Abstract
Background
Plasma concentrations of N-terminal pro-B type natriuretic peptide (NT-proBNP) have been found to predict risk of sudden cardiac death (SCD) in patients with known cardiac disease, and C-Reactive Protein (CRP) levels have been found to predict risk among apparently healthy men; but currently there are no data on SCD risk prediction for either of these markers in a population of women unselected on the basis of CVD.
Methods and Results
In a prospective, nested case-control analysis within the 121,700 participant Nurses’ Health Study, 99 cases of definite or probable SCD were identified and matched to 294 controls. In multivariable models that adjusted for CHD risk factors, GFR, and other biomarkers, the trend across quartiles approached significance for NT-proBNP (RR = 2.37 for comparison of the highest versus lowest quartile; P, trend =0.05) but not for hsCRP (P, trend=0.60). When examined continuously, both NT-proBNP and hsCRP were significantly associated with SCD risk in age and fasting-adjusted models (P-for linear trend 0.04 and 0.03). Adjustment for CHD risk factors and other biomarkers strengthened the relationship with NT-proBNP and SCD (relative risk for 1-SD increment=1.49; 95% CI, 1.09 – 2.05; P =0.01), but eliminated the relationship with hsCRP (P =0.34). Women with NT-proBNP levels above the prespecified cut-point of 389 pg/mL were at a markedly increased risk of SCD in both models (RR =5.68; 95% CI, 1.78–18.2; P =0.003).
Conclusions
In this population of women, baseline levels of NT-proBNP were associated with subsequent risk of SCD. If this association is confirmed in larger prospectively studied populations, these findings might provide another useful marker contributing to efforts to screen and prevent SCD among women.
Keywords: death,sudden; natriuretic peptides; women; epidemiology; risk factors
Introduction
Sudden cardiac death (SCD) is one of the most common causes of death in developed countries. Estimates approach 450,000 deaths per year1, and these deaths account for over 50% of CHD deaths and 15–20% of all deaths2. The vast majority of patients who suffer SCD do not fit into high-risk subsets, and over 50% have no known history of heart disease at the time of their death3. In women, this percentage appears even higher with estimates as high as 69%.3,4 Despite this, approximately 90% of women who suffer a cardiac arrest or die suddenly will have some sort of structural heart disease documented on evaluation or autopsy5,6. Since SCD is often the first manifestation of clinically undetected structural heart disease, improved methods to detect structural heart disease may better identify women who are at risk.
N-terminal pro-B type natriuretic peptide (NT-proBNP), released primarily under conditions of volume and pressure overload in the ventricles7, is a sensitive marker of several forms of structural heart disease8, each of which are important components of arrhythmic risk. Concentrations of NT-proBNP have been found to independently predict SCD risk among patients with heart failure due to systolic dysfunction9 and among patients with acute myocardial infarction10, but currently there are no data for SCD risk prediction in a general population. Concentrations of C-reactive protein (CRP), a marker of inflammation, have been found to predict SCD in one population of apparently healthy men11, possibly through the detection of clinically unrecognized atherosclerosis. Several small studies also suggest that both NT-proBNP and CRP may specifically predict ventricular arrhythmias in patients with implantable cardioverter defibrillators12, 13.
In order to address the hypothesis that these markers of sub-clinical cardiovascular disease (CVD) might predict risk of sudden arrhythmic death among women, we performed a prospective nested case-control analysis within the Nurses’ Health Study.
Methods
Study Population
The Nurses’ Health Study (NHS) is a prospective cohort investigation among 121,700 female US registered nurses aged 30 to 55 years at baseline in 1976 (NHS). Information about medical history, lifestyle choices, and incident disease is assessed biennially by self-administered questionnaires. The validity and reproducibility of the data collected have been reported in detail previously4. Between 1989 and 1990, 32,826 women provided a blood sample, and these women served as a source population for this case-control analysis. Participants who provided blood samples were similar to those who did not14. Blood samples were collected in tubes treated with liquid sodium heparin and were placed on ice packs, stored in Styrofoam containers, and returned to our laboratory by overnight courier. The majority of blood samples (97%) arrived within 26 hours of phlebotomy. Immediately upon arrival, samples were centrifuged (1200 × g for 15 min at room temperature) and divided into aliquots of plasma, erythrocytes, and buffy-coat fractions, which were then placed in liquid nitrogen freezers at −130 °C or colder until analysis. Informed consent was obtained from all subjects, and the researchprotocol was approved by the institutional review board at Brighamand Women’s Hospital.
Endpoint Confirmation and Selection of Controls
The study end points included incident cases of sudden arrhythmic cardiac death that occurred after return of the blood sample and before June 1, 2006. The specific details regarding the classification of sudden and arrhythmic cardiac death in this cohort have been described in detail elsewhere.4 A cardiac death was considered a definite SCD if the death or cardiac arrest that precipitated death occurred within one hour of symptom onset as documented by medical records or next-of-kin reports. Deaths were also classified as arrhythmic or non-arrhythmic based on the definition of Hinkle and Thaler15. An arrhythmic death was defined as an abrupt spontaneous collapse of the circulation (pulse disappeared) without evidence of prior circulatory impairment (shock, congestive heart failure) or neurologic dysfunction (change in mental status, loss of consciousness, or seizure). Deaths where the pulse gradually disappeared and/or those preceded by circulatory or neurologic impairment were considered non-arrhythmic deaths, and these deaths were excluded from the SCD endpoint. Unwitnessed deaths or deaths that occurred during sleep where the participant was documented to be symptom free when last observed within the preceding 24 hours, and circumstances suggested that the death could have been arrhythmic were considered probable SCDs (n=36)16, 17.
Using risk-set sampling18, we randomly selected three controls for each case matched for age (+/− 1 year), ethnicity, smoking status (current, never, past), time and date of blood sampling, fasting status, and presence or absence of reported CVD (MI, angina, CABG, or stroke) at the time of blood draw. For cases that reported developing CVD after the blood draw, a second set of three controls who reported CVD after the blood draw were obtained to further control for the development of CVD prior to SCD.
Measurement of Biochemical Variables
All testing was done on the Dimension Vista® 1500 System from Dade Behring (now Siemens Healthcare Diagnostics Inc.). The total cholesterol, high density lipoprotein (HDL) and directly obtained low density lipoprotein (LDL) cholesterol and triglycerides were measured with spectrophotometric assays. NT-proBNP was measured with a one-step sandwich chemiluminescent immunoassay based on LOCI® technology, while CRP was measured with using the CardioPhase® high sensitivity CRP assay, a nephelometric assay that utilizes monoclonal antibodies specific to human CRP. Typical coefficients of variation (20-day ANOVA) for the lipid assays are <5%. Study samples were sent to the laboratory for analysis in randomly ordered batches, and laboratory personnel were unaware of the samples case-control status. Within-run coefficients of variation percent were assessed by analyzing quality control samples repeatedly. The coefficient of variation percents for the NT-proBNP assay was 6.33, and 7.71% for the hsCRP assay.
Assessment of other Factors
Anthropometric, lifestyle, and cardiac risk factors status were self-reported on questionnaires administered in 1990, with missing information substituted from previous questionnaires. Body mass index is calculated as the weight in kilograms divided by the square of the height in meters. Physical activity was expressed in terms of metabolic equivalent (MET) hours. The validity and reproducibility of these measurements have been described previously4, 19. GFR was estimated using the Modification of Diet in Renal Disease (MDRD) formula20
Statistical analysis
Means or proportions for baseline cardiac risk factors were calculated for cases and controls. The significance of associations between cases and controls were tested with the generalized estimating equations for categorical variables and with repeated measures analysis using Proc Mixed in SAS for continuous variables after natural logarithmic transformation to normalize their distribution. The raw values for continuous variables were also compared between case and control groups using conditional logistic regression. We analyzed the association between biomarker levels and the risk of sudden cardiac arrhythmic death using conditional logistic regression. With risk-set analysis, the odds ratio derived from the conditional logistic regression directly estimates the hazard ratio, and thus, the rate ratio or relative risk18.
To determine whether a gradient of risk was present across plasma values, subjects were first divided into quartiles based on the distribution of control values. To test for a linear trend across quartiles, the median value was assigned to each quartile and then modeled as a continuous variable in separate conditional regression models. Plasma biomarker levels were analyzed as continuous variables and as categorical values. In order to minimize the influence of outliers and to encourage linearity, biomarkers not normally distributed were log-transformed to improve the normality of their distributions.
For categorical analyses, we used pre-specified cut-points for each biomarker. For NT-proBNP and hsCRP, we used a cutpoint corresponding to the 80th percentile of the study population to facilitate comparisons with previous studies21, 22. In addition, we analyzed proposed clinical cut-points for hsCRP (>3.0 mg per liter)23 and NT-pro-BNP (≥ 389 pg/mL)24. We also examined clinical cutpoints for lipid values (LDL > 160 mg/dL, HDL<40 mg/dL, triglycerides > 200 mg/dL, total cholesterol > 240 mg/dL).
For each analysis, three multivariable conditional logistic regression models were performed. The first adjusted for age and fasting status (imperfectly matched variables). Fasting status was discordant from the case for 32 controls, and the maximum age difference between a case and control was 2.08 years, with 95% matched within one-year. The second multivariable model further adjusted for body-massindex (<25, 25–30, 30+); history of diabetes, hypertension, or hyperlipidemia; parental history of premature myocardial infarction before the age of 60; alcohol intake (<0.1 g, 0.1–15g, 15–29.9g, or at least 30 g per day); physical activity (quintiles from lowest to highest level); and use of postmenopausal hormone therapy (yes, no) and aspirin (< or = 22d/month). The third additionally simultaneously adjusted for other plasma biomarker levels (hsCRP, NT-proBNP, triglyceride, and TC/HDL ratio).
Three sensitivity analyses were performed. The first sensitivity analysis utilized an alternative set of three controls matched for the development of CVD after the blood draw (n=10 SCD cases) to explore the sensitivity of our results to the development of interim CVD. The second excluded women who reported CVD events prior to or at the time of the blood draw. The third excluded probable SCDs (n=36) and matched controls from the analysis to determine the sensitivity of the result to the expanded definition of SCD. All analyzes were carried out using SAS version 9.1 (SAS Institute Inc, Cary, NC). A two-tailed p value <0.05 was considered to indicate statistical significance. Dr. Albert and MV Moorthy had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written
Results
Baseline Characteristics and Traditional Risk Factors
Table 1 shows baseline characteristics at the time of blood draw for the 99 study subjects who died suddenly (63 definite and 36 probable) and the 294 matched controls. The mean time from study enrollment to SCD was 120.5 months. Risk factors significantly associated with SCD in this dataset include parental history of MI prior to age 60, history of hypertension and history of diabetes mellitus. Women who died suddenly also more frequently used aspirin. There were no significant associations between body-mass index, hormone replacement therapy use, alcohol intake, or physical activity.
Table 1.
Baseline Characteristics of the Study Participants
| Characteristic | SCD Cases N= 99 |
Controls N= 294 |
P-value |
|---|---|---|---|
| Age (yr), mean ± SD* | 60.6 ± 6.2 | 60.5 ± 6.1 | Matching Factor |
| Caucasian (n,%) | 99 (100) | 294 (100) | Matching Factor |
| Current Smoker (n,%) | 23 (23.2) | 69 (23.5) | Matching Factor |
| Body-mass index, mean ± SD* | 27.1 ± 5.5 | 26.4 ± 4.9 | 0.24 |
| Parental History of MI <60 yrs (n,%) | 28 (28.3) | 52 (17.7) | 0.04 |
| HRT use (n,%) | 32 (32.3) | 102 (34.7) | 0.64 |
| Aspirin use > 22 days per month (n,%) | 25 (25.3) | 49 (16.7) | 0.04 |
| History of Hypertension†(n,%) | 60 (60.6) | 128 (43.5) | 0.005 |
| History of Diabetes (n,%) | 21 (21.2) | 21 (7.14) | <0.001 |
| Alcohol, g/d, Median (IQR)‡ | 1.10 (0–7.60) | 1.0 (0–6.60) | 0.70 |
| Physical activity, MET-hrs/wk, Median (IQR) ‡ | 10.9 (3.2 –24.0) | 9.3 (3.5–24.9) | 0.94 |
| History of CVD(n,%) § | 30 (30.3) | 89 (30.3) | Matching factor |
| Cholesterol (mg/dL) | |||
| Total | 234.3 ± 39.9 | 229.2± 41.3 | 0.27 |
| LDL | 155.8 ± 37.1 | 150.4 ± 37.8 | 0.20 |
| HDL | 64.6 ± 16.8 | 65.2 ± 15.3 | 0.76 |
| Total-to-HDL cholesterol ratio | 3.81 ± 0.98 | 3.66 ± 0.87 | 0.14 |
| Triglycerides (mg/dL) | 177.6 ± 95.1 | 158.8± 81.3 | 0.06 |
| NT-proBNP, pg/mL | 94.0 (47.0–194.0) | 79.0 (45.0–148.0) | 0.03 |
| hsCRP (mg/L) | 3.62 (1.50–7.60) | 3.08 (1.22 –6.52) | 0.04 |
SD denotes standard deviation
Self-reported systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or taking antihypertensive medication.
IQR denotes interquartile range
Cardiovascular disease includes a history of MI, angina, CABG, or stroke
Serum Biomarker and Lipid Levels
The median levels of hs-CRP and NT-pro-BNP were 3.36 mg/L and 82.0 pg/mL respectively in this population. The distribution of unadjusted hsCRP and NT-proBNP levels among cases and controls is demonstrated in Figure 1. Median levels of hs-CRP and NT-proBNP tended to be slightly higher in cases versus controls, but the unadjusted continuous values did not achieve significance (P=0.09 and P=0.08 respectively). When the logarithmically transformed means of hs-CRP and NT-proBNP were compared, these differences were significant (P=0.03 and P=0.04, respectively). Of the lipid parameters, only the logarithmically transformed triglyceride levels were marginally higher in SCD cases than in the matched controls (P=0.06).
Figure 1.
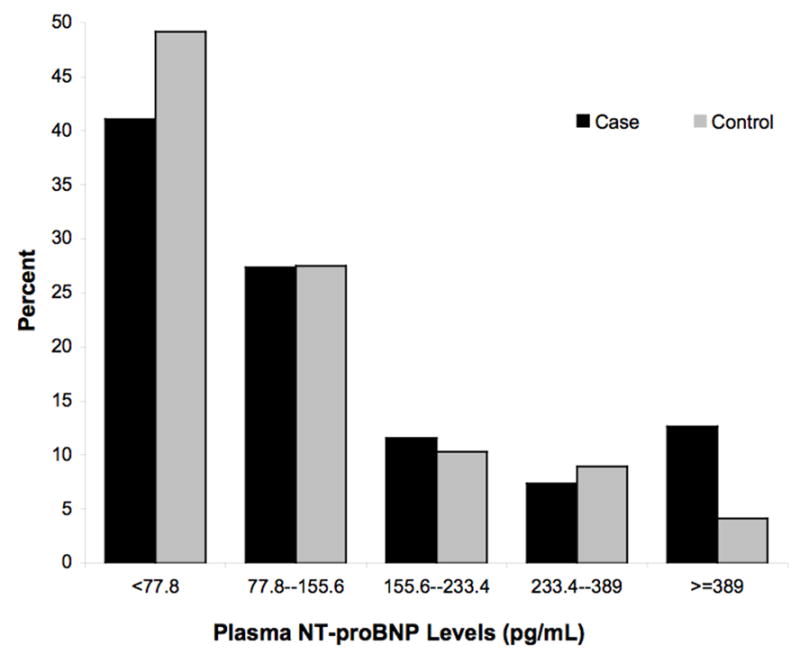
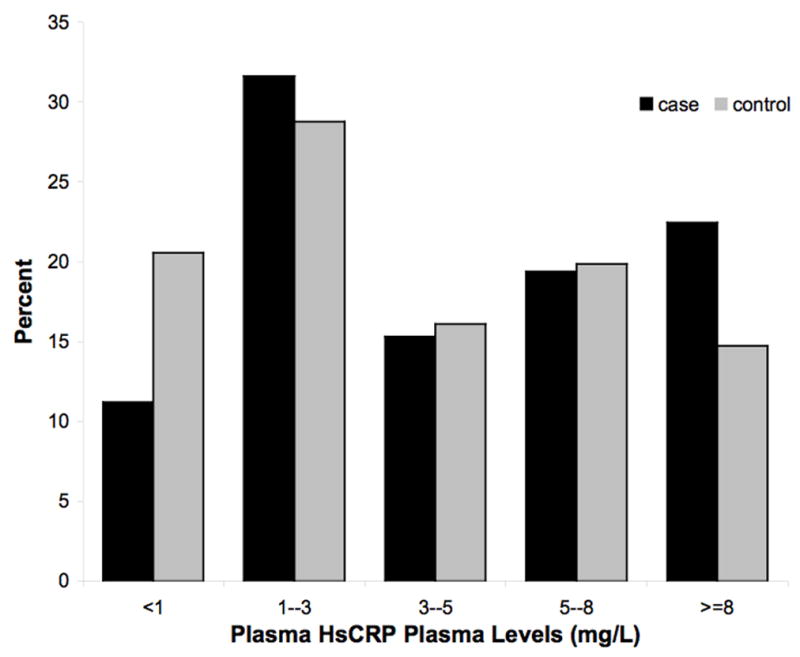
A and B: Distributions of non-transformed NT-proBNP (A) and Hs CRP levels (B) in cases and controls
Table 2 displays the relationship between quartiles of plasma lipid, NT-proBNP, and hsCRP levels, with the combined primary endpoint of probable and definite SCD. Baseline levels of hsCRP, LDL, HDL, triglycerides, and total cholesterol/HDL ratio were not significantly associated with SCD in age and fasting-adjusted or multivariable-adjusted models in the quartile analysis. In multivariable models that adjusted for CHD risk factors, GFR, and other biomarkers (hsCRP, triglyceride, and TC/HDL ratio: Table 2, Model 3), the trend across quartiles approached significance for NT-proBNP (P=0.05). Compared to women in the lowest quartile, those in the highest quartile of NT-proBNP had a rate ratio (RR) of 2.37 (95% CI, 0.97to 5.80; P=0.06). As demonstrated in figure 2, the percentage of cases and controls reporting a history of prior CVD was highest in the top quartile for both NT-proBNP and hsCRP.
Table 2.
Relative risks of Sudden Arrhythmic Death during Follow-up, According to Quartile of Plasma Biomarker Levels at Baseline
| Quartiles of Plasma Level |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | P for trend | |
| NT-proBNP | |||||
| Median (pg/mL) | 32.5 | 61 | 102.5 | 235 | |
| Quartile range- | 6 – 44 | 45 – 78 | 79 – 146 | 148 – 3126 | |
| Model 1 (age, fasting) * | 1.0 | 1.08 (0.53 –2.19) | 1.33 (0.65 –2.74) | 1.66 (0.79 –3.47) | 0.15 |
| Multivariable Model 2† | 1.0 | 1.34 (0.61 – 2.97) | 1.37 (0.61 – 3.08) | 2.07 (0.88 – 4.88) | 0.10 |
| Multivariable Model 3‡ | 1.0 | 1.33 (0.58 – 3.02) | 1.34 (0.58 – 3.10) | 2.37 (0.97 – 5.80) | 0.05 |
| hsCRP | |||||
| Median (mg/L) | 0.68 | 1.99 | 4.61 | 9.34 | |
| Quartile range- | 0.27 – 1.21 | 1.22 – 3.06 | 3.08 – 6.45 | 6.59 – 143 | |
| Model 1* | 1.0 | 1.31 (0.65 –2.61) | 1.43 (0.72 –2.86) | 1.91 (0.97–3.77) | 0.06 |
| Multivariable Model 2† | 1.0 | 1.07 (0.49 – 2.35) | 1.09 (0.48 – 2.49) | 1.32 (0.58 – 3.02) | 0.46 |
| Multivariable Model 3‡ | 1.0 | 1.18 (0.51 – 2.72) | 1.10 (0.46 – 2.65) | 1.30 (0.54 – 3.14) | 0.60 |
| Total Cholesterol-HDL Ratio | |||||
| Median- | 2.67 | 3.28 | 3.82 | 4.73 | |
| Quartile range- | 1.67 – 3.06 | 3.06 – 3.55 | 3.56 – 4.20 | 4.20 – 8.00 | |
| Model 1* | 1.0 | 1.18 (0.59 –2.34) | 0.92 (0.45 –1.90) | 1.56 (0.82 –2.98) | 0.18 |
| Multivariable Model 2† | 1.0 | 1.26 (0.58 – 2.76) | 1.04 (0.46 – 2.36) | 1.15 (0.51 – 2.58) | 0.89 |
| Multivariable Model 3‡ | 1.0 | 1.01 (0.42 – 2.43) | 1.38 (0.54 – 3.53) | 1.34 (0.49 – 3.66) | 0.50 |
| LDL-cholesterol | |||||
| Median (mg/dL) | 108.5 | 139 | 160 | 190.5 | |
| Quartile range- | 49 – 123 | 124 – 149 | 150 – 173 | 174 – 296 | |
| Model 1* | 1.0 | 1.92 (0.97 –3.80) | 1.46 (0.70 –3.03) | 1.79 (0.87 –3.68) | 0.22 |
| Multivariable Model 2† | 1.0 | 1.63 (0.76 – 3.51) | 1.44 (0.62 – 3.35) | 1.36 (0.59 – 3.13) | 0.66 |
| Multivariable Model 3‡ | 1.0 | 1.37 (0.59 – 3.20) | 1.66 (0.66 – 4.20) | 1.36 (0.53 – 3.51) | 0.57 |
| Triglycerides | |||||
| Median (mg/dL) | 82 | 122 | 166 | 239 | |
| Quartile range- | 7 – 101 | 102 – 141 | 142 – 193 | 194 – 759 | |
| Model 1* | 1.0 | 1.02 (0.51 –2.04) | 1.05 (0.51 –2.18) | 1.66 (0.84 –3.28) | 0.10 |
| Multivariable Model 2† | 1.0 | 0.90 (0.43 – 1.91) | 0.87 (0.39 – 1.97) | 1.52 (0.71 – 3.25) | 0.19 |
| Multivariable Model 3‡ | 0.85 (0.37 – 1.93) | 0.81 (0.32 – 2.03) | 1.46 (0.56 – 3.83) | 0.31 | |
| HDL-cholesterol | |||||
| Median (mg/dL) | 49 | 59 | 68 | 82.5 | |
| Quartile range- | 24 – 54 | 55 – 62 | 63 – 73 | 74 – 135 | |
| Model 1* | 1.0 | 0.66 (0.34 –1.31) | 0.89 (0.48 –1.65) | 0.92 (0.49 –1.72) | 0.95 |
| Multivariable Model 2† | 1.0 | 0.72 (0.35 – 1.51) | 1.03 (0.51 – 2.10) | 1.29 (0.61 – 2.73) | 0.40 |
| Multivariable Model 3‡ | 1.0 | 0.81 (0.37 – 1.76) | 1.08 (0.49 – 2.39) | 1.25 (0.53 – 2.93) | 0.53 |
Model 1: Controlled simultaneously for age and fasting status
Multivariable Model 2: Controlled for variables listed above in Model 1 and history of hypertension, history of diabetes, alcohol consumption (<0.1 g, 0.1–14.9, 15.0 to 29.9, 30+), parental history of myocardial infarction prior to age 60, body-massindex (<25 kg/m2, 25–30 kg/m2, >=30 kg/m2), physical activity (quintiles of metabolic equivalent (MET-hours)), current postmenopausal hormone use, GFR, and aspirin use>=22 days/month.
Multivariable Model 3: Controlled for variables listed above in multivariable Model 2 and simultaneously for plasma NT-proBNP, hsCRP, triglyceride, and TC/HDL-Ratio levels.
Figure 2.
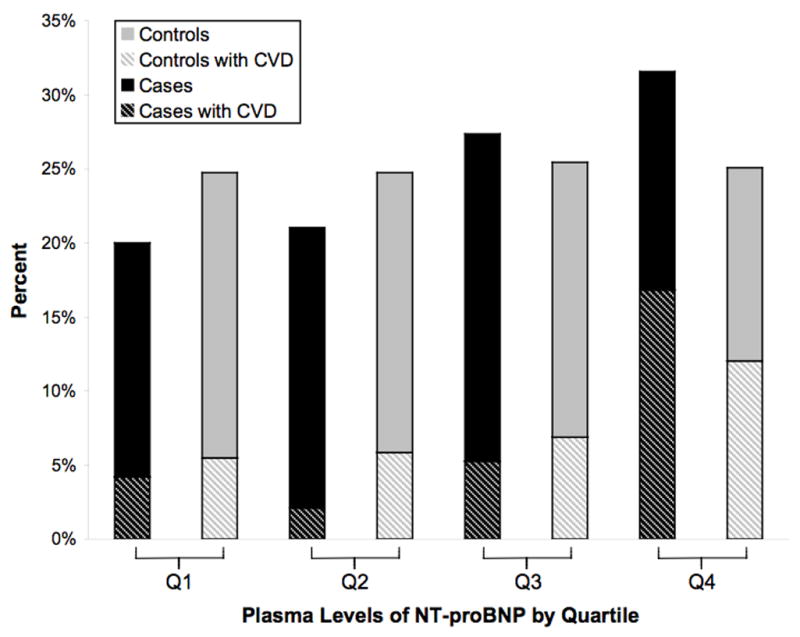
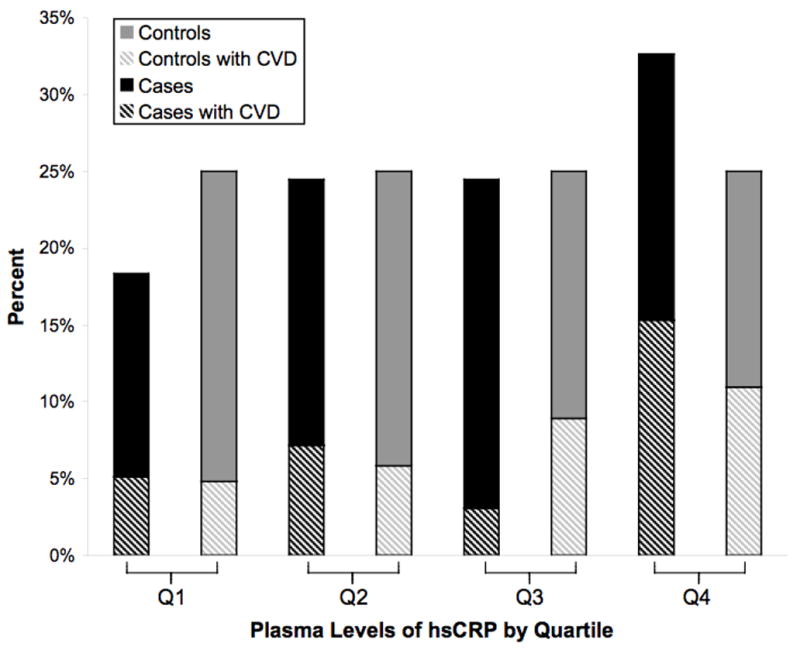
A and B: Proportion of SCD cases and controls within each quartile of NT-proBNP (A) and hsCRP levels (B) in patients with CVD at baseline. The dark bars represent SCD cases and the light bars represent controls, and the hatched bars denote the proportion who reported a prior history of CVD
When examined continuously, logarithmically transformed NT-proBNP levels and hsCRP levels were significantly associated with SCD risk in age and fasting-adjusted models, with a P-value equal to 0.04 and 0.03 respectively (Table 3). Further adjustment for CHD risk factors and GFR (Model 2) as well as simultaneous control for other biomarkers (hsCRP, triglyceride, and TC/HDL ratio; Model 3) strengthened the relationship with NT-proBNP and SCD (RR for 1-SD increment in log NT-proBNP level = 1.49; 95% CI, 1.09 – 2.05; P=0.01), while the relationship for hsCRP was attenuated and no longer significant (RR for 1-SD in log hs-CRP (RR= 1.17, 95% CI, 0.85 –1.61, P=0.34). Results were similar when the non-transformed continuous biomarker levels were entered in the above multivariable models (RR for 1-SD increment in NT-proBNP level = 1.34; 95% CI, 1.02–1.74; P=0.03). None of the lipid levels were significantly associated with SCD in the continuous analysis in either age-adjusted or multivariable models (data not shown).
Table 3.
Primary Analysis: Relative risks of Sudden Arrhythmic Death during Follow-up, According to Log-transformed Continuous values or Pre-specified Threshold levels of NT-proBNP and hsCRP.
| Biomarker | RR (95% CI) per 1-SD in Log Variable | P-value | RR (95%CI) for values above the 80th percentile | P-value | RR (95%CI) for Clinical Cut-Points* | P-value |
|---|---|---|---|---|---|---|
| NT-proBNP | ||||||
| Model 1 (age, fasting) † | 1.32 (1.01 – 1.71) | 0.04 | 1.50 (0.83 – 2.71) | 0.18 | 3.60 (1.43 – 9.10) | 0.007 |
| Multivariable Model 2‡ | 1.45 (1.07 – 1.97) | 0.02 | 1.77 (0.88 – 3.55) | 0.11 | 5.45 (1.77 –16.8) | 0.003 |
| Multivariate Model 3§ | 1.49 (1.09 –2.05) | 0.01 | 1.99 (0.97 – 4.12) | 0.06 | 5.68 (1.78 –18.2) | 0.003 |
| hsCRP | ||||||
| Model 1 (age, fasting) † | 1.33 (1.03 – 1.70) | 0.03 | 1.57 (0.92 – 2.68) | 0.10 | 1.40 (0..87–2.24) | 0.17 |
| Multivariate Model 2† | 1.18 (0.87 – 1.59) | 0.28 | 1.39 (0.74 – 2.61) | 0.33 | 1.12 (0.65–1.94) | 0.69 |
| Multivariate Model 3 § | 1.17 (0.85 – 1.61) | 0.34 | 1.49 (0.76 – 2.93) | 0.25 | 1.05 (0.58–1.91) | 0.86 |
Model 1: Controlled simultaneously for age and fasting status
Multivariable Model 2: Controlled for variables listed above in Model 1 and history of hypertension, history of diabetes, alcohol consumption (<0.1 g, 0.1–14.9, 15.0 to 29.9, 30+), parental history of myocardial infarction prior to age 60, body-massindex (<25 kg/m2, 25–30 kg/m2, >=30 kg/m2), physical activity (quintiles of metabolic equivalent (MET-hours)), current postmenopausal hormone use, GFR, and aspirin use>=22 days/month. ‡Multivariate
Model 3: Controlled for variables listed above in multivariable Model 2 and simultaneously for triglycerides, TC/HDL-Ratio, and for NTproBNP or hsCRP.
When pre-specified cutoffs were examined (table 3), there was a trend toward a higher risk among those with NT-proBNP levels above the 80th percentile (≥187 pg/mL) in the fully adjusted multivariable model (RR=1.99; 95% CI, 0.97–4.12; P=0.06). Women with NT-proBNP levels above the proposed clinical cut-point of 389 pg/mL (n=24, 6.1%) were at a markedly increased risk of SCD in both age-adjusted and multivariable adjusted models (RR=5.68; 95% CI, 1.78–18.2; P=0.003) as compared to women with NT-proBNP levels below this cutpoint. The proportion of cases with NT-proBNP levels > 389 pg/mL was 12.6%, and the two-thirds of cases and controls above this cutpoint had a history of prior CVD The corresponding categorical analyses for hsCRP utilizing the 80th percentile or a clinical cutpoint of > 3.0mg/L were not significant in either the age and fasting-adjusted or the multivariable adjusted models. Similarly, lipid levels above the 80th percentile and/or above accepted clinical cut-points were not significantly associated with SCD risk in the age and fasting-adjusted or multivariable adjusted model (data not shown).
Sensitivity Analyses (Table 4)
Table 4.
Sensitivity Analyses: Relative risks of Sudden Cardiac Death during Follow-up, according to Log- transformed Continuous values or Pre-specified Threshold levels of NT-proBNP and hsCRP.
| A. Analyses utilizing Alternative Controls matched for Interim Development of CVD | ||||
|---|---|---|---|---|
| Biomarker | RR (95% CI) per 1-SD in Log Variable | P-value | RR (95%CI) for Clinical Cut-Points* | P-value |
| NT-proBNP | ||||
| Model 1 (age, fasting) † | 1.22 (0.94–1.57) | 0.13 | 3.25 (1.33 – 7.92) | 0.01 |
| Multivariate Model‡ | 1.35 (1.00–1.95) | 0.05 | 4.35 (1.49 – 12.6) | 0.01 |
| hsCRP | ||||
| Model 1 (age, fasting) † | 1.30 (1.01–1.68) | 0.04 | 1.36 (0.85–2.17) | 0.21 |
| Multivariate Model‡ | 1.26 (0.92 –1.74) | 0.15 | 1.26 (0.71–2.22) | 043 |
| B. Analyses limited to Women without CVD at baseline (n=68 cases) | ||||
| Biomarker | RR (95% CI) per 1-SD in Log Variable | P-value | RR (95%CI) for Clinical Cut-Points* | P-value |
| NT-proBNP | ||||
| Model 1 (age, fasting) † | 1.32 (0.97 – 1.78) | 0.01 | 6.01 (1.33 – 27.2) | 0.02 |
| Multivariate Model† | 1.54 (1.02 – 2.33) | 0.04 | 4.51 (0.77 – 26.3) | 0.09 |
| hsCRP | ||||
| Model 1 (age, fasting) † | 1.38 (1.07 – 1.78) | 0.03 | 1.73 (0.98 – 3.08) | 0.06 |
| Multivariate Model‡ | 0.96 (0.61 – 1.48) | 0.84 | 0.80 (0.36 – 1.81) | 0.59 |
| C. Analyses limited to Definite SCDs (n=63 cases) | ||||
| Biomarker | RR (95% CI) per 1-SD in Log Variable | P-value | RR (95%CI) for Clinical Cut-Points* | P-value |
| NT-proBNP* | ||||
| Model 1 (age, fasting) † | 1.43 (1.04 –1.97) | 0.03 | 4.68 (1.48–14.8) | 0.009 |
| Multivariate Model‡ | 1.71 (1.12–2.63) | 0.01 | 19.9 (2.67–149) | 0.004 |
| hsCRP | ||||
| Model 1 (age, fasting) † | 1.46 (1.06 –2.01) | 0.02 | 1.72 (0.93 –3.17) | 0.09 |
| Multivariate Model‡ | 1.48 (0.94–2.33) | 0.09 | 2.01 (0.80 –5.04) | 0.14 |
hsCRP (>3.0 mg per liter)32 and NT-pro-BNP (> 389 pg/mL)33.
Model 1: Controlled simultaneously for age and fasting status
Multivariable Model 2: Controlled for variables listed above in Model 1 and history of hypertension, history of diabetes, alcohol consumption (<0.1 g, 0.1–14.9, 15.0 to 29.9, 30+), parental history of myocardial infarction prior to age 60, body-mass index (<25 kg/m2, 25–30 kg/m2, >=30 kg/m2), physical activity (quintiles of metabolic equivalent (MET-hours)), current postmenopausal hormone use, GFR, aspirin use>=22 days/month, triglycerides, TC/HDL-Ratio, and for NTproBNP or hsCRP
When analyses were further adjusted for the development of CVD after the blood draw by utilizing the alternate set of controls, relationships for NT-proBNP were attenuated slightly. The RR was 1.35 (95% CI, 1.00–1.95; P =0.05) for 1-SD increment in log NT-proBNP level and 4.35 (95% CI, 1.49–12.6; P =0.01) for NT-proBNP levels above the 389 pg/mL cutpoint in the fully adjusted model. When analyses were limited to the 68 women without reported CVD at baseline, relationships for NT-proBNP were not materially altered (RR=1.54; 95% CI, 1.02 –2.33; P = 0.04 for 1-SD increment in log NT-proBNP level), however, only eight women had NT-proBNP levels above 389 pg/mL cutpoint (RR= 4.51; 95% CI, 0.77 – 26.3; P = 0.09). In contrast, relationships for hsCRP were attenuated further.
When analyses were limited to the definite SCDs (n=63), results for NT-proBNP and hsCRP were stronger. For each 1-SD increment in log NT-proBNP level, the RR for definite SCD was 1.71 (95% CI, 1.12 – 2.63; P=0.01) in the fully adjusted multivariable model, and women above the 389 pg/mL cutpoint were at a markedly elevated risk (RR=19.9; 95% CI, 2.67–149; P=0.004). For hsCRP, the multivariable continuous relationship between log transformed hsCRP and definite SCD was strengthened but remained non-significant (RR= 1.48; 95% CI, 0.94 – 2.33 for 1 SD increment in log hsCRP; P = 0.09).
Discussion
In this prospective nested case-control study of women, baseline blood levels of NT-proBNP were associated with SCD risk over the 16 years of follow-up. These relationships were significant in the primary analyses when analyzed as continuous variables or when pre-specified cutoffs were utilized. Relationships strengthened when adjusted for CHD risk factors and in analysis limited to definite SCDs, and the continuous relationship remained significant even after patients who reported CVD at baseline were excluded from the analysis. In the primary multivariable analyses including definite and probable SCD events, each increment of 1-SD in log NT-proBNP was associated with an approximate 50% increase in the risk of SCD, and levels above the pre-specified cut-point of 389pg/mL, where women with a history of CVD predominated, were associated with a five-fold increased risk of SCD. In contrast to the findings for NT-proBNP, log hsCRP levels were not significantly associated with SCD risk after multivariable adjustment. There were no significant associations observed between any of the lipid values and SCD among women in any of the primary or secondary analyses.
Previously, elevated levels of natriuretic peptides including NT-proBNP have been shown to be associated with risk of SCD or ventricular arrhythmias in high-risk patient populations. In a study of 452 ambulatory patients with chronic heart failure and left ventricular ejection fraction (LVEF) less than 35%, elevated BNP levels were shown to be a strong, independent predictor of SCD9. In another series of 521 patients status post acute MI, elevated BNP (HR=3.9, 95% CI 1.2 to 12.3, P=0.02) remained a significant predictor of SCD risk even after adjusting for clinical variables and LVEF10. In addition, several small studies suggest that these markers may predict appropriate shocks for ventricular tachycardia/fibrillation in ICD patients12, 13 In population based studies, BNP and NT-proBNP levels have been associated with total cardiovascular events and overall mortality 21, 22, 25. This study extends these findings by establishing a relationship between NT-proBNP levels and the specific endpoint of SCD in an unselected population of women with and without CVD.
These data offers potential insights into the pathophysiology underlying SCD in women. Elevated natriuretic peptide levels are thought to be reflective of increased myocardial wall stress and filling pressures, and chronic exposure to these hemodynamic stressors may result in significant left ventricular dilation, hypertrophy, and/or fibrosis altering the electrophysiologic substrate and increasing cardiac vulnerability to malignant arrhythmias26–28. Alternatively, increased ventricular filling pressures due to already established, but as yet unrecognized, structural heart disease might be responsible for the elevated SCD risk associated with high levels of NT-proBNP. Consistent with this possibility, relationships were attenuated slightly with further control for the development of CVD detected after the NT-proBNP measurement.
The consistently observed association between NT-proBNP and SCD in all analyses, as compared to the relative lack of an association between the other well-established CHD risk factors, namely HDL-C, LDL-C, triglycerides, total cholesterol/HDL ratio, and hsCRP and SCD, suggests that elevated filling pressures and associated mechanical-electrical disturbances may play a greater role then occult CHD in SCD risk among women. In support of this hypothesis, women who suffer SCD appear to have a lower prevalence of underlying CHD as compared to men in several series.5, 6
This potential sex difference in the underlying prevalence of CHD may account for the relative lack of prediction of hsCRP as compared to that previously observed among apparently healthy men11. In the previous study, CRP level was significantly associated with SCD risk even after controlling for lipid levels over 17 years of follow-up. An alternative explanation for these apparent sex differences may be the expanded definition of SCD. Since women are more likely to be unwitnessed at the time of their SCD29, we included unwitnessed deaths that were known to be alive and well without symptoms in the preceding 24 hours similar to other epidemiologic studies16, 17. The inclusion of unwitnessed deaths increasesthe sensitivity for arrhythmic death15, but also reduces the proportion of all sudden natural deathsthat are due to cardiac causes30. In support of this possible explanation for the discrepant results, associations were stronger for hsCRP in analyses excluding these un-witnessed deaths. However, the smaller number of events limits our power and confidence regarding these associations.
The positive relationships observed here for NT-proBNP may have potential clinical implications as well. Marked elevations in NT-pro-BNP levels may be useful in identifying women at higher risk for SCD many years prior to the fatal event. However, the relationship observed here in the small subgroup with high NT-proBNP levels would need to be confirmed in a much larger population of women with high levels of NT-proBNP. If confirmed, documentation of high NT-proBNP levels may provide the impetus to screen for structural heart disease and to initiate specific therapy, such as beta-blockade, to prevent SCD.
Several limitations of the present study warrant consideration. First, our analysis was based on a single baseline determination of each plasma marker. Therefore, we were unable to account for changes in these markers over time and are limited in our ability to assess effects on short-term risk. This limitation could account for the relatively null results observed for lipid parameters and hsCRP if the majority of their effects are on short-term risk or if levels changed significantly over the course of the study due to changes in dietary habits and/or lipid lowering agents. Second, the selective nature of the cohort, U.S. female registered nurses who were entirely Caucasian, may limit the generalizability of the findings to other groups of women, ethnicities, or men. The healthy nature of this cohort is demonstrated by the low SCD rate, and the population effect observed here may or may not prove to have clinical applicability for individual subjects.
Even though this study is the largest we know of to examine the association between these plasma markers and risk of SCD in women, our power to detect small-to-moderate effects is still limited by the small number of cases, especially for analyses limited to definite SCD cases and among women without CVD. Therefore, it is possible that associations for the other markers would have emerged in a larger sample size. Finally, although the primary and secondary analyses were pre-specified, concern for multiple comparisons resulting in spurious significant results is warranted given the large number of comparisons. However the consistency of the association between NT-proBNP and SCD across analyses supports the validity of the results.
In summary, in this population of women, higher baseline blood levels of NT-proBNP were associated with the subsequent development of SCD, and this relationship was independent of established risk factors for CHD and/or SCD. NT-proBNP, when used in combination with other cardiac risk factors, may be a useful indicator of long-term SCD risk among women.
Clinical Summary
Plasma concentrations of N-terminal pro-B type natriuretic peptide (NT-proBNP) predict risk of sudden cardiac death (SCD) in patients with known cardiac disease, but currently there are no data for SCD risk prediction in populations unselected on the basis of cardiac disease. In this nested case-control analysis within the 121,700 person Nurses’ Health Study, 99 cases of definite or probable SCD were identified and matched to 294 controls on the basis of age, smoking, and prior cardiovascular disease. Baseline plasma NT-proBNP levels, but not C-reactive protein or lipid levels, were significantly associated with SCD using a multivariable model adjusting for coronary heart disease (CHD) risk factors and other biomarkers. After multivariable adjustment, each increment of 1-SD in log NT-proBNP was associated with an approximate 50% increase in the risk of SCD, and levels above the pre-specified cut-point of 389pg/mL were associated with a five-fold increased risk of SCD. These findings offer potential insights into the pathophysiology underlying SCD in women. Specifically, they point to increased myocardial wall stress, along with associated left ventricular dilatation, hypertrophy, and/or fibrosis, as potential mediators of electrical instability leading to SCD in women. The lack of association of other well-established CHD risk factors (including hsCRP) suggest that mechanical-electrical disturbances might play a greater role than undetected atherosclerosis in SCD risk among women. If confirmed in larger studies, marked elevations in NT-pro-BNP levels might also be useful in identifying women at higher risk for SCD many years prior to the fatal event.
Acknowledgments
The Nurses’ Health Study is located at the Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School. We are indebted to the participants in the Nurses’ Health Study for their outstanding commitment and cooperation, and to Julie Pester, Martin Van Denburgh, Lisa Dunn, Barbara Egan, and Helena Judge Ellis for their expert assistance.
Funding:
The study was supported by a research grant from Siemens Healthcare Diagnostics to Dr. Albert. The Nurses’ Health Study is supported by Grants CA-87969, HL-34594, and HL-03783 from the National Institutes of Health. The study sponsor did not influencethe study design, analysis, interpretation, and presentationof data.
Footnotes
Disclosures:
Dr. Januzzi reports receiving grant support, speaker’s fees, and/or consulting income from Roche Diagnostics, Dr. Januzzi reports receiving grant support, speaker’s fees, and/or consulting income from Roche Diagnostics, Siemens Healthcare Diagnostics, Ortho Clinical Diagnostics, Inverness Medical Innovations, BG Medicine, and Critical Diagnostics.
Dr. Gantzer is employed by the study sponsor, Siemens Healthcare Diagnostics.
Dr Albert reports receiving grant support from Siemens Healthcare Diagnostics.
The assays used in this study were paid for and manufactured by Siemens Healthcare Diagnostics, the study sponsor. In addition to Siemens Healthcare Diagnostics, the following companies that Dr. Januzzi consults for also make assays for natriuretic peptides and/or hsCRP: Roche Diagnostics, Ortho Clinical Diagnostics, and Inverness Medical Innovations.
References
- 1.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 2.Gillum RF. Geographic variation in sudden coronary death. Am Heart J. 1990;119:380–389. doi: 10.1016/s0002-8703(05)80031-6. [DOI] [PubMed] [Google Scholar]
- 3.Cupples LA, Gagnon DR, Kannel WB. Long- and short-term risk of sudden coronary death. Circulation. 1992;85:I11–18. [PubMed] [Google Scholar]
- 4.Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, Stampfer MJ, Manson JE. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–2101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 5.Albert CM, McGovern BA, Newell JB, Ruskin JN. Sex differences in cardiac arrest survivors. Circulation. 1996;93:1170–1176. doi: 10.1161/01.cir.93.6.1170. [DOI] [PubMed] [Google Scholar]
- 6.Chugh SS, Kelly KL, Titus JL. Sudden cardiac death with apparently normal heart. Circulation. 2000;102:649–654. doi: 10.1161/01.cir.102.6.649. [DOI] [PubMed] [Google Scholar]
- 7.Wiese S, Breyer T, Dragu A, Wakili R, Burkard T, Schmidt-Schweda S, Fuchtbauer EM, Dohrmann U, Beyersdorf F, Radicke D, Holubarsch CJ. Gene expression of brain natriuretic peptide in isolated atrial and ventricular human myocardium: influence of angiotensin II and diastolic fiber length. Circulation. 2000;102:3074–3079. doi: 10.1161/01.cir.102.25.3074. [DOI] [PubMed] [Google Scholar]
- 8.Wang TJ, Levy D, Benjamin EJ, Vasan RS. The epidemiology of “asymptomatic” left ventricular systolic dysfunction: implications for screening. Ann Intern Med. 2003;138:907–916. doi: 10.7326/0003-4819-138-11-200306030-00012. [DOI] [PubMed] [Google Scholar]
- 9.Berger R, Huelsman M, Strecker K, Bojic A, Moser P, Stanek B, Pacher R. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002;105:2392–2397. doi: 10.1161/01.cir.0000016642.15031.34. [DOI] [PubMed] [Google Scholar]
- 10.Tapanainen JM, Lindgren KS, Makikallio TH, Vuolteenaho O, Leppaluoto J, Huikuri HV. Natriuretic peptides as predictors of non-sudden and sudden cardiac death after acute myocardial infarction in the beta-blocking era. J Am Coll Cardiol. 2004;43:757–763. doi: 10.1016/j.jacc.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 11.Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105:2595–2599. doi: 10.1161/01.cir.0000017493.03108.1c. [DOI] [PubMed] [Google Scholar]
- 12.Blangy H, Sadoul N, Dousset B, Radauceanu A, Fay R, Aliot E, Zannad F. Serum BNP, hs-C-reactive protein, procollagen to assess the risk of ventricular tachycardia in ICD recipients after myocardial infarction. Europace. 2007;9:724–729. doi: 10.1093/europace/eum102. [DOI] [PubMed] [Google Scholar]
- 13.Yu H, Oswald H, Gardiwal A, Lissel C, Klein G. Comparison of N-terminal pro-brain natriuretic peptide versus electrophysiologic study for predicting future outcomes in patients with an implantable cardioverter defibrillator after myocardial infarction. Am J Cardiol. 2007;100:635–639. doi: 10.1016/j.amjcard.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 14.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 15.Hinkle LE, Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65:457–464. doi: 10.1161/01.cir.65.3.457. [DOI] [PubMed] [Google Scholar]
- 16.Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 17.de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, van Ree JW, Daemen MJ, Houben LG, Wellens HJ. Out-of-hospital cardiac arrest in the 1990’s: a population-based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997;30:1500–1505. doi: 10.1016/s0735-1097(97)00355-0. [DOI] [PubMed] [Google Scholar]
- 18.Prentice RL, Breslow NE. Retrospective studies and failure time models. Biometrika. 1978;65:153–158. [Google Scholar]
- 19.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 21.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 22.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. Jama. 2005;293:1609–1616. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49:2129–2138. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 24.Emberson JR, Ng LL, Armitage J, Bowman L, Parish S, Collins R. N-terminal Pro-B-type natriuretic peptide, vascular disease risk, and cholesterol reduction among 20,536 patients in the MRC/BHF heart protection study. J Am Coll Cardiol. 2007;49:311–319. doi: 10.1016/j.jacc.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 25.Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, Venge P, Arnlov J. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 26.Hansen DE, Craig CS, Hondeghem LM. Stretch-induced arrhythmias in the isolated canine ventricle. Evidence for the importance of mechanoelectrical feedback. Circulation. 1990;81:1094–1105. doi: 10.1161/01.cir.81.3.1094. [DOI] [PubMed] [Google Scholar]
- 27.Franz MR, Cima R, Wang D, Profitt D, Kurz R. Electrophysiological effects of myocardial stretch and mechanical determinants of stretch-activated arrhythmias. Circulation. 1992;86:968–978. doi: 10.1161/01.cir.86.3.968. [DOI] [PubMed] [Google Scholar]
- 28.Kowey PR, Friechling TD, Sewter J, Wu Y, Sokil A, Paul J, Nocella J. Electrophysiological effects of left ventricular hypertrophy. Effect of calcium and potassium channel blockade. Circulation. 1991;83:2067–2075. doi: 10.1161/01.cir.83.6.2067. [DOI] [PubMed] [Google Scholar]
- 29.Straus SM, Bleumink GS, Dieleman JP, van der Lei J, Stricker BH, Sturkenboom MC. The incidence of sudden cardiac death in the general population. J Clin Epidemiol. 2004;57:98–102. doi: 10.1016/S0895-4356(03)00210-5. [DOI] [PubMed] [Google Scholar]
- 30.Kuller L, Lilienfeld A, Fisher R. An epidemiological study of sudden and unexpected deaths in adults. Medicine (Baltimore) 1967;46:341–361. doi: 10.1097/00005792-196707000-00003. [DOI] [PubMed] [Google Scholar]


