Abstract
IP3 receptors (IP3Rs) regulate the release of Ca++ from intracellular stores in response to IP3. Little is known about the regulation of IP3R expression and their role during the activation of CD4 T cells. In this study we show that mouse naïve CD4 T cells express IP3R1, IP3R2 and IP3R3, but gene expression of IP3R3, primarily, is downregulated upon activation due to loss of the Ets-1 transcription factor. Downregulation of IP3R expression in activated CD4 T cells is associated with the failure of T cell receptor ligation to trigger Ca++ release in these cells. We also show that downregulation of specific IP3Rs in activated CD4 T cells correlates with the requirement of IP3R-mediated Ca++ release only for the induction, but not for the maintenance of IL-2 and IFNγ expression. Interestingly, while inhibition of IP3R function early during activation blocks IL-2 and IFNγ production, it promotes the production of IL-17 by CD4 T cells. Thus, IP3Rs play a key role in the activation and differentiation of CD4 T cells. The immunosuppressive effect of pharmacological blockers of these receptors may be complicated by promoting the development of inflammatory CD4 T cells.
Keywords: T cells, Cell activation, Cytokines, Transcription factors, Gene regulation
INTRODUCTION
The regulation of Ca++ is a critical step in T cell activation. Signaling through the T cell receptor (TCR) and activation of adapter proteins results in the activation of phospholipase Cγ1 (PLCγ1). PLCγ1 hydrolyzes phosphotidylinositol 4,5-bisphosphate (PIP2) to generate inositol 1,4,5 triphosphate (IP3) and diacylglycerol. IP3 triggers Ca++ release from intracellular stores through IP3 receptors (IP3R) in the endoplasmic reticulum (ER) (1). Upon ER Ca++ depletion, a Ca++ release activated Ca++ (CRAC) channel is activated leading to massive Ca++ influx. STIM1, a type I transmembrane protein on the ER that functions as a Ca++ sensor, acts synergistically with the plasma membrane Ca++ channel, Orai1 (or CRACM1). This interaction is thought to function as the long-sought CRAC channel that activates store operated Ca++ (SOC) entry (2–5). The overall influx of Ca++ leads to activation of several Ca++-dependent pathways including the phosphatase calcineurin (6). The role of IP3Rs in Ca++-mediated signaling and T cell function has been largely ignored, due to the prominent importance given to the CRAC channels in these signals. Little is therefore known about the contribution of IP3R-mediated Ca++ release to cytokine production by primary CD4 T cells.
Three types of IP3Rs (IP3R1, IP3R2, and IP3R3) which exhibit different expression pattern and regulation by IP3 and Ca++ have been identified (7). IP3R1 is most abundant in brain, but it can also be detected in a variety of tissues (8). IP3R2 and IP3R3 are also widely distributed, but spleen expresses primarily IP3R3 (9). T cell lines appear to express all the three IP3Rs (10). The three IP3Rs share the capacity to release Ca++ upon binding IP3, albeit with different sensitivity to IP3 with IP3R2 being the most sensitive and IP3R3 the least sensitive (11). IP3Rs activity is regulated by Ca++ (12, 13), phosphorylation (14–17) and free nucleotides (18). IP3Rs are involved in TCR-induced Ca++ flux in Jurkat T cells (19, 20) and have been implicated in promoting cell death in T and B cell lines (21–23), but no reports have demonstrated the role of these receptors during T cell activation or effector functions. T cells from IP3R1-deficient mice exhibit normal activation in response to TCR stimulation (24). Although IP3R2-or IP3R3- deficient mice have been reported, their immune phenotype has not yet been characterized (25, 26). Furthermore, little is known about the regulation of IP3R gene expression. In this study, we show that although three IP3Rs are expressed in naïve CD4 T cells, the expression of IP3R3 gene is strongly downregulated during activation due to loss of the transcription factor Ets-1. We also show that IP3R-mediated Ca++ release is required for early production of IL-2 and IFNγ, but negatively regulates IL-17 production in CD4 T cells.
MATERIALS AND METHODS
Mice
Wildtype B10.BR mice (Jackson Laboratory, Bar Harbor, ME) were used for most of the experiments. Ets-1 deficient mice (27, 28) and AND TCR transgenic mice (29) have been previously described. Experimental procedures used in this study were reviewed and approved by the Animal Care and Use committee of the University of Vermont.
Cell preparation and activation
Total CD4 T cells were prepared from mouse spleen and lymph nodes by negative selection as previously described (30, 31). Isolation of naïve (CD44low) and memory (CD44high) CD4 T cells was performed by FACS-sorting as we previously described (32). Cells were activated with plate-bound anti-CD3 mAb (5 μg/ml; 2C11) and soluble anti-CD28 mAb (1 μg/ml; BD biosciences, San Diego, CA) monoclonal antibodies (mAbs). 2-APB (2-aminoethoxydiphenyl borate; 15 μM; Tocris, Ellisville, MO), Xe-C (Xestospongin-C; 5 μM; Calbiochem, Gibbstown, NJ) or recombinant human IL-2 (20 ng/ml; R & D systems, Minneapolis, MN) were added at different periods of time during activation. CD4 T cells from AND TCR transgenic mice were activated with pigeon cytochrome C peptide (5 μM) in the presence of mitomycin-C treated DCEK-ICAM cells (antigen presenting cells (APCs)) as previously described (33). Analysis of TCRβ levels was performed by flow cytometry using FITC-conjugated anti-TCRβ (eBiosciences, San Diego, CA) and hamster IgG isotype control (eBiosciences).
Western blot analysis
Cells were lysed and whole cell lysates were examined by Western blot analysis as we previously described (32) using the anti-IP3R1 (Affinity Bioreagents, Golden, CO), anti-IP3R2 (Santa Cruz biotechnology, Santa Cruz, CA), anti-IP3R3 (BD Transduction Labs, San Diego, CA), anti-STAT1 (BD Transduction Labs), anti-ERK (Cell signaling, Danvers, MA), anti-phospho ERK (Cell signaling) or anti-Actin (Santa Cruz biotechnology) antibodies.
Conventional and quantitative reverse transcriptase PCR
Total RNA was isolated from cells using the RNeasy RNA isolation kit (Qiagen, Valencia, CA) as recommended by the manufacturer. RNA was reverse transcribed to cDNA and used for conventional RT-PCR using the oligos, IP3R1 (5′-ctcaccagttggctcggcataa-3′& 5′-cggagcgcaggaagaagtcatt-3′), IP3R2 (5′-ggcgaagaggcaaatgaggaatc-3′ & 5′-ccaggaggccaggagttaggaa-3′) and IP3R3 (5′-gtgccccatgaaccgc tactctgc-3′ & 5′-tcccccacgaccacattatcc-3′). The PCR products were visualized in a 2.5% agarose gel. The same primers were also used to detect the expression of IP3R1, IP3R2 and IP3R3 by quantitative RT-PCR using the SYBR green method. IL-17 analysis was performed by real-time RT-PCR, using the assay on demand method (Applied Biosystems, Foster City, CA). β2m was used as house keeping gene. Relative mRNA levels were determined using comparative threshold cycle (CT) method.
FACS analysis of Ca++ flux
CD4 or CD8 T cells were loaded for 45 minutes at 37°C with 10 μM Indo-1 (34) (Molecular Probes, Inc., Carlsbad, CA), harvested, washed, transferred to a standard extracellular solution (SES) (140 mM NaCl, 4 mM KCl, 1 mM CaCl2, 2 mM MgCl2, 1 mM KH2PO4, 10 mM glucose, 10 mM HEPES, pH 7.4) and stimulated with anti-CD3 mAb (30 μg/ml) and anti-hamster Ab (50 μg/ml). Ionomycin (500 ng/ml) and EGTA (50 μg/ml) were used as positive and negative controls. The ratio of bound Indo-1 fluorescence to unbound Indo-1 fluorescence was determined for baseline using Flow LSRII (Becton-Dickinson, Franklin Lakes, NJ) as previously described (35).
Determination of cytokine production by ELISA
ELISAs were performed using the purified anti-IL-2, anti-IFNγ and anti-IL-17 mAb (2 μg/ml) as capture antibody, the corresponding biotinylated anti-IL-2, anti-IL-17 and anti-IFNγ mAb (1 μg/ml; BD Pharmingen), horseradish peroxidase-conjugated streptavidin (Sigma), and the TMB microwell peroxidase substrate and stop solution (KPL, Inc., Gaithersburg, MD) according to the recommended protocol as described earlier (36).
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared from freshly isolated and activated CD4 T cells and used for EMSA as we previously described (32). The oligos specific for IP3R3 gene (NCBI accession no. NT_039649) correspond to the positions − 121 (5′-gctgggggtcgtccggtggcaag-3′), −183 (5′-gggagagccccgaag tgcagcgc-3′), −784 (5′-ggctctaggaggaagcaaacgcc-3′) and −949 (5′-gctgggtctcttcctgcttctgt-3′). For AP-1 DNA binding, AP-1 consensus oligo (37, 38) was used. Cold competition was performed in the presence of non-labeled oligo containing a consensus EBS (Ets-1 binding sites). Anti-Ets-1 Ab (Santa Cruz Biotechnology) was used for supershift analysis.
Chromatin Immunoprecipitation (ChIP) assay
ChIP assay was performed using the ChIP-IT kit (Active motif, Inc., Carlsbad, CA) as recommended by the manufacturer. Anti-Ets-1 Ab was used to immunoprecipitate Ets-1. Detection of IP3R3 promoter in Ets-1 immunoprecipitation was performed by the real-time PCR (Applied Biosystems) using the oligos for −784 position (For-5′-tcaaaccaaagctctaggaggaa-3′, Rev 5′-cgcccactgaaacaagttctc-3′ & probe [6FAM]5′-aaacgcccagcctccgtggc-3′[BHQ1]) and −949 position (For 5′-gcaggtcagcagctgtctca-3′, Rev 5′-cgtttgtccctgggagaaaa-3′ & probe [6FAM]5′-ccctcctgggtctcttcctgcttctgt-3′[BHQ1]. Fold differences were then determined by using the comparative CT method (39).
RNase protection assay
Ribonuclease protection assay (RPA) was performed using the mCK-1 template kit (BD biosciences) according to the manufacturer’s protocol. Briefly, 3 μg of total RNA was hybridized overnight with [32P]UTP radiolabeled in vitro-transcribed RNA probes. Overlapping single stranded RNA on hybridized double-stranded RNAs was digested with RNases A and T1 and the protected dsRNA duplexes were purified and resolved on urea-denaturing gels. Gels were dried and exposed to film for autoradiographic analysis.
RESULTS
Inhibition of IP3R3 gene expression in naïve CD4 T cells upon activation
Although IP3Rs are believed to be required for the release of Ca++ from intracellular stores and activation of CRAC channels in T cells, little is known about the relative expression of the different types of IP3Rs in naive CD4 T cells. We therefore examined the regulation of IP3R1, IP3R2, and IP3R3 prior to and during activation of CD4 T cells with anti-CD3 and anti-CD28 mAbs by Western blot analysis. The three types of IP3Rs were present in freshly isolated CD4 T cells with the levels of IP3R1 remaining constant during the activation period analyzed (Fig. 1A). In contrast, the levels of IP3R2 and IP3R3 were decreased upon activation of CD4 T cells (Fig. 1A). Analysis of mRNA levels by RT-PCR showed no changes in IP3R1, decreased IP3R2 and almost undetectable IP3R3 mRNA levels in CD4 T cells activated for 24h and 48h (Fig. 1B), indicating that activation downregulates the expression of the IP3R2 and IP3R3 genes. Analysis by real-time RT-PCR in CD4 T cells activated for several periods of time further confirmed the downregulation of IP3R2 and IP3R3 gene expression as early as 6h of activation (Fig. 1C). To rule out that this effect was due to the presence of a small fraction of non-CD4 T cells (e.g. macrophages) in our CD4 T cell preparation, we examined the expression of IP3Rs in CD44low (naïve) and CD44high (memory) CD4 T cells purified by cell sorting. Prior to activation, the levels of IP3R1, IP3R2 and IP3R3 were comparable in naïve and memory CD4 T cells (Fig. 1D). As seen with total CD4 T cells, the levels of IP3R1 were not affected by the activation status of naïve CD4 T cells (representing over 90% of the CD4 T cells). Although the IP3R2 levels were slightly reduced in activated naïve CD4 T cells, the most striking change was the abrogation of IP3R3 expression with activation (Fig. 1D). IP3R3 expression was also strongly downregulated in activated memory cells (Fig. 1E). To further demonstrate that downregulation of IP3Rs is not just the result of the strong signals provided by anti-CD3/anti-CD28 mAbs stimulation, we examined IP3Rs levels in antigen specific CD4 T cells. CD4 T cells from AND TCR transgenic mice (29) were activated with pigeon cytochrome C peptide in the presence of APCs for 24h and 48h. The levels of IP3R2 and IP3R3, but not IP3R1, were also highly reduced upon antigen specific stimulation (Fig. 1F).
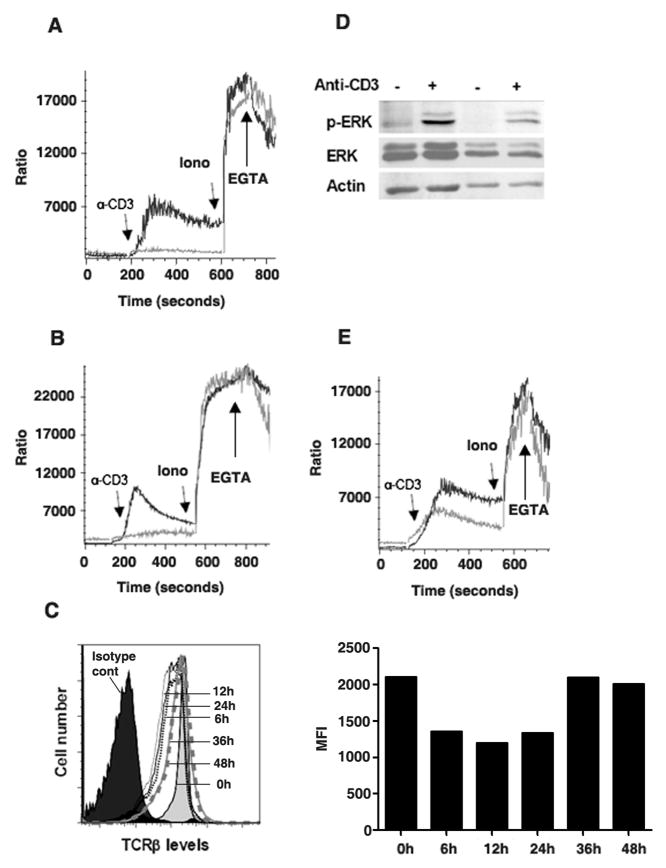
Fig. 1. Regulation of IP3Rs expression during the activation of CD4 and CD8 T cells.
(A) Freshly isolated CD4 T cells (day 0) or activated with plate bound anti-CD3 and soluble anti- CD28 mAbs for 1 or 2 days were analyzed for expression of IP3R1, IP3R2 and IP3R3 by Western blot analysis. STAT1 protein levels were analyzed as a loading control. Results are representative of at least three independent experiments. (B) RT-PCR was performed for IP3Rs in CD4 T cells that were activated as in (A). β-actin was used as house keeping gene. (C) Relative mRNA levels of IP3Rs in freshly isolated or CD4 T cells that were activated with anti-CD3 and anti-CD28 mAbs for 0, 6, 12, 24 and 48h were examined by real-time RT-PCR. (D) IP3Rs expression in unstimulated (day 0) naïve CD4 T cells, naïve cells activated with anti- CD3/anti-CD28 mAbs (day 2) and unstimulated memory CD4 T cells were examined by Western blot analysis. Densitometric analysis was performed and relative values for IP3R1, IP3R2, IP3R3 and STAT1 are shown (lower panel). (E) IP3R3 expression in unstimulated or activated memory CD4 T cells was examined by Western blot analysis. (F) IP3Rs expression was examined by Western blot analysis in CD4 T cells from AND TCR transgenic mice upon antigen stimulation. (G) Unstimulated or activated CD8 T cells were analyzed for expression of IP3Rs by RT-PCR analysis.
Analysis of the expression of IP3Rs in CD8 T cells showed that the expression of IP3R1 gene was not affected, but the expression of IP3R3 and IP3R2 genes was also downregulated upon activation (Fig. 1G), further confirming that TCR-mediated signals repress the expression of these IP3Rs.
Expression of IP3R3 in CD4 T cells requires the Ets-1 transcription factor
Although little is known about the regulation of IP3Rs gene expression, a previous study has identified a regulatory element at position −121 in the IP3R3 promoter for the Ets-1 transcription factor (9). Using Transcription Element Search Software (TESS) (40), we identified three other potential Ets-1 binding sites in the IP3R3 promoter (−183, −784 and −949) (Fig. 2A). Interestingly, Ets-1 has been shown to be expressed in naïve CD4 T cells, but its expression is downregulated upon activation (41, 42), similar to the downregulation of IP3R3 that we observed (Fig. 1). We therefore examined whether the potential Ets-1 regulatory elements identified in the IP3R3 promoter were able to bind Ets-1 from CD4 T cells by EMSA. Ets-1 binding to the oligos corresponding to the −784 and −949 positions in the IP3R3 promoter was readily evident (Fig. 2B). A weaker Ets-1 binding was also detected for the previously described −121 position oligo (Fig. 2B). The specificity of Ets-1 binding was shown using cold oligos and an anti-Ets-1 antibody (Fig. 2B). No Ets-1 binding to the oligo corresponding to the −183 position was detected (data not shown). Thus, Ets-1 present in unstimulated CD4 T cells primarily binds the − 784 and −949 Ets-1 binding sites of the IP3R3 promoter. In correlation with the downregulation of Ets-1 (41, 42) and IP3R3 (Fig. 1) upon activation, Ets-1 binding to -784 and −949 oligos was practically undetectable in activated CD4 T cells (Fig. 2C). As a control we examined AP-1 DNA binding, which was strongly upregulated in activated CD4 T cells (Fig. 2C) as previously reported (31). No Ets-1 binding sites were identified in the IP3R1 promoter and a poorly conserved Ets-1 site was identified in the IP3R2 promoter (data not shown). To demonstrate binding of Ets-1 to the endogenous promoter of IP3R3 in vivo, we performed ChIP assay in combination with real-time PCR. Similar to the in vitro results, Ets-1 binding to −784 and −949 positions of the endogenous IP3R3 promoter was readily detectable in freshly isolated CD4 T cells, but almost undetectable in activated CD4 T cells (Fig. 2D).
Fig. 2. Ets-1 is required for the expression of IP3R3.
(A) Promoter of IP3R3 gene was analyzed for Ets-1 binding sites (EBS). Consensus EBS (Cons) and EBS at positions −121, −183, −784 and −949 are underlined. (B) Nuclear extracts prepared from naïve CD4 T cells were incubated with oligos containing EBS at −121, −784 and −949 positions in the presence or absence (-) of cold Ets-1 consensus oligo (oli) or anti-Ets-1 Ab (Ab) and EMSA was performed. (C) Nuclear extracts (1.8 μg for each sample) prepared from CD4 T cells prior to or after activation with anti-CD3/anti-CD28 mAbs were incubated with Ets-1 (−784), Ets-1 (−949) or consensus AP-1 oligos and EMSA was performed. (D) ChIP assay was performed to examine the association between the Ets-1 transcription factor and the Ets-1 binding sites at −784 and −949 positions of IP3R3 promoter using freshly isolated or activated CD4 T cells. Relative abundance of IP3R3 promoter in anti-Ets-1/chromatin immunoprecipitation was expressed as fold increase with relative to abundance of IP3R3 promoter in no antibody/chromatin immunoprecipitation control. (E) IP3Rs expression in unstimulated wildtype and Ets-1 deficient CD4 T cells was examined by Western blot analysis.
To show that Ets-1 contributes to the IP3R3 expression, we examined IP3R3 levels in freshly isolated CD4 T cells from the previously described Ets-1 deficient mice (27, 28). IP3R3 levels were almost undetectable in Ets-1 deficient CD4 T cells compared to wildtype CD4 T cells (Fig. 2E). The levels of IP3R2 were only slightly reduced, and the levels of IP3R1 were not affected in Ets-1 deficient CD4 T cells (Fig. 2E). Thus, Ets-1 binds to the IP3R3 promoter and is required for the expression of IP3R3 in CD4 T cells. These data suggest that the downregulation of Ets-1 during activation causes the downregulation of IP3R3.
Downregulation of IP3R expression correlates with the inability of TCR-ligation to mobilize Ca++ in activated T cells
We examined whether the lack of sufficient IP3R levels in activated CD4 T cells interfered with Ca++ mobilization triggered by TCR-ligation. As reported (43), TCR cross-linking induced a rapid rise in intracellular Ca++ levels in freshly isolated CD4 T cells (Fig. 3A). In contrast, TCR cross-linking did not induce Ca++ flux in activated CD4 T cells (Fig. 3A). Similarly, TCR cross-linking induced a rapid raise in intracellular Ca++ levels in freshly isolated, but not in activated CD8 T cells (Fig. 3B).
Fig. 3. TCR ligation fails to induce Ca++ flux in activated CD4 T cells.
(A) Unstimulated CD4 T cells (black line), or CD4 T cells activated with anti-CD3/anti-CD28 mAbs for two days (gray line), were loaded with Indo-1 and examined for Ca++ flux by flow cytometry in response to anti-CD3 mAb (α-CD3), Ionomycin (Ion) or EGTA. Arrows indicate the time when each reagent was added. (B) Unstimulated CD8 T cells (black line) or CD8 T cells activated (gray line) as in (A) were loaded with Indo-1 and examined for Ca++ flux by flow cytometry in response to anti-CD3 mAb. (C) TCRβ expression in CD4 T cells unstimulated (0h), activated for 6, 12, 24, 36 or 48h with anti-CD3 and anti-CD28 mAbs was examined by flow cytometry. Mean fluorescence intensity (MFI) of the TCR is also shown (right panel). (D) Unstimulated or activated CD4 T cells were treated with medium or anti-CD3 mAb for 5 min and phosphorylated ERK (p-ERK), total ERK and actin were examined by Western blot analysis. (E) CD4 T cells isolated from wildtype (black line) or Ets-1 deficient (gray line) mice were loaded with Indo-1 and examined for Ca++ flux by flowcytometry in response to anti-CD3 mAb.
Analysis of cell surface expression of TCR showed that there was only a slight downregulation early during activation, but the TCR levels remain high even after 48h of activation (Fig. 3C). Thus, the failure of TCR ligation to induce Ca++ flux in activated T cells was not due to the lack of TCR cell surface expression. In addition, ERK phosphorylation was induced at a similar level in both freshly isolated and activated CD4 T cells (Fig. 3D), indicating that the lack of Ca++ mobilization in activated CD4 T cells was not due to the absence of cell surface TCR or a global impairment of TCR signaling, but likely due to insufficient levels of IP3Rs to mediate Ca++ release. To further support this model, we examined Ca++ mobilization in Ets-1 deficient CD4 T cells, since these cells lack IP3R3 as shown above (Fig. 2E). The Ca++ flux triggered by TCR-ligation was impaired in freshly isolated Ets-1 deficient CD4 T cells compared with wildtype CD4 T cells (Fig. 3E).
IP3R-mediated Ca++ release is required for the initial IL-2 and IFNγ gene expression
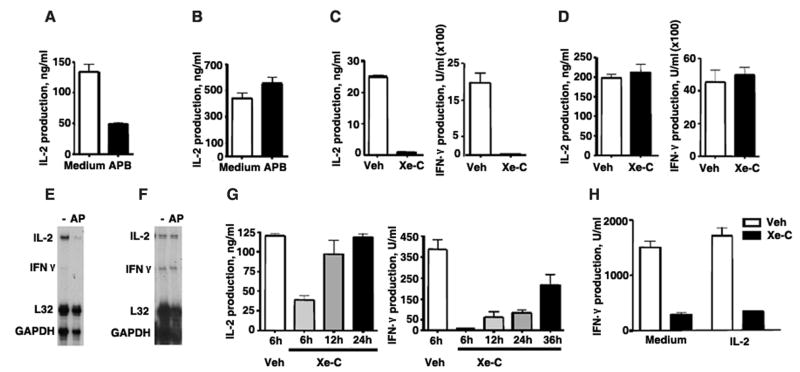
The downregulation of IP3R during activation of CD4 T cells suggested a differential contribution of IP3R-mediated Ca++ signals during the early versus the late phase of activation. We therefore examined the effect of the pharmacological inhibitor, 2-APB on cytokine production. Although at high concentration (>20 μM) 2-APB can inhibit other channels, at lower concentrations it has been shown to more selectively inhibit IP3Rs (44, 45). CD4 T cells were activated with anti-CD3 and anti-CD28 mAbs in the presence or absence of 2-APB and IL-2 production was measured after 48h. 2-APB caused a strong inhibition of IL-2 production (Fig. 4A). In contrast, when 2-APB was added 48h after the initial activation and IL-2 production was determined two days later (day 4), the levels of IL-2 were not affected (Fig. 4B). To further show the role of IP3R in cytokine gene expression we also examined the effect of Xe-C, a more specific pharmacological blocker of IP3R. Addition of Xe-C at the time of activation abrogated the production of IL-2 and IFNγ (Fig. 4C). In contrast, the levels of IL-2 and IFNγ (Fig. 4D) were not affected by Xe-C when added two days after the initial activation.
Fig. 4. IP3R-mediated Ca++ release is required for the initial IL-2 and IFNγ gene expression.
(A) CD4 T cells were activated with anti-CD3 and anti-CD28 mAbs in medium alone or in the presence (APB) of 2-APB (15 μM) and IL-2 production was examined by ELISA on day 2. (B) CD4 T cells were activated with anti-CD3/anti-CD28 mAbs and after two days medium or 2-APB was added to the culture. IL-2 production was examined by ELISA two days later (day 4). Results are representative of at least three experiments. (C) CD4 T cells were activated with anti-CD3 and anti-CD28 mAbs in the presence of DMSO as a vehicle (Veh) or Xe-C (5 μM) and after two days IL-2 and IFNγ production were examined by ELISA. (D) CD4 T cells were activated with anti-CD3 and anti-CD28 mAbs. After two days, vehicle or Xe-C was added to the cells and IL-2 and IFNγ production was examined by ELISA two days later. (E) CD4 T cells were activated with anti-CD3/anti-CD28 mAbs in the presence (AP) or absence (−) of 2-APB and RNA was examined 24h later by RPA. L32 and GAPDH were used as loading controls. (F) CD4 T cells were activated with anti-CD3/anti-CD28 mAbs. Medium alone (-) or 2-APB (AP) was added on day 2 and RNA was analyzed by RPA on day 3. L32 and GAPDH were used as loading controls. (G) CD4 T cells were activated with anti-CD3 and anti-CD28 mAbs and after 6, 12 or 24h Xe-C or vehicle was added to the culture. IL-2 and IFNγ production were examined by ELISA at 48h from initial activation. (H) CD4 T cells were activated in the presence of IL-2 (20 ng/ml) or medium alone. Xe-C or vehicle was added at 24h of activation and supernatants were analyzed for IFNγ production at 48h
We also examined the contribution of IP3R-mediated Ca++ flux on cytokine gene expression during activation of CD4 T cells by performing RNase protection assay (RPA). The expression of IL-2 and IFNγ were practically abrogated in cells activated for 24h in the presence of 2-APB (Fig. 4E). In contrast, when 2-APB was added after two days of activation and mRNA levels were examined 24h later, no effect on either IL-2 or IFNγ mRNA levels was observed (Fig. 4F). Together, these results indicate that IP3R-mediated Ca++ release is not required for cytokine gene expression after two days of activation, correlating with the downregulation of IP3R3 expression in naïve CD4 T cells.
To further dissect for how long after TCR ligation IP3R-mediated Ca++ flux was required for optimal cytokine production, we examined the effect of inhibiting IP3R at different times after activation. Addition of Xe-C 6h after activation still caused a substantial inhibition of IL-2 production, but it had almost no effect when added 12h or 24h after activation (Fig. 4G). Addition of Xe-C after 6h of activation also abrogated IFNγ production (Fig. 4G). However, unlike IL-2, IFNγ production was also strongly inhibited when Xe-C was added even 24h after activation and substantially reduced when added at 36h (Fig. 4G). The effect of Xe-C on IFNγ production was not due to insufficient IL-2 production, since addition of exogenous IL-2 did not restore IFNγ levels (Fig. 4H). Since the induction of IFNγ expression in CD4 T cells upon activation occurs later than IL-2 expression, these results indicate that IP3R-mediated Ca++ release is required for initial induction of both IL-2 and IFNγ gene expression but not for the sustained expression of these cytokines.
IP3R-mediated Ca++ release suppresses IL-17 production
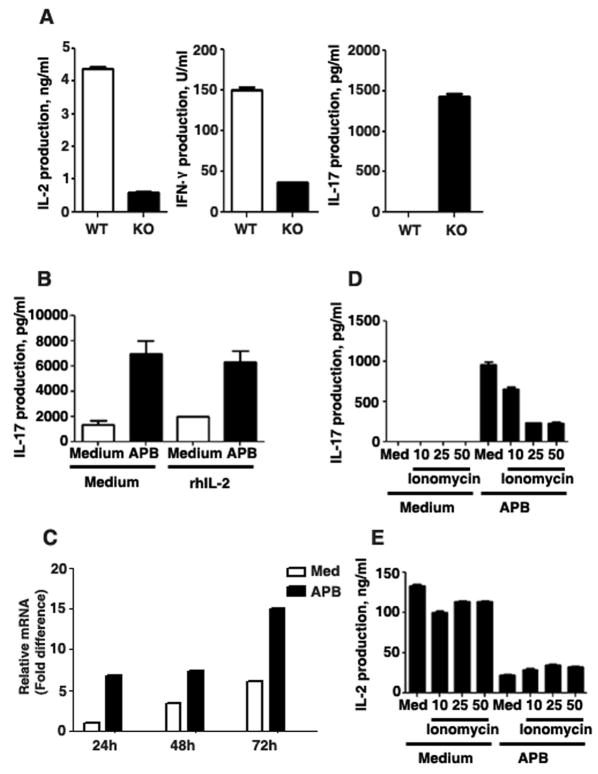
Although Ets-1 deficiency has been shown to impair IL-2 and IFNγ production in Th1 cells (42), a recent study has showed increased levels of IL-17 in Th17 cells from Ets-1 deficient mice (46). We have also confirmed that CD4 T cells from Ets-1 deficient mice produced substantially lower amounts of IL-2 and IFNγ, but increased IL-17 levels upon activation with anti-CD3 and anti-CD28 mAbs in the absence of polarizing cytokines (Fig. 5A). Since Ets-1 is required for IP3R3 expression (Fig. 2E) and Ca++-responses to TCR-ligation (Fig. 3E), we examined whether an impaired IP3R-mediated Ca++ response could affect IL-17 production. The addition of 2-APB during the activation of wildtype CD4 T cells increased IL-17 production (Fig. 5B) and IL-17 mRNA levels (Fig. 5C), in contrast to its effect on IL-2 and IFNγ production. It has been proposed that IL-2 suppresses IL-17 production (47). However, exogenous IL-2 could not overcome the stimulatory effect of 2-APB on IL-17 production (Fig. 5B) indicating that 2-APB-induced IL-17 production is not an indirect effect of reduced IL-2 production. To further demonstrate that the enhanced IL-17 production was due to decreased intracellular Ca++ levels, CD4 T cells were activated with anti-CD3 and anti-CD28 mAbs in the presence of 2-APB and a low dose of Ionomycin, a Ca++ -ionophore that enhances Ca++ -influx from extracellular stores. Ionomycin, in a dose dependent manner, decreased the production of IL-17 induced by 2-APB (Fig. 5D). In contrast, Ionomycin had no effect on IL-2 production either in the presence or absence of 2-APB (Fig. 5E). Thus, while intracellular Ca++ is required for the expression of IL-2, IFNγ and, presumably other cytokines, inhibition of Ca++-signals selectively promotes the IL-17 production.
Fig. 5. IP3R-mediated Ca++ release suppresses IL-17 expression.
(A) CD4 T cells from wildtype (WT) or Ets-1 deficient (KO) mice were activated with anti-CD3/anti-CD28 mAbs and supernatant was analyzed for IL-2, IFNγ and IL-17 production by ELISA. (B) CD4 T cells were activated with anti-CD3/anti-CD28 mAbs for 4 days in the presence or absence of 2-APB (15 μM). Medium or rhIL-2 (100 ng/ml) was added to culture on day 0 of activation. On day 4, supernatant was analyzed for IL-17 production. (C) CD4 T cells were activated with anti-CD3/anti-CD28 mAbs for 1, 2 or 3 days in the presence or absence of 2-APB (15 μM). Relative mRNA levels of IL-17 were examined by real-time RT-PCR. (D & E) CD4 T cells were activated with anti-CD3/anti-CD28 mAbs in the presence or absence of 2-APB for 2 or 4 days. Ionomycin was added at 10, 25 or 50 ng/ml concentrations to control or 2-APB treated cells at the time of activation. Supernatants were analyzed for IL-17 production (D) on day 4 or IL-2 production (E) on day 2.
DISCUSSION
Although in most instances IP3Rs are expressed constitutively, the protein levels of IP3Rs are shown to be regulated by degradation (48). Previous studies have shown that the binding of IP3 can lead to a conformational change in IP3Rs rendering them susceptible to protein degradation that is mediated by the ubiquitin/proteasome pathway (48–51). IP3R3 has been shown to be downregulated upon stimulation with retinoic acid during differentiation of embryonal carcinoma cells into neural cells (9). TNFα induces the degradation of IP3R1 and IP3R2 by caspases (52, 53), and IP3R3 by calpains (52) in Jurkat T cells. Less is known about the regulation of IP3Rs at the level of gene expression. NFAT has been involved in the promoter activity of and expression of IP3R1 (23, 54). We show here that expression of IP3R3 and IP3R2 genes, but not IP3R1 is downregulated in CD4 and CD8 T cells upon activation. An Ets-1 binding site was previously reported in the promoter of IP3R3 (9), but there was no evidence for a role of Ets-1 in the expression of this receptor. We show that Ets-1 is required for the expression of IP3R3 in CD4 T cells and that downregulation of Ets-1 during activation is associated with the downregulation of IP3R3 expression. Ets-1 is not required for IP3R1 and has little effect on IP3R2 expression. Furthermore, Ets-1 deficiency in CD4 T cells results in an impaired Ca++ mobilization in response to TCR-ligation. Although it has been reported that Ets-1 regulates the expression of different cytokines (e.g. IL-2, GM-CSF, IL-5), receptors (IL-2Rβ chain) and transcription factors (T-bet) (42, 55–59), this may not be due to a direct effect of Ets-1 on these genes, but an indirect effect of Ets-1 on IP3R expression and impaired Ca++ accumulation.
TCR ligation induces Ca++ release from intracellular stores as well as extracellular Ca++ influx (1). However, we show here that TCR-ligation fails to induce intracellular Ca++ accumulation in activated CD4 and CD8 T cells, while retaining its ability to induce other signals such as ERK activation. Although additional studies are required, we propose that this inability of the TCR to trigger Ca++ mobilization could be due to the insufficient levels of IP3R2 and IP3R3 to initiate the release of Ca++ from intracellular stores. Although IP3R1 remains present in activated cells, it does not appear to compensate the absence of IP3R2 and IP3R3. It is possible that IP3R1 may be mislocalized in the intracellular compartments. Since we also show that expression of IL-2 and IFNγ is independent of IP3R activity later during the activation, downregulation of IP3Rs could be a mechanism to turn off this pathway.
Herein we show that full cytokine gene expression requires IP3R-mediated Ca++ release for a relatively longer period of time (6–24h) that depends on the kinetics of specific cytokine. IL-2 is one of the earliest genes induced upon TCR activation and is cell-cycle independent, whereas IFNγ gene expression is delayed and cell-cycle dependent (60). In correlation, we show that IL-2 production requires IP3R-mediated Ca++ release for up to first 6–12h, while IFNγ requires it for up to 24–36h, the time when its transcription probably starts. Thus, IP3R-mediated Ca++ is essential not only for IL-2 production and proliferation, but also for the expression of effector cytokines in CD4 T cells. In contrast, we show that Ca++ signal seems to suppress IL-17 production, since Ca++ blockers induced IL-17 production while Ca++ ionophores reversed this effect. Th17 cells are currently considered to be involved in the inflammatory process during autoimmune diseases (61). Our results suggest that therapy that interferes with the Ca++ signaling pathway may not be totally immunosuppressive, but promotes the generation of inflammatory cells. Interestingly, it has long been described that treatment with the immunosuppressive drug, cyclosporine-A, which interferes with the Ca++ dependent phosphatase calcineurin, can lead to autoimmune diseases (62). Although this effect has been associated with the appearance of autoreactive cells from the thymus, our results suggest that it could also be due to increased survival of Th17 cells. Overall, our results situate IP3R activity and Ca++ signaling control as a critical step not only during the activation, but also the differentiation of CD4 T cells with the capacity to modulate the ensuing immune response.
Acknowledgments
We thank Tim Hunter and the personnel in the DNA sequencing facility (Vermont Cancer Center) for real-time RT-PCR analysis.
Footnotes
This work was supported by a National Institutes of Health Program Project grant (P02AI045666) (M.R.) and the COBRE Program of the National Center for Research Resources (RR15557) (M.R.).
DISCLOSURES
Authors have no financial conflict of interest.
References
- 1.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annual Review of Immunology. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 2.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 4.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109 Suppl:S67–79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 7.Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-triphosphate receptors as signal integrators. Annual Review of Biochemistry. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- 8.Ross CA, Danoff SK, Schell MJ, Snyder SH, Ullrich A. Three Additional Inositol 1,4,5-Trisphosphate Receptors: Molecular Cloning and Differential Localization in Brain and Peripheral Tissues. PNAS. 1992;89:4265–4269. doi: 10.1073/pnas.89.10.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamura T-a, Hashimoto M, Aruga J, Konishi Y, Nakagawa M, Ohbayashi T, Shimada M, Mikoshiba K. Promoter structure and gene expression of the mouse inositol 1,4,5-trisphosphate receptor type 3 gene. Gene. 2001;275:169–176. doi: 10.1016/s0378-1119(01)00658-8. [DOI] [PubMed] [Google Scholar]
- 10.Sugiyama T, Yamamoto-Hino M, Miyawaki A, Furuichi T, Mikoshiba K, Hasegawa M. Subtypes of inositol 1,4,5-trisphosphate receptor in human hematopoietic cell lines: Dynamic aspects of their cell-type specific expression. FEBS Letters. 1994;349:191–196. doi: 10.1016/0014-5793(94)00662-8. [DOI] [PubMed] [Google Scholar]
- 11.Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. Embo J. 1999;18:1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boehning D, Joseph SK, Mak DOD, Foskett JK. Single-Channel Recordings of Recombinant Inositol Trisphosphate Receptors in Mammalian Nuclear Envelope. Biophys J. 2001;81:117–124. doi: 10.1016/s0006-3495(01)75685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaznacheyeva E, V, Lupu D, Bezprozvanny I. Single-Channel Properties of Inositol (1,4,5)-Trisphosphate Receptor Heterologously Expressed in HEK-293 Cells. J Gen Physiol. 1998;111:847–856. doi: 10.1085/jgp.111.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferris CD, Huganir RL, Bredt DS, Cameron AM, Snyder SH, Snyder H. Inositol Trisphosphate Receptor: Phosphorylation by Protein Kinase C and Calcium Calmodulin-Dependent Protein Kinases in Reconstituted Lipid Vesicles. PNAS. 1991;88:2232–2235. doi: 10.1073/pnas.88.6.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jayaraman T, Ondrias K, Ondriasová E, Marks AR. Regulation of the Inositol 1,4,5-Trisphosphate Receptor by Tyrosine Phosphorylation. Science. 1996;272:1492–1494. doi: 10.1126/science.272.5267.1492. [DOI] [PubMed] [Google Scholar]
- 16.Khan MT, Wagner L, II, Yule DI, Bhanumathy C, Joseph SK. Akt Kinase Phosphorylation of Inositol 1,4,5-Trisphosphate Receptors. J Biol Chem. 2006;281:3731–3737. doi: 10.1074/jbc.M509262200. [DOI] [PubMed] [Google Scholar]
- 17.deSouza N, Cui J, Dura M, McDonald TV, Marks AR. A function for tyrosine phosphorylation of type 1 inositol 1,4,5-trisphosphate receptor in lymphocyte activation. J Cell Biol. 2007;179:923–934. doi: 10.1083/jcb.200708200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mak DOD, McBride S, Foskett JK. ATP Regulation of Type 1 Inositol 1,4,5-Trisphosphate Receptor Channel Gating by Allosteric Tuning of Ca2+ Activation. J Biol Chem. 1999;274:22231–22237. doi: 10.1074/jbc.274.32.22231. [DOI] [PubMed] [Google Scholar]
- 19.Jayaraman T, Ondriasova E, Ondrias K, Harnick DJ, Marks AR. The inositol 1,4,5-trisphosphate receptor is essential for T-cell receptor signaling. Proc Natl Acad Sci U S A. 1995;92:6007–6011. doi: 10.1073/pnas.92.13.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dadsetan S, Zakharova L, Molinski TF, Fomina AF. Store-operated Ca2+ influx causes Ca2+ release from the intracellular Ca2+ channels that is required for T cell activation. J Biol Chem. 2008;283:12512–12519. doi: 10.1074/jbc.M709330200. [DOI] [PubMed] [Google Scholar]
- 21.Khan AA, Soloski MJ, Sharp AH, Schilling G, Sabatini DM, Li SH, Ross CA, Snyder SH. Lymphocyte apoptosis: mediation by increased type 3 inositol 1,4,5-trisphosphate receptor. Science. 1996;273:503–507. doi: 10.1126/science.273.5274.503. [DOI] [PubMed] [Google Scholar]
- 22.Jayaraman T, Marks AR. T cells deficient in inositol 1,4,5-trisphosphate receptor are resistant to apoptosis. Mol Cell Biol. 1997;17:3005–3012. doi: 10.1128/mcb.17.6.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Fox CJ, Master SR, Bindokas VP, Chodosh LA, Thompson CB. Bcl-XL affects Ca2+ homeostasis by altering expression of inositol 1,4,5-trisphosphate receptors. PNAS. 2002;99:9830–9835. doi: 10.1073/pnas.152571899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirota J, Baba M, Matsumoto M, Furuichi T, Takatsu K, Mikoshiba K. T-cell-receptor signalling in inositol 1,4,5-trisphosphate receptor (IP3R) type-1-deficient mice: is IP3R type 1 essential for T-cell-receptor signalling? Biochem J. 1998;333(Pt 3):615–619. doi: 10.1042/bj3330615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Zima AV, Sheikh F, Blatter LA, Chen J. Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate(IP3)-receptor type 2-deficient mice. Circ Res. 2005;96:1274–1281. doi: 10.1161/01.RES.0000172556.05576.4c. [DOI] [PubMed] [Google Scholar]
- 26.Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi-Iwanaga H, Noda T, Aruga J, Mikoshiba K. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309:2232–2234. doi: 10.1126/science.1114110. [DOI] [PubMed] [Google Scholar]
- 27.Muthusamy N, Barton K, Leiden JM. Defective activation and survival of T cells lacking the Ets-1 transcription factor. Nature. 1995;377:639–642. doi: 10.1038/377639a0. [DOI] [PubMed] [Google Scholar]
- 28.Barton K, Muthusamy N, Fischer C, Ting CN, Walunas TL, Lanier LL, Leiden JM. The Ets-1 transcription factor is required for the development of natural killer cells in mice. Immunity. 1998;9:555–563. doi: 10.1016/s1074-7613(00)80638-x. [DOI] [PubMed] [Google Scholar]
- 29.Kaye J, Hsu ML, Sauron ME, Jameson SC, Gascoigne NR, Hedrick SM. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 30.Diehl S, Chow CW, Weiss L, Palmetshofer A, Twardzik T, Rounds L, Serfling E, Davis RJ, Anguita J, Rincon M. Induction of NFATc2 expression by interleukin 6 promotes T helper type 2 differentiation. J Exp Med. 2002;196:39–49. doi: 10.1084/jem.20020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rincon M, Derijard B, Chow CW, Davis RJ, Flavell RA. Reprogramming the signalling requirement for AP-1 (activator protein-1) activation during differentiation of precursor CD4+ T-cells into effector Th1 and Th2 cells. Genes Funct. 1997;1:51–68. doi: 10.1046/j.1365-4624.1997.00007.x. [DOI] [PubMed] [Google Scholar]
- 32.Dienz O, Eaton SM, Krahl TJ, Diehl S, Charland C, Dodge J, Swain SL, Budd RC, Haynes L, Rincon M. Accumulation of NFAT mediates IL-2 expression in memory, but not naive, CD4+ T cells. Proc Natl Acad Sci U S A. 2007;104:7175–7180. doi: 10.1073/pnas.0610442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swain SL, Hu H, Huston G. Class II-Independent Generation of CD4 Memory T Cells from Effectors. Science. 1999;286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 34.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 35.Anguita J, Ramamoorthi N, Hovius JW, Das S, Thomas V, Persinski R, Conze D, Askenase PW, Rincon M, Kantor FS, Fikrig E. Salp15, an ixodes scapularis salivary protein, inhibits CD4(+) T cell activation. Immunity. 2002;16:849–859. doi: 10.1016/s1074-7613(02)00325-4. [DOI] [PubMed] [Google Scholar]
- 36.Diehl S, Krahl T, Rinaldi L, Norton R, Irvin CG, Rincon M. Inhibition of NFAT Specifically in T Cells Prevents Allergic Pulmonary Inflammation. J Immunol. 2004;172:3597–3603. doi: 10.4049/jimmunol.172.6.3597. [DOI] [PubMed] [Google Scholar]
- 37.Angel P, Imagawa M, Chiu R, Stein B, Imbra RJ, Rahmsdorf HJ, Jonat C, Herrlich P, Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987;49:729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- 38.Lee W, Mitchell P, Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987;49:741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- 39.Chakrabarti SK, James JC, Mirmira RG. Quantitative Assessment of Gene Targeting in Vitro and in Vivo by the Pancreatic Transcription Factor, Pdx1. Importance of chromatin structure in direct promoter binding. J Biol Chem. 2002;277:13286–13293. doi: 10.1074/jbc.M111857200. [DOI] [PubMed] [Google Scholar]
- 40.Schug Jonathan, Christian Overton G. Technical Report CBIL-TR-1997–1001-v0.0. Computational Biology and Informatics Laboratory School of Medicine University; Pennsylvania: 1997. TESS: Transcription Element Search Software on the WWW. [Google Scholar]
- 41.Bhat NK, Thompson CB, Lindsten T, June CH, Fujiwara S, Koizumi S, Fisher RJ, Papas TS. Reciprocal Expression of Human ETS1 and ETS2 Genes During T-Cell Activation: Regulatory Role for the Protooncogene ETS1. PNAS. 1990;87:3723–3727. doi: 10.1073/pnas.87.10.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grenningloh R, Kang BY, Ho IC. Ets-1, a functional cofactor of T-bet, is essential for Th1 inflammatory responses. J Exp Med. 2005;201:615–626. doi: 10.1084/jem.20041330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss A, Imboden J, Hardy K, Manger B, Terhorst C, Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- 44.Simkus CR, Stricker C. The contribution of intracellular calcium stores to mEPSCs recorded in layer II neurones of rat barrel cortex. J Physiol. 2002;545:521–535. doi: 10.1113/jphysiol.2002.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prakriya M, Lewis RS. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J Physiol (Lond) 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moisan J, Grenningloh R, Bettelli E, Oukka M, Ho IC. Ets-1 is a negative regulator of Th17 differentiation. J Exp Med. 2007;204:2825–2835. doi: 10.1084/jem.20070994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’Shea J. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Bhanumathy CD, Nakao SK, Joseph SK. Mechanism of Proteasomal Degradation of Inositol Trisphosphate Receptors in CHO-K1 Cells. J Biol Chem. 2006;281:3722–3730. doi: 10.1074/jbc.M509966200. [DOI] [PubMed] [Google Scholar]
- 49.Zhu CC, Furuichi T, Mikoshiba K, Wojcikiewicz RJ. Inositol 1,4,5-trisphosphate receptor down-regulation is activated directly by inositol 1,4,5-trisphosphate binding. Studies with binding-defective mutant receptors. J Biol Chem. 1999;274:3476–3484. doi: 10.1074/jbc.274.6.3476. [DOI] [PubMed] [Google Scholar]
- 50.Zhu CC, Wojcikiewicz RJ. Ligand binding directly stimulates ubiquitination of the inositol 1, 4,5-trisphosphate receptor. Biochem J. 2000;348(Pt 3):551–556. [PMC free article] [PubMed] [Google Scholar]
- 51.Oberdorf J, Webster JM, Zhu CC, Luo SG, Wojcikiewicz RJ. Down-regulation of types I, II and III inositol 1,4,5-trisphosphate receptors is mediated by the ubiquitin/proteasome pathway. Biochem J. 1999;339(Pt 2):453–461. [PMC free article] [PubMed] [Google Scholar]
- 52.Diaz F, Bourguignon LYW. Selective down-regulation of IP3receptor subtypes by caspases and calpain during TNF [alpha]-induced apoptosis of human T-lymphoma cells. Cell Calcium. 2000;27:315–328. doi: 10.1054/ceca.2000.0126. [DOI] [PubMed] [Google Scholar]
- 53.Hirota J, Furuichi T, Mikoshiba K. Inositol 1,4,5-Trisphosphate Receptor Type 1 Is a Substrate for Caspase-3 and Is Cleaved during Apoptosis in a Caspase-3-dependent Manner. J Biol Chem. 1999;274:34433–34437. doi: 10.1074/jbc.274.48.34433. [DOI] [PubMed] [Google Scholar]
- 54.Graef IA, Mermelstein PG, Stankunas K, Neilson JR, Deisseroth K, Tsien RW, Crabtree GR. L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature. 1999;401:703–708. doi: 10.1038/44378. [DOI] [PubMed] [Google Scholar]
- 55.Romano-Spica V, Georgiou P, Suzuki H, Papas TS, Bhat NK. Role of ETS1 in IL-2 gene expression. J Immunol. 1995;154:2724–2732. [PubMed] [Google Scholar]
- 56.Lin JX, Bhat NK, John S, Queale WS, Leonard WJ. Characterization of the human interleukin-2 receptor beta-chain gene promoter: regulation of promoter activity by ets gene products. Mol Cell Biol. 1993;13:6201–6210. doi: 10.1128/mcb.13.10.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas RS, Tymms MJ, Seth A, Shannon MF, Kola I. ETS1 transactivates the human GM-CSF promoter in Jurkat T cells stimulated with PMA and ionomycin. Oncogene. 1995;11:2135–2143. [PubMed] [Google Scholar]
- 58.Blumenthal SG, Aichele G, Wirth T, Czernilofsky AP, Nordheim A, Dittmer J. Regulation of the human interleukin-5 promoter by Ets transcription factors. Ets1 and Ets2, but not Elf-1, cooperate with GATA3 and HTLV-I Tax1. J Biol Chem. 1999;274:12910–12916. doi: 10.1074/jbc.274.18.12910. [DOI] [PubMed] [Google Scholar]
- 59.Ji HB, Gupta A, Okamoto S, Blum MD, Tan L, Goldring MB, Lacy E, Roy AL, Terhorst C. T cell-specific expression of the murine CD3delta promoter. J Biol Chem. 2002;277:47898–47906. doi: 10.1074/jbc.M201025200. [DOI] [PubMed] [Google Scholar]
- 60.Bird JJ, Brown DR, Mullen AC, Moskowitz NH, Mahowald MA, Sider JR, Gajewski TF, Wang CR, Reiner SL. Helper T Cell Differentiation Is Controlled by the Cell Cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 61.Bettelli E, Oukka M, Kuchroo VK. TH-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 62.Bucy RP, Xu XY, Li J, Huang G. Cyclosporin A-induced autoimmune disease in mice. J Immunol. 1993;151:1039–1050. [PubMed] [Google Scholar]