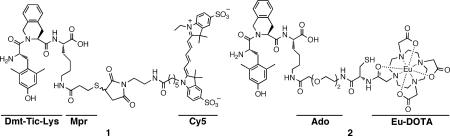
Abstract
A general solid-phase synthetic strategy is developed to prepare fluorescent and/or lanthanide labeled derivatives of the δ-opioid receptor (δOR) ligand H-Dmt-Tic-Lys(R)-OH. The high δ-OR affinity (Ki = 3 nM) and desirable in vivo characteristics of the Cy5 derivative 1 suggests its usefulness for structure-function studies, receptor localization and as a high-contrast non-invasive molecular marker for live imaging ex vivo or in vivo.
Labeling of opioid peptides remains an active area of research as pharmacological tools to study opioid receptor structure and function, as well as for imaging.1 Most labeled opioids for in vivo imaging have been designed to be lipophilic to permit passage across the blood-brain barrier (BBB). However, opioid receptors have also been implicated to play a role in a variety of cancers,2 cardiovascular diseases,3 and gastrointestinal disorders.4 Further, recent research promises newer paradigms of opioid analgesia acting outside the central nervous system.5 Therefore, there is an increasing need to develop labeled opioid ligands and establish synthetic strategies, especially solid-phase approaches, for in vivo imaging of peripherally as well as centrally restricted opioid receptors.6 These ligands could also be useful for in vitro studies such as altered opioid receptor expression profiles observed in patients with morphine dependence and in hypertrophic scars with associated nociceptive pain.7
Among the wide variety of opioid ligands known, the dipeptide Dmt-Tic represents the minimal peptide sequence that selectively interacts with δ-opioid receptor as a potent antagonist (Kiδ = 0.022 nM; pA2 = 8.2).8 Numerous derivatives of Dmt-Tic have been reported with either agonist or antagonist properties, μ- or δ-opioid selectivities, or mixed μ,δ activities, which makes it an ideal candidate for labeling.9 For example, H-Dmt-Tic-Lys(Ac)-OH exhibits high δ-receptor affinity (Ki = 0.047 nM) in radioligand binding assays.10 Opioid peptide ligands with fluorescent functionalities such as rhodamine, pyrene, dansyl, biotin and fluorescein have been described before.1,11 We chose a cyanine (Cy) dye as it is highly fluorescent and water soluble, as well as provides a significant advantage over other optical labels for in vivo imaging. For example, the Cy5 fluoresces in the far-red region of the visible spectrum and is, thus, ideal for minimizing background artifacts. Labeling of opioid peptides generally cannot involve the Nα-terminus since this is critical for the opioid receptor affinity (“message region”).12 Further, a free carboxylic function at the C-terminus is important to maintain high δ-receptor selectivity.8b,10,12 Thus, the label must be attached on the side chain, often a C-terminal residue, in a manner that has minimal influence on the ligand-binding domain. Here, we describe a solid-phase strategy to prepare Dmt-Tic ligands and their labeled analogues linked at the C-terminus lysine side chain via small linkers. In this context, hydrophilic components such as spacers and labels were employed for peripheral retention, lower non-specific uptake, and faster blood clearance of the ligand.13 The bioevaluation of the Cy5 probe 1 as a representative example is described, and the flexibility of the synthetic scheme to prepare dual-modality agents is highlighted.
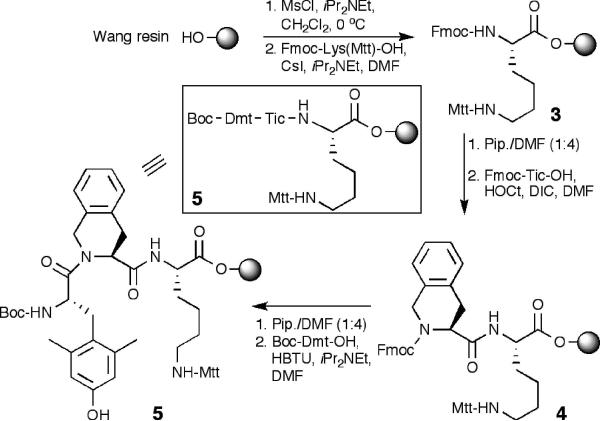
An easy, robust and scaleable synthetic route to label Dmt-Tic ligands was developed based only on commercially available reagents, and a labeling scheme that could be performed on-resin or in solution as desired. Many commercially available labeling moieties contain an activated carboxylic acid (e.g., N-hydroxy succinimide (NHS) ester derivative), or a maleimide that readily react with an amine or a thiol, respectively. Therefore, the synthetic scheme was designed for labeling H-Dmt-Tic-Lys(R)-OH by a maleimide derivative of Cy5 and an NHS ester of the lanthanide chelator DOTA (1,4,7,10-tetraazacyclododecane-N,N’,N”,N”’-tetracetic acid). For optimal spacing between the ligand and the probe, small linkers such as 3-mercaptopropionyl (Mpr) and 8-amino-3,6-dioxaoctanyl (Ado) were employed. The synthesis was started with esterification of Nα-Fmoc-Lys(Nε-Mtt)-OH onto Wang resin (Scheme 1). This was achieved from Wang resin, which was mesylated using an 8-fold excess of MsCl at 0 °C to activate OH groups, followed by coupling with Fmoc-Lys(Mtt)-OH.14 The Nα-Fmoc protection from 3 was removed with piperidine in DMF and Fmoc-Tic-OH was coupled using standard Nα-Fmoc/tBu strategy of solid-phase peptide synthesis to give intermediate 4.15 For final coupling, Boc-Dmt-OH was used since a free N-terminal peptide can be directly obtained after final acidic cleavage. Additionally, a choice of Nα-terminal Boc protection prevents against premature Fmoc deprotection by free NH2 groups released on side-chain of lysine and any consequent cyclative elimination (dioxopiperazine) of Dmt-Tic from Dmt-Tic-Lys(R)-resin.16 Lastly, Boc is smaller than Fmoc, facilitating a faster coupling rate. Despite that, Boc-Dmt coupling to the sterically hindered Tic-Lys(R)-resin was challenging. The coupling has to be mediated via strong HBTU activation accelerated by microwave.17 Also, there is a conceptual disadvantage of using an unprotected phenolic group on Dmt. The reaction leads to Dmt self-condensation, forming small amounts of Dmt-oligomers. Nonetheless, the formed phenolic esters are susceptible to mild aminolysis, and can be selectively removed by treatment with 50% piperidine in CH2Cl2:MeOH (5:1) before acidic cleavage.
Scheme 1.
Synthesis of resin intermediate 3a
a Abbreviations: Mtt: methyltrityl; Pip. = piperidine; HOCt = 6-chloro-1-hydroxybenzotriazole; DIC = diisopropylcarbodiimide HBTU: 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium hexafluorophosphate)
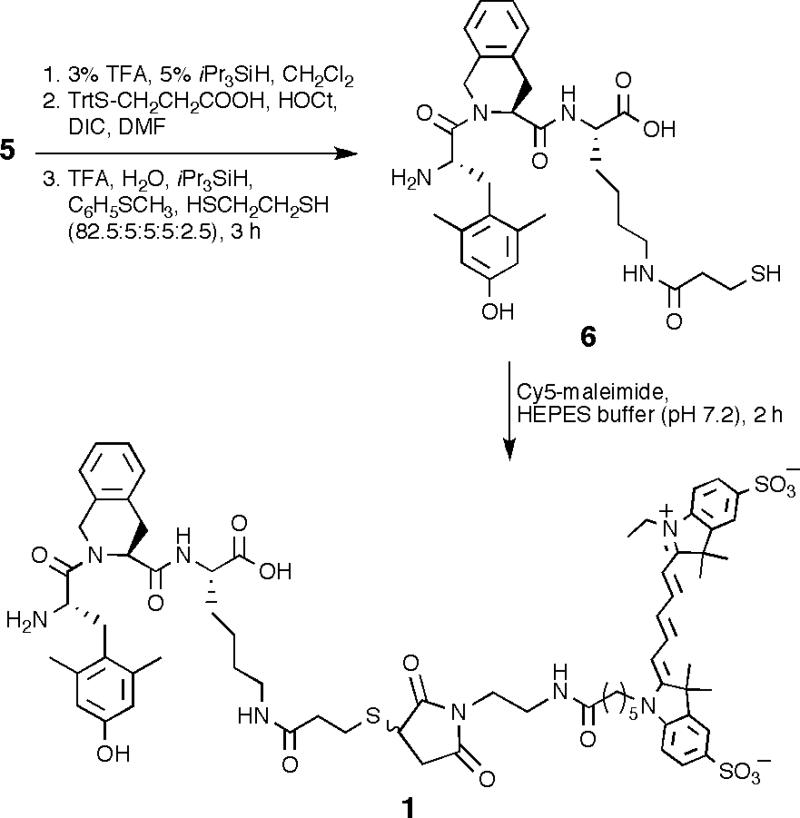
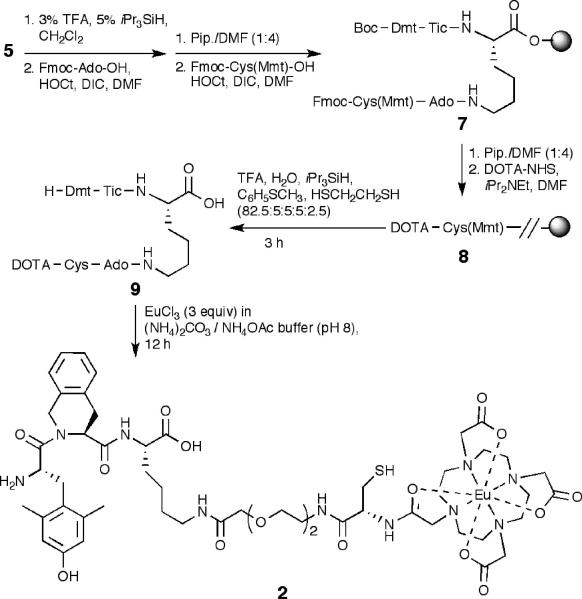
Following Dmt coupling to give intermediate 5, the coupling of dyes and chelating agents can be performed on the resin or in solution. For Cy5 labeling, more cost-effective conjugation of dye in solution was preferred via a thiol-maleimide reaction. 3-Mercaptopropionyl was chosen as a small linker for C-terminal attachment as the dye possesses a 12-atom linker with maleimide at the end. Thus, TrtS-CH2CH2COOH was coupled to 5 using HOCt/DIC protocol and then cleaved with acidic cocktail (82.5% TFA, 5% H2O, 5% iPr3SiH, 5% thioanisole and 2.5% ethanedithiol) to give ligand 6 (Scheme 2). The compound was purified by preparative HPLC and the Cy5 dye was conjugated to the peptide in solution to give ligand 1.18 The on-resin labeling was tested by synthesizing a DOTA chelate as shown in Scheme 3. The Mtt protection on lysine was removed with 3% TFA and 5% iPr3SiH in CH2Cl2. Here we employed a bifunctional handle to investigate its utility for coupling commonly available labeling moieties, for dual-modality labeling (e.g., optical/magnetic), for coupling to nanoparticles with lanthanide labels, and for preparing dimeric ligands at a later stage (unpublished data). For this purpose, the synthetic scheme was designed to incorporate orthogonally protected Fmoc-Cys(Mmt)-OH at the end of the Ado linker and coupled using standard Nα-Fmoc/tBu strategy to give intermediate 7. The amine group of Cys was then chosen to couple DOTA on-resin using DOTA-NHS ester (2 equiv) and iPr2NEt (8 equiv) in DMF for overnight to give 8.19 The peptide was then cleaved from the resin, and europium chelation carried out in solution to yield ligand 2.20 The purified peptides were dissolved in DMSO:H2O (3:2) for bioassay purposes.21
Scheme 2.
Synthesis of Mpr derivative 6 and Cy5 ligand 1
Scheme 3.
Syntheses of Eu-DOTA labeled ligand 2a
a Abbreviations: Mmt = methoxytrityl.
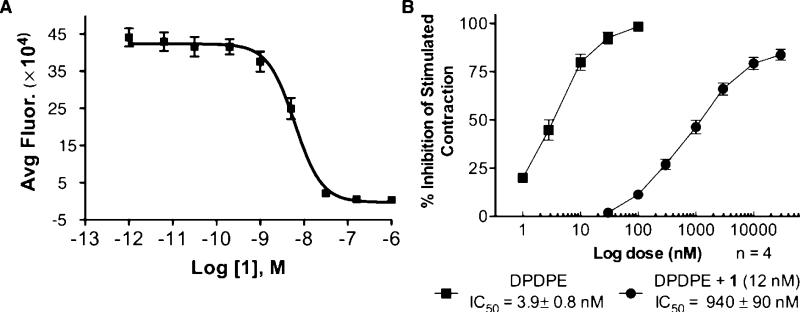
Purified ligands 1 and 6 were evaluated for their binding affinity for the δOR in a competitive binding assay using HCT116 colon carcinoma cells engineered to express the δOR (Figure 1). Europium labeled opioid peptide DPLCE was used as a competing ligand in a time-resolved fluorescence (TRF) assay of our own design.22 Peptide 6 retained high δ receptor affinity (Ki = 2.5 ± 0.8 nM), which was only slightly lower than that reported for Dmt-Tic-Lys-NH2 (Ki = 0.71 nM) and significantly lower than Dmt-Tic-Lys(Ac)-OH (Ki = 0.047 nM).3,4 However, a direct comparison between these TRF results and reported radioligand binding assays is not wholly instructive due to inherent difference in the assay methods. Ligand 1, with a Cy5 label, exhibited a similar bioactivity profile as ligand 6 and retained high δOR affinity (Ki = 3 ± 0.1 nM), which is equipotent to its unlabeled counterpart. Thus, attachment of the Mpr spacer and the Cy5 label did not interfere in any significant way in the ligand-receptor interaction. This is in sharp contrast to many labeled opioid peptides (including Tic based analogues) with remarkably high loss in affinity for δOR.23 Further, ligand 1 exhibited high inhibitory potency (Ke = 37 ± 9 pM for n=8) in the mouse-isolated vas deferens (MVD) assay against δ-agonist DPDPE (Figure 1B),24 which clearly demonstrates it as one of the best labeled δOR ligands.
Figure 1.
A) Competitive binding assay of Cy5 labeled ligand 1 (Ki = 3 nM; R2 = 0.96). B) Mean concentration-response data for DPDPE from MVD assay (n=4 shown here).
For in vivo studies, SCID mice were xenografted bilaterally with HCT116/δOR, and parental HCT116 tumors, which do not express δOR. Mice then were tail vein injected with 10 μg of ligand 1 and images were acquired at different times post-injection using a VersArray 1300B cooled CCD camera, a filtered fiberoptic light source and a tunable emission filter (CRI, Inc) (see supporting information for methods). After 15 min post-injection, the fluorescence intensity maps indicated systemic presence of the compound with high intensity throughout the animal (not shown). At 24 h, the compound was systemically cleared and retained in the δOR-containing but not the parental tumors, as shown in Figure 2 (A&B). Images were analyzed using Image-Pro® Plus 5 by drawing regions-of-interest (ROIs) over each tumor and non-involved muscle tissue. Histograms were generated for each ROI, and mean fluorescence intensities were determined for each time point. After 72 h, all δOR(+) tumors had elevated fluorescence intensities compared to the corresponding δOR(-) tumors (Figure 2C). Notably, this intensity differential was independent of dose, although higher contrast was observed at low dose (10μg) after 24 h (cf. high dose (100 μg) animals). The Cy5 labeled opioid peptide provided a high-constrast non-invasive molecular marker for live imaging of cultured cells or in vivo imaging.
Figure 2.
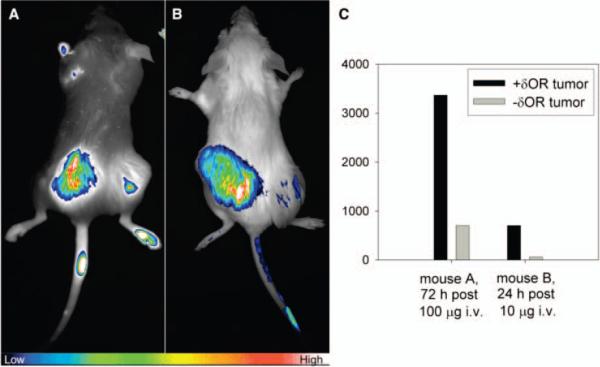
Fluorescence imaging of targeted agent. Mice bilaterally implanted with HCT116 xenografts of (right) control cells, and (left) cells over-expressing δOR. A) Mouse imaged 72 h post-intravenous (i.v.) injection of 100 μg of ligand 1; B) Mouse imaged 24 h post-i.v. injection of 10 μg of ligand 1; C) Fluorescence intensity values over whole tumor regions of interest (#1) after 72 h of clearance of 100 μg and (#2) after 24 h of clearance of 10 μg of ligand 1.
In summary, we have described a solid-phase synthetic methodology for derivatization of the highly potent δ-opioid ligand, Dmt-Tic-Lys(R)-OH. We sought easy modification with fluorescent dyes and/or chelating labels either on-resin or in solution. The applicability of this synthetic approach was demonstrated by derivatizing a Dmt-Tic ligand with the lanthanide chelator on a solid-phase support, and the Cy5 label in solution. Finally, bioevaluation of the Cy5 labeled compound demonstrated its potential utility in in vitro studies and in vivo imaging of the peripherally expressed δ-opioid receptor.
Supplementary Material
Acknowledgment
This research was supported by NCI grants R01 CA123547 and R01 CA097360 and ABRC Grant ABRC06-006.
References
- (1).(a) Lipkowski AW, Misicka A, Kosson D, Kosson P, Lachwa-From M, Brodzik-Bienkowska A, Hruby VJ. Life Sci. 2002;70:893–897. doi: 10.1016/s0024-3205(01)01467-9. [DOI] [PubMed] [Google Scholar]; (b) Navratilova E, Waite S, Stropova D, Eaton MC, Alves ID, Hruby VJ, Roeske WR, Yamamura HI, Varga EV. Mol. Pharmacol. 2007;71:1416–1426. doi: 10.1124/mol.106.030023. [DOI] [PubMed] [Google Scholar]; (c) Lever JR. Curr. Pharm. Des. 2007;13:33–49. doi: 10.2174/138161207779313821. [DOI] [PubMed] [Google Scholar]
- (2).(a) Zagon IS, McLaughlin PJ, Goodman SR, Rhodes RE. J. Natl. Cancer Inst. 1987;79:1059–1065. [PubMed] [Google Scholar]; (b) Schreiber G, Campa MJ, Prabhakar S, O’Briant K, Bepler G, Patz EF. Anticancer Res. 1998;18:1787–1792. [PubMed] [Google Scholar]; (c) Fichna J, Janecka A. Cancer Metastasis Rev. 2004;23:351–366. doi: 10.1023/B:CANC.0000031773.46458.63. [DOI] [PubMed] [Google Scholar]; (d) Debruyne D, Oliveira MJ, Bracke M, Mareel M, Leroy A. Int. J. Biochem. Cell Biol. 2006;38:1231–1236. doi: 10.1016/j.biocel.2006.01.003. [DOI] [PubMed] [Google Scholar]
- (3).Villemagne PS, Dannals RF, Ravert HT, Frost JJ. Eur. J. Nucl. Med. Mol. Imaging. 2002;29:1385–1388. doi: 10.1007/s00259-002-0897-z. [DOI] [PubMed] [Google Scholar]
- (4).Pol O, Palacio JR, Puig MM. J. Pharmacol. Exp. Ther. 2003;306:455–462. doi: 10.1124/jpet.103.049346. [DOI] [PubMed] [Google Scholar]
- (5).Stein C, Schafer M, Machelska H. Nat. Med. 2003;9:1003–1008. doi: 10.1038/nm908. [DOI] [PubMed] [Google Scholar]
- (6).Ryu EK, Wu Z, Chen K, Lazarus LH, Marczak ED, Sasaki Y, Ambo A, Salvadori S, Ren C, Zhao H, Balboni G, Chen X. J. Med. Chem. 2008;51:1817–1823. doi: 10.1021/jm7014765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) Narita M, Funada M, Suzuki T. Pharamaol. Ther. 2001;89:1–15. doi: 10.1016/s0163-7258(00)00099-1. [DOI] [PubMed] [Google Scholar]; (b) Cheng B, Liu HW, Fu XB, Sheng ZY, Li JF. Br. J. Dermatol. 2008;158:713–720. doi: 10.1111/j.1365-2133.2008.08449.x. [DOI] [PubMed] [Google Scholar]
- (8).(a) Dmt: 2′,6′-dimethyl-L-tyrosine; Tic: 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid;Salvadori S, Attila M, Balboni G, Bianchi C, Bryant SD, Crescenzi O, Guerrini R, Picone D, Tancredi T, Temussi PA, Lazarus LH. Mol. Med. 1995;1:678–689.
- (9).Bryant SD, Jinsmaa Y, Salvadori S, Okada Y, Lazarus LH. Biopolymers (Pept. Sci.) 2003;71:86–102. doi: 10.1002/bip.10399. [DOI] [PubMed] [Google Scholar]
- (10).(a) Balboni G, Onnis V, Congiu C, Zotti M, Sasaki Y, Ambo A, Bryant SD, Jinsmaa Y, Lazarus LH, Trapella C, Salvadori S. J. Med. Chem. 2006;49:5610–5617. doi: 10.1021/jm060741w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Balboni G, Salvadori S, Guerrini R, Negri L, Giannini E, Bryant SD, Jinsmaa Y, Lazarus LH. J. Med. Chem. 2004;47:4066–4071. doi: 10.1021/jm040033f. [DOI] [PubMed] [Google Scholar]
- (11).(a) Hazum E, Chang K-J, Shechter Y, Wilkinson S, Cuatrecasas P. Biochem. Biophys. Res. Commun. 1979;88:841–846. doi: 10.1016/0006-291x(79)91485-2. [DOI] [PubMed] [Google Scholar]; (b) Mihara H, Lee S, Shimohigashi Y, Aoyagi H, Kato T, Izumiya N, Costa T. FEBS Lett. 1985;193:35–38. doi: 10.1016/0014-5793(85)80074-0. [DOI] [PubMed] [Google Scholar]; (c) Berezowska I, Chung NN, Lemieux C, Zelent B, Szeto HH, Schiller PW. Peptides. 2003;24:1195–1200. doi: 10.1016/j.peptides.2003.07.004. [DOI] [PubMed] [Google Scholar]; (d) Kumar V, Aldrich JV. Org. Lett. 2003;5:613–616. doi: 10.1021/ol027044s. [DOI] [PubMed] [Google Scholar]; (e) Balboni G, Salvadori S, Piaz AD, Bortolotti F, Argazzi R, Negri L, Lattanzi R, Bryant SD, Jinsmaa Y, Lazarus LH. J. Med. Chem. 2004;47:6541–6546. doi: 10.1021/jm040128h. [DOI] [PubMed] [Google Scholar]
- (12).(a) Hruby VJ, Gehrig CA. Med. Res. Reviews. 1989;9:343–401. doi: 10.1002/med.2610090306. [DOI] [PubMed] [Google Scholar]; (b) Aldrich JV, Vigil-Cruz SC. In: Burger’s Medicinal Chemistry and Drug Discovery. 6th ed. Abraham DJ, editor. Vol. 6. Wiley & Sons; New York: 2003. p. 329. Chapter 7. [Google Scholar]
- (13).(a) Calculated logD of compound 1 reveals a value of 1.01 at pH 7.4 (logD: 2.6, 1.35, 1.16 at pH 1.5, 5.0, 6.5, respectively).Duval RA, Allmon RL, Lever JR. J. Med. Chem. 2007;50:2144–2156. doi: 10.1021/jm0700013.
- (14).In a 50 mL bottle containing 1g of Wang resin (0.93 mmol/g) with a magnetic stir bar, dry CH2Cl2 was added to swell the resin for 1 h. The solvent was removed, the bottle closed with a septum and flushed with nitrogen and iPr2NEt (9 equiv, 1.4 mL) in 15 mL of CH2Cl2 was added. The resin slurry was cooled to 0 ° C followed by dropwise addition of MsCl (8 equiv, 0.57 mL) in 2 mL of CH2Cl2. The reaction was stirred for 20 min, the ice-bath removed and the stirring continued for another 20 min (rt). The resin was then transferred to a syringe reactor and washed with dry CH2Cl2, and dry DMF. Fmoc-Lys(Mtt)-OH (2 equiv, 1.2g), CsI (2 equiv, 0.5g), iPr2NEt (2 equiv, 0.32 mL) in ca 10 mL of dry DMF were added and the reaction stirred overnight at rt.
- (15).The resin was Nα-Fmoc deprotected with piperidine/DMF (1:4) and then washed with DMF, CH2Cl2, 0.2 M HOBt/DMF, and DMF. Fmoc-Tic-OH (3 equiv), HOCt (3 equiv) and DIC (6 equiv) in DMF were then added and the reaction was stirred for 2 h.
- (16).Caspasso S, Sica F, Mazzarella L, Balboni G, Guerrini R, Salvadori S. Int. J. Pep. & Prot. Res. 1995;45:567–573. doi: 10.1111/j.1399-3011.1995.tb01321.x. [DOI] [PubMed] [Google Scholar]
- (17).Boc-Dmt-OH (3 equiv), HBTU (3 equiv) and iPr2NEt (6 equiv) in DMF were added to the resin and the reaction was heated in a household microwave for 3 sec. The reaction was stirred until it cooled to rt; the heating was repeated (5x), and the resin stirried for another 2 h.
- (18).Ligand 8 was dissolved in HEPES buffer (pH 7.2) and 1.3 equivof Cy5-maleimide was added in aliquots until full conversion was achieved as monitored on analytical HPLC. The labeled ligand was then separated with SPE. (See Supporting Information)
- (19).Note that commercially available DOTA-NHS (Macrocyclics, TX) contains an estimated 3 equiv of TFA by weight. Also, DOTA can alternatively be coupled using a maleimide derivative, following selective cleavage of Mmt group with 3% TFA, 5% iPr3SiH in CH2Cl2.
- (20).The purified compound was dissolved in (NH4)2CO3/NH4OAc buffer (pH 8) and 3 equiv of EuCl3.6H2O was added. The reaction stirred overnight in inert atmosphere (to prevent disulfide formation by air oxidation). The excess salts were then removed by solid-phase extraction (SPE). (See Supporting Information)
- (21).No diketopiperazine formation was observed in this solvent system for one month. SeeJ. Am. Chem. Soc. 1994;116:8450–8458.
- (22).(a) Handl HL, Vagner J, Yamamura HI, Hruby VJ, Gillies RJ. Anal. Biochem. 2005;343:299–307. doi: 10.1016/j.ab.2005.05.040. [DOI] [PubMed] [Google Scholar]; (b) Handl HL, Vagner J, Yamamura HI, Hruby VJ, Gillies RJ. Anal. Biochem. 2004;330:242–250. doi: 10.1016/j.ab.2004.04.012. [DOI] [PubMed] [Google Scholar]
- (23).Arttamangkul S, Alvarez-Maubecin V, Thomas G, Williams JT, Grandy DK. Mol. Pharmacol. 2000;58:1570–1580. doi: 10.1124/mol.58.6.1570. [DOI] [PubMed] [Google Scholar]
- (24).Kramer TH, Davis P, Hruby VJ, Burks TF, Porreca F. J. Pharmacol. Exp. Ther. 1993;266:577–584. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.