Abstract
SPL7013 is the sodium salt of a sulfonated dendrimer that has potent antiviral properties. VivaGel®, a topical gel containing 3% w/w SPL7013, has been shown to be safe and well-tolerated in human clinical studies. BufferGel® is a Carbopol®-based acidic buffering gel that enhances the natural protective action of the vagina to produce a broad-spectrum microbicidal environment. The positive attributes of both gels were combined into a combination vaginal microbicidal gel having dual mechanisms of action. A 3% w/w SPL7013 combination gel, pH 3.7, was developed and fully characterized, and was shown to have more than 2-fold greater acidic buffering capacity than BufferGel. Ultracentrifugation experiments demonstrated that SPL7013 was not sequestered or entropically trapped in the viscous gel, thereby confirming, along with viral challenge studies, that SPL7013 has sufficient mobility in the viscous gel to exert antiviral properties.
Keywords: microbicide, vaginal, dendrimer, ultracentrifugation, diffusion, Carbopol®
INTRODUCTION
The incidence of HIV infection continues to grow rapidly, with the most recent data from the United Nations showing that 42 million people worldwide are HIV positive (Tanne, 2006; Zarocostas, 2005). Of particular concern is the high percentage of women who have become infected with HIV. In those parts of the world where the primary means of infection is through heterosexual contact, women comprise up to 60% of the total infected population. An additional concern with the increasing number of HIV infected women is the increased rate of transmission of HIV to the newborn. Thus, there is an urgent need for strategies to prevent HIV infection during sexual contact. One strategy is the use of microbicides, which are chemical entities that can prevent or reduce transmission of sexually transmitted infections (STIs) when applied to the vagina or rectum (Ndesendo 2008). Microbicidal candidates have included, among others, 1) macromolecular polyanions such as PRO 2000 (naphthalene 2-sulphonate polymer), cellulose sulfate, dextran sulfate, and carrageenan, (Cummins 2007; Maguire 2001; Neurath 2002; Turpin, 2002) or 2) acidic buffering gels, which exert their activity by inducing or maintaining low vaginal pH, wherein viruses such as HIV and HSV, cellular vectors such as HIV-infected cells, and pathogenic bacteria are inactivated (Olmsted 2005; Patton 2004; Williams 2007; Zeitlin 2001)
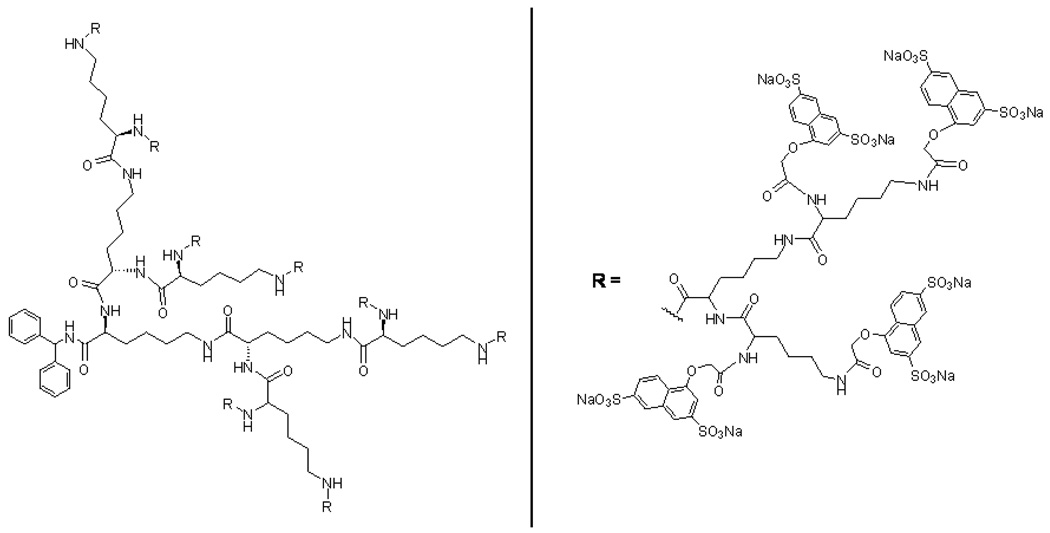
Dendrimers are a relatively new class of macromolecule characterized by highly branched, three-dimensional architectures that offer an alternative to polyanionic microbicidal polymers (McCarthy 2005). The controlled synthesis of dendrimers allows the assembly of highly defined structures that radiate out in generations from a central initiator core. Several anionic dendrimers have been synthesized and tested in-vitro and in-vivo for their anti-viral properties. In fact, selected anionic dendrimers are potent inhibitors of a range of STI’s, including HIV-1, HSV-1, and HSV-2. One such polyanionic dendrimer being developed by Starpharma Pty. Ltd, SPL7013 (16,581 Da), is built up from a divalent core, the benzhydrylamine amide of L-lysine (Figure 1). Successive additions of four L-lysine layers leads to a dendrimer with 32 amine groups on the surface; 16 α-amines and 16 ε-amines from the outer L-lysine layer. The final step in the synthesis involves the last amide-bond-forming reaction to attach 32 sodium (1-naphthyleneyl-3,6-disulphonic acid)-oxyacetamido groups to the surface via amide linkers. The EC50 of SPL7013 against HIV-1 (Strain IIIB) was <1 µg/ml (Bernstein 2003; Gong 2005). In addition, SPL7013 was found to be highly active against CCR5- and CXCR4-tropic HIV-1 infection (Lackman-Smith 2008). A 5% w/w SPL7013 vaginal gel was found to completely prevent vaginal transmission of SHIV89.6P in female pigtailed macaques (Jiang 2005). In June 2003, the SPL7013 gel (termed VivaGel) was the first submission to the US FDA for a dendrimer-based drug. Initial human trials have shown VivaGel to be safe and well-tolerated (Rupp 2007), and additional clinical studies are on-going. Roth et al. recently showed that a SPL7013 gel significantly reduced the amount of HIV1 bound to epithelial cells in-vitro versus placebo gel (Roth 2007). In addition, Abner et al. showed that SPL7013 gel significantly reduced HIV-1 infection in human colorectal explant tissue versus placebo (Abner 2005). Rupp provides an excellent review of the HIV and HSV antiviral activity of SPL7013 and SPL7013-containing gels in in-vitro and animal models (Rupp 2007).
Figure 1.
Structure of SPL7013 dendrimer (MW 16,581 Da). Successive additions of four L-lysine layers to the divalent core of benzhydrylamine amide of L-lysine leads to a final dendrimer molecule having 32 amide-linked 1-naphthyleneyl-3,6-disulphonic acid)-oxyacetamido groups on the surface.
BufferGel is a broad-spectrum microbicide/spermicide gel being developed by ReProtect Inc. that enhances the protective action of vaginal acidity (Ballagh 2008; Patton 2004; Van De Wijgert 2001; Williams 2007). BufferGel is a non-toxic lubricant gel formulated at pH 3.8, the pH of the healthy vagina. BufferGel is made with a strongly buffering polymer, Carbopol 974P, that releases protons to acidify the ejaculate, thereby enhancing the protective acidity of the vagina and complementing the acidifying action of lactobacilli, the vaginal flora that create the protective acidity of the vagina (Olmsted 2000; Olmsted 2005). Human clinical trials have shown BufferGel to be safe, acceptable, and less inflammatory and tissue-destructive than detergent-based spermicides (Cone 2006; Mayer 2001; Patton 2004; Van De Wijgert 2001), and it is currently being investigated in Phase 3 clinical trials.
The overall goal of the present studies was to combine SPL7013 dendrimer in a Carbopol-based acidic buffering gel to create a microbicidal gel having dual mechanisms of action. Various combination vaginal gels were prepared and tested for pH, osmolality, buffering capacity, and viscosity. The effect of temperature, pH, and SPL7013 concentration on gel viscosity was also determined. Since SPL7013 dendrimer is a polyanionic macromolecule, there was some concern that its diffusion would be constrained in a viscous Carbopol-based gel thereby reducing its antiviral activity. Thus, an ultracentrifugation method was developed to ascertain the tendency of SPL7013 to be sequestered or trapped in the viscous gel.
MATERIALS AND METHODS
Materials
Carbopol 974P NF and Carbopol 971P NF were obtained from Lubrizol Corporation (Wickliff, OH). Lactic acid, USP, methyl paraben NF, propyl paraben NF, Edetate Disodium USP (ethylenediaminetetraacetic acid disodium; EDTA disodium), glacial acetic acid, potassium hydroxide NF, sodium chloride (0.9% w/v), and sodium hydroxide NF were purchased from Spectrum Quality Products, Inc. (New Brunswick, NJ). SPL7013 dendrimer (lot #08-036-026- 02) was provided by Starpharma Pty. Ltd. (Melbourne, Australia). The SPL7013 synthetic process is monitored by HPLC and LC/MS in-process controls and delivers SPL7013 as a single molecular entity as determined by HPLC, capillary electrophoresis (CE), and electrospray mass spectral analysis.
Gel Preparation
The gels were prepared using a modification of a previously published method (Bernstein 2003) using a Caframo Ultrahigh-Torque Stirrer Model BDC1850 (Wiarton, Ontario, Canada) with a 5.1 cm stainless steel propeller blade. A general formula and method is provided for preparing 200 g batches based on Carbopol 974P. Specifically, MilliQ water (167 g) was added to the stirring vessel with stirring at 800 rpm. Next, 300 mg EDTA disodium USP was added and allowed to dissolve (2 min). Next, 8.68 g Carbopol 974P was then added slowly over 15–20 min while stirring at 1000 rpm to allow for homogeneous hydration of the polymer. Next, 360 mg methyl paraben and 40 mg propyl paraben were added and allowed to dissolve over 5 min. Lactic acid (1000 µL) and acetic acid (166 µL) were then added and allowed to mix for 2 min. SPL7013 dendrimer (6.795 g) was then added and allowed to completely dissolve. An additional 3.72 g of Carbopol 974P was then added while stirring at 1200 rpm over 15–20 minutes. Finally, the pH was adjusted to the target pH of 3.7–3.8 using 2N KOH (~7.3 mL) and the batch was then q.s. to 200 g with MilliQ water. Additionally, in order to test the effect of final gel pH on viscosity, gels were made having final pHs of 3.4, 3.6, 3.8, 4.0, and 4.2. Finally, to determine the effect of SPL7013 dendrimer on gel viscosity, four gels were made having a final pH 3.7–3.8 with 6.2% w/w Carbopol 974P and lactic and acetic acids and containing final SPL7013 concentrations of 3%, 4%, 5%, and 6%.
Gel Characterization Methods
The viscosity of the gels was determined using a Brookfield Cone and Plate Rheometer RVDV-III+ (Brookfield Engineering; Middleborro, MA) at 25°C at 5 min at an RPM of 1.7 using Spindle CPE-52. For some gel samples, the viscosity was tested at various temperatures (in the range of 4–37°C) to ascertain the effect of temperature on gel viscosity. For these measurements, the rheometer was pre-equilibrated to the desired temperature using a circulating water bath. Osmolality was determined using a FISKE 110 Freezing-Point Depression Osmometer. The pH of the gels was determined using an Orion pH Meter Model 520A. The buffer capacity was determined by measuring the number of mEq of 0.5N NaOH titrametric standard needed to bring 2.5 g of the formulation from its starting pH of approximately 3.8 to a final pH of 5.0. Specifically, approximately 2.5 g of the sample was deposed in a beaker. The sample was mixed with 20.0 mL of 0.9% NaCl solution using a stir bar for 5 min at a temperature of 23–25°C. The homogeneously mixed sample was then titrated with standardized 0.5 N NaOH solution until the pH of the mixture was 5.0. The volume of the NaOH needed to raise the pH to 5.0 was recorded and the buffer capacity of the sample was calculated using the following equation:
SPL7013 Dendrimer Sequestration Experiments
Experiments were conducted in order to determine the relative extent of sequestration of SPL7013 in centrifuged prototype combination vaginal gels. One (1) gram samples of the indicated 3% w/w gels were ultracentrifuged using a Beckman TL-100 Ultracentrifuge with T100.3 rotor at 25° C at 50,000 rpm for 30 min, along with 1 g samples of a 3% w/w SPL7013 solution, in order to assess settling and sedimentation, if any, of the SPL7013 in solution, as well as to serve as a control for assaying SPL7013 concentrations. After centrifugation for 30 min, a 100 µL aliquot part of the supernatant was carefully aspirated from each sample and diluted in deionized water to a fixed final volume of 20 mL. In order to determine the concentration of SPL7013 retained in the dense gel layers, the remaining supernatant was carefully decanted, and a 100 mg sample of the gel layer was removed with a spatula, placed in a flask, and similarly diluted. Standard curves of each gel and the control solution were prepared by assaying the UV absorbance (332 nm) of serial dilutions of the 3%w/w SPL7013 control solution and the two 3%w/w starting control gels. UV spectroscopy at 332 nm was selected as the analytical method, since the Carbopol polymers showed no absorbance at this wavelength, obviating the need for chromatographic separation of components, while SPL7013 displayed an absorption peak at 332 nm. No interference from gel components was noted at 332 nm using this assay, as all three curves were nearly superimposable, with nearly identical linearity and linear equations (data not shown).
RESULTS AND DISCUSSION
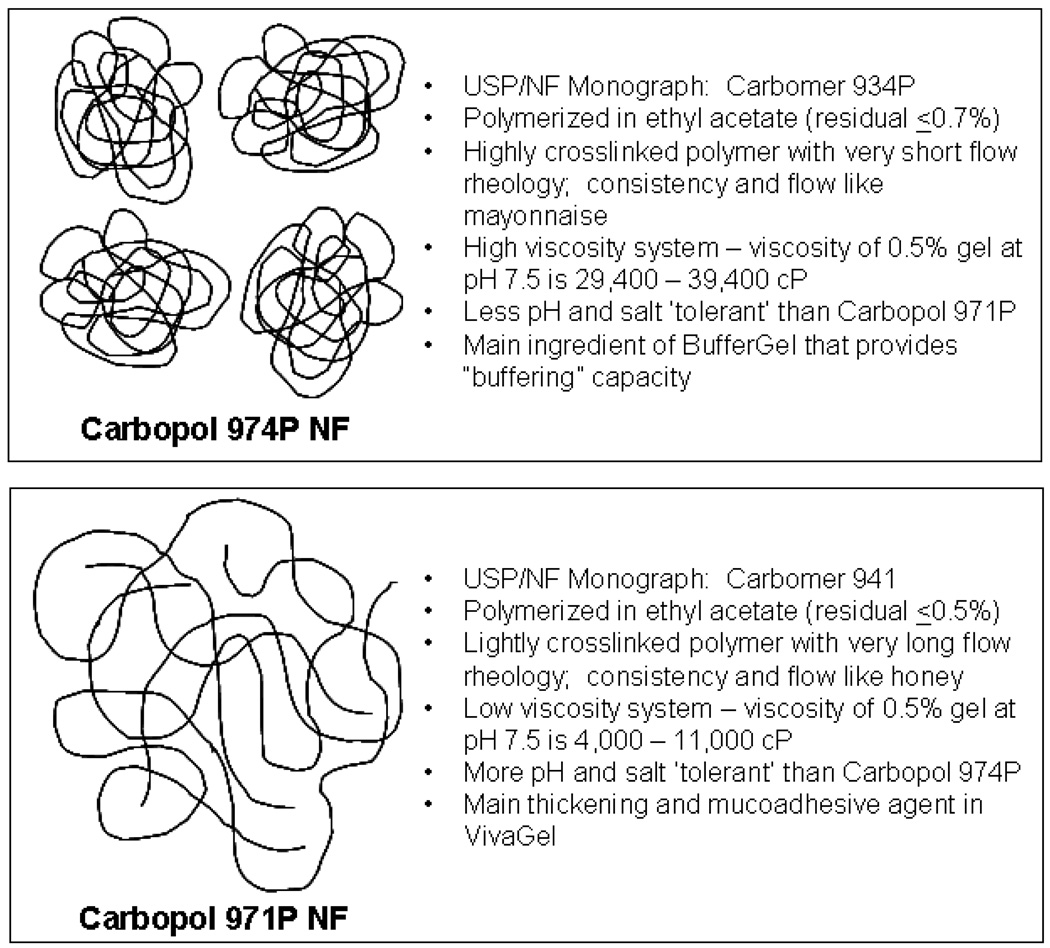
Background and Target Composition for Prototype Combination Vaginal Gels
The compositions of BufferGel and VivaGel are shown in Table 1. All excipients for both BufferGel and VivaGel are generally regarded as safe (GRAS) when used in the amounts listed for vaginal administration. It should be noted that the concentration of BufferGel excipients is not publicly disclosed. Both gels are currently under human clinical testing for safety and efficacy. The gels are aqueous Carbopol-based gels with the notable difference that BufferGel contains Carbopol 974P while VivaGel contains Carbopol 971P. Carbopol 974P and Carbopol 971P have some important similarities and differences, as shown in Figure 2. Whereas Carbopol 974P is a highly crosslinked, high viscosity polymer with a consistency and flow like mayonnaise, Carbopol 971P is a lightly crosslinked low viscosity polymer with a consistency and flow like honey. Carbopol 971P is both more pH- and salt-tolerant, meaning that the viscosity of Carbopol 971P-containing gels is less affected by changes in pH and salt concentration. This fact was considered in the initial development of VivaGel, since the addition of 3% w/w SPL7013 resulted in a sodium concentration of 115 mM, which significantly affected gel viscosity. Specifically, the addition of 3% w/w SPL7013 to an otherwise viscous Carbopol-based gel made with 3–5% w/w Carbopol in the pH range of pH 3–3.8 caused a substantial if not complete loss of viscosity for both Carbopol 971P and Carbopol 974P-based gels. Interestingly, magnesium sulfate is added to BufferGel to reduce the viscosity of the formulation to that acceptable for vaginal administration and product use.
Table 1.
Compositions of BufferGel and VivaGel, and Target Composition for Novel Prototype Combination Vaginal Gels (Prototypes 1 and 2) Containing SPL7013 Dendrimer
| Ingredient (all values in % w/w) |
BufferGel | VivaGel | Target Prototype Combination Vaginal Gels |
|---|---|---|---|
| Mucoadhesive/ Thickening/ Buffering agent |
Carbopol 974P | 5.0% Carbopol 971P | Carbopol 974P or 971P |
| pH adjustment | Dibasic potassium phosphate |
9% 2N Sodium hydroxide | KOH |
| Preservative | Sorbic acid | 0.18% methyl paraben and 0.02% propyl paraben |
0.18% methyl paraben and 0.02% propyl paraben |
| Osmolality adjustment |
Monobasic Sodium Phosphate |
None | None |
| Antioxidant | EDTA disodium | 0.1% EDTA disodium | 0.15% EDTA disodium |
| Emollient | None | 1.0% propylene glycol and 1.0% glycerin |
None |
| Viscosity reducer | Magnesium sulfate | None | Not needed due to Na from Na SPL7013 |
| Inactivation of bacterial pathogens (GC and BV) |
None | None | Lactic and acetic acids |
| SPL7013 Dendrimer (active) | None | 3% | 3% |
Figure 2.
Structure and physical/chemical properties of Carbopol 974P and Carbopol 971P. Both polymers are Generally Regarded as Safe (GRAS) and strongly mucoadhesive at lower pH due to H-bonding of the repeating carboxylic moiety (pKa 6 ± 0.5). In addition, aqueous-based gels made of each polymer are non-Newtonian fluids. Differences between the two polymers are listed adjacent to their structures.
Carbopol-based gels are known to be comprised of swollen microgel particles having both a macroviscosity and microviscosity. As shown in Figure 2, highly cross-linked polymers such as Carbopol 974P have more rigid gel microparticles (globules) when hydrated, while lightly cross-linked polymers such as Carbopol 971P are extended and flexible, thereby creating a more cage-like structure. As a consequence, the release rate of drugs from Carbopol 974P-based gels is usually greater than those from Carbopol 971P-based gels. For example, MacLean et al., using x-ray fluorescence, showed that the release rate of iodine-labeled bovine serum albumin from subcutaneously injected Carbopol-based gels was significantly greater for highly-crosslinked Carbopol 940 gels as compared to less crosslinked Carbopol-based gels (MacLean-McDavitt 2003).
As described in the Introduction, SPL7013 is a high molecular weight dendrimer, with a multi-layered lysine backbone and a polyvalent naphthalene sulphonic acid surface. The size of the dendrimer and the large number of degrees of freedom in the scaffold result in a highly mobile structure comprising both hydrophilic (i.e. lysine moieties, and charged sulphonic acid groups) and hydrophobic (i.e. naphthyl rings) regions, in addition to the association of 64 Na+ ions per molecule. Both Carbopol 971P and 974P are formed by crosslinking acrylic acid with allyl ethers of pentaerythritol, giving a saturated structure rich in ether, carbonyl and hydroxyl moieties. Carbopol might form intermolecular interactions with SPL7013 (via hydrogen bonding of dendrimer NH groups with Carbopol carbonyl groups, or dendrimer carbonyls and Carbopol hydroxyls). Additionally, encapsulation of SPL7013 in the polymer matrix might arise due to the size, shape and flexibility of the compound in solution versus the matrix of polymer chains formed by the Carbopol. Moreover, the differing extent of crosslinking between Carbopol 971P and 974P could result in varied sequestration behavior of SPL7013 in these two gelling agents.
To achieve a prototype combination vaginal gel product with desirable product features, a series of 974P and 971P gels containing SPL7013 were produced and evaluated both qualitatively and quantitatively. Although each of the Carbopol types provide some theoretical advantages, Carbopol 974P-based gels were pursued for the combination vaginal gel since placebo Carbopol 971P-based gels tended to be more ‘sticky’ to the touch with a consistency like ‘honey’.
The assessment of the prototype gels lubricity (or conversely, stickiness) is primarily determined by touch. However, as this evaluation is conducted external to the body it is acknowledge that internal application of the gel (into a warmer, higher humidity environment) may result in slightly different feel. In order to simplify the prototype formulations, it was elected to omit any emollient (e.g. the 1% w/w propylene glycol or glycerin that are included in VivaGel) unless required to enhance the physical feel. If the prototype formulations were judged to need an emollient, then low levels (<10% w/w) would be included to avoid any potential mucosal irritation that has been observed at high concentrations with such excipients and which is caused by the high osmolality of the ensuing gels (Adriaens 2008). In addition to the in-vivo (slug) evaluation of toxicity related to high glycerol content, the use of a hyperosmolar gel formulation in a Phase I clinical trial resulted in unacceptable adverse events (i.e. abnormal transudation of physiologic fluid across the cervico-vaginal mucosae) due to the high glycerin content (Lacy 2008).
Both lactic and acetic acid were included in the combination vaginal gel product (Prototype 1), as these cell-permeant species, naturally occurring in the vagina, have been shown to inactivate both viral and bacterial pathogens (Dimitonova 2007). Methyl and propyl parabens were chosen as the preservative in the prototype combination vaginal gels. The levels of the parabens are standard in topical products for providing antimicrobial properties, but further confirmed by antimicrobial effectiveness testing (AET) on the prototype gel formulations (data not shown).
Prototype Combination Vaginal Gels
Gel composition and characterization
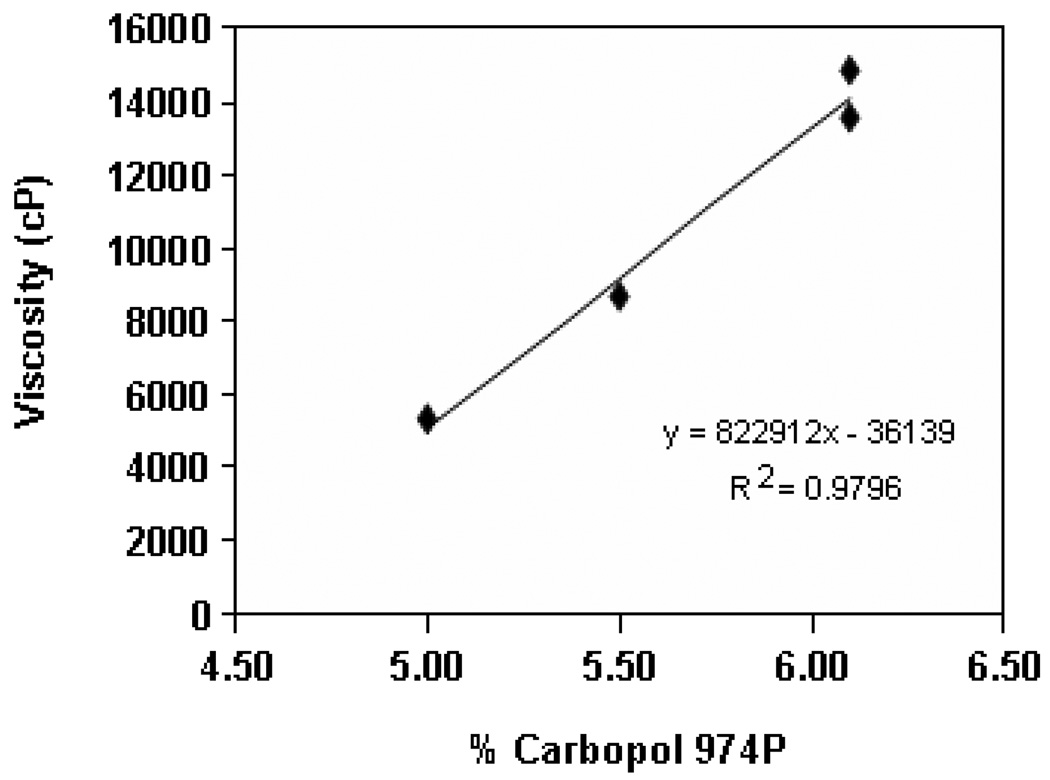
The composition and characterization of combination vaginal gel prototypes is shown in Table 2. The final prototypes with lactic and acetic acids (termed “Prototype 1”) and without lactic and acetic acid (termed “Prototype 2”) were prepared using 6.2% w/w Carbopol 974P at a pH of 3.7. This relatively high Carbopol content was required in order to maintain sufficient viscosity of the vaginal gel, since the formulation of 3% w/w SPL7013 introduced a final sodium concentration of ~115 mM, which substantially reduced the gel viscosity at the relatively low pH of the prototype formulations. The reduction of viscosity of Carbopol-based gels by sodium is well known. Moreover, it was independently confirmed that the addition of 57.5 mM NaCl to a pH of 3.7, and concentration of 3.8% w/w Carbopol 974P gel without SPL7013 reduced the viscosity to nearly that of water. In Figure 3, the effect of percent Carbopol 974P on the resulting viscosity of 3% w/w SPL7013 gels is plotted. There was a linear [R2 = 0.9796] relationship between Carbopol content and resulting gel viscosity. Interestingly, it was very difficult to produce a gel containing 6.2% w/w Carbopol 974P in the absence of SPL7013 (and its associated viscosity-reducing sodium ions), since the extremely high viscosity hindered mixing. These findings helped in defining a suitable manufacturing process for the gel wherein roughly half of the Carbopol is added first to make a gel base, and the remainder is added after the SPL7013 dendrimer is added. Coincidentally, the necessity of increasing the Carbopol 974P content in the prototype combination vaginal gels due to the inclusion of SPL7013 also resulted in gels having significantly increased acidic buffering capacity as compared to that of BufferGel. Whereas BufferGel and VivaGel had acidic buffering capacity of 0.15 mEq/g and 0.12 mEq/g, respectively, Prototype 1 and Prototype 2 had acidic buffering capacities more than 2-fold greater of 0.32 mEq/g and 0.29 mEq/g, respectively. The addition of physiological levels of lactic (0.6% w/w; 67 mM) and acetic acid (0.087% w/w; 15 mM) in Prototype 1 resulted in about a 90% increase in the osmolality of Prototype 1 versus Prototype 2 with an increase in the acidic buffering capacity of 10%. In comparison, the inclusion of 1% w/w propylene glycol and 1% w/w glycerin in the VivaGel formulation contributed to its increased osmolality of 380 mOsm/kg H2O. With reference to the above discussion on hyperosmolar formulations inducing mucosal toxicity (Adriaens 2008; Lacy 2008), it should be noted that adverse toxicological behavior is observed with osmolality values greater than 2000 mOsm/kg H2O, i.e. substantially greater than those measured for formulations described here.
Table 2.
Final Composition of Prototype Combination Vaginal Gels Containing SPL7013 Dendrimer
| Ingredients | Prototype 1 Vaginal Gel (% w/w) |
Prototype 2 Vaginal Gel (%w/w) |
||
|---|---|---|---|---|
| Purified Water | q.s. to 100% | q.s. to 100% | ||
| Methyl Paraben NF | 0.18 | 0.18 | ||
| Propyl Paraben NF | 0.02 | 0.02 | ||
| EDTA Disodium USP | 0.15 | 0.15 | ||
| Carbopol 974P NF | 6.2 | 6.2 | ||
| Lactic Acid USP | 0.6 | None | ||
| Acetic Acid USP | 0.087 | None | ||
| 2N KOH NF | q.s. to pH 3.7 | q.s. to pH 3.7 | ||
| SPL7013 Dendrimer | 3.0 | 3.0 | ||
| Characterization (n = 3.5 batches) | ||||
| pH | 3.7 ± 0.08 | 3.7 ± 0.03 | ||
| Viscosity | 9484 ± 1344 cP | 8245 ± 1693 cP | ||
| Buffering Capacity | 0.31 ± 0.02 mEq/g | 0.28 ± 0.01 mEq/g | ||
| Osmolality | 202 ± 13 mOsm/kg | 102 ± 4 mOsm/kg | ||
Figure 3.
The effect of Carbopol 974P concentration on the resulting viscosity (cP) of Prototype 1 Combination Vaginal Gel made with 3% w/w SPL7013 dendrimer.
Gel Characterization
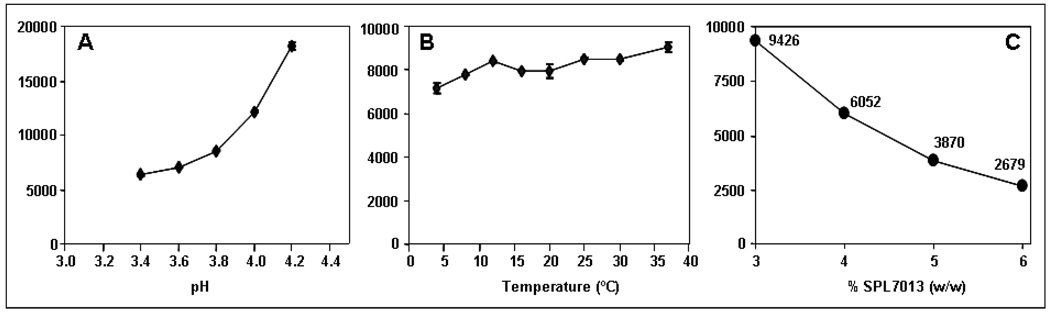
Effect of pH, temperature, and Percent SPL7013 on Gel Viscosity
In order to address concerns over the effects of pH and temperature on the viscosity of the combination vaginal gel prototypes, gels containing 5.5% w/w Carbopol 974P and SPL7013 at various pHs were prepared. As shown in Figure 4A, there was a relatively small increase in viscosity in gels having a pH at or below pH 3.8. However, above pH 3.8, there was an exponential increase in viscosity which is consistent with what is known about Carbopol-based gels alone in aqueous vehicles. These results suggested that target pH of the gel could be lowered to pH 3.7 and allow for even greater buffering capacity. The effect of temperature on the viscosity of a 3% w/w SPL7013 Prototype 1 (pH 3.7) was also investigated. As shown in Figure 4B, a plot of viscosity versus temperature demonstrated that the viscosity of Prototype 1 varied only modestly across this relatively broad temperature range. The information from these studies should prove extremely useful for large scale manufacturing of the Prototype 1 and subsequent storage. Lastly, the effect of SPL7013 concentration on Prototype 1 gel viscosity in plotted in Figure 4C. As expected, as the SPL7013 concentration increased, the resulting gel viscosity decreased in an almost linear manner. It was concluded that a maximum of 4% w/w SPL7013 could be formulated in the Prototype 1 gel allowing the vaginal gel formulation to have sufficient viscosity for administration (i.e. >5,000 cP using this method).
Figure 4.
The influence of pH (A), temperature (B) and % SPL7013 dendrimer concentration (C) on the resulting viscosity of Prototype 1 Combination Vaginal Gel. Y-axis for A-C is Viscosity (cP).
Ultracentrifugation of SPL7013 Gels
The phenomenon of ‘entropic trapping’ of macromolecules in the void space of polymer hydrogels has previously been described (Liu 1999). Entropic trapping is influenced by molecular size dependent diffusion of the macromolecule. The mean (z-average) diameter of SPL7013 as determined by dynamic light scattering measurements (at 1 mg/ml in 10−2M NaCl) (Prestidge 2005) is 5 nm. This correlates well to the Stokes diameter of approximately 3–4 nm which may be calculated based on its molecular weight of 16,581 Da. It was therefore hypothesized that Carbopol 974P in the viscous gel might bind or trap SPL7013 by either entropic trapping or hydrogen bonding, thereby leaving some SPL7013 less available for interaction with HIV, HSV, or semen. This would be an obvious concern for a vaginal microbicidal gel product containing an anti-viral compound that required the compound to physically interact with the virus in order to exert activity. Importantly, previous studies have suggested that the rate of diffusion of molecules decreases with increasing size (Flemstrom 1999). For example, Flemstrom et al. showed that the mucus gel layer in the duodenum significantly restricts migration of macromolecules to the duodenal surface. However, Olmsted et al. later showed, using improved analytical methods and employing fluorescent recovery after photobleaching (FRAP) and fluorescent imaging, that large proteins (15–650 kDa) and even small viruses such as the human papilloma virus (55 nm) and Norwalk virus (38 nm) could diffuse as rapidly in mucus as in saline (Olmsted 2001). It was demonstrated that the mesh spacing between mucin fibers (20–200 nm) was large enough to allow these macromolecules to diffuse unconstrained. Still, the diffusion of larger proteins as well as that of the herpes simplex virus (180 nm) were hindered by the viscous mucin.
Analytical ultracentrifugation is a common and powerful method employed to quantify the size and shape of macromolecules and their interactions (Howlett 2006). This method has many different applications, including the study of molecular associations and reactions under equilibrium conditions, as well as to ascertain macromolecule size and shape. For example, Howlett et al. review the uses of ultracentrifugation to study protein association and assembly (Howlett 2006). In addition, Joralemon et al. used analytical ultracentrifugation to study the sedimentation equilibrium of formed nanostructures resulting from polymeric micelles crosslinked with divergently synthesized dendrimers (Joralemon 2005).
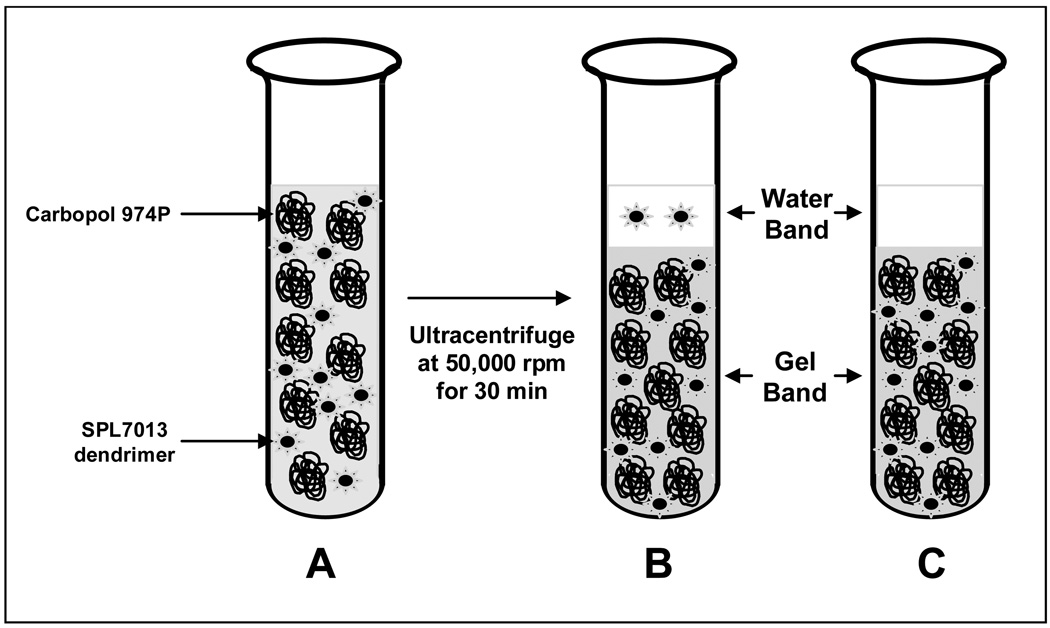
In the present studies, we intended to adapt ultracentrifugation to determine if SPL7013 dendrimer was sequestered in Carbopol 974P and Carbopol 971P-based aqueous gels. As shown in Figure 5A, tubes containing SPL7013 or SPL7013-containing gels were subjected to ultracentrifugation for 30 min at 50,000 rpm to phase separate the gels into a water-rich band (“water-band”) and gel-rich band (“gel-rich band”). Sequestration of SPL7013 by Carbopol 974P would result in a lower than theoretical SPL7013 concentration (e.g., 3% w/w) in the water-band as shown in Figure 5C. In contrast, concentrations of SPL7013 in the water-band that were close to 3% w/w (as indicated by Figure 5B) would suggest no discernable binding or trapping of SPL7013 by Carbopol 974P.
Figure 5.
Schematic depicting the physical disposition of SPL7013 dendrimer in Prototype Combination Vaginal Gels before ultracentrifugation (A), and after ultracentrifugation (B,C). Ultracentrifugation of the viscous Carbopol-based gel produces a water-rich band (“Water Band”) on top and a gel-rich band (“Gel Band”) on the bottom of the tube. B shows a water band with equal concentration of SPL7013 in the Water Band and the Gel Band as would be expected in the absence of sequestration. C shows a water band with reduced SPL7013 concentration as would be expected if SPL7013 dendrimer were sequestered by the Carbopol-based gel.
The results showed that ultracentrifugation of an aqueous SPL7013 solution (i.e. absent of any Carbopol or other excipients) resulted in no significant sedimentation of SPL7013 under the conditions used for ultracentrifugation (Table 3). The upper layer of a centrifuged SPL7013 solution contained 99.0% ± 0.03% (n=6) of the theoretical SPL7013 concentration. Ultracentrifugation of both Prototype Vaginal Gels resulted in the formation of distinct watery supernatants and dense gel-rich bands. Qualitative examination revealed that the water-band comprised approximately 20% of the volume of the entire gel sample. The aqueous supernatant was sampled once per vial in order to avoid puncturing or disturbing the gel band. The total water band volume was not measured, since droplets stuck irregularly to the inside of the vial and to the pipette tips. Thus, a complete mass balance of SPL7013 in the water and the gel bands was not performed. As summarized in Table 3, SPL7013 was not sequestered in the Carbopol-rich band after ultracentrifugation of both the Prototype 1 and Prototype 2 gels. Instead, the SPL7013 readily diffused from the gel region and concentrated in the watery supernatant. The concentration of SPL7013 in the water band after ultracentrifugation was approximately 50% higher than that in the starting prototype combination vaginal gels, and significantly lower in the remaining compressed gel bands.
Table 3.
Concentration of SPL7013 Dendrimer in Water-Band and Gel-Band after Ultracentrifugation of 3% w/w Prototype 1 and Prototype 2 Vaginal Gels at 50,000 rpm for 30 min
| SPL7013 Concentration (% w/w) (n=6) | ||||
|---|---|---|---|---|
| Material | Water-Band | Gel-Band | ||
| SPL7013 | ||||
| Aqueous solution, untreated control | 3.00 ± 0.03 | N/A | ||
| Aqueous solution, ultracentrifuged | 2.97± 0.01 | N/A | ||
| Prototype 1 Vaginal Gel | ||||
| Control, untreated | 3.03 ± 0.01 | 3.03 ± 0.01 | ||
| Ultracentrifuged | 4.54 ± 0.07 | 2.67 ± 0.06 | ||
| Prototype 2 Vaginal Gel | ||||
| Control, untreated | 3.00 ± 0.02 | 3.00 ± 0.02 | ||
| Ultracentrifuged | 4.46 ± 0.03 | 2.49 ± 0.01 | ||
| Viscosity (cP) | pH | |||
| VivaGel | ||||
| Ultracentifuged | 10,183 | 4.80 | No Band | 3.00 ± 0.05 |
| Ultracentrifuged | ||||
| after diluting 10% with water | 8,830 | 4.47 | No Band | 2.74 ± 0.11 |
| after diluting 5% with water | 9,525 | 4.37 | No Band | 2.87 ± 0.06 |
| after diluting 2.5% with water | 9,872 | 4.34 | No Band | 2.91 ± 0.04 |
| after diluting 10% with 6N HCl | 695 | 0.66 | 2.93 ± 0.10 | 3.56 ± 0.26 |
| after diluting 5% with 6N HCl | 2,009 | 1.22 | 2.90 ± 0.08 | 3.48 ± 0.11 |
| after diluting 2.5% with water | 3,188 | 1.96 | 2.84 ± 0.07 | 3.29 ± 0.12 |
| after diluting 10% with 5M NaCl | 2,914 | 4.27 | 3.67 ± 0.08 | 2.46 ± 0.16 |
| after diluting 5% with 5M NaCl | 3,237 | 4.23 | 3.50 ± 0.09 | 2.62 ± 0.06 |
| after diluting 2.5% with 5M NaCl | 6,598 | 4.21 | No Band | 2.97 ± 0.11 |
Interestingly, VivaGel (10,183 cP) and a neutral 2.7% w/w hydroxyethyl cellulose (HEC) placebo gel (otherwise known as the “Universal” placebo, which is commonly used in topical microbicide development as a comparator formulation) (Tien 2005) could not form discernible water and gel-rich layers when ultracentrifuged, even after 100,000 rpm for 45 min. Further examination revealed that viscosity and pH most influenced the ability of the gel to form bands after ultracentrifugation. For example, VivaGel could be compressed into a water band and a gel layer if it was acidified with HCl, or if sodium chloride was added into the gel to yield gels having viscosities between 3,000–4,000 cP. Specifically, when 25 mg 6N HCl or 50 mg 5M NaCl was added to 1 g VivaGel, ultracentrifugation at 50,000 rpm for 30 min led to banding and the SPL7013 concentration in the water-band was essentially equivalent to that in the initial VivaGel formulation. Interestingly, ultracentrifugation did not lead to banding when only 25 mg 5M NaCl was added to 1 g VivaGel. This gel had a viscosity of 6,598 cP, which was actually lower than the value measured for either of the prototype combination vaginal gels. However, the pH of this modified VivaGel sample was 4.21, which was still a half pH unit higher than that of the prototype combination vaginal gels. Thus, the influence of lower pH on reduced Carbopol ionization and subsequent reduced electrostatic repulsion in promoting gel compression and SPL7013 diffusion may be particularly important at moderate viscosity levels. In cases of relatively higher pH, where the Carbopol residues would tend to be deprotonated and anionic, such as with the prototype combination vaginal gels or the high salt mixtures, electrostatic repulsion between polymer and SPL7013 may contribute to higher SPL7013 diffusion and subsequent SPL7013 concentration in the water bands.
In summary, novel combination vaginal gel formulations have been successfully developed which aimed to combine design and formulation criteria from two leading topical microbicides currently in human clinical trials. Both prototype gel formulations have been fully characterized, and demonstrated to have potential utility as topical vaginal microbicide products. Finally, ultracentrifugation studies have shown that the anti-viral macromolecule SPL7013 dendrimer is not entropically trapped in Carbopol-based gels and would in turn be available to physically interact with virus.
ACKNOWLEDGMENTS
Supported by National Institutes of Health (U19 AI060598), National Institute of Child Health/Human Development of the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
REFERENCES
- Abner SR, Guenthner PC, Guarner J, Hancock KA, Cummins JE, Jr, Fink A, Gilmore GT, Staley C, Ward A, Ali O, Binderow S, Cohen S, Grohskopf LA, Paxton L, Hart CE, Dezzutti CS. A human colorectal explant culture to evaluate topical microbicides for the prevention of HIV infection. J Infect Dis. 2005;192:1545–1556. doi: 10.1086/462424. [DOI] [PubMed] [Google Scholar]
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512–516. doi: 10.1097/OLQ.0b013e3181644669. [DOI] [PubMed] [Google Scholar]
- Ballagh SA, Brache V, Mauck C, Callahan MM, Cochon L, Wheeless A, Moench TR. A Phase I study of the functional performance, safety and acceptability of the BufferGel Duet. Contraception. 2008;77:130–137. doi: 10.1016/j.contraception.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Bernstein DI, Stanberry LR, Sacks S, Ayisi NK, Gong YH, Ireland J, Mumper RJ, Holan G, Matthews B, McCarthy T, Bourne N. Evaluations of unformulated and formulated dendrimer-based microbicide candidates in mouse and guinea pig models of genital herpes. Antimicrob Agents Chemother. 2003;47:3784–3788. doi: 10.1128/AAC.47.12.3784-3788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RA, Hoen T, Wong X, Abusuwwa R, Anderson DJ, Moench TR. Vaginal microbicides: detecting toxicities in vivo that paradoxically increase pathogen transmission. BMC Infect Dis. 2006;6:90. doi: 10.1186/1471-2334-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins JE, Jr, Guarner J, Flowers L, Guenthner PC, Bartlett J, Morken T, Grohskopf LA, Paxton L, Dezzutti CS. Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob Agents Chemother. 2007;51:1770–1779. doi: 10.1128/AAC.01129-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitonova SP, Danova ST, Serkedjieva JP, Bakalov BV. Antimicrobial activity and protective properties of vaginal lactobacilli from healthy Bulgarian women. Anaerobe. 2007;13:178–184. doi: 10.1016/j.anaerobe.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Flemstrom G, Hallgren A, Nylander O, Engstrand L, Wilander E, Allen A. Adherent surface mucus gel restricts diffusion of macromolecules in rat duodenum in vivo. Am J Physiol. 1999;277:G375–G382. doi: 10.1152/ajpgi.1999.277.2.G375. [DOI] [PubMed] [Google Scholar]
- Gong E, Matthews B, McCarthy T, Chu J, Holan G, Raff J, Sacks S. Evaluation of dendrimer SPL7013, a lead microbicide candidate against herpes simplex viruses. Antiviral Res. 2005;68:139–146. doi: 10.1016/j.antiviral.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Howlett GJ, Minton AP, Rivas G. Analytical ultracentrifugation for the study of protein association and assembly. Curr Opin Chem Biol. 2006;10:430–436. doi: 10.1016/j.cbpa.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Emau P, Cairns JS, Flanary L, Morton WR, McCarthy TD, Tsai CC. SPL7013 gel as a topical microbicide for prevention of vaginal transmission of SHIV89.6P in macaques. AIDS Res Hum Retroviruses. 2005;21:207–213. doi: 10.1089/aid.2005.21.207. [DOI] [PubMed] [Google Scholar]
- Joralemon MJ, O'reilly RK, Hawker CJ, Wooley KL. Shell click-crosslinked (SCC) nanoparticles: a new methodology for synthesis and orthogonal functionalization. J Am Chem Soc. 2005;127:16892–16899. doi: 10.1021/ja053919x. [DOI] [PubMed] [Google Scholar]
- Lackman-Smith C, Osterling C, Luckenbaugh K, Mankowski M, Snyder B, Lewis G, Paull J, Profy A, Ptak RG, Buckheit RW, Jr, Watson KM, Cummins JE, Jr, Sanders-Beer BE. Development of a comprehensive human immunodeficiency virus type 1 screening algorithm for discovery and preclinical testing of topical microbicides. Antimicrob Agents Chemother. 2008;52:1768–1781. doi: 10.1128/AAC.01328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy C. Unacceptable side effects of a hyperosmolar vaginal microbicide in a Phase I trial. Microbicides 2008. 2008 doi: 10.1258/ijsa.2010.010215. Abstract B08-B527. [DOI] [PubMed] [Google Scholar]
- Liu L, Li P, Asher SA. Entropic trapping of macromolecules by mesoscopic periodic voids in a polymer hydrogel. Nature. 1999;397:141–144. doi: 10.1038/16426. [DOI] [PubMed] [Google Scholar]
- MacLean-McDavitt DS, Robertson JD, Jay M. Monitoring the in vivo delivery of proteins from carbomer hydrogels by X-ray fluorescence. Pharm Res. 2003;20:435–441. doi: 10.1023/a:1022612422769. [DOI] [PubMed] [Google Scholar]
- Maguire RA, Bergman N, Phillips DM. Comparison of microbicides for efficacy in protecting mice against vaginal challenge with herpes simplex virus type 2, cytotoxicity, antibacterial properties, and sperm immobilization. Sex Transm Dis. 2001;28:259–265. doi: 10.1097/00007435-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Mayer KH, Peipert J, Fleming T, Fullem A, Moench T, Cu-Uvin S, Bentley M, Chesney M, Rosenberg Z. Safety and tolerability of BufferGel, a novel vaginal microbicide, in women in the United States. Clin Infect Dis. 2001;32:476–482. doi: 10.1086/318496. [DOI] [PubMed] [Google Scholar]
- McCarthy TD, Karellas P, Henderson SA, Giannis M, O'Keefe DF, Heery G, Paull JR, Matthews BR, Holan G. Dendrimers as drugs: discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol Pharm. 2005;2:312–318. doi: 10.1021/mp050023q. [DOI] [PubMed] [Google Scholar]
- Ndesendo VM, Pillay V, Choonara YE, Buchmann E, Bayever DN, Meyer LC. A Review of Current Intravaginal Drug Delivery Approaches Employed for the Prophylaxis of HIV/AIDS and Prevention of Sexually Transmitted Infections. AAPS PharmSciTech. 2008 doi: 10.1208/s12249-008-9073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath AR, Strick N, Li YY. Anti-HIV-1 activity of anionic polymers: a comparative study of candidate microbicides. BMC Infect Dis. 2002;2:27. doi: 10.1186/1471-2334-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted SS, Dubin NH, Cone RA, Moench TR. The rate at which human sperm are immobilized and killed by mild acidity. Fertil Steril. 2000;73:687–693. doi: 10.1016/s0015-0282(99)00640-8. [DOI] [PubMed] [Google Scholar]
- Olmsted SS, Khanna KV, Ng EM, Whitten ST, Johnson ON, 3rd, Markham RB, Cone RA, Moench TR. Low pH immobilizes and kills human leukocytes and prevents transmission of cell-associated HIV in a mouse model. BMC Infect Dis. 2005;5:79. doi: 10.1186/1471-2334-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys J. 2001;81:1930–1937. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DL, Sweeney YC, Cummings PK, Meyn L, Rabe LK, Hillier SL. Safety and efficacy evaluations for vaginal and rectal use of BufferGel in the macaque model. Sex Transm Dis. 2004;31:290–296. doi: 10.1097/01.olq.0000124614.91448.d4. [DOI] [PubMed] [Google Scholar]
- Prestidge C, Ametov I, Barnes T. Characterization of Starpharma Dendrimers by Dynamic and Static Light Scattering. Private Communication. 2005 [Google Scholar]
- Roth S, Monsour M, Dowland A, Guenthner PC, Hancock K, Ou CY, Dezzutti CS. Effect of topical microbicides on infectious human immunodeficiency virus type 1 binding to epithelial cells. Antimicrob Agents Chemother. 2007;51:1972–1978. doi: 10.1128/AAC.01358-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp R, Rosenthal SL, Stanberry LR. VivaGel (SPL7013 Gel): a candidate dendrimer--microbicide for the prevention of HIV and HSV infection. Int J Nanomedicine. 2007;2:561–566. [PMC free article] [PubMed] [Google Scholar]
- Tanne JH. Nearly 40 million people worldwide are infected with HIV. Bmj. 2006;332:1289. doi: 10.1136/bmj.332.7553.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin JA. Considerations and development of topical microbicides to inhibit the sexual transmission of HIV. Expert Opin Investig Drugs. 2002;11:1077–1097. doi: 10.1517/13543784.11.8.1077. [DOI] [PubMed] [Google Scholar]
- Van De Wijgert J, Fullem A, Kelly C, Mehendale S, Rugpao S, Kumwenda N, Chirenje Z, Joshi S, Taha T, Padian N, Bollinger R, Nelson K. Phase 1 trial of the topical microbicide BufferGel: safety results from four international sites. J Acquir Immune Defic Syndr. 2001;26:21–27. doi: 10.1097/00126334-200101010-00003. [DOI] [PubMed] [Google Scholar]
- Williams DL, Newman DR, Ballagh SA, Creinin MD, Barnhart K, Weiner DH, Bell AJ, Jamieson DJ. Phase I safety trial of two vaginal microbicide gels (Acidform or BufferGel) used with a diaphragm compared to KY jelly used with a diaphragm. Sex Transm Dis. 2007;34:977–984. doi: 10.1097/olq.0b013e31813347e9. [DOI] [PubMed] [Google Scholar]
- Zarocostas J. Number of people infected with HIV worldwide reaches 40m. Bmj. 2005;331:1224. doi: 10.1136/bmj.331.7527.1224-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin L, Hoen TE, Achilles SL, Hegarty TA, Jerse AE, Kreider JW, Olmsted SS, Whaley KJ, Cone RA, Moench TR. Tests of Buffergel for contraception and prevention of sexually transmitted diseases in animal models. Sex Transm Dis. 2001;28:417–423. doi: 10.1097/00007435-200107000-00010. [DOI] [PubMed] [Google Scholar]