Abstract
Pavlovian conditioning paradigms have become important model systems for understanding the neuroscience of behavior. In particular, studies of the extinction of Pavlovian fear responses are yielding important information about the neural substrates of anxiety disorders in humans. These studies are germane to understanding the neural mechanisms underlying behavioral interventions that suppress fear, including exposure therapy. This chapter described detailed behavioral protocols for examining the nature and properties of fear extinction in laboratory rodents.
Extinction of classical fear conditioning refers to a reduction in conditional responding after the repeated presentation of a conditioned stimulus (CS, usually a tone) in the absence of the unconditioned stimulus (US, usually a footshock) with which it was previously paired [see Wehner and Radcliffe (2004) for a chapter concerning fear conditioning procedures]. Interestingly, extinguished fear can be recovered in a number of situations. It can re-emerge with the passage of time after extinction (spontaneous recovery), as a result of a change in experimental context (renewal effect), or from an unsignaled presentation of the US (reinstatement effect). These observations suggest that extinction is a new learning process, and the fear reduction results from inhibition rather than erasure of the original fear memory. Moreover, unlike fear conditioning, extinction is highly context specific (for reference see Bouton et al., 2006; Ji and Maren, 2007).
Because knowledge of the conditions that facilitate extinction learning may help to optimize extinction-based exposure therapies for the treatment of anxiety disorders, such as panic disorder and posttraumatic stress disorder (PTSD, the behavioral and neural mechanisms of fear extinction have attracted increasing attention over the last 20 years (Hermans et al., 2006). It has been shown that fear extinction engages several brain structures, including the amygdala, hippocampus and prefrontal cortex (see Ji and Maren, 2007; Quirk and Mueller, 2008). Typical methods for conducting an extinction experiment are presented below. Procedures for fear extinction are first described (see “Basic Protocol”), followed by alternative procedures including renewal (see “Alternative Protocol 1”), spontaneous recovery (see “Alternative Protocol 2”), and reinstatement (see “Alternative Protocol 3”).
Basic Protocol: Fear extinction in rats
This protocol uses freezing behavior in rats to index the acquisition and extinction of learned fear. It is important to point out that the conditioning and extinction of fear exhibits similar properties in several species (e.g., rats, mice, rabbits, cats) and using many different response measures (e.g., acoustic startle, bar press suppression, heart rate, etc.). Typically, extinction experiments require 8 to 16 subjects for appropriate statistical power. The protocol we describe makes use of automated methods for assessing freezing behavior, although visual observation is a reliable and less expensive alternative (but requires that observers be blind to the experimental conditions). The system described in this protocol was manufactured by MED-Associates, Inc. (St. Albans, VT) and makes use of force transducers underneath the conditioning chambers to detect motion in the experimental subjects (see Fig. 1). In the description of the extinction protocols below, behavioral sessions are commonly denoted by a three-letter code, with each letter corresponding to the context used for the conditioning, extinction and testing sessions. A basic protocol for an ABB design (i.e. one in which the context is the same during extinction and testing but different from the context in which fear is first acquired) is outlined here (Table 1). In this particular protocol, 24 hours is interposed between each behavioral session and the entire protocol requires three days to complete.
Figure 1.
Photographs of typical equipment used for fear conditioning, extinction and testing. A, A set of 8 experimental chambers (MED-Associates) are situated in sound-attenuating cabinets and located in an isolated room. B, Components of a typical conditioning chamber and cabinet.
Table 1.
Experimental Design (Basic Protocol)
| Group | Conditioning | Extinction | Test |
|---|---|---|---|
| EXT | AT+ | BT− | BT− |
| NO-EXT | AT+ | B− | BT− |
NOTE: A and B refer to contexts; T = auditory CS; Plus and minus signs indicate footshock or the lack of footshock, respectively; EXT: extinction group, NO-EXT: no-extinction control.
Materials
Rats (e.g. adult, male Long-Evans)
1% ammonium and 1% acetic acid solution for cleaning chambers (odors from these solutions are also used to establish unique olfactory contexts)
Observation chambers (30 × 24 × 21 cm; MED-Associates): The chambers are constructed of aluminum (side walls) and Plexiglas (rear wall, ceiling, and hinged front door) and are situated in sound-attenuating cabinets located in a brightly lit and isolated room. The floor of each chamber consists of 19 stainless steel rods (4 mm in diameter) spaced 1.5 cm apart (center-to-center). Rods are wired to a shock source and solid-state grid scrambler (MED-Associates) for the delivery of foot shock US (0.1 to 1.0 mA). A speaker mounted outside a grating in one wall of the chamber is used for the delivery of acoustic CS (pure tones or white noise).
Each conditioning chamber rests on a load-cell platform that is used to record chamber displacement in response to eachrat’s motor activity and is acquired online via Threshold-Activity software (MED-Associates). The output of each chamber’s load cell is set to a gain that is optimized for detecting freezing behavior (somatomotor immobility, except that necessitated by breathing). Load-cell amplifier output (−10 to +10 V) from each chamber is digitized and absolute values of the load-cell voltages are computed and multiplied by 10 to yield a scale that ranges from 0 to 100. For each chamber, load-cell voltages are digitized at 5 Hz, yielding one observation every 200 msec. Freezing is quantified by computing the number of observations for each rat that has a value less than the freezing threshold (load-cell activity = 10). We score an observation as freezing if it falls within a continuous group of at least five observations that are all less than the freezing threshold. Thus, freezing is only scored if the rat is immobile for at least 1 sec.
A computer that will run MED-PC software and interface with the chambers for stimulus delivery and data acquisition.
Voltmeter and sound meter to check stimulus intensities.
Creating unique experimental contexts
Three distinct contexts are used in these protocols (Figure 2). For the first context (context A), a 15 W houselight mounted opposite the speaker is turned on, and room lights remain on. The chambers are cleaned with a 1% acetic acid solution. To provide a distinct odor, stainless steel pans containing a thin layer of this solution are placed underneath the grid floors before the rats are placed inside. Ventilation fans in each chest supplies background noise (65 dB). Rats are transported from their home cages to this context in white plastic boxes. For the second context (context B), all room and chamber houselights are turned off. Red fluorescent lights are on providing illumination. Additionally, the doors on the sound-attenuating cabinets are closed, the ventilation fans are turned off, and the chambers are cleaned with 1% ammonium hydroxide solution. Also, stainless steel pans containing a thin layer of the same solution are placed underneath the grid floors before the rats are placed inside to provide a distinct odor. Rats are transported to this context in black plastic boxes.
Figure 2.
Representative photographs of each experimental context. The contexts differ not only visually, but also in terms of the olfactory, auditory, tactile, and transport cues.
In the renewal design described below (Alternative Protocol 1), a third context (context C) is used in which all chamber houselights and ventilation fans are turned off. The chambers are cleaned with 70% ethanol. Also, stainless steel pans containing a thin layer of the same solution are placed underneath the grid floors before the rats are placed inside to provide a distinct odor. Black, plastic floor mats are placed above grid floors. Rats are transported to this context in while plastic boxes with bedding.
Day 1: Conditioning to an auditory CS in a novel context
On this day the animals are conditioned to the auditory CS in context A.
Turn on all equipment.
Calibrate the shock intensity, the loudness of the auditory cue, and load-cell platforms (See Commentary: Calibration & Scoring” for more detail).
Clean all chambers thoroughly with 1% acetic acid solution.
Setup computers for stimulus delivery and data acquisition.
Transport the animals from their home cages in the vivarium into the conditioning room.
Place each subject into the conditioning chamber.
Activate the computer programs.
Three minutes after placement in the chambers, present five conditioning trials (70 sec intertrial interval) in which and auditory CS (2 kHz, 80 dB, 10 sec) co-terminates with a footshock US (1.0 mA, 1 sec).
Remove the animals 60 sec after the final shock.
Transport the animals back to their home cages.
Clean each chamber with 1% acetic acid solution thoroughly and reset all computer programs.
Obtain the next set of animals to be conditioned and repeat steps 5 to 11.
Day 2: Extinction to an auditory CS
Twenty-four hours after conditioning, animals are extinguished to the auditory CS in context B.
13. Turn on all equipment and calibrate again.
14. Clean all chambers thoroughly with 1% ammonium hydroxide solution.
15. Setup computers for stimulus delivery and data acquisition.
16. Transport the animals from their home cages into the conditioning room.
17. Place each subject into the conditioning chamber.
18. Activate the computer programs.
19. Three minutes after placement in the chambers present 30 auditory CSs (10 sec; 80dB, 70 sec intertrial interval); no USs are delivered during this session.
20. Remove the animals 60 sec after the final CS.
21. Transport the animals back to their home cages.
22. Clean each chamber with 1% ammonium hydroxide solution thoroughly and reset all computer programs.
23. Obtain the next set of animals to be extinguished and repeat steps 16 to 22.
NOTE: For NO-EXT controls, the animals are exposed to context B for equivalent amount of time without tone presentations. For experiments with multiple groups per extinction condition (EXT and NO-EXT), the order to the groups should be counterbalanced (for further explanation see “Commentary” section).
Day 3: Retention test to an auditory CS
Twenty-four hours after extinction, all of the animals are tested to the auditory CS in context B.
24. Turn on all equipment and calibrate again.
25. Clean all chambers thoroughly with 1% ammonium hydroxide solution.
26. Setup computers for stimulus delivery and data acquisition.
27. Transport the animals from their home cages into the conditioning room.
28. Place each subject into the conditioning chamber.
29. Activate the computer programs.
30. Two minutes after placement in the chambers, present 30 auditory CSs (10 sec; 80dB, 70 sec intertrial interval); no USs are delivered during this session.
31. Transport the animals back to their home cages.
32. Clean each chamber with 1% ammonium hydroxide solution thoroughly and reset all computer programs.
33. Obtain the next set of animals to be tested and repeat steps 27 to 32.
Alternative Protocol 1: Context-specific extinction and renewal
After extinction, rats exhibit little fear to a CS in the extinction context, but show a return of fear to that CS in a context in which the CS has not been extinguished. This renewal of fear indicates that extinction is context-specific. There are several different ways one can arrange the conditioning, extinction, and test contexts to observe renewal (i.e., ABA, ABC, AAB). The design of each type of renewal procedure is shown in Table 2. It takes three days to perform these procedures. A group of rats that does not undergo extinction can serve as a control to demonstrate the degree of extinction and renewal in the extinguished groups tested inside or outside, respectively, their extinction contexts.
Table 2.
Experimental Design (Alternative Protocol 1: Renewal))
| ABA vs AAA | |||
| Design | Conditioning | Extinction | Test |
| ABA (DIFF) | AT+ | BT−, A− | AT− |
| AAA (SAME) | AT+ | AT−, B− | AT− |
| AAB vs ABB | |||
| Design | Conditioning | Extinction | Test |
| AAB (DIFF) | AT+ | AT−, B− | BT− |
| ABB (SAME) | AT+ | BT−, A− | BT− |
| ABC vs ACC | |||
| Design | Conditioning | Extinction | Test |
| ABC (DIFF) | AT+ | BT−, C− | CT− |
| ACC (SAME) | AT+ | CT−, B− | CT− |
NOTE 1: SAME or DIFF denote to whether extinction and test occur in the same or different contexts. Other notations are the same as previously stated. For the “Extinction” session, animals are extinguished in one context and exposed to the other context. The order of context exposure should be counterbalanced. A no-extinction control can be included in any of the designs.
Alternative Protocol 2: Spontaneous recovery of fear memory in rats
Extinguished fear memory recovers with the passage of time. It can be easily conducted with any extinction design. Simply repeat retention test procedures in the extinction context 7, 14 or 21 days after the first test session.
Alternative Protocol 3: Reinstatement of fear memory after extinction
Exposure to a single presentation of the US after extinction can cause a recovery of conditioned fear to the CS during a later retention test. This is a 4-day procedure with the reinstatement shock session on Day 3 and the retention test on Day 4. The general design of the experiment is summarized in Table 3. A group of rats that is extinguished, but does not receive reinstatement shock can be used to control for nonassociative sensitization of shock.
Table 3.
Experimental Design (Alternative Protocol 3: Reinstatement)
| Design | Conditioning | Extinction | Reinstatement | Test |
|---|---|---|---|---|
| DIFF | AT+ | AT−, B− | B+ | AT− |
| SAME | AT+ | AT−, B− | A+ | AT− |
| NO-SHOCK | AT+ | AT−, B− | A− or B− | AT− |
NOTE: SAME and DIFF denote to whether reinstatement and test occur in the same or different contexts. Other notations are the same as previously stated.
Background Information
In the laboratory, memories of traumatic experiences can be modeled using Pavlovian fear conditioning in rats. For this procedure, an innocuous conditioned stimulus (CS), such as a tone, is presented with an aversive unconditioned stimulus (US), such as a mild electric shock. After conditioning, the CS elicits a learned fear response, which includes increases in heart rate, blood pressure, and stress hormone release (Maren, 2001; Fanselow and Poulos, 2005). A unique feature of fear memory is that it can be acquired with as little as one exposure and can persist for a lifetime (Maren, 2005). However, if the CS is repeatedly presented in the absence of the US, conditioned fear responses begin to diminish, a phenomenon known as extinction. Rather than an erasure of the previous CS-US memory, a new association is formed between the CS and absence of US during extinction that effectively suppresses the previously acquired CR (Bouton, 1993; Ji and Maren, 2007). Evidence of fear renewal in which fear memories can be unmasked in a context different than that of the extinction context, supports the extinction association (Bouton et al., 2006). In addition, the extinguished CRs can be recalled over time (Quirk, 2002) or if exposed to the US without the CS (Ji and Maren, 2007).
It is widely accepted that the amygdala is the site of the synaptic plasticity that mediates both the acquisition and the inhibition of new fears after conditioning and extinction, respectively (Myers and Davis, 2002; Maren and Quirk, 2004; Bruchey et al., 2007; Corcoran and Quirk, 2007). The amygdala consists of several nuclei, including the central nucleus (CEA) and the basolateral complex (BLA), which itself is composed of the lateral (LA), basomedial (BM) and basolateral (BL) nuclei (Maren, 2001). According to the current model of fear circuitry, the BLA is the site at which sensory information about the CS and US converge during initial fear learning and the acquisition of fear responses occurs. Receiving input both directly and indirectly from the BLA, the CEA primarily mediates the expression of the fear response; however the CEA is also necessary for acquisition and long-term storage of fear memories (LeDoux et al., 1988; Wilensky et al., 2006; Zimmerman et al., 2007).
As with fear conditioning, the BLA is also involved in forming extinction memories (Bruchey et al., 2007). However, unlike fear conditioning, extinction may involve local inhibitory neurons in the BLA and the CEA as well as GABAergic inhibitory interneurons located between the two nuclei through which suppression of BLA excitatory projection neurons occurs (Royer and Pare, 2002). As such, extinction can be viewed as parallel learning that does not affect previous plasticity but rather reflects an increase in plasticity in the GABAergic inhibitory interneurons due to glutamatergic input from the BLA. Additionally, the amygdala receives information regarding the auditory CS during extinction (Teich et al., 1989), although the route by which this information reaches the amygdala is not as clear as it is for fear conditioning. Beyond the amygdala, other neural structures may be involved in extinction such as the ventromedial prefrontal cortex (vmPFC) and the hippocampus, both of which have interconnections with the amygdala (Quirk and Mueller, 2008). These structures appear to modulate the inhibition of CRs and the specifics of the surrounding environment, respectively (Bruchey et al., 2007; Ji and Maren, 2007).
Considerable evidence has shown that extinction memories are labile; they dissipate with time and changes in context. In fact, although extinction effectively retards conditional responding, it is a relatively transitory phenomenon that is context-specific. Various studies have demonstrated that if an animal is trained in context A, extinguished in context B and tested in context C, the animal will show a reliable increase in freezing during testing relative to extinction training (Bouton et al., 2006). This renewal effect clearly illustrates that the environment is important in determining whether the extinction memory is retrieved. The role of context in the regulation of CR suppression is also evident in another context effect: spontaneous recovery, where the fear response will gradually appear after the passage of time when tested in the same context that extinction occurred (Quirk, 2002). Moreover, a final example of the context specificity of extinction is reinstatement, or the appearance of the CR in response to the US alone in a related context. All of these effects suggest that excitatory and inhibitory memories are established during conditioning and extinction, respectively, and that context serves as a retrieval cue or occasion setter do determine which memory is expressed when the CS is presented.
Not surprisingly, the hippocampus, a structure that encodes contextual and temporal information, may be critical in mediating the context’s control on extinction and therefore have a role in the consolidation of extinction memories (Ji and Maren, 2007; Quirk and Mueller, 2008). Inactivation of the hippocampus prevents the typical renewal effect to the CS when presented in a context different than that in which extinction took place (Corcoran and Maren, 2001; Corcoran and Maren, 2004; Corcoran et al., 2005). As mentioned previously, the vmPFC may be involved in the contextual modulation of extinction insofar as it receives projections from the hippocampus (Bruchey et al., 2007). In turn, the vmPFC regulates neuronal activity in the amygdala through two distinct pathways that act in an opposing manner: the infralimbic (IL) pathway, which inhibits CR expression and the prelimbic (PL) pathway, which drives the expression of CRs (Vidal-Gonzalez et al., 2006; Corcoran and Quirk, 2007; Quirk and Mueller, 2008). These two pathways may provide a way in which the hippocampus can dictate whether the fear responses are either inhibited or expressed, depending on the context.
It is apparent that extinction forms a separate memory from conditioning yet both learning processes undergo similar plastic changes resulting in stable memories. Understanding the neural structures and processes involved in extinction is critical for developing effective treatments for inhibiting pathological fear in humans.
Commentary
Critical Parameters/Troubleshooting
The context specificity of extinction
As mentioned previously, extinction is highly context dependent, as demonstrated by renewal experiments (for a review see Bouton, 1993). It is therefore essential to ensure that all of the contexts are very distinct from one another. If the distinction between contexts is inadequate, the rats will often display generalized fear when placed into a context that should not be innately fearful. For example, when completing a renewal experiment with the ABA design, following conditioning (AT+), when rats are placed into the B context for extinction (BT−), they may freeze before any tones are presented, even though they have no previous experience with this context. The likely cause of this is context generalization. This is easily eliminated by ensuring that the contexts are sufficiently different and by cleaning the chambers thoroughly between tests.
Timing
The delay between conditioning and extinction can have dramatic consequences on the effectiveness of extinction. Maren and Chang (2006) have shown that extinction only 15 min after conditioning drastically reduces the retention of extinction. In fact, the effect is so dramatic that the animals receiving extinction 15 min after conditioning show levels of fear that are not significantly different from the unextinguished (NO-EXT) controls. It is therefore essential to consider the timing delay between conditioning and extinction when designing experiments.
Counterbalancing
As noted in the renewal protocol above, it is important to counterbalance the contexts (A or B), the order of groups in each phase (SAME or DIFF), and the chambers that the rats are trained, tested, and extinguished in. Counterbalancing the contexts ensures that the measured level of fear is not being differentially influenced by the contexts. For the ABA (DIFF) renewal protocol described above, counterbalancing entails running half of your animals in the ABA design and half of them in a BAB design. The results can then be collapsed across those groups.
The time of day can influence context (Bouton, 1993). Counterbalancing the order of groups eliminates the variable of time. To do this, simply alternate groups (DIFF and SAME).
If multiple chambers are being used for the experiment, it is essential to ensure that the chambers that each experimental group is in are counterbalanced. In other words, make sure each chamber is used by each group of animals so that all of the SAME animals are not in one chamber, and all of the DIFF animals are not in another chamber. This will control for any variable differences between the chambers.
Within-subjects experimental design
To this point, only between-subjects experimental designs have been discussed. Under certain circumstances a within-subjects experimental design may be more appropriate. In such an extinction protocol, the rats would be trained to multiple discrete CSs such as a pure tone and white noise burst. The discrete CSs would then be extinguished in different contexts and a retrieval test would be performed for each CS in both of the extinction contexts (eg. train the rats to both cues in context A, extinguish the pure tone CS in context B and the white noise CS in context C, finally test for retrieval to each CS in both contexts B and C). Such a protocol allows for the direct comparison of the CR between tests within the same subjects. For a more detailed example of a within-subjects protocol see Hobin et al. (2003).
Calibration and Scoring
Inter-chamber reliability is essential to minimizing error within experimental groups. It is therefore necessary to calibrate the conditioning chambers before each experiment. Variations in tone intensity (CS), footshock intensity (US), or load cell output (activity or freezing [CR]) between chambers add additional variables that make interpreting the data difficult.
Calibrating the tone intensity requires a dB meter and the Med-Test software (MED-Associates or equivalent from another manufacturers). The Med-Test software plays pure tones or white noises of a set frequency and intensity through a given chamber’s speaker. Using the dB meter, measure the speaker output in each chamber (from the same location in every chamber). The reading of the dB meter should match the selected intensity output. If necessary the hardware for each chamber can be independently adjusted so that the actual output matches the selected output.
Calibrating the footshock intensity is also essential as increased footshock intensity leads to increased levels of fear and consequently higher levels of freezing. Calibrating the footshock requires the Med-Test software (MED-Associates or equivalent from another manufacturer) and either a shock calibration tool (MED-Associates or equivalent from another manufacturer) or an oscilloscope with alligator clips. The Med-Test software will deliver a shock of selected intensity (current) to the grid of a given chamber. With the alligator clips connected to two different bars of the same grid floor measure the actual shock current using the oscilloscope or the shock test tool. If the selected output and actual output differ, adjust the shock generator for that chamber until the outputs are equivalent.
When using the load cells described in the protocol for automated scoring, it is essential that each load cell outputs the same voltage for the same amount of transduced movement in each of the conditioning chambers. To calibrate the load cells you will need an oscillator that attaches firmly to the grid floor (MED-Associates or equivalent from another manufacturer) and the Threshold-Activity Software. The oscillator will oscillate at a set frequency causing the Threshold-Activity software to record a waveform. This waveform should be the same for all conditioning chambers and can be adjusted if necessary on the load cell hardware. Additionally, it is imperative that the load cells be set so that the sensitivity is ideal for recording freezing behavior. In other words, the freezing recorded by the computer cannot be different from that recorded by a trained observer hand scoring the data [for more information on hand scoring data, see Wehner and Radcliffe (2004)]. Additionally, when using automated scoring, video recording the rats throughout the experiment is recommended as a backup. If for some reason the automated scoring fails, the videotapes can always be used to hand score all the behavior at a later time.
Pavlovian Fear Conditioning
For a review of the critical parameters for Pavlovian fear conditioning, please see Wehner and Radcliffe (2004).
Anticipated Results
Basic Protocol
During conditioning, both groups (EXT and NO-EXT) should show very low levels of freezing before the first tone-shock presentation. Following tone-shock presentations the levels of freezing should increase significantly in both groups (see Fig. 3a). During extinction, the EXT group should show low levels of freezing before the first tone presentation. Following the first tone presentation the EXT group should show a drastically enhanced freezing response. Following each subsequent tone presentation the levels of freezing should slowly decrease until they reach near baseline levels by the end of the extinction session. The NO-EXT group should show low levels of freezing throughout the extinction session. The level of freezing recorded by the load cells may start to increase slightly for the NO-EXT group towards the end of the extinction session as animals become less active (this does not look like freezing when watching the rats behavior) (see Fig. 3b). During the test phase of the experiment both EXT and NO-EXT groups should show low levels of fear before the tone onset. Following the tone onset the EXT group may show a slight increase in freezing but it will rapidly return to baseline. The NO-EXT group however should show very robust levels of freezing following the tone onset and remain significantly higher than the EXT group through the majority of the test (see Fig. 3c).
Figure 3.
Idealized graph showing the anticipated results of the Basic Protocol. Average percentage of freezing during (A) training, (B) extinction and(C) retention testing ; EXT -extinction group, NO-EXT - no-extinction control group.
Alternative Protocol 1: Renewal of Fear
Results during the conditioning session should be identical to those described above. Results for the ABA (DIFF) group during the extinction phase should be the same as those described above. Freezing levels for the AAA (SAME) group should be the same as those described above, for the ABA (DIFF) group, except that the rats will show high levels of freezing even before the first tone presentation arising from their fear to the conditioning context (see Fig. 4a). During the exposure to the other context rats in the ABA (DIFF) group will show high levels of fear when returned A-, but will show extinction throughout the session eventually returning to near baseline levels of freezing. Rats in the AAA (SAME) group will show low levels of freezing when placed in the B-context, similar to the NO-EXT group during extinction in the “Basic Protocol” above (see Fig. 4b). During the test phase, both groups should show low levels of freezing when initially placed in the chamber. Following the tone onset, rats in the ABA (DIFF) groups should show significantly more freezing than rats in the AAA (SAME) group (rats in the AAA (SAME) group should look similar to the EXT group during test in the “Basic Protocol”.
Figure 4.
Idealized graph showing the anticipated results of Alternative Protocol 1. Average percentage of freezing during (A) extinction, (B) context exposure and (C) retention testing; DIFF - ABA group, SAME - AAA group. A non-extinguished control group (NO-EXT) is shown for comparison during the retention test.
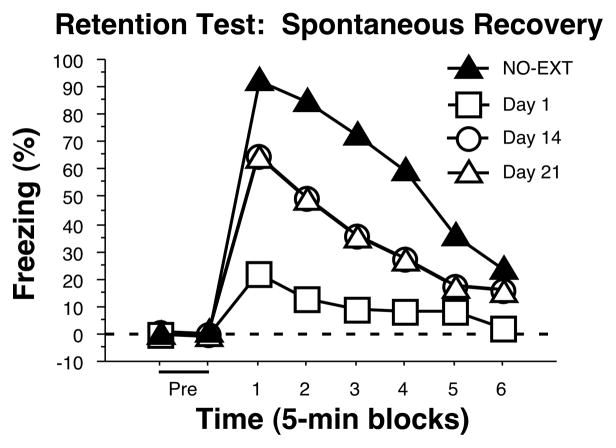
Alternative Protocol 2: Spontaneous Recovery of Fear
Results during the conditioning, extinction, and first retention test should look identical to those described above in the “Basic Protocol” results section. When tested for retention 14 or 21 days after the initial retention test, rats in the EXT group should show higher levels of freezing than they did during the initial retention test (see Fig. 5).
Figure 5.
Idealized graph showing the anticipated results of Alternative Protocol 2. Average percentage of freezing during the retention test performed after 1, 14, or 21 days. A non-extinguished control group (NO-EXT) is shown for comparison during the retention test.
Alternative Protocol 3: Reinstatement of Fear
Conditioning and extinction in both groups (DIFF and SAME) should look identical to that described for the AAA (SAME) group in “Alternative Protocol 1.” During test, both groups should show low levels of freezing. Following tone onset, rats in the SAME group should show substantially more freezing than rats in the DIFF group (see Fig. 6). Rats in the DIFF group may exhibit more freezing than NO-SHOCK rats due to nonassociative sensitization of fear.
Figure 6.
Idealized graph showing the anticipated results of Alternative Protocol 3. Percentage of freezing during the tone retention test in rats tested in the same (SAME) or different (DIFF) context as the one in which reinstatement shock was delivered. An extinguished control group that did not receive reinstatement shocks (NO-SHOCK) is shown for comparison.
Time Considerations
The number of days required for each protocol is listed within the protocol. When designing an experiment and the number of animals to run each day, keep in mind that the extinction sessions will take the longest and the amount of time between sessions for each animal should be kept constant.
Acknowledgments
This chapter was supported by grants from the NIH (R01MH065961; R01MH073655) to SM.
Literature
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Bruchey AK, Shumake J, Gonzalez-Lima F. Network model of fear extinction and renewal functional pathways. Neuroscience. 2007;145:423–437. doi: 10.1016/j.neuroscience.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn Mem. 2004;11:598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Hermans D, Craske MG, Mineka S, Lovibond PF. Extinction in human fear conditioning. Biol Psychiatry. 2006;60:361–368. doi: 10.1016/j.biopsych.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Goosens KA, Maren S. Context-dependent neuronal acticity in the lateral amygdala represents fear memories after extinction. J Neurosci. 2003;23:8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Maren S, Chang CH. Recent fear is resistant to extinction. Proc Natl Acad Sci U S A. 2006;103:18020–18025. doi: 10.1073/pnas.0608398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem. 2002;9:402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Pare D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience. 2002;115:455–462. doi: 10.1016/s0306-4522(02)00455-4. [DOI] [PubMed] [Google Scholar]
- Teich AH, McCabe PM, Gentile CC, Schneiderman LS, Winters RW, Liskowsky DR, Schneiderman N. Auditory cortex lesions prevent the extinction of Pavlovian differential heart rate conditioning to tonal stimuli in rabbits. Brain Res. 1989;480:210–218. doi: 10.1016/0006-8993(89)91584-9. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner JM, Radcliffe RA. Cued and contextual fear conditioning in mice. In: Crawley JN, et al., editors. Current Protocols in Neuroscience. Vol. 2. Wiley; New York: 2004. Unit 8.5C. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JM, Rabinak CA, McLachlan IG, Maren S. The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learn Mem. 2007;14:634–644. doi: 10.1101/lm.607207. [DOI] [PMC free article] [PubMed] [Google Scholar]