Abstract
Objective
The objective of this study was to use MR imaging to investigate long-term changes in muscle and tendon morphology following a hamstring strain injury.
Materials and Methods
MR images were obtained from 14 athletes who sustained a clinically diagnosed grade I/II hamstring strain injury between 5-23 months prior as well as five healthy controls. Qualitative bilateral comparisons were used to assess the presence of fatty infiltration and changes in morphology that may have arisen as a result of the previous injury. Hamstring muscle and tendon/scar volumes were quantified in both limbs for the biceps femoris long head (BFLH), biceps femoris short head (BFSH), the proximal semimembranosus tendon (PSMT) and the proximal conjoint biceps femoris and semitendinosus tendon (PBFT). Differences in muscle and tendon volume between limbs were statistically compared between the previously injured and healthy control subjects.
Results
Increased low-intensity signal was present along the musculotendon junction adjacent to the site of presumed prior injury for 11 of the 14 subjects, suggestive of persistent scar tissue. The thirteen subjects with biceps femoris injuries displayed a significant decrease in BFLH volume (p<0.01), often accompanied by an increase in BFSH volume. Two of these subjects also presented with fatty infiltration within the previously injured BFLH.
Conclusion
The results of this study provide evidence of long-term musculotendon remodeling following a hamstring strain injury. Additionally, many athletes are likely returning to sport with residual atrophy of the BFLH and/or hypertrophy of the BFSH. It is possible that long-term changes in musculotendon structure following injury alters contraction mechanics during functional movement, such as running, and may contribute to re-injury risk.
Keywords: MRI, hamstrings, muscle injury, repair, remodeling
INTRODUCTION
Muscle strain injuries may account for ∼30% of sports medicine practice [1], with hamstring injuries being particularly frequent among individuals participating in high speed running [2, 3, 4, 5]. The treatment and rehabilitation of hamstring injuries remains challenging, as evidenced by approximately 30% of individuals experiencing a re-injury within the first year after initial injury [6-8]. Of particular clinical concern is the observation that subsequent injuries are often more severe and require more time away from sport than the initial injury [9, 10].
Magnetic resonance (MR) imaging provides an objective standard for confirming the presence of an acute muscle strain injury [11, 12]. Recent studies have shown that the location and extent of abnormalities (e.g. edema and hemorrhage) on MR images not only confirm the presence and severity of initial muscle fiber damage, but can also provide a reasonable estimate of the rehabilitation period [13-15]. In addition, re-injury rates have been shown to be higher among individuals that sustain a more severe original injury, as determined by the length of muscle damage present on MR images obtained at the time of injury [10, 16].
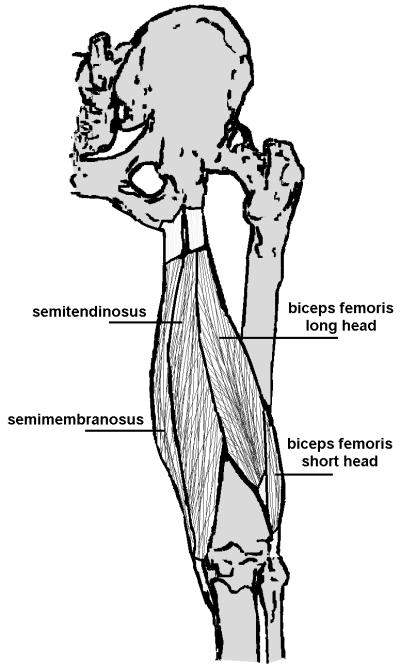
Various studies have investigated structural changes immediately following an acute hamstring strain injury [10, 13, 15, 17]. However, it is unclear how repair processes may alter musculotendon morphology in the months following return to sport. It has been hypothesized that scar tissue formation, along with weakness or atrophy of the previously injured muscle may be contributing factors to re-injury [8]. Indeed, prior animal models of injury have shown that muscle tissue may not be able to fully regenerate to pre-injury state, with connective scar tissue persisting at the site of injury indefinitely [18, 19]. Given that most hamstring strain injuries occur along the proximal musculotendon junction [20], where the muscle fibrils intersect with the tendon [17] (Fig. 1), it is likely that remodeling takes place near this region. In addition, atrophy and fatty replacement within the previously injured or surrounding muscles may also occur as part of the remodeling process [11, 21, 22].
Fig. 1.
The hamstring muscles consist of the semimembranosus, semitendinosus, and biceps femoris muscles. They originate from an incompletely separated tendon on the lateral and proximal aspect of the ischial tuberosity. Of the three hamstring muscles, the long head of the biceps femoris is the most commonly injured [1, 17, 20, 26].
The purpose of this study was to use MR imaging to qualitatively and quantitatively investigate evidence of long-term changes in muscle and tendon morphology after a hamstring strain injury. We hypothesized that scar tissue would be present near the site of prior injury, with accompanying atrophy of the previously injured muscle. MR images from healthy control subjects were also obtained in order to provide insights into normal variations in muscle and tendon morphology between limbs.
MATERIALS AND METHODS
Fourteen previously injured athletes were recruited (Table 1). All had experienced a clinically diagnosed prior hamstring strain injury (grade I or II) that required a minimum of two weeks away from sport, and were free of other current or history of musculoskeletal impairments. Each subject participated in a supervised rehabilitation program, and since returned to sport for at least one month prior to this study. An additional five healthy athletes were recruited with no current or history of musculoskeletal impairments. In accordance with the UW Health Sciences Human Subjects’ Committee, each subject or parent provided written informed consent prior to participation in the investigation.
Table 1.
Demographics and information regarding the prior injury/injuries of the subjects participating in this study
| Subject | Gender | Age | Months from Injury | Side of Injury | Location of Injury | Number of Injuries | Activity at Time of Injury |
|---|---|---|---|---|---|---|---|
| 1 | Male | 44 | 23 | Right | Proximal SM | 1 | Soccer |
| 2 | Male | 18 | 5 | Right | Proximal BF | 1 | Soccer |
| 3 | Female | 43 | 9 | Right | Proximal BF | 2 | Softball |
| 4 | Male | 31 | 5 | Left | Proximal BF | 2 | Football (sprinting) |
| 5 | Male | 19 | 13 | Left (both) | Proximal BF (both) | 2 | Track (sprinting) |
| 6 | Female | 19 | 7 | Left (both) | Proximal BF (both) | 2 | Track (hurdling) |
| 7 | Male | 18 | 8 | Right (all) | Proximal BF (all) | 3 | Track (sprinting) |
| 8 | Female | 45 | 6 | Right | Distal BF | 1 | 10k race |
| 9 | Male | 47 | 19 | Left | Distal BF | 1 | Track (sprinting) |
| 10 | Male | 56 | 18 | Right | Distal BF | 1 | Sprinting |
| 11 | Male | 20 | 7 | Right | Distal BF | 1 | Track (sprinting) |
| 12 | Female | 17 | 5 | Right (both) | Distal BF (both) | 2 | Track (sprinting) |
| 13 | Male | 38 | 5 | Left (recent) Right (prior) | Distal ST (recent) Proximal BF (prior) | 2 | Soccer |
| 14 | Male | 23 | 5 | Right (recent) Right (prior) | Distal BF (recent) Proximal BF (prior) | 2 | Track (sprinting) |
Abbreviations: SM = semimembranosus; BF = biceps femoris, ST = semitendinosus.
MR images were obtained of both limbs on a 1.5 Tesla MR scanner (General Electric Healthcare, Milwaukee, Wisconsin) using a phased-array torso coil. T2-weighted fast spin-echo coronal images were obtained for each subject (TR/TEeff, 2200/70; matrix, 512×512; 2 NEX; 44cm FOV; 4/0.4mm thickness). T1- weighted fast-spin echo axial and coronal images were acquired for the first five previously injured subjects (TR/TEeff, 550/17; matrix, 512×512; 1 NEX; 5mm axial with no gap, and 4.0/0.4mm coronal slice thickness). Thereafter, an iterative decomposition algorithm of water and fat with echo asymmetric and least-squares estimation (IDEAL) spoiled gradient echo sequence [23] was used for the subsequent nine previously injured and five healthy subjects (TR/TEeff, 12.7/4.4; 15° flip angle; matrix, 512×512; 1.5 NEX; 1.4mm coronal slice thickness, no gap). The change from a T1-weighted to IDEAL sequence was made because IDEAL uses a water-fat separation algorithm, such that reconstructed images do not suffer from water-fat chemical shift artifacts, thereby eliminating the need to manually account for this artifact, as was done for the T1-weighted images. Bilateral images were acquired from the ischial tuberosity to just below the knee, which encompassed the entire length of the biarticular hamstrings.
Qualitative bilateral comparisons were used to assess the presence of any fatty infiltration and differences in structure that may have arisen as a result of the previous injury. These qualitative comparisons were conducted by the same experienced musculoskeletal radiologist (M.J.T) for all subjects, who was blinded of the injury location during all assessments. An increased amount of high-intensity signal within muscle on T1-weighted sequences, compared to the contralateral limb, was indicative of fatty replacement [22]. Scar tissue is represented as low-intensity signal on both T1- and T2-weighted MR images [18, 22, 24, 25]. We considered increased low-intensity signal adjacent to tendon on the previously injured limb to be potential evidence of scarring [21] as a result of the prior injury.
Hamstring muscle and tendon/scar volumes were determined for both limbs using manual segmentation. We quantified volumes of the biceps femoris long head (BFLH), biceps femoris short head (BFSH), proximal conjoint biceps femoris and semitendinosus tendon (PBFT) and proximal semimembranosus tendon (PSMT) (Mimics Software; Materialize Corp.; Ann Arbor, MI) (Fig. 2). For individuals with distinguishable proximal biceps femoris and semitendinosus tendons, only the biceps femoris tendon volume was quantified. Muscle or tendon boundaries were identified and manually outlined on each image in which the desired structure was present. Volumes were calculated as the product of the inter-slice distance and the summed cross-sectional areas from all slices containing the muscle or tendon of interest. All measurements were conducted for both the previously injured subjects and the healthy controls by the same investigator (A.S.), blinded to the site of injury. In addition, we conducted an inter-observer reliability test during which one of the investigators (D.G.T) manually segmented the muscles and tendons of the fourth control subject but was blinded to the subject being investigated during the segmentation process. Percent difference in muscle and tendon volumes between limbs were then calculated all subjects. Differences are reported relative to the un-injured limb for the previously injured subjects and relative to the right limb for the control subjects. Volume difference measures were statistically evaluated between the previously injured and control subjects using t-tests (α=0.05).
Fig. 2.
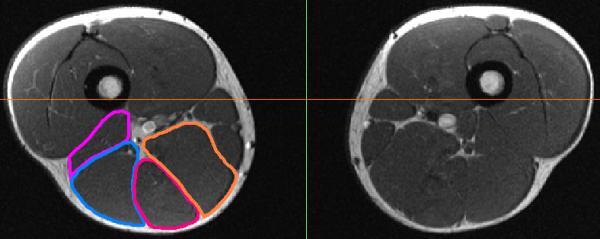
Hamstring muscles and tendons were manually outlined on each slice and used to estimate the muscle and tendon volumes of each limb. The right (left image) biceps femoris short head (purple), biceps femoris long head (blue), semimembranosus (orange), and semitendinosus (red) were outlined on this image, obtained from a previously injured subject.
RESULTS
Clinical notes indicated that seven subjects (1-7) sustained only proximal injuries, five subjects (8-12) sustained only distal injuries, and two subjects sustained both a proximal and distal injury (13,14) (Table 1). Twelve subjects injured only the biceps femoris, one subject injured the semimembranosus, and the remaining subject injured both the biceps femoris and semitendinosus (separate limbs on different occasions). Six of the subjects sustained a single injury, seven subjects sustained two injuries, and one subject experienced three injuries.
Differences in muscle and tendon volumes computed by the two investigators during the inter- observer reliability test revealed good agreement. BFLH and BFSH volume differences between limbs were identical for both investigators. Some disagreement in tendon volume differences was present, with a 3% and 10% discrepancy between investigators for the PBFT and PSMT, respectively.
Biceps Femoris Injuries
Significant differences were observed between limbs for the BFLH volume of the 13 subjects with biceps femoris injuries, compared to the healthy controls (p<0.01) (Table 2). These differences were represented by atrophy of the BFLH, often accompanied by hypertrophy of the BFSH (Fig. 3). Qualitative assessment identified that 10 of the 13 subjects presented with visual evidence of muscle volume differences. Additionally, the differences in PBFT volumes between limbs was larger for the previously injured subjects, compared to the controls, although not significant (p=0.07). The measured differences for the previously injured subjects were detected by qualitative assessment in 11 of the subjects (Fig. 4). The mean percent difference between limbs in the BFLH, BFSH, and PBFT volumes for the 13 previously injured subjects was -10%, +13% and, +85%, respectively.
Table 2.
Percent change in muscle and tendon volumes between the injured and non-injured limbs for the previously injured subjects and between the right and left limbs for the healthy controls. Negative values indicate the injured limb to be smaller than the non-injured limb, or that the right limb is smaller than the left limb
| Muscle volume | Tendon volume | |||||
|---|---|---|---|---|---|---|
| Case | BFLH | BFSH | SM | PBFT | PSMT | |
| Proximal SM injury | ||||||
| 1 | -2 | +4 | +10 | -32 | +52 | |
| Proximal BF injury | ||||||
| 2 | -4 | +23 | --- | +218 | +8 | |
| 3 | -20 | +30 | --- | 0 | -4 | |
| 4 | -23 | +1 | --- | +114 | -5 | |
| 5 | -12 | +17 | --- | +124 | +12 | |
| 6 | -16 | +46 | --- | +105 | +27 | |
| 7 | +5 | +16 | --- | -7 | -1 | |
| mean (SD) | -12 (11) | +22 (15) | --- | +92 (85) | +6 (12) | |
| p-value* | <0.01 | 0.06 | --- | 0.06 | 0.66 | |
| Distal BF injury | ||||||
| 8 | -26 | +25 | --- | --- | --- | |
| 9 | -29 | +19 | --- | --- | --- | |
| 10 | +1 | +9 | --- | --- | --- | |
| 11 | -4 | -3 | --- | --- | --- | |
| 12 | -6 | +9 | --- | --- | --- | |
| mean (SD) | -13 (14) | +12 (11) | --- | --- | --- | |
| p-value* | 0.01 | 0.38 | --- | --- | --- | |
| Proximal & distal injury | ||||||
| 13 | +3 | -21 | --- | -4 | -46 | |
| 14 | -1 | -1 | --- | +132 | +7 | |
| Healthy Controls | ||||||
| 1 | -13 | -6 | --- | +9 | +10 | |
| 2 | +2 | +7 | --- | +12 | +1 | |
| 3 | +2 | +4 | --- | +7 | +6 | |
| 4 | +14 | +12 | --- | +18 | +20 | |
| 5 | +18 | +7 | --- | -4 | +8 | |
| absolute mean (SD) | 10 (7) | 2 (3) | --- | 8 (8) | 9 (7) | |
compared to healthy controls
Abbreviations: BFLH = biceps femoris long head muscle; BFSH = biceps femoris short head muscle; PBFT = combined proximal biceps femoris and semitendinosus tendon; PSMT = proximal semimembranosus tendon; SM = semimembranosus muscle
Fig. 3.
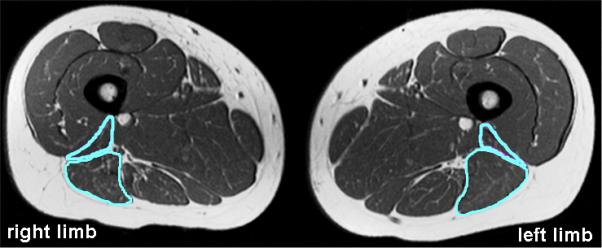
Moderate to substantial atrophy of the previously injured biceps femoris long head (BFLH) was present with corresponding hypertrophy of the biceps femoris short head (BFSH) in seven of the 13 subjects with biceps femoris injuries. Four of the remaining six subjects presented with either BFLH hypertrophy (2 subjects) or BFSH atrophy (2 subjects). Shown here, atrophy of the right BFLH along with hypertrophy of the right BFSH.
Fig. 4.
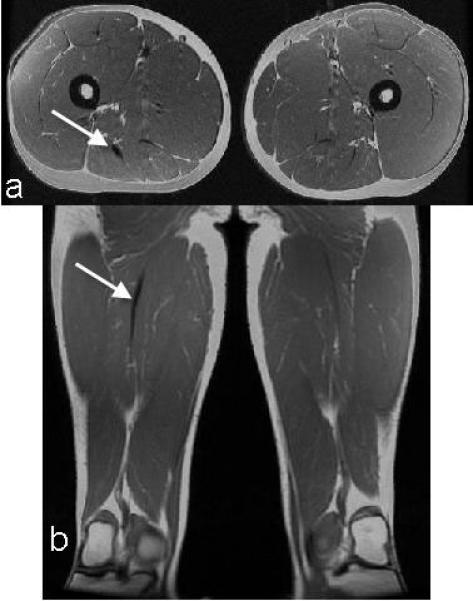
Scarring was present along the proximal musculotendon junction in four of the six subjects with proximal biceps femoris injuries. The arrow denotes an increased region of low-intensity signal along the proximal musculotendon junction of the biceps femoris in the axial (a) and coronal (b) planes.
Proximal Injuries
Seven subjects (1-7) sustained only proximal injuries. Four of these subjects experienced two injuries, two sustained one injury, and one subject had three injuries. Six of the seven subjects injured the proximal biceps femoris, while one injured the semimembranosus.
An increase in PBFT volume (p=0.06), atrophy of the BFLH (p<0.01), and hypertrophy of the BFSH (p=0.06) were present for the six subjects with proximal biceps femoris injuries, compared to the healthy controls (Table 2). Increased low-intensity signal was qualitatively observed along the musculotendon junction of the PBFT in four of these six subjects (Fig. 4). The PBFT volumes for these four subjects were, on average, 140% (range 105-218%) larger than the un-injured limb. Atrophy of the BFLH was qualitatively characterized on the axial images as decreased cross-sectional area of the proximal BFLH relative to the contralateral muscle (within 14-16 mm of the ischial tuberosity). Hypertrophy of the BFSH generally occurred near its origin, with the largest measured and observed differences occurring along the proximal lateral supracondylar line. Finally, subject 3 presented with substantial fatty infiltration within both the long and short heads of the biceps femoris on the previously injured limb (Fig. 5), with no visible or measurable scarring present.
Fig. 5.
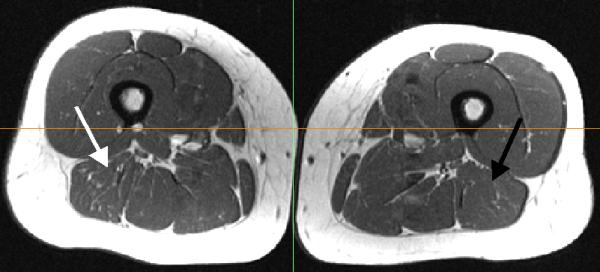
Fatty infiltration was observed within the long and short heads of the biceps femoris. The white arrow denotes the previously injured BFLH, while the black arrow designates the BFLH on the un-injured limb.
The single subject with a semimembranosus injury exhibited a 52% increase in PSMT volume compared to the contralateral limb. A moderate increase in semimembranosus muscle volume (+10%) was found with no measurable or observable difference in biceps femoris muscle size.
Distal Injuries
Five subjects sustained only distal injuries, all to the biceps femoris. Four of these subjects (8-11) experienced single injuries, while one subject incurred two injuries. Atrophy of the BFLH (p=0.01) was present, compared to the healthy controls, with three of the subjects demonstrating moderate to substantial atrophy of the BFLH and/or hypertrophy of the BFSH (Fig. 3, Table 2). PBFT volume was similar (p=0.38) between subjects with and without prior injury. However, increased low-intensity signal was qualitatively observed along the distal musculotendon junction of the biceps femoris for all of these subjects. One of the subjects with a distal injury also presented with considerable fatty infiltration within both the long and short heads of the biceps femoris on the previously injured limb (Fig. 5).
Proximal and Distal Injuries
Two subjects experienced both a proximal and distal injury (Table 1). Subject 13 injured his right proximal biceps femoris and left distal semitendinosus. Subject 14 injured both his right proximal and distal biceps femoris, but exhibited a much more substantial increase in tendon volume proximally, than distally (Table 2).
Healthy Controls
Three males and two females were recruited. The mean (SD) age, height, and weight of these subjects was 28 (5) years, 1.73 (0.06) m, 68 (4) kg, respectively. Some degree of asymmetry between the right and left limbs was observed for each subject (Table 2). Four of the five subjects exhibited larger BFLH and BFSH muscles in the right limb, while one subject had a larger left BFLH and BFSH. Four of the five subjects also had larger PBFT and PSMT tendons on the right limb, while one subject had a larger right PSMT and larger left PBFT.
DISCUSSION
We qualitatively and quantitatively investigated long-term changes (5-23 months) in muscle and tendon morphology following a hamstring strain injury. Despite the diversity within our subject population (Table 1), we observed some consistency in the injuries sustained and the morphological changes that ensued. The BFLH was the most commonly injured muscle, consistent with the observations of prior studies [1, 17, 20, 26]. Evidence of scar tissue was often observed along the musculotendon junction adjacent to the site of prior injury. For those subjects with biceps femoris injuries, we also observed atrophy of the BFLH on the side of injury, often accompanied by hypertrophy of the BFSH.
In order to gain further insights into the natural variations that exist in muscle and tendon morphology in healthy individuals, we obtained images of five healthy control subjects. These subjects also showed some variability in muscle and tendon volumes between limbs. It is interesting to note that the previously injured subjects exhibited a significantly smaller BFLH and larger BFSH on the previously injured limb. In contrast, both the BFLH and BFSH were either smaller or larger than the contralateral limb for all five healthy subjects. We also observed substantial variations in tendon sizes between the limbs of healthy subjects, although to a much lesser degree than the previously injured subjects, particularly in the PBFT (PBFT: healthy, 14%; previously injured, 92%).
Scar tissue has been observed as early as six weeks after an initial injury [13], and the degree of PBFT asymmetry present in the previously injured subjects in this study demonstrate that scarring likely persists on a more long-term basis (5-23 months post injury). Unfortunately, we cannot definitively conclude if this persists indefinitely, as shown in animal models [18, 19]. It is important to recognize that, while asymmetries exist in healthy individuals, apparent thickening of a tendon, compared to the contralateral limb, on MR images may be indicative of a prior musculotendon injury. Scar tissue adjacent to the site of prior injury may alter in-vivo muscle contraction mechanics. In particular, the collagen fibers comprising remodeled tendon tend to be less well organized, with different stiffness properties than normal tendon [27]. Specifically, scar tissue may increase the overall mechanical stiffness of the myofibrous tissue it replaces, which may require the muscle fibers to lengthen a greater amount to achieve the same overall musculotendon length relative to a pre-injury state. Finally, six of the eight subjects in this study that sustained multiple injuries and incurred those injuries in the same leg and location (i.e. proximal or distal) as the previous injury, as noted on clinical exam. This supports previous hypotheses [11, 28], that believe recurrent strain injuries likely occur near these regions of scarring, where the normal contractility mechanics are likely impaired.
The extent of the observed morphological changes showed large variations across subjects. Qualitative assessment of the MR images was able to identify morphological changes that agreed with quantitative measures for 11 of the 14 subjects, with manual segmentation techniques detecting more subtle differences in overall muscle and tendon morphology. A prior study that investigated the reliability of MR imaging and clinical assessment with regards to the evaluation of acute hamstring strain injuries [29]. It was found that in 18 of the 58 cases studied, a clinical diagnosis of hamstring injury was made with no positive identification of injury on MR images [29]. As a result, it should be expected that a large variation in the extent of remodeling would be present on long-term MR images, as we indeed observed in this study. Because we did not obtain MR images at the time of injury, we cannot definitively prove that the observed differences between limbs are a direct result of a hamstring injury. However, given the similarity of our long-term findings to those observed shortly after original injuries [13], we believe it is likely that the differences observed in our study are attributable to the initial hamstring injury. Our observation that asymmetries in morpohology between limbs in the control subjects were much smaller than those observed in the previously injured subjects lends further credence to this conclusion.
The morphological changes that take place following injury may be influenced by a number of factors, including severity of the initial injury, the rehabilitation exercises employed, and the frequency and intensity of training upon return to sport. For example, the hypertrophy present in the short head of the biceps femoris may be an exercise-induced compensation for atrophy of the injured long head. Such a compensation, which is enabled by the separate innervations of the long and short heads, may allow for the preservation of overall knee flexion strength. Periodic MR imaging of individuals from the time of original injury through the return to sport may enable a better understanding of the morphological changes attributable to individual factors during the initial healing process.
All subjects underwent supervised rehabilitation. However, the rehabilitation program was not standardized across subjects. As a result, the observed differences between subjects may be influenced by specific aspects of the rehabilitation strategies employed. Further, the determination of the original injury was performed by a variety of health care providers, as documented in their medical records. Because of this, we cannot report definitively the muscle injured. Additionally, health records could not be obtained for three of the 14 subjects. Thus, the date and location of injury for these subjects was based on direct subject questioning, rather than clinical diagnosis.
Manual segmentation techniques were required to quantify muscle and tendon volumes. Muscle volume estimates obtained from MR image data have previously been shown to have intra-observer reliability of 4-5% [30, 31], while accuracy of volume measures estimated from femoral cartilage using 2mm slice thickness resulted in a coefficient of variation for intra-observer variability of 1.2 to 1.3% [32]. Cartilage volume measures are smaller than hamstring muscle volumes and more comparable to tendon. We conducted an inter-observer reliability test for this study, which resulted in identical agreement in muscle volumes differences between investigators. Discrepancies in tendon volume differences were observed and likely a result of the amount of aponeurosis included in the segmentation process. This was standardized by the investigator that segmented all of the subjects, providing us with confidence in the consistency of the results reported. Manual segmentation takes 10-12 hours to complete for each subject. Thus, an intra-observer reliability analysis was not conducted. Finally, we were unable to accomplish consistent segmentation of the distal hamstring aponeuroses and tendons for volume calculations. This was due to the relatively small thickness of the distal aponeurosis and the intersection of the distal tendon with other low-intensity signal structures that cross the knee.
In conclusion, we have provided evidence of long-term muscle remodeling following a hamstring strain injury. Morphological differences between the limbs of the previously injured subjects were substantially greater than the natural variations occurring in healthy, un-injured athletes. Seventy-nine percent of the previously injured subjects presented with apparent residual scarring at the presumed injury site that persisted a minimum of five months after injury. Of the 13 subjects with biceps femoris injuries, 85% likely returned to sport with residual atrophy of the BFLH and/or hypertrophy of the BFSH. It is possible that these long-term changes to the musculotendon structure alter the in-vivo contraction mechanics during functional movement, such as running, and may contribute to re-injury risk.
Acknowledgments
Acknowledgment of funding sources: National Football League Medical Charities, National Institutes of Health Grant Number R01 AR56201, National Science Foundation pre-doctoral fellowship (A.S.)
REFERENCES
- 1.Garrett WE., Jr. Muscle strain injuries. American Journal of Sports Medicine. 1996;24(6 Suppl):S2–8. [PubMed] [Google Scholar]
- 2.Kujala UM, Orava S, Jarvinen M. Hamstring injuries. Current trends in treatment and prevention. Sports Medicine. 1997;23(6):397–404. doi: 10.2165/00007256-199723060-00005. [DOI] [PubMed] [Google Scholar]
- 3.Seward H, Orchard J, Hazard H, Collinson D. Football injuries in Australia at the elite level. Med J Aust. 1993;159:298–301. doi: 10.5694/j.1326-5377.1993.tb137863.x. [DOI] [PubMed] [Google Scholar]
- 4.Orchard JW. Intrinsic and extrinsic risk factors for muscle strains in Australian football. American Journal of Sports Medicine. 2001;29(3):300–303. doi: 10.1177/03635465010290030801. [DOI] [PubMed] [Google Scholar]
- 5.Arnason A, Sigurdsson SB, Gudmundsson A, Holme I, Engebretsen L, Bahr R. Risk factors for injuries in football. Am J Sports Med. 2004;32(1 Suppl):5S–16S. doi: 10.1177/0363546503258912. [DOI] [PubMed] [Google Scholar]
- 6.Woods C, Hawkins RD, Maltby S, Hulse M, Thomas A, Hodson A. The Football Association Medical Research Programme: an audit of injuries in professional football--analysis of hamstring injuries. Br J Sports Med. 2004;38(1):36–41. doi: 10.1136/bjsm.2002.002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orchard J, Seward H. Epidemiology of injuries in the Australian Football League, seasons 1997-2000. Br J Sports Med. 2002;36(1):39–44. doi: 10.1136/bjsm.36.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orchard J, Best TM. The management of muscle strain injuries: an early return versus the risk of recurrence. Clinical Journal of Sport Medicine. 2002;12(1):3–5. doi: 10.1097/00042752-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Brooks JH, Fuller CW, Kemp SP, Reddin DB. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am J Sports Med. 2006;34(8):1297–1306. doi: 10.1177/0363546505286022. [DOI] [PubMed] [Google Scholar]
- 10.Koulouris G, Connell DA, Brukner P, Schneider-Kolsky M. Magnetic resonance imaging parameters for assessing risk of recurrent hamstring injuries in elite athletes. Am J Sports Med. 2007;35(9):1500–1506. doi: 10.1177/0363546507301258. [DOI] [PubMed] [Google Scholar]
- 11.Koulouris G, Connell D. Hamstring Muscle Complex: An Imaging Review 10.1148/rg.253045711. Radiographics. 2005;25(3):571–586. doi: 10.1148/rg.253045711. [DOI] [PubMed] [Google Scholar]
- 12.Verrall GM, Slavotinek JP, Barnes PG, Fon GT, Spriggins AJ. Clinical risk factors for hamstring muscle strain injury: a prospective study with correlation of injury by magnetic resonance imaging. British Journal of Sports Medicine. 2001;35(6):435–439. doi: 10.1136/bjsm.35.6.435. discussion 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connell DA, Schneider-Kolsky ME, Hoving JL, Malara F, Buchbinder R, Koulouris G, et al. Longitudinal study comparing sonographic and MRI assessments of acute and healing hamstring injuries. AJR American Journal of Roentgenology. 2004;183(4):975–984. doi: 10.2214/ajr.183.4.1830975. [DOI] [PubMed] [Google Scholar]
- 14.Gibbs NJ, Cross TM, Cameron M, Houang MT. The accuracy of MRI in predicting recovery and recurrence of acute grade one hamstring muscle strains within the same season in Australian Rules football players. Journal of Science & Medicine in Sport. 2004;7(2):248–258. doi: 10.1016/s1440-2440(04)80016-1. [DOI] [PubMed] [Google Scholar]
- 15.Slavotinek JP, Verrall GM, Fon GT. Hamstring injury in athletes: using MR imaging measurements to compare extent of muscle injury with amount of time lost from competition. AJR American Journal of Roentgenology. 2002;179(6):1621–1628. doi: 10.2214/ajr.179.6.1791621. [DOI] [PubMed] [Google Scholar]
- 16.Verrall GM, Slavotinek JP, Barnes PG, Fon GT, Esterman A. Assessment of physical examination and magnetic resonance imaging findings of hamstring injury as predictors for recurrent injury. J Orthop Sports Phys Ther. 2006;36(4):215–224. doi: 10.2519/jospt.2006.36.4.215. [DOI] [PubMed] [Google Scholar]
- 17.Garrett WE, Jr., Rich FR, Nikolaou PK, Vogler JB., 3rd Computed tomography of hamstring muscle strains. Medicine and science in sports and exercise. 1989;21(5):506–514. [PubMed] [Google Scholar]
- 18.Best TM, Shehadeh SE, Leverson G, Michel JT, Corr DT, Aeschlimann D. Analysis of changes in mRNA levels of myoblast- and fibroblast-derived gene products in healing skeletal muscle using quantitative reverse transcription-polymerase chain reaction. Journal of Orthopaedic Research. 2001;19(4):565–572. doi: 10.1016/S0736-0266(00)00067-X. [DOI] [PubMed] [Google Scholar]
- 19.Kaariainen M, Jarvinen T, Jarvinen M, Rantanen J, Kalimo H. Relation between myofibers and connective tissue during muscle injury repair. Scandinavian Journal of Medicine & Science in Sports. 2000;10(6):332–337. doi: 10.1034/j.1600-0838.2000.010006332.x. [DOI] [PubMed] [Google Scholar]
- 20.De Smet AA, Best TM. MR imaging of the distribution and location of acute hamstring injuries in athletes. AJR Am J Roentgenol. 2000;174(2):393–399. doi: 10.2214/ajr.174.2.1740393. [DOI] [PubMed] [Google Scholar]
- 21.Bordalo-Rodrigues M, Rosenberg ZS. MR imaging of the proximal rectus femoris musculotendinous unit. Magn Reson Imaging Clin N Am. 2005;13(4):717–725. doi: 10.1016/j.mric.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Tuite MJ, DeSmet AA. MRI of selected sports injuries: muscle tears, groin pain, and osteochondritis dissecans. Seminars in Ultrasound, CT & MR. 1994;15(5):318–340. doi: 10.1016/s0887-2171(05)80002-2. [DOI] [PubMed] [Google Scholar]
- 23.Reeder SB, Pineda AR, Wen Z, Shimakawa A, Yu H, Brittain JH, et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med. 2005;54(3):636–644. doi: 10.1002/mrm.20624. [DOI] [PubMed] [Google Scholar]
- 24.Drape JL, Silbermann-Hoffman O, Houvet P, Dubert T, Thivet A, Benmelha Z, et al. Complications of flexor tendon repair in the hand: MR imaging assessment. Radiology. 1996;198(1):219–224. doi: 10.1148/radiology.198.1.8539383. [DOI] [PubMed] [Google Scholar]
- 25.May DA, Disler DG, Jones EA, Balkissoon AA, Manaster BJ. Abnormal signal intensity in skeletal muscle at MR imaging: patterns, pearls, and pitfalls. Radiographics. 2000;20:S295–315. doi: 10.1148/radiographics.20.suppl_1.g00oc18s295. [DOI] [PubMed] [Google Scholar]
- 26.Speer KP, Lohnes J, Garrett WE., Jr. Radiographic imaging of muscle strain injury. Am J Sports Med. 1993;21(1):89–95. doi: 10.1177/036354659302100116. discussion 96. [DOI] [PubMed] [Google Scholar]
- 27.Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303–329. doi: 10.1146/annurev.bioeng.6.040803.140240. [DOI] [PubMed] [Google Scholar]
- 28.Nikolaou PK, Macdonald BL, Glisson RR, Seaber AV, Garrett WE., Jr. Biomechanical and histological evaluation of muscle after controlled strain injury. Am J Sports Med. 1987;15(1):9–14. doi: 10.1177/036354658701500102. [DOI] [PubMed] [Google Scholar]
- 29.Schneider-Kolsky ME, Hoving JL, Warren P, Connell DA. A comparison between clinical assessment and magnetic resonance imaging of acute hamstring injuries. Am J Sports Med. 2006;34(6):1008–1015. doi: 10.1177/0363546505283835. [DOI] [PubMed] [Google Scholar]
- 30.Holzbaur KR, Murray WM, Gold GE, Delp SL. Upper limb muscle volumes in adult subjects. Journal of biomechanics. 2007;40(4):742–749. doi: 10.1016/j.jbiomech.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Tingart MJ, Apreleva M, Lehtinen JT, Capell B, Palmer WE, Warner JJ. Magnetic resonance imaging in quantitative analysis of rotator cuff muscle volume. Clinical orthopaedics and related research. 2003;(415):104–110. doi: 10.1097/01.blo.0000092969.12414.e1. [DOI] [PubMed] [Google Scholar]
- 32.Gold GE, Hargreaves BA, Vasanawala SS, Webb JD, Shimakawa AS, Brittain JH, et al. Articular Cartilage of the Knee: Evaluation with Fluctuating Equilibrium MR Imaging--Initial Experience in Healthy Volunteers. Radiology. 2006;238(2):712–718. doi: 10.1148/radiol.2381042183. [DOI] [PubMed] [Google Scholar]