Abstract
BACKGROUND
Members of the Six family of homeoproteins are expressed in numerous tissues during vertebrate embryogenesis, and are critical regulators of both cell proliferation and survival. Here we report the temporal and spatial expression of Six1 during maturation of the mouse submandibular salivary gland (SSG) from embryonic day 18.5 (E18.5) to postnatal day 28. Additionally, we examine the role of Six1 during SSG development using Six1-deficient mice.
METHODS
Six1 expression was assessed by RT-PCR, western blot, and immunofluorescence. Proliferation was measured by bromodeoxyuride (BrdU) incorporation index, and apoptosis was evaluated by TUNEL assay.
RESULTS
Six1 mRNA and protein levels are high in the epithelial SSG cells at E18.5 and decrease progressively in the postnatal maturing SSG. Although SSGs from Six1-/- embryos are significantly smaller than wild type SSGs, the histological structures of the SSG acini and ducts are similar. Six1-/- salivary epithelial cells exhibit an intrinsic defect in cell proliferation accompanied by a significant reduction in the Six1 target gene cyclin A1, previously shown to be a critical mediator of Six1-induced proliferation.
CONCLUSION
Our results suggest that the reduction in size of Six1-/- SSGs is result of a decrease in cell proliferation during development/maturation.
Keywords: submandibular salivary gland, Six1, development, maturation, proliferation, cyclin A1
Introduction
Homeobox genes encode a large and diverse group of DNA binding proteins that act as “master regulators” of development, performing critical functions in specifying cell proliferation, differentiation, survival, and migration (Pearson et al., 2005). The Six family of homeoproteins (Six1-6) are characterized structurally by a divergent homeodomain that is involved in DNA binding, and an N-terminally localized Six domain, that confers cooperative interactions with co-factors (Kawakami et al., 2000, Christensen et al., 2008). Animal studies demonstrate that the Six family members play critical roles in organogenesis via regulating cell growth and survival, as well as tissue specification (Christensen et al., 2008). Importantly, mutations in Six family members are found in numerous human genetic disorders, underscoring their importance in human embryonic development (Christensen et al., 2008).
Recently, Six1-/- mice were generated, which exhibit perinatal death due to severe muscle hypoplasia that results in inadequate diaphragm muscle development and suffocation at birth. In addition, these animals exhibit hypoplastic or completely lacking kidneys and thymus, craniofacial structure malformations, and defects in neurogenesis (Laclef et al., 2003, Xu et al., 2003, Zheng et al., 2003, Ozaki et al., 2004, Li et al., 2003, Zou et al., 2004, Ikeda et al., 2007). Interestingly, cells in the Six1-/- affected organs exhibit an increase in apoptosis and a decrease in proliferation (Li et al., 2003, Xu et al., 2003, Ozaki et al., 2004), suggesting that Six1 is important for the expansion of tissue-specific progenitor cell populations in early development. This expansion is believed to occur because Six1 homeoprotein directly activates numerous cell cycle regulators, including gdnf, c-myc, and cyclin D1 during development (Li et al., 2003, Yu et al., 2006). Recently, our group identified the tissue-restricted cyclin A1 as a transcriptional target of Six1, uncovering yet another mechanism by which Six1 promotes cell cycle progression (Ford et al., 1998, Coletta et al., 2004a).
Salivary gland development is a dynamic process in which cellular proliferation and survival are carefully controlled during branching morphogenesis, directed, in part, by transcriptional regulation (Melnick & Jaskoll, 2000). Although a reduction in salivary gland size has been observed in Six1-deficient mice (Laclef et al., 2003), the mechanism by which Six1 influences submandibular salivary gland (SSG) development/maturation is unknown. To address this question, we have analyzed the expression profile of the Six1 gene during normal SSG development/maturation, and have characterized the effect of Six1 deficiency in this gland.
Material and methods
Animals
Generation and characterization of Six1 null mice have previously been described (Ozaki et al., 2004). The mice were housed at the Center for Laboratory Animal Care at the University of Colorado Denver (UCD) and treated in accordance with the NIH Guide to Humane Use of Animals in Research. All animal protocols were approved by the UCD-Institutional Animal Care and Use Committee (IACUC). To determine the normal temporal expression pattern of Six1, wild type C57Bl6/J mice were used. For Six1, cyclin A1 and cyclin A2 expression analysis, at each timepoint, SSG from 3 animals were sampled.
Reverse transcription-polymerase chain reaction (RT-PCR)
RNA was isolated from SSGs according to the manufacturer’s protocol for Trizol reagent (Invitrogen, Carlsbad, CA, USA). Before the RT reactions, all RNA samples were treated with DNaseI for 10 min at room temperature in order to eliminate genomic DNA contamination. Two micrograms of total RNA per sample were used to generate cDNA using random primers and Superscript II RNase H-reverse transcriptase (Invitrogen, Carlsbad, CA, USA). The resulting cDNAs were subsequently amplified in a 50 μl reaction mixture containing 1 μM of each primer, 2 mM MgCl2, 0.8 mM dNTPs, and 0.025 U/μl Taq DNA polymerase. Actin was used as a housekeeping control. Primer pairs used to amplify mouse Six1 (WT allele) were 5′ GAA TCA ACT CTC TCC TCT GG 3′ and 5′TTA GGA ACC CAA GTC CAC CA 3′; EGFP (mutated allele) primers were 5′ CTG GTG ACC ACC CTG ACC TAC 3′ and 5′ TGA TCC CGG CGG CGG TCA CGA A 3′; and actin primers were 5′ TAT CCT GAC CCT GAA GTA CC 3′ and 5′ GGT CAG GAT CTT CAT GAG GT 3′. After denaturation for 2 min at 94°C, 30 cycles of amplification were performed using a thermocycler, followed by a final extension of 10 min at 72°C. The amplification cycling parameters were: denaturation for 30 sec at 94°C, annealing for 1 min at 55°C, and extension for 2 min at 72°C. After amplification, 20 μl of PCR products were electrophoresed on a 1% agarose gel containing 0.5 μg/ml of ethidium bromide.
Quantitative real-time PCR (qPCR)
qPCR was performed using a model 7000 instrument (Applied Biosystems, Foster City, CA, USA). Amplicons were detected using Taqman fluorescence probes as described elsewhere (Lie & Petropoulos, 1998). The primers and probes used for this study were as follows: for Six1 5′ AAC TGC AGC AGC TGT GGC T 3′, 5′ GTC GGC CGC GAA GTT TC 3′, and 5′ AAA GCG CAC TAC GTG GAG GCC G 3′ (probe), and for cyclin A1 5′ TTT CCC CAA TGC TGG TTG A 3′, 5′ AAC CAA AAT CCG TTG CTT CCT 3′, and 5′ CCC ACC ACC CAT GCC CAG TCA 3′ (probe). The cyclin A2 and 18S rRNA primers and probes were purchased as Assays-on-Demand gene expression from Applied Biosystems. Target genes were analyzed using standard curves to determine relative levels of gene expression, and individual cDNA samples were normalized according to the levels of 18S rRNA.
Western blot analysis
SSGs were washed with cold phosphate-buffered saline (PBS) and lysed in RIPA buffer (50mM Tris-HCl pH7.4, 150mM NaCl, 1mM EDTA, 0.5% NP-40, 0.5% deoxycholic acid, 0.5% SDS, 1mM PMSF, 10mM NaF, 1mM Na3VO4 and 1μg/ml leupeptin). After centrifugation, protein concentrations were measured using a protein assay (Bio Rad, Hercules, CA, USA) according to the manufacturer’s instructions. Western blot analysis was performed using anti-Six1 and anti-actin antibodies as described (Ford et al., 2000).
Immunofluorescence
SSGs from Six1-/- animals at embryonic day 18.5 (E18.5) were fixed in 4% paraformaldehyde in PBS for 16 h at 4°C. Following fixation, the tissues were washed in PBS, paraffin-embedded, and sectioned at 3 μm. After dewaxing and hydrating in graded alcohol solutions, the sections were treated with 10 mM citric acid pH6.0 in a microwave for 20 min. To prevent non-specific binding, the tissues were blocked with the M.O.M. mouse Ig blocking reagent (Vector Labs, Burlingame, CA, USA) in PBS for 16 h at 4°C. The tissues were then incubated with monoclonal mouse anti-GFP antibodies (Chemicon Int., Temecula, CA), washed with PBS, and incubated with goat anti-mouse IgG conjugated with fluorescein (Calbiochem, San Diego, CA, USA). Tissues were examined under an Olympus BX51 fluorescence microscope equipped with a Penguin 600CL camera. Tissues untreated with primary antibodies were used as negative controls.
SSG measurement, histology, cell proliferation and cell death assays
Pregnant female mice at day 18.5 of gestation were intraperitoneally injected with 100 mg of bromodeoxyuridine (BrdU) per kg body weight. Embryos were collected 2 h later and the SSG volumes were calculated using the formula: volume=0.5×Length×Width2. After measurement, the SSGs from wild Six1+/+ and Six1-/- embryos were dissected with the aid of a stereoscopic microscope, fixed in 70% ethanol, embedded in paraffin, and sectioned at 3 μm. The sections were either hematoxylin & eosin (H&E) stained or analyzed for cell proliferation or cell death. Cell proliferation was measured via BrdU incorporation using an immunohistochemical analysis kit (GE Healthcare, Piscataway, NJ, USA), whereas cell death assays were performed by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) analysis using an apoptosis detection kit (Intergen, Norcross, GA).
Statistical analysis
All assays were performed at least twice. Student’s T-test was used for statistical analysis, and p≤0.05 was considered to indicate statistical significance.
Results
Six1 is dynamically expressed in the maturing SSG
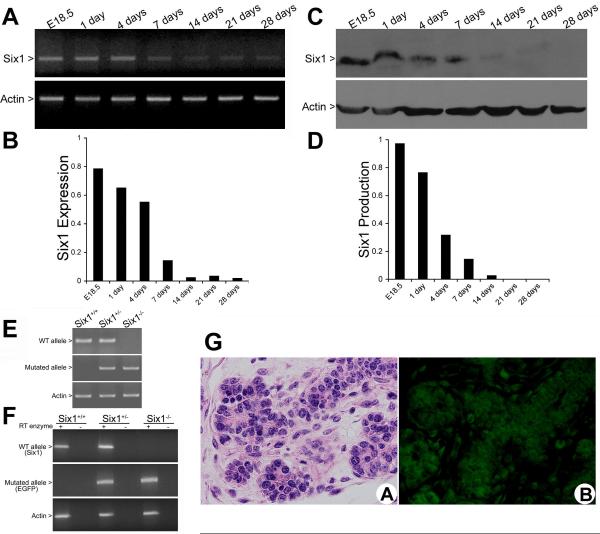
To elucidate the potential role of Six1 in SSG maturation, we first evaluated the temporal and spatial expression of Six1 in wild-type mouse SSGs from E18.5 to postnatal day 28. Six1 mRNA levels were high in SSGs from E18.5 to postnatal day 4, after which they declined progressively (Fig. 1A and B). In accordance with Six1 mRNA expression levels, the amount of Six1 protein decreased steadily in the developing postnatal SSG as shown by western blot analysis (Fig. 1C and D). To determine which cell types within the salivary gland express Six1, immunofluorescence was performed on E18.5 SSGs isolated from Six1 heterozygote embryos, in which enhanced green fluorescent protein (EGFP) had been knocked into the Six1 locus (Fig. 1E and F). EGFP expression was strongly observed in the developing ductal and acinar cells, with some mesenchymal cells showing very low levels of expression (Fig. 1G).
Figure 1.
Temporal and spatial expression of Six1 during SSG late embryogenesis is dynamic. (A) RNA isolated from wild-type SSGs was subjected to RT-PCR assays using specific primers for Six1 (actin was used as a control). (B) Comparison of Six1 expression throughout SSG development by densitometric analysis indicates that Six1 mRNA levels progressively decrease from E18.5 to postnatal day 28. Values are expressed in arbitrary units as the ratio of the optical density of Six1/actin. (C and D) Western blot and densitometric analyses demonstrate that the Six1 protein is present at high levels in SSG development, and levels decrease throughout the course of development. (E) PCR analyses used to genotype Six1+/+, Six1+/-, and Six1-/- neonates. Tail DNA was isolated and PCR was performed using specific primer pairs as described. (F) RT-PCR analyses of SSG from Six1+/+, Six1+/-, and Six1-/- at day E18.5 confirms the absence of Six1 mRNA and the expression of EGFP in the Six1-/- SSG. (G) H&E staining and immunostaining against EGFP, which was knocked in to the Six1 locus, demonstrate the expression of Six1 in the ductal and acinar cells of the SSG at E18.5. (Magnification, ×40)
Six1-/- SSGs are significantly smaller than wild-type littermates as a consequence of decreased proliferation
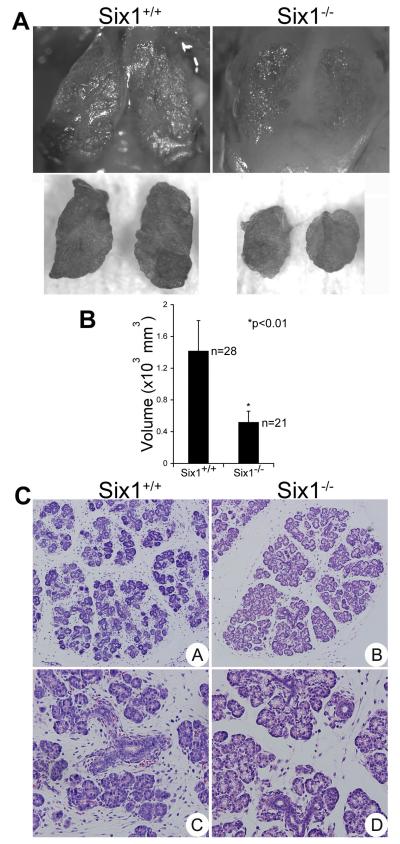
Our results confirm those from previous findings (Laclef et al., 2003) revealing that Six1-/- SSGs are significantly smaller than Six1+/+ SSGs (Fig. 2A). The SSG volume in Six1+/+ animals (n=28) ranged from 0.67 to 2.45×103 mm3, with a mean of 1.41±0.38×103 mm3, whereas the SSG volume in Six1-/- animals (n=21) ranged from 0.24 to 0.86×103 mm3, with a mean of 0.51±0.14×103 mm3 (Fig. 2B). Although the Six1-/- SSGs were significantly smaller than wild-type SSGs, the histological structures of the Six1-/- SSG acini and ducts showed normal morphogenesis (Fig. 2C).
Figure 2.
SSGs are reduced in size in Six1-deficient embryos. (A) Photographs of SSGs from a Six1+/+ embryo (left) and from a Six1-/- littermate (right) at E18.5. Top panels represent SSG within the mouse, whereas bottom panels represent glands after dissection from the mouse. (B) Measurements of SSG size demonstrate that Six1-/- SSGs are significantly smaller than Six1+/+ SSGs. (C) E18.5 SSGs from Six1-/- mice are completely normal in structure when compared with litter-matched wild type mice. (Magnification: A, B ×25; C, D ×40)
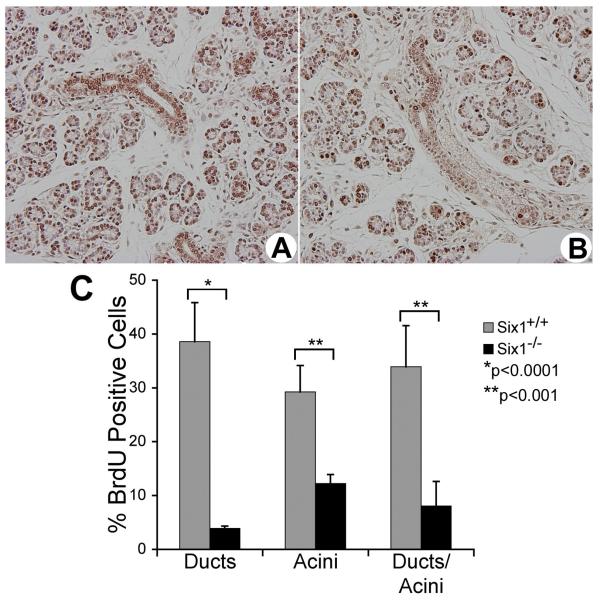
Six1 stimulates proliferation and inhibits apoptosis in both cell culture and in mouse model systems (Coletta et al., 2004a, Ozaki et al., 2004, Xu et al., 2003, Li et al., 2003, Ikeda et al., 2007), suggesting that the loss of Six1 leads to an exit from the cell cycle and to the premature death of epithelial cells. Thus, BrdU incorporation and TUNEL assays were performed to determine whether the decreased size of Six1-/- SSGs was the result of attenuated cell proliferation and/or increased cell elimination by apoptosis. The percentage of total BrdU-positive cells in Six1+/+ and Six1-/- SSGs was 33.92±8.05 and 7.62±4.54% at E18.5, respectively, demonstrating a marked decrease in the number of proliferating epithelial cells in the Six1-/- SSGs (Fig. 3). However, the number of TUNEL-positive cells was very low in both Six1+/+ and Six1-/- SSG epithelial cells, and no significant difference was observed (data not shown).
Figure 3.
Proliferation is dramatically decreased in Six1-/- SSG epithelial cells. BrdU-staining in SSGs from Six1+/+ (A) and Six1-/- (B) embryos at E18.5. (C) The BrdU indix, expressed as the percentage of positive cells, was determined by counting 1,500 cells in five independent samples for each genotype.
Six1 transcriptional target cyclin A1 is decreased in Six1-/- SSGs
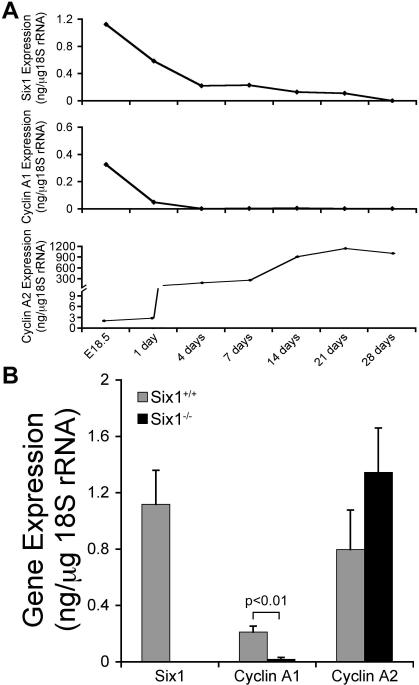
To investigate the molecular mechanism underlying the proliferation defect in Six1-/- SSG epithelial cells, expression levels of cyclin A1, a known transcriptional target of Six1 that mediates its effects on proliferation, was analyzed. First, the temporal expression of cyclin A1 and its functionally related gene, cyclin A2, was determined throughout SSG development. The temporal expression of cyclin A1 was very similar to that of Six1, with high levels of expression in the embryonic SSG. In contrast, cyclin A2 was most highly expressed in the postnatal SSG, although its levels were significantly higher than cyclin A1 throughout the course of SSG development (Fig. 4A). Expression of cyclin A1 was significantly lower in Six1-/- SSGs as compared to Six1+/+ SSGs (p<0.01), whereas cyclin A2 levels were not changed in a statistically significant manner (Fig. 4B). Thus, the proliferation defect observed in Six1-/- SSG cells was accompanied by a downregulation of the Six1 transcriptional target cyclin A1, which is highly expressed in the SSG during late embryogenesis.
Figure 4.
Six1 and cyclin A1 expression are coordinately regulated during embryogenesis, and expression of cyclin A1 is significantly reduced in Six1-/- SSG. SSGs were dissected, RNA isolated and converted into cDNA, and Six1, cyclin A1, and cyclin A2 expression levels determined by qPCR. (A) Representative expression of Six1, cyclin A1, and cyclin A2 during different developmental stages of the mouse SSG. Note that Six1 and cyclin A1 have a similar temporal expression during late development of SSG. (B) Expression levels of Six1, cyclin A1, and cyclin A2 in the SSG from Six1+/+ and Six1-/- embryos at day 18.5. Results represent the average ± SD of 3 independent samples, each sample containing the pooled SSGs of 5 mice for each genotype. SSGs from Six1-/- mice demonstrated a significant reduction in cyclin A1 expression as compared to Six1+/+.
Discussion
The SSG develops through a process of branching morphogenesis, undergoing complex stages of cell proliferation and differentiation, which are tightly coordinated by transcriptional regulatory pathways (Melnick & Jaskoll, 2000). However, the molecular mechanisms underlying these events within the SSG remain largely unknown. The homeobox gene family encodes proteins with DNA-binding and transcriptional activities that are critical in normal development (Pearson et al., 2005). To date, from a family with more than 200 members, only few homeobox genes are reported to be expressed in the mouse SSG (Biben et al., 2002, Jaskoll et al., 1998, Raju et al., 1993, Tanaka et al., 2000). In this study we have identified the expression of another homeoprotein in the mouse SSG, and we have examined the dependence of SSG maturation on this important developmental molecule. Expression of Six1 is high during late stages of embryonic development (E18.5), and its levels progressively decline during postnatal SSG development. These findings are consistent with the general role of Six family members, which function in regulating cell proliferation and in specifying cell fate in the developing embryo (Christensen et al., 2008).
Studies examining Six1 deficiency in mice suggest that it is a key participant in development by controlling the expansion of progenitor cell populations in specific organs (Li et al., 2003, Xu et al., 2003, Ozaki et al., 2004). Inner ear development in Six1-/- embryos arrests at the otic vesicle stage and all components of the inner ear fail to form due to an increase in apoptosis and a decrease in proliferation of the cells of the otic epithelium (Zheng et al., 2003, Ozaki et al., 2004). In the kidney, loss of Six1 leads to a failure of ureteric bud proliferation and invasion, with subsequent apoptosis of mesenchymal cells (Xu et al., 2003). Recently, it has been shown that Six1 stimulates proliferation and tumor growth through direct activation of cyclin A1 (Coletta et al., 2004a). In contrast to studies which demonstrated that Six1 alters both cell proliferation and apoptosis during inner ear and kidney development, no alterations in cell death were observed in Six1-/- SSGs. However, Six1 deficiency in the mouse SSG resulted in a profound decrease in proliferation. Therefore, we conclude from our studies that the significant reduction in size of Six1-/- SSGs occurs as a result of decreased proliferation. Importantly, the lack of an apoptotic effect may be due to the developmental stages analyzed in this study. During SSG development, proliferation seems to be a constant event, whereas apoptosis is rare, except for a few apoptotic cells observed in a large duct at E15 in mouse (Melnick & Jaskoll, 2000), a stage which we did not include in our analysis. Interestingly, apoptosis is associated with rat intercalated duct development (Hayashi et al., 2000, Hecht et al., 2000).
Although our data clearly demonstrate that Six1 deficiency decreases cell proliferation, the SSG is still properly formed and no difference in the degree of maturity of the gland was observed. The absence of morphological alterations in Six1-/- SSGs confirms that the primary role of Six1 in the SSG is to expand the SSG cell population and is not to influence differentiation or morphogenesis per se. It is likely that the SSG is not completely absent in Six1-/- mice because additional factors are involved in the proliferation of SSG cells during development, and these can, in part, compensate for the loss of Six1. One possibility is that other Six family members compensate for the loss of Six1. At this time, the expression pattern of the other four Six family members throughout SSG development has not been investigated. Given the documented redundancy between Six family members during development (Grifone et al., 2005, Konishi et al., 2006, Kobayashi et al., 2007), another Six family member might compensate for the loss of Six1 and promote proliferation to establish the SSG. Redundancy between Six1 targets might also compensate for the loss of Six1. For example, the Six1 transcriptional target cyclin A1 is, at least in part, functionally redundant with cyclin A2 during embryogenesis (Winston, 2001). Both A-type cyclins bind to and activate the cyclin-dependent kinases 1 and 2 and are required at critical points in the cell cycle, including the progression though S phase and the G2/M transition (Yang et al., 1999, Romanowski et al., 2000, Liu et al., 2000). Here we demonstrate that cyclin A1 expression is dramatically reduced in the Six1-/- SSG, whereas cyclin A2 levels remain unchanged. Thus, it is possible that cyclin A2 partially compensates for the loss of cyclin A1 in the Six1-deficient SSG. Furthermore, as no SSG size alterations have been reported in cyclin A1 null mice (Liu et al., 1998), it is likely that other cyclin A1-independent proliferative pathways important for SSG development are affected by Six1.
In summary, we demonstrate that Six1 is important for SSG development by controlling the proliferation that is necessary for the expansion of SSG epithelial cells. Recent evidence suggests that Six1 may play important roles in multiple tumor types, as its overexpression has been observed in breast (Reichenberger et al., 2005, Coletta et al., 2004b, Ford et al., 1998), ovarian (Behbakht et al., 2007), hepatocellular carcinoma (Ng et al., 2006), cervical carcinoma (Wan et al., 2008), Wilms’ tumor (Li et al., 2002), and alveolar rhabdomyosarcoma (RMS) (Yu et al., 2004, Khan et al., 1999). Interestingly, in some cases, Six1 expression is high in cancers derived from tissues where it is normally expressed and where it plays a functional role during development, including kidney and muscle (Khan et al., 1999, Li et al., 2002). As the molecular pathways involved in carcinogenesis often represent aberrations of normal processes that control embryogenesis (Abate-Shen, 2002), it is of interest to determine whether Six1 can contribute to salivary gland tumorigenesis.
Acknowledgments
This work was supported by grants from the NIH (R01CA095277), Susan G. Komen Breast Cancer Foundation (9862), American Cancer Society/UCCC, and Avon Foundation for H.L.F. R.D.C. was supported by fellowships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil, University of Colorado Cancer Center, Colorado Cancer League, and W.M. Thorkildsen Foundation. E.L.M. was funded by a predoctoral fellowship from the Department of Defense Breast Cancer Research Program (W81XWH-06-1-0409).
Footnotes
Conflict of interest There is no conflict of interest.
References
- Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer. 2002;2:777–85. doi: 10.1038/nrc907. [DOI] [PubMed] [Google Scholar]
- Behbakht K, Qamar L, Aldridge CS, Coletta RD, Davidson SA, Thorburn A, Ford HL. Six1 overexpression in ovarian carcinoma causes resistance to TRAIL-mediated apoptosis and is associated with poor survival. Cancer Res. 2007;67:3036–42. doi: 10.1158/0008-5472.CAN-06-3755. [DOI] [PubMed] [Google Scholar]
- Biben C, Wang CC, Harvey RP. NK-2 class homeobox genes and pharyngeal/oral patterning: Nkx2-3 is required for salivary gland and tooth morphogenesis. Int J Dev Biol. 2002;46:415–22. [PubMed] [Google Scholar]
- Christensen KL, Patrick AN, McCoy EL, Ford HL. The Six Family of Homeobox Genes in Development and Cancer. Adv Cancer Res. 2008;101 doi: 10.1016/S0065-230X(08)00405-3. [DOI] [PubMed] [Google Scholar]
- Coletta RD, Christensen K, Reichenberger KJ, Lamb J, Micomonaco D, Huang L, Wolf DM, Müller-Tidow C, Golub TR, Kawakami K, Ford HL. The Six1 homeoprotein stimulates tumorigenesis via reactivation of cyclin A1. Proc Natl Acad Sci U S A. 2004a;101:6478–83. doi: 10.1073/pnas.0401139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coletta RD, Christensen K, Reichenberger KJ, Lamb J, Micomonaco D, Huang L, Wolf DM, Muller-Tidow C, Golub TR, Kawakami K, Ford HL. The Six1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1. Proc Natl Acad Sci U S A. 2004b;101:6478–83. doi: 10.1073/pnas.0401139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford HL, Kabingu EN, Bump EA, Mutter GL, Pardee AB. Abrogation of the G2 cell cycle checkpoint associated with overexpression of HSIX1: a possible mechanism of breast carcinogenesis. Proc Natl Acad Sci U S A. 1998;95:12608–13. doi: 10.1073/pnas.95.21.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford HL, Landesman-Bollag E, Dacwag CS, Stukenberg PT, Pardee AB, Seldin DC. Cell cycle-regulated phosphorylation of the human SIX1 homeodomain protein. J Biol Chem. 2000;275:22245–54. doi: 10.1074/jbc.M002446200. [DOI] [PubMed] [Google Scholar]
- Grifone R, Demignon J, Houbron C, Souil E, Niro C, Seller MJ, Hamard G, Maire P. Six1 and Six4 homeoproteins are required for Pax3 and Mrf expression during myogenesis in the mouse embryo. Development. 2005;132:2235–49. doi: 10.1242/dev.01773. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Ozono S, Watanabe K, Nagatsu I, Onozuka M. Morphological aspects of the postnatal development of submandibular glands in male rats: involvement of apoptosis. J Histochem Cytochem. 2000;48:695–8. doi: 10.1177/002215540004800513. [DOI] [PubMed] [Google Scholar]
- Hecht R, Connelly M, Marchetti L, Ball WD, Hand AR. Cell death during development of intercalated ducts in the rat submandibular gland. Anat Rec. 2000;258:349–58. doi: 10.1002/(SICI)1097-0185(20000401)258:4<349::AID-AR3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Ookawara S, Sato S, Ando Z, Kageyama R, Kawakami K. Six1 is essential for early neurogenesis in the development of olfactory epithelium. Dev Biol. 2007;311:53–68. doi: 10.1016/j.ydbio.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Jaskoll T, Luo W, Snead ML. Msx-2 expression and glucocorticoid-induced overexpression in embryonic mouse submandibular glands. J Craniofac Genet Dev Biol. 1998;18:79–87. [PubMed] [Google Scholar]
- Kawakami K, Sato S, Ozaki H, Ikeda K. Six family genes--structure and function as transcription factors and their roles in development. Bioessays. 2000;22:616–26. doi: 10.1002/1521-1878(200007)22:7<616::AID-BIES4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Khan J, Bittner ML, Saal LH, Teichmann U, Azorsa DO, Gooden GC, Pavan WJ, Trent JM, Meltzer PS. cDNA microarrays detect activation of a myogenic transcription program by the PAX3-FKHR fusion oncogene. Proc Natl Acad Sci U S A. 1999;96:13264–9. doi: 10.1073/pnas.96.23.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Kawakami K, Asashima M, Nishinakamura R. Six1 and Six4 are essential for Gdnf expression in the metanephric mesenchyme and ureteric bud formation, while Six1 deficiency alone causes mesonephric-tubule defects. Mech Dev. 2007;124:290–303. doi: 10.1016/j.mod.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Ikeda K, Iwakura Y, Kawakami K. Six1 and Six4 promote survival of sensory neurons during early trigeminal gangliogenesis. Brain Res. 2006;1116:93–102. doi: 10.1016/j.brainres.2006.07.103. [DOI] [PubMed] [Google Scholar]
- Laclef C, Souil E, Demignon J, Maire P. Thymus, kidney and craniofacial abnormalities in Six 1 deficient mice. Mech Dev. 2003;120:669–79. doi: 10.1016/s0925-4773(03)00065-0. [DOI] [PubMed] [Google Scholar]
- Li CM, Guo M, Borczuk A, Powell CA, Wei M, Thaker HM, Friedman R, Klein U, Tycko B. Gene expression in Wilms’ tumor mimics the earliest committed stage in the metanephric mesenchymal-epithelial transition. Am J Pathol. 2002;160:2181–90. doi: 10.1016/S0002-9440(10)61166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, Rosenfeld MG. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–54. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- Lie YS, Petropoulos CJ. Advances in quantitative PCR technology: 5′ nuclease assays. Curr Opin Biotechnol. 1998;9:43–8. doi: 10.1016/s0958-1669(98)80082-7. [DOI] [PubMed] [Google Scholar]
- Liu D, Liao C, Wolgemuth DJ. A role for cyclin A1 in the activation of MPF and G2-M transition during meiosis of male germ cells in mice. Dev Biol. 2000;224:388–400. doi: 10.1006/dbio.2000.9776. [DOI] [PubMed] [Google Scholar]
- Liu D, Matzuk MM, Sung WK, Guo Q, Wang P, Wolgemuth DJ. Cyclin A1 is required for meiosis in the male mouse. Nat Genet. 1998;20:377–80. doi: 10.1038/3855. [DOI] [PubMed] [Google Scholar]
- Melnick M, Jaskoll T. Mouse submandibular gland morphogenesis: a paradigm for embryonic signal processing. Crit Rev Oral Biol Med. 2000;11:199–215. doi: 10.1177/10454411000110020401. [DOI] [PubMed] [Google Scholar]
- Ng KT, Man K, Sun CK, Lee TK, Poon RT, Lo CM, Fan ST. Clinicopathological significance of homeoprotein Six1 in hepatocellular carcinoma. Br J Cancer. 2006;95:1050–5. doi: 10.1038/sj.bjc.6603399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Nakamura K, Funahashi J, Ikeda K, Yamada G, Tokano H, Okamura HO, Kitamura K, Muto S, Kotaki H, Sudo K, Horai R, Iwakura Y, Kawakami K. Six1 controls patterning of the mouse otic vesicle. Development. 2004;131:551–62. doi: 10.1242/dev.00943. [DOI] [PubMed] [Google Scholar]
- Pearson JC, Lemons D, McGinnis W. Modulating Hox gene functions during animal body patterning. Nat Rev Genet. 2005;6:893–904. doi: 10.1038/nrg1726. [DOI] [PubMed] [Google Scholar]
- Raju K, Tang S, Dube ID, Kamel-Reid S, Bryce DM, Breitman ML. Characterization and developmental expression of Tlx-1, the murine homolog of HOX11. Mech Dev. 1993;44:51–64. doi: 10.1016/0925-4773(93)90016-q. [DOI] [PubMed] [Google Scholar]
- Reichenberger KJ, Coletta RD, Schulte AP, Varella-Garcia M, Ford HL. Gene amplification is a mechanism of Six1 overexpression in breast cancer. Cancer Res. 2005;65:2668–75. doi: 10.1158/0008-5472.CAN-04-4286. [DOI] [PubMed] [Google Scholar]
- Romanowski P, Marr J, Madine MA, Rowles A, Blow JJ, Gautier J, Laskey RA. Interaction of Xenopus Cdc2 × cyclin A1 with the origin recognition complex. J Biol Chem. 2000;275:4239–43. doi: 10.1074/jbc.275.6.4239. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Komuro I, Inagaki H, Jenkins NA, Copeland NG, Izumo S. Nkx3.1, a murine homolog of Ddrosophila bagpipe, regulates epithelial ductal branching and proliferation of the prostate and palatine glands. Dev Dyn. 2000;219:248–60. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1054>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- Wan F, Miao X, Quraishi I, Kennedy V, Creek KE, Pirisi L. Gene expression changes during HPV-mediated carcinogenesis: a comparison between an in vitro cell model and cervical cancer. Int J Cancer. 2008;123:32–40. doi: 10.1002/ijc.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston N. Regulation of early embryo development: functional redundancy between cyclin subtypes. Reprod Fertil Dev. 2001;13:59–67. doi: 10.1071/rd00042. [DOI] [PubMed] [Google Scholar]
- Xu PX, Zheng W, Huang L, Maire P, Laclef C, Silvius D. Six1 is required for the early organogenesis of mammalian kidney. Development. 2003;130:3085–94. doi: 10.1242/dev.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Muller C, Huynh V, Fung YK, Yee AS, Koeffler HP. Functions of cyclin A1 in the cell cycle and its interactions with transcription factor E2F-1 and the Rb family of proteins. Mol Cell Biol. 1999;19:2400–7. doi: 10.1128/mcb.19.3.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Davicioni E, Triche TJ, Merlino G. The Homeoprotein Six1 Transcriptionally Activates Multiple Protumorigenic Genes but Requires Ezrin to Promote Metastasis. Cancer Res. 2006;66:1982–1989. doi: 10.1158/0008-5472.CAN-05-2360. [DOI] [PubMed] [Google Scholar]
- Yu Y, Khan J, Khanna C, Helman L, Meltzer PS, Merlino G. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med. 2004;10:175–81. doi: 10.1038/nm966. [DOI] [PubMed] [Google Scholar]
- Zheng W, Huang L, Wei ZB, Silvius D, Tang B, Xu PX. The role of Six1 in mammalian auditory system development. Development. 2003;130:3989–4000. doi: 10.1242/dev.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Silvius D, Fritzsch B, Xu PX. Eya1 and Six1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes. Development. 2004;131:5561–72. doi: 10.1242/dev.01437. [DOI] [PMC free article] [PubMed] [Google Scholar]