Abstract
Endothelial-dysfunction, oxidative stress and inflammation are associated with vascular aging and promote the development of cardiovascular-disease. Caloric restriction (CR) mitigates conditions associated with aging, but its effects on vascular dysfunction during aging remain poorly defined. To determine whether CR exerts vasoprotective effects in aging, aortas of ad libitum (AL) fed young and aged and CR-aged F344 rats were compared. Aging in AL-rats was associated with impaired acetylcholine-induced relaxation, vascular oxidative stress and increased NF-κB-activity. Lifelong CR significantly improved endothelial function, attenuated vascular ROS production, inhibited NF-κB activity and down-regulated inflammatory genes. To elucidate the role of circulating factors in mediation of the vasoprotective effects of CR, we determined whether sera obtained from CR-animals can confer anti-oxidant and anti-inflammatory effects in cultured coronary-arterial endothelial cells (CAECs), mimicking the effects of CR. In CAECs cultured in the presence of AL-serum TNFα elicited oxidative-stress, NF-κB-activation and inflammatory gene expression. By contrast, treatment of CAECs with CR-serum attenuated TNFα-induced ROS generation and prevented NF-κB-activation and induction of inflammatory genes. siRNA-knockdown of SIRT1 mitigated the anti-oxidant and anti-inflammatory effects of CR-serum. CR exerts anti-oxidant and anti-inflammatory vascular effects, which are likely mediated by circulating factors, in part, via a SIRT1-dependent pathway.
Keywords: aging, oxidative stress, inflammation, calorie restriction, resveratrol
Introduction
Caloric restriction (CR) is a dietary regimen recognized to delay aging and extend lifespan in evolutionary distant organisms (including the invertebrate C. elegans, F. pyramitela and D. melanogaster as well as laboratory rodents). CR also slows the functional decline associated with aging in various organ systems, such as skeletal muscle, heart and the immune system and delays early onset of age-related diseases (e.g. cancer and cataract formation) in mammals(Pearson et al., 2008a; Pearson et al., 2008b). Despite the fact that cardiovascular disease is the primary cause for age-related mortality and morbidity in elderly humans(Lakatta and Levy, 2003), little information is available whether CR can prevent or delay development of cardiovascular disease.
CR may improve vascular health by attenuating systemic risk factors for atherosclerosis or by enhancing cellular functions and impacting gene expression in vascular endothelial and smooth muscle cells that creates a microenvironment, which offsets atherogenesis (e.g. attenuation of ROS production, anti-inflammatory effects). Accordingly, CR diminished systemic risk factors of atherosclerosis normalizing serum cholesterol, triglycerides, fasting glucose and fasting insulin levels and decreasing blood pressure both in obese and non-obese individuals (Fontana et al., 2004; Fontana et al., 2007; Jung et al., 2007; Meyer et al., 2006; Miyaki et al., 2008; Pereira et al., 2004; Pierce et al., 2008; Walford et al., 1992). Despite these advances, the effects of chronic CR on pro-atherogenic vascular alterations during aging remain poorly understood.
Vacular aging is associated with pro-oxidant and pro-inflammatory phenotypic and functional changes, that promote the development of cardiovascular disease(Csiszar et al., 2008b). Previous studies have demonstrated that increased production of reactive oxygen species (ROS) in the aged vasculature results in endothelial dysfunction decreasing the bioavailability of vasodilator and vasoprotective nitric oxide (Csiszar et al., 2002; van der Loo et al., 2000). Current views of vascular aging are consistent with chronic low-grade vascular inflammation(Csiszar et al., 2003; Csiszar et al., 2008b; Franceschi et al., 2000), up-regulation of adhesion molecules, iNOS and enhanced production of chemokines and pro-inflammatory cytokines. Increasing evidence supports a key role for ROS-dependent activation of the redox sensitive transcription factor, NF-κB, in vascular pro-inflammatory phenotypic alterations during aging (Csiszar et al., 2008b; Ungvari et al., 2007b). Numerous studies on CR show that oxidative stress (De Cabo et al., 2004; Hyun et al., 2006; Kim et al., 2008) and inflammatory gene expression (Higami et al., 2006; Lee et al., 2002) in a variety of tissues are attenuated. Collectively, these studies serve the basis of our hypothesis (Ungvari et al., 2008a) that CR has the potential to attenuate aging-induced pathophysiological alteration in the vasculature by counteracting vascular oxidative stress and inflammation.
The mechanisms underlying the beneficial effects of CR on mammalian healthspan are undoubtedly multifaceted, involving both cell-autonomous effects (e.g. changes in mitochondrial function), changes in paracrine regulation (altered cytokine microenvironment) and effects mediated by neuroendocrine factors (recently reviewed elsewhere(Ungvari et al., 2008a)). Important to the present study are observations by de Cabo et al(de Cabo et al., 2003) that in vitro treatment of cultured hepatocytes with sera from CR animals mimics phenotypic effects observed in vivo during CR. These studies support a key role of neuroendocrine factors in phenotypic responses due to CR. Neuroendocrine mediators present in the circulation reach endothelial cells and elicit a variety of responses, however, no studies have investigated whether these circulating factors mediate vasoprotective effects during CR. Based on the aforementioned studies, we further hypothesize that circulating factors triggered by CR activate endogenous cytoprotective mechanisms in endothelial cells, thus contributing to the anti-oxidative and anti-inflammatory vasoprotective effects of CR.
To test these hypotheses we initially determined whether CR in aged rat arteries attenuates oxidative stress, improves endothelial function, and inhibits NF-κB-driven inflammatory gene expression. We next tested whether sera obtained from CR animals can confer anti-oxidant and anti-inflammatory effects in cultured coronary arterial endothelial cells by activating SIRT1 and mimicking the effects observed in CR animals.
Methods
Animal models
Animal use protocols were approved by the Institutional Animal Care and Use Committee of the New York Medical College, Valhalla, NY. Male Fisher 344 rats were purchased from the National Institute of Aging and kept under pathogen free conditions (Csiszar et al., 2003; Csiszar et al., 2004; Ungvari et al., 2008b; Ungvari et al., 2007b). The following experimental groups were used: a) 3 month old [“young”] ad libitum (AL) fed, b) 28 month old [“aged”] AL fed and c) 28 month old with lifelong 40% CR (CR). All animals were disease free and exhibited no signs of systemic inflammation and/or neoplastic alterations. Upon sacrifice the aortas were isolated as reported (Csiszar et al., 2007a; Ungvari et al., 2008b; Ungvari et al., 2007b). Sera were collected from AL fed and CR fed rats for cell culture experiments as described (de Cabo et al., 2003).
Functional studies
Endothelial function was assessed by measuring relaxation of aortic ring preparations to acetylcholine as previously described(Csiszar et al., 2008a; Csiszar et al., 2007a). In brief, aortas of each animal were cut into ring segments 2 mm in length and mounted on 40 µm stainless steel wires in the myograph chambers (Danish Myo Technology A/S, Inc., Denmark) for measurement of isometric tension. The vessels were superfused with Krebs buffer solution (118 mM NaCl, 4.7 mM KCl, 1.5 mM CaCl2, 25 mM NaHCO3, 1.1 mM MgSO4, 1.2 mM KH2PO4, and 5.6 mM glucose; at 37°C; gassed with 95% air and 5% CO2). After an equilibration period of 1 hour during which an optimal passive tension was applied to the rings (as determined from the vascular length-tension relationship), relaxations of pre-contracted (by 10−6 mol/L phenylephrine) vessels to acetylcholine (ACh; from 10−9 to 10−5 mol/L) were obtained. The effects of the O2 ·− scavenger PEG-SOD (200 U/mL) on ACh-induced vascular responses were also tested to assess the role of increased O2 ·− in endothelial dysfunction. To assess the relaxant properties of the vascular smooth muscle, relaxations to endothelium-independent vasodilator S-nitroso-N-acetyl-DL-penicillamine (SNAP), which releases NO+, were also obtained.
Studies on endothelial cells: effects of AL and CR sera treatment, SIRT1 induction and SIRT1 knockdown
To study the role of circulating factors that may mediate effects of CR, primary human coronary artery endothelial cells (CAECs; Cell Applications, Inc., San Diego, CA; after passage 4) were cultured as described(Csiszar et al., 2008a; Csiszar et al., 2006; Ungvari et al., 2008b) in Endothelial Cell Growth Media (Beckton Dickinson) supplemented with 10% fetal calf serum (FCS) and 1% antibiotics (Gibco,Gaithersburg,MD) until the time of treatment. For treatment, FCS was replaced with serum collected from AL or CR rats, as previously described(Allard et al., 2008; de Cabo et al., 2003). To determine whether SIRT1 mediates the endothelial protective effects of CR serum factors downregulation of SIRT1 in CAECs was achieved by RNA interference, using the proprietary SIRT1 siRNA sequences (Origen) and the electroporation-based Amaxa Nucleofector technology (Amaxa, Gaithersburg, MD), as we have previously reported(Csiszar et al., 2008a). Cell density at transfection was 30%. Specific gene silencing was verified with QRT-PCR and Western blotting (at the mRNA and protein level, respectively) as described(Csiszar et al., 2008a). Transfection efficiency (determined by analyzing co-expression of GFP by flow cytometry) was over 70%. On day 2 after transfection, when gene silencing was optimal, cells were treated with CR sera. To determine whether SIRT1 induction mimics the protective effects of CR sera, CAECs were treated with resveratrol (10 µmol/L, for 24 h), a pharmacological activator of SIRT1(Baur and Sinclair, 2006). In other experiments SIRT1 was overexpressed (~7 fold) in CAECs using a proprietary cDNA construct (Starategen), as previously described(Csiszar et al., 2008a). Thereafter, cells were treated with TNFα (10 ng/mL) or vehicle for 24 h. After the culture period 1) cellular ROS production, 2) NF-κB activation and 3) NF-κB-driven gene expression were analyzed (see below).
Measurements of ROS production
In freshly isolated aortic segments cellular O2 ·− production was assessed using the SOD-inhibitable lucigenin (10 µmol/L) chemiluminescence (CL) method, as described(Csiszar et al., 2008a; Csiszar et al., 2007a; Csiszar et al., 2002). In brief, vessels from young AL, aged AL and aged CR rats were placed in scintillation vials containing HEPES-buffered (pH 7.4) Krebs solution and lucigenin (10 µmol/L) CL was measured in a liquid scintillation counter (Beckman LS-6000IC) in the absence and presence of superoxide dismutase (SOD, 200 U/mL). Scintillation counts were obtained 15 to 20 minutes after addition of vessels (averaged) and background-corrected values were normalized to tissue weight.
In other experiments, dihydroethidine (DHE), an oxidative fluorescent dye, was used to assess vascular ROS production in segments of en face preparations of the aortas as we have previously reported(Csiszar et al., 2008a; Csiszar et al., 2007a; Pearson et al., 2008a). In brief, vessels were incubated with DHE (3×10−6 mol/L; at 37 °C) and for quantitative measurements the time course of the build-up of ethidium fluorescence in en face preparations of the aortas was recorded for 30 min(Csiszar et al., 2007b). The slope factor was calculated and normalized to tissue mass. Unstained aortas and vessels pre-incubated with PEG-SOD were used for background correction and negative control, respectively.
In experiments using CAECs production of O2 ·− was assessed using a flow cytometer-based modified DHE assay(Csiszar et al., 2008a). This method enabled us to specifically assess oxidative stress in cells which expressed GFP co-transfected with the SIRT1 siRNA expressing vector. H2O2 production in CAECs was measured fluorometrically using the Amplex Red/horseradish peroxidase assay as described(Csiszar et al., 2007b; Ungvari et al., 2008b; Ungvari et al., 2007b).
Determination of endogenous glutathione and ascorbate using HPLC electrochemical detection
Concentrations of redox-active GSH and ascorbate were measured in aorta homogenates using a Perkin-Elmer HPLC equipped with an eight-channel coulometric array detector (ESA, Inc., Chelmsford, MA) as described(Cho et al., 2007). In brief, 10 mg aliquots of tissue samples were washed with ice-cold PBS and homogenized in 5% (w/v) metaphosphoric acid. Samples were centrifuged at 10,000-× g for 10 min to sediment protein and the supernatant fraction was saved for analysis of redox sensitive compounds. Precipitated proteins were dissolved in 0.1 N NaOH and saved for protein determinations by a spectrophotometric quantitation method using BCA reagent (Pierce Chemical Co., Rockford, IL). Concentrations of GSH and ascorbic acid in saved supernatant fractions were determined by injecting 5 µL aliquots onto an Ultrasphere 5 u, 4.6 × 250 mm, C18 column and eluting with mobile phase of 50 mM NaH2PO4, 0.05 mM octane sulfonic acid, 1.5% acetonitrile (pH 2.62) at a flow rate of 1 mL/min. The 8-channel CoulArray detectors were set at 200, 350, 400, 450, 500, 550, 600 and 700 mV, respectively. Peak areas were analyzed using ESA, Inc. software and concentrations of GSH and ascorbate are reported as nmol/mg protein.
SIRT1 activity assay
Nuclear SIRT1 activity was measured in cells treated with AL sera or CR sera. In brief, cells were suspended in lysis buffer (10 mM Tris HCl pH 7.5, 10 mM NaCl, 15 mM MgCl2, 250 mM sucrose, 0.5% NP-40 and 0.1 mM EGTA), vortexed for 10 seconds followed by incubation for 15 min on ice. The cells were spun through 4 mL of sucrose cushion (30% sucrose, 10 mM Tris HCl pH 7.5, 10 mM NaCl, 3 mM MgCl2) at 1,300 × g for 10 min at 4°C. The nuclear pellet was washed once with cold 10 mM Tris HCl pH 7.5, 10 mM NaCl. The isolated nuclei were suspended in 50 µL of extraction buffer (50 mM HEPES KOH, pH 7.5, 420 mM NaCl, 0.5 mM EDTA Na2, 0.1 mM EGTA, 10% glycerol), sonicated for 30 s and incubated on ice for 30 min, followed by centrifugation (15,000 rpm for 10 min). The nuclear extract was collected and the protein concentration was determined by the Bradford method. SIRT1 was immunoprecipitated from the samples using a rabbit polyclonal antibody directed against the C-terminus of SIRT1 (Abcam #ab28170). SIRT1 activity in the samples was measured using the Cyclex SIRT1 Deacetylase Fluorimetric Assay Kit according to the manufacturer’s protocol (CycLex Ltd., Nagano, Japan). In brief, this assay is based on the principle that upon NAD-dependent deacetylation of the specific substrate by SIRT1 (in the presence of trichostatin A, a potent inhibitor of SIRT1-independent histone deacetylases), the fluoro-substrate peptide is cleaved by a lysyl endopeptidase, separating the quencher from the fluorophore. Specific activity of SIRT1 was assessed by measuring time-dependent changes in fluorescence intensity, normalized to protein concentration. To demonstrate the specificity of the assay, we assessed resveratrol- (from 10−6 to 10−4 mol/L) induced increases in the specific activity of recombinant SIRT1 in the presence and absence of the specific SIRT1 activator sirtinol (10−4 mol/L). Standard assay controls included the use of a fluoro-deacetylated peptide (to control for lysyl endopeptidase activity), no enzyme control, no NAD+ control and no inhibitor control.
Nuclear extraction and NF-κB binding activity assay
To determine whether CR inhibits NF-κB activity, nuclei were isolated from freshly isolated aortic segments using the Nuclear Extraction kit from Active Motif (Carlsbad, CA) as reported(Csiszar et al., 2005; Ungvari et al., 2007b). In brief, arteries of young AL, aged AL and aged CR rats were homogenized with a dounce tissue homogenizer in 500 mL ice-cold hypotonic lysis buffer followed by two centrifugation steps (500 g, for 30 s, 4°C) to exclude tissue debris. Then, nuclear proteins (~10 µg/vessel segment) were extracted according to the manufacturer's protocol. Protein concentrations in samples were equalized using a Bradford protein assay (Bio-Rad). Using the nuclear extract obtained, NF-κB binding activity was assayed using the TransAM NF-κB ELISA kit (Active Motif) according to the manufacturer's guidelines (Ungvari et al., 2007b).
Transient transfection and luciferase assays
To elucidate the anti-inflammatory action of circulating CR factors, TNFα-induced NF-κB activation was compared in AL and CR sera-treated CAECs by a reporter gene assay as described(Csiszar et al., 2008a; Csiszar et al., 2006; Ungvari et al., 2007b). We used a NF-κB reporter comprised of an NF-κB response element upstream of firefly luciferase (NF-κB-Luc, Stratagene) and a renilla luciferase plasmid under the control of the CMV promoter (as an internal control). Transfections in CAECs were performed using the Amaxa Nucleofector technology (Amaxa, Gaithersburg, MD) (Csiszar et al., 2008a; Csiszar et al., 2006; Ungvari et al., 2007b). Firefly and renilla luciferase activities were assessed after 24 h using the Dual Luciferase Reporter Assay Kit (Promega) and a luminometer.
Quantitative real-time RT-PCR
We have used a quantitative real time RT-PCR technique to analyze mRNA expression of TNFα, NF-κB-driven genes (ICAM-1, iNOS, BMP2) and SIRT1 in aorta samples as well as CAECs, as previously reported(Csiszar et al., 2007a; Csiszar et al., 2002; Ungvari et al., 2008b; Ungvari et al., 2007b). In brief, total RNA was isolated with Mini RNA Isolation Kit (Zymo Research, Orange, CA) and was reverse transcribed using Superscript III RT (Invitrogen) as described previously(Csiszar et al., 2006; Csiszar et al., 2002). Real time RT-PCR technique was used to analyze mRNA expression using the Strategen MX3000, as reported(Csiszar et al., 2006). Amplification efficiencies were determined using dilution series of a standard vascular sample. Quantification was performed using the efficiency-corrected ΔΔCq method. The relative quantities of the reference genes GAPDH, HPRT, YWHAZ and β-actin were determined and a normalization factor was calculated based on their geometric mean for internal normalization. Oligonucleotides used for quantitative real-time RT-PCR are listed in Table 1. Fidelity of the PCR reaction was determined by melting temperature analysis and visualization of product on a 2% agarose gel.
Table 1.
Oligonucleotides for real-time RT-PCR
| mRNA targets |
Species | Sense | Antisense |
|---|---|---|---|
| SIRT1 | rat | CGCCTTATCCTCTAGTTCCTGTG | CGGTCTGTCAGCATCATCTTCC |
| ICAM-1 | rat | CACAGCCTGGAGTCTC | CCCTTCTAAGTGGTTGGAA |
| iNOS | rat | TCCCGAAACGCTACACT | CAATCCACAACTCGCT |
| TNFα | rat | AACCACCAAGCAGAGGAG | CTTGATGGCGGAGAGGAG |
| YWHAZ | rat | ACGAGTTGCTTGGTGTTCC | AGCCGTCATCTCAAGTTATTTCC |
| GAPDH | rat | CCAAGGAGTAAGAAACCC | TTGATGGTATTCGAGAGAAGG |
| HPRT | rat | AAGACAGCGGCAAGTTGAATC | AAGGGACGCAGCAACAGAC |
| β-actin | rat | GAAGTGTGACGTTGACAT | ACATCTGCTGGAAGGTG |
| SIRT1 | human | CTTGTGGCAGTAACAGTGATAGTG | TCATCTCCATCAGTCCCAAATCC |
| ICAM-1 | human | CTCTCGCTCTGTCACC | GGAAGTCTGGGCAATGT |
| iNOS | human | GGATTGATCGGAGCCT | ATGGGGAACAGACTGG |
| IL-6 | human | CCACCCCTGACCCAAC | AGTGTCCTAACGCTCATAC |
| BMP2 | human | GGTGGAATGACTGGATTG | GCATCGAGATAGCACTG |
| HPRT | human | CCGTGTGTTAGAAAAGTAAGAAGC | AACTGCTGACAAAGATTCACTGG |
| GAPDH | human | AACGAATTTGGCTACAGC | AGGGTACTTTATTGATGGTACAT |
| β-actin | human | CGGTGAAGGTGACAGCAG | TGTGTGGACTTGGGAGAGG |
Flow cytometry
CAECs forming a 90% confluent monolayer in a six-well plate were cultured with TNFα (10 ng/ml) for 24 hr at 37°C, detached by cell dissociation buffer (Invitrogen Corporation; Grand Island, NY), fixed in 5% formalin–PBS for 5 min at 20°C, washed in ice-cold PBS by centrifugation (200 × g for 8 min at 4°C), and resuspended in 2 ml PBS containing mouse monoclonal antibody for human ICAM-1 (R&D Systems). After reacting for 1 hr at 20°C, cells were washed in PBS by centrifugation and exposed to AF488-conjugated goat anti-mouse IgG. To minimize nonspecific binding of antibodies, incubation was conducted in the presence of isotype-matched mouse IgG (Sigma). Cells treated with only a second antibody served as controls. Flow cytometry was performed by a Guava Easycyte flow cytometer. The percentage of cells expressing ICAM-1 was determined according to a forward light scatter/side light scatter gating combined with an FL-1 channel for immunostaining.
Western blotting
To analyze protein expression of SIRT1 Western blotting was performed as described(Csiszar et al., 2007b; Ungvari et al., 2008b), using a rabbit polyclonal antibody diirected against the C-terminus of SIRT1 (Abcam #ab28170). The antibody detects a band of approximately 91 kDa, which is significantly attenuated after knockdown of SIRT1 by siRNA (Fig. 2C), showing the specificity of the antibody binding. Anti-β-actin (Novus Biologicals, Littletown, CO) was used for normalization purposes.
Figure 2.
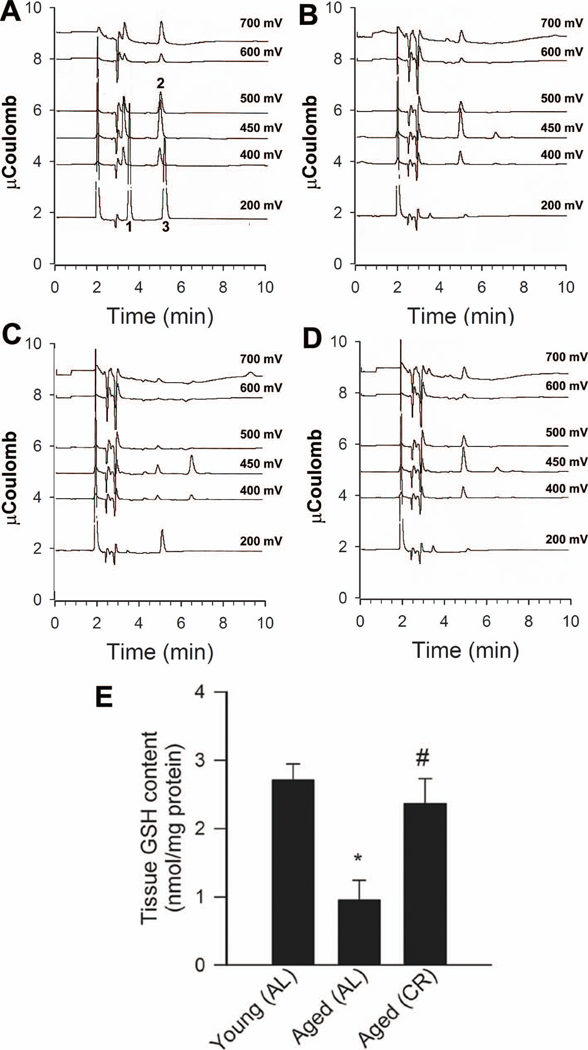
HPLC coulometric analysis of glutathione (GSH) content in homogenates of the aortas of young (B), aged AL fed (C) and aged CR fed (D) rats. The peak elution times and patterns of oxidation of a standard mixture of 50 nmol/mL ascorbate (peak 1), 25 nmo/L GSH (peak 2), and 50 nmol/L uric acid (peak 3) are shown in panel A. Only channels 1,3,4,5,7,8 are displayed, corresponding to 200, 400, 450, 500, 600 and 700 mV potentials, respectively. See Methods for further details on analytical conditions. F: Bar graphs represent summary data for GSH content in aortic segments of young and aged AL fed and aged CR fed F344 rats. *P<0. 05 vs. young AL, #P<0. 05 vs. aged AL.
Monocyte adhesion assay
We measured adhesion of fluorescently-labeled human monocytic (THP-1) cells to confluent monolayers of CAECs using a microplate-based assay as reported(Csiszar et al., 2006). In brief, CAECs were grown to confluence in 96-well plates and were treated with TNFα (10 ng/mL; incubation time: 2 h, at 37 °C). THP-1 cells were labeled with the fluorescent dye calcein (5 µmol/L final concentration; Molecular Probes, Eugene. OR; in serum-free RPMI medium for 30 min at 37 °C). Cells were then washed twice with prewarmed (37°C) RPMI. PMA (phorbol myristate acetate, 10−6 mol/L)-pretreated fluorescently labeled THP-1 cells (5 × 105 /well) were added the microplate wells containing confluent CAECs (medium removed; incubation time: 120 min, at 37 °C). Nonadherent THP-1 cells were removed by careful washing (three times with pre-warmed RPMI). PBS (200 µL) was added to each well and fluorescence was measured using a Tecan Infinite M200 plate reader (excitation : 485 nm; emission: 528 nm). Controls included measurement of total fluorescence of labeled cells before adhesion, controls for measuring autofluorescence of unlabeled cells, and measurement of monocyte adhesion to microplate wells in the absence of CAECs.
Apoptosis assays
To determine whether CR circulating factors exert anti-apoptotic effects, CAECs were pre-treated with AL and CR sera and apopotosis was induced by administration of oxidized LDL (ox-LDL; 40 µg/mL, for 24 h; purchased from Biomedical Technologies Inc., Stoughton, MA) or tumor necrosis factor (TNFα, 10 ng/mL, for 24 hours). Apoptotic cell death was assessed using a flow cytometer-based TUNEL assay(Ungvari et al., 2007a).
To corroborate the results of the TUNEL assay, endothelial cell samples were homogenized in lyses buffer and caspase activities were measured using Caspase-Glo 3/7 assay kit according to the manufacturer’s instruction (Promega, Madison, WI). In 96-well plates 50 µl sample was mixed for 30 sec with 50 µl Caspase-Glo 3/7 reagent and incubated for 2 h at room temperature. Lyses buffer with the reagent served as blank. Luminescence of the samples was measured using an Infinite M200 plate reader (Tecan, Research Triangle Park, NC). Luminescent intensity values were normalized to the sample protein concentration.
Data analysis
Data were normalized to the respective control mean values and expressed as means ± S.E.M. Statistical analyses of data were performed using Student’s t-test or two-way ANOVA followed by the Tukey’s post hoc test, as appropriate. P<0.05 was considered statistically significant.
Results
CR improves endothelial function and attenuates oxidative stress in aged rat arteries
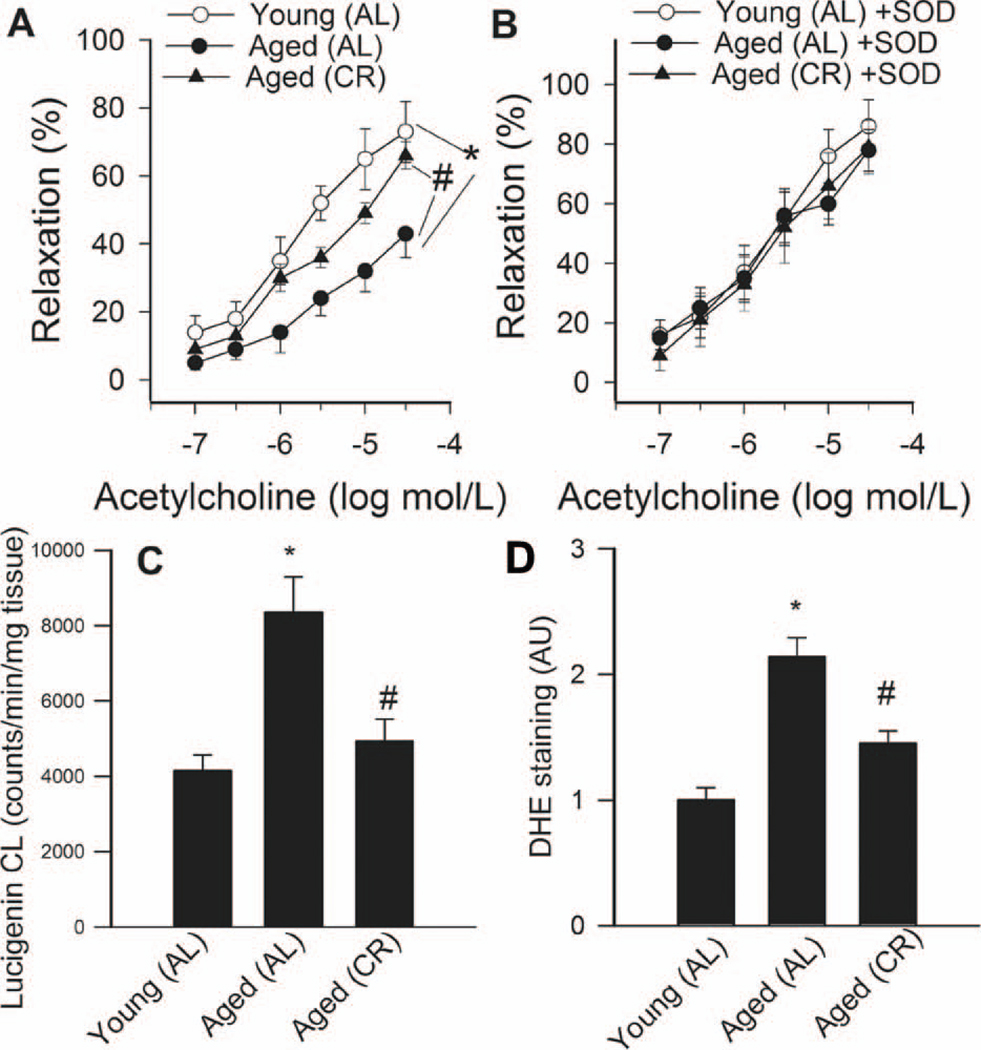
Advancing age resulted in a significant decline in acetylcholine-induced vascular relaxations in AL fed rats, which was significantly improved by chronic CR (Fig. 1A). Administration of PEG-SOD significantly improved acetylcholine-induced relaxation of arteries from AL fed rats, minimizing the differences between the groups (Fig. 1B). Aging or CR did not affect significantly SNAP-induced vascular relaxations (not shown).
Figure 1.
A: Relaxation to acetylcholine in vessels of young and aged AL fed and aged CR F344 rats. Administration of PEG-SOD (200 U/mL; Panel B) improves endothelial function in vessels of aged AL rats and abolished the differences between the groups. Data are mean ± S. E. M. (n=5–7). *P<0. 05 vs aged AL. C: Superoxide production in aortic segments of young and aged AL fed and aged CR fed F344 rats, as measured by the lucigenin chemiluminescence (CL) method. *P<0. 05 vs. young AL, #P<0. 05 vs. aged AL. D: Superoxide production in aortic segments of young and aged AL fed and aged CR fed F344 rats, as measured by the DHE fluorescence method. *P<0. 05 vs. young AL, #P<0. 05 vs. aged AL.
Lucigenin chemiluminescence (Fig. 1C) and DHE fluorescence (Fig. 1D) measurements showed that O2 ·− production was increased in arteries of aged AL fed rats compared to the arteries from young AL fed rats. Vascular O2 ·− generation was significantly reduced in aged CR rats (Fig. 1C,D). Consistent with the presence of age-related oxidative stress, aortic GSH content (Fig 2) and ascorbate concentrations (in nmol/mg protein, young: 0.35±.02; aged AL: 0.13±0.04*, aged CR: 0.28±0.05#, *P<0.05 vs. young, #P<0.05 vs. AL) were significantly reduced in aged AL rats and were normalized by CR.
Treatment of CAECs with CR sera attenuates oxidative stress: role of SIRT1
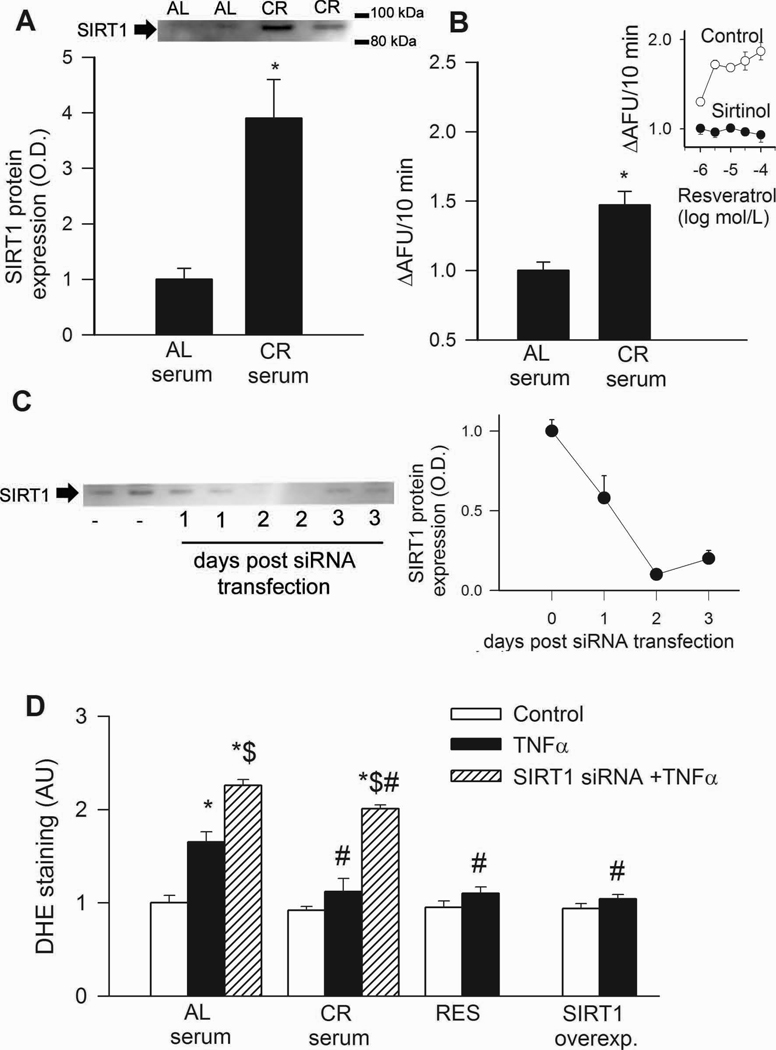
In CAECs, treatment with CR sera significantly up-regulated SIRT1 protein expression and activity (Fig. 2A,B). Endothelial SIRT1 expression was effectively down-regulated by siRNA (Fig. 2C).
In CAECs cultured in the presence of AL sera, addition of TNFα significantly increased ROS production (Fig. 2D). By contrast, treatment of CAECs with sera from CR rats significantly attenuated TNFα-induced oxidative stress (Fig. 2D). Knockdown of SIRT1 significantly increased ROS production in activated CAECs treated with sera from either AL or CR animals (Fig. 2D), significantly minimizing the difference between the two groups. Resveratrol treatment or overexpression of SIRT1 also significantly decreases oxidative stress in activated CAECs (Fig. 2D) mimicking the effects of CR serum treatment. Using the Amplex Red assay we also found that CR serum treatment significantly decreased H2O2 production in CAECs (by ~33%). By contrast, knockdown of SIRT1 elicited significant increases in H2O2 production in CAECs treated with AL or CR sera (23% and 47%, respectively). GSH content was also increased in CR serum-treated CAECs (in nmol/mg; AL: 0.85±0.03, CR: 1.23±0.12, P<0.05).
CR inhibits NF-κB activation and NF-κB-driven inflammatory gene expression in aged rat arteries
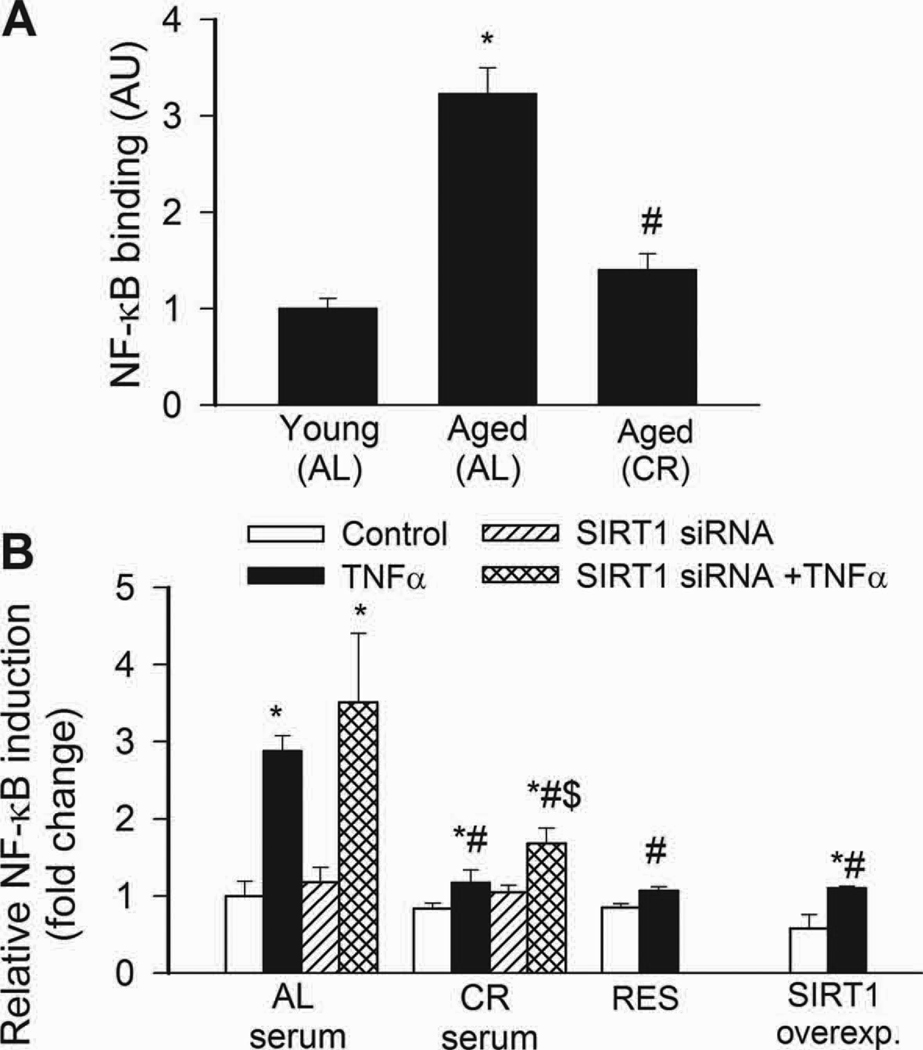
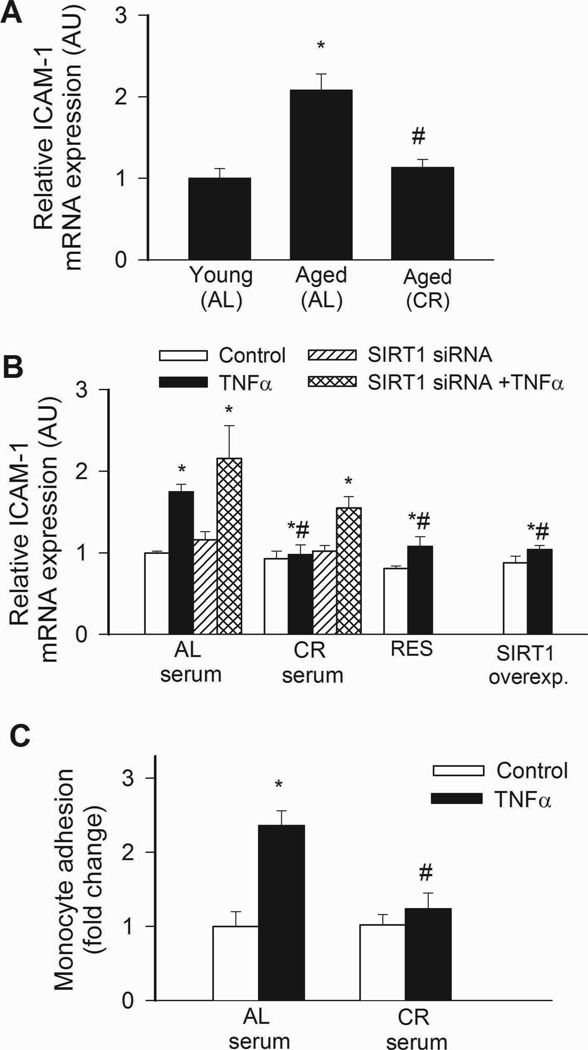
NF-κB binding activity was increased in nuclei extracted from freshly isolated arteries of aged AL rats, as compared to those isolated from young rats (Fig. 3A). Aging in AL rats was also associated with an increased vascular expression of NF-κB –driven inflammatory genes, including ICAM-1 (Fig. 4A) and iNOS (not shown). By contrast, vascular NF-κB activation (Fig. 4A) and inflammatory gene expression (Fig. 4A) were attenuated by CR in aged rats. TNFα and SIRT1 are important regulators of NF-κB activity in the aged vasculature(Csiszar et al., 2008b). Expression of TNFα was up-regulated in arteries of aged AL rats, whereas CR significantly reduced vascular TNFα expression (relative mRNA expression, young: AL: 1.0±0.2, aged AL: 5.0±1.5, aged CR: 1.2±0.5, P<0.05). Conversely, expression of SIRT1 tended to be decreased with age in AL rats and to be increased by CR (young AL: 1.0±0.03, aged AL: 0.86±0.03, aged CR: 1.74±0.48, n.s.).
Figure 3.
Treatment of CAECs with sera collected from CR rats up-regulated SIRT1 protein expression (A; Western blotting) and activity (B; data from fluorimetric SIRT1 activity assay). Inset: effect of resveratrol on the activity of recombinant SIRT1 in the presence and absence of sirtinol (fold change). C: Time course for siRNA knockdown of SIRT1 in CAECs (Western blotting). D: In CAECs cultured in the presence of AL sera TNFα (10 ng/mL) significantly increases ROS production (measured by flow cytometer-based DHE fluorescence method). By contrast, CR serum treatment significantly attenuates TNFα-induced oxidative stress. Knockdown of SIRT1 (siRNA) significantly increases ROS production both in AL and CR sera-treated activated CAECs, significantly decreasing the difference between the two groups. Resveratrol treatment or overexpression of SIRT1 also significantly decreases oxidative stress in activated CAECs mimicking the effects of CR serum treatment. *P<0. 05 vs. no TNFα; #P<0. 05 vs. AL serum treated; $P<0. 05 vs. TNFα. Data are mean ± S. E. M. (n=5 in each group).
Figure 4.
A: Increased NF-κB binding activity in nuclear extracts from arteries of aged AL rats. NF-κB activity was significantly reduced in CR rats. Data are mean ± S. E. M. (n=5 in each group). *P<0. 05 vs. young, #P<0. 05 vs. aged AL. B: Reporter gene assay showing that in CAECs cultured in the presence of AL sera TNFα (10 ng/mL) significantly increased NF-κB activity (*P<0. 05 vs. no TNFα). By contrast, treatment with CR serum significantly (#P<0. 05 vs. AL serum treated) attenuates activation of NF-κB by TNFα. Knockdown of SIRT1 (siRNA) significantly increased NF-κB activation both in CR sera-treated activated CAECs, decreasing the difference between the two groups ($P<0.05 vs. TNFα). Resveratrol treatment or overexpression of SIRT1 also significantly decreased oxidative stress in activated CAECs mimicking the effects of CR serum treatment. Data are mean ± S. E. M. (n=5 in each group).
Treatment of CAECs with CR sera inhibits NF-κB activation: role of SIRT1
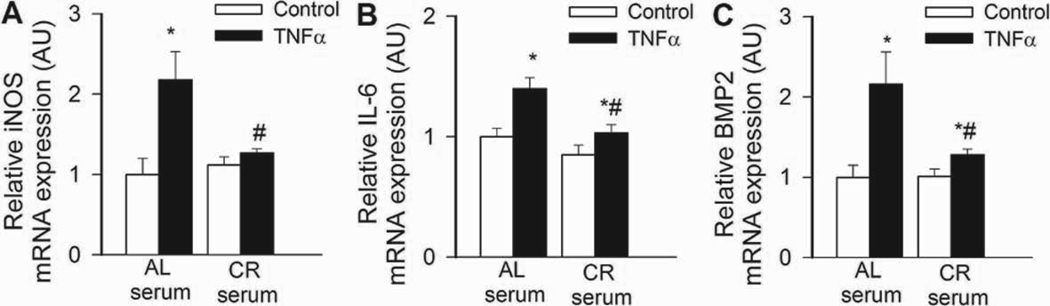
In CAECs cultured in the presence of AL sera, TNFα significantly increased transcriptional activity of NF-κB (Fig. 4B) and expression of ICAM-1 (Fig. 5B). By contrast, treatment of CAECs with CR sera significantly attenuated both TNFα-induced NF-κB activation (Fig. 4B) and NF-κB –driven ICAM-1 mRNA expression (Fig. 5B). TNF- stimulation of AL sera-treated CAECs induced surface expression of ICAM-1 in 95 ± 1% of CAECs, compared to only 5 % positive cells without TNFα- stimulation. Treatment with CR sera decreased the percentage of stimulated ICAM-1 positive cells to 15 ± 3% (p < 0.001). The decrease in ICAM-1 positive cells was paralleled by a reduction in the relative expression levels of ICAM-1 (Fig. 5B) and attenuated monocyte adhesiveness to CR sera treated CAECs (Fig. 5C). Treatment of CAECs with CR sera significantly attenuated TNFα-induced expression of other NF-κB –driven genes, including iNOS, IL-6 and BMP2 (Figure 6.A,B,C, respectively). Knockdown of SIRT1 significantly increased TNFα-induced NF-κB activation (Fig. 4B) and ICAM-1 expression (Fig. 5B) both in AL and CR sera-treated CAECs, significantly decreasing the difference between the two groups. Resveratrol treatment or overexpression of SIRT1 also significantly decreased NF-κB activation (Fig. 4B) and ICAM-1 induction (Fig. 5B) in TNFα-treated CAECs, thus mimicking the effects of CR serum treatment.
Figure 5.
A: Expression of ICAM-1 mRNA in arteries of young and aged AL fed and aged CR F344 rats. Analysis of mRNA expression was performed by real-time QRT-PCR. β-actin was used for normalization. *P<0. 05 vs. young, #P<0. 05 vs. aged AL. Data are mean ± S. E. M. (n=5 for each group). B: In CAECs cultured in the presence of AL sera TNFα (10 ng/mL) significantly increased ICAM-1 expression (*P<0. 05 vs. no TNFα). By contrast, treatment with CR serum significantly (#P<0. 05 vs. AL serum treated) attenuated TNFα-induced ICAM-1 expression. Knockdown of SIRT1 (siRNA) significantly increased ICAM both in CR sera-treated activated CAECs, decreasing the difference between the two groups ($P<0. 05 vs. TNFα). Resveratrol treatment or overexpression of SIRT1 also significantly attenuated ICAM-1 expression in activated CAECs mimicking the effects of CR serum treatment. Data are mean ± S.E.M. (n=5 in each group). C: Results of monocyte adhesion assay (see Methods). In CAECs cultured in the presence of AL sera TNFα (10 ng/mL) significantly increased the adhesion of fluorescently labeled PMA-stimulated THP-1 monocytic cells. By contrast, treatment with CR serum significantly (#P<0. 05 vs. AL serum treated) attenuated TNFα-induced monocyte adhesiveness.
Fig. 6.
In CAECs cultured in the presence of AL sera TNFα (10 ng/mL) significantly (*P<0. 05 vs. no TNFα) increased the expression of the NF-κB –driven genes iNOS (A), IL-6 (B) and BMP2 (C). By contrast, CR serum treatment significantly (#P<0. 05 vs. AL serum treated) attenuated TNFα-induced inflammatory gene expression. Data are mean ± S. E. M. (n=5 for each group).
Treatment of CAECs with CR sera inhibits apoptosis
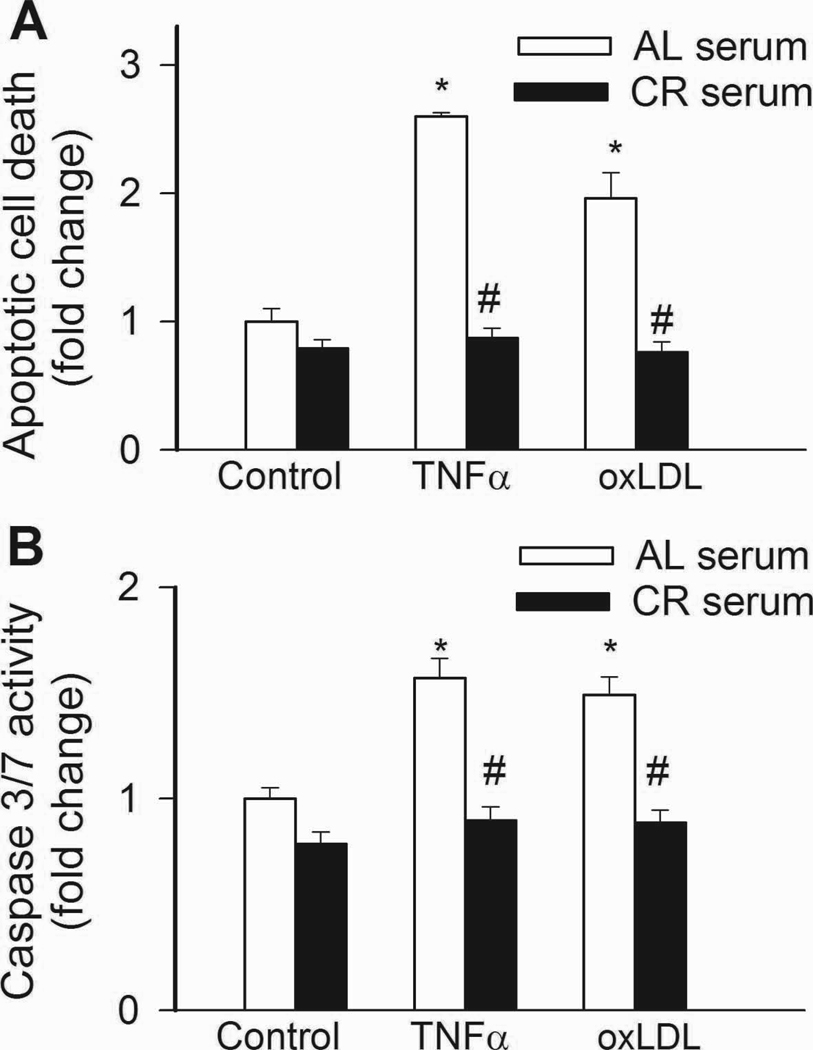
In CAECs cultured in the presence of AL sera, treatment with TNFα or oxLDL promoted apoptotic cell death, as shown by the increased ratio of TUNEL positive cells (Fig. 7A) and increased caspase 3/7 activity (Fig. 7B). By contrast, treatment of cells with CR sera prevented TNFα- and oxLDL-induced endothelial apoptosis (Fig. 7A,B).
Fig. 7.
In CAECs cultured in the presence of AL sera TNFα (10 ng/mL) and ox-LDL (40 µg/mL) significantly (P<0. 05 vs. no TNFα) increased apoptotic cell death as shown by the increased TUNEL positivity (Panel A; flow cytometry) and increased caspase 3/7 activity (Panel B). By contrast, treatment with CR serum prevented TNFα- and oxLDL-induced endothelial apoptosis. #P<0. 05 vs. AL serum treated, Data are mean ± S. E. M. (n=5 for each group).
Discussion
Caloric restriction is a dietary strategy shown to have anti-aging effects in a variety of tissues across an array of mammalian and non-mammalian species. Our studies show that CR attenuates oxidative stress and improves endothelial function in arteries of aged rats. This effect on vascular aging is associated with decreases in NF-κB activity and down-regulation of NF-κB dependent genes. We also show that treatment of cultured endothelial cells with sera from CR animals inhibited TNFα-induced ROS production, NF-κB activation and inflammatory gene expression, recapitulating in vitro the effects observed in vivo on CR animals.
Previous studies focused on the effects of CR on systemic cardiovascular risk factors (recently reviewed elsewhere(Ungvari et al., 2008a)) but provided little information on vascular effects directly affected by CR. Our data support the finding that lifelong CR prevents endothelial dysfunction in aging (Fig. 1A), extending the findings of recent studies of obese humans(Miyaki et al., 2008). Exogenous administration of SOD improved endothelial function in aged AL arteries whereas little improvement was observed in arteries of aged CR rats and those of young animals (Fig. 1B). These results suggest that endothelial protective effect of CR is due to the attenuation of the age-associated increase in vascular O2 ·− generation. Indeed, measurements of lucigenin chemiluminescence and ethidium fluorescence (Fig. 1C,D) as well as measurements of GSH (Fig. 2) and ascorbate content indicate that CR elicits significant reduction of vascular oxidative stress in aged animals. Because rats can synthesize ascorbate, we interpret the simultaneous age-related decline in tissue ascorbate(van der Loo et al., 2003) and GSH levels as a sign of age-related oxidative stress, rather than a sign of dietary vitamin deficiency. Our findings provide direct support for and extend the hypothesis of Sohal and Weindruch(Sohal and Weindruch, 1996) that anti-aging action of CR is derived from the ability of cells to attenuate oxidative stress associated with aging. In addition to its anti-oxidant effects, CR also up-regulates eNOS(Ungvari et al., 2008a), which can contribute to improved endothelial function.
Earlier studies by our laboratories have demonstrated that vascular oxidative stress in aging is due, at least in part, to a TNFα-mediated up-regulation of NADPH oxidases(Csiszar et al., 2007a; Csiszar et al., 2002). Support for this finding was obtained using etanercept that prevents TNFα binding to its receptor, and thus attenuates vascular oxidative stress by down-regulating NADPH oxidase and improves endothelial function in aged rats(Csiszar et al., 2007a). Our studies demonstrate that CR significantly decreases vascular TNFα expression and attenuates vascular ROS production, effects that contribute significantly to the endothelial protective effects. Increased ROS generation by mitochondria also contributes to vascular oxidative stress in aging(Ungvari et al., 2008b; Ungvari et al., 2007b). Since mitochondria isolated from CR rats produce significantly less ROS than those from AL controls(Lambert and Merry, 2004), we consider that decreases in mitochondrial ROS generation may also contribute to the anti-oxidative effects of calorie restriction in the vasculature.
We devised an in vitro approach to determine the role of circulating factors that mediate anti-oxidant effects of CR. Our data show for the first time that circulating factors within the plasma of CR animals significantly attenuate TNFα-induced ROS production (Fig. 3D) in human coronary arterial endothelial cells. These findings support the view that neuroendocrine factors mediate, at least in part, the anti-oxidant effects of CR.
The free radical theory of aging postulates that increased ROS production with age results in a variety of macromolecular oxidative modifications and accumulation of such oxidative damage is fundamental to the aging process. ROS, in addition to causing macromolecular damage, have important signaling roles. Recent studies show that increased ROS, derived from mitochondria and NADPH oxidases, lead to chronic low-grade vascular inflammation in aging(Csiszar et al., 2008b; Ungvari et al., 2008a). This process seems to be a direct pathophysiological link to atherogenesis. We have extended our previous findings that aging is associated with increases in NF-κB activation(Ungvari et al., 2007b) (Fig. 4A) and an up-regulation of NF-κB-driven gene expression (Fig. 5A) in rat arteries. CR significantly attenuates both NF-κB activation and vascular ICAM-1 expression in aged rats (Fig. 4A and Fig. 5A). Scavenging of ROS attenuates NF-κB activation in aged vessels(Ungvari et al., 2007b). This strongly suggest a direct causal relationship between oxidative stress and endothelial activation in aging. These studies in their aggregate suggest that attenuation of vascular oxidative stress (Fig. 1) contributes to the anti-inflammatory action of CR.
In apoE-deficient mice CR attenuates atherogenesis (Guo et al., 2002). If CR exerts similar anti-inflammatory vascular effects in humans (especially, un-related to weight reduction), protection against the development of vascular disease may be observed in the elderly as well. NF-κB activation and chronic low grade inflammation seem to be a generalized phenomenon in aging, because increases in NF-κB activity and a pro-inflammatory shift in gene expression profile have been observed recently in skeletal muscle, liver, brain, cardiac muscle and adipose tissue of aged rodents(Higami et al., 2006; Lee et al., 2002). In this regard it is noteworthy that CR also attenuates inflammatory gene expression in the heart(Lee et al., 2002) and adipose tissue(Higami et al., 2006) of aged rodents, suggesting that anti-inflammatory effects of CR may contribute to its anti-aging protective effects in multiple organs.
As noted earlier, anti-oxidant effects in the vasculature of CR animals are, at least in part, mediated by circulating factors (Fig. 3D). We hypothesized that if reduced ROS generation contributes to the attenuation of NF-κB activation in CR than the same circulating factors should inhibit NF-κB activation as well. In support of this view, our data show that circulating factors in sera of CR animals convey significant anti-inflammatory effects in cultured endothelial cells and mimic the vascular phenotypic changes induced in animals by CR in vivo. Accordingly, treatment of CAECs with sera from CR animals effectively prevents TNFα-induced NF-κB activation (Fig. 4B), induction of NF-κB-driven gene expression (including that of ICAM-1 [Fig. 5B], iNOS, IL-6 and BMP2 [Figure 6]) and endothelial activation (Fig. 5C). The actual circulating factor (s) which mediate the anti-oxidant and anti-inflammatory effects of CR are presently unknown. Because serum adiponectin levels increase in CR rats (Shinmura et al., 2008; Shinmura et al., 2007) and previous studies demonstrate that adiponectin can inhibit NF-κB activation in cultured endothelial cells (Ouchi et al., 2000), future studies will examine the role of adiponectin in the anti-inflammatory vasoprotective effects of CR in aging.
Multiple lines of evidence indicate that homologs of the NAD+-dependent protein deacetylase SIRT1 mediate the lifespan extension by CR in lower organisms (Wood et al., 2004). In mammals SIRT1 is also inducible by CR(Cohen et al., 2004), suggesting a central role for this enzyme in mammalian physiology and stress response. SIRT1 is expressed in the cardiovascular system(Csiszar et al., 2008a; Shinmura et al., 2008) and is induced by CR(Shinmura et al., 2008), yet, its physiological/pathophysiological role is incompletely understood. Our studies indicate that the anti-oxidant and anti-inflammatory effects induced by circulating factors during CR are in part mediated by pathways that involve SIRT1. Accordingly, we found that sera from CR animals induced SIRT1 in endothelial cells (Fig. 3A,B) extending previous observations in other cell types (Allard et al., 2008; Cohen et al., 2004). Knockdown of SIRT1 prevented, at least in part, the reduction of cellular ROS production elicited by treatment with CR sera (Fig. 3D).
Our studies show that knockdown of SIRT1 also enhances NF-κB activation (Fig. 4B) and ICAM-1 expression (Fig. 5B) in endothelial cells treated with sera from CR animals. By contrast, pharmacological activation (resveratrol) or overexpression of SIRT1 in endothelial cells attenuates oxidative stress (Fig. 3D) and inhibites endothelial activation(Csiszar et al., 2008a) (Fig. 4B and 5B), mimicking the effects of CR sera. Accordingly, resveratrol treatment also mimics transcriptional aspects of CR improving endothelial function, decreasing oxidative stress and inhibiting NF-κB –driven inflammatory gene expression in aged mice(Pearson et al., 2008a). Of note, a portion of the phenotypic and functional alterations elicited by CR sera in endothelial cells appears to be independent of SIRT1 (Fig. 4B and Fig. 5B). Further studies are evidently needed to identify these parallel vasoprotective pathway (s) which are activated by neuroendocrine factors in CR(Pearson et al., 2008b).
Another potentially important finding is that treatment of cells in vitro with sera from CR animals also mimics the known anti-apoptotic effects of CR in vivo(Edwards et al., 2007; Shinmura et al., 2008), preventing the induction of apoptosis in endothelial cells by multiple pro-apoptotic stimuli (TNFα, oxLDL; Figure 7). In comparable fashion, resveratrol confers significant anti-apoptotic effects in endothelial cells both in vitro (Csiszar et al., 2008a; Ungvari et al., 2007a) and in vivo (Pearson et al., 2008a), mimicking the effects of CR.
In conclusion, CR significantly attenuates age-related vascular oxidative stress and inflammation and improves endothelial function. We propose that neuroendocrine factors are key mediators of the vascular effects of CR, in part, via activating SIRT1. Because serum obtained from caloric restricted humans also activates SIRT1 in human cells in vitro(Allard et al., 2008), we suggest that CR may also promote vascular health in the elderly.
Acknowledgement
This work was supported by grants from the American Diabetes Association (to ZU), the American Federation for Aging Research (to AC), the NIH (HL077256 and HL43023,to ZU, CA111842 to JP), the Hungarian Science Fund (OTKA 68758 to GL) and Proyecto de Excelencia (P06-CTS-01555,to RJ) and by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III (Red HERACLES RD06/0009,Spain; to RJ) and the Intramural Research Program of the National Institute on Aging, NIH (to RDC).
List of abbreviations
- GSH
reduced glutathione
- siRNA
small interfering RNA
- AL
ad libitum fed
- CR
caloric restriction
- iNOS
inducible nitric oxide synthase
- GFP
green fluorescent protein
- qRT-PCR
quatitative real-time PCR
- PMA
phorbol 12-myristate 13-acetate
- BMP-2
bone morphogenetic protein-2
- SIRT1
silent mating type information regulation 2 homolog, S. cerevisiae
- ROS
reactive oxygen species
- DHE
dihydroethidine
- CAECs
coronary arterial endothelial cells
- oxLDL
oxidized low density lipoprtotein
- TUNEL assay
Terminal deoxynucleotidyl transferase mediated dUTP Nick End Labeling assay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allard JS, Heilbronn LK, Smith C, Hunt ND, Ingram DK, Ravussin E, de Cabo R. In vitro cellular adaptations of indicators of longevity in response to treatment with serum collected from humans on calorie restricted diets. PLoS ONE. 2008;3:e3211. doi: 10.1371/journal.pone.0003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nature reviews. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Cho S, Szeto HH, Kim E, Kim H, Tolhurst AT, Pinto JT. A novel cell-permeable antioxidant peptide, SS31, attenuates ischemic brain injury by down-regulating CD36. J Biol Chem. 2007;282:4634–4642. doi: 10.1074/jbc.M609388200. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008a;294:H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-TNFalfa treatment in aging. The American journal of pathology. 2007a;170:388–698. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell. 2007b;6:783–797. doi: 10.1111/j.1474-9726.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-{alpha}-induced activation of coronary arterial endothelial cells: role of NF-{kappa}B inhibition. Am J Physiol. 2006;291:H1694–H1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG, Ungvari Z. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation. 2005;111:2364–2372. doi: 10.1161/01.CIR.0000164201.40634.1D. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in rat coronary arteries. 2003. Faseb J;17:1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Wang M, Lakatta EG, Ungvari ZI. Inflammation and endothelial dysfunction during aging: role of NF-{kappa}B. J Appl Physiol. 2008b;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cabo R, Cabello R, Rios M, Lopez-Lluch G, Ingram DK, Lane MA, Navas P. Calorie restriction attenuates age-related alterations in the plasma membrane antioxidant system in rat liver. Exp Gerontol. 2004;39:297–304. doi: 10.1016/j.exger.2003.12.003. [DOI] [PubMed] [Google Scholar]
- de Cabo R, Furer-Galban S, Anson RM, Gilman C, Gorospe M, Lane MA. An in vitro model of caloric restriction. Experimental gerontology. 2003;38:631–639. doi: 10.1016/s0531-5565(03)00055-x. [DOI] [PubMed] [Google Scholar]
- Edwards MG, Anderson RM, Yuan M, Kendziorski CM, Weindruch R, Prolla TA. Gene expression profiling of aging reveals activation of a p53-mediated transcriptional program. BMC Genomics. 2007;8:80. doi: 10.1186/1471-2164-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans 1. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JO. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. American journal of physiology. 2007;293:E197–E202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Guo Z, Mitchell-Raymundo F, Yang H, Ikeno Y, Nelson J, Diaz V, Richardson A, Reddick R. Dietary restriction reduces atherosclerosis and oxidative stress in the aorta of apolipoprotein E-deficient mice. Mechanisms of ageing and development. 2002;123:1121–1131. doi: 10.1016/s0047-6374(02)00008-8. [DOI] [PubMed] [Google Scholar]
- Higami Y, Barger JL, Page GP, Allison DB, Smith SR, Prolla TA, Weindruch R. Energy restriction lowers the expression of genes linked to inflammation, the cytoskeleton, the extracellular matrix, and angiogenesis in mouse adipose tissue. J Nutr. 2006;136:343–352. doi: 10.1093/jn/136.2.343. [DOI] [PubMed] [Google Scholar]
- Hyun DH, Emerson SS, Jo DG, Mattson MP, de Cabo R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19908–19912. doi: 10.1073/pnas.0608008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SH, Park HS, Kim KS, Choi WH, Ahn CW, Kim BT, Kim SM, Lee SY, Ahn SM, Kim YK, Kim HJ, Kim DJ, Lee KW. Effect of weight loss on some serum cytokines in human obesity: increase in IL-10 after weight loss. J Nutr Biochem. 2007 doi: 10.1016/j.jnutbio.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kwak HB, Leeuwenburgh C, Lawler JM. Lifelong exercise and mild (8%) caloric restriction attenuate age-induced alterations in plantaris muscle morphology, oxidative stress and IGF-1 in the Fischer-344 rat. Exp Gerontol. 2008;43:317–329. doi: 10.1016/j.exger.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Merry BJ. Effect of caloric restriction on mitochondrial reactive oxygen species production and bioenergetics: reversal by insulin. Am J Physiol Regul Integr Comp Physiol. 2004;286:R71–R79. doi: 10.1152/ajpregu.00341.2003. [DOI] [PubMed] [Google Scholar]
- Lee CK, Allison DB, Brand J, Weindruch R, Prolla TA. Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proc Natl Acad Sci U S A. 2002;99:14988–14993. doi: 10.1073/pnas.232308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- Miyaki A, Maeda S, Yoshizawa M, Misono M, Saito Y, Sasai H, Endo T, Nakata Y, Tanaka K, Ajisaka R. Effect of weight reduction with dietary intervention on arterial distensibility and endothelial function in obese men. Angiology. 2008 doi: 10.1177/0003319708325449. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol Delays Age-Related Deterioration and Mimics Transcriptional Aspects of Dietary Restriction without Extending Life Span. Cell Metab. 2008a;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, Cascajo MV, Tamashiro KL, Poosala S, Csiszar A, Ungvari Z, Kensler TW, Yamamoto M, Egan JM, Longo DL, Ingram DK, Navas P, de Cabo R. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci U S A. 2008b;105:2325–2330. doi: 10.1073/pnas.0712162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira MA, Swain J, Goldfine AB, Rifai N, Ludwig DS. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA. 2004;292:2482–2490. doi: 10.1001/jama.292.20.2482. [DOI] [PubMed] [Google Scholar]
- Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension. 2008;52:72–79. doi: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol. 2008;295:H2348–H2355. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. American journal of physiology. 2007a;292:H2417–H2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008a;102:519–528. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari ZI, Labinskyy N, Gupte SA, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008b;294:H2121–H2128. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007b;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- van der Loo B, Bachschmid M, Spitzer V, Brey L, Ullrich V, Luscher TF. Decreased plasma and tissue levels of vitamin C in a rat model of aging: implications for antioxidative defense. Biochem Biophys Res Commun. 2003;303:483–487. doi: 10.1016/s0006-291x(03)00360-7. [DOI] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Lüscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walford RL, Harris SB, Gunion MW. The calorically restricted low-fat nutrient-dense diet in Biosphere 2 significantly lowers blood glucose, total leukocyte count, cholesterol, and blood pressure in humans. Proc Natl Acad Sci U S A. 1992;89:11533–11537. doi: 10.1073/pnas.89.23.11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]