Abstract
Background
Two macrophage ABC transporters, ABCA1 and ABCG1 have a major role in promoting cholesterol efflux from macrophages. Peritoneal macrophages deficient in ABCA1 and/or ABCG1 show enhanced expression of inflammatory and chemokine genes. This study was undertaken to elucidate the mechanisms and consequences of enhanced inflammatory gene expression in ABC transporter deficient macrophages.
Methods and Results
Basal and LPS-stimulated thioglycollate-elicited peritoneal macrophages showed increased inflammatory gene expression in the order Abca1−/−Abcg1−/−>Abcg1−/−>Abca1−/−>WT. The increased inflammatory gene expression was abolished in macrophages deficient in TLR4 or MyD88/TRIF. TLR4 cell surface concentration was increased in Abca1−/−Abcg1−/−>Abcg1−/−>Abca1−/− or WT macrophages. Treatment of transporter deficient cells with cyclodextrin reduced, while cholesterol-cyclodextrin loading increased inflammatory gene expression. Abca1−/−Abcg1- bone marrow-derived macrophages showed enhanced inflammatory gene responses to TLR2,3 and 4 ligands. To assess in vivo relevance we injected IP thioglycollate in Abcg1−/− bone marrow transplanted Western diet-fed Ldlr deficient mice. This resulted in a profound inflammatory infiltrate in the adventitia and necrotic core region of atherosclerotic lesions, consisting primarily of neutrophils.
Conclusions
The results suggest that HDL and apoA-1 exert anti-inflammatory effects by promoting cholesterol efflux via ABCG1 and ABCA1 with consequent attenuation of signaling via Toll-like receptors. In response to a peripheral inflammatory stimulus, atherosclerotic lesions containing Abcg1−/− macrophages experience an inflammatory “echo” suggesting a possible mechanism of plaque destabilization in subjects with low HDL.
Keywords: Atherosclerosis, ABC transporters, cholesterol, Toll-receptors, inflammation
Introduction
HDL levels are inversely related to the incidence of cardiovascular disease,1 and increasing HDL can reduce atherosclerosis in animal models.2,3 A principal anti-atherogenic property of HDL is thought to be its ability to promote cholesterol efflux from macrophage foam cells. In addition, HDL has been described to have various anti-inflammatory properties, including its ability to bind lipopolysaccharide, to remove oxidized lipids from LDL and to carry enzymes such as platelet-activating factor acetylhydrolase (PAFAH) and paraoxonase (PON) which inactivate oxidized phospholipids.4
The ability of HDL and its apolipoproteins to mediate macrophage cholesterol efflux depends in large part on the macrophage ABC transporters, ABCA1 and ABCG1. Whereas ABCA1 promotes cholesterol efflux to lipid-poor apoA-1, ABCG1 induces cholesterol efflux to HDL particles.5,6 Thus, these two transporters have complementary activity in mediating cholesterol efflux.7,8 Accordingly, in vivo measurements of macrophage reverse cholesterol transport have shown additive effects of ABCA1 and ABCG1,9 and genetic knock-out of both ABCA1 and ABCG1 in hematopoietic cells leads to massive cholesteryl ester accumulation in peritoneal macrophages, prominent foam cell accumulation in organs and accelerated atherogenesis in a susceptible background.10,11 In double knock-out bone marrow recipients atherosclerotic lesions and heart showed increased numbers of apoptotic cells, as well as an infiltration of inflammatory cells and foam cells.11 Peritoneal macrophages from Abca1−/−Abcg1−/− mice and Abcg1−/− mice and to a lesser extent Abca1−/− mice showed increased expression and secretion of a variety of inflammatory cytokines (TNFα, IL-6, IL-1β and IL-12p70) and chemokines (MIP-1α, MIP-2, MCP-1).11–13 Abcg1−/− mice accumulate foam cells and other inflammatory cells in the lung,14–16 but, surprisingly, transplantation of Abcg1−/− bone marrow into atherosclerosis susceptible recipients has resulted in either no change or reduced atherosclerosis in most studies.10,17,18 These observations suggested that accumulation of cholesterol, oxysterols or other lipids in ABC transporter deficient macrophages leads to increased inflammatory gene expression.11,16 The purpose of the present study was to determine the mechanisms responsible for the increased expression of inflammatory genes in Abcg1−/− and Abca1−/−Abcg1−/− macrophages, and to further explore the consequences of ABCG1 deficiency in the inflammatory response of atherosclerotic lesions.
Methods
For details, see the online data supplement
Animals and diets
Abca1−/−, Abcg1−/− and Abca1−/−Abcg1−/− littermates in a mixed C57BL/6 × DBA background were as previously described.11 C3H/HeJ mice carrying a toll-like receptor 4 mutation were obtainedfrom The Jackson Laboratory. Bone marrow (BM) transplantation was performed as previously described.19 The atherosclerosis studies were conducted in female C57BL/6 Ldlr−/− mice transplanted with C57BL/6 Abcg1−/− bone marrow fed a Western diet (TD 88137, Harlan Teklad) for 12 weeks.18 At the end of the study, Abcg1−/− recipients were challenged for 3 days with a single i.p injection of thioglycollate (38g/L) and one group of mice were allowed to recover for 2 weeks after injection. All mice were housed at Columbia University Medical Center according to animal welfare guidelines. Animals had ad libitum access to both food and water.
“The authors had full access to and take responsibility for the integrity of the data. All authors have read and agree to the manuscript as written”.
Results
Macrophages deficient in ABCA1 and/or ABCG1 show increased expression of inflammatory cytokines in response to LPS
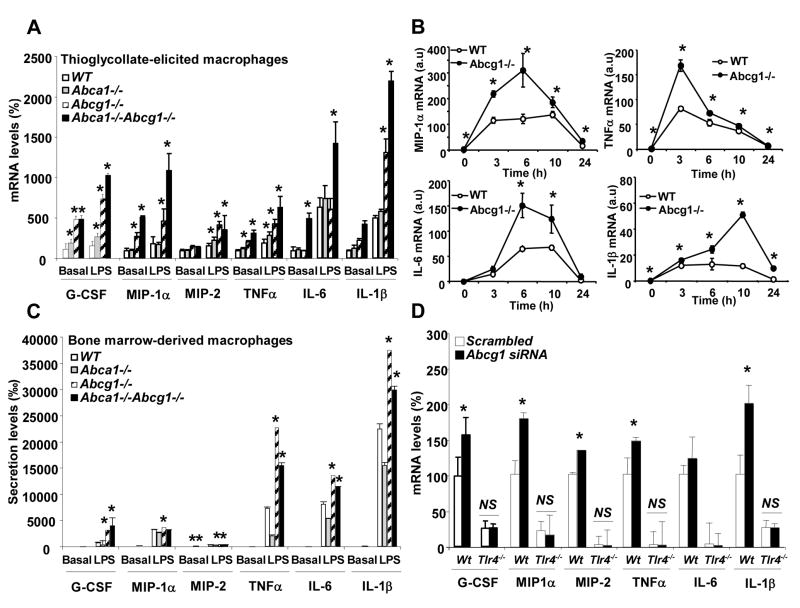
Following exposure of elicited macrophages to LPS there was an exaggerated cytokine response in Abca1−/−Abcg1−/− macrophages (G-CSF, MIP-1α, MIP-2, TNFα, IL-6, IL-1β) and Abcg1−/− macrophages (all cytokines tested except IL-6), while Abca1−/− macrophages showed slight increases only in some cytokines (G-CSF, MIP-2 and TNFα) (Fig. 1A). Although different macrophage preparations showed different degrees of increase in cytokine gene expression, the pattern was always: Abca1−/−Abcg1−/−>Abcg1−/−>Abca1−/−>WT. Secretion patterns of cytokines were parallel to mRNA responses (not shown). A time course experiment showed a greater initial increase as well as more sustained cytokine gene expression in Abcg1−/− compared to WT macrophages following LPS exposure (Fig. 1B.) We also evaluated inflammatory cytokine secretion in bone marrow-derived macrophages after 10 days in cell culture. An enhanced response to LPS was seen in Abcg1−/− and double KO macrophages but not in Abca1−/− macrophages (Fig. 1C). Notably, under these conditions there was no detectable basal secretion of cytokines in either WT or Abc transporter deficient macrophages (Fig. 1C), suggesting that the exaggerated cytokine expression of thioglycollate-elicited macrophages represented a response to exogenous factors such as LPS. LPS is a known contaminant of thioglycollate and is likely present in trace amounts in freshly elicited peritoneal macrophages.20 These observations indicated a markedly increased cytokine response to LPS in Abca1−/−Abcg1−/− and Abcg1−/− macrophages, with a much smaller effect in Abca1−/− cells.
Figure 1. Enhanced inflammatory and chemokine gene expression in Abca1−/−, Abcg1−/− and Abca1−/−Abcg1−/− compared to WT macrophages in response to LPS.
Thioglycollate-elicited or bone marrow-derived macrophages from mice on mixed background C57BL/6 × DBA were incubated in 0.2% BSA DMEM in the presence of 50ng/mL LPS for 4h (A and C, respectively). C57BL/6 WT and Abcg1−/− thioglycollate-elicited macrophages were incubated for various times with 50ng/mL LPS (B). Thioglycollate-elicited macrophages from WT and Tlr4−/− mice were treated with scrambled or ABCG1 siRNA before LPS stimulation for 4h (D). At the end of the incubation, transcript or secretion levels were determined. Expression of mRNA was normalized to β-actin. mRNA and secretion levels were expressed as percentage over untreated WT macrophages. Results are mean ± SEM of three independent experiments. *P< 0.05 vs. WT.
Knock-down of Abc transporters increases signaling via TLR4 and MyD88/TRIF in macrophages
LPS is known to signal via Toll Receptor 4 (TLR4). In order to test the hypothesis that the exaggerated cytokine response of elicited Abcg1−/− peritoneal macrophages was due to enhanced TLR4 signaling, we carried out an siRNA knockdown of Abcg1,6 in macrophages obtained from mice with genetic deficiency of TLR4. This genetic deficiency resulted in almost undetectable basal cytokine expression and prevented the inflammatory response induced by Abcg1 knockdown (Fig. 1D). Deficiency of MyD88, a downstream signaling molecule of TLR4 also abolished the LPS response in WT and Abcg1 deficient macrophages (Supplemental Fig. 1A and 1B). Similar results were obtained after knockdown of both Abcg1 and Abca1 by siRNA,18 in Myd88−/− Trif−/− macrophages (Supplemental Fig. 1C). Moreover, an inhibitor of LPS activation of TLR4 markedly reduced the increase in cytokine expression and secretion in freshly isolated thioglycollate macrophages from Abcg1−/−, Abca1−/− and Abca1−/−Abcg1−/− macrophages (not shown). Consistent with a role of TLR4 signaling, Abcg1−/− macrophages exhibited an increased content of nuclear p65 NFκB following LPS exposure (Supplemental Fig. 2A) and inhibitors of NFκB or p38 MAP kinase markedly reduced the increased cytokine gene expression in these cells (Supplemental Fig. 2B and 2C). These studies show that increased LPS signaling via TLR4 is responsible for increased expression of inflammatory cytokines in elicited Abcg1−/− macrophages. However, total levels of TLR4 in membranes and cytosol were not altered in Abcg1−/− macrophages (Supplemental Fig. 2A).
Increased inflammatory gene expression does not involve LXR transrepression, ER stress or activation of inflammasome
We carried out further studies to determine the mechanism of the increase in NFκB-mediated gene expression in Abcg1−/− macrophages. An important form of signaling pathway cross-talk involves transrepression of NFκB responses as a result of activation of nuclear receptors such as LXRs or PPARs.21 Abcg1−/− macrophages accumulate sterols, notably 7-ketocholesterol and desmosterol after feeding a high cholesterol diet (Table 1) as well as 27-OH cholesterol (0.26±0.04 vs. 0.44±0.04 ng/mg protein in macrophages from WT and Abcg1−/− mice fed a chow diet respectively, P<0.05). Thus, we considered the possibility that Abcg1−/− macrophages might accumulate endogenous sterols that bind LXRs but fail to mediate transrepression of NFκB responses. The synthetic LXR activator T-0901317 was able to reduce expression of inflammatory cytokines in WT and Abcg1−/− macrophages, indicating that the transrepression mechanism by LXR was intact in these cells (Supplemental Fig. 3A). Similar findings were observed after treatment of macrophages with natural LXR ligands such as 27-OH cholesterol or desmosterol (Supplemental Fig. 4A). By contrast, addition of 7-ketocholesterol to WT and Abcg1−/− cells reduced the mRNA levels of some cytokines but did not abolish the differential response of Abcg1−/− and WT macrophages, inconsistent with a transrepression mechanism involving this particular oxysterol (Supplemental Fig. 4B). We also assessed the effects of exposure of macrophages to oxPL or oxLDL before LPS treatment. However, oxPL and oxLDL both caused a reduced inflammatory response in WT and Abcg1−/− macrophages (Supplemental Fig. 4B) along with an induction of the PPAR-gamma target gene, CD36 (data not shown). These responses most likely reflect PPAR-gamma activation and transrepression of NFkB responses.21 Finally, knock-down of both LXRalpha and LXRbeta by siRNA (about 70% efficiency) did not alter the profile of inflammatory cytokine expression in wild-type or Abcg1−/− macrophages (Supplemental Fig. 3B), although it was sufficient to reduce expression of LXR target genes such as SREBP-1c (data not shown). These data are inconsistent with a mechanism involving failure of transrepression by LXRs.
Table 1.
Macrophage sterol levels from Ldlr−/− mice transplanted with Abcg1+/+ or Abcg1−/− BM.
| Cholesterol ug/mg protein | Desmosterol ng/mg protein | Lanosterol ng/mg protein | 7-ketocholesteol ng/mg protein | |
|---|---|---|---|---|
| Abcg1+/+Ldlr−/− | 82±5 | 563±41 | 765±56 | 156±27 |
| Abcg1−/−Ldlr−/− | 135±18* | 751±38* | 843±76 | 323±60* |
Thioglycollate-elicited macrophages were obtained from 4–5 recipient mice fed a Western diet for 12 weeks and briefly cultured (1h) before sterol composition analysis by gas chromatography. Value are means ± SEM.
P < 0.05, significant difference vs. control mice.
We considered two additional mechanisms for increased NFκB responses in Abcg1−/− macrophages. First, Abcg1−/− macrophages have increased ACAT activity, suggesting an increased content of free cholesterol in the ER,11,22 and FC accumulation in the ER can lead to an ER stress response with subsequent NFκB activation.23 However, there was no increase in free cholesterol in the ER of Abcg1−/− macrophages (Supplemental Fig. 5B) and no induction of CHOP or other ER stress markers (Supplemental Fig. 5A). Finally, some cytokines such as IL-1β are secreted in increased amounts as a result of activity of the inflammasome.24 However, the inflammasome activator nigericin, did not modify IL-1β or other cytokine secretion (Supplemental Fig. 5C) and caspase-1 cleavage, a hallmark of inflammasome activation, was unchanged in Abcg1−/− macrophages (Supplemental Fig. 5D).
Modulation of macrophage membrane cholesterol modulates inflammatory response: possible role of lipid rafts
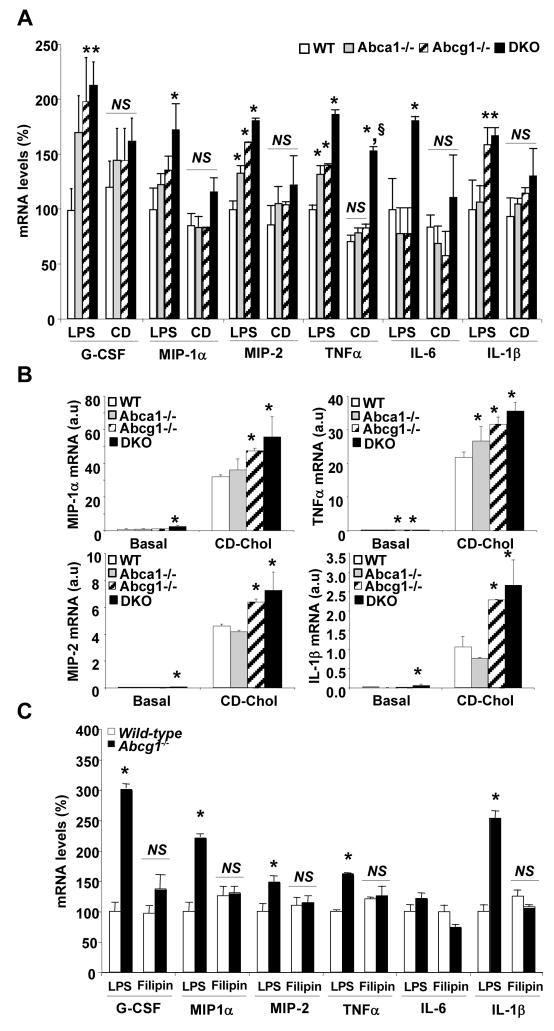
ABCG1 promotes efflux of cholesterol, 7-oxysterols and possibly phospholipids from cells.6,25 To test the hypothesis that increased inflammatory gene expression was secondary to cholesterol accumulation, we treated macrophages with cyclodextrin (CD) to deplete membrane cholesterol,26 and then exposed them to LPS. This treatment either abolished or reduced the increase in cytokine gene expression seen in ABC transporter deficient macrophages compared to WT (Fig. 2A). In contrast, CD-cholesterol loading led to a marked increase in cytokine gene expression in macrophages in the order Abca1−/−Abcg1−/−>Abcg1−/−>Abca1−/−>WT (Fig. 2B), i.e. the same pattern as observed in basal or LPS treated elicited macrophages (Fig 1A). Furthermore, treatment of macrophages with filipin, an agent that binds cholesterol in the plasma membrane,27 abolished the increased inflammatory cytokine expression in Abcg1−/− cells (Fig. 2C).
Figure 2. Modulation of the inflammatory response in Abca1−/−, Abcg1−/− and Abca1−/− Abcg1−/− macrophages after manipulation of plasma membrane cholesterol.
Thioglycollate-elicited macrophages from mice on mixed background C57BL/6 × DBA were incubated with 5mM cyclodextrin (CD) for 30 min before treatment with 50ng/mL LPS for 4h (A) or incubated with CD-cholesterol (2.5:1 molar ratio) for 4 hours (B). Filipin was pre-incubated at 3μg/mL for 30 min before addition of LPS to the cells and during the 4 hours-LPS treatment (C). Inflammatory transcript levels were quantified and normalized to β-actin RNA amount. mRNA levels were expressed as percentage over LPS-treated WT macrophages or as arbitrary units (a.u). Values are mean ± SEM. *P< 0.05 vs. WT.
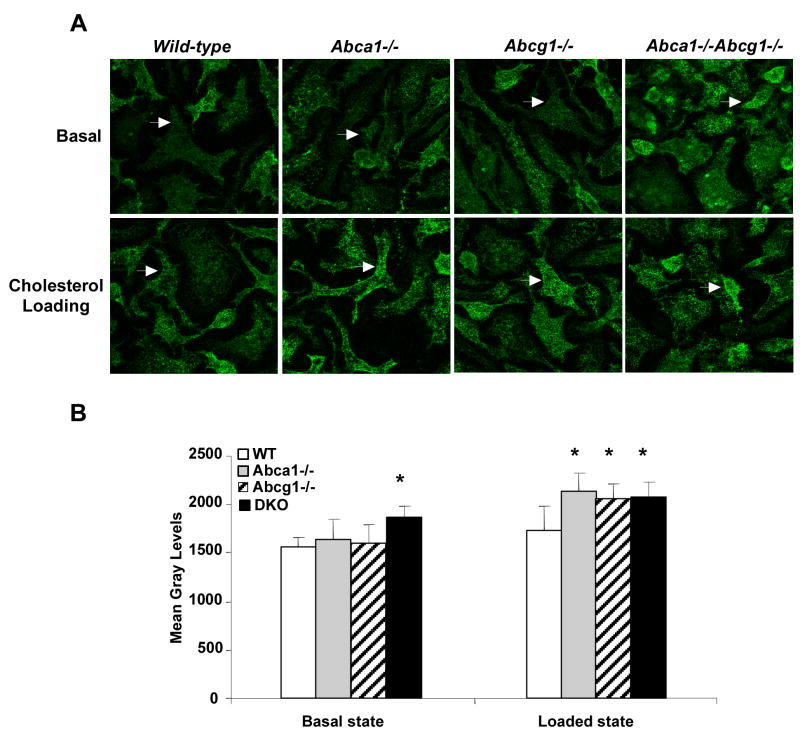
To assess the possibility that cholesterol accumulation in plasma membrane might lead to formation of increased liquid ordered domains in their plasma membranes, we incubated cells with fluorescent cholera toxin, which binds GM1 gangliosides in membrane liquid ordered domains. Confocal microscopy demonstrated significantly increased fluorescence in the plasma membrane of Abca1−/−Abcg1−/− macrophages in the basal state (Fig. 3A shows representative images and Fig 3B shows quantification of fluorescence intensity from 3-D reconstruction of confocal images). After cholesterol loading, increased fluorescence was also seen in Abca1−/− and Abcg1−/− knockout macrophages (Fig. 3A and 3B). Together this data suggest that increased inflammatory responses are due to an increased content of unesterified cholesterol in the plasma membrane, and may reflect increased lipid raft formation.
Figure 3. Visualized lipid rafts in Abca1−/−, Abcg1−/− and Abca1−/−Abcg1−/− macrophages.
Bone marrow-derived macrophages from mice on mixed background C57BL/6 × DBA were cultured in 0.2% BSA DMEM (Basal) or loaded overnight with 50μg/mL acLDL plus 3μmol/L TO901317 compound (Loaded state). Plasma membrane raft structures were labeled for ganglioside GM1 and visualized by confocal microscopy as described in methods. Images represent a 3D re-construction from the z-stack of image slices (A). Quantification was performed using ImageJ software and expressed as mean gray levels (B). Values are mean ± SD. *P< 0.05 vs. WT.
Increased cell surface expression of TLR4
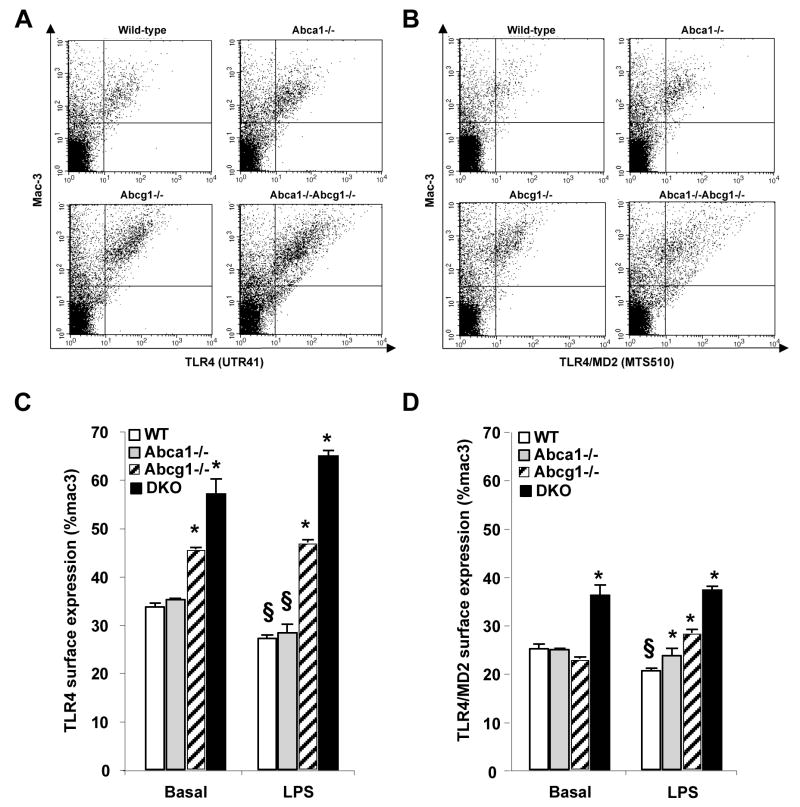
Although overall expression levels of TLR4 mRNA and protein were unaltered (Supplemental Fig. 2A and data not shown), FACS analysis showed an increased cell surface expression of TLR4 in Abcg1−/− and Abca1−/−Abcg1−/− macrophages, both under basal conditions and after treatment with LPS (Fig. 4A and 4C). Treatment with LPS resulted in a small but significant reduction in cell surface TLR4 concentration in WT and Abca1−/− macrophages, but not in Abcg1−/− or Abca1−/−Abcg1−/− macrophages (Fig. 4C). Optimal activation of the TLR4 signaling pathway by LPS involves the formation of an LPS signaling complex consisting of surface molecules such as CD14 and MD2.28 Using an antibody that selectively recognizes the TLR4/MD2 complex,29 we showed increased cell surface TLR4/MD2 complex formation in basal Abca1−/−Abcg1−/− macrophages, and following LPS treatment complex formation in the order Abca1−/−Abcg1−/−>Abcg1−/−>Abca1−/−>WT (Fig. 4B and 4D).
Figure 4. Increased surface expression of TLR4/MD2 complex in Abca1−/−, Abcg1−/−and Abca1−/−Abcg1−/− macrophages.
Thioglycollate-elicited macrophages from mice of each genotype were were cultured for 1h in suspension in presence or absence of 50ng/mL LPS. Cell surface expression of TLR4 (A and C) or TLR4/MD2 complex (B and D) was detected by flow cytometry of live cells stained with Alexa Fluor 488 anti-TLR4 (UT41), FITC anti-mouse TLR4/MD2 complex (MTS510) and PE anti-mouse Mac-3 staining (A and D). Profiles of fluorescence intensity of TLR4, TLR4/MD2 complex and Mac-3 staining in LPS-treated condition (A and B, respectively). Change in TLR4 or TLR4/MD2 surface expression in the different conditions (C and D, respectively). Data are mean ± SEM and expressed as % of Mac-3. *P< 0.05 vs. WT cells in the same condition. §P< 0.05 vs. untreated condition.
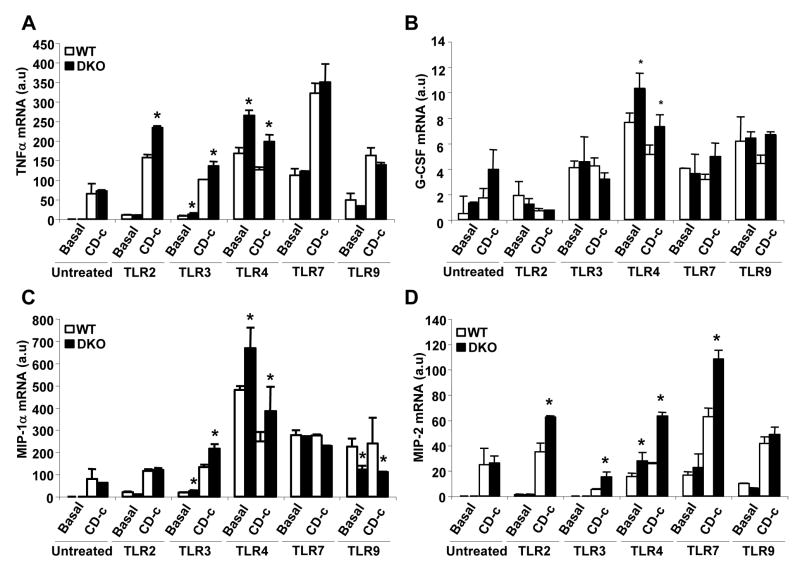
Increased response to TLR2, TLR3 and TLR4 ligands in Abca1−/−Abcg1−/− cells
In order to assess responses to ligands of various TLRs, we treated basal or CD-cholesterol loaded WT or Abca1−/−Abcg1−/− bone marrow-derived macrophages with ligands to different TLRs (Fig. 5). Treatment with ligands to TLR2, 3 and 4 but not TLR7 or 9 resulted in increased TNFα mRNA expression in Abca1−/−Abcg1−/− cells (Fig. 5A). The pattern was similar for other inflammatory genes, with more restricted responses for some genes (MIP1α, G-CSF) and broader responses including to TLR7 ligand for MIP-2 (Fig. 5B, 5C and 5D). Interestingly, in the absence of TLR ligands, there was no increase in inflammatory gene expression in ABC transporter deficient cells, even though there was a response to cholesterol loading. For TLR2 and 3 ligands, effects of cholesterol loading and transporter deficiency were more than additive, while they were not for TLR4 ligand. While this appears different to earlier results where clear synergy was seen (Fig. 2D), this likely reflects a much smaller TLR4 stimulus due to only trace LPS in this earlier experiment.
Figure 5. Increased response to TLR2, TLR3 and TLR4 ligands in Abca1−/−, Abcg1−/−and Abca1−/−Abcg1−/− macrophages.
Bone marrow-derived macrophages from mice on mixed background C57BL/6 × DBA were incubated in 10% FBS DMEM (basal) or with CD-cholesterol (CD-c, 2.5:1 molar ratio) for 4 hours in presence of different TLR ligands: TLR2 ligand (peptidoglycan, PGN, 2.5μg/mL), TLR3 ligand (PolyI:C, 2.5μg/mL), TLR4 ligand (LipidA, 100ng/mL), TLR7 ligand (Gardiquimod, 2.5μg/mL) and TLR9 (Bacterial CpG-DNA, 2.5μg/mL). Inflammatory transcript levels were quantified and normalized to β-actin RNA amount. mRNA levels of TNFα (A), G-CSF (B), MIP-1α (C) and MIP-2 (D) were expressed as arbitrary units (a.u). Values are mean ± SEM. *P< 0.05 vs. WT.
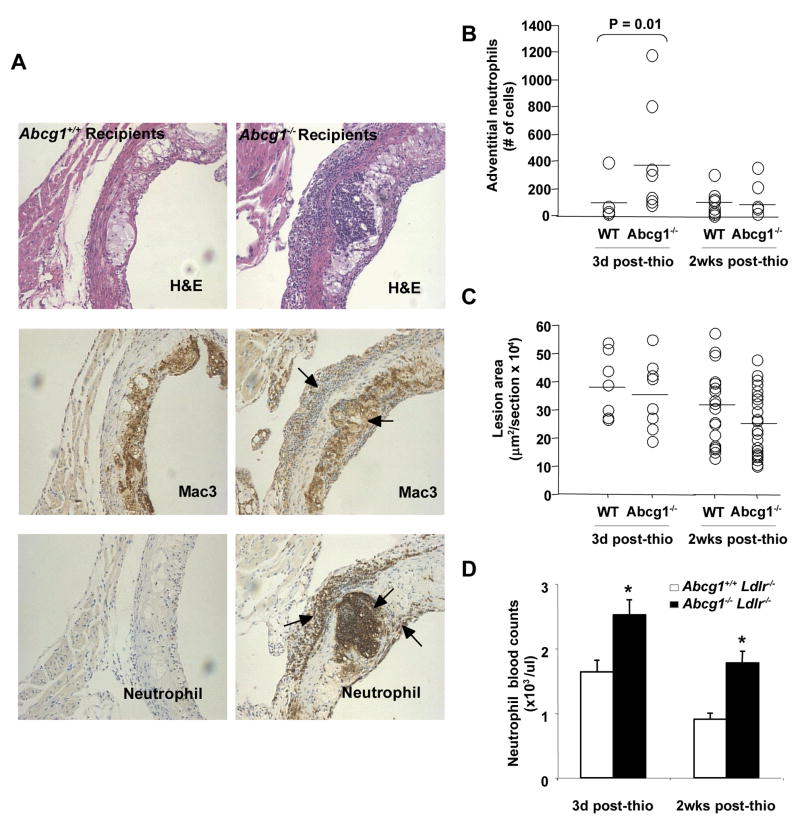
Marked inflammatory cell infiltration in lesions of Ldlr−/− mice transplanted with Abcg1−/− bone marrow
Although double KO bone marrow recipients showed increased atherosclerosis, Abcg1−/− bone marrow transplanted mice showed either no change or decreased atherosclerosis.10,11,17,18 This seemed to be discordant with our observations showing increased inflammatory response in thioglycollate-elicited Abcg1−/− macrophages. To further assess relevance of the inflammatory response to atherosclerosis, we examined atherosclerotic lesions of Abcg1−/− bone marrow recipients 3 days after intraperitoneal thioglycollate injection. Interestingly, there was a marked inflammatory cell infiltrate in mice transplanted with Abcg1−/− bone marrow compared to WT bone marrow transplanted mice (Fig. 6A). This was most prominent in the adventitia and in the necrotic core region of plaques. Microscopic examination of H&E sections indicated that many of the infiltrating cells appeared to be neutrophils with multi-lobed nuclei (not shown). The infiltrating cells showed abundant immunostaining for neutrophils (MCA771G) and to a much lesser extent with macrophages (Mac-3) (Fig. 6A) and monocytes (CD68+) and lymphocytes (CD3+) (not shown). The neutrophil stain also reacted strongly with necrotic debris in the necrotic core region of Abcg1−/− bone marrow recipients but not in Abcg1+/+ recipients. A systematic quantification suggested an approximate 4-fold increase in neutrophil content in the adventitia of lesions of Abcg1−/− bone marrow recipients compared to controls (Fig. 6B). In order to determine if there were longer-range consequences on atherosclerosis, lesions were analyzed 2 weeks after an intraperitoneal thioglycollate injection (Fig. 6C). At this time there was no difference in the neutrophil content of lesions (Fig. 6B), in necrotic core staining or macrophage content (not shown), and lesions if anything were somewhat smaller in Abcg1−/− bone marrow recipients (Fig. 6C). These observations suggest a transient intense predominantly neutrophilic inflammatory cell infiltrate associated with accumulation of necrotic debris probably derived from dead neutrophils in Abcg1−/− bone marrow recipients without an effect on lesion area. An examination of peripheral blood count in mice injected with IP thioglycollate showed an increased neutrophil count in Abcg1−/− bone marrow recipients; 2 weeks after IP thioglycollate, neutrophil levels had returned towards baseline but were still higher in Abcg1−/− bone marrow recipients (Fig. 6D).
Figure 6. Increased neutrophil infiltration in the proximal aorta of Ldlr−/− mice transplanted with Abcg1−/− bone marrow but no significant change in atherosclerotic lesion development following an acute inflammatory stimulus.
H&E staining in the proximal aorta of C57BL/6 Abcg1−/− recipients challenged for 3 days with a single i.p injection of thioglycollate (A). Mac-3 and MCA771G immunostaining (brown) revealed typical accumulations of foam cell macrophages under fibrous caps as well as prominent accumulations of neutrophils in the adventicia underlying plaques, smaller numbers of cells underneath fibrous caps, and neutrophil debris within necrotic cores (A). Quantification of the neutrophil infiltration in aortas. The increased neutrophil infiltration observed in Abcg1−/− recipients 3 days after thioglycollate injection was transient since no significant differences were observed 2 weeks post-injection (B). Quantification of proximal aortic root lesion area in wild-type and Abcg1−/− recipients revealed no apparent effect of the neutrophil infiltration on lesion areas (C). Increased peripheral blood neutrophil counts in Abcg1−/− recipients may contribute to increased neutrophil infiltration of the aortic root (D). *P<0.05 vs. WT recipients.
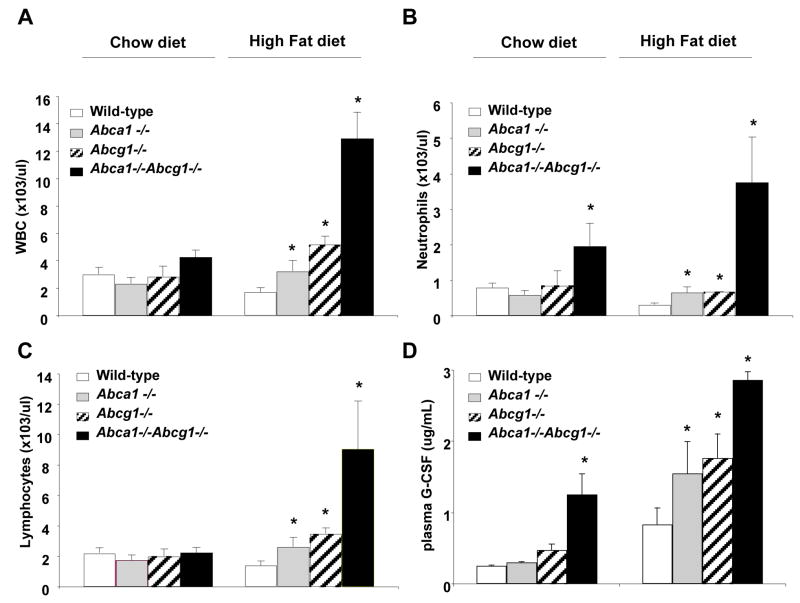
Increased plasma G-CSF and neutrophilia
The increased neutrophil count led us to systematically assess cell blood counts in mice of the four different genotypes. On a chow diet Abca1−/−Abcg1−/− mice had about a 2-fold increase in the neutrophil count (Fig. 7B). In response to a high fat diet, double KO mice and to a lesser extent single KO mice developed an increase in total WBC count that was not observed in control mice (Fig. 7A), suggesting an interaction between hypercholesterolemia and Abc transporter deficiency. The increase in total WBC count was due to an increase in both neutrophils and lymphocytes (Fig. 7B and 7C). Our studies in macrophages had shown a prominent increased in G-CSF mRNA and secretion in Abcg1−/− and Abca1−/−Abcg1−/− macrophages (Fig. 1A and 1C). Plasma G-CSF levels were prominently increased in double KO mice, and to a lesser extent in single KO mice, especially on the western diet (Fig. 7D).
Figure 7. Increased peripheral blood neutrophils and plasma G-CSF in Abca1−/−, Abcg1−/− and Abca1−/−Abcg1−/− total knockout female mice fed a high fat diet for 11 weeks.
Peripheral white blood count, WBC (A), blood neutrophil (B), and lymphocyte counts (C). Increased plasma G-CSF levels closely paralleled the neutrophilia observed in mice carrying the different genotypes (D). Data are mean ± SEM. *P<0.05 vs. WT
Discussion
Lipid accumulation and increased expression of inflammatory genes are central events in atherogenesis, but specific molecular mechanisms linking lipid accumulation to inflammation in macrophages are poorly understood. Seminal studies have indicated roles of TLR2, TLR4 and MyD88 in atherogenesis in hypercholesterolemic animal models, suggesting a link between hyperlipidemia and TLR signaling.28–32 The present study suggests that cholesterol accumulation in the plasma membrane of Abcg1−/− and Abca1−/−Abcg1−/− macrophages leads to increased levels and signaling of TLR4 and an increased inflammatory response following exposure to LPS. While these findings are consistent with a recent study in Abca1−/− macrophages,33 our studies suggest that ABCG1 has a larger role in modulating macrophage inflammatory responses than ABCA1 and that ABCA1 has a compensatory role when ABCG1 is deficient.
Abca1−/−Abcg1−/− macrophages also showed increased expression of several inflammatory cytokines when treated with ligands to TLR2 and TLR3 but not TLR7 or TLR9, indicating a widespread but not general enhancement of innate immune responses. This suggests that loading of plasma and endosomal membranes with free cholesterol results in enhanced inflammatory response to several TLR ligands, a situation that may occur when macrophages ingest apoptotic cells containing bacterial or viral products. The ability of HDL and apoA-1 to promote cholesterol efflux via ABCA1 and ABCG1 may have a down-modulating effect on these innate immune responses, also likely relevant to inflammation in atherogenesis.
We initially reported increased expression and secretion of inflammatory cytokines and chemokines in peritoneal macrophages from mice deficient in Abca1 and/or Abcg1.11 Baldan et al,16 found increased inflammatory gene expression in lungs and in peritoneal macrophages of Abcg1−/− mice following treatment with LPS, and Wojcik et al,15 showed increased inflammatory gene expression in alveolar macrophages of Abcg1−/− mice. The present study has provided new insights into the mechanism of increased inflammatory gene expression in Abcg1−/− and double knock-out macrophages. Several potential mechanisms such as inflammasome activation, reduced LXR transrepression of NFκB responses, ER stress and accumulation of specific sterols such as 7-oxysterols or 27-OH cholesterol, and inflammatory responses to oxidized phospholipids were largely excluded. Our data suggest that free cholesterol accumulation in the plasma membrane leads to increased levels and signaling of TLR4 via MyD88/TRIF. This may be related to increased formation of liquid ordered domains in the plasma membrane, resulting in increased cell surface expression and/or dimerization and activation of TLR4 in the presence of LPS. The increased TLR4/MD2 complex observed in all genotypes in the order Abca1−/−Abcg1−/−>Abcg1−/−>Abca1−/−>WT closely paralleled the inflammatory response to LPS and most likely reflected clustering of the TLR4/MD2 complex in rafts and increased TLR4 signaling.29,34 The LPS-mediated down-regulation of TLR4 seen in wild type and Abca1−/− cells did not occur in Abcg1−/− and Abca1−/−Abcg1−/− macrophages (Fig. 4C), suggesting that reduced TLR4 internalization could also contribute to the exaggerated inflammatory response in these cells.
Deficiency of TLR2, 4 and MyD88 reduces atherosclerosis in susceptible backgrounds,30–32 suggesting that TLR ligands are activating macrophages in atherosclerotic lesions. While the nature of the relevant ligands in atheroma remains unclear,35 this indicates that our observation of enhanced signaling via TLR2, 3 and 4 in Abca1−/−Abcg1−/− macrophages is likely relevant to accelerated atherosclerosis in mice with deficiency of these two transporters in hematopoietic cells. One consequence of increased inflammatory gene expression was an increase in neutrophils in blood. Abca1−/−Abcg1−/− mice and to a lesser extent single KO mice developed a remarkable neutrophilia after feeding a high cholesterol diet. Neutrophilia likely reflected increased production of G-CSF by hematopoietic cells, as levels of G-CSF and degree of neutrophilia appeared well correlated. The mechanism of neutrophilia likely involved at least in part increased G-CSF secretion by macrophages secondary to increased TLR4/MyD88 signaling. Interestingly, there was also lymphocytosis in high cholesterol diet-fed transporter deficient mice (Fig. 5C), consistent with a recent report showing enhanced lymphocyte proliferative responses in mice deficient in LXRβ or ABCG1.36 Together these findings emphasize the role of HDL and LXRs in dampening innate and acquired immune responses as a result of promotion of sterol efflux via ABCA1 and ABCG1.
We made the surprising observation that neutrophils became prominent in lesions of Abcg1−/− bone marrow transplanted mice after a peripheral inflammatory stimulus (IP thioglycollate injection), most likely reflecting exposure to trace lipopolysaccharide in the thioglycollate.20 Marked neutrophil accumulation in plaques appeared to reflect the underlying neutrophilia in these mice. In addition, macrophages of Abcg1−/− mice show increased secretion of MIP-1α and MIP-2, both potent neutrophil chemokines.37 Neutrophils are present in small numbers in atherosclerotic lesions of Ldlr−/− and apoE−/− mice, especially in the surface and adventitia of the lesion.38,39 Remarkably, a recent study showed that neutrophils accounted for about one fifth of the phagocytic capacity of leukocytes in lesions of apoE−/− mice.40 Neutrophils are prominent in human lesions in the context of acute coronary syndromes but it is not clear whether they are present secondary to athero-thrombosis as part of the cleaning up process, or whether they have a causative role, involving secretion of elastase, myeloperoxidase and H2O2.41 In prospective studies the neutrophil count is predictive of CHD, and also of the outcome after an ACS event.42 Although the neutrophil infiltration did not result in a plaque disruption in our study, such effects may have been limited since mice are resistant to plaque breakdown and thrombus formation. We speculate that similar changes in human atherosclerotic plaques could be involved in plaque destabilization i.e. after a peripheral inflammatory stimulus, a subject with low HDL levels and low ABCG1 activity might be more susceptible to an enhanced inflammatory “echo” response in lesions, leading to plaque destabilization. Our study suggests that one of the basic anti-atherogenic properties of HDL, suppression of inflammatory responses may be in part secondary to its ability to promote cholesterol efflux via ABCG1 and to a lesser extent ABCA1. A variety of treatments that raise HDL levels, such as niacin and CETP inhibitors, may promote cholesterol efflux via ABCG1,43,44 and thus suppress inflammatory and chemokine gene responses in macrophage foam cells.
Supplementary Material
Acknowledgments
Funding sources: This work was supported by grants from the NIH (HL54591).
Footnotes
Disclosures: Conflict of interest, Alan R. Tall reports being a consultant to Pfizer, Merck, Boehringer-Ingelheim and Takeda Pharmaceuticals.
References
- 1.Gordon DJ, Rifkind BM. High-density lipoprotein-the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 2.Plump AS, Scott CJ, Breslow JL. Human apolipoprotein A–I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci USA. 1994;91:9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okamoto H, Yonemori F, Wakitani K, Minowa T, Maeda K, Shinkai H. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature. 2000;406:203–207. doi: 10.1038/35018119. [DOI] [PubMed] [Google Scholar]
- 4.Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev. 2006;58:342–374. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
- 5.Oram JF, Lawn RM, Garvin MR, Wade DP. ABCA1 is the cAMP- inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J Biol Chem. 2000;275:34508–34511. doi: 10.1074/jbc.M006738200. [DOI] [PubMed] [Google Scholar]
- 6.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci USA. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelissen IC, Harris M, Rye KA, Quinn C, Brown AJ, Klockx M, Cartland S, Packianathan M, Kritharides L, Jessup W. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler Thromb Vasc Biol. 2006;26:534–40. doi: 10.1161/01.ATV.0000200082.58536.e1. [DOI] [PubMed] [Google Scholar]
- 8.Vaughan AM, Oram JF. ABCA1 and ACG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J Lipid Res. 2006;47:2433–43. doi: 10.1194/jlr.M600218-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Out R, Hoekstra M, Habets K, Meurs I, de Waard V, Hildebrand RB, Wang Y, Chimini G, Kuiper J, Van Berkel TJ, Van Eck M. Combined Deletion of Macrophage ABCA1 and ABCG1 Leads to Massive Lipid Accumulation in Tissue Macrophages and Distinct Atherosclerosis at Relatively Low Plasma Cholesterol Levels. Arterioscler Thromb Vasc Biol. 2008;28:258–64. doi: 10.1161/ATVBAHA.107.156935. [DOI] [PubMed] [Google Scholar]
- 11.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koseki M, Hirano KI, Masuda D, Ikegami C, Tanaka M, Ota A, Sandoval JC, Nakagawa-Toyama Y, Sato SB, Kobayashi T, Shimada Y, Ohno-Iwashita Y, Matsuura F, Shimomura I, Yamashita S. Increased lipid rafts and accelerated lipopolysaccharide-induced tumor necrosis factor-αsecretion in Abca1-deficient macrophages. J Lipid Res. 2007;48:299–306. doi: 10.1194/jlr.M600428-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Francone OL, Royer L, Boucher G, Haghpassand, Freeman A, Brees D, Aiello RJ. Increased cholesterol deposition, expression of scavenger receptors, and response to chemotactic factors in Abca1-deficient macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1198–1205. doi: 10.1161/01.ATV.0000166522.69552.99. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Wojcik AJ, Skaflen MD, Srinivasan S, Hedrick CC. A critical role for ABCG1 in macrophage inflammation and lung homeostasis. J Immunol. 2008;180:4273–4282. doi: 10.4049/jimmunol.180.6.4273. [DOI] [PubMed] [Google Scholar]
- 16.Baldan A, Gomes AV, Ping P, Edwards PA. Loss of ABCG1 results in chronic pulmonary inflammation. J Immunol. 2008;180:3560–3568. doi: 10.4049/jimmunol.180.5.3560. [DOI] [PubMed] [Google Scholar]
- 17.Baldan A, Pei L, Lee R, Tarr P, Tangirala RK, Weinstein NM, Frank J, Li AC, Tontonoz P, Edwards PA. Impaired development of atherosclerosis in hyperlipidemia Ldlr−/− and ApoE−/− mice transplanted with Abcg1−/− bone marrow. Arterioscler Thromb Vasc Biol. 2006;26:2301–2307. doi: 10.1161/01.ATV.0000240051.22944.dc. [DOI] [PubMed] [Google Scholar]
- 18.Ranalletta M, Wang N, Han S, Yvan-Charvet L, Welch C, Tall AR. Decreased atherosclerosis in low-density lipoprotein receptor knockout mice transplanted with Abcg1−/− bone marrow. Arterioscler Thromb Vasc Biol. 2006;26:2308–2315. doi: 10.1161/01.ATV.0000242275.92915.43. [DOI] [PubMed] [Google Scholar]
- 19.Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Marley SB, Hadley CL, Wakelin D. Effect of genetic variation on induced neutrophilia in mice. Infect Immun. 1994;62:4304–4309. doi: 10.1128/iai.62.10.4304-4309.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghisletti S, Huang W, Ogawa S, Pascual G, Lin ME, Willson TM, Rosenfeld MG, Glass CK. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang N, Ranalletta M, Matsuura F, Peng F, Tall AR. LXR-induced redistribution of ABCG1 to plasma membrane in macrophages enhances cholesterol mass efflux to HDL. Arterioscler Thromb Vasc Biol. 2006;26:1310–6. doi: 10.1161/01.ATV.0000218998.75963.02. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Schwabe RF, De Vries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, Davis RJ, Flavell R, Brenner DA, Tabas I. Free cholesterol-loaded macrophages are an abundant source of tumour necrosis factor-alpha and interleukin-6: model of NF-kappaB-and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem. 2005;280:21763–72. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- 24.Lamkanfi M, Kanneganti TD, Franchi L, Nunez G. Caspase-1 inflammasomes in infection and inflammation. J Leukoc Biol. 2007;82:220–5. doi: 10.1189/jlb.1206756. [DOI] [PubMed] [Google Scholar]
- 25.Terasaka N, Wang N, Yvan-charvet L, Tall AR. HDL protects macrophages from oxidized LDL-induced apoptosis by promoting efflux of 7- ketocholesterol via ABCG1. Proc Natl Acad Sci USA. 2007;104:15093–8. doi: 10.1073/pnas.0704602104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christian AE, Haynes MP, Phillips MC, Rothblat GH. Use of cyclodextrins for manipulating cellular cholesterol content. J Lipid Res. 1997;38:2264–72. [PubMed] [Google Scholar]
- 27.Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–32. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoll LL, Denning GM, Weintraub NL. Endotoxin, TLR4 signaling and vascular inflammation: potential therapeutic targets in cardiovascular disease. Curr Pharm Des. 2006;12:4229–45. doi: 10.2174/138161206778743501. [DOI] [PubMed] [Google Scholar]
- 29.Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, Miyake K. Cutting edge: Cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol. 2000;164:3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- 30.Bjorkbacka H, Kunjathoor V, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–420. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 31.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–56. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased cellular free cholesterol in macrophage-specific Abca1 knockout mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008 doi: 10.1074/jbc.M801408200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115:2603–2611. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- 35.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–9. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 36.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolpe SD, Cerami A. Macrophage inflammatory protein 1 and 2: members of a novel supergene of cytokines. Faseb J. 1989;3:2765–2773. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]
- 38.van Leeuwen M, Gijbels MJ, Duijvestijn A, Smook M, van de Gaar MJ, Heeringa P, de Winther MP, Tervaert JW. Accumulation of myeloperoxidase-positive neutrophils in atherosclerosis lesions in LDLR−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:85–9. doi: 10.1161/ATVBAHA.107.154807. [DOI] [PubMed] [Google Scholar]
- 39.Zernecke A, Bot I, Djalali-Talab Y, Shagdarsuren E, Bidzhekov K, Meiler S, Krohn R, Schober A, Sperandio M, Soehnlein O, Bornemann J, Tacke F, Biessen EA, Weber C. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ Res. 2008;102:209–17. doi: 10.1161/CIRCRESAHA.107.160697. [DOI] [PubMed] [Google Scholar]
- 40.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Nanoparticles PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–87. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–7. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 42.Coller BS. Leukocytosis and ischemic vascular disease morbidity and mortality: is time to intervene? Arterioscler Thromb Vasc Biol. 2005;25:658–70. doi: 10.1161/01.ATV.0000156877.94472.a5. [DOI] [PubMed] [Google Scholar]
- 43.Rader DJ. Mechanisms of disease: HDL metabolism as a target for novel therapies. Nat Clin Pract Cardiovasc Med. 2007;4:102–109. doi: 10.1038/ncpcardio0768. [DOI] [PubMed] [Google Scholar]
- 44.Tall AR, Yvan-Charvet L, Terasaka N, Paglet T, Wang N. HDL, ABC transporters, and cholesterol efflux: Implications for the treatment of atherosclerosis. Cell Metab. 200(7):365–75. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.