid and nonopioid conditional analgesia: the role of spinal opioid, noradrenergic, and serotonergic systems. Behav Neurosci. 1991;105:687–698. doi: 10.1037//0735-7044.105.5.687. [DOI] [PubMed] [Google Scholar]
- Linden W, Hogan BE, Rutledge T, Chawla A, Lenz JW, Leung D. There is more to anger coping than “in” or “out”. Emotion. 2003;3:12–29. doi: 10.1037/1528-3542.3.1.12. [DOI] [PubMed] [Google Scholar]
- Linton SJ. A review of psychological risk factors in back and neck pain. Spine. 2000;25:1148–1156. doi: 10.1097/00007632-200005010-00017. [DOI] [PubMed] [Google Scholar]
- Linton SJ. Do psychological factors increase the risk for back pain in the general population in both a cross-sectional and prospective analysis? Eur. J. Pain. 2005;9:355–361. doi: 10.1016/j.ejpain.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Lombardo ER, Tan G, Jensen MP, Anderson KO. Anger management style and associations with self-efficacy and pain in male veterans. J. Pain. 2005;6:765–770. doi: 10.1016/j.jpain.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Lotsch J, Skarke C, Grosch S, Darimont J, Schmidt H, Geisslinger G. The polymorphism A118G of the human mu-opioid receptor gene decreases the pupil constrictory effect of morphine-6-glucuronide but not that of morphine. Pharmacogenetics. 2002;12:3–9. doi: 10.1097/00008571-200201000-00002. [DOI] [PubMed] [Google Scholar]
- Lotsch J, Stuck B, Hummel T. The human mu-opioid receptor gene polymorphism 118A > G decreases cortical activation in response to specific nociceptive stimulation. Behav. Neurosci. 2006;120:1218–1224. doi: 10.1037/0735-7044.120.6.1218. [DOI] [PubMed] [Google Scholar]
- Maclean PD. Some psychiatric implications of physiological studies on frontotemporal portion of limbic system (visceral brain). Electroencephalography Clin Neurophys. 1952;4:407–418. doi: 10.1016/0013-4694(52)90073-4. [DOI] [PubMed] [Google Scholar]
- Margas W, Zubkoff I, Schuler G, Janicki P, Ruiz-Velasco V. Modulation of Ca2+ channels by heterologously expressed wild-type mutant human mu opioid receptors (hMORs) containing the A118G single nucleotide polymorphism. J. Neurophysiol. 97:1058–1067. doi: 10.1152/jn.01007.2006. [DOI] [PubMed] [Google Scholar]
- Martin R, Wan CK, David JP, Wegner EL, Olson BD, Watson D. Style of anger expression: relation to expressivity, personality, and health. Person. Soc. Psych. Bull. 1999;25:1196–1207. [Google Scholar]
- Martin R, Watson D, Wan CK. A three-factor model of trait anger: dimensions of affect, behavior, and cognition. J. Personal. 2000;68:869–897. doi: 10.1111/1467-6494.00119. [DOI] [PubMed] [Google Scholar]
- Martin del Campo AF, Dowson JH, Herbert J, Paykel ES. Effects of naloxone on diurnal rhythms in moods and endocrine function: a dose-response study in man. Psychopharm. 1994;114:583–590. doi: 10.1007/BF02244988. [DOI] [PubMed] [Google Scholar]
- Materazzo F, Cathart S, Pritchard D. Anger, depression, and coping interactions in headache activity and adjustment: a controlled study. J. Psychosom. Res. 2000;49:69–75. doi: 10.1016/s0022-3999(00)00144-6. [DOI] [PubMed] [Google Scholar]
- McCubbin JA, Surwit RS, Williams RB. Opioid dysfunction and risk for hypertension: Naloxone and blood pressure responses during different types of stress. Psychosom. Med. 1988;50:8–14. doi: 10.1097/00006842-198801000-00002. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog. Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Miller GM, Bendor J, Tiefenbacher S, Yang H, Novak MA, Madras BK. A muopioid receptor single nucleotide polymorphism in rhesus monkey: association with stress response and aggression. Mol. Psychiatry. 2004;9:99–108. doi: 10.1038/sj.mp.4001378. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn. Affect. Behav. Neurosci. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ohira H, Nomura M, Ichikawa N, Isowa T, Idaka T, Sato A, Fukuyama S, Nakajima T, Yamada J. Association of neural and physiological responses during voluntary emotion suppression. Neuroimage. 2006;29:721–733. doi: 10.1016/j.neuroimage.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia--imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Peyron R, Faillenot I, Mertens P, Laurent B, García-Larrea L. Motor cortex stimulation in neuropathic pain. Correlations between analgesic effect and hemodynamic changes in eth brain. A PET study. Neuroimage. 2007;34:310–321. doi: 10.1016/j.neuroimage.2006.08.037. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, García-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol. Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol. Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Pickar D, Cohen MR, Naber D, Cohen RM. Clinical studies of the endogenous opioid system. Biol. Psychiatry. 1982;17:1243–1276. [PubMed] [Google Scholar]
- Pietrini P, Guazzelli M, Basso G, Jaffe K, Grafman J. Neural correlates of imaginal aggressive behavior assessed by positron emission tomography in healthy subjects. Am. J. Psychiatry. 2000;157:1772–1781. doi: 10.1176/appi.ajp.157.11.1772. [DOI] [PubMed] [Google Scholar]
- Poon CS, Siniaia MS. Plasticity of cardiorespiratory neural processing: classification and computational functions. Respir. Physiol. 2000;122:83–109. doi: 10.1016/s0034-5687(00)00152-3. [DOI] [PubMed] [Google Scholar]
- Porter LS, Stone AS, Schwartz JE. Anger expression and ambulatory blood pressure: a comparison of state and trait measures. Psychosom. Med. 1999;61:454–463. doi: 10.1097/00006842-199907000-00009. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn. Affect. Behav. Neurosci. 2007;7:391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- Powell JE, Taylor D. Anger, depression, and anxiety following heroin withdrawal. Int. J. Addict. 1992;27:25–35. doi: 10.3109/10826089109063460. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Ramzy I, Wallerstein RS. Pain, fear, and anxiety: a study in their interrelationships. Psychoanal. Study Child. 1958;13:147–189. doi: 10.1080/00797308.1958.11823179. [DOI] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Rhudy JL, Williams AE, McCabe KM, Nguyen MA, Rambo P. Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology. 2005;42:579–587. doi: 10.1111/j.1469-8986.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- Rhudy JL, Williams AE, McCabe KM, Rambo PL, Russell JL. Emotional modulation of spinal nociception and pain: the impact of predictable noxious stimulation. Pain. 2006;126:221–233. doi: 10.1016/j.pain.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Ribeiro SC, Kennedy SE, Smith YR, Stohler CS, Zubieta JK. Interface of physical and emotional stress regulation through the endogenous opioid system and muopioid receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:1264–1280. doi: 10.1016/j.pnpbp.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Sayar K, Gulec H, Topbas M. Alexithymia and anger in patients with fibromyalgia. Clin. Rheumatol. 2004;23:441–448. doi: 10.1007/s10067-004-0918-3. [DOI] [PubMed] [Google Scholar]
- Schachter H. Pain, fear, and anger in hypertensives and normotensives: a psychophysiological study. Psychosom. Med. 1957;19:17–29. doi: 10.1097/00006842-195701000-00003. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Strain EC, Greenberg BD, Preston KL, Lancaster E, Bigelow GE, Barta PE, Pearlson GD. Site of opioid action in the human brain: mu and kappa agonsits’ subjective and cerebral blood flow effects. Am. J. Psychiatry. 1998;155:470–473. doi: 10.1176/ajp.155.4.470. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch. Gen. Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- Sewards TV, Sewards MA. Fear and power-dominance drive motivation: neural representations and pathways mediating sensory and mnemonic inputs, and outputs to premotor structures. Neurosci. Biobeh. Rev. 2002;26:553–79. doi: 10.1016/s0149-7634(02)00020-9. [DOI] [PubMed] [Google Scholar]
- Siegel J. The Multidimensional Anger Inventory. J. Personal. Soc. Psychol. 1986;51:191–200. doi: 10.1037/0022-3514.51.1.191. [DOI] [PubMed] [Google Scholar]
- Skarke C, Darimont J, Schmidt H, Geisslinger G, Lotsch J. Analgesic effects of morphine and morphine-6-glucuronide in a transcutaneous electrical pain model in healthy volunteers. Clin. Pharmacol. Ther. 2003;73:107–121. doi: 10.1067/mcp.2003.5. [DOI] [PubMed] [Google Scholar]
- Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta JK. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J. Neurosci. 2006;26:5777–5785. doi: 10.1523/JNEUROSCI.5223-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Johnson EH, Russell SF, Crane RJ, Jacobs GA, Worden TJ. The experience and expression of anger: construction and validation of an anger expression scale. In: Chesney MA, Rosenman RH, editors. Anger and hostility in cardiovascular and behavioral disorders. Hemisphere Publishing Corp.; Washington, D.C.: 1985. pp. 5–30. [Google Scholar]
- Speilberger CD. State-Trait Anger Expression Inventory Professional Manual. Psychological Assessment Resources; Odessa, FL: 1999. [Google Scholar]
- Sprenger T, Valet M, Boecker H, Henriksen G, Spilker ME, Willoch F, Wagner KJ, Wester HJ, Tölle TR. Opioidergic activation in the medial pain system after heat pain. Pain. 2006;122:63–67. doi: 10.1016/j.pain.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Staud R. Predictors of clinical pain intensity in patients with fibromyalgia syndrome. Curr. Rheumatol. Rep. 2004;6:281–286. doi: 10.1007/s11926-004-0036-x. [DOI] [PubMed] [Google Scholar]
- Suchday S, Larkin KT. Biobehavioral responses to interpersonal conflict during anger expression among anger-in and anger-out men. Ann. Behav. Med. 2001;23:282–290. doi: 10.1207/S15324796ABM2304_7. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biol. Psychiatry. 2006;60:296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. J. Neurosci. 2002;22:2748–2752. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, Erhard P, Tolle TR. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain--an fMRI analysis. Pain. 2004;109:399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Venable VL, Carlson CR, Wilson J. The role of anger and depression in recurrent headache. Headache. 2001;41:21–30. doi: 10.1046/j.1526-4610.2001.111006021.x. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Derbyshire S, Jones AK. Pain processing in four regions of human cingulate cortex localized with co-registered PET and MR imaging. Eur. J. Neurosci. 1996;8:1461–1473. doi: 10.1111/j.1460-9568.1996.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J. Comp. Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Voulgari A, Lykouras L, Papanikolaou M, Tzonou A, Danou-Roussaki A, Christodoulou G. Influence of psychological and clinical factors on postoperative pain and narcotic consumption. Psychother. Psychosom. 1991;55:191–196. doi: 10.1159/000288429. [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc. Natl. Acad. Sci. USA. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KJ, Sprenger T, Kochs EF, Tölle TR, Valet M, Willoch F. Imaging human cerebral pain modulation by dose-dependent opioid analgesia: a positron emission tomography activation study using remifentanil. Anesthesiology. 2007;106:548–556. doi: 10.1097/00000542-200703000-00020. [DOI] [PubMed] [Google Scholar]
- Webb HE, Lascelles RG. Treatment of facial and head pain associated with depression. Lancet. 1962;1:355–356. doi: 10.1016/s0140-6736(62)91305-3. [DOI] [PubMed] [Google Scholar]
- Wildmann J, Kruger A, Schmole M, Niemann J, Matthaei H. Increase of circulating beta-endorphin-like immunoreactivity correlates with the change in feeling of pleasantness after running. Life Sci. 1986;38:997–1003. doi: 10.1016/0024-3205(86)90233-x. [DOI] [PubMed] [Google Scholar]
- Young EA, Lopez JF, Murphy-Weinburg V, Watson SJ, Akil H. Hormonal evidence for altered responsiveness to social stress in major depression. Neuropsychopharm. 2000;23:411–418. doi: 10.1016/S0893-133X(00)00129-9. [DOI] [PubMed] [Google Scholar]
- Zald DH, Kim SW. Anatomy and function of the orbital frontal cortex, I: anatomy, neurocircuitry; and obsessive-compulsive disorder. J. Neuropsychiatry Clin. Neurosci. 1996a;8:125–138. doi: 10.1176/jnp.8.2.125. [DOI] [PubMed] [Google Scholar]
- Zald DH, Kim SW. Anatomy and function of the orbital frontal cortex, II: Function and relevance to obsessive-compulsive disorder. J. Neuropsychiatry Clin. Neurosci. 1996b;8:249–261. doi: 10.1176/jnp.8.3.249. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J. Biol. Chem. 2005;280:32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, DeRubeis RJ, Redei E. High self-esteem, hardiness and affective stability are associated with higher basal pituitary-adrenal hormone levels. Psychoneuroendocrin. 1995;20:591–601. doi: 10.1016/0306-4530(95)00005-9. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, Koeppe RA. Regulation of human affective responses by anterior cingulate and limbic muopioid neurotransmission. Arch. Gen. Psychiatry. 2003;60:1145–1153. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]