Abstract
Human pluripotent stem cells, including embryonic and induced pluripotent stem cells, hold enormous potential for the treatment of many diseases, owing to their ability to generate cell types useful for therapeutic applications. Currently, many stem cell culture propagation and differentiation systems incorporate animal-derived components for promoting self-renewal and differentiation. However, use of these components is labor intensive, carries the risk of xenogeneic contamination and yields compromised experimental results that are difficult to duplicate. From a biomaterials perspective, the generation of an animal- and cell-free biomimetic microenvironment that provides the appropriate physical and chemical cues for stem cell self-renewal or differentiation into specialized cell types would be ideal. This review presents the use of natural and synthetic polymers that support propagation and differentiation of stem cells, in an attempt to obtain a clear understanding of the factors responsible for the determination of stem cell fate.
Keywords: biomaterials, differentiation, extracellular matrix, microenvironment, pluripotent, polymers, self-renewal, stem cells
Over the last decade, human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) [1,2] and, more recently, induced pluripotent stem cells (iPSCs) [3-5] have garnered a lot of attention owing to their inherent self-renewal and pluripotent capabilities. The pluripotent nature of these cells, that is, the ability to differentiate into all somatic cell types, has opened avenues for potential stem cell-based regenerative therapies, for development of drug discovery platforms and as unique in vitro models for the study of early human development. To meet specific needs for cell-based therapies, some of the key research questions that need to be addressed include:
The elucidation of molecular mechanisms that determine the stem cell fates of self-renewal, differentiation, apoptosis and quiescence;
Bioprocessing strategies to generate bankable and uniform populations of undifferentiated cells.
The key variables in the development of bioprocessing strategies for propagation of pluripotent stem cells involve medium formulations and biomaterials as substrates. In this review, we present an overview of the potential for different biomaterials in determining pluripotent stem cell fate relating to self-renewal and differentiation. Biomaterials have been investigated extensively as substrates for cell propagation, scaffolds for various organs and as delivery vehicles for drugs, growth factors and cells in many regenerative biomedical paradigms. One of the potential benefits of using biomaterials for pluripotent stem cell propagation is the elimination of direct coculture with a supportive feeder layer that has been an integral component of pluripotent stem cell culture. This removes the risk of contamination with xenogeneic pathogens and reduces variability in experimental outcomes due to feeder layer contribution. In addition, specialized biomaterials that include appropriate chemical (incorporation of growth factors) and physical (topographic features) modifications, have contributed to successful differentiation of embryonic stem cells (ESCs) to multiple cell types. In this review, we attempt to present the knowledge acquired from the integration of biomaterial engineering and pluripotent stem cell biology to chalk out standardized routes for stem cell self-renewal and/or directed differentiation.
Mouse embryonic stem cells (mESCs) were one of the first ESC types derived from the inner cell mass of pre-implantation blastocysts, and cultured in direct coculture conditions on mitotically inactivated mouse embryonic fibroblast (MEF) feeder layers [6]. This culture methodology was primarily adopted to provide the cells with the appropriate conditions conducive to self-renewal rather than differentiation. This methodology has since been employed by many laboratories to isolate and culture hESCs and stem cells from other species. In primates, ESCs were first isolated from rhesus monkeys and subsequently from humans, and maintained for extended periods without undergoing differentiation in direct coculture with MEFs, with removal of MEF feeders in the culture system triggering spontaneous differentiation into many somatic cell types [2,7].
From a cell-based regenerative therapy perspective, the use of MEFs for the propagation of hPSCs is recognized as a potential hurdle by many researchers. It is widely acknowledged that mice carry pathogenic parasites, bacteria and viruses, as well as endogenous retroviruses, in their genome. Some endogenous retroviruses even have the capability to infect foreign species and have been shown to cause diseases, such as leukemia in immunosuppressed primates [8]. The use of MEFs thus poses a potential risk of contamination of the hPSCs. Researchers in the stem cell community are thus presented with opportunities and challenges towards the development of hPSC propagation systems that do not involve the use of other species-derived feeders.
Hydrogels have been used in mammalian cell culture for over 30 years, because of their high water absorbability and tissue-like texture. Polymethylmethacrylate and polylactic glycolic acid (PLGA) are examples of a few biocompatible materials used in various tissue culture-based applications [9,10]. In ESC research, polymeric substrates have largely been used as carrier systems for lineage-specific differentiated cells. Instances of the use of polymeric-based biomaterials employed in maintenance of self-renewal capabilities of ESCs are few in number. The use of a polymer-based substrate that can be synthesized with ‘off-the shelf’ constituents for hPSC culture has several advantages, which include:
Economic feasibility
Reduction in the labor involved to maintain an additional cell line as feeders
Elimination of the source of potential xenogeneic contamination
It is expected that advances in biomaterial-based approaches will contribute immensely to standardization of culture methodologies, leading to development of bioprocesses for hPSC propagation and differentiation.
Substrates for pluripotent stem cell self-renewal
The need for an unlimited supply of starting hPSC populations for current research and future therapeutic applications fuels this area of research. In this section, we address the application of different substrates that are both feeder-dependent and -independent systems and have contributed to successful long-term propagation of pluripotent stem cells in their undifferentiated state.
Feeder-dependent propagation systems
Mitotically inactivated MEFs have been the traditional ‘feeder-layer’ of choice ever since the isolation and propagation of mESCs [6]. They have served as ideal substrates as they provide appropriate physical and chemical cues conducive to the maintenance of self-renewal in pluripotent stem cell populations. In a few cases, such as those involving rat ESCs and American mink ESC propagation, it was demonstrated that the best feeder layer involved fibroblasts derived from the host animal [11,12]. However, the isolation and propagation of several rodent, nonrodent ESCs [13-15] and nonhuman primate ESCs [7,16] required MEFs as feeders for long-term propagation. MEFs have also been used as the feeder of choice for culture systems that were initially developed for propagating hESCs and were shown to be capable of maintaining hESCs in their undifferentiated state for more than 250 population doublings [1,2]. The microenvironment suitable for successful self-renewal and proliferation of ESCs on MEFs comprise unidentified physical cues based on matrix deposited and chemical cues based on nutrients released into the culture medium. However, the use of animal cells as a substrate poses risks of xenogeneic contamination by animal pathogens, which in turn could be transmitted to patients. Recent findings have revealed the presence of a nonhuman carbohydrate moiety on the surface of hESCs with the potential of eliciting an immune reaction from humans [17].
Extensive research to propagate hESCs in culture conditions with reduced or zero animal-derived products are being actively pursued. One of the earliest advances towards a xeno-free environment was the elimination of serum, specifically fetal bovine serum, in hESC cultures. Fetal bovine serum has been extensively used in mammalian cell cultures; however, the composition of serum is not entirely known and different batches vary in their capabilities to support undifferentiated proliferation. A serum replacement, knockout serum replacement (KSR) [201], optimized for mESC cultures, was successfully introduced in hESC cultures [18], and is now routinely used as a serum substitute in many laboratories to propagate hESCs [19,20]. In an attempt to make the hESC culture conditions animal-free, several human fibroblast cell lines have been tested and proven to be suitable feeders. Propagation conditions involving human foreskin fibroblasts as feeders in KSR-supplemented growth medium have been used to maintain hESCs for 70 passages [21], and in the derivation of new hESC lines [19]. An advantage to using human foreskin fibroblasts as a feeder layer is in its ability to be continuously cultured for approximately 42 passages, unlike MEFs, which senesce in five to seven passages. There was no significant difference found in the propagation of hESCs on different lines of foreskin fibroblasts obtained from several newborns, leading to the conclusion that the source of the foreskin fibroblasts does not affect the undifferentiated propagation of hESCs [22,23].
Other human fibroblast feeders tested included fetal skin and muscle cells, adult fallopian tubal epithelial cells, adult skin, muscle, glandular and stromal endometrial cells, and commercially available fetal skin, lung and neonatal foreskin cell lines. One general observation made by the investigators was that fetal lines were more supportive of long-term hESC propagation compared with adult lines [24,25]. Examples of other human feeders tested for hESC propagation were human marrow stromal cells [26], human adult uterine endometrial cells, human adult breast parenchymal cells, embryonic fibroblasts [27] and human placental fibroblasts [28,29]. To test the efficiency of hESC-derived fibroblasts as possible feeders, two different hESC lines (NCLI and H1) were grown on their autogenic as well as allogeneic feeders from fibroblast derivatives. These studies demonstrated that hESC-derived feeders were capable of maintaining self-renewal in both the parent and a foreign hESC line [30]. Cynomolgus monkey ESCs maintained on human placental feeder layers, amniotic epithelial plate cells or chorionic plate cells remained pluripotent for at least 18 passages [31]. These studies demonstrated that regardless of the source of the feeder layer, certain cell types were more capable of maintaining self-renewal capabilities of pluripotent stem cells than others. Detailed analyses of the similarities and differences between such feeders will provide valuable insights into key factors that maintain self-renewal capabilities and prevent differentiation of pluripotent stem cells.
Feeder-independent propagation systems
A few groups have demonstrated that hESCs can be maintained without feeders or feeder-conditioned medium, relying on growth medium supplemented with high concentrations of basic FGF. Many of these studies helped ascertain the molecular mechanisms involved in hESC self-renewal, such as the central role played by inhibition of components of the BMP signaling pathway [32,33]. Many other studies have explored the role of ‘natural biopolymers’, such as complex protein gels: Gelatin, Matrigel™ and naturally derived polymers; alginate, agarose and hyaluronic acid (HA) in the maintenance of pluripotent stem cells. Other approaches have focused on replacing natural materials with synthetic polymers that permit controlled modifications and incorporation of a synergy of physical, chemical and mechanical cues for self-renewal of pluripotent stem cells.
Natural biopolymers for pluripotent stem cell self-renewal
Gelatin is thermally denatured collagen derived from animal skin and bones, and has long been used as a promoter of cell adhesion and proliferation in various cell lines [34-36]. MESCs, in the absence of a feeder layer, have been maintained on gelatin-coated dishes and growth medium supplemented with IL-6 family member cytokine leukemia inhibitory factor (LIF) for extended periods [37-39]. The binding of LIF receptor β/gp130 heterodimer and the activation of the JAK/STAT3 signaling pathway has been implicated in mESC self-renewal [40]. As a cost-effective alternative to periodic LIF supplementation, propagation of mESCs on LIF-immobilized substrates, such as photoreactive gelatin, demonstrated that mESCs maintained pluripotent characteristics even after 6 days of culture [41]. Another study explored some of the molecular mechanisms involved in mESC self-renewal, in which the cells were grown on gelatin-coated polyamide nanofibers that had been electrospun onto glass coverslips. The cells had enhanced proliferation and self-renewal capabilities when compared with cells grown on gelatin-coated glass coverslips, and these effects were correlated to an increase in the expression of Nanog, the activation of the small GTPase Rac, and the activation of the PI3K pathway. The relationship between the PI3K pathway and regulation of STAT3 and ERK make it likely that micro-environmental cues are a major factor in control of mESC self-renewal [42,43].
In an attempt to move away from MEF-based feeder layers, an extracellular matrix (ECM)-based substrate, Matrigel, has been used to support hESC cultures. Matrigel is an ECM protein-based gel derived from the basement membrane of Engelberth—Holm—Swarm mouse sarcoma. Engelberth—Holm—Swarm tumor basement membrane has been found to be rich in collagen, laminin, heparin sulfate proteoglycans and growth factors such as TGF-β, EGF and FGF [44-46]. Extensive studies on the efficacy of Matrigel and its individual components, such as laminin, collagen IV and fibronectin, have been investigated for hESC propagation. Collagen IV and fibronectin did not individually support hESC propagation, whereas laminin was successful in maintaining long-term undifferentiated hESC cultures. However, all of these hESCs were propagated in MEF-conditioned medium (MEF-CM), with the absence of MEF-CM leading to extensive spontaneous differentiation of the hESCs [47]. Another study demonstrated the successful maintenance of hESC cultures in growth medium containing KSR supplemented with high concentrations of bFGF, TGF-β1 and LIF, on a fibronectin matrix [20]. The premise for the addition of these growth factors was that:
Basic FGF had been used to maintain hESC cultures [18];
TGF-β1 found in Matrigel supported hESC proliferation [47];
Fibronectin promotes cell adhesion [48];
LIF activates the JAK/STAT3 pathway and is sufficient for the self-renewal of mESCs [37-39,49], and might elicit the same response in hESCs.
However, in hESCs it was found that neither the addition of LIF nor the activation of the STAT3 pathway contributed to self-renewal [2,50].
Apart from the use of Matrigel, very few studies have demonstrated the use of ECM-based substrates for hESC maintenance, with one study reporting the use of human serum as a substrate to maintain hESCs for over 27 passages [51]. Preliminary data from our laboratory have also demonstrated the usefulness of certain fibroblast-derived ECMs for long-term propagation of hESCs and iPSCs. In MEF feeder layer-based culture systems, it has been observed that ESCs and iPSCs push aside the feeders to settle and expand on the ECM laid out on the tissue-culture dish. To further study this phenomenon and investigate whether deposited ECM is sufficient for hPSC propagation, acellular substrates derived from mouse feeder layers were examined. Our studies demonstrate the ability of acellular MEF substrates to support propagation of WA09 and BG01v hESCs for extended periods of time (Figure 1). A recent study that is extremely promising has focused on the usefulness of HA-based hydrogels for propagation of hESCs [52]. HA is a nonsulfated linear polysaccharide of (1-β-4) D-glucuronic acid and (1-β-3) N-acetyl-D-glucosamine found largely in the ECM of undifferentiated cells during early embryogenesis, and shows reduced expression on differentiation [53]. HA is also present in the body as a major constituent of the ECM in connective, neural and epithelial tissue. Large-scale microbial production of HA has allowed for the use of this polymer in different therapeutic applications. Such naturally derived polysaccharides are also considered as synthetic ECM hydrogels, which can substitute natural ECM. The premise for the use of ECM-based protein hydrogels is to create a microenvironment that mimics the physiological milieu of the inner cell mass, by acting as a host that provides the resident cells with 3D architecture and mechanical support for structured organization and easy diffusibility for nutrients and metabolites [54-56]. hESCs that were encapsulated within the HA construct in MEF-CM maintained their undifferentiated state for 20 days. The cells were released from the hydrogel by treating the constructs with hyalurodinase to digest the HA hydrogel; the cells showed no adverse effects to extended exposure (24 h) to the enzyme. Although natural biopolymers such as polysaccharides, ECM-based substrates and other hydrogels hold great promise as biomaterials for pluripotent stem cell propagation and presenting cells with familiar microenvironments, new strategies are required for greater control to promote uniform proliferation of stem cells in their undifferentiated state.
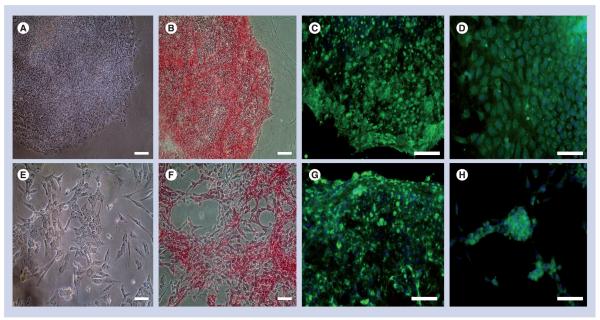
Figure 1. Pluripotent characteristics of WA09 (top panel) and BG01v (bottom panel) human embryonic stem cells are maintained on naturally derived mouse embryonic fibroblast-secreted extracellular matrix-based substrates.
Phase contrast micrographs (A & E) depict the presence of undifferentiated cells that exhibit high nuclear:cytoplasmic ratio. Cells also exhibit positive expression for alkaline phosphatase activity (B & F), cell surface marker stage-specific embryonic antigen 4 (C & G) and transcription factor OCT 4 (D & H). Scale bar = 100 μm.
Synthetic biomaterials for pluripotent stem cell self-renewal
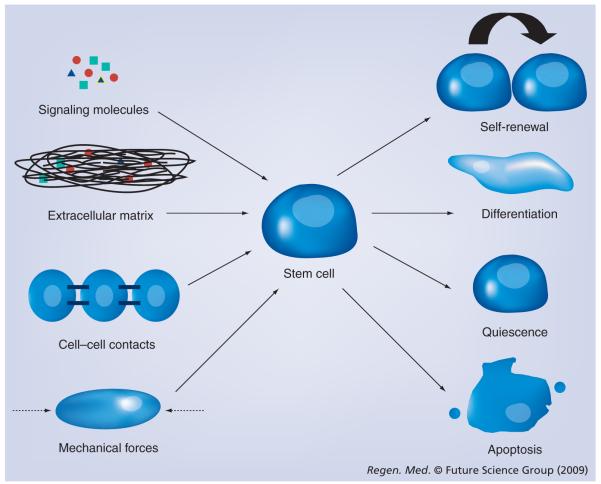
Replacing natural materials with synthetic polymers allows for controlled modifications, such as the addition of relevant embryological growth factors. It is useful to note that mammalian cell culture propagation has traditionally been conducted on flat, rigid substrates. However, the 3D microenvironments that the cells encounter in the body have a combination of physical, chemical and mechanical cues (Figure 2) [57,58]. Consequently, the ease with which polymers can be engineered into complex 3D structures is a key benefit of using polymers instead of feeder cells in pluripotent propagation systems. Poly(α-hydroxy esters) and polyurethane are two examples of synthetic polymers that have been used as part of pluripotent stem cell propagation systems. Poly(α-hydroxy esters) with enhanced surface hydrophilicity and roughness properties [59], and fluorinated hydroxyapatites have been identified as substrates that can maintain mESCs as colonies [60]. Poly(hydroxyethyl methacrylate) (p[HEMA]), a widely used nondegradable biomaterial, is another synthetic polymer that supports mESC proliferation [61,62]. mESCs were maintained in their undifferentiated state for a limited time period of 4 days, which was confirmed by replating the cells extracted from the hydrogel slabs onto gelatincoated dishes. Cells that were allowed to proliferate further in the p(HEMA) slabs formed large clusters (6–8 days) and showed reduced expression of pluripotent markers on replating compared with the 4-day cultures on gelatin-coated dishes.
Figure 2. Components of the cellular microenvironment in vitro need to mimic in vivo conditions.
Synergy of biochemical cues such as signaling molecules and growth factors; biophysical cues such as substrates; mechanical cues such as hemodynamic forces, shear forces and periodic strains; and cell—cell interactions are required for determination of the fate of pluripotent stem cells.
Mouse ESCs grown on nonwoven polyester fabrics from polyethylene terphthalate (PET) initially exhibited high expression of the pluripotent markers alkaline phosphatase and stage-specific embryonic antigen-1 [63]. However, reduced immunoreactivity to stage-specific embryonic antigen-1 was seen in mESCs after 72–96 h, indicating that PET substrates did not facilitate long-term undifferentiated propagation and would be best suited for differentiation studies rather than for maintaining self-renewal. However, another study involving fibrous PET matrices was shown to sustain long-term growth of mESCs [64], where matrices with pores 30–60 μm wide caused more rapid cell growth than pores of 60–130 μm in width. This was attributed to greater contact between the cells and PET fibers within smaller pores, leading to increased attachment and better support. Large pores allowed cells to aggregate more easily, which induced mESC differentiation. Gelatin coating of the polymers did not improve mESC proliferation and self-renewal, though such effects could be attributed to blocking and partial destruction of the pore structure of the PET matrices. One drawback of such a 3D fibrous matrix construct for ESC culture is poor oxygen supply at high cell densities. The effects of poor oxygen transfer could be seen when cell proliferation slowed and cellular death was seen at or near day 14.
A 3D porous polymer scaffold of the elastomer poly-(glycerolcosebacate)-acrylate (PGSA) supports undifferentiated hESC culture for 1 week [65]. PGSA is an elastic hydrogel that is known to elicit low inflammation in response to in vivo transplantation [66,67] and allows for future applications as a biocompatible and biodegradable scaffold. Polyurethane microwells coated with Matrigel demonstrated the ability to maintain hESCs for a few weeks without the need for routine passaging. The cells grown in these conditions maintained their pluripotency and differentiated into the three germ layers when cultured in suspension. The use of mouse-derived Matrigel as a substrate indicated that an ECM modification of the synthetic polymer was essential for propagating hESCs [68]. A synthetic 3D ECM-based semi-interpenetrating polymer network of poly(N-isopropyl acrylamide-co-acrylic acid) was reported to support short-term hESC self-renewal. Modification of the hydrogel polymer with the incorporation of synthetic RGD peptides to present the cells with adhesion sites improved cell attachment, allowed the formation of colonies and maintained pluripotency for a limited period of 5 days [69]. Recent studies have also investigated the feasibility of culturing hESCs on microcarriers in feeder-free, 3D suspension culture [70,71]. These studies suggest that suspension-based propagation of hESCs on microcarriers may provide unique opportunities for bulk hESC production and storage.
The culture of pluripotent stem cells on polymeric surfaces with or without immobilized proteins opens up avenues for identification of synthetic microenvironments that can be easily synthesized and modified to form scaffolds that support the differentiation of ESCs into highly ordered 3D structures. In ESC studies, biomaterials have been frequently used to direct differentiation to specific lineages, in the presence of appropriate growth factors. However, maintenance of its undifferentiated state has proven to be a challenge. The use of native and synthetic polymers in promoting ESC self-renewal is still in its nascent phase and has great potential, as demonstrated by the various studies conducted to date (Table 1). Combinatorial and microarray approaches have been adopted recently in high-throughput screening of materials to control ESC fate on polymer surfaces. Such high-throughput approaches also hold much promise in 3D screening of libraries of biofunctional groups such as morphogenetic proteins incorporated within synthetic 3D materials. A vast number of possible combinations of polymers, surface treatments, surface-bound ligands and other substrate modifications need to be investigated in ESC propagation systems.
Table 1.
Summary of substrates used for propagation of mouse and human pluripotent stem cells.
| Substrate type | Cell source | Substrate | Timeline of study | Ref. |
|---|---|---|---|---|
| Feeder layer based | hESCs | Human foreskin fibroblasts | >70 passages | [21–23] |
| Fetal skin cells | 20 passages | [24,25] | ||
| Fetal muscle cells | >50 passages | |||
| Adult skin fibroblasts | >30 passages | |||
| Adult muscle fibroblasts | >30 passages | |||
| Commercially available fetal skin fibroblasts | >25 passages | |||
| Adult fallopian tubal epithelial fibroblasts | >20 passages | |||
| Adult marrow cells | 13 passages | [26] | ||
| Human adult uterine endometrial cells | 90 passages | [27] | ||
| Human adult breast parenchymal cells | 50 passages | |||
| Human embryonic fibroblasts | 80 passages | |||
| Human placental fibroblasts | >25 passages | [28,29] | ||
| hESC-derived fibroblasts | 44 passages | [30,154] | ||
| 30–52 passages | ||||
| Natural substrates | mESCs | LIF immobilized on gelatin | 6 days | [41] |
| Gelatin-coated polyamide nanofibers | 3 days | [155] | ||
| hESCs | Matrigel™ | ∼130 passages | [47] | |
| Human serum | >27 passages | [51] | ||
| Mouse embryonic fibroblasts ECM | >30 passages | [156] | ||
| Hyaluronic acid | 20 days | [52] | ||
| Synthetic substrates | mESCs | Hydroxyapatite polymers | 48 h (time required for colonization) |
[59,60] |
| Poly(hydroxyethyl methacrylate) | 4 days | [61] | ||
| LIF-immobilized PET fibers | 72–96 h | [63] | ||
| Gelatin-immobilized PET fibers | 15 days | [64] | ||
| hESCs | Poly-(glycerolcosebacate)-acrylate | 1 week | [65] | |
| Polyurethane microwells | >21 days | [68] | ||
| Poly(N-isopropyl acrylamide-co-acrylic acid) SIPN | 5 days | [69] | ||
ECM: Extracellular matrix; hESC: Human embryonic stem cell; LIF: Leukemia inhibitory factor; mESC: Mouse embryonic stem cell; PET: Polyethylene terphthalate; SIPN: Semi-interpenetrating polymer network.
The controlled microenvironment that is synthesized to support ESC adhesion and propagation is heavily dependent on the physical and chemical cues that will be incorporated. Studies have demonstrated that presentation of various cell adhesion ligands, introduction of roughness and patterned features can influence stem cell fate by stimulating cell attachment, proliferation and differentiation [72,73]. Patterned surfaces of hyaluronan and poly-L-lysine were shown to maintain mESCs in their undifferentiated state within a spatially controlled culture system with fibroblasts. In these studies, hyaluronan was lithographically patterned on glass surfaces to create parallel strips alternating with glass; fibronectin was selectively deposited on the glass, with poly-L-lysine on hyaluronan permitting differential cell adhesion on the two surfaces. Fibroblasts were grown on glass surfaces treated with fibronectin and mESCs on poly-L-lysine surfaces. Such systems have a potential advantage over random coculture, where mixing of feeder cells with ESCs has to be avoided [74]. Comparative studies of superporous polymers with varying pore sizes, surface charges and hydrophobicity characteristics have shown that substrates with smaller pore sizes, in the range of 30 μm wide, supported proliferation of mESCs better than pores of larger dimensions (100 μm). Neutral or cationic substrates as compared with a negatively charged substrate, and a hydrophobic surface compared with a hydrophilic one allowed for greater mESC adhesion and aggregation for colony formation [61,62]. The effect of mESC culture surface roughness on poly(α-hydroxy esters) substrates was studied, with results demonstrating that, within a certain range, an increase in roughness with a corresponding reduction in hydrophilicity enhanced mESC attachment and proliferation [59]. These informative studies demonstrate that surface texture modifications can play a role in influencing the self-renewal properties of stem cells.
A recent study has demonstrated the potential of high-throughput arrays in studying biomaterial—ESC interactions and provided insights into the role of specific polymers in promoting cell attachment, proliferation and differentiation [75]. Another combinatorial approach focused on the effects of 18 different laminin-derived peptides on proliferation and self-renewal of hESCs. Self-assembled monolayers were designed, which allowed the formation of array elements of well-defined shapes and sizes. Five out of the 18 peptides tested were effective in maintaining the undifferentiated state of hESCs for 5–7 days. Further characterization of the five peptides with a nanofiberbased peptide (RNIAEIIKDI)-amphiphile containing 3D-hydrogel demonstrated their potential in promoting proliferation and maintaining undifferentiated hESCs [76]. A recent research development involving transductions of somatic cells to induce pluripotency has been heralded as one of the major breakthroughs in stem cell research; primarily due to its potential to advance customized cell-based therapies and the evasion of embryo-related ethical debates [3-5]. Although the use of synthetic materials for promoting iPSC fate from somatic cells has not been explored, the use of synthetic polymers as gene or protein delivery vehicles for inducing pluripotency is a definite possibility. Recent studies have demonstrated the potential of poly(amino esters)-based biodegradable nanoparticulate vectors for gene delivery to efficiently transfect ESCs with low cytotoxicity [77,78]. These methodologies have significant implications in the generation of iPSC lines that are free of exogenous viral additions to the genome. Ultimately, the ideal feeder- and animal-free culture environment to maintain stable hESC cultures or to induce pluripotency from somatic cells would depend on the development of completely synthetic substrates.
Substrates for pluripotent stem cell differentiation
The excitement in the biomedical community with regard to pluripotent stem cells centers on their ability to differentiate into any somatic cell type. This unique property makes it possible to derive rare tissue types in unlimited quantities, which would be of immense use in areas such as in vitro drug screening and regenerative medicine. Differentiation of ESCs occurs spontaneously either in prolonged cultures or in the absence of feeder layers, leading to populations consisting of multiple cell types from many different lineages. A more controlled method would be to induce the formation of embryoid bodies (EBs), which are 3D ESC aggregates grown in suspension or in an environment that does not support cell adhesion [79]. Heterogeneous subpopulations that are enriched for a cell type of interest are then often isolated and replated [80-85], with induction of differentiation by the addition of growth factors to the medium [86,87]. Thus, directed differentiation involves:
Exposure of ESCs to growth factors that mimic natural pathways in embryonic development
Coculture with suitable cytokine-secreting feeders
Replating enriched populations derived from EBs
Cell types that have been derived through use of EBs include those of chondrogenic, adipogenic, neurogenic, cardiogenic, hematopoietic and myogenic lineages. However, thus far, the resulting populations have been largely random in their phenotype and organization. Consequently, there exists a need for protocols that can differentiate ESCs to highly specific derivatives with increased reproducibility and capacity for scale-up. The challenges in directed differentiation protocols are a direct result of the basic lack of understanding of the mechanisms involved in lineage specification. However, advances in biomaterial-based approaches have contributed to development of feeder-dependent and feeder-free (natural and synthetic polymers) culture systems that have contributed to differentiation into multiple cell types from the three different germ layers (ectoderm, endoderm and mesoderm).
Endodermal differentiation
Of the several cell types that originate from the endoderm, differentiation strategies for hepatic and pancreatic cell types have been extensively developed. Induction of hepatic and pancreatic differentiation from ESCs has garnered a lot of attention due to possible therapeutic applications for diseases such as liver damage and diabetes. Studies of the differentiation of ESCs into the pancreatic lineage largely involves formation of EBs followed by a sequential and elaborate exposure to endodermal lineage-promoting growth factors and ECM proteins [88,89]. Currently, there is no significant report that points to the use of polymeric substrates for induction of pancreatic differentiation of mouse or human ESCs. However, there have been reports published on the use of both feeder layers and polymers in differentiation towards the hepatic lineage. Coculture of mESCs with primary adult rat hepatocytes has been shown to induce hepatocyte differentiation, with enhancement upon addition of hepatotrophic growth factors [90]. Additional studies for hepatic differentiation have focused on the use of natural polymers, such as collagen and alginate [91]. Alginate is a biocompatible hydrophilic viscous gum derived from algae and has been used in various biomedical applications due to its hydrogel and ECM gel-like properties [92,93]. The use of alginate beads for EB formation allows for scale-up of hepatocyte derivation and acts as a source of cells for a bioartifical liver system [94]. Naturally derived alginate beads were used to form EBs from mESCs, followed by replating of dissociated cells in medium supplemented with appropriate growth factors inducing hepatocyte differentiation.
Synthetic polymers such as polyurethane have also been used to promote hepatogenesis. For example, a polyurethane foam (PUF) as part of a hepatocyte culture system was presented as a hybrid artificial liver [95], with subsequent replacement of hepatocytes with ESCs as a cell source within this module. When mESCs were placed in the porous PUF, they aggregated into spherical multicellular structures and on further exposure to a cocktail of growth factors, differentiated into mature hepatocytes, and expressed endoderm-specific late-stage markers of hepatic differentiation [96]. An improvement to the culture system for hepatic differentiation was later introduced by the same group of researchers and included a bioreactor consisting of a PUF block with capillaries to aid growth medium flow, to effect hepatic lineage induction in mouse and cynomolgus monkey ESCs [97,98].
Hepatocytes have commonly been derived from hESCs by either replating cells from EBs or by sequential exposure of ESCs to different growth factors [99-101]. Similar to mESCs, natural ECM-based polymers have been used in hepatic differentiation of hESCs. A comparative study between 2D and 3D culture systems that utilized collagen demonstrated that 3D collagen scaffolds induced specification in 5-day-old EBs. Furthermore, addition of exogenous growth factors to 3D collagen scaffold allowed for better hepatocyte differentiation than collagen-coated 2D dishes [102]. Unlike mESCs, no reported studies of synthetic polymers as substrates in hESC hepatogenesis have been identified.
Mesodermal differentiation
Derivatives of the mesoderm that have been successfully differentiated from ESC cultures include cardiomyocytes, cells of hematopoietic lineage, endothelial cells and germ-line cells [103-109]. The utilization of biomaterials as part of differentiation strategies for specialized cells from the mesodermal lineages — hematopoietic, osteogenic, chondrogenic and adipogenic — have been extensively studied. Feeder-dependent and feeder-free systems have been extensively used in hematopoietic differentiation of mESCs. Cells of the hematopoietic lineage were the first reported cell types derived by direct differentiation of mESCs using a feeder layer, which included OP9 cells [110] and RP010 [111]; bone marrow stromal cell lines in growth medium conditioned by fetal liver stromal cells, supplemented with IL-6.
Natural polymers, such as Matrigel, fibrin, dextran and collagen, have been used as substrates for differentiation of mESCs to both the hematopoietic and endothelial lineage. Researchers achieved hematopoietic differentiation of mESCs by culture on collagen type IV, through a chain of intermediate differentiation steps, with mESCs first forming cells from the proximal lateral mesoderm, followed by formation of hemoangiogenic progenitors, differentiation into hematopoietic precursors and ultimately mature blood cells. This study also identified the progression of lineage commitment for hematopoietic precursor cells and noted that hemoangiogenic progenitor constituted a critical stage for the divergence of the endothelial and hematopoietic lineage [112,113]. Differentiation of mESCs on collagen IV substrate was later adapted to generate a pure endothelial progenitor population [114,115]. However, collagen-based substrates were found to be inefficient in differentiating rhesus monkey ESCs to endothelial cells [116]. The same study also demonstrated that ESCs required endogenous matrix for survival and function, even when grown on collagen or extracellular protein-rich Matrigel.
Endothelial and hematopoietic differentiation of ESCs have also been demonstrated within 3D fibrin constructs [117]. In a specific study, researchers demonstrated that fibrin polymers reinforced with poly(ethylene glycol) (PEG), promoted a high expression of the marker vascular endothelial-cadherin in encapsulated mESC-derived EBs. It has been postulated that PEGylation of fibrin blocks antigens, resulting in a reduction of cell—matrix interactions, as fibrin gels have the capacity to engage cell-adhesion molecules [118] involved in modulating differentiation in the EB [119]. 3D dextran constructs modified with bioactive ECM molecules have also been used to generate EBs from mESCs. Specifically, addition of VEGF within the dextran constructs yielded cells with increased expression of endothelial markers KDR/Flk-1 and decreased expression of ectodermal and endodermal markers. These cells also showed lower ectodermal (Nestin) and endodermal (α-fetoprotein) marker expression, indicating a preference for mesodermal differentiation of ESCs within the VEGF—dextran culture environment. Further endothelial differentiation was achieved by removing the cells from the hydrogels and propagating on gelatin-coated dishes in endothelial differentiation medium [120]. Another study that further highlighted the importance of ECM components in the differentiation of mESC-derived EBs involved the used of 3D constructs that consisted of collagen-based semi-interpenetrating polymer networks (SIPNs) with varying concentrations of fibronectin and laminin [121]. EBs differentiating within the fibronectin-loaded SIPNs formed cord-like structures indicative of endothelial differentiation, while laminin-loaded SIPNs produced beating cells that provided evidence of increased differentiation towards cardiomyocytes.
Synthetic polymers have also been used to direct differentiation of ESCs to hematopoietic cells based on use of Cytomatrix®, a porous tan-talum-based scaffold synthesized using chemical vapor deposition of metals onto an open-pore carbon scaffold, creating a mechanically strong and highly porous scaffold. EBs generated in this scaffold produced hematopoietic progenitor cells with a greater efficiency, formed more hematopoietic colonies and exhibited greater propensity to produce dendritic cell-like myeloid cells than EBs made on traditional 2D culture dishes. Dynamic culture conditions using spinner flask technology increased the efficiency of hematopoietic differentiation within the scaffolds, with EBs within the scaffolds less likely to aggregate when covered with an ECM coating. These studies demonstrated that differentiation within a 3D micro-environment enhanced ECM production, which in turn increased cell—cell and cell—substrate interactions and contributed to more efficient hematopoietic differentiation [117,122].
Recent studies that have focused on differentiation of hESCs to hematopoietic cells have utilized human feeders and other natural polymers. Direct coculture of hESCs with stromal cells from mouse hematopoietic tissue, bone marrow cell line S17 or a yolk sac endothelial line C166 induced differentiation into the hematopoietic lineage [123]. An example of the application of natural polymers in mesodermal differentiation involved a study that compared three models for differentiation: 2D culture conditions, EBs formed within a polymeric (alginate) scaffold and a slow-turning lateral vessel bioreactor. Undifferentiated hESCs seeded into the porous scaffolds formed EBs similar to those produced by a slow-turning lateral vessel bioreactor, in that they were well rounded and did not form aggregates. However, alginate scaffolds induced vascular differentiation to a greater extent than either the 2D culture plates or the bioreactor [124]. Unlike mESCs, no reported studies of synthetic polymers as substrates in hESC differentiation to hematopoietic and endothelial cells have been identified.
Feeder layers of similar lineage have been shown to support and induce osteogenic differentiation in mESCs and hESCs. For example, fetal murine osteoblasts with mESCs [81] and human periodontal ligament fibroblasts with hESCs have been used in coculture systems to enhance osteogenic differentiation [125]. The natural polymer alginate was initially postulated to support chondrogenic proliferation [126,127], but studies demonstrated that EBs formed from mESCs encapsulated within an alginate construct with dexamethasone supplementation showed no enhanced chondrogenic potential when compared with intact EBs [128]. In addition, there was no stimulatory effect of dexamethasone on chondrogenic differentiation. However, this report was one of the first to demonstrate chondrogenic potential of EBs within a 3D construct. Successful examples of the application of a scaffold for osteogenic differentiation from mESCs include the use of a 3D self-assembling peptide substrate [129] and alginate beads loaded into a bioreactor fed with osteogenic-inducing growth medium [130].
Synthetic polymers such as PEG have been successfully used in a recent study that reported the induction of chondrogenic differentiation in mESCs within a PEG-based polymeric 3D environment. In these studies, 5-day-old EBs prepared in suspension cultures were encapsulated in a photopolymerizable PEG polymer, with addition of TGF-β1 and glucosamine to the growth medium, enhancing the chondrogenic differentiation capabilities of EBs [131,132]. Such 3D systems exhibit potential for ESC delivery to the site of injury, circumventing issues that arise with low proliferative capacity of differentiated chondrocytes as well as loss of phenotype during ex vivo expansion [133]. An inorganic substrate derived from bioactive sol-gel glass initially used for the differentiation and proliferation of human and murine osteoblasts was also used to promote osteogenic differentiation from mESCs. Temporal- and dose-dependent manipulations of growth factor supplementation further enhanced the positive effect of this sol gel [134,135]. For osteogenic and chondrogenic induction from hESCs, researchers have demonstrated that predifferentiated hESCs (5-day-old EBs) replated in the presence of osteogenic supplements induced differentiation. In vivo implantation of these predifferentiated cells within poly(D,L-lactide) scaffolds in severely combined immunodeficient mice allowed for further differentiation to specialized mineralizing tissue [136]. Substrate-based adipogenic differentiation of mESCs has only been demonstrated on polycaprolactone synthetic polymer. These studies reported the use of nanoscale fibers from electrospun polycaprolactone scaffolds to mimic ECM architecture and induce mESC differentiation. In these studies, mESCs were directly seeded onto the scaffolds and treated with insulin, tri-iodothyronine and retinoic acid (RA) to induce adipogenesis. On comparing cells derived from this 3D culture system with those derived from a 2D system that involved prespecification with EB formation, it was noted that optimal results were achieved when the cellular microenvironments closely resembled in vivo conditions [137]. A high-throughput screening approach utilized microprinting technology to present pluripotent cells with combinations of ECM proteins in a microarray [138] and in a multiwell format [139]. A commercial arrayer was used to deposit mixtures of varying concentrations of collagen I, collagen III, collagen IV, fibronectin and laminin onto a thin custom-made acrylamide gel pad. The ECM was deposited in a highly controlled manner, and there were no detected instances of cross-contamination between spots. A multiwell format was generated using a slide carrier and gaskets, followed by seeding of mESCs within these wells and presentation of 12 different combinations of four growth factors known to induce cardiac differentiation. A total of 240 ECM and growth factor signaling environments were studied and the results acquired during this expansive run were consistent with published data on cardiogenesis. The choices of cells that are generated from the mesoderm are a clear indicator of the choice of substrate for inducing differentiation from pluripotent stem cells. The nature of the microenvironment that comprises of specific physical, chemical and mechanical cues need to be taken into account, prior to determining the biomaterial to be used in specific directed differentiation strategies.
Ectodermal differentiation
Ectodermal derivatives are often seen in spontaneously differentiating cultures and are considered as the ‘default pathway’ for differentiation of pluripotent stem cells [140]. Several ectodermal derivatives such as oligodendrocytes, dopaminergic neurons and motor neurons have been produced using a cocktail of growth factors including FGF2, RA, epidermal growth factor, brain- and glial-derived neurotrophic factor and sonic hedgehog [51,141-144]. Direct coculture of mouse and primate ESCs with PA6 stromal cells induced differentiation into dopaminergic neurons and circumvented the formation of multicellular aggregates (EBs) in the differentiation process. These studies determined that stromal cell-derived inducing activity was responsible for the differentiation, but little was ascertained of its molecular nature or induction mechanism [145,146]. Naturally derived ECMs and synthetic polymers have both been used to differentiate mESCs into ectodermal derivatives. A comparative study was conducted to determine the effects of 2D and 3D fibrin-based constructs on neural differentiation of EBs from mESCs. These studies demonstrated that intact EBs encapsulated with the 3D fibrin gel differentiated more readily than those on 2D fibrin gel into mature neurons and astrocytes under specific culture conditions [147]. mESCs cultured in hollow fibers made of cellulose triacetate polymer and ethylene vinyl alcohol copolymer in the presence of stromal cell-conditioned medium differentiated into dopaminergic neurons [148]. This study presents a model system for in vivo delivery of mESCs within scaffolds that promote differentiation. Such transplantation strategies might avoid obstacles that arise with rejection of implanted cells by the host, with added value of the semipermeable membrane permitting the influx of nutrients for the survival of the implanted cells and the efflux of dopamine.
Few studies have reported the application of synthetic biomaterials for neural differentiation of hESCs. When predifferentiated hESCs as 8-day-old EBs were dissociated and seeded in a 50/50 blend of poly(L-lactic acid) and PLGA-based biomaterial, multilayered rosette-like bodies with epithelial cell-lined narrow lumen were induced by supplementing the growth medium with RA. However, in the same construct, mesodermal differentiation was induced by addition of TGF-β and endodermal differentiation was induced by addition of activin A and IGF [149]. Enhanced neural differentiation of hESCs was achieved in the construct by addition of RA, NGF and neurotrophins [150]. As part of a teratoma formation protocol used to investigate pluripotent potential of hESCs, researchers investigated the differentiation potential of hESCs seeded in laminin-coated PLGA scaffolds [151]. Further analysis of the teratomas provided evidence of different cell types that originated from the three germ layers. However, close to 38% of the cells exhibited increased expression of the neuronal marker, nestin, thereby indicating that the nature of pretransplantation treatment, the site of transplantation and the ECM components in the scaffold could selectively enhance neural phenotypes within the teratoma [152].
Future perspective
Recent advances demonstrate the usefulness of different biomaterials in affecting the fate of pluripotent stem cells. Specific combinations of biomaterials promote the maintenance of the undifferentiated state of pluripotent stem cells (Table 1), while others have been found to induce differentiation into specific lineages from the three different germ layers (Table 2). Among the many different possibilities, uses of naturally derived biomaterials based on HA-based hydrogels in combination with specific ECMs appear most promising in maintaining the undifferentiated state of hESCs. It seems likely that use of synthetic mimics of these naturally derived biomaterials that offer user-controllable composition and compliance will provide the best substrate for propagating stable populations of stem cells [153]. Results from these ongoing experiments will contribute to the development of an integrated p ropagation system that will meet specific expectations that aim to:
Be efficient, producing high densities and quantities of human pluripotent stem cells;
Maintain genotypic and phenotypic integrity over several generations;
Use only defined, animal-product-free culture components.
From a differentiation perspective, a greater understanding of the cell—cell and cell—matrix interactions in specific microenvironments is needed to assist in the development of directed differentiation strategies towards specialized cell types. A better understanding of components of the in vivo cellular microenvironment is needed to develop in vitro biomimetic approaches that will constitute a synergy of biochemical, biophysical and mechanical cues in the determination of pluripotent stem cell fate. Advances in technologies that facilitate high-throughput experimentation and analysis, such as material-based arrays, will provide unique opportunities for the identification of the best biomaterial combinations that can be used to determine stem cell fate of self-renewal or differentiation.
Table 2.
Substrate types used to induce differentiation into different cell types from mouse and human pluripotent stem cells.
| Substrate type | Cell source | Substrate | Differentiation lineage |
Ref. |
|---|---|---|---|---|
| Feeder layer based | mESCs | Primary rat hepatocyte | Hepatic | [90] |
| Mouse stromal cells OP9 and RP010 | Hematopoietic | [110,111] | ||
| Fetal murine osteoblasts | Osteogenic | [81] | ||
| Stromal cells PA6 | Neuronal | [145] | ||
| hESCs | Mouse bone marrow cells line S17, yolk sac endothelial line C166 | Hematopoietic | [123] | |
| Human periodontal ligament fibroblasts | Osteogenic | [125] | ||
| Natural substrates | mESCs | Collagen-coated plates | Hepatic | [91] |
| Alginate beads | Hepatic | [94] | ||
| Collagen type IV | Hematopoietic | [112] | ||
| Fibrin polymers reinforced with poly(ethylene glycol) | Endothelial and hematopoietic |
[117] | ||
| Dextran constructs | Endothelial | [120] | ||
| Semi-interpenetrating polymer networks with fibronectin and laminin |
Endothelial and cardiac | [121] | ||
| Alginate construct | Chondrogenic | [128–130] | ||
| Self-assembling peptide construct | Osteogenic | [129] | ||
| Fibrin polymer construct | Neuronal | [147] | ||
| hESCs | Collagen scaffolds | Hepatic | [102] | |
| Alginate scaffolds | Hematopoietic | [124] | ||
| Synthetic substrates | mESCs | Polyurethane foam | Hepatic | [97,98] |
| Cytomatrix | Hematopoetic | [117,122] | ||
| Poly-(ethylene glycol) | Osteogenic | [131,132] | ||
| Nanoscale fiber-based electrospun polycaprolactone scaffolds | Adipogenic | [137] | ||
| Copolymer of cellulose triacetate polymer and ethylene vinyl alcohol |
Neuronal | [148] | ||
| hESCs | Poly(D,L-lactide) scaffolds | Osteogenic | [136] | |
| Poly(L-lactic acid) and poly(lactic-co-glycolic acid) | [149] | |||
| + retinoic acid | Neuronal | |||
| + TGF-β | Chondrogenic | |||
| + activin A and IGF | Pancreatic | |||
hESC: Human embryonic stem cell; mESC: Mouse embryonic stem cell.
Executive summary.
Substrates for self-renewal of pluripotent stem cells
Biomaterials that have been successfully used in the maintenance of embryonic stem cells include natural polymers, such as gelatin and hyaluronic acid, and synthetic polymers, such as poly(hydroxyethyl methacrylate), poly(glycerosebacate) acrylate and polyurethane.
The importance of cell—cell interactions in human embryonic stem cell cultures and during passaging is demonstrated in the study of polyurethane microwells. Within these microwells, the cells were forced to create and maintain clusters, providing a unique microenvironment for maintaining pluripotency of the human embryonic stem cells for 3 weeks without the need for passaging.
Substrates for differentiation of pluripotent stem cells
The choice of the polymer is expected to be directly related to the choice of the differentiated cell type from pluripotent stem cells. There is no single polymer that supports differentiation of stem cells into all three lineages. Natural biomaterials that promote differentiation include alginate and collagen scaffolds for endodermal differentiation, dextran for mesodermal differentiation and fibrin for ectodermal differentiation. Synthetic substrates such as polyurethane, poly(ethylene glycol) and poly(lactic co-glycolic acid) have also been used in feasibility studies.
Unresolved issues in pluripotent stem cell propagation & differentiation
Conventional culture conditions involve animal-derived components, feeder layers, serum and/or conditioned medium. The use of such products poses risks of contamination of pluripotent stem cells and their derivatives with animal pathogens.
In the case of human pluripotent stem cells, serum replacement, human feeders and increased amounts of growth factors in medium formulations provide acceptable results in terms of extended propagation without the direct involvement of animal-derived products. However, these methodologies involve the use of conditioned medium, are cost-inefficient and do not translate across multiple cell lines.
The advantages of using biomaterials to develop directed differentiation strategies from pluripotent stem cells as compared with uncontrolled differentiation in an embryoid body are; greater control of the microenvironment and the ability to observe the progression of differentiation leading to a specialized end-point cell type. This allows for a better understanding of the mechanisms and the different intermediate stages involved in lineage specification.
Future perspective
Moving towards an animal-free environment for culturing human pluripotent stem cells would require the identification of polymeric substrates that support long-term proliferation and self-renewal. A wide array of polymers in addition to the various extracellular matrix modifications possible creates an expansive library of polymer—protein combinations that need to be tested for human pluripotent stem cell compatibility. High throughput analyses of various combinations are necessary to identify supportive materials and characterize cell—polymer interaction.
An ideal substrate would address the issues of xenogeneic contamination, labor intensiveness of maintaining two cell lines, maintenance of genomic and phenotypic integrity and provide a microenvironment that mimics the stem cell niche.
Development of controlled microenvironments that can induce pluripotent characteristics in somatic cells and simultaneous determination of stem cell fate of self-renewal and/or differentiation has potential for customized patient-specific therapies.
The integration of the fields of biomaterials science and stem cell biology would benefit from an off-the-shelf product that synthesizes a hydrogel providing the appropriate physical (3D architecture and support) and chemical cues (modification with growth factors).
Acknowledgments
Financial & competing interests disclosure
This work was supported in part by funding provided by NSF-CAREER grant 0744556 and from NIH T15-HL074303. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as: • of interest •• of considerable interest
- 1.Reubinoff BE, Pera MF, Fong C-Y, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 2.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 6.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 7.Thomson JA, Kalishman J, Golos TG, et al. Isolation of a primate embryonic stem cell line. Proc. Natl Acad. Sci. USA. 1995;92:7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donahue RE, Kessler SW, Bodine D, et al. Helper virus induced T cell lymphoma in nonhuman primates after retroviral mediated gene transfer. J. Exp. Med. 1992;176:1125–1135. doi: 10.1084/jem.176.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frondoza CG, Tanner KT, Jones LC, Hungerford DS. Polymethylmethacrylate particles enhance DNA and protein synthesis of human fibroblasts in vitro. J. Biomed. Mater. Res. 1993;27:611–617. doi: 10.1002/jbm.820270508. [DOI] [PubMed] [Google Scholar]
- 10.Newman KD, McBurney MW. Poly(D,L-lactic-co-glycolic acid) microspheres as biodegradable microcarriers for pluripotent stem cells. Biomaterials. 2004;25:5763–5771. doi: 10.1016/j.biomaterials.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Iannaccone PM, Taborn GU, Garton RL, Caplice MD, Brenin DR. Pluripotent embryonic stem cells from the rat are capable of producing chimeras. Dev. Biol. 1994;163:288–292. doi: 10.1006/dbio.1994.1146. [DOI] [PubMed] [Google Scholar]
- 12.Sukoyan MA, Golubitsa AN, Zhelezova AI, et al. Isolation and cultivation of blastocyst-derived stem cell lines from American mink (Mustela vison) Mol. Reprod. Dev. 1992;33:418–431. doi: 10.1002/mrd.1080330408. [DOI] [PubMed] [Google Scholar]
- 13.Notarianni E, Galli C, Laurie S, Moor RM, Evans MJ. Derivation of pluripotent, embryonic cell lines from the pig and sheep. J. Reprod. Fertil. Suppl. 1991;43:255–260. [PubMed] [Google Scholar]
- 14.Graves KH, Moreadith RW. Derivation and characterization of putative pluripotential embryonic stem cells from preimplantation rabbit embryos. Mol. Reprod. Dev. 1993;36:424–433. doi: 10.1002/mrd.1080360404. [DOI] [PubMed] [Google Scholar]
- 15.Doetschman T, Williams P, Maeda N. Establishment of hamster blastocyst-derived embryonic stem (ES) cells. Dev. Biol. 1988;127:224–227. doi: 10.1016/0012-1606(88)90204-7. [DOI] [PubMed] [Google Scholar]
- 16.Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Hearn JP. Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biol. Reprod. 1996;55:254–259. doi: 10.1095/biolreprod55.2.254. [DOI] [PubMed] [Google Scholar]
- 17.Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat. Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 18.Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 19••.Inzunza J, Gertow K, Stromberg MA, et al. Derivation of human embryonic stem cell lines in serum replacement medium using postnatal human fibroblasts as feeder cells. Stem Cells. 2005;23:544–549. doi: 10.1634/stemcells.2004-0201.First study to replace mouse embryonic fibroblasts as a feeder layer. Human foreskin fibroblast lines support human embryonic stem cell (hESC) self-renewal with no reduction in functionality of the cells in the long term.
- 20.Amit M, Shariki C, Margulets V, Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol. Reprod. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- 21.Amit M, Margulets V, Segev H, et al. Human feeder layers for human embryonic stem cells. Biol. Reprod. 2003;68:2150–2156. doi: 10.1095/biolreprod.102.012583. [DOI] [PubMed] [Google Scholar]
- 22.Choo AB, Padmanabhan J, Chin AC, Oh SK. Expansion of pluripotent human embryonic stem cells on human feeders. Biotechnol. Bioeng. 2004;88:321–331. doi: 10.1002/bit.20247. [DOI] [PubMed] [Google Scholar]
- 23.Hovatta O, Mikkola M, Gertow K, et al. A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Hum. Reprod. 2003;18:1404–1409. doi: 10.1093/humrep/deg290. [DOI] [PubMed] [Google Scholar]
- 24.Richards M, Fong C-Y, Chan W-K, Wong P-C, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat. Biotechnol. 2002;20:933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- 25.Richards M, Tan S, Fong C-Y, Biswas A, Chan W-K, Bongso A. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells. 2003;21:546–556. doi: 10.1634/stemcells.21-5-546. [DOI] [PubMed] [Google Scholar]
- 26.Cheng L, Hammond H, Ye Z, Zhan X, Dravid G. Human adult marrow cells support prolonged expansion of human embryonic stem cells in culture. Stem Cells. 2003;21:131–142. doi: 10.1634/stemcells.21-2-131. [DOI] [PubMed] [Google Scholar]
- 27.Lee JB, Song JM, Lee JE, et al. Available human feeder cells for the maintenance of human embryonic stem cells. Reproduction. 2004;128:727–735. doi: 10.1530/rep.1.00415. [DOI] [PubMed] [Google Scholar]
- 28.Genbacev O, Krtolica A, Zdravkovic T, et al. Serum-free derivation of human embryonic stem cell lines on human placental fibroblast feeders. Fertil. Steril. 2005;83:1517–1529. doi: 10.1016/j.fertnstert.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 29.Kim SJ, Song CH, Sung HJ, et al. Human placenta-derived feeders support prolonged undifferentiated propagation of a human embryonic stem cell line, SNUhES3: comparison with human bone marrow-derived feeders. Stem Cells Dev. 2007;16:421–428. doi: 10.1089/scd.2006.0098. [DOI] [PubMed] [Google Scholar]
- 30•.Stojkovic P, Lako M, Stewart R, et al. An autogeneic feeder cell system that efficiently supports growth of undifferentiated human embryonic stem cells. Stem Cells. 2005;23:306–314. doi: 10.1634/stemcells.2004-0137.Autogenic and allogeneic hESC fibroblasts successfully maintained self-renewal in both parent and foreign hESC lines.
- 31.Miyamoto K, Hayashi K, Suzuki T, et al. Human placenta feeder layers support undifferentiated growth of primate embryonic stem cells. Stem Cells. 2004;22:433–440. doi: 10.1634/stemcells.22-4-433. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Powell S, Brunette E, Lebkowski J, Mandalam R. Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnol. Bioeng. 2005;91:688–698. doi: 10.1002/bit.20536. [DOI] [PubMed] [Google Scholar]
- 33.Xu R-H, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat. Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 34.Zimrin AB, Pepper MS, McMahon GA, Nguyen F, Montesano R, Maciag T. An antisense oligonucleotide to the notch ligand jagged enhances fibroblast growth factor-induced angiogenesis in vitro. J. Biol. Chem. 1996;271:32499–32502. doi: 10.1074/jbc.271.51.32499. [DOI] [PubMed] [Google Scholar]
- 35.Guo D, Jia Q, Song H-Y, Warren RS, Donner DB. Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains. J. Biol. Chem. 1995;270:6729–6733. doi: 10.1074/jbc.270.12.6729. [DOI] [PubMed] [Google Scholar]
- 36.Ernst M, Oates A, Dunn AR. gp130-mediated signal transduction in embryonic stem cells involves activation of JAK and Ras/mitogen-activated protein kinase pathways. J. Biol. Chem. 1996;271:30136–30143. doi: 10.1074/jbc.271.47.30136. [DOI] [PubMed] [Google Scholar]
- 37.Smith AG, Heath JK, Donaldson DD, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 38.Nichols J, Evans EP, Smith AG. Establishment of germ-line-competent embryonic stem (ES) cells using differentiation inhibiting activity. Development. 1990;110:1341–1348. doi: 10.1242/dev.110.4.1341. [DOI] [PubMed] [Google Scholar]
- 39.Williams RL, Hilton DJ, Pease S, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 40.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Makino H, Hasuda H, Ito Y. Immobilization of leukemia inhibitory factor (LIF) to culture murine embryonic stem cells. J. Biosci. Bioeng. 2004;98:374–379. doi: 10.1016/S1389-1723(04)00298-1. [DOI] [PubMed] [Google Scholar]
- 42.Nur-E-Kamal A, Ahmed I, Kamal J, Schindler M, Meiners S. Three-dimensional nanofibrillar surfaces promote self-renewal in mouse embryonic stem cells. Stem Cells. 2006;24:426–433. doi: 10.1634/stemcells.2005-0170. [DOI] [PubMed] [Google Scholar]
- 43.Paling NRD, Wheadon H, Bone HK, Welham MJ. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J. Biol. Chem. 2004;279:48063–48070. doi: 10.1074/jbc.M406467200. [DOI] [PubMed] [Google Scholar]
- 44.Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 45.Kleinman HK, McGarvey ML, Hassell JR, et al. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 46.Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. Identification of multiple active growth factors in basement membrane matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp. Cell Res. 1992;202:1–8. doi: 10.1016/0014-4827(92)90397-q. [DOI] [PubMed] [Google Scholar]
- 47.Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 48.Takashima A, Grinnell F. Human keratinocyte adhesion and phagocytosis promoted by fibronectin. J. Invest. Dermatol. 1984;83:352–358. doi: 10.1111/1523-1747.ep12264522. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida K, Chambers I, Nichols J, et al. Maintenance of the pluripotential phenotype of embryonic stem cells through direct activation of gp130 signalling pathways. Mech. Dev. 1994;45:163–171. doi: 10.1016/0925-4773(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 50.Humphrey RK, Beattie GM, Lopez AD, et al. Maintenance of pluripotency in human embryonic stem cells is STAT3 independent. Stem Cells. 2004;22:522–530. doi: 10.1634/stemcells.22-4-522. [DOI] [PubMed] [Google Scholar]
- 51.Stojkovic P, Lako M, Przyborski S, et al. Human-serum matrix supports undifferentiated growth of human embryonic stem cells. Stem Cells. 2005;23:895–902. doi: 10.1634/stemcells.2004-0326. [DOI] [PubMed] [Google Scholar]
- 52••.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc. Natl Acad. Sci. 2007;104:11298–11303. doi: 10.1073/pnas.0703723104.Encapsulated hESCs within cross-linked hyaluronic acid hydrogels supported hESC expansion and maintained self-renewal in a 3D environment. This system can also be used to induce differentiation by the addition of appropriate factors.
- 53.Toole BP. Hyaluronan in morphogenesis. J. Intern. Med. 1997;242:35–40. doi: 10.1046/j.1365-2796.1997.00171.x. [DOI] [PubMed] [Google Scholar]
- 54.Jen AC, Wake MC, Mikos AG. Review: hydrogels for cell immobilization. Biotechnol. Bioeng. 1996;50:357–364. doi: 10.1002/(SICI)1097-0290(19960520)50:4<357::AID-BIT2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 55.Putnam AJ, Mooney DJ. Tissue engineering using synthetic extracellular matrices. Nat. Med. 1996;2:824–826. doi: 10.1038/nm0796-824. [DOI] [PubMed] [Google Scholar]
- 56.Shoichet MS, Li RH, White ML, Winn SR. Stability of hydrogels used in cell encapsulation: an in vitro comparison of alginate and agarose. Biotechnol. Bioeng. 1996;50:374–381. doi: 10.1002/(SICI)1097-0290(19960520)50:4<374::AID-BIT4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 57.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 58.Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr. Opin. Cell Biol. 2002;14:633–640. doi: 10.1016/s0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- 59.Harrison J, Pattanawong S, Forsythe JS, et al. Colonization and maintenance of murine embryonic stem cells on poly(α-hydroxy esters) Biomaterials. 2004;25:4963–4970. doi: 10.1016/j.biomaterials.2004.01.054. [DOI] [PubMed] [Google Scholar]
- 60.Harrison J, Melville AJ, Forsythe JS, et al. Sintered hydroxyfluorapatites—IV: the effect of fluoride substitutions upon colonisation of hydroxyapatites by mouse embryonic stem cells. Biomaterials. 2004;25:4977–4986. doi: 10.1016/j.biomaterials.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 61.Horak D, Kroupova J, Slouf M, Dvorak P. Poly(2-hydroxyethyl methacrylate)-based slabs as a mouse embryonic stem cell support. Biomaterials. 2004;25:5249–5260. doi: 10.1016/j.biomaterials.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 62.Kroupova J, Horak D, Pachernik J, Dvorak P, Slouf M. Functional polymer hydrogels for embryonic stem cell support. J. Biomed. Mater. Res. B Appl. Biomater. 2006;76:315–325. doi: 10.1002/jbm.b.30366. [DOI] [PubMed] [Google Scholar]
- 63.Cetinkaya G, Turkoglu H, Arat S, et al. LIF-immobilized nonwoven polyester fabrics for cultivation of murine embryonic stem cells. J. Biomed. Mater. Res. A. 2007;81:911–919. doi: 10.1002/jbm.a.31107. [DOI] [PubMed] [Google Scholar]
- 64.Ouyang A, Ng R, Yang S-T. Long-term culturing of undifferentiated embryonic stem cells in conditioned media and three-dimensional fibrous matrices without extracellular matrix coating. Stem Cells. 2007;25:447–454. doi: 10.1634/stemcells.2006-0322. [DOI] [PubMed] [Google Scholar]
- 65.Gerecht S, Townsend SA, Pressler H, et al. A porous photocurable elastomer for cell encapsulation and culture. Biomaterials. 2007;28:4826–4835. doi: 10.1016/j.biomaterials.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat. Biotechnol. 2002;20:602–606. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Kim YM, Langer R. In vivo degradation characteristics of poly(glycerol sebacate) J. Biomed. Mater. Res. A. 2003;66:192–197. doi: 10.1002/jbm.a.10534. [DOI] [PubMed] [Google Scholar]
- 68••.Mohr JC, de Pablo JJ, Palecek SP. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006;27:6032–6042. doi: 10.1016/j.biomaterials.2006.07.012.Microwells supported viable hESC cultures within a defined 3D system for 2–3 weeks. Cell—cell interactions within the hESC cluster maintain undifferentiated state of cells.
- 69.Li YJ, Chung EH, Rodriguez RT, Firpo MT, Healy KE. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J. Biomed. Mater. Res. A. 2006;79:1–5. doi: 10.1002/jbm.a.30732. [DOI] [PubMed] [Google Scholar]
- 70.Nie Y, Bergendahl V, Hei DJ, Jones JM, Palecek SP. Scalable culture and cryopreservation of human embryonic stem cells on microcarriers. Biotechnol. Prog. 2009;25:20–31. doi: 10.1002/btpr.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Phillips BW, Horne R, Lay TS, Rust WL, Teck TT, Crook JM. Attachment and growth of human embryonic stem cells on microcarriers. J. Biotechnol. 2008;138:24–32. doi: 10.1016/j.jbiotec.2008.07.1997. [DOI] [PubMed] [Google Scholar]
- 72.Massia SP, Hubbell JA. An RGD spacing of 440 nm is sufficient for integrin αVβ3-mediated fibroblast spreading and 140 nm for focal contact and stress fiber formation. J. Cell Biol. 1991;114:1089–1100. doi: 10.1083/jcb.114.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 74.Khademhosseini A, Suh KY, Yang JM, et al. Layer-by-layer deposition of hyaluronic acid and poly-L-lysine for patterned cell co-cultures. Biomaterials. 2004;25:3583–3592. doi: 10.1016/j.biomaterials.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 75••.Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat. Biotechnol. 2004;22:863–866. doi: 10.1038/nbt981.Large-scale screening techniques were used to characterize 1700 materials and study the cell—material interactions as it pertains to stem cell fate.
- 76.Derda R, Li L, Orner BP, Lewis RL, Thomson JA, Kiessling LL. Defined substrates for human embryonic stem cell growth identified from surface arrays. ACS Chem. Biol. 2007;2:347–355. doi: 10.1021/cb700032u. [DOI] [PubMed] [Google Scholar]
- 77.Green JJ, Zhou BY, Mitalipova MM, et al. Nanoparticles for gene transfer to human embryonic stem cell colonies. Nano. Lett. 2008;8:3126–3130. doi: 10.1021/nl8012665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang F, Green JJ, Dinio T, et al. Gene delivery to human adult and embryonic cell-derived stem cells using biodegradable nanoparticulate polymeric vectors. Gene Ther. 2009;16:533–546. doi: 10.1038/gt.2008.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martin GR, Evans MJ. Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc. Natl Acad. Sci. USA. 1975;72:1441–1445. doi: 10.1073/pnas.72.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev. Biol. 1995;168:342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- 81.Buttery LDK, Bourne S, Xynos JD, et al. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng. 2001;7:89–99. doi: 10.1089/107632700300003323. [DOI] [PubMed] [Google Scholar]
- 82.Dani C, Smith AG, Dessolin S, et al. Differentiation of embryonic stem cells into adipocytes in vitro. J. Cell. Sci. 1997;110(Pt 11):1279–1285. doi: 10.1242/jcs.110.11.1279. [DOI] [PubMed] [Google Scholar]
- 83.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J. Embryol. Exp. Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 84.Fraichard A, Chassande O, Bilbaut G, Dehay C, Savatier P, Samarut J. In vitro differentiation of embryonic stem cells into glial cells and functional neurons. J. Cell. Sci. 1995;108(Pt 10):3181–3188. doi: 10.1242/jcs.108.10.3181. [DOI] [PubMed] [Google Scholar]
- 85.Kramer J, Hegert C, Guan K, Wobus AM, Muller PK, Rohwedel J. Embryonic stem cell-derived chondrogenic differentiation in vitro: activation by BMP-2 and BMP-4. Mech. Dev. 2000;92:193–205. doi: 10.1016/s0925-4773(99)00339-1. [DOI] [PubMed] [Google Scholar]
- 86.Smith AG. Embryo-derived stem cells: of mice and men. Annu. Rev. Cell Dev. Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 87.Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc. Natl Acad. Sci. USA. 2000;97:11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang J, Au M, Lu K, et al. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940–1953. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- 89.Schroeder IS, Rolletschek A, Blyszczuk P, Kania G, Wobus AM. Differentiation of mouse embryonic stem cells to insulin-producing cells. Nat. Protocols. 2006;1:495–507. doi: 10.1038/nprot.2006.71. [DOI] [PubMed] [Google Scholar]
- 90.Moore RN, Dasgupta A, Rajaei N, et al. Enhanced differentiation of embryonic stem cells using co-cultivation with hepatocytes. Biotechnol. Bioeng. 2008 doi: 10.1002/bit.21987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Teratani T, Yamamoto H, Aoyagi K, et al. Direct hepatic fate specification from mouse embryonic stem cells. Hepatology. 2005;41:836–846. doi: 10.1002/hep.20629. [DOI] [PubMed] [Google Scholar]
- 92.Atala A, Kim W, Paige KT, Vacanti CA, Retik AB. Endoscopic treatment of vesicoureteral reflux with a chondrocyte-alginate suspension. J. Urol. 1994;152:641–643. doi: 10.1016/s0022-5347(17)32671-x. discussion 644. [DOI] [PubMed] [Google Scholar]
- 93.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 94.Fang S, Qiu YD, Mao L, Shi XL, Yu DC, Ding YT. Differentiation of embryoid-body cells derived from embryonic stem cells into hepatocytes in alginate microbeads in vitro. Acta Pharmacol. Sin. 2007;28:1924–1930. doi: 10.1111/j.1745-7254.2007.00713.x. [DOI] [PubMed] [Google Scholar]
- 95.Yamashita Y, Shimada M, Tsujita E, et al. Polyurethane foam/spheroid culture system using human hepatoblastoma cell line (Hep G2) as a possible new hybrid artificial liver. Cell Transplant. 2001;10:717–722. [PubMed] [Google Scholar]
- 96.Matsumoto K, Mizumoto H, Nakazawa K, Ijima H, Funatsu K, Kajiwara T. Hepatic differentiation of mouse embryonic stem cells in a three-dimensional culture system using polyurethane foam. J. Biosci. Bioeng. 2008;105:350–354. doi: 10.1263/jbb.105.350. [DOI] [PubMed] [Google Scholar]
- 97.Mizumoto H, Aoki K, Nakazawa K, Ijima H, Funatsu K, Kajiwara T. Hepatic differentiation of embryonic stem cells in HF/organoid culture. Transplant. Proc. 2008;40:611–613. doi: 10.1016/j.transproceed.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 98.Matsumoto K, Mizumoto H, Nakazawa K, Ijima H, Funatsu K, Kajiwara T. Hepatic differentiation of mouse embryonic stem cells in a bioreactor using polyurethane/spheroid culture. Transplant. Proc. 2008;40:614–616. doi: 10.1016/j.transproceed.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 99.Shirahashi H, Wu J, Yamamoto N, et al. Differentiation of human and mouse embryonic stem cells along a hepatocyte lineage. Cell Transplant. 2004;13:197–211. doi: 10.3727/000000004783984016. [DOI] [PubMed] [Google Scholar]
- 100.Rambhatla L, Chiu CP, Kundu P, Peng Y, Carpenter MK. Generation of hepatocyte-like cells from human embryonic stem cells. Cell Transplant. 2003;12:1–11. doi: 10.3727/000000003783985179. [DOI] [PubMed] [Google Scholar]
- 101.Lavon N, Yanuka O, Benvenisty N. Differentiation and isolation of hepatic-like cells from human embryonic stem cells. Differentiation. 2004;72:230–238. doi: 10.1111/j.1432-0436.2004.07205002.x. [DOI] [PubMed] [Google Scholar]
- 102.Baharvand H, Hashemi SM, Ashtiani S Kazemi, Farrokhi A. Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro. Int. J. Dev. Biol. 2006;50:645–652. doi: 10.1387/ijdb.052072hb. [DOI] [PubMed] [Google Scholar]
- 103.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc. Natl Acad. Sci. USA. 2002;99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Toyooka Y, Tsunekawa N, Akasu R, Noce T. Embryonic stem cells can form germ cells in vitro. Proc. Natl Acad. Sci. USA. 2003;100:11457–11462. doi: 10.1073/pnas.1932826100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhan X, Dravid G, Ye Z, et al. Functional antigen-presenting leucocytes derived from human embryonic stem cells in vitro. Lancet. 2004;364:163–171. doi: 10.1016/S0140-6736(04)16629-4. [DOI] [PubMed] [Google Scholar]
- 106.Chadwick K, Wang L, Li L, et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906–915. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 107.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ. Res. 2002;91:501–508. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 108.Kehat I, Kenyagin-Karsenti D, Snir M, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hubner K, Fuhrmann G, Christenson LK, et al. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300:1251–1256. doi: 10.1126/science.1083452. [DOI] [PubMed] [Google Scholar]
- 110.Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 111.Palacios R, Golunski E, Samaridis J. In vitro generation of hematopoietic stem cells from an embryonic stem cell line. Proc. Natl Acad. Sci. USA. 1995;92:7530–7534. doi: 10.1073/pnas.92.16.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage ana lysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125:1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 113.Hirashima M, Kataoka H, Nishikawa S, Matsuyoshi N, Nishikawa S-I. Maturation of embryonic stem cells into endothelial cells in an in vitro model of vasculogenesis. Blood. 1999;93:1253–1263. [PubMed] [Google Scholar]
- 114.McCloskey KE, Stice SL, Nerem RM. In vitro derivation and expansion of endothelial cells from embryonic stem cells. Methods Mol. Biol. 2006;330:287–301. doi: 10.1385/1-59745-036-7:287. [DOI] [PubMed] [Google Scholar]
- 115.Nishikawa S-I, Hirashima M, Nishikawa S, Ogawa M. Cell biology of vascular endothelial cells. Ann. NY Acad. Sci. 2001;947:35–41. doi: 10.1111/j.1749-6632.2001.tb03928.x. [DOI] [PubMed] [Google Scholar]
- 116.Chen SS, Fitzgerald W, Zimmerberg J, Kleinman HK, Margolis L. Cell—cell and cell—extracellular matrix interactions regulate embryonic stem cell differentiation. Stem Cells. 2007;25:553–561. doi: 10.1634/stemcells.2006-0419. [DOI] [PubMed] [Google Scholar]
- 117.Liu H, Collins SF, Suggs LJ. Three-dimensional culture for expansion and differentiation of mouse embryonic stem cells. Biomaterials. 2006;27:6004–6014. doi: 10.1016/j.biomaterials.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 118.Mosesson MW, Amrani DL. The structure and biologic activities of plasma fibronectin. Blood. 1980;56:145–158. [PubMed] [Google Scholar]
- 119.Francis SE, Goh KL, Hodivala-Dilke K, et al. Central roles of α5β1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler. Thromb. Vasc. Biol. 2002;22:927–933. doi: 10.1161/01.atv.0000016045.93313.f2. [DOI] [PubMed] [Google Scholar]
- 120.Ferreira LS, Gerecht S, Fuller J, Shieh HF, Vunjak-Novakovic G, Langer R. Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials. 2007;28:2706–2717. doi: 10.1016/j.biomaterials.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Battista S, Guarnieri D, Borselli C, et al. The effect of matrix composition of 3D constructs on embryonic stem cell differentiation. Biomaterials. 2005;26:6194–6207. doi: 10.1016/j.biomaterials.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 122.Liu H, Roy K. Biomimetic three-dimensional cultures significantly increase hematopoietic differentiation efficacy of embryonic stem cells. Tissue Eng. 2005;11:319–330. doi: 10.1089/ten.2005.11.319. [DOI] [PubMed] [Google Scholar]
- 123.Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc. Natl Acad. Sci. USA. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gerecht-Nir S, Cohen S, Ziskind A, Itskovitz-Eldor J. Three-dimensional porous alginate scaffolds provide a conducive environment for generation of well-vascularized embryoid bodies from human embryonic stem cells. Biotechnol. Bioeng. 2004;88:313–320. doi: 10.1002/bit.20248. [DOI] [PubMed] [Google Scholar]
- 125.Inanc B, Elcin AE, Elcin YM. Effect of osteogenic induction on the in vitro differentiation of human embryonic stem cells cocultured with periodontal ligament fibroblasts. Artif. Organs. 2007;31:792–800. doi: 10.1111/j.1525-1594.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- 126.Hauselmann HJ, Fernandes RJ, Mok SS, et al. Phenotypic stability of bovine articular chondrocytes after long-term culture in alginate beads. J. Cell. Sci. 1994;107:17–27. doi: 10.1242/jcs.107.1.17. [DOI] [PubMed] [Google Scholar]
- 127.Petit B, Masuda K, D’Souza AL, et al. Characterization of crosslinked collagens synthesized by mature articular chondrocytes cultured in alginate beads: comparison of two distinct matrix compartments. Exp. Cell Res. 1996;225:151–161. doi: 10.1006/excr.1996.0166. [DOI] [PubMed] [Google Scholar]
- 128.Tanaka H, Murphy CL, Murphy C, Kimura M, Kawai S, Polak JM. Chondrogenic differentiation of murine embryonic stem cells: effects of culture conditions and dexamethasone. J. Cell. Biochem. 2004;93:454–462. doi: 10.1002/jcb.20171. [DOI] [PubMed] [Google Scholar]
- 129.Garreta E, Genove E, Borros S, Semino CE. Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Eng. 2006;12:2215–2227. doi: 10.1089/ten.2006.12.2215. [DOI] [PubMed] [Google Scholar]
- 130.Randle WL, Cha JM, Hwang Y-S, et al. Integrated 3-dimensional expansion and osteogenic differentiation of murine embryonic stem cells. Tissue Eng. 2007;13:2957–2970. doi: 10.1089/ten.2007.0072. [DOI] [PubMed] [Google Scholar]
- 131.Hwang NS, Kim MS, Sampattavanich S, Baek JH, Zhang Z, Elisseeff J. Effects of three-dimensional culture and growth factors on the chondrogenic differentiation of murine embryonic stem cells. Stem Cells. 2006;24:284–291. doi: 10.1634/stemcells.2005-0024. [DOI] [PubMed] [Google Scholar]
- 132.Hwang NS, Varghese S, Theprungsirikul P, Canver A, Elisseeff J. Enhanced chondrogenic differentiation of murine embryonic stem cells in hydrogels with glucosamine. Biomaterials. 2006;27:6015–6023. doi: 10.1016/j.biomaterials.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 133.Homicz MR, Schumacher BL, Sah RL, Watson D. Effects of serial expansion of septal chondrocytes on tissue-engineered neocartilage composition. Arch. Otolaryngol. Head Neck Surg. 2002;127:398–408. doi: 10.1067/mhn.2002.129730. [DOI] [PubMed] [Google Scholar]
- 134.Bielby RC, Christodoulou IS, Pryce RS, Radford WJ, Hench LL, Polak JM. Time- and concentration-dependent effects of dissolution products of 58S sol-gel bioactive glass on proliferation and differentiation of murine and human osteoblasts. Tissue Eng. 2004;10:1018–1026. doi: 10.1089/ten.2004.10.1018. [DOI] [PubMed] [Google Scholar]
- 135.Bielby RC, Pryce RS, Hench LL, Polak JM. Enhanced derivation of osteogenic cells from murine embryonic stem cells after treatment with ionic dissolution products of 58S bioactive sol-gel glass. Tissue Eng. 2005;11:479–488. doi: 10.1089/ten.2005.11.479. [DOI] [PubMed] [Google Scholar]
- 136.Bielby RC, Boccaccini AR, Polak JM, Buttery LDK. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng. 2004;10:1518–1525. doi: 10.1089/ten.2004.10.1518. [DOI] [PubMed] [Google Scholar]
- 137.Kang X, Xie Y, Powell HM, et al. Adipogenesis of murine embryonic stem cells in a three-dimensional culture system using electrospun polymer scaffolds. Biomaterials. 2007;28:450–458. doi: 10.1016/j.biomaterials.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 138••.Flaim CJ, Chien S, Bhatia SN. An extracellular matrix microarray for probing cellular differentiation. Nat. Methods. 2005;2:119–125. doi: 10.1038/nmeth736.Extracellular matrix microarray platform for the culture of patterned cells atop combinatorial matrix mixtures enables the study of differentiation in response to a multitude of microenvironments.
- 139.Flaim CJ, Teng D, Chien S, Bhatia SN. Combinatorial signaling microenvironments for studying stem cell fate. Stem Cells Dev. 2008;17:29–40. doi: 10.1089/scd.2007.0085. [DOI] [PubMed] [Google Scholar]
- 140.Reubinoff BE, Itsykson P, Turetsky T, et al. Neural progenitors from human embryonic stem cells. Nat. Biotechnol. 2001;19:1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- 141.Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 142.Park S, Lee KS, Lee YJ, et al. Generation of dopaminergic neurons in vitro from human embryonic stem cells treated with neurotrophic factors. Neurosci. Lett. 2004;359:99–103. doi: 10.1016/j.neulet.2004.01.073. [DOI] [PubMed] [Google Scholar]
- 143.Perrier AL, Tabar V, Barberi T, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc. Natl Acad. Sci. USA. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wiles MV, Johansson BM. Embryonic stem cell development in a chemically defined medium. Exp. Cell Res. 1999;247:241–248. doi: 10.1006/excr.1998.4353. [DOI] [PubMed] [Google Scholar]
- 145.Kawasaki H, Mizuseki K, Nishikawa S, et al. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28:31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- 146.Kawasaki H, Suemori H, Mizuseki K, et al. Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc. Natl Acad. Sci. USA. 2002;99:1580–1585. doi: 10.1073/pnas.032662199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Willerth SM, Arendas KJ, Gottlieb DI, Sakiyama-Elbert SE. Optimization of fibrin scaffolds for differentiation of murine embryonic stem cells into neural lineage cells. Biomaterials. 2006;27:5990–6003. doi: 10.1016/j.biomaterials.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yamazoe H, Iwata H. Efficient generation of dopaminergic neurons from mouse embryonic stem cells enclosed in hollow fibers. Biomaterials. 2006;27:4871–4880. doi: 10.1016/j.biomaterials.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 149.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc. Natl Acad. Sci. USA. 2003;100:12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Levenberg S, Burdick JA, Kraehenbuehl T, Langer R. Neurotrophin-induced differentiation of human embryonic stem cells on three-dimensional polymeric scaffolds. Tissue Eng. 2005;11:506–512. doi: 10.1089/ten.2005.11.506. [DOI] [PubMed] [Google Scholar]
- 151.Cao Y, Croll TI, Cooper-White JJ, O’Connor AJ, Stevens GW. Production and surface modification of polylactide-based polymeric scaffolds for soft-tissue engineering. Methods Mol. Biol. 2004;238:87–112. doi: 10.1385/1-59259-428-x:87. [DOI] [PubMed] [Google Scholar]
- 152.Lees JG, Lim SA, Croll T, et al. Transplantation of 3D scaffolds seeded with human embryonic stem cells: biological features of surrogate tissue and teratoma-forming potential. Regen. Med. 2007;2:289–300. doi: 10.2217/17460751.2.3.289. [DOI] [PubMed] [Google Scholar]
- 153.Zhang J, Skardal A, Prestwich GD. Engineered extracellular matrices with cleavable crosslinkers for cell expansion and easy cell recovery. Biomaterials. 2008;29:4521–4531. doi: 10.1016/j.biomaterials.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang Q, Fang ZF, Jin F, Lu Y, Gai H, Sheng HZ. Derivation and growing human embryonic stem cells on feeders derived from themselves. Stem Cells. 2005;23:1221–1227. doi: 10.1634/stemcells.2004-0347. [DOI] [PubMed] [Google Scholar]
- 155.Nur EKA, Ahmed I, Kamal J, Schindler M, Meiners S. Three-dimensional nanofibrillar surfaces promote self-renewal in mouse embryonic stem cells. Stem Cells. 2006;24:426–433. doi: 10.1634/stemcells.2005-0170. [DOI] [PubMed] [Google Scholar]
- 156.Klimanskaya I, Chung Y, Meisner L, Johnson J, West MD, Lanza R. Human embryonic stem cells derived without feeder cells. Lancet. 2005;365:1636–1641. doi: 10.1016/S0140-6736(05)66473-2. [DOI] [PubMed] [Google Scholar]
- 201.Invitrogen WO9830679716 1998