Abstract
We describe the design and report baseline results of a cluster-randomized intervention to determine the importance of bovines for Schistosoma japonicum transmission in southern China. The study involves four matched village pairs in Hunan and Jiangxi Provinces, with a village within each pair randomly selected as intervention (human and bovine praziquantel treatment) or control (human praziquantel treatment only). Total study population prevalences at baseline were 12.4% (n = 5,390) and 15.2% (n = 1,573) for humans and bovines, respectively; village prevalences were similar within pairs. Bovine contamination index calculations showed that bovines less than 24 months of age were responsible for 74% of daily bovine environmental contamination with S. japonicum eggs. The village characteristics and baseline results underpin a rigorous study, which has major implications for deployment of a transmission-blocking bovine vaccine against S. japonicum. The combination of such a vaccine with other control strategies could potentially eliminate S. japonicum from southern China.
INTRODUCTION
Schistosomiasis japonica, which is caused by infection with Schistosoma japonicum, is a zoonosis of major public health importance in China, with approximately one million people infected.1,2 Locations with high endemicity are around the Dongting and Poyang Lakes, where the bulk of transmission occurs.2–5
We undertook a drug-based intervention study (1998–2003) around the Poyang Lake in Jiangxi Province, to test the hypothesis that buffaloes are major reservoirs for human infection in the marshlands and lake regions of southern China.6 The rationale behind this study was that in these areas, environmental contamination with schistosome eggs is largely due to water buffaloes because of their high fecal output (approximately 25–50 kg/day).4 Bovines, particularly water buffaloes, had been previously reported as being important reservoirs for S. japonicum transmission,1,7 although proof of this hypothesis had not been established.
The results from the drug intervention study showed that human incidence decreased from 8.9% to 5.6% over a four-year period. It was concluded that buffalo chemotherapy was responsible for a significant reduction in human infection due to the prevention of an increase in the human infection rates, and that buffaloes are the major reservoir hosts for schistosomiasis transmission to humans in this area. Mathematical modeling supported this conclusion, which predicted that buffaloes are responsible for approximately 75% of human transmission.1,8 However, these results were only for one pair of villages around Poyang Lake and may not be completely representative of all the lake and marshland areas of southern China.
This paper describes the design and reports on the baseline results of a more extensive cluster-randomized intervention trial (2004–2008) currently underway around both the Poyang and Dongting Lakes. Furthermore, the paper justifies the selected study design and uses the baseline results to validate the chosen methodology. Specifically, the trial aims are to 1) reproduce and validate the results of the drug intervention study on a larger, more rigorous and generalizable scale; 2) examine the efficacy of bovine chemotherapy on human infection and reinfection rates so as to provide insight into the potential effectiveness of an anti-schistosome vaccine targeting buffaloes;9,10 3) assess bovine chemotherapy as a plausible schistosomiasis control method, particularly in combination with human treatment; and 4) integrate the empirical data collected from the study into our previously developed mathematical model.8
METHODS
Study design
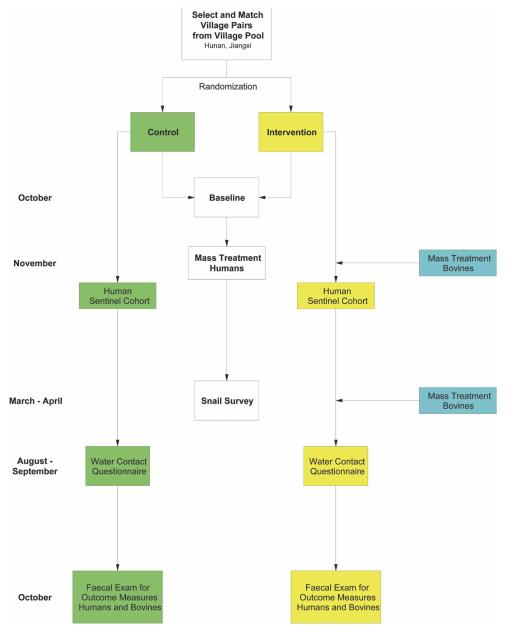
An overview of the cluster-randomized design11,12 is shown in (Figure 1). The trial commenced in 2004 and will end in 2008. Administrative villages were selected as the clusters; these were matched into pairs to reduce confounding and to increase statistical efficiency, and were then randomized into control and intervention villages within each pair. All humans (but no bovines) are treated in the control villages, and humans and bovines are treated in the intervention villages. The primary end point of the trial will be human infection incidences. The efficacy of bovine treatment will be measured by the accumulated comparison of human infection incidences across the intervention and control villages, and will be reflected in the predicted percentage of human infection-years prevented by the intervention.
FIGURE 1.
Trial profile. November to October equals one year of the trial and is repeated for four years. This figure appears in color at www.ajtmh.org.
Village selection
Candidate villages with a human and bovine schistosome prevalence of 10–15% were identified from Jiangxi and Hunan Provinces. Four village pairs were then selected, two pairs from Hunan and two pairs from Jiangxi. Within each pair, villages were matched as closely as possible on the basis of criteria related to transmission (infection levels, transmission ecology) and force of infection (proximity to water contact site, bovine:human ratio). One village in each pair was randomly chosen as the intervention village (human and bovine treatment), leaving the other as the control (human treatment only).
Study sites
Four pairs of villages were selected for the trial: two pairs from Hunan Province (Pair 1: Yongxiang and Mengjiang; Pair 2: Yongfu and Jizhong) and two pairs from Jiangxi Province (Pair 3: Aiguo and Dingshan; Pair 4: Fuqian and Xindong). Table 1 shows the characteristics of the village pairs and their randomly assigned status as either a control or intervention village. This table highlights the similarities of the village pairs as per the study design. For example, all villages have grass and reeds as the vegetation on the marshland; each pair has similar human and bovine populations as well as geographic size and distance to water contact site. Types of marshland within the village pairs are the same but differ between the provinces.3,13 Schistosoma japonicum transmission occurs in two seasons around the Poyang and Dongting Lakes, with the first generally from April to June and the second generally from August to October.
TABLE 1.
Village pair characteristics (October 2004), People’s Republic of China
| Province | Hunan | Jiangxi | ||||||
| Pair | Pair 1 | Pair 2 | Pair 3 | Pair 4 | ||||
| Village status | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention |
| Administrative village | Yongxiang | Mengjiang | Jizhong | Yongfu | Dingshan | Aiguo | Fuqian | Xindong |
| Location | Li Xian County, Mengjiang Township |
Li Xian County, Juiyuan Township |
Yongxiu County, Wucheng Township |
Jinxian County, Sanli Township |
Yugan County, Kangshan Township |
Yugan County, Dongtang Township |
||
| Area (km2) | 1.34 | 1.23 | 2.8 | 2.4 | 6 | 4 | 4 | 5 |
| Distance between villages within pairs (km) |
9 | 14 | 50 | 35 | ||||
| Distance between village and water contact site (Lake) (meters) |
100–500 | 30 | 50 | 300 | 100 | |||
| Type of marshland | Beach and dyke type | Lake type | ||||||
| Vegetation on marshland | Grass and reeds | |||||||
| Registered human population |
1,176 | 888 | 1,581 | 918 | 1,091 | 1,548 | 1,512 | 1,649 |
| Actual population residing |
550 | 573 | 731 | 523 | 614 | 870 | 971 | 981 |
| Bovine (water buffalo) population |
68 | 61 | 97 | 112 | 569 | 265 | 257 | 250 |
| Goats present | Yes | Yes | Yes | Yes | No | No | No | No |
Baseline
At baseline, two stool samples were collected and a questionnaire was administered to all residents who usually lived in the villages. Stool samples were examined microscopically using the Kato-Katz thick smear technique, with three slides per stool read blind, to determine S. japonicum prevalence and intensity of infection.14 The questionnaire consisted of questions relating to demographics, medical history, and water contact.
A stool sample was also collected from all bovines in the study villages and examined for S. japonicum prevalence using the miracidial hatching test (3 individual hatches read blind [50 grams of feces/hatching]) and intensity of infection, using a traditional Chinese sedimentation method.4 A snail survey was performed to measure the prevalence of infection in snails and the density of infected snails per unit area. This was conducted using the Chinese method of random quadrant sampling (0.11 meters2-sized frames, 20 meters between frames) of the marshland areas relevant to each village.15
Follow-up and study procedure
After the baseline survey, a sentinel cohort of people, to be monitored for new infections for the duration of the study, was selected from each village. The inclusion criteria were that an individual must be 1) a resident of the selected village, 2) a resident of the village for more than 12 months, 3) 5–60 years of age, 4) not intending to migrate out of the village for the next four years, and 5) continuously residing in the study area over the study period.
Mass treatment of residents is carried out in all villages annually in November. In the intervention villages, bovines are treated twice annually in April and November. One month after their respective mass treatments, stool samples are collected from the sentinel cohort and bovines (intervention villages only) to test treatment efficacy. Any positive persons or bovines are re-treated and retested until stool samples show negative results.
A snail survey is performed each year in March/April using the Chinese traditional method described above. A water contact questionnaire is administered to the sentinel cohort members annually in August in Hunan Province and in September in Jiangxi Province. The questionnaire covers general questions relating to participant yearly water exposure by season; a month-long water contact diary is also kept.16
In October to November each year, stool samples are collected from sentinel cohort members and all bovines to determine outcome measures, which include incidence and intensity of infection for sentinel cohort members and infection rates and intensity of infection for bovines.
Treatment regimen
All village residents are treated annually with a single dose of praziquantel (40 mg/kg) as recommended by the World Health Organization (WHO).17 Transient fishermen and boatmen are treated annually with praziquantel (40 mg/kg).
All bovines in the intervention villages are treated twice annually with praziquantel (buffaloes, 25 mg/kg; cattle, 30 mg/kg) until cured. Interloping bovines from neighboring villages are carefully monitored by local field staff and treated as part of the study group, but are not included in prevalence calculations. All new bovines entering the intervention villages are reported to local field staff, recorded, tested, and treated as above. Newborn bovines are recorded and treated three months after birth. Previous studies in Poyang and Dongting Lakes showed 85–95% efficacy on a single praziquantel dose in humans (40 mg/kg) and water buffaloes (25 mg/kg), with 100% efficacy after re-examination and retreatment.18–20
In China, it is estimated that up to 40 species of wild and domestic animals are infected with schistosomes but many of these are not considered to act as reservoirs.6,21 Sheep and goats are not considered to be significant/major reservoirs,1,6,21 but to reduce confounding they are treated twice annually in all villages with a single dose of praziquantel (20 mg/kg). Any dogs or pigs found roaming the marshland similarly receive praziquantel treatment.
Power
The power for best-case and worst-case scenarios in terms of bovine effect size and bovine treatment coverage was calculated for the first two years of the study (bovine effect size = environmental contamination leading to S. japonicum transmission to humans).
The calculations used the following assumptions: 1) 4 village pairs; 2) target sentinel cohort size of 300 subjects per village; 3) total village population of 1,000; 4) the study works on treating the whole village; 5) design effect of 1.5; 6) an attrition rate between years 1 and 2 of 20%; 7) years 1 and 2 are independent for the cumulative power; 8) target sample size is the number obtained in each village for testing (after excluding non-compliers); and 9) significance level of 1% in year 1 and 5% in year 2.
The power calculated for the worst-case scenario of 60% bovine effect size and 80% bovine treatment coverage was 38.1% for year 1 and 88.7% for year 2, and the cumulative power was 95.7% for years 1 and 2. The power calculated for the best-case scenario of 70% bovine effect size and 90% bovine treatment coverage was 68.6% for year 1 and 98% for year 2, and the cumulative power was 99.8% for years 1 and 2.
Data management and statistics: baseline
A Microsoft (Redmond, WA) ACCESS based database was designed specifically for this project and was used for data management. SAS software (SAS Institute, Cary, NC) was used for statistical analysis and the Cochran-Mantel-Haenszel test was used to calculate odds ratios. The bovine contamination index (BCI) was derived using the formula22 BCI = [arithmetic mean eggs per gram [epg] (of infected bovines) × number of infected bovines] × 2,500. A conservative approach was used when deriving the BCI by using the lower end of the 25–50 kg of bovine feces excreted per day; thus, the value 25,000.
Statistical analysis of end points
Statistical analysis of study end points will be similar to published procedures.6 Logistic regression will be used for formal analyses of human and bovine infection rates and intensities. Snail infections will be analyzed by calculating prevalence and the density of infected snails per 100 meters2.
Ethical considerations
Written ethical approval for this study was obtained from the national, provincial, and village levels within China, and the Human Research Ethics Committee of the Queensland Institute of Medical Research. Oral informed consent was obtained from all adults and from parents or guardians of minors who were involved in the project. Study participants identified as stool egg-positive for schistosomiasis were treated with 40 mg/kg of praziquantel, the current dosage recommended by WHO.17
RESULTS
Baseline human prevalence and intensity of infection
At baseline, 5,390 of 5,813 residents were surveyed across the four village pairs (coverage rate = 93%). Stool examinations showed a S. japonicum prevalence of 12.4% (95% confidence interval [CI] = 11.5–13.3%) across the total study population.
Baseline human prevalence and intensity of infection (geometric mean epg in the infected individuals) for S. japonicum within the study villages are shown with 95% CIs in Table 2. The baseline human prevalences were similar within village pairs and the odds ratios were not significant, although CIs were wide (Table 2).
TABLE 2.
Village pair characteristics at baseline (October 2004), People’s Republic of China*
| Province | Hunan | Jiangxi | ||||||
| Pair | Pair 1 | Pair 2 | Pair 3 | Pair 4 | ||||
| Village status | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention |
| Administrative village | Yongxiang | Mengjiang | Jizhong | Yongfu | Dignshan | Aiguo | Fuqian | Xindong |
| Human | ||||||||
| Sample size | 520 | 528 | 707 | 519 | 607 | 762 | 810 | 937 |
| Prevalence | 7.7% (5.4–10.0) | 9.5% (7.0–12.0) | 14.9% (12.2–17.5) | 17.0% (13.7–20.2) | 13.0% (10.3–15.7) | 11.5% (9.3–13.8) | 11.0% (8.8–13.1) | 14.0% (11.8–16.2) |
| Geometric mean epg in infected animals |
7.3 (5.9–9.0) | 8.6 (6.9–10.6) | 9.2 (7.7–11.1) | 12.4 (9.8–15.7) | 26.3 (21.3–32.5) | 12.2 (9.7–15.2) | 10.8 (8.8–13.3) | 27.6 (21.4–35.6) |
| Sex ratio (F/M) | 252/268 | 258/270 | 349/358 | 246/273 | 298/309 | 375/387 | 350/451 | 424/512 |
| Prevalence by sex (F/M) | 6.0/9.3% | 4.7/14.1% | 10.3/19.3% | 17.5/16.5% | 11.8/14.2% | 8.0/15.0% | 8.1/13.3% | 10.6/16.8% |
| Mean age (years) | 41.0 | 42.5 | 40.5 | 41.4 | 36.5 | 35.6 | 37.3 | 35.3 |
| Sentinel cohort no. | 363 | 335 | 467 | 334 | 400 | 467 | 671 | 751 |
| Sentinel cohort prevalence Bovine |
7.7% (5.0–10.5) | 8.7% (5.6–11.7) | 13.9% (10.8–17.1) | 18.9% (14.6–23.1) | 14.2% (10.7–17.6) | 13.1% (10.0–16.1) | 11.9% (9.5–14.4) | 13.8% (11.4–16.3) |
| Sample size | 63 | 59 | 82 | 88 | 564 | 254 | 233 | 230 |
| Prevalence | 25.4% (14.3–36.4) | 28.8% (16.9–40.7) | 29.3% (19.2–39.3) | 18.2% (10.0–26.4) | 11.5% (8.9–14.2) | 14.2% (9.9–18.5) | 15.9% (11.2–20.6) | 12.2% (7.9–16.4) |
| Geometric mean epg in infected animals |
4.9 (2.6–9.3) | 5.5 (2.6–11.7) | 7.2 (5–10.4) | 3.4 (1.8–6.7) | 0.53 (0.43–0.66) | 2.3 (1.8–3) | 1.9 (1.4–2.5) | 0.8 (0.45–1.5) |
| Snail | ||||||||
| Prevalence | 7.04% | 2.2% | 19.65% | 1.24% | 0.87% | 1.4% | 4.05% | 0.24% |
| Density of infected snails per 100 m2 |
5.23 | 14.51 | 56.26 | 46.73 | 6.91 | 35.36 | 18.50 | 2.68 |
| Odds ratio | ||||||||
| Human prevalence | 0.80 (0.52–1.23) | 1.17 (0.86–1.59) | 1.15 (0.83–1.59) | 1.32 (0.99–1.76) | ||||
| Bovine prevalence | 0.84 (0.38–1.87) | 0.54–0.26–1.10) | 0.79 (0.51–1.22) | 0.73 (0.43–1.25) | ||||
Values in parentheses are 95% confidence intervals. epg = eggs per gram (of feces).
There were slightly more males than females within the study villages (Table 2). Males also had a higher schistosome prevalence then females (Table 2), except for Yongfu where females had a slightly higher prevalence (17.5% versus 16.5%).
Baseline human prevalence by sex, age, and occupation for the total study population
There were more males (2,828) than females (2,561) in the total study population. Also, the prevalence was higher in males (15.0%) than females (9.6%).
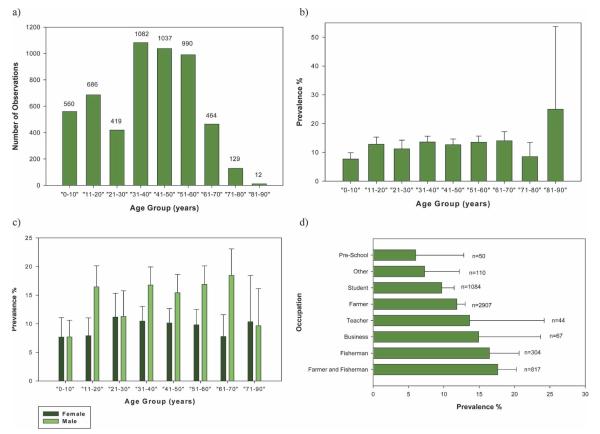
Most of the people in the total study population were 31–60 years of age (Figure 2a) with a mean age of 38 years. Prevalences within the age groups are shown in Figure 2b. Most groups had a prevalence between 11% and 14%, and those 0–10 and 71–80 years of age had prevalences of 7.7% and 8.5%, respectively. Those persons 81–90 years of age had a prevalence of 25%, the highest of all age groups. Prevalence by age and sex is also shown in Figure 2c; except for females 71–90 years of age, all males per age group had a higher prevalence than females.
FIGURE 2.
Baseline human prevalence by sex, age, and occupation for the total study population. a, Age group versus number of observations; b, age group versus prevalence; c, age group versus sex versus prevalence; d, occupation versus prevalence. Error bars show upper 95% confidence intervals. This figure appears in color at www.ajtmh.org.
The main occupation was farmer (2,907), followed by student (1,084), farmer and fisherman (817), and fisherman (304) (Figure 2d). Prevalence by occupation is shown in Figure 2d. The highest prevalences were found in farmer and fisherman (17.6%) and fisherman only (16.5%); the lowest was in pre-school children (6%).
Baseline bovine prevalence and intensity of infection
At baseline, 1,573 of 1,679 bovines were examined for S. japonicum across the total study population (coverage rate = 94%) and had a prevalence of 15.2% (95% CI = 13.4–17.0%). Of these, 60 were cattle with a baseline prevalence of 21.7% (95% CI = 10.9–32.4%) and 1,513 were water buffaloes with a baseline prevalence of 14.9% (95% CI = 13.1–16.7%).
The baseline bovine prevalence and intensity of infection (epg) in infected bovines for S. japonicum within the study villages are shown with 95% CIs in Table 2. Baseline bovine prevalences were similar within the village pairs and the odds ratios were not statistically significant although CIs were wide (Table 2).
Baseline bovine prevalence, intensity of infection, and contamination index by age for the total study population
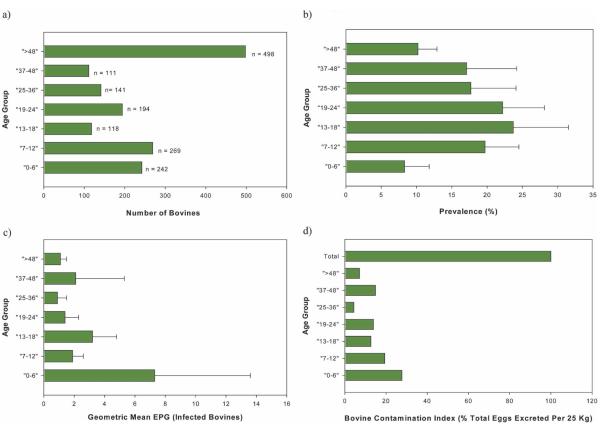
Bovine ages (in months) were divided into age groups and are shown with the respective numbers of animals in Figure 3a. Most (1,075 [68.3%] of 1,5673) bovines were less than of 48 months of age. The prevalence and intensity of infection (epg of infected bovines) by age group is shown in Figure 3b and c. The highest prevalences occurred in bovines between 6 and 48 months of age, with the highest prevalence (23.7%) in those 13–18 months of age. The intensity of infection fluctuated between 1.3 and 3.6 epg among the age groups, except for bovines 0–6 months of age, where it was much higher (7.3 epg).
FIGURE 3.
Baseline bovine prevalence, intensity of infection, and contamination index by age group for total study population. a, Age group versus number of observations; b, age group versus prevalence; c, age group versus intensity of infection; d, age group versus bovine contamination index. EPG = egg per gram (of feces). Error bars show upper 95% confidence intervals. This figure appears in color at www.ajtmh.org.
The BCI is the total number of eggs excreted by the infected bovines per day. Figure 3d shows the BCI by age group. The total BCI for all the infected bovines was 28.7 million, of which 92.9% and 73.6% were attributed to those less than 48 months of age and those less than 24 months of age, respectively.
Baseline snail prevalence and density of infected snails
The prevalence of snails at baseline (Table 2) fluctuated substantially across the study villages, with Jizhong village showing a high prevalence (19.65%) and Xindong showing a low prevalence (0.24%). The density of infected snails at baseline (Table 2) also fluctuated, although the differences were not as pronounced. Because snail measures are variable across seasons,23 they were not used to match the village pairs.
DISCUSSION
A cluster-randomized design was selected for this study because it enables assessment of community-based interventions by comparing discrete populations and allows for measurement of the direct and indirect effects on trial endpoints. Use of cluster-randomized trials in infectious disease research, particularly when evaluating interventions on the community level, has been shown by Hayes and other12 to be effective. They highlight four main reasons relevant to the current study that necessitate the use of a cluster-randomized trial. First, the intervention is applicable at the community level; second, it is logistically more feasible; third, contamination may occur if performed as an individually randomized trial; and fourth, a cluster-randomized trial is able to measure both direct and indirect effects of the intervention.
In this trial, the treatment of bovines will have an indirect or herd effect on human S. japonicum incidence. Through the comparison of control (human treatment) and intervention (human and bovine treatment) villages within the matched pairs, the impact of bovine chemotherapy on human incidence can be measured. This design also reduces the risk of contamination between control and intervention groups because they are independent communities. Furthermore, confounding is reduced through the matching of village pairs and their randomly assigned status as either control or intervention villages. This design also makes treatment delivery easier and avoids treatment inequity.
The characteristics of the selected village pairs and the human and bovine prevalences within pairs (Tables 1 and 2) were similar, which indicated our success in carefully matching the pairs and subsequently reducing confounding. Odds ratios supported these similarities within the village pairs and although they were not significant, the CIs were wide. The density of infected snails and snail prevalences are highly variable because snail numbers and infection are subject to environmental change and thus fluctuate from season to season.23 Because of this variability, it is not a useful criterion for matching and was not used here to match village pairs.
In contrast to other cluster-randomized trials, four village pairs were selected here for follow-up over a four-year period resulting in the collection of four end point measures per village, which is the equivalent of 16 pairs of villages. Both the human and bovine baseline prevalences (Table 2) are close to or, in some instances, higher than the range (10–15%) set by the study design. As the trial proceeds, the effect of bovine chemotherapy on human incidence can thus be determined satisfactorily.
The power of the study has been shown to be as high as 99.8% for the first two years of the study at a significance level of 5% for a cohort size of 300 for the best-case scenario. The actual sentinel cohort sizes selected for the villages exceeds 300 and will increase the actual observed power of this study even in the worst case scenario, which still produces a power of 95.7% for the first two years of the study at a 5% significance level. Coverage rates of 93% and 94% for humans and bovines surveyed, respectively, at baseline add to the sensitivity of the study.
The age of the total study population followed a normal distribution with a slight skew to the left, which was possibly due to a lower life expectancy in the study communities. The prevalence was essentially equal (11–14%) among the most active age groups (given the need of water exposure to become infected); however, the prevalence of the group 21–30 years of age was lower than expected possibly because of lower numbers of this age group present in the population due to individuals leaving the village to seek work or undertake higher education. The group 81–90 years of age had the highest prevalence (25%) but because the number of individuals was small they were grouped with persons 71–80 years of age (Figures 2b and c).
The occupations with the highest S. japonicum prevalence were those that involved water contact, notably farmers and fishermen. Teachers and those involved in business also had a high prevalence, although numbers were small and given the close proximity of the villages to water, schistosome exposure to most groups was likely. Prevalence in farmers alone, who were thought to have much contact with water, was not as high as expected. However, this was the most common occupation (2,907 individuals) and there may have been some misclassification of occupation between farmers and farmers and fishermen.
There is evidence that bovine prevalence and intensity of infection decreases with age and that there may be a self-cure effect.1,24 The baseline results shown here indicate that prevalence was higher in bovines less than 48 months of age, and although intensity of infection fluctuated among the age groups, it was generally higher in younger bovines, in particular those less than six months of age.
The calculated BCI (Figure 3d) also shows that at baseline the bulk of environmental contamination due to bovines was from those less than 48 months of age, particularly those less than 24 months of age. This is, in part, influenced by the higher number of bovines 48 months of age (Figure 3a). However, the BCI is driven mainly by mean egg counts, and in infected bovines the geometric mean epg was considerably higher in bovines less than 24 months of age (Figure 3c). This indicates that younger bovines appear to be more important in schistosome transmission. Longitudinal analyses we will be undertaking during the trial will further explore the relationship of age and S. japonicum infection.
Proof of principle for the importance of bovines, particularly water buffaloes, as major reservoir hosts for S. japonicum transmission had previously been established,6 but this new intervention trial aims to reproduce those results on a larger, more generalizable scale, particularly for the lake and marshland areas in southern China where most infections occur.6 This is particularly important for underpinning the rationale for the development of a transmission-blocking bovine vaccine against S. japonicum.9,10 This trial will also assess bovine chemotherapy as a plausible schistosomiasis control method and the empirical results obtained will be incorporated into our previously developed transmission dynamics mathematical model for further validation.8
Acknowledgments
We thank the staff at the Jiangxi and Hunan Provincial Institutes of Parasitic Diseases–Chinese Centre for Disease Control and Prevention and the villagers who participated in the study.
Financial support: This study was supported by the National Health and Medical Research Council of Australia and a Wellcome Trust (United Kingdom) International Collaborative Research Grants Scheme Award. Darren J. Gray is a National Health and Medical Research Council Postgraduate Public Health Scholar.
Footnotes
Disclosure: None of the authors have any conflicts of interest.
REFERENCES
- 1.Ross AG, Sleigh AC, Li Y, Davis GM, Williams GM, Jiang Z, Feng Z, McManus DP. Schistosomiasis in the People’s Republic of China: prospects and challenges for the 21st Century. Clin Microbiol Rev. 2001;14:270–295. doi: 10.1128/CMR.14.2.270-295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng J. Schistosomiasis control in China: progress and challenges. Chin J Parasitic Dis Parasitol. 2003;21:4–5. [PubMed] [Google Scholar]
- 3.Li YS, Sleigh AC, Ross AGP, Williams GM, Tanner M, McManus DP. Epidemiology of Schistosoma japonicum in China: morbidity and strategies for control in the Dongting Lake of region. Int J Parasitol. 2000;30:273–281. doi: 10.1016/s0020-7519(99)00201-5. [DOI] [PubMed] [Google Scholar]
- 4.Guo JG, Ross AG, Lin DD, Williams GM, Chen HG, Li Y, Davis GM, Feng Z, McManus DP, Sleigh AC. A baseline study on the importance of bovines for human Schistosoma japonicum infection around Poyang Lake, China. Am J Trop Med Hyg. 2001;65:272–278. doi: 10.4269/ajtmh.2001.65.272. [DOI] [PubMed] [Google Scholar]
- 5.Davis GM, Wu WP, Chen HG, Liu HY, Guo JG, Lin DD, Lu SB, Williams G, Sleigh A, Feng Z, McManus DP. A baseline study of importance of bovines for human Schistosoma japonicum infections around Poyang lake, China: villages studied and snail sampling strategy. Am J Trop Med Hyg. 2002;66:359–371. doi: 10.4269/ajtmh.2002.66.359. [DOI] [PubMed] [Google Scholar]
- 6.Guo J, Li Y, Gray D, Ning A, Hu G, Chen H, Davis GM, Sleigh AC, Zheng F, McManus DP, Williams GM. A drug-based intervention study on the importance of buffaloes for human Schistosoma japonicum infection around Poyang Lake, People’s Republic of China. Am J Trop Med Hyg. 2006;74:335–341. [PubMed] [Google Scholar]
- 7.He YK, Zhou SJ, Wu ZW. Role of farm cattle, water buffaloes and pigs in transmission of schistosomiasis japonica in the Dongting Lake region. Chin J Schistosomiasis Control. 1991;3:44–45. [Google Scholar]
- 8.Williams GM, Sleigh AC, Li Y, Feng Z, Davis GM, Chen H, Ross AG, Bergquist R, McManus DP. Mathematical modelling of schistosomiasis japonica: comparison of control strategies in the Peoples’ Republic of China. Acta Trop. 2002;82:253–262. doi: 10.1016/s0001-706x(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 9.McManus DP. Prospects for development of a transmission blocking vaccine against Schistosoma japonicum. Parasite Immunol. 2005;27:297–308. doi: 10.1111/j.1365-3024.2005.00784.x. [DOI] [PubMed] [Google Scholar]
- 10.Wu ZD, Lu ZY, Yu XB. Development of a vaccine against Schistosoma japonicum in China: a review. Acta Trop. 2005;96:106–116. doi: 10.1016/j.actatropica.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Donner A, Klar N. Design and Analysis of Cluster Randomisation Trials in Health Research. Arnold Publishers; London: 2000. [Google Scholar]
- 12.Hayes RJ, Alexander NDE, Bennett S, Cousens SN. Design and analysis issues in cluster-randomized trials of interventions against infectious diseases. Stat Methods Med Res. 2000;9:95–116. doi: 10.1177/096228020000900203. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Lin D. The prevalence and control of schistosomiasis in Poyang Lake region, China. Parasitol Int. 2004;53:115–125. doi: 10.1016/j.parint.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique for schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 15.World Bank Loan Program Completion Report on Infectious and Endemic Disease Control Project: Schistosomiasis Control Component (1992–2001) Department of Diseases Control and Foreign Loan Office, Ministry of Health, People’s Republic of China; Bejing, People’s Republic of China: 2002. [Google Scholar]
- 16.Ross AG, Yuesheng L, Sleigh AC, Williams GM, Hartel GF, Forsyth SJ, Yi L, McManus DP. Measuring exposure to Schistosoma japonicum in China. I. Activity diaries to assess water contact and comparison to other measures. Acta Trop. 1998;71:213–228. doi: 10.1016/s0001-706x(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . WHO Preventive Chemotherapy in Human Helminthiasis. World Health Organization; Geneva: 2006. [Google Scholar]
- 18.Kenworthy JD, Ye P, Wu GC, Yu H, Shi YJ, Li H, Coles GC. Field evaluation of a test for praziquantel resistance in Schistosoma sp. Vet Parasitol. 2003;113:83–87. doi: 10.1016/s0304-4017(03)00036-0. [DOI] [PubMed] [Google Scholar]
- 19.Liang YS, Dai JR, Ning A, Yu DB, Xu XJ, Zhu YC, Coles GC. Susceptibility of Schistosoma japonicum to praziquantel in China. Trop Med Int Health. 2001;6:707–714. doi: 10.1046/j.1365-3156.2001.00772.x. [DOI] [PubMed] [Google Scholar]
- 20.Li YS, Sleigh AC, Ross AGP, Li Y, Williams GM, Forsyth SJ, Tanner M, McManus DP. A two-year epidemiological survey in China provides epidemiological evidence for human resistance to re-infection by Schistosoma japonicm. Ann Trop Med Parasitol. 1999;93:629–642. doi: 10.1080/00034989958131. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization The control of schistosomiasis: second report of the WHO Expert Committee. World Health Organ Tech Rep Ser. 1993;830:1–86. [PubMed] [Google Scholar]
- 22.Tian-Ping W, Johanson MV, Shi-Qing Z, Feng-Feng W, Wei-Duo W, Gong-Hua Z, Xin-Ping P, Yang J, Ornbjerg N. Transmission of Schistosoma japonicum by humans and domestic animals in the Yangtze River valley, Anhui province, China. Acta Trop. 2005;96:198–204. doi: 10.1016/j.actatropica.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Davis GM, Wu WP, Williams G, Liu H-Y, Lu SB, Chen HG, Zheng F, McManus DP, Guo JG. Schistosomiasis japonica intervention study on Poyang Lake, China: the snail’s tale. Malacologia. 2006;49:79–105. [Google Scholar]
- 24.Wang WQ, Liu SX. The self-cure phenomenon in definitive hosts infected with schistosomes. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2003;21:179–182. [PubMed] [Google Scholar]