Abstract
Novel methodology for the formation of amide bonds under neutral conditions is described. Evidence is presented that the active acyl donors are thio FCMA intermediates, generated from the reactions of thioacids with isonitriles.
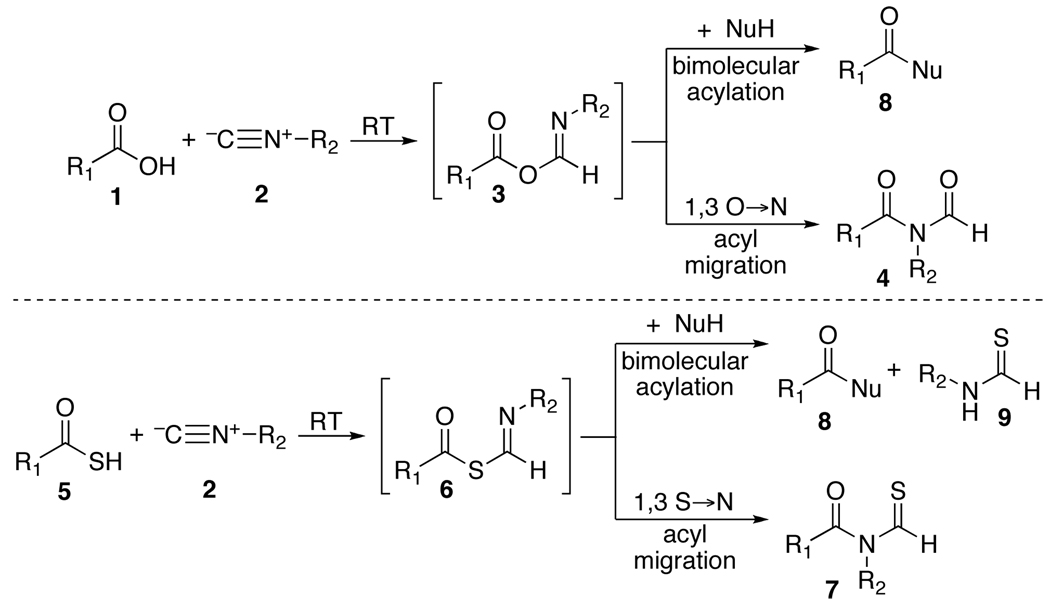
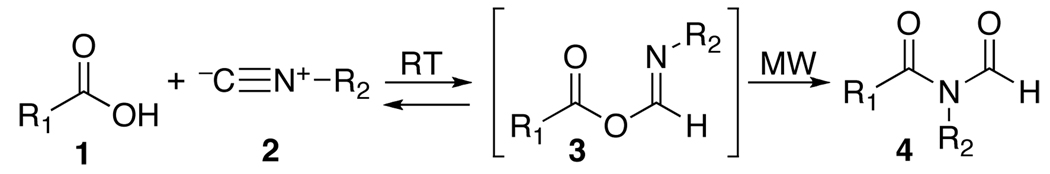
Following explorations into the chemistry of isonitriles,1 we were recently able to develop a new line of methodology which we consolidate in the expression, two-component coupling (2CC).2 The 2CC progression presumably commences via the reaction of an isonitrile (2) with a carboxylic acid (1), thereby generating a formimidate carboxylate mixed anhydride (3).3 For ease of communication, we refer to such a structure as a “FCMA”. While no FCMA has been fully characterized, Rebek has brought to bear impressive evidence as to their existence4 in special settings. It seems that under the usual reaction conditions, the oxy FCMA (3) is produced slowly and is disfavored at equilibrium. However, at higher temperatures under microwave mediation, the oxy FCMA, as its Z stereoisomer (3-Z), apparently undergoes a 1,3-O→N acyl transfer to generate an N-formylamide (4),2b the end point of the 2CC reaction.
In the short time since its discovery, the 2CC reaction has been adapted to the generation of a variety of biology related structures including secondary and tertiary amides and is already a valuable resource in the increasingly important field of chemoglycobiology.5
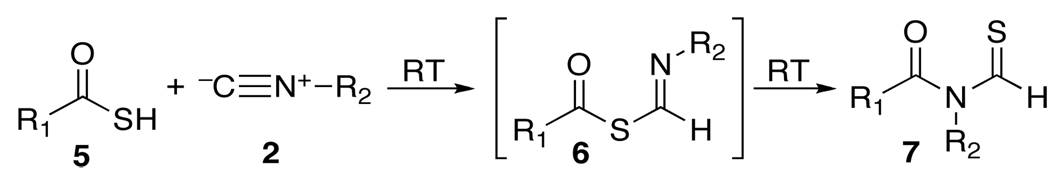
In probing the matter further, it was discovered that the overall thio counterpart of the 2CC reaction, unlike the oxa version, occurs at room temperature.2d,6 The throughput of thio FCMA (6) intermediates generated from reactions of 2 with thioacids (5),7 is much greater than is the case for their oxy FCMA (3) counterparts. Moreover, the corresponding S→N rearrangement (see 6→7) is far more rapid than is its oxy counterpart (3→4). Indeed transformation 6→7 apparently takes place at room temperature. Early findings concerning the scope and limitations of the thio FCMA version of the 2CC reaction have been charted.2d
The value of the above described 2CC and thio 2CC chemistry was much enhanced as we learned how to synthesize, often for the first time, high value added isonitriles.2f Application of the 2CC chemistry to merge “valuable” acids (as well as thioacids) with “valuable” isonitriles, provides access to useful peptide and glycopeptide substructural motifs.2a–c
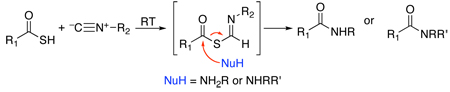
Earlier we had considered the possibility of exploiting oxy FCMA intermediates (3) as potential bimolecular acylating agents (see 3 + NuH→8).2d,e For such a prospectus to be feasible, bimolecular acylation had to dominate over monomolecular 1,3-O→N acyl transfer (leading to 4). In our initial reaction survey, conducted at room temperature, interdiction was, indeed, faster than 1,3 O→N intramolecular acyl transfer (i.e. 8 dominates over 4). However in the oxyacid experiments, the interdiction leading to 8 was quite slow and inefficient.2b As the temperature was raised, the apparent rates of intramolecular 1,3-O→N acyl transfer become competitive with interdiction and a mixture of products ensued. Accordingly, attempts at efficient bimolecular acylation via oxy FCMA intermediates (3) were frustrated. Thus in our current modalities, the ultimate product of the 2CC reaction reflects only the functionality of the acid and that of the isonitrile in the end products delivered; i.e. N-formylamide 4 or N-thioformylamide 7.2a,d
As described above, both the formation of thio FCMA 6 and its 1,3-S→N intermolecular acyl transfer leading to 7 are much more rapid than for their oxy counterparts (3→4).2d Accordingly, a rather fascinating possibility presented itself. To evaluate this matter, it would first be necessary to explore the relative rates of rearrangement of the thio FCMA intermediates (see 6→7 vs 6→8). If per chance, interdiction (6→8) were faster than its already rapid rearrangement possibility (6→7), an opportunity for accomplishing bimolecular acylation via an isonitrile would be at hand. In such a setting, we could envision a new acylation reaction where the formation of the active acylating agent (6) and its intramolecular acyl transfer product (see 8) were conducted under particularly mild conditions, giving rise to neutral “debris” (i.e. thioformamide 9).
In this proposed new bimolecular acylation format, structure 2 would now represent a simple, “throwaway” isonitrile (vide infra).8 This isonitrile would serve as a neutral dehydrothiolating agent enabling an acylation event (see 8) with extrusion of the neutral debris product 9. It was hoped that the proposed chemistry advanced in Figure 3 would be applicable to the establishment of sensitive acyl products such as are found in biological level target systems.
Figure 3.
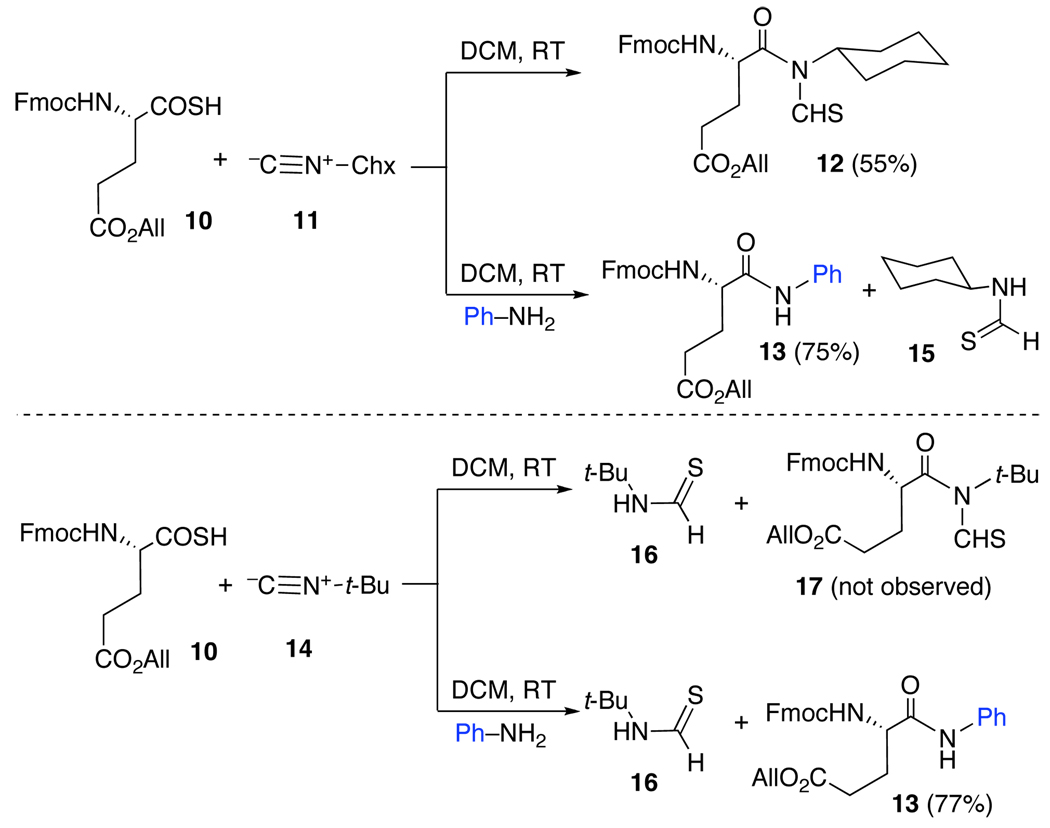
We began the exploration by investigating the reaction of differentiated thioacid 10 and cyclohexylisonitrile 11. Indeed, as anticipated by precedent, a two component coupling (2CC) reaction occurred to afford the expected product 12 in 55% yield. We next investigated the reaction of 10 and 11, but now in the presence of aniline (2eq) as the hypothetical nucleophile (NuH). It was found that anilide 13 was produced in 75% yield.9 Indeed under these conditions, we could find no clear indication for the formation of the previously observed 2CC product 12.Accordingly, we had to surmise that, at least with aniline as the nucleophile, interdiction was much faster than intramolecular 1,3-S→N transfer, which would have led to the 2CC product 12.
We also addressed the potential competition between intramolecular 1,3-S→N acyl transfer and intermolecular acyl transfer as a function of the structure of the isonitrile. In an introductory experiment,2h it was found that the recourse to t-butylisonitrile (14) strongly retarded the formation of 2CC coupling product. In fact, with 14 as the governing isonitrile, there was obtained a high yield of t-butylthioformamide 16, rather than 17. Presumably compound 16 arose from the reaction of the corresponding thio FCMA with unreacted acid (10). This proposal as to the origin of 16 further served to re-enforce the notion that thio FCMA intermediates (6) are very strong bimolecular acylating agents.10
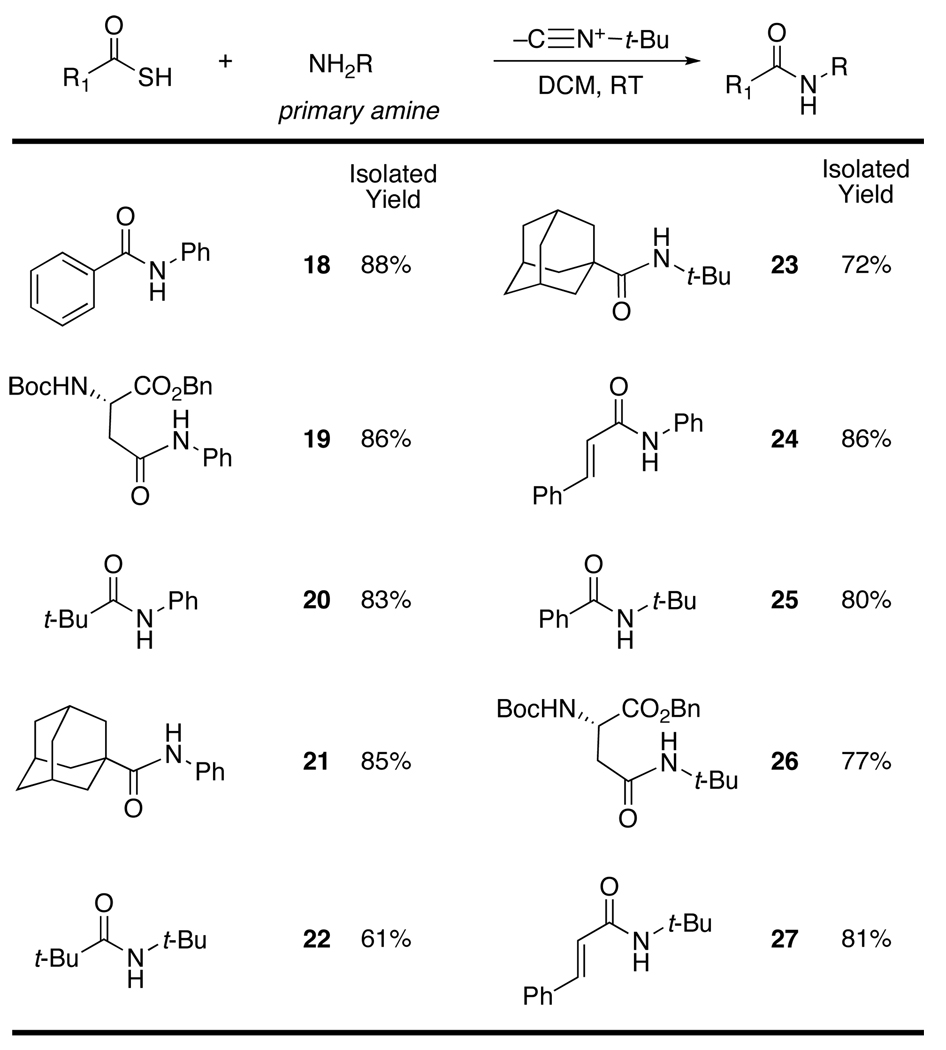
We now launched our proposed program in earnest. When the t-butylisonitrile (14) mediated reaction was conducted in the presence of primary amine nucleophiles, anilides were smoothly produced. The formation of anilides 18 and 19, notwithstanding the relatively weak reactivity of anilino nucleophiles, was encouraging. Even more impressive was the success of the method in producing hindered anilides 20 and 21. Remarkably, the method works well for the formation of doubly hindered amides (see 22 and 23).9 The formation of cinnamanilide 24 demonstrated that the potential difficulty of conjugate addition need not be a complication.
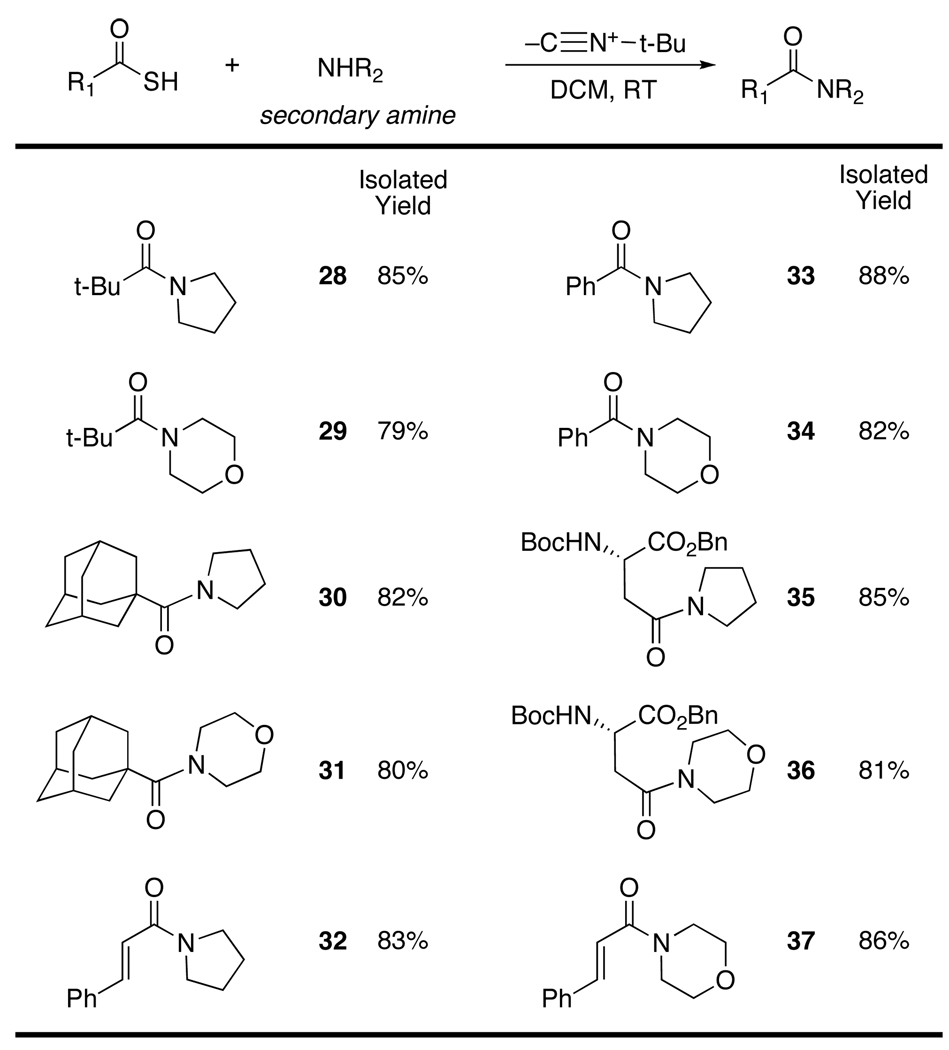
The power of the new acylation method is perhaps best seen by the fact that it can be used to produce tertiary amides (see compounds 28 to 37).11 Particular attention should be drawn to the formation of products 28 and 29 wherein both flanking sides of the amide bond are highly hindered. The ability to produce such products under mild neutral conditions is a strong virtue of the method. Finally, we also note the formation of cinnamic acid amides 32 and 37 without any apparent complication from Michael additions.
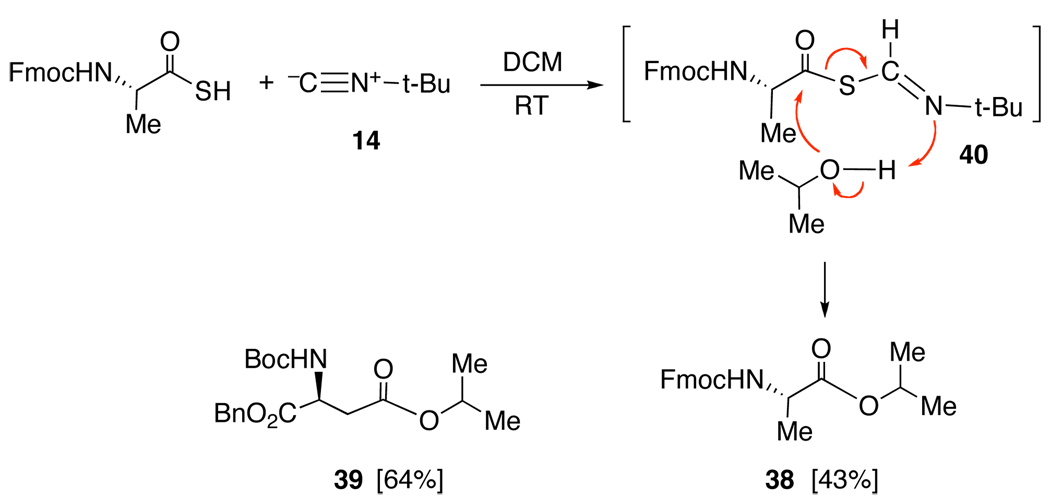
Remarkably as shown in Figure 7, the chemistry also works very nicely for the production of esters. Even isopropyl esters 38 and 39 are produced smoothly, from the reactions of the corresponding thioacids with isopropanol. These data strongly support the notion that an intermediate with exceptionally powerful acyl donor capacities is generated in the reaction of thioacid and isonitrile. Thus, in the formation of 38, it is proposed, but not proven, that the Fmoc-alanine thioacid reacts with t-butylisonitrile to give 40, which acylates isopropanol to yield ester 38. It will be noted that the transformation is achieved without recourse to the usual acylation catalysis by tertiary amines.12 In Figure 7, we suggest that, perhaps, concurrent proton transfer from the alcohol to the nitrogen of the emerging t-butylthioformamide helps to drive the acylation.
Figure 7.
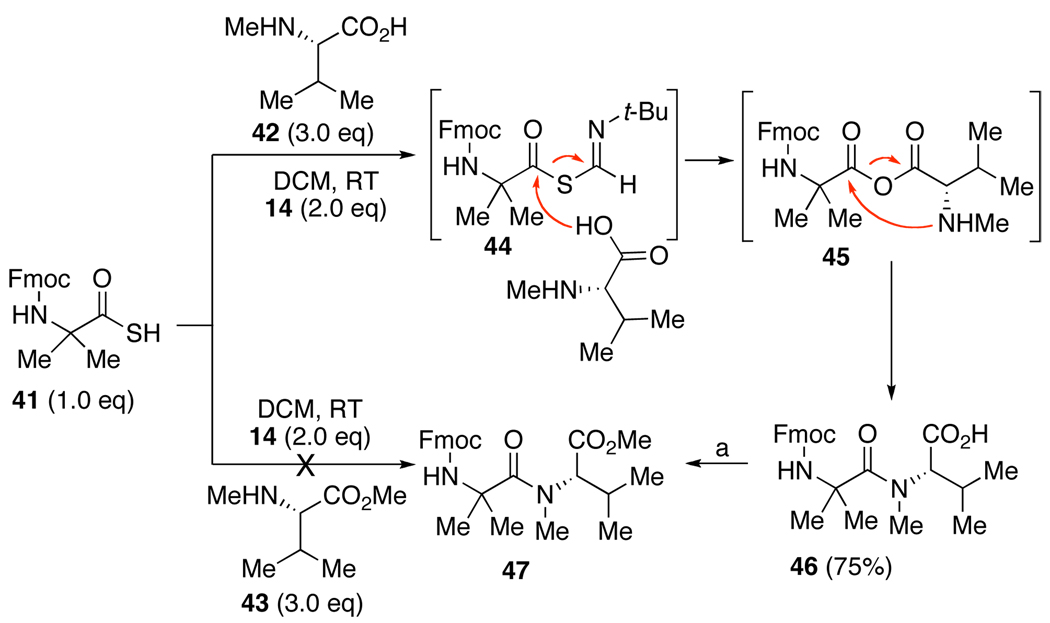
Finally we emphasize the straightforward formation of the highly hindered tertiary amide 46. This product must have arisen by interdiction of thio FCMA intermediate 44 with N-methyl valine 42.13 We further note that this reaction essentially fails when conducted on N-methyl valine methyl ester 43. Accordingly it is proposed that the thio FCMA 44 first acylates the carboxyl group of 42, giving rise to mixed anhydride 45. The latter proceeds to product 46 by a novel O→N acyl transfer.14
In summary, we have presented above a notable advance in the construction of amide bonds, including highly hindered cases.15,16 The chemistry is easily executed. The reactions are currently conducted under neutral conditions at room temperature in dichloromethane. Subsequent disclosures will deal with the applicability of this chemistry to the fashioning of biologic-level molecules. The prognosis, at this time, is quite promising.
In this and earlier papers, we have attempted to delineate the various conjectures, mechanistic analyses and experiments, which enabled these discoveries. We note in passing that amines, isonitriles and thioacids are very old functional groups which go back to the beginnings of organic chemistry. The amide forming construction described here could, in principle, have been conducted in 1909 without difficulty. It is not unlikely that careful mechanistically based revisitation of the foundations of organic chemistry might yield additional surprises of considerable value.
Supplementary Material
Figure 1.
Figure 2.
Figure 4.
Figure 5.
Figure 6.
Figure 8a.
aKey: (a) TMSCH2N2, THF/MeOH, RT, 90%.
Acknowledgement
Support was provided by the NIH (CA103823 to SJD). We thank Prof. W. F. Berkowitz for helpful discussions. Special thanks to Ms. Rebecca Wilson for editorial advice and consultation and to Ms. Dana Ryan for assistance with the preparation of the manuscript. We thank Dr. George Sukenick, Ms. Hui Fang, and Ms. Sylvi Rusli of the Sloan-Kettering Institute’s NMR core facility for mass spectral and NMR spectroscopic analysis
Footnotes
Supporting Information Available: Experimental procedures, copies of spectral data, and characterization (PDF). This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.(a) Passerini M. Gazz. Chim. Ital. 1921;51:181. [Google Scholar]; (b) Ugi I, Meyr R, Fetzer U, Steinbrückner C. Angew. Chem. 1959;71:386. [Google Scholar]
- 2.(a) Li X, Danishefsky SJ. J. Am. Chem. Soc. 2008;130:5446. doi: 10.1021/ja800612r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li X, Yuan Y, Berkowitz WF, Todaro LJ, Danishefsky SJ. J. Am. Chem. Soc. 2008;130:13222. doi: 10.1021/ja8047078. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Li X, Yuan Y, Kan C, Danishefsky SJ. J. Am. Chem. Soc. 2008;130:13225. doi: 10.1021/ja804709s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yuan Y, Zhu J, Li X, Wu X, Danishefsky SJ. Tetrahedron Lett. 2009;50:2329. doi: 10.1016/j.tetlet.2009.02.205. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wu X, Li X, Danishefsky SJ. Tetrahedron Lett. 2009;50:1523. doi: 10.1016/j.tetlet.2009.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zhu J, Wu X, Danishefsky SJ. Tetrahedron Lett. 2009;50:577. doi: 10.1016/j.tetlet.2008.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Stockdill JL, Wu X, Danishefsky SJ. Tetrahedron Lett. 2009;50:5152. doi: 10.1016/j.tetlet.2009.06.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones GO, Li X, Hayden AD, Houk KN, Danishefsky SJ. Org. Lett. 2008;10:4093. doi: 10.1021/ol8016287. [DOI] [PubMed] [Google Scholar]
- 4.(a) Hou J-L, Ajami D, Rebek J., Jr. J. Am. Chem. Soc. 2008;130:7810. doi: 10.1021/ja802288k. [DOI] [PubMed] [Google Scholar]; (b) Restorp P, Rebek J., Jr. J. Am. Chem. Soc. 2008;130:11850. doi: 10.1021/ja803854r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Bernardes GJL, Castagner B, Seeberger PH. ACS Chemical Biology. 2009;4 doi: 10.1021/cb900014n. [DOI] [PubMed] [Google Scholar]; (b) Yang Y, Ficht S, Brik A, Wong C. J. Am. Chem. Soc. 2007;129:7690. doi: 10.1021/ja0708971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chupp JP, Leschinsky KL. J. Org. Chem. 1975;40:66. [Google Scholar]
- 7.Fu X, Jiang S, Li C, Xin J, Yang Y, Ji R. Bioorg. Med. Chem. Lett. 2007;17:465. doi: 10.1016/j.bmcl.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Of course, the resulting formamide can be recycled to amine and isonitrile, thus completing the cycle.
- 9.This compound was purified and its structure is defined by infrared, NMR (1H and 13C), as well as high resolution mass spectra. All data are provided in the Supplementary Information.
- 10.The cognate product, which we don’t isolate, is almost certainly the (syn) mono thio anhydride. It seems unlikely (but not impossible) that such an anhydride is performing acylation. The rapidity of our observed acylation in less hindered settings renders such an interpretation highly unlikely.
- 11.Chatterjee J, Gilon C, Hoffman A, Kessler H. Acc. Chem. Res. 2008;41:1331. doi: 10.1021/ar8000603. [DOI] [PubMed] [Google Scholar]
- 12.The electronic depiction suggested in going from 40→38 is not intended to address the issue of timing (concertedness).
- 13.The initially low solubility of compound 42 in DCM did not affect the reactivity of this substrate. The reaction efficiently proceeded in either DCM, DMF or DCM/DMF (1:1/v:v) solvent systems without additional base.
- 14.Brown ZZ, Schafmeister CE. J. Am. Chem. Soc. 2008;130:14382. doi: 10.1021/ja806063k. [DOI] [PubMed] [Google Scholar]
- 15.For reviews describing recent advances in amide bond formation, see: Valeur E, Bradley M. Chem. Soc. Rev. 2009;38:606. doi: 10.1039/b701677h. El-Faham A, Khattab SN. Synlett. 2009;6:886. Kent SBH. Chem. Soc. Rev. 2009;38:338. doi: 10.1039/b700141j. Hackenberger CPR, Schwarzer D. Angew. Chem., Int. Ed. Engl. 2008;47:10030. doi: 10.1002/anie.200801313. Bode JW. Current Opinion in Drug Discovery and Development. 2006;9:765. Montalbetti CAGN, Falque V. Tetrahedron. 2005;61:10827. Albericio F. Curr. Opin. Chem Biol. 2004;8:211. doi: 10.1016/j.cbpa.2004.03.002. Han S, Kim Y. Tetrahedron. 2004;60:2447.
- 16.While quantitative comparisons are not yet available, we believe that the rate of interdiction relative to rearrangement of the thio FCMA renders it a superior acylating device relative to intermediates arising in carbodiimide methodology.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.