Abstract
Studies in healthy human subjects and patients with irritable bowel syndrome suggest sex differences in cerebral nociceptive processing. Here we examine sex differences in functional brain activation in the rat during colorectal distention (CRD), a preclinical model of acute visceral pain. [14C]-iodoantipyrine was injected intravenously in awake, nonrestrained female rats during 60-mmHg or 0-mmHg CRD while electromyographic abdominal activity (EMG) and pain behavior were recorded. Regional cerebral blood flow related tissue radioactivity was analyzed by statistical parametric mapping from autoradiographic images of 3-dimensionally reconstructed brains. Sex differences were addressed by comparing current data with our previously published data collected from male rats. While sex differences in EMG and pain scores were modest, significant differences were noted in functional brain activation. Females showed widespread changes in limbic (amygdala, hypothalamus) and paralimbic structures (ventral striatum, nucleus accumbens, raphe), while males demonstrated broad cortical changes. Sex differences were apparent in the homeostatic afferent network (parabrachial nucleus, thalamus, insular and dorsal anterior cingulate cortices), in an emotional-arousal network (amygdala, locus coeruleus complex), and in cortical areas modulating these networks (prefrontal cortex). Greater activation of the ventromedial prefrontal cortex and broader limbic/paralimbic changes in females suggest greater engagement of affective mechanisms during visceral pain. Greater cortical activation in males is consistent with the concept of greater cortical inhibitory effects on limbic structures in males, which may relate to differences in attentional and cognitive attribution to visceral stimuli. These findings show remarkable similarities to reported sex differences in brain responses to visceral stimuli in humans.
Keywords: visceral pain, colorectal distension, cerebral blood flow, brain mapping, sex difference
Introduction
Women are at greater risk for experiencing many forms of functional pain disorders, including irritable bowel syndrome (IBS) [25,44]. The belief that sex differences in visceral nociception stems, at least in part, from differences in neural processing of the nociceptive input has initiated examination of sex differences in IBS patients [1,22,29], and in healthy human subjects [2,20] during noxious visceral stimulation using functional brain imaging.
In a H215O positron emission tomography (15O-PET) study, Naliboff et al. [29] reported that in response to 45-mmHg rectal distension, female IBS patients show greater activation in limbic areas (including the ventromedial prefrontal, anterior and infragenual cingulate cortices, and amygdala), whereas males showed greater activation in the dorsolateral prefrontal and insular cortices, and dorsal pons. The pattern is consistent with greater cognitive processing in males, but greater emotional responses in females. In an earlier 15O-PET study, Berman et al. [1] also noted greater activation of the insula in male than in female IBS patients.
Two functional magnetic resonance imaging (fMRI) studies have been published examining sex differences in regional brain response to rectal distension in healthy human subjects. Berman et al. [2] reported trend of more extensive activations in men than women in the insular, anterior cingulate and midcingulate cortices. In contrast, Kern et al. [20] reported that women, but not men, showed activation in the insular and anterior cingulate/prefrontal region in response to rectal distension.
Despite the discrepancies in results that may be attributable to differences in experimental paradigms, these studies collectively provide evidence for sex differences in neural processing of nociceptive visceral afferent information. Recently, a framework has been proposed to understand at the network level visceral nociceptive processing [22,27,29] consisting of: (a) a “homeostatic–afferent” network central to processing of visceral afferent information [8,27]; (b) an “emotional-arousal” network involved in arousal and emotion-related pain amplification and autonomic responses [30,40,46]; (c) a “cortical-modulatory” network mediating cortical modulation of (a) and (b) [26]. In an 15O-PET study of IBS patients, Labus et al. [22] showed that sex differences in brain responses are largely due to alterations in the effective connectivity of the emotional-arousal network, including the amygdala, locus coeruleus complex, rostral and subgenual cingulate areas.
In contrast to these human studies, animal studies on sex differences in visceral pain have relied on measuring visceromotor responses (VMR) [14,17] to noxious colorectal distension (CRD), an animal model of acute visceral pain [31]. Compared to male rats, female rats have been shown to have lower CRD threshold for VMR [14] and greater VMR response to noxious CRD [17].
We recently reported functional brain activation to noxious CRD in awake, nonrestraint male rats, and proposed to use the autoradiographic cerebral perfusion method of functional brain mapping in rodent to bridge the gap between preclinical and clinical study of visceral pain [50]. In the current study, we extended the investigation to groups of female rats to examine sex differences in functional brain activation in the above-mentioned circuits during noxious CRD.
Materials and Methods
Animals
Twenty-four adult, female Wistar rats (3 months old, Harlan Inc., Indianapolis, IN, USA) were randomized into two groups: distension and control (n = 12 each group). Rats were individually housed on a 12 hour light/12 hour dark cycle with free access to water and rodent chow. Experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee of the University of Southern California and in accordance with ethical guidelines for investigations of experimental pain in conscious animals provided by IASP. Results were compared with those from male rats we published before [50] to address sex differences.
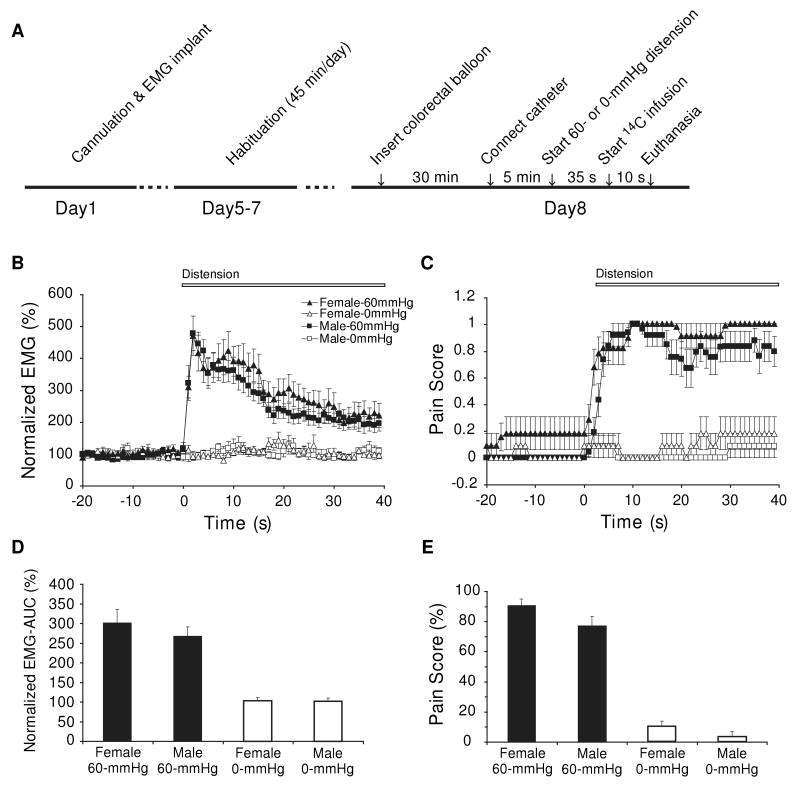
Experimental Design (Fig. 1A)
Figure 1. Comparison of EMG and behavioral responses to 60-mmHg colorectal distension in female and male rats.
(A) Experimental protocol. Time course of EMG activity (B) and behavioral pain posturing (C) before and during colorectal distension (CRD) in female and male rats (n = 10 - 12). (D) Area under curve computed over the 40-s period after the onset of CRD for EMG activity normalized to the 40-s baseline before CRD. Two-way ANOVA revealed significant main effect of CRD (F1,41 = 63.6, P < 0.0005), but not of Sex (F1,41 = 0.6, P = 0.5) or of the Sex × CRD interaction (F1,41 = 0.5, P = 0.5). (D) Percentage of time animals spent in pain postures during the 40-s period after CRD onset. Rats exposed to 60-mmHg CRD spent significantly more time in pain postures than 0-mmHg controls (CRD effect: F1,42 = 299.0, P < 0.0005, two-way ANOVA). Pain scores were significantly higher in female than in male rats (Sex effect: F1,42 = 5.1, P < 0.05). There was no significant Sex × CRD interaction (F1,42 = 0.5, P = 0.5). Results of the two male groups were reproduced from Wang et al. [50] with permission.
As previously reported [50], the right external jugular vein was cannulated and the distal end of the catheter externalized dorsally in the region rostral to the scapula. Electrodes were implanted in the left external oblique musculature and connected to a telemetry transmitter (TA10ETA-F20, Data Sciences Intl., St. Paul, MN, USA) to measure abdominal electromyographic activity (EMG). The transmitter was implanted subcutaneously in the infrascapular region. Animals were allowed to recover for seven days before the cerebral perfusion experiments. Rats were habituated to an uninflated colorectal balloon and the experiment cage for 45 min per day for three days prior to cerebral perfusion.
CRD and injection of the cerebral perfusion tracer
Under isoflurane sedation, a latex balloon (length = 6 cm) was inserted intra-anally such that its caudal end was 1 cm proximal to the anus. The silicone tubing connecting the balloon and the barostat (Distender Series II, G&J Electronics, Toronto, Canada) was fixed to the base of the tail with adhesive tape and covered by a protective stainless steel spring. Animals were allowed to recover for 30 min in the experiment cage, the floor of which was covered with bedding from the animal's home cage. At the end of the recovery period, a piece of tubing filled with the radiotracer, [14C]-iodoantipyrine (125 μCi/kg in 300 μl of 0.9% saline, ARC, St. Louis, MO, USA) was connected to the animal's cannula on one end, and to a syringe filled with euthanasia agent (pentobarbital 75 mg/kg, 3 mol/L potassium chloride) on the other. The nonrestrained animal was allowed to rest for 5 min before receiving a distension of 60-mmHg (CRD) or 0-mmHg (control). Thirty-five seconds after the onset of distension, radiotracer was infused by a motorized pump at 2.25 mL/min, followed immediately by euthanasia, which resulted in cardiac arrest within 8 - 10 s, termination of brain perfusion, and death. This 8 - 10 s time window provided the temporal resolution during which the distribution of regional cerebral blood flow related tissue radioactivity (rCBF) was mapped.
Brain slicing and autoradiography
Brains were rapidly removed, flash frozen in methylbutane/dry ice, and cryosectioned into 20-μm-thick coronal slices (inter-slice spacing 300 μm). Slices were exposed for 2 weeks to Ektascan Diagnostic Film (Eastman Kodak, Rochester, NY, USA), and images were digitized on an 8-bit gray scale.
Pseudoaffective responses
Abdominal EMG analysis
EMG signals were recorded telemetrically (sampling rate 1kHz), low-cut filtered at 20 Hz and full-wave rectified [39]. Each rat's rectified EMG signal was averaged over consecutive 1-s intervals for the 40-s period before (baseline) and 40-s after the onset of CRD. Each data point was normalized to average baseline amplitude (defined as 100%). Area under the curve (AUC) was calculated for the 40-s period after CRD onset. EMG data were lost due to transmitter failure on 1 rat in the control group.
Behavioral analysis of nociception
Behavior was recorded with two digital camcorders placed horizontally at a 90° angle. Video was digitized and scored offline using the Observer XT software (Noldus, Leesburg, VA, USA). Postures indicative of visceral pain were defined as arching (convex back and retraction of the abdomen from the floor) and stretching (whole body stretched horizontally) [39]. Presence or absence of either pain posture (1 = present, 0 = absent) was scored continuously in 1-s intervals over the 40-s baseline and 40-s after the onset of CRD.
Group differences in the EMG and behavioral curves were separately tested with two-way ANOVA using the between-subject factors of sex (male, female) and CRD (0- or 60-mmHg). We also performed repeated measures ANOVA with the between-subject factors of sex and CRD, and the within-subject factor of time. Data were presented as mean ± SEM.
Statistical parametric mapping
rCBF was quantified by autoradiography and analyzed on a whole-brain basis using statistical parametric mapping (SPM, version SPM2, Wellcome Centre for Neuroimaging, University College London, London, UK). SPM, a software package developed for analysis of imaging data in humans [11] has recently been adapted by us [15,32] for use in autoradiographs of the rat brain. A 3D reconstruction of each animal's brain was conducted using 57 serial coronal sections. Adjacent sections were aligned both manually and using TurboReg, an automated pixel-based registration algorithm [42]. A typical reconstructed brain had ∼ 2,750,000 voxels (voxel size: 40 μm × 40 μm × 300 μm). After 3D reconstruction, one “artifact free” brain was selected as reference and smoothed with a Gaussian kernel (FWHM = 3 × voxel dimension). Brains from both groups were spatially normalized to the smoothed reference brain. Following spatial normalization, normalized images were averaged to create a mean image, which was then smoothed to create the final female brain template. Each original 3D reconstructed brain was then spatially normalized into the standard space defined by the template. An unbiased, voxel-by-voxel analysis of whole-brain activation using SPM was used for detection of significant changes in functional brain activation between rats exposed to CRD and controls. Global differences in the absolute amount of radiotracer delivered to the brain were adjusted in SPM for each animal by scaling the voxel intensities so that the mean intensity for each brain was the same (proportional scaling). We implemented a Student t test at each voxel, testing the null hypothesis that there was no effect of group. To control both Type I and Type II errors, we set a significance threshold of P < 0.05 for individual voxels within clusters of at least 100 contiguous voxels (extent threshold). Brain regions were identified using a rat brain atlas [34]. One brain from the control group was not included for analysis due to major cryosection artifacts.
Staging of the estrus cycle
The VMR response to CRD has been shown to fluctuate with the estrus cycle in anesthetized [14], and awake, restrained rats [18] (but see [17]). Vaginal cytology was obtained from vaginal lavage daily over 1 week prior to the start of the experiment [16]. Staging of the estrus cycle was also confirmed by postmortem uterine weights, which change between ∼ 325 and 525 mg during the 4- to 5-day estrus cycle of the rat [12].
Results
EMG
60-mmHg CRD evoked significant EMG responses in female rats (307 ± 34%, normalized AUC, vs. 267 ± 24% in males [50]), compared to 0-mmHg controls (103 ± 8%, vs. 103 ± 8% in males)(Fig. 1B, 1D). Two-way ANOVA revealed significant main effect of CRD (F1,41 = 63.6, P < 0.0005), but not of Sex (F1,41 = 0.6, P = 0.5) or of the Sex × CRD interaction (F1,41 = 0.5, P = 0.5). EMG changed significantly with time during CRD (Time × CRD: F1,39 = 13.8, P < 0.0005). However, the Time × Sex and Time × Sex × CRD interactions showed no significant effect.
Behavior
Sex differences in pain posturing during the period of CRD were modest (female vs. male:0-mmHg control, 10 ± 3% vs. 3 ± 3%; 60-mmHg CRD: 90 ± 6% vs. 77 ± 6%. Percentage of time animal spent in pain postures during 40-s of CRD)(Fig. 1E). Rats exposed to 60-mmHg CRD spent significantly more time in pain postures than 0-mmHg controls (CRD: F1,42 = 299.0, P < 0.0005). Pain posture scores were significantly higher in female than in male rats (Sex effect: F1,42 = 5.1, P < 0.05). There was no significant Sex × CRD interaction (F1,42 = 0.5, P = 0.5). Pain behavior changed significantly with time during CRD (Time × CRD: F1,39 = 8.0, P < 0.0005). However, the Time × Sex and Time × Sex × CRD interactions were nonsignificant (Fig. 1C).
Estrus Cycle
There was no significant difference between the postmortem uterine weights of the two female groups (0-mmHg controls: 0.36 ± 0.03 g, 60-mmHg CRD: 0.42 ± 0.03 g, P = 0.2), and no significant correlation between uterin weights (normalized by body mass) and EMG-AUC (R2 = 0.02) or pain score (R2 = 0.02) within the CRD group. At the time of CRD and perfusion experiments, stages of the estrus cycle as determined by cytology were represented equivalently across groups (0-mmHg: 6 diestrus/metestrus, 3 estrus, 3 proestrus; 60-mmHg: 4 diestrus/metestrus, 3 estrus, 5 proestrus). Females exposed to 60-mmHg CRD showed a non-significant trend toward increases in EMG-AUC across diestrus/metestrus, proestrus, and estrus (EMG-AUC: 253.5 ± 58.4%, 289.5 ± 40.8%, 379.6 ± 99.4%, respectively. F2,9 = 0.68, P = 0.5, one-way ANOVA). Data from female rats within each group were pooled for SPM analysis.
Functional brain response to CRD (60-mmHg vs. 0-mmHg)
Cerebral Cortex
Relevant to the known circuitry of regions involved in the processing and modulation of visceral pain, female CRD rats compared to controls showed increases in rCBF in the ventral anterior cingulate cortex, infralimbic cortex, and right mid/posterior insula, and decreases in rCBF in lateral and ventral orbitofrontal cortex, secondary somatosensory and dorsal anterior cingulate cortex (Fig. 2). In addition, female CRD rats showed increased rCBF in auditory, piriform and temporal association cortices, with decreases noted in parietal association cortex. In contrast, male CRD rats showed widespread cortical activation in the dorsal anterior cingulate, insular (both anterior and mid/posterior), prelimbic, primary and secondary somatosensory, primary and secondary motor, visual, auditory, ectorhinal, parietal association, and temporal association cortices [50].
Figure 2. Comparison of changes in regional cerebral blood flow related tissue radioactivity in response to 60-mmHg colorectal distension in female and male rats.
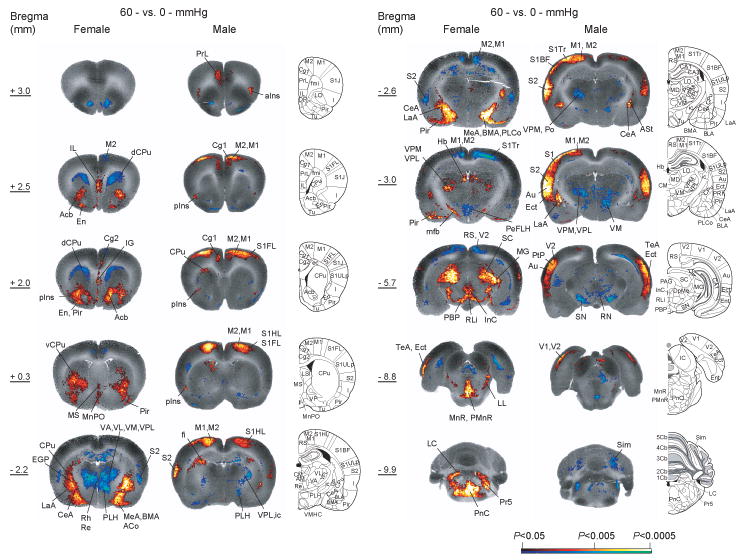
Statistically significant positive (red scale) and negative (blue scale) differences of the 60-mmHg distension groups compared to 0-mmHg controls (voxel level P < 0.05, cluster > 100 contiguous voxels) as determined by statistical parametric mapping are shown on representative coronal slices with anterior-posterior coordinates given relative to bregma (n = 11 for female control, n = 12 for the other groups). Abbreviations [34]: ACo/PLCo (anterior/posterolateral cortical amygdala), Au (auditory cortex), BMA (basomedial amygdala), CA1 (hippocampus CA1 region), CeA (central n. of the amygdala), Cg1, Cg2 (cingulate cortex, areas 1 and 2), dCPu, vCPu (dorsal and ventral caudate-putamen), Ect (ectorhinal cortex), EGP (external globus pallidus), En (endopiriform n.), Hb (habenula), aIns, pIns (anterior, mid/posterior insular cortex), LaA (lateral n. of the amygdala), LC (locus coeruleus), LL (lateral lemniscus), M1, M2 (primary and secondary motor cortex), MD (mediodorsal thalamic n.), Me (medial amygdala), mfb (medial forebrain bundle), MG (medial geniculate), MnPO (medial preoptic n.), MnR/PMnR (median, paramedian raphe), MS (medial septum), Acb (n. accumbens), PBP (parabrachial pigmented n.), PeFLH (perifornical lateral hypothalamus), Pir (piriform cortex), PrL (prelimbic cortex), PnC (pontine reticular caudal n.), Po (posterior thalamic n.), PtA (parietal association area), Re, Rh (reuinens, rhomoboid thalamic n.), RLi (rostral linear raphe), SC (superior colliculus), Sim (simple lobule), S1BF, S1FL, S1HL, S1Tr (primary somatosensory cortex: barrel field, forelimb, hindlimb, trunk), S2 (secondary somatosensory cortex), TeA (temporal association cortex), VA, VL, VM, VPL, VPM, (ventral anterior, ventrolateral, ventromedial, ventral posterolateral, ventral posteromedial thalamic n.), V1, V2 (primary, secondary visual cortex), Pr5 (trigeminal n.). The male results were reproduced from Wang et al. [50] with permission. Rat brain atlas figures were reproduced with modification from Paxinos and Watson [34] with permission.
Limbic and paralimbic areas
Female rats in response to CRD showed significant increases in rCBF broadly across the amygdala. Activated amygdala regions included the central, medial, and basomedial nuclei, as well as the cortical amygdala and small portions of the lateral amygdala and amygdalostriatal transition area. In contrast, male rats in response to CRD demonstrated more limited increases in rCBF in the amygdala that were restricted to the central nucleus and small portions of the lateral amygdala and amygdalostriatal transition area [50]. In females, but not males, increased rCBF was noted also in the medial preoptic area of the hypothalamus, nucleus accumbens and ventral tegmental area, while significant decreases were noted in the hippocampus, and posterior and dorsomedial hypothalamus.
Thalamus
Within the thalamus, females showed both significant increases and decreases in rCBF, whereas males showed only decreases. Specifically, females showed significant increases in rCBF in response to CRD in the lateral and medial geniculate, mediodorsal, ventral posterolateral, ventral posteromedial, posterior, and posterior triangular thalamic nuclei, whereas males showed a decrease or no change. Females showed decreases in rCBF in the ventrolateral, ventral anterior, ventromedial, and anterior (anteriodorsal, anterioventral, anteriomedial) thalamic nuclei, as well as the midline nuclear group (paraventricular, paratenial, reuinens, rhomboidal, submedius), whereas males showed significant decrease in rCBF only in the ventromedial thalamic nucleus [50].
Basal ganglia and associated nuclei
Within the basal ganglia and associated nuclei, females showed significant increases in rCBF in the accumbens nucleus, posterior ventral caudate-putamen, the external and internal globus pallidus, red nucleus, pararubral nucleus, prerubral field, and substantia nigra. Decreases were noted in the anterior dorsal caudate-putamen. Males showed significant increases in rCBF in the anterior dorsal caudate-putamen and decreases in the lateral caudate-putamen, red nucleus, pararubral nucleus, prerubral field, and substantia nigra [50].
Other subcortical regions
Other subcortical regions showing increases in rCBF in female rats in response to CRD were the anterior pretectal area, endopiriform nucleus, gigantocellular reticular nucleus, habenula, interstitial nucleus of Cajal, locus coeruleus, interpeduncular nucleus, parabrachial area (pigmented, lateral, medial nuclei), reticular intermediate nucleus, caudal pontine reticular nucleus, raphe (dorsal, median, paramedian, interpositus nucleus, rostral linear), subbrachial nucleus, superior and inferior colliculi, and trigeminal lateral sensory nucleus. In these areas, males showed no change or individual significant increases (inferior colliculus), or decreases (parabrachial area, pons, trigeminal lateral sensory nucleus) [50].
Discussion
In this rat model of acute visceral pain, substantial sex differences in functional brain activation were observed despite only modest sex differences in visceromotor and behavioral responses to noxious CRD. Females showed more widespread activation of subcortical structures, in particular limbic and paralimbic structures, including the amygdala and raphe nuclei, as well as the thalamus. Males, in contrast, showed broader cortical activation. Remarkable similarities in sex-related brain activation patterns in response to a noxious visceral stimulus were observed to previously published findings in human subjects [22,29].
The concept of several interacting networks (a homeostatic afferent network, an emotional-arousal network and a cortical modulatory network) in the processing and modulation of visceral pain has recently been reviewed [27]. We use this framework to discuss our main findings (Fig. 3).
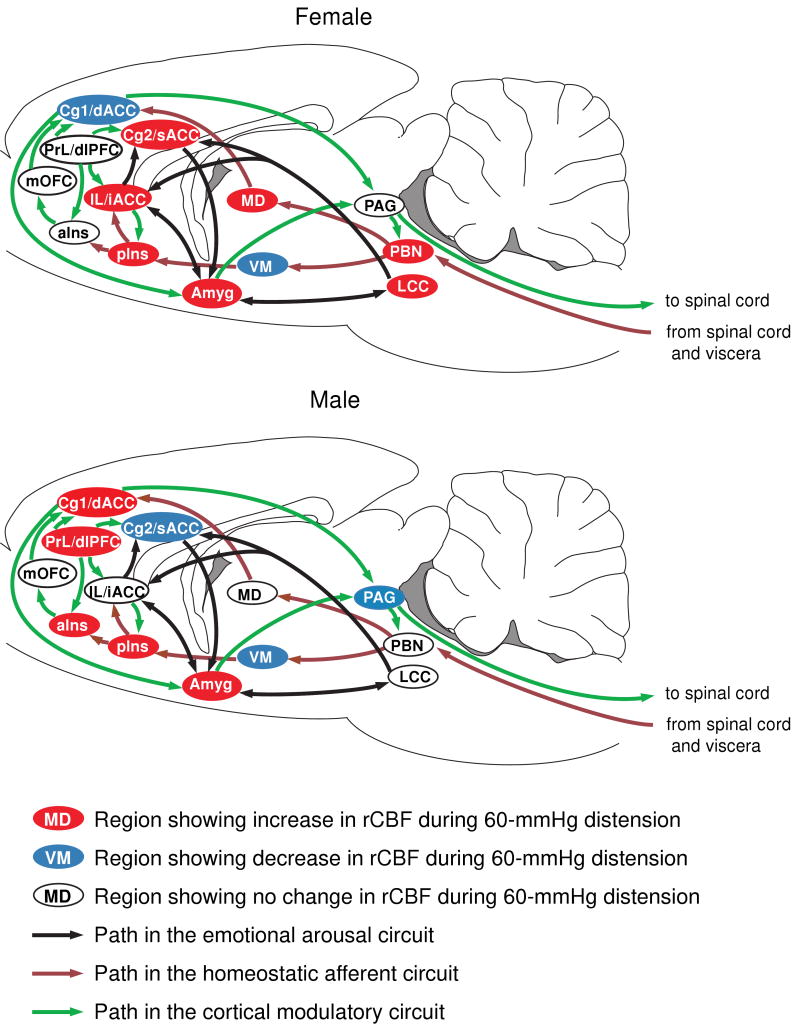
Figure 3. Sex differences in activation of the homeostatic afferent, emotional-arousal, and cortical modulatory networks in response to noxious visceral stimulation.
Regions are highlighted as showing significant increases (red), decreases (blue) or no significant change (white) in regional cerebral blood flow during 60-mmHg CRD. Abbreviations: aIns (anterior insula), Cg1 (cingulate cortex, area 1, BA24b [49]), Cg2 (cingulate cortex, area 2, BA24a [49]), dACC (dorsal anterior cingulate cortex in human), dlPFC (dorsolateral prefrontal cortex in human), iACC (infragenual anterior cingulate cortex in human), IL (infralimbic cortex, BA25 [49]), LCC (locus coeruleus complex), MD (mediodorsal thalamic nucleus), mOFC (medial orbital frontal cortex), PAG (periaqueductal gray), PBN (parabrachial nucleus), pIns (mid/posterior insula), PrL (prelimbic cortex, BA32 [49]), sACC (supragenual anterior cingulate cortex in human), VM (ventromedial thalamic nucleus). Rat brain atlas figures were reproduced with modification from Paxinos and Watson [34] with permission.
The homeostatic afferent network allows the brain to respond to a variety of non-painful and painful visceral, somatic, and emotional stimuli [8,27]. Nodes of the network include subregions of the insula and the dorsal anterior cingulate areas (dACC), mediodorsal and ventromedial thalamic nuclei (MD, VM), and the parabrachial nucleus with projections to sensory and limbic cortices. Our data showed striking sex differences in activation of this network. In response to CRD, females, but not males, showed activation of the parabrachial nucleus, a finding consistent with recent work showing sex differences in activation of spinoparabrachial neurons [28]. Males showed activation of the dACC (Cg1 area) and both anterior and mid/posterior insula (aIns, pIns), whereas females showed activation only in pIns, and decrease of rCBF in dACC. The insular cortex, often referred to as interoceptive cortex [8], has consistently been identified as being activated during CRD. Greater activation in the anterior insula in men than in women has been previously reported in healthy human subjects [2], as well as in IBS patients [1,29]. The functional implication of these sex differences remain unclear, but may relate to reported sex differences in cardiovascular responses [33,52,55]. In the thalamus, MD and ventral posterolateral nuclei (VPL) have been reported to show graded electrophysiologic responses to graded CRD pressures [53,54]. Female rats showed significant activation in these thalamic nuclei, with no significant somatosensory cortical activation. Males, on the other hand, showed decrease in rCBF in the VPL and ventral posteromedial nuclei (VPM), but strong activation in somatosensory cortex at the same time. VPM and VPL have been implicated in some forms of sensory gating within corticothalamic and thalamocortical pathways [5,21]. Future electrophysiologic work will need to evaluate the possibility of sex differences in sensory gating between the thalamus and cerebral cortex, something that has been suggested in a prior imaging study [43].
The emotional-arousal circuit, including the locus coeruleus complex (LCC), amygdala, infragenual and supragenual cingulate cortices in humans, plays an important role in the emotional arousal associated with perceived or real perturbation of homeostasis [30,40,46]. Reciprocal feedforward connections between amygdala and locus coeruleus via noradrenergic and corticotropin releasing factor containing projections play a central role in activation of the stress response, and activation of this system has been implicated in the stress-induced modulation of gastrointestinal motility and stress-induced visceral hyperalgesia [41]. Recently, Labus et al. applied effective connectivity analysis to 15O-PET data and showed sex differences in the effective connectivity of this circuit in IBS patients during expectation of visceral pain [22]. In particular, women showed consistently strong positive connectivity between amygdala and LCC, whereas men showed weaker, and sometimes negative connections. In the current study, female rats, but not males, showed activation of LCC during CRD. These results are consistent with evidence for sexual dimorphism of the locus coeruleus [24,37], as well as sex differences in the stress responsiveness of the brain noradrenergic system [9]. In addition, females showed activation in broad areas of the amygdala (cortical, basomedial, central, lateral, amygdala-striatal transition), while males showed activation of a more circumscribed region of the amygdala (central, lateral, amygdala-striatal transition). Greater activation of the amygdala in women in response to visceral pain in clinical studies has been reported by some investigators [23,29] but not by others [3,4,51]. Such differences may reflect the fact that cognitive mechanisms under voluntary control may regulate the amygdala's output and these may be differentially altered by what the subject anticipates [35].
Activity within the homeostatic afferent and the emotional arousal network are modulated by cortical regions including the dorsolateral prefrontal cortex (dlPFC) and medial orbitofrontal cortex (mOFC)[27]. These regions modulate activity in limbic and paralimbic areas (amygdala, dACC, ventral cingulate cortex), which in turn regulate activity of descending inhibitory and facilitatory pathways through the periaqueductal gray and pontomedullary nuclei. It is important to note that while there is significant homology between rats and humans at the level of limbic circuits and the emotional arousal and homeostatic afferent networks, there are likely major differences at the cortical level, particularly in the prefrontal areas [38,45]. Although prefrontal cortex in rats is significantly less developed than in primates, anatomical and functional dlPFC-like features are present in rats in dACC and prelimbic areas [45]. The infralimbic cortex of the rat is considered to be homologous to the infragenual cingulate cortex (BA 25) in human [38], and also shows features of mOFC [47].
Female rats in response to CRD showed significant increases in rCBF in the infralimbic and ventral anterior cingulate cortices (Cg2), but decreases in dACC. In constrast, males showed activation in the prelimbic cortex and dACC (Cg1). These results show remarkable similarity to sex differences reported in IBS patients [29]: whereas women showed greater response in the infragenual cingulate cortex and ventromedial prefrontal cortex (a subsector of mOFC), men showed greater response in the dlPFC. Together with the observed sex differences in the emotional arousal and homeostatic afferent networks, these findings are consistent with greater cortical processing and cortico-limbic inhibition in males and greater emotional responses in females.
Our results are also consistent with the idea proposed by Phillips et al. [36] of the existence of a dorsal and a ventral stream of emotional cognition. The ventral stream, consisting of the amygdala, insula, ventral striatum, and ventral regions of the anterior cingulate gyrus and prefrontal cortex, is posited to appraise emotional behavior and produce an affective state. The dorsal stream, consisting of dorsal regions of the anterior cingulate gyrus, prefrontal cortex, and hippocampus, acts as a regulatory mechanism for the ventral stream. Our study shows clear sex differences in these two neural systems in the rat in response to CRD, with a predominant activation of the ventral system in females and of the dorsal system in the males.
In response to CRD, female but not male rats showed significant changes in rCBF in the raphe nucleus, as well as in many target regions of its serotonergic efferent projections (medial forebrain bundle, midline and intralaminar nuclei of the thalamus, amygdala, posteromedial regions of the striatum, endopiriform nucleus, ventral pallidum, inferior colliculus, lateral and dorsal hypothalamus, globus pallidus, accumbens nucleus, piriform and frontal cortices) [48]. These findings are consistent with the idea of sexual dimorphism of the raphe nuclei [6,7], and its projections [19], as well as sex differences in the functional response of the serotonergic system to stress [10,13].
We observed significant sex differences in pain posture, but a non-significant trend in VMR. A non-significant trend in VMR fluctuation was also noted during the estrus cycle. These trends are in general agreement with reports by others [14,18,28], with differences in the VMR responses likely related to differences in the rat strain and experimental protocol (nonrestrained vs. restrained animals, single CRD vs. repetitive CRD, awake vs. anesthetized animals). While our study had insufficient power to detect significant differences in the visceral pain response across the estrus cycle, our results suggest that sex differences in the visceral pain perception are related to differences in central processing of visceral stimuli that persist when female subjects are pooled across the estrus cycles.
Conclusion
Our results in rodents are consistent with the notion that women show greater affective aspects of the visceral pain response, while men show greater recruitment of corticolimbic inhibitory structures [27]. Sex differences in the functional brain response to visceral pain are apparent in nodes of the homeostatic afferent and the emotional arousal network, as well as at the level of cortical modulation of these networks. The striking similarity between these findings in a rodent acute visceral pain model and those from human brain imaging studies supports the potential usefulness of rodent brain imaging studies to investigate chronic visceral pain syndromes, such as IBS.
Table 1. Regions showing significant s in functional brain activation in response to acute colorectal distension (60-mmHg) compared to 0-mmHg controls in female and male rats.
| Brain region | 60- vs. 0-mmHg (Left / Right) |
|
|---|---|---|
| Female | Male | |
| Cerebral cortex | ||
| Auditory (Au) | + / + | + / + |
| Cingulate, dorsal anterior (Cg1) | - / - | + / + |
| ventral anterior (Cg2) | / + | -/ - |
| Ectorhinal (Ect) | + / + | |
| Infralimbic (IL) | + / + | |
| Insular, anterior (aIns) | + / + | |
| mid/posterior dysgranular (pIns) | / + | + / + |
| Motor, primary (M1) | + / + | |
| secondary (M2) | - / - | + / + |
| Orbital, lateral, ventral (LO, VO) | - / - | |
| Parietal, association (PtA) | - / - | + / + |
| Piriform (Pir) | + / + | |
| Prelimbic (PrL) | + / + | |
| Somatosensory, primary, barrel field (S1BF) | + / + | |
| forelimb (S1FL) | + / + | |
| hindlimb (S1HL) | + / + | |
| trunk (S1Tr) | / - | + / + |
| Somatosensory, secondary (S2) | - / - | + / + |
| Temporal association (TeA) | + / + | + / + |
| Visual, primary (V1) | + / + | |
| secondary (V2) | + / + | |
| Subcortical regions | ||
| Accumbens n., core, shell (Acb) | + / + | |
| Amygdala, cortical, basomedial, medial (AA, ACo, BMA, MeA) | + / + | |
| central n., amygdalostriatal transition area (CeA, AStr) | + / + | + / + |
| lateral n.(LaA) | + / | + / + |
| Anterior pretectal area (APT) | + / + | |
| Caudate putamen, ventral posterior (vCPu) | + / + | |
| dorsal anterior (dCPu) | - / - | + / + |
| lateral (lCPu) | - / - | |
| Endopiriform n., dorsal, intermed (DEn, IEn) | + / + | |
| Gigantocellular reticular n. (Gi) | + / + | |
| Globus pallidus, external (EGP) | + / + | |
| internal (IGP) | + / + | |
| Habenula (Hb) | + / + | |
| Hippocampus, CA1-3, anterior | - / - | |
| CA1, posterior | - / | - / |
| Hypothalamus, medial preoptic (MnPO) | + / + | |
| posterior, dorsomedial (PH, DM) | - / - | |
| peduncular part of lateral (PLH) | - / - | - / - |
| Inferior colliculus (IC) | + / + | + / + |
| Interpeduncular n. (IP) | + | |
| Interstitial nucleus of Cajal (InC) | + / + | |
| Locus coeruleus (LC) | + / + | |
| Parabrachial, pigmented n. of the VTA, lateral n., medial n. (PBP, LPB, MPB) | + / + | - / - |
| Periaqueductal gray (PAG) | - / - | |
| Pontine reticular nucleus, caudal (PnC) | + / + | - / - |
| Raphe, dorsal, median, paramedian, interpositus n., rost linear (DR, MnR, PMnR, RIP, RLi) | + | |
| Red nucleus (RN), pararubral n. (PaR), prerubral field (PR) | + / + | - / - |
| Reticular n., intermediate (Irt) | + / + | |
| Subbrachial n. (SubB) | + / + | |
| Substantia nigra (SN) | + / + | - / - |
| Superior colliculus (SC) | + / + | - / - |
| Thalamus, lateral geniculate (LG) | + / + | - / - |
| medial geniculate (MG) | + / + | |
| mediodorsal (MD) | + / + | |
| ventroposterior (VPM,VPL) | + / + | - / - |
| ventrolateral, ventral anterior (VL, VA) | - / - | |
| ventromedial nucleus (VM) | - / - | - / - |
| posterior group (Po, PoT) | + / + | - / - |
| anterior nuclei (anterodorsal, AD; anteroventral, AV; anteriomedial, AM) | - / - | |
| midline nuclear group (paraventricular, PVA, paratenial, PT; reuinens, Re; rhomboidal, Rh; submedius, Sub) | - / - | |
| Trigeminal nucleus, sensory, lateral (Pr5) | + / + | - / |
| Ventral tegmental area, rostral (VTAR) | + / + | |
Significant increases or decreases are noted with ‘+’ and ‘-’, respectively. Significance is shown at the voxel level (P < 0.05) for clusters of >100 contiguous voxels. Male results were reproduced from Wang et al. [52] with permission. Abbreviations are taken from the Paxinos and Watson (2005) rat atlas [35].
Acknowledgments
Grant support from GlaxoSmithKline, and the Animal Models Core and the Neuroimaging Core of the Center for Neurobiology of Stress, UCLA (NIDDK P50DK064539, NCCAM AT00268). There are no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berman S, Munakata J, Naliboff BD, Chang L, Mandelkern M, Silverman D, Kovalik E, Mayer EA. Gender differences in regional brain response to visceral pressure in IBS patients. Eur J Pain. 2000;4:157–172. doi: 10.1053/eujp.2000.0167. [DOI] [PubMed] [Google Scholar]
- 2.Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Bueller JA, Ruby K, Mayer EA. Sex differences in regional brain response to aversive pelvic visceral stimuli. Am J Physiol Regul Integr Comp Physiol. 2006;291:R268–276. doi: 10.1152/ajpregu.00065.2006. [DOI] [PubMed] [Google Scholar]
- 3.Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Ohning G, Kilpatrick L, Bueller JA, Ruby K, Jarcho J, Mayer EA. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonaz B, Baciu M, Papillon E, Bost R, Gueddah N, Le Bas JF, Fournet J, Segebarth C. Central processing of rectal pain in patients with irritable bowel syndrome: an fMRI study. Am J Gastroenterol. 2002;97:654–661. doi: 10.1111/j.1572-0241.2002.05545.x. [DOI] [PubMed] [Google Scholar]
- 5.Castro-Alamancos MA. Dynamics of sensory thalamocortical synaptic networks during information processing states. Prog Neurobiol. 2004;74:213–247. doi: 10.1016/j.pneurobio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Cordero ME, Rodriguez A, Torres R, Valenzuela CY. Human raphe magnus nucleus: a morphometric Golgi-Cox study with emphasis on sex differences. Brain Res Dev Brain Res. 2001;131:85–92. doi: 10.1016/s0165-3806(01)00266-8. [DOI] [PubMed] [Google Scholar]
- 7.Cordero ME, Valenzuela CY, Torres R, Rodriguez A. Sexual dimorphism in number and proportion of neurons in the human median raphe nucleus. Brain Res Dev Brain Res. 2000;124:43–52. doi: 10.1016/s0165-3806(00)00104-8. [DOI] [PubMed] [Google Scholar]
- 8.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature reviews. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 9.Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31:544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez R, Cruz-Morales SE, Carvalho MC, Xavier M, Brandao ML. Sex differences in serotonergic activity in dorsal and median raphe nucleus. Physiol Behav. 2003;80:203–210. doi: 10.1016/j.physbeh.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab. 1991;11:690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- 12.Goodman RL. A quantitative analysis of the physiological role of estradiol and progesterone in the control of tonic and surge secretion of luteinizing hormone in the rat. Endocrinology. 1978;102:142–150. doi: 10.1210/endo-102-1-142. [DOI] [PubMed] [Google Scholar]
- 13.Heinsbroek RP, van Haaren F, Feenstra MG, van Galen H, Boer G, van de Poll NE. Sex differences in the effects of inescapable footshock on central catecholaminergic and serotonergic activity. Pharmacol Biochem Behav. 1990;37:539–550. doi: 10.1016/0091-3057(90)90025-d. [DOI] [PubMed] [Google Scholar]
- 14.Holdcroft A, Sapsed-Byrne S, Ma D, Hammal D, Forsling ML. Sex and oestrous cycle differences in visceromotor responses and vasopressin release in response to colonic distension in male and female rats anaesthetized with halothane. Br J Anaesth. 2000;85:907–910. doi: 10.1093/bja/85.6.907. [DOI] [PubMed] [Google Scholar]
- 15.Holschneider DP, Maarek JM. Brain maps on the go: functional imaging during motor challenge in animals. Methods (San Diego, Calif. 2008;45:255–261. doi: 10.1016/j.ymeth.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins WJ, Becker JB. Sex. In: Whishaw IQ, Kolb B, editors. The behavior of the laboratory rat: a handbook with tests. New York: Oxford University Press; 2004. pp. 307–320. [Google Scholar]
- 17.Ji Y, Murphy AZ, Traub RJ. Sex differences in morphine-induced analgesia of visceral pain are supraspinally and peripherally mediated. Am J Physiol Regul Integr Comp Physiol. 2006;291:R307–314. doi: 10.1152/ajpregu.00824.2005. [DOI] [PubMed] [Google Scholar]
- 18.Ji Y, Tang B, Traub RJ. The visceromotor response to colorectal distention fluctuates with the estrous cycle in rats. Neuroscience. 2008;154:1562–1567. doi: 10.1016/j.neuroscience.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jovanovic H, Lundberg J, Karlsson P, Cerin A, Saijo T, Varrone A, Halldin C, Nordstrom AL. Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. Neuroimage. 2008;39:1408–1419. doi: 10.1016/j.neuroimage.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Kern MK, Jaradeh S, Arndorfer RC, Jesmanowicz A, Hyde J, Shaker R. Gender differences in cortical representation of rectal distension in healthy humans. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1512–1523. doi: 10.1152/ajpgi.2001.281.6.G1512. [DOI] [PubMed] [Google Scholar]
- 21.Kim D, Park D, Choi S, Lee S, Sun M, Kim C, Shin HS. Thalamic control of visceral nociception mediated by T-type Ca2+ channels. Science. 2003;302:117–119. doi: 10.1126/science.1088886. [DOI] [PubMed] [Google Scholar]
- 22.Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41:1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu CL, Wu YT, Yeh TC, Chen LF, Chang FY, Lee SD, Ho LT, Hsieh JC. Neuronal correlates of gastric pain induced by fundus distension: a 3T-fMRI study. Neurogastroenterol Motil. 2004;16:575–587. doi: 10.1111/j.1365-2982.2004.00562.x. [DOI] [PubMed] [Google Scholar]
- 24.Luque JM, de Blas MR, Segovia S, Guillamon A. Sexual dimorphism of the dopamine-beta-hydroxylase-immunoreactive neurons in the rat locus ceruleus. Brain Res Dev Brain Res. 1992;67:211–215. doi: 10.1016/0165-3806(92)90221-h. [DOI] [PubMed] [Google Scholar]
- 25.Mayer EA, Berman S, Chang L, Naliboff BD. Sex-based differences in gastrointestinal pain. European journal of pain (London, England) 2004;8:451–463. doi: 10.1016/j.ejpain.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, Chang L. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Murphy AZ, Suckow SK, Johns M, Traub RJ. Sex differences in the activation of the spinoparabrachial circuit by visceral pain. Physiol Behav. 2009;97:205–212. doi: 10.1016/j.physbeh.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naliboff BD, Berman S, Chang L, Derbyshire SW, Suyenobu B, Vogt BA, Mandelkern M, Mayer EA. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738–1747. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 30.Naliboff BD, Berman S, Suyenobu B, Labus JS, Chang L, Stains J, Mandelkern MA, Mayer EA. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006;131:352–365. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen PT, Holschneider DP, Maarek JM, Yang J, Mandelkern MA. Statistical parametric mapping applied to an autoradiographic study of cerebral activation during treadmill walking in rats. Neuroimage. 2004;23:252–259. doi: 10.1016/j.neuroimage.2004.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- 34.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Elsevier Academic Press; 2005. [Google Scholar]
- 35.Petrovic P, Carlsson K, Petersson KM, Hansson P, Ingvar M. Context-dependent deactivation of the amygdala during pain. J Cogn Neurosci. 2004;16:1289–1301. doi: 10.1162/0898929041920469. [DOI] [PubMed] [Google Scholar]
- 36.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 37.Pinos H, Collado P, Rodriguez-Zafra M, Rodriguez C, Segovia S, Guillamon A. The development of sex differences in the locus coeruleus of the rat. Brain Res Bull. 2001;56:73–78. doi: 10.1016/s0361-9230(01)00540-8. [DOI] [PubMed] [Google Scholar]
- 38.Preuss TM. Do Rats Have Prefrontal Cortex - The Rose-Woolsey-Akert Program Reconsidered. J Cogn Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Stam R, van Laar TJ, Wiegant VM. Physiological and behavioural responses to duodenal pain in freely moving rats. Physiol Behav. 2004;81:163–169. doi: 10.1016/j.physbeh.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 40.Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. NeuroImage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 41.Tache Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thevenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- 43.Tomasi D, Chang L, Caparelli EC, Ernst T. Sex differences in sensory gating of the thalamus during auditory interference of visual attention tasks. Neuroscience. 2008;151:1006–1015. doi: 10.1016/j.neuroscience.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 45.Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 46.Valentino RJ, Miselis RR, Pavcovich LA. Pontine regulation of pelvic viscera: pharmacological target for pelvic visceral dysfunctions. Trends in pharmacological sciences. 1999;20:253–260. doi: 10.1016/s0165-6147(99)01332-2. [DOI] [PubMed] [Google Scholar]
- 47.Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 48.Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- 49.Vogt BA, Peters A. Form and distribution of neurons in rat cingulate cortex: areas 32, 24, and 29. J Comp Neurol. 1981;195:603–625. doi: 10.1002/cne.901950406. [DOI] [PubMed] [Google Scholar]
- 50.Wang Z, Bradesi S, Maarek JM, Lee K, Winchester WJ, Mayer EA, Holschneider DP. Regional brain activation in conscious, nonrestrained rats in response to noxious visceral stimulation. Pain. 2008;138:233–243. doi: 10.1016/j.pain.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong SW, Kimmerly DS, Masse N, Menon RS, Cechetto DF, Shoemaker JK. Sex differences in forebrain and cardiovagal responses at the onset of isometric handgrip exercise: a retrospective fMRI study. J Appl Physiol. 2007;103:1402–1411. doi: 10.1152/japplphysiol.00171.2007. [DOI] [PubMed] [Google Scholar]
- 53.Yang SW, Follett KA, Piper JG, Ness TJ. The effect of morphine on responses of mediodorsal thalamic nuclei and nucleus submedius neurons to colorectal distension in the rat. Brain Res. 1998;779:41–52. doi: 10.1016/s0006-8993(97)01053-6. [DOI] [PubMed] [Google Scholar]
- 54.Yang SW, Follett KA, Piper JG, Ness TJ. The effect of morphine on responses of nucleus ventroposterolateralis neurons to colorectal distension in the rat. Brain Res Bull. 1999;48:609–614. doi: 10.1016/s0361-9230(99)00042-8. [DOI] [PubMed] [Google Scholar]
- 55.Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol. 1991;303:355–374. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]