Abstract
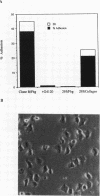
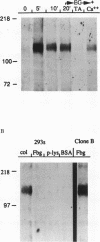
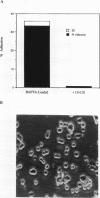
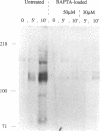
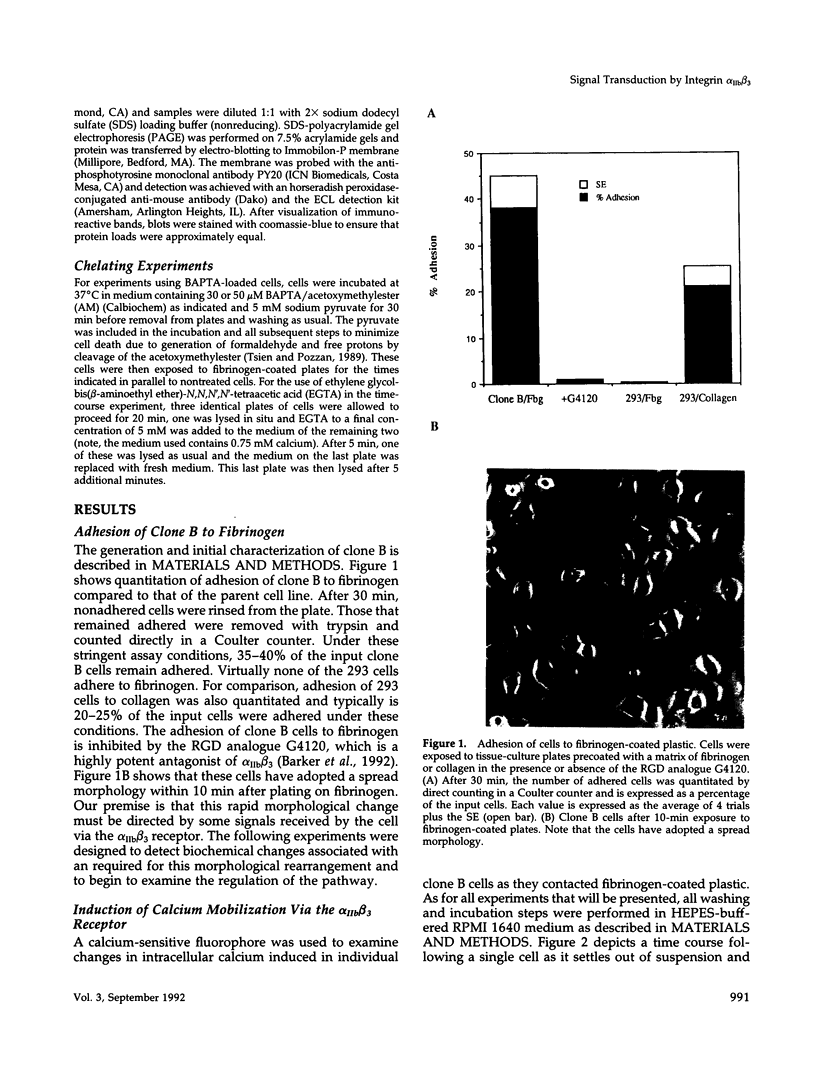
We demonstrate an example of signal transduction by an integrin and have begun to define the pathway through which this signaling is achieved. We constructed a stably transfected derivative of 293 cells (ATCC 1573) that expresses the platelet integrin GPIIbIIIa (alpha IIb beta 3). This cell line, clone B, adheres to and spreads on fibrinogen, a ligand for alpha IIb beta 3, while the parent cell line does not. Stimulation of these cells either by adhesion to fibrinogen or with antiserum directed against alpha IIb beta 3 results in induction of calcium oscillations, followed by tyrosine phosphorylation of at least one protein of molecular weight approximately 125 kDa. We establish that this phosphorylation, as well as the morphological rearrangements, requires the mobilization of calcium.
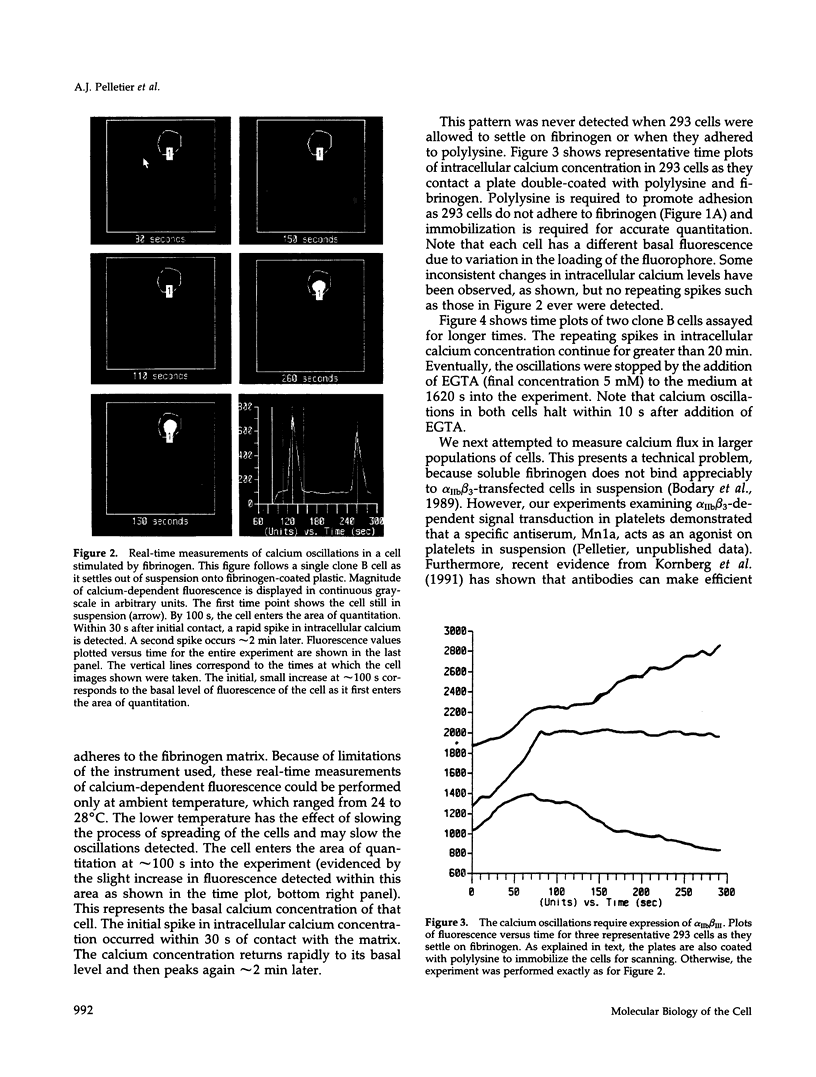
Full text
PDF

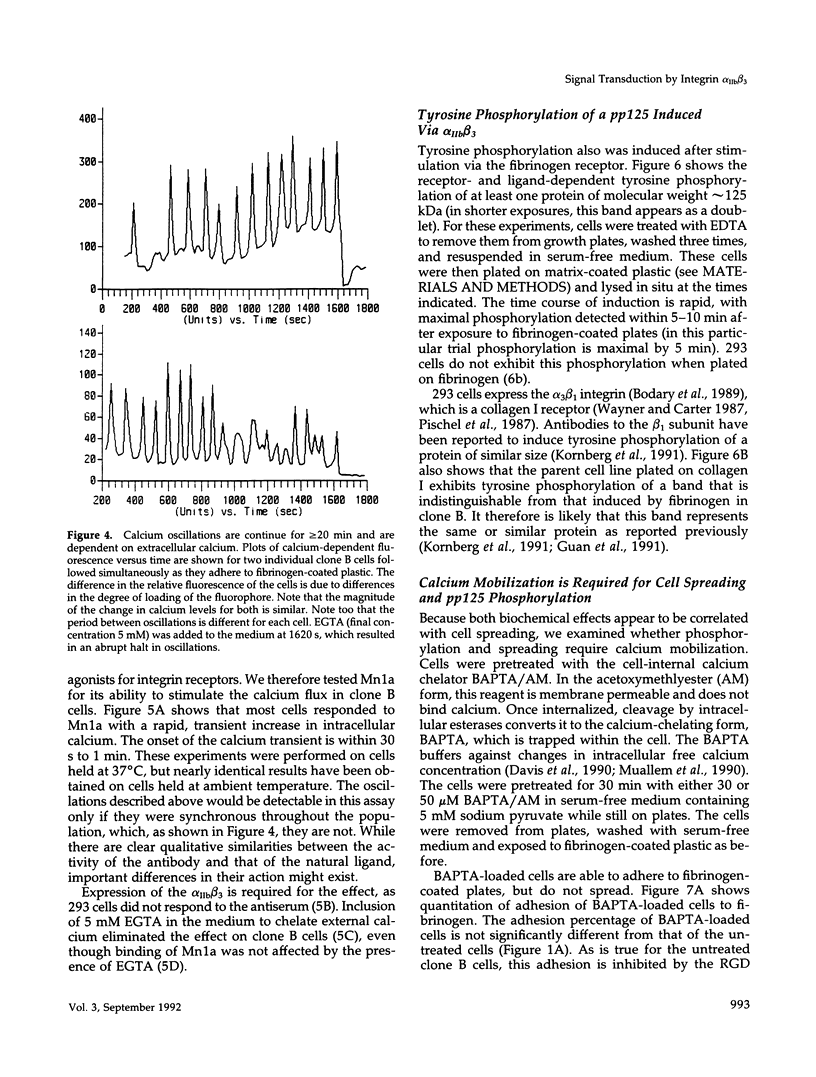



Images in this article
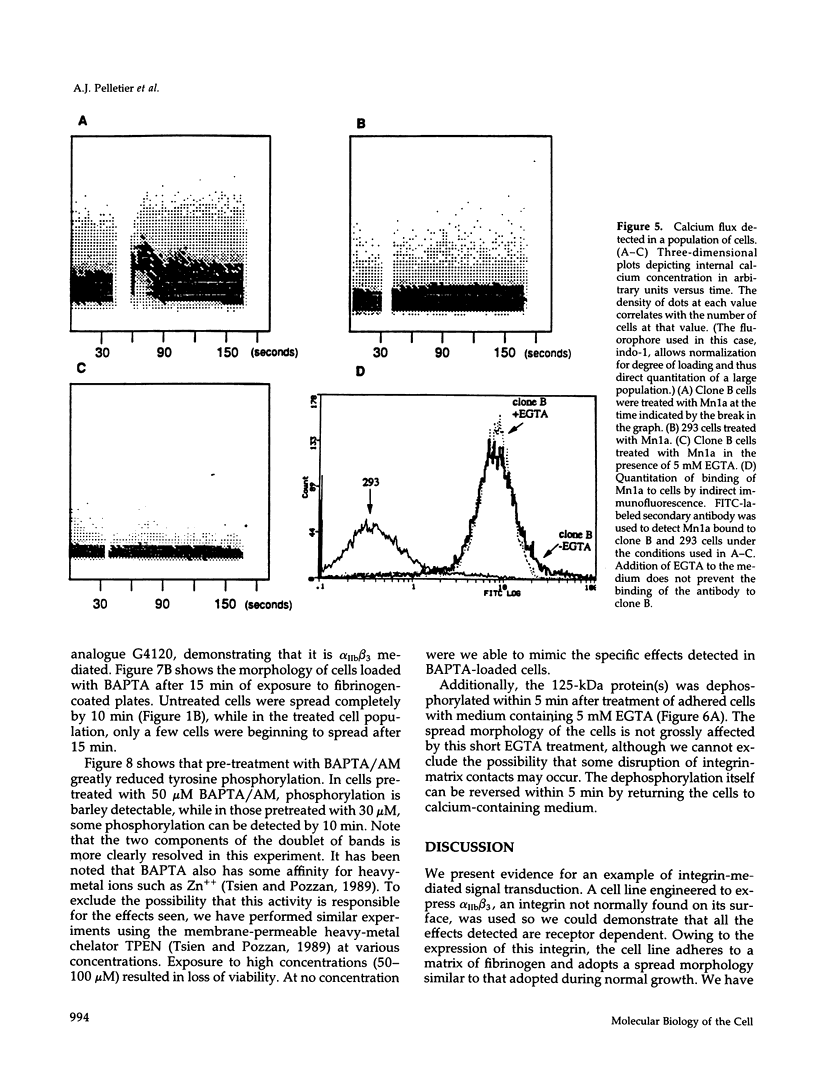
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Barker P. L., Bullens S., Bunting S., Burdick D. J., Chan K. S., Deisher T., Eigenbrot C., Gadek T. R., Gantzos R., Lipari M. T. Cyclic RGD peptide analogues as antiplatelet antithrombotics. J Med Chem. 1992 May 29;35(11):2040–2048. doi: 10.1021/jm00089a014. [DOI] [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Bodary S. C., Napier M. A., McLean J. W. Expression of recombinant platelet glycoprotein IIbIIIa results in a functional fibrinogen-binding complex. J Biol Chem. 1989 Nov 15;264(32):18859–18862. [PubMed] [Google Scholar]
- Davies T. A., Drotts D. L., Weil G. J., Simons E. R. Cytoplasmic Ca2+ is necessary for thrombin-induced platelet activation. J Biol Chem. 1989 Nov 25;264(33):19600–19606. [PubMed] [Google Scholar]
- Dedhar S. Signal transduction via the beta 1 integrins is a required intermediate in interleukin-1 beta induction of alkaline phosphatase activity in human osteosarcoma cells. Exp Cell Res. 1989 Jul;183(1):207–214. doi: 10.1016/0014-4827(89)90430-8. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Tyrosine-specific protein phosphorylation is regulated by glycoprotein IIb-IIIa in platelets. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2234–2238. doi: 10.1073/pnas.86.7.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A., Brugge J. S., Shattil S. J. Role of platelet membrane glycoprotein IIb-IIIa in agonist-induced tyrosine phosphorylation of platelet proteins. J Cell Biol. 1990 Dec;111(6 Pt 2):3117–3127. doi: 10.1083/jcb.111.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A., Nemeth S. P., Brugge J. S. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Feb;83(4):852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Kim J. W., Machesky L. M., Rhee S. G., Pollard T. D. Regulation of phospholipase C-gamma 1 by profilin and tyrosine phosphorylation. Science. 1991 Mar 8;251(4998):1231–1233. doi: 10.1126/science.1848725. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Machesky L. M., Baldassare J. J., Pollard T. D. The actin-binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science. 1990 Mar 30;247(4950):1575–1578. doi: 10.1126/science.2157283. [DOI] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Grant D. S., Tashiro K., Segui-Real B., Yamada Y., Martin G. R., Kleinman H. K. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989 Sep 8;58(5):933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Trevithick J. E., Hynes R. O. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991 Nov;2(11):951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harootunian A. T., Kao J. P., Paranjape S., Tsien R. Y. Generation of calcium oscillations in fibroblasts by positive feedback between calcium and IP3. Science. 1991 Jan 4;251(4989):75–78. doi: 10.1126/science.1986413. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ingber D. E. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc Natl Acad Sci U S A. 1990 May;87(9):3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaconi M. E., Theler J. M., Schlegel W., Appel R. D., Wright S. D., Lew P. D. Multiple elevations of cytosolic-free Ca2+ in human neutrophils: initiation by adherence receptors of the integrin family. J Cell Biol. 1991 Mar;112(6):1249–1257. doi: 10.1083/jcb.112.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg L. J., Earp H. S., Turner C. E., Prockop C., Juliano R. L. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specificity of the interaction between phosphatidylinositol 4,5-bisphosphate and the profilin:actin complex. J Cell Biochem. 1988 Jul;37(3):255–267. doi: 10.1002/jcb.240370302. [DOI] [PubMed] [Google Scholar]
- Muallem S., Khademazad M., Sachs G. The route of Ca2+ entry during reloading of the intracellular Ca2+ pool in pancreatic acini. J Biol Chem. 1990 Feb 5;265(4):2011–2016. [PubMed] [Google Scholar]
- Ng-Sikorski J., Andersson R., Patarroyo M., Andersson T. Calcium signaling capacity of the CD11b/CD18 integrin on human neutrophils. Exp Cell Res. 1991 Aug;195(2):504–508. doi: 10.1016/0014-4827(91)90402-g. [DOI] [PubMed] [Google Scholar]
- Pidard D., Montgomery R. R., Bennett J. S., Kunicki T. J. Interaction of AP-2, a monoclonal antibody specific for the human platelet glycoprotein IIb-IIIa complex, with intact platelets. J Biol Chem. 1983 Oct 25;258(20):12582–12586. [PubMed] [Google Scholar]
- Pischel K. D., Hemler M. E., Huang C., Bluestein H. G., Woods V. L., Jr Use of the monoclonal antibody 12F1 to characterize the differentiation antigen VLA-2. J Immunol. 1987 Jan 1;138(1):226–233. [PubMed] [Google Scholar]
- Plow E. F., Ginsberg M. H. Cellular adhesion: GPIIb-IIIa as a prototypic adhesion receptor. Prog Hemost Thromb. 1989;9:117–156. [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariff A., Luna E. J. Diacylglycerol-stimulated formation of actin nucleation sites at plasma membranes. Science. 1992 Apr 10;256(5054):245–247. doi: 10.1126/science.1373523. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Tsien R., Pozzan T. Measurement of cytosolic free Ca2+ with quin2. Methods Enzymol. 1989;172:230–262. doi: 10.1016/s0076-6879(89)72017-6. [DOI] [PubMed] [Google Scholar]
- Vukicevic S., Luyten F. P., Kleinman H. K., Reddi A. H. Differentiation of canalicular cell processes in bone cells by basement membrane matrix components: regulation by discrete domains of laminin. Cell. 1990 Oct 19;63(2):437–445. doi: 10.1016/0092-8674(90)90176-f. [DOI] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987 Oct;105(4):1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A., Tanoue K., Yamazaki H. Secondary signals mediated by GPIIb/IIIa in thrombin-activated platelets. Biochim Biophys Acta. 1990 Aug 13;1054(1):8–13. doi: 10.1016/0167-4889(90)90198-m. [DOI] [PubMed] [Google Scholar]