Abstract
Contusive spinal cord injury (SCI) is the most common type of spinal injury seen clinically. Several rat contusion SCI models have been described, and all have strengths and weaknesses with respect to sensitivity, reproducibility, and clinical relevance. We developed the Louisville Injury System Apparatus (LISA), which contains a novel spine-stabilizing device that enables precise and stable spine fixation, and is based on tissue displacement to determine the severity of injury. Injuries graded from mild to moderately severe were produced using 0.2-, 0.4-, 0.6-, 0.8-, 1.0-, and 1.2-mm spinal cord displacement in rats. Basso, Beattie, and Bresnahan (BBB) and Louisville Swim Score (LSS) could not significantly distinguish between 0.2-mm lesion severities, except those of 0.6- and 0.8-mm BBB scores, but could between 0.4-mm injury differences or if the data were grouped (0.2–0.4, 0.6–0.8, and 1.0–1.2). Transcranial magnetic motor evoked potential (tcMMEP) response amplitudes were decreased 10-fold at 0.2-mm displacement, barely detected at 0.4-mm displacement, and absent with greater displacement injuries. In contrast, somatosensory evoked potentials (SSEPs) were recorded at 0.2- and 0.4-mm displacements with normal amplitudes and latencies but were detected at lower amplitudes at 0.6-mm displacement and absent with more severe injuries. Analyzing combined BBB, tcMMEP, and SSEP results enabled statistically significant discrimination between 0.2-, 0.4-, 0.6-, and 0.8-mm displacement injuries but not the more severe injuries. Present data document that the LISA produces reliable and reproducible SCI whose parameters of injury can be adjusted to more accurately reflect clinical SCI. Moreover, multiple outcome measures are necessary to accurately detect small differences in functional deficits and/or recovery. This is of crucial importance when trying to detect functional improvement after therapeutic intervention to treat SCI.
Key words: BBB, spinal cord injury, SSEP, tcMMEP, tissue displacement
Introduction
Studies examining the pathophysiology of and recovery from spinal cord injury (SCI) are dependent on animal models that are both clinically relevant and highly reproducible. Convincingly demonstrating the effect of a therapeutic strategy is dependent on a controlled graded injury animal model based on strict biomechanical parameters (Gruner, 1992; Jakeman et al., 2000; Ramer et al., 2000). Based on the seminal work by Allen in dogs (Allen, 1911), a number of techniques have been used to induce graded contusive injuries in rodents with each subsequent device/technique relying on the most up-to-date technologies available at the time (Behrmann et al., 1992; Falconer et al., 1996; Gruner, 1992; Jakeman et al., 2000; Scheff et al., 2003; Soblosky et al., 2001; Wrathall et al., 1985). The devices employed were designed to create mild, moderate, and severe injuries which in turn were characterized using standardized histological and behavioral assessments. Table 1 provides an overview of the different principles on which these devices were designed.
Table 1.
Comparison of Widely Used SCI Devices
| Device name | Rat/mouse | Injury parameter (accuracy) | Calibration method | Dwell-time | Velocity (m/sec) | Target stabilization | Monitors | Reference |
|---|---|---|---|---|---|---|---|---|
| LISA | Y/Y | Displacement (±0.007 mm) | Non-contact laser sensor | 50 msec to 5 sec (±5 msec) | 0.5–2.0 | Mouse or rat stabilizer | Velocity Force Duration of compression | Present study |
| NYU | Y/N | Velocity (±5%) | Contact (closed circuit) | Variable, depends on operator release | 0.3–0.9 | Two pairs of clamps on spinous processes | Velocity Height Movement of vertebra | Gruner et al., 1992 |
| ESCID | Y/Y | Displacement (±0.008 mm) | Contact (Ling vibrator)a | 0.148 | Two pairs of forceps on spinous processes | Velocity Force Momentum | Stokes et al., 1992 | |
| IH | Y/Y | Force (depends on background noise) | Internal | Immediately <5 msec | 0.1–0.13 | Two pairs of forceps on spinous processes | Force Displacement | Scheff et al., 2003 |
| Weight drop | Y/Y | Velocity (NA) | Contact (visual observation) | Variable, depends on operator release | 0.3–0.9 | Two pairs of forceps on spinous processes | Strain Force | Gale et al., 1985 |
| Modified aneurysm clips | Y/Y | Clip tension (depends on location of blades on the cord)a | NA | Variable, depends on operator release | NA | None needed | None | Rivlin et al., 1978 |
The importance of the closing velocity of the clip blades on the spinal cord was ignored. The outcome of injury can be dramatically different using the same clip but with dissimilar releasing methods (unpublished data).
Clinically, SCI impact velocities are greater than 1.0 m/sec (Sances et al., 1984). Velocity-based contusion devices generate different injury severities by dropping a fixed weight onto the spinal cord from different heights (Allen, 1911; Constantini et al., 1994; Falconer et al., 1996; Gruner, 1992; Panjabi et al., 1988; Soblosky et al., 2001; Wrathall et al., 1985). Using a rod with a mass of 10 g, the impact velocity of the NYU system is 0.33–0.9 m/sec creating mild (6.25 g-cm) to severe (50 g-cm) injuries, respectively (Basso et al., 1995; Constantini et al., 1994; Gruner, 1992). In devices employing a pneumatic piston, impact velocities can be adjusted over the wider range of 0.5–3.0 m/sec, thereby creating a more clinically relevant injury (Seki et al., 2002; Yeo, 2004). However, impact velocity varies from that predicted as the spinal column yields during impact, thereby, reducing the effective velocity. Most systems attempt to overcome this problem by placing clamps on the spinous processes adjacent to the injury level. However, motion between adjacent segmental levels induces variability and inconsistency in injury parameters and, thus, behavioral and histological measurements. To minimize the effect of spinal column yield, we have developed a spine stabilizer that fixes the spinal column at the level of injury as part of the Louisville Injury System Apparatus (LISA).
The Infinite Horizons (IH) impactor (Scheff et al., 2003) was designed to use force as the major injury parameter. The advantage of this approach is that the impactor does not need to touch the spinal cord for calibration prior to impact. The IH impactor shows excellent correlation between force and displacement of the spinal cord in both rats (Scheff et al., 2003) and mice (Ghasemlou et al., 2005). However, its maximal injury velocity is 0.13 m/sec, which is necessitated by the fact that the force transducer is mounted on the impactor. Using this configuration, it is difficult to use force as the dependent variable at higher injury velocities. When the force transducer is mounted on the dynamic impactor, injury velocities of >0.2 m/sec result in noise levels that are equal to or higher than the signal (unpublished data).
At higher impact velocities, tissue displacement is thought to be the single most important parameter for generating a graded contusive injury model (Grill, 2005; Jakeman et al., 2000; Stokes, 1992). The severity of injury is determined largely by the distance that the spinal cord is rapidly displaced, which can be accurately controlled and measured. The electromagnetic SCI device (ESCID), a tissue displacement system with a fixed impact velocity of 0.148 m/sec, also demonstrates excellent correlation between injury severity, histology, and behavior in the rat (Stokes, 1992) and mouse (Jakeman et al., 2000). This system also measures many related biomechanical parameters such as force, velocity, power, impulse/momentum, and energy, but cannot be used at higher impact velocities.
Here, we report on the LISA, a novel SCI device that also uses tissue displacement as the major determinant to create a precise lesion and has the capability to adjust impact velocities between 0.5 and 2 m/sec. Behavioral and electrophysiological assessments and their combination are used to determine the discriminability of very small differences in injury severity.
Methods
LISA contusion device
The pneumatically powered impactor of the LISA is attached to a laser sensor that emits a laser beam that detects the reflection from the spinal cord to determine the distance to a mechanical accuracy of ±0.005 mm (Fig. 1). The laser sensor measures the position to the target tissue, determines the magnitude of cord displacement, and measures impactor velocity. The LISA impactor is accelerated using compressed air on its downward movement, and withdrawal is controlled using an electric valve (Mac 45A AA1-DDBA-1BA; Air Hydro Power, Louisville, KY). Two sensors monitor the injury parameters: (1) The laser distance sensor (OADM 12 U6430; Baumer Ltd., Southington, CT) is attached to the impactor complex and emits a laser beam focused on the target (impactor or the dura) as well as receiving the laser reflection. The laser beam measures the distance from the laser sensor to the impactor tip, as well as the distance to the dural surface over the targeted spinal cord. It then calculates the difference between these two distances that represents the degree of tissue displacement that is a measure of the injury severity (Fig. 1). The laser sensor also measures the impactor velocity by monitoring changes in impactor distance against time. (2) The second sensor, placed beneath the fixation device, is a piezoelectric sensor (209C11 PCB; Piezotronics, Inc. Depew, NY). Analog output voltage signals from the OADM12 laser distance sensor and the 209C11 Quartz Impact Force Sensor are connected to the analog input channels on the Universal Serial Bus (USB; DT USB-9805) board. The USB is used to record data acquisition. An air control valve that triggers the impactor movement is controlled either by software through the USB board control channels or by switch buttons located on the top of the control box. The Time Delay Relay (TR-51526–04; Micrometic, Milwaukee, WI) switch can be set to open the air valve for a predefined duration (0.05–5 sec) that controls contact duration of the impactor against the spinal cord. Three-dimensional movement of the stage is controlled by three microdrivers in the X, Y, and Z planes (7VT174–10 X2 and 7T174–13; STANDA, Vilnius, Lithuania). These microdrivers provide a high degree of accuracy and stability. The software was designed using DT measure Foundry 4.0.7 visual software environment for Microsoft Windows. The software provides a Graphical User Interface (GUI) allowing the operator to set up injury parameters and to accomplish the procedures, such as calibration of “zero” position of the impactor, detection of the dural surface with the laser distance sensor, and setting up the injury severity levels. Impactor velocity, force absorbed by the target tissue, and impactor-tissue contact duration are represented in a one-dimensional waveforms. Data are stored on a PC in-.text file format which is suitable for data analysis environments such as MatLab. The LISA is commercially available through LIS Inc. (Louisville, KY).
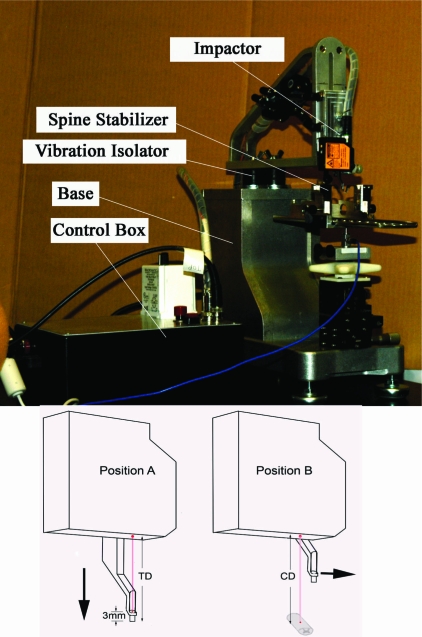
FIG. 1.
(Top) The LISA consists of the following: (1) impactor complex that measures tissue displacement and causes the contusion, (2) spine stabilizer that secures the spinal cord, (3) vibration isolator that prevents vibration from being transmitted from the impactor complex to the force sensor located below the spine stabilizer, (4) base, and (5) control box that contains circuits that control the impactor position and its duration of contact with the spinal cord. It also conveys biomechanical information to the impactor complex. (Bottom) Cartoons of the impactor complex illustrate the mechanism of tissue displacement control. In position A, the distance from the laser sensor (pink vertical line) to the reflecting surface (DRS) of the impactor is calibrated in its fully extended position (vertical large arrow). Tip distance (TD) = tip thickness (3 mm) + DRS. In position B, the impactor is withdrawn and moved laterally away from the laser beam path (horizontal large arrow). The distance from the laser sensor to the spinal cord is measured (cord distance [CD]). The desired tissue displacement is determined by the difference between the cord distance and tip distance (CD-TD). The impactor is returned back to the laser beam path and is ready for impact.
The LISA produces the contusion injury by the following steps: (1) The distance from the laser sensor to the tip of the impactor (TD) is determined in the extended position (Fig. 1, bottom left). (2) The distance from the laser sensor to the dural overlying the spinal cord (CD) is measured by retracting the impactor and swinging the tip laterally to expose the spinal cord under the laser beam (Fig. 1, bottom right). (3) Tissue displacement is determined by setting the difference between the TD and CD to the desired level. If the TD = CD, release of the impactor would not contuse the cord as it would stop at the cord surface. Tissue displacement can be adjusted to ± 0.007 mm. The impactor is powered by compressed gas, and velocity is measured by monitoring the distance and duration of the impactor tip movement. The injury force is measured using a quartz force sensor that is placed under the spine stabilizer. The duration of tissue compression is controlled by a time relay (between 0.05 and 5 sec) that controls the time at which the impactor tip is lifted from the cord surface.
Force calibration experiment on the LISA
In the extended position, the impactor just touches the surface of the force sensor and the stage is gradually elevated (Z microdrive; arrow) to create pressure on the force sensor at 100-, 200-, and 300-g levels independently. The impactor is withdrawn at 1.5 m/sec from the sensor that generates a reading (Volts) that represents force. Peak force readings from the sensor can be converted to Newtons (N), which correspond to the various loads. A 100-g weight correlated with a gravity factor of 0.98 (approximately = 1 N). For the sensor, calibrations were 0.48 ± 0.02, 0.94 ± 0.01, and 1.47 ± 0.01 V for 100, 200, and 300 g by each unloading weight, respectively. A 1.0-V reading using this sensor was equal to 2.08 N.
Animals
Animal care, handling, and surgery were conducted in accordance with Guidelines for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, 1996) and with the approval of the University of Louisville Institutional Animal Care and Use Committee (IACUC). Eighty-four adult female Sprague-Dawley (SD) rats weighing 200–225 g were used. Three injury factors believed to affect lesion severity were studied. First, evaluation of defined tissue displacement was conducted: a 1.0 m/sec impactor velocity was used to deform the spinal cord from 0.2 mm to 1.2 mm in 0.2-mm displacement increments (normal diameter of the thoracic spinal cord is approximately 2.5 mm) to induce a mild to moderately severe SCI. More severe injury groups (1.4- and 1.6-mm displacements) were not studied. Second, the effect of injury velocity was examined by doubling the velocity from 1.0 to 2.0 m/sec using 0.6-mm tissue displacement for both velocities. Third, to assess the effect of dwell time of impactor/cord compression, we utilized a 0.6-mm cord displacement and an injury velocity of 1.0 m/sec with increased compression times from 0.2 to 5.0 sec. Final animal group sizes analyzed for all injury severities was 6–7 rats/group.
Surgical procedures
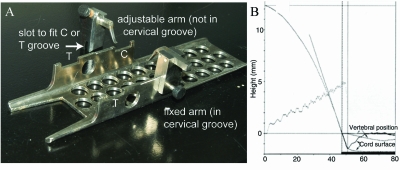
Rats were anesthetized with sodium pentobarbital 45 mg/kg, i.p. (Nembutal; Abbott Laboratories, Chicago, IL). Under sterile conditions, a midline incision was made overlying the T6-11 vertebral levels. The skin flaps and paraspinal muscles were retracted laterally. The animal was secured into the rat spine stabilizer (Fig. 2A,B) by inserting the stainless steel arms of the stabilizer bilaterally on the facets of T8 and then locking the set screws. This device suspends the spine to prevent respiration and other movements from influencing the spine position. A T8 laminectomy was performed to expose the dura overlying the spinal cord. After hemostasis, the ligamentum flavum was completely removed at T8-9. The dura mater remained intact. This spine stabilizer provides excellent immobilization in the NYU system, which directly measures vertebral movement (Fig. 2C), and in the LISA. Following SCI, the rat stabilizer was detached from the LISA and the incision was closed in layers. Following surgery, all rats were given a 10-mL bolus injection of 0.9% saline subcutaneously, returned to their cages, and provided with water and food ad lib. Rats were placed on a 37°C heating blanket overnight. All animals received 0.05 mg/kg BID buprenorphine given subcutaneously for pain relief for the first 48 h following SCI.
FIG. 2.
(A) Picture of the rat spine stabilizer. Note the shape of the two arms (one is fixed and the other adjustable), which provides excellent bone fixation. The three main components of the spine stabilizing device include a U-shaped steel channel to hold the animal, a fixed arm, and an adjustable arm. In this figure, the fixed arm is set in one of the cervical (C) grooves while the adjustable arm is not yet attached. For thoracic injuries, the thoracic (T) grooves are utilized. The animal is secured into the rat spine stabilizer by inserting the stainless steel arms of the stabilizer bilaterally on the facets of T8 and then locking the set screws. This device suspends the spine to prevent respiration and other movements from influencing the spine position. (B) The effectiveness of vertebral stabilization was studied on the NYU device that monitors the vertebral position and the spinal cord surface. Using the LISA spine stabilization device, a 12.5-g-cm injury did not cause bone movement and, after impact, the deformed spinal cord recoiled back to the original position (solid lines). However, attaching forceps to the spinous processes as the fixation method results in significant vertebral and spinal cord shift (dotted lines).
Behavioral assessment
All animals were handled daily for 2 weeks pre-injury, and baseline Basso, Beattie, and Bresnahan (BBB) scores were obtained. Animals were tested following surgery at week 2 and weekly thereafter until week 5 (Basso et al., 1995). Two observers trained in performing the BBB assessment at Ohio State University carried out the assessments and were blinded to the injury severities. Hindlimb function was also assessed using the Louisville Swim Score (LSS) once at week 5 following injury (Smith et al., 2006a,b). The LSS is an 18-point scale (0–17) based on five components of swimming and the scores of each component are summed. Those components are (1) forelimb dependency (0–4); (2) hindlimb activity (0–4); (3) hindlimb alternation (0–3); (4) trunk stability (0–4); and (5) body position (0–2). A score of 0–5 indicates a poor swimmer that lacks hindlimb movement and is totally dependent on forelimb activity. A score of 12–17 defines a good swimmer that utilized the hindlimbs with rhythmic alternative movement and good trunk stability.
Electrophysiological assessment
TcMMEPs were obtained by stimulating awake, non-anesthetized, restrained rats as described previously (Cao et al., 2005; Glassman et al., 1995; Hadi et al., 2000; Loy et al., 2002; Zhang et al., 2007). Briefly, rats were restrained in a cloth stockinet tacked to a wooden board. Hindlimbs were exposed to enable insertion of recording electrodes into the gastrocnemius muscles bilaterally, with the active electrode placed into the muscle belly and the reference electrode placed near the distal tendon. The ground electrode was placed subcutaneously between the coil and recording electrodes. TcMMEP responses were elicited by activating sub-cortical structures with a 5-cm electromagnetic coil placed over the cranium. A single magnetic pulse at 60% of maximal output intensity was used. Depending on the amplitude of the responses, a gain of 500–5000 was used to record the compound muscle action potentials. Data obtained from four responses recorded at intervals of 1 min were averaged. Onset latencies and amplitudes were recorded. All animals were tested weekly for 5 weeks.
Somatosensory evoked potentials (SSEPs) were also recorded from non-sedated animals on week 5 (Zhang et al., 2007). During initial electrode placement, animals were anesthetized with 50 mg/kg pentobarbital sodium (Abbott Laboratories, Chicago, IL). Three 1.2-mm-diameter holes were bored in the skull. A 0.8–1.0-mm ball-tipped electrode was made by melting the end of a 0.1-mm-diameter silver wire (Goodfellow Corp., Devon, PA). The ball of the electrode was placed epidurally through the holes. Active recording electrodes were placed over the sensory cortex 2 mm lateral to the midline and 2 mm posterior to the bregma. The reference electrode was placed epidurally over the olfactory bulb. Dental cement was used to seal the holes and anchor the recording electrodes. SSEPs were induced by percutaneous electrical stimulation through a ring electrode at the posterior tibial nerve (PTN). The electrode is a cuff electrode with a fixed distance (7 mm) of the two metal bands, one being the anode and the other the cathode shaped in a ring contour as described previously (Zhang et al., 2007). Stimulation intensities were 1.2–1.5 mA, with a 100-μsec pulse width at 0.3 Hz. SSEP responses were recorded over the left cerebral cortex following right hindlimb stimulation and vice versa for 15 stimulations on each side. The bandpass filter setting was 10–3,000 Hz. Evoked potentials were recorded on a Cadwell machine (Cadwell Laboratories, Kennewick, WA).
Histopathology and lesion morphology
On post-injury week 5, rats were re-anesthetized with an overdose of sodium pentobarbital and perfused transcardially with 50 mL of phosphate-buffered saline (PBS) followed by 300 mL of 4% paraformaldehyde (pH 7.4) in PBS. The spinal cord was removed and postfixed by immersion in the same fixative for 4 h, and then cryoprotected by overnight immersion in 30% sucrose in PBS at 4°C. Following cryoprotection, a 4-mm section of cord spanning the lesion epicenter was frozen, blocked, and embedded in Tissue-Tek (Tissue-Tek, Sukura, Torrance, CA). The epicenter was visually defined as the site of the T8 spinal cord that exhibited maximal tissue destruction. Serial sections of 30 μm were cut on a Leica CM3050 cryostat and stained with ironeriochrom cyanine R to identify areas of myelin. Quantification of the spared white matter at the lesion epicenter was performed as previously described (Magnuson et al., 2005). We chose to quantify epicenter white matter as it is the variable that best correlates with the functional outcome (Basso et al., 1996a).
Statistical analysis
Injury parameters
One-way analysis of variance (ANOVA), followed by Tukey Honestly Significant Differences (HSD) post hoc t-tests were performed to examine the amount of force used to produce the injury and whether there were differences among the injury displacement groups. The two velocities (1 and 2 m/sec), duration of contact between impactor and spinal cord (0.2 and 5.0 sec), force, and LSS scores were compared using independent t-tests for means with equal or unequal (F test of variance between two samples) variance, as appropriate.
Behavioral assessments
Weekly assessments of BBB locomotor scores were compared using repeated-measures ANOVA with the between-groups factor followed by Tukey HSD post hoc t-tests. A one-way ANOVA followed by Tukey HSD post hoc t-tests were performed to examine whether there were differences in LSS scores among the injury displacement groups at week 5 post-injury.
Electrophysiological assessments
Normal SSEPs were obtained from the six rats in the 0.2-mm displacement group prior to injury and were compared to post-injury SSEPs. Oneway ANOVA and Tukey HSD post hoc t-tests were used to compare the normative SSEP P1 and N1 latencies and P1-N1 amplitude with the other injury displacement groups at week 5 post-injury. The 0.2 mm pre- and post-injury SSEPs were compared with paired t-tests. After injury, a measurable tcMMEP could be elicited from only a small number of animals. Thus, the number of response rates following injury in each displacement group was examined.
Spared white matter area
The amount of spared white matter at the epicenter of injury in the displacement groups was compared with one-way ANOVA followed by Tukey HSD post hoc t-tests. Velocity and the area of spared white matter of the duration groups were compared using independent t-tests (for means with equal or unequal variance, as appropriate).
Correlations
The correspondence between the various measures was examined using Pearson correlations. When the correlation included a categorical measure, the non-parametric Spearman rank correlation was used. Correlations among the following outcome measures were examined: tissue displacement, force of impact, behavioral assessments (LSS and BBB scores at week 5), and spared white matter.
Combined outcome measures
To determine the overall status of injury and/or recovery of the rat's locomotion in conjunction with the degree of motor and sensorimotor conduction through the spinal cord, outcome measure responses from the behavior and electrophysiology assessments on week 5 were combined in a manner similar to that of the Combined Behavior Score (CBS) (Gale, 1985). BBB scores were categorized in accordance with the stages of recovery designated in the BBB scale scoring system that ranges from early (0–7), intermediate (8–13) to late (14–21), with a 1, 2, or 3, respectively (Basso et al., 1996a). The quality and quantity of the tcMMEP and SSEP responses were taken into account by dividing electrophysiological responses into the following three scoring categories: 0 = no response; 1 = response is 2 SD from the mean; and 2 = normal response within 1 SD of the mean. The sum of these scores was calculated for each of the six 0.2-mm-distance displacement groups and compared. Although the average combined scores of the milder injury groups (0.2, 0.4, and 0.6 mm) were relatively normal in distribution, the more severely injured groups (0.8, 1.0, and 1.2 mm) had no tcMMEP or SSEP response, and the combined scores, therefore, primarily reflected the BBB category. Non-parametric statistical analyses (Wilcoxon and Mann-Whitney U) were used to rank the combined scores and compare the displacement groups. We sought to ensure that all variables would be comparable by normalizing values before determining the category to which they belonged so that each was equally contributing to the final summed value.
Results
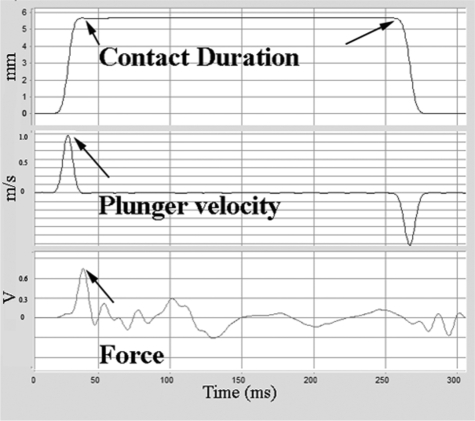
The spine stabilizer of the LISA (Fig. 2A) secured the target spinal cord horizontally (without angulation) under the impactor tip enabling precise tissue displacement during impact. The spinal cord of the animals did not noticeably move (Fig. 2B), nor was any bone fracture observed during contusion. Tissue displacement, dwell time of impactor tip/spinal cord tissue contact, impactor velocity at impact, and force were measured (Fig. 3). The relationship of tissue displacement and force is documented in Table 2. Regulating the air pressure of the piston accurately controlled impactor velocities at 1.0 and 2.0 m/sec. At 12 psi, the velocity of the impactor was 1.000 ± 0.026 m/sec (n = 25) and at 60 psi was 2.000 ± 0.030 m/sec (n = 25). The two dwell times of impactor tip/spinal cord contact (0.2 and 5.0 sec) were controlled within a range of ± 0.015 sec. Initially, we encountered severe interference during force monitoring when the quartz sensor was attached to the dynamic impactor. When the 1.5 m/sec impactor suddenly stopped without contact, a noise equivalent to 18.72 Newtons (on the piezoelectric force sensor) was generated that was much larger than the signal from the sensor itself. One way to avoid this noise artifact is to decrease the impactor velocity. At 0.1 m/sec, noise is at an acceptable level, equivalent to 0.41 Newtons. However, by attaching the quartz sensor to a static portion of the LISA (under the rat trough) and dampening vibration with shock absorbing materials (Fig. 1), noise was reduced to 0.25 ± 0.02 Newtons, allowing force to be monitored for an impactor velocity of 1.0 m/sec. However, at a velocity of 2 m/sec, force could not be measured because the noise was 1.0 Newton, a signal-to-noise ratio below our required minimum of 4 (data not shown).
FIG. 3.
Injury parameters were monitored including impactor/cord contact duration (msec), impactor velocity at impact (m/sec), and force (V) at impact.
Table 2.
Force Recordings at Different Levels of Displacements
| Displacement (mm) (n = 6) | Newtons (mean ± SD) |
|---|---|
| 0.0a | 0.25 ± 0.02 |
| 0.2 | 1.02 ± 0.06 |
| 0.4 | 1.25 ± 0.50 |
| 0.6 | 1.49 ± 0.35 |
| 0.8 | 1.52 ± 0.31 |
| 1.0 | 1.73 ± 0.44 |
| 1.2 | 1.80 ± 0.33 |
Force reading from no tissue contact during the impact cycle was defined as noise.
Operative outcome and monitoring injury parameters
A 4% mortality was related to anesthetic complications. All remaining animals survived the 35 days of the study and experienced no autophagia, bladder infection, or wound infection. The LISA apparatus delivered an accurate spinal cord displacement as the reading obtained from the laser sensor corresponded to the reading from the vertical micro-driver. The gross external appearance of the contused spinal cords differed according to the injury severity, with more severe injuries causing greater vascular damage and hemorrhage. In the 0.2-mm displacement injuries, the spinal cord dorsal vein was preserved, but as the displacement increased to 1.0 and 1.2 mm, greater hemorrhage and contusion obscured the spinal cord surface (data not shown).
Hindlimb locomotor outcomes
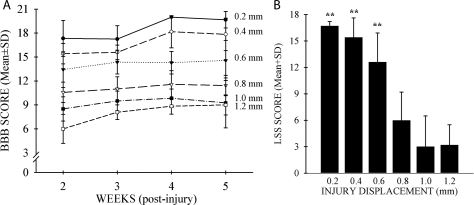
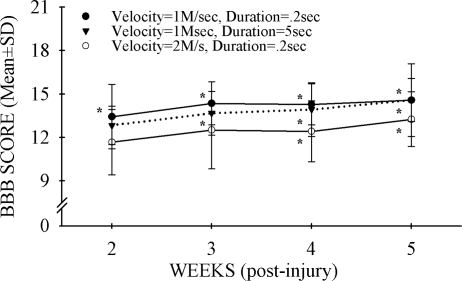
All injured animals showed functional impairment during the first few days after surgery. Weekly BBB locomotor tests were initiated at 2 weeks following SCI, and LSS was assessed once at week 5. Both BBB (Fig. 4A: F = 20.8, df = 2.3, 73.8, p < 0.001) and LSS (Fig. 4B: F = 30, df = 5.31, p < 0.001) showed statistically significant changes over time. Only BBB scores could distinguish between successive 0.2-mm injury severities: 0.6- and 0.8-mm displacement (p < 0.05, all weeks). However, if the 0.2–0.4-mm, 0.6–0.8-mm, and 1.0–1.2-mm displacement groups were combined, both the BBB and LSS tests detected statistically significant differences between these groups. These results suggest that detecting functional differences of a given therapeutic intervention at mild-moderate injury levels using only BBB or LSS analysis would require large effects to be seen or a large number of animals used per experimental group (data not shown). The BBB and LSS scores are highly correlated (r = 0.85, p < 0.001, n = 37). At the same level of spinal cord displacement (0.6 mm), neither doubling the impactor velocity to 2 m/sec nor increasing the dwell time of displacement from 0.2 to 5.0 sec influenced the outcome of hindlimb locomotor function as assessed by BBB (Fig. 5) or LSS (data not shown; both: t = 1.1, df = 11, p > 0.05).
FIG. 4.
(A) Open field locomotor function was evaluated using the Basso, Beattie, and Bresnahan (BBB) score for each group subjected to the displacement injury with a velocity of 1 m/sec and impactor/cord contact time of 0.2 sec. Greater tissue displacements led to poorer functional recovery. At each greater displacement level, the BBB score was progressively lower which continued over 5 weeks. (B) The Louisville Swim Score (LSS) was used to test rats at 5 weeks after spinal cord injury (SCI). Swimming performance worsened with increasing cord displacement. After 5 weeks, the milder injury displacement groups were able to swim much better than those injured at 0.8 mm or higher displacement levels (***p = 0.001). Data are the mean ± SD (n = 6–7/group).
FIG. 5.
Neither increased velocity nor increased duration of cord-impactor contact had an effect on the outcome of locomotor function. All three injury conditions (velocity 1 m/sec, duration 0.2 sec [filled circles]; velocity 1 m/sec, duration 5.0 sec [inverted filled triangles]; velocity 2 m/sec, duration 0.2 sec [open circles]) showed improvement over time (*p > 0.05). There were no significant differences at any time points between the three groups. Data are the mean ± SD (n = 6/group).
Electrophysiological outcomes
We investigated whether tcMMEP and SSEP responses could objectively predict the severity of tissue damage following controlled displacement SCI and whether these electrophysiological parameters would correlate with functional improvement. TcMMEP responses recorded in all rats prior to SCI (Fig. 6A) were multiphasic muscle compound action potentials with latencies of 6.16 ± 0.16 msec and peak-to-peak amplitudes of 18.96 ± 4.59 mV. In the 0.2-mm displacement injury group, 67% of rats showed normal latencies 1 week following injury, but the amplitudes were only 25% of normal (4.65 ± 6.57 mV; data not shown). By week 5, the number of animals with normal latencies increased to 83.3%, but the amplitudes remained significantly below normal (F = 14.5, df = 1.3, p < 0.05). In the 0.4-mm displacement SCI group, only 20% of the week 1 and 40% of the week 5 animals regained normal latencies, with the amplitudes being significantly lower than the 0.2-mm displacement SCI rats by week 5 (3.9 ± 6.4 mV; t = 4.3, df = 5, p < 0.01; Table 3). TcMMEP responses were absent in the 0.6-mm displacement SCI group as well as in all animals subjected to greater displacement injuries (Table 3). The recovery pattern of tcMMEP responses did not parallel the rapid rate of behavioral recovery over the same time course.
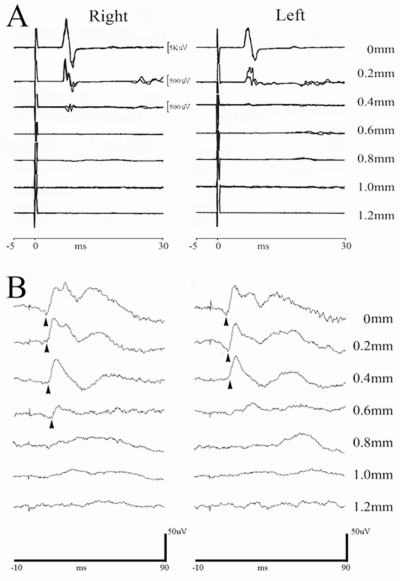
FIG. 6.
Transcranial magnetic motor evoked potentials (tcMMEPs) and somatosensory evoked potentials (SSEPs) could be recorded in all animals before surgery. (A) Representative tcMMEP waveforms of a normal animal (displacement = 0 mm) are compared to responses following graded spinal cord injury (SCI). Responses are very sensitive to injury severity (displacement > 0.2 mm). (B) SSEPs were recorded in all rats using low-intensity electrical stimulation to the posterior tibial nerve (PTN). Representative waveforms of the responses show deterioration after spinal cord contusion. Compared with the uninjured spinal cord (displacement = 0 mm), the P1 latency (arrowhead) was longer in all injury groups with a response (p < 0.01). A decreased amplitude was noted in the 0.6-mm displacement group (p = 0.058; n = 5–6/group).
Table 3.
TcMMEP Responses on Week 5
| Displacement | Response rate (%) | Onset latency (msec) | Peak-to-peak amplitude (mV) |
|---|---|---|---|
| 0 mm | 100 | 6.3 ± 0.21 | 15.9 ± 5.3 |
| 0.2 mm | 100 | 6.3 ± 0.24 | 3.9 ± 6.4** |
| 0.4 mm | 33.3*** | 6.2 ± 0.16 | 0.9 ± 0.8** (**) |
| 0.6–1.2 mm | 0***(***) | N/A | N/A |
Data are the mean ± SD; n = 5–6/group. For response rate, ***p < 0.001 as compared to 0 and 0.2 mm displacement; (***)p < 0.001 as compared to 0.4 mm displacement. For amplitude, **p < 0.01 as compared to 0 displacement, (**)p < 0.01 for 0.4 mm compared to 0.2 mm displacement.
Cortical SSEPs from control rats demonstrated a biphasic wave (Fig. 6B) with the P1 latency at 11.62 ± 0.37 msec and the P1-N1 amplitude at 55.41 ± 17.33 μV (Table 4). Although SSEP impulses are transmitted through the dorsal funiculus that was directly contused, there was a surprising degree of preservation of the SSEP response following injury. SSEPs were elicited from all rats with 0.2- and 0.4-mm displacement SCI, with no SSEP responses obtained from the 0.8-, 1.0-, and 1.2-mm displacement SCI groups (Table 4). In the 0.6-mm SCI displacement group, SSEP responses were preserved in 67% of the animals. Furthermore, at the 0.6-mm displacement SCI, impactor velocity significantly affected SSEP responses. When the impact velocity was increased to 2.0 m/sec, SSEP responses could be detected in only 33% of the animals (data not shown). Alternatively, as the dwell time between impactor and cord increased from 0.2 to 5.0 sec, the SSEP responses were not affected (data not shown). In addition to increased latency, the quality of SSEP also deteriorated with increased tissue damage. There were no significant amplitude changes in the 0.2-mm (53.75 ± 16.50 μV) and 0.4-mm (55.50 ± 27.50 μV) groups compared with control, but they were decreased in the 0.6-mm group (29.9 ± 8.0 μV) compared to the 0.2- and 0.4-mm groups (F = 4.1, df = 2,15, p < 0.05) and post-injury compared with baseline (p < 0.05).
Table 4.
SSEP Responses on Week 5
| Displacement | Response rate (%) | P1 onset latency (msec) | P1–N1 amplitude (uV) |
|---|---|---|---|
| 0 mm | 100 | 11.6 ± 0.34 | 55.4 ± 14.0 |
| 0.2 mm | 100 | 12.8 ± 0.47 | 53.8 ± 16.5 |
| 0.4 mm | 100 | 13.2 ± 0.90* | 55.5 ± 27.5 |
| 0.6 mm | 66.7*** | 13.3 ± 0.73* | 26.9 ± 6.8* |
| 0.8–1.2 mm | 0***(***) | N/A | N/A |
Data are the mean ± SD; n = 5–6/group. For response rate, ***p < 0.001 as compared to 0, 0.2, and 0.4 mm displacement; (***)p < 0.001 as compared to 0.6 mm displacement. For onset latency, *p < 0.05 as compared to 0.0 mm displacement; for P1–N1 amplitude, *p < 0.05 compared to all other groups.
Histological outcome
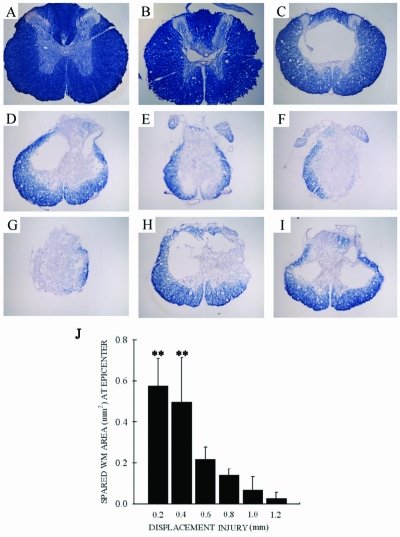
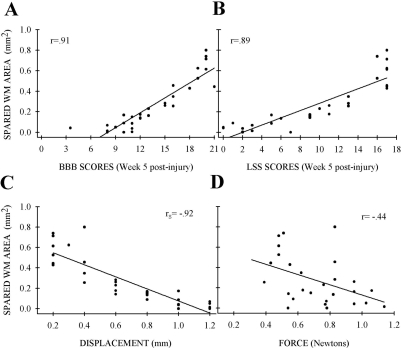
As with all contusive SCI in rats, the injury epicenter appeared as a central cavity with a gliotic scar and an outer rim of spared white matter. The amount of spared white matter at the lesion epicenter was significantly reduced with increasing displacement (Fig. 7A–G). However, post-hoc analysis could not show statistical significance between successive 0.2-mm injury severities, only that 0.2- and 0.4-mm injuries were different than all other lesion severities (Fig. 7J). As with the BBB and LSS scores, combining injury severities into three groups did enable significant differences to be elucidated (data not shown). Similar to the behavioral data, neither doubling the injury velocity to 2 m/sec (0.22 ± .06 vs. 0.23 ± 0.08) nor increasing the duration of displacement from 0.2 to 5.0 msec (0.22 ± 0.06 vs. 0.23 ± 0.07; Fig. 7H,I) influenced the amount of spared white matter. The amount of spared white matter correlated significantly with 5-week BBB (r = 0.91, p < 0.001; Fig. 8A) and LSS (r = 0.89, p < 0.001; Fig. 8B) scores, displacement (rs = −0.92, p < 0.001; Fig. 8C), and force (r = 0.44, p < 0.05; Fig. 8D), although the correlation with force was not as strong as with the other parameters.
FIG. 7.
There was greater tissue loss as displacement injury increased: 0-mm (A), 0.2-mm (B), 0.4-mm (C), 0.6-mm (D), 0.8-mm (E), 1.0-mm (F), and 1.2-mm (G) displacement (iron-eriochrome cyanine RC staining). Higher velocity (2 m/sec; H) and longer duration of cord-impactor contact (5 sec; I) did not show a difference in white matter (WM) sparing compared with the 0.6-mm displacement. (J) There was a greater degree of white matter sparing among the 0.2- and 0.4-mm groups compared to the higher displacement groups (p < 0.001). Data are the mean ± SD (n = 5–6/group).
FIG. 8.
The amount of spared white matter at the epicenter correlated to behavior outcome and injury parameters. Spared white matter at the epicenter was plotted against week 5 post-injury results: (A) Basso, Beattie, and Bresnahan (BBB) scores. (B) Louisville Swim Score (LSS) scores. (C) Tissue displacement. (D) Impact force. BBB (p < 0.001, n = 31) and LSS (p < 0.001, n = 31) correlated positively, and tissue displacement (p < 0.001, n = 31) and impact force (p < 0.05, n = 27) correlated inversely with white matter sparing (rs, Spearman rank; r, Pearson correlation). Individual data points are shown.
Combined behavioral analysis
The combination of the categorized BBB behavior and electrophysiology assessments yielded a procedure with the ability to discriminate and detect differences between most of the 0.2-mm displacement injury groups. Combining BBB, tcMMEP, and SSEP P1 onset latency category responses showed significant differences between all of the milder to moderate severity levels from 0.2 to 0.8 mm (0.2–0.4 mm [p < 0.005], 0.4–0.6 mm [p < 0.05] and 0.6–0.8 mm [p < 0.005]), revealing the detection of minor differences in conduction time through the spinal cord. Only the two pairs at the most severe injury levels were not significantly different (0.8–1.0 or 1.0–1.2 mm), indicative of the severity of injury throughout the cord. Furthermore, analyses of two other combinations of the category responses of the BBB with either (1) both of the amplitude measures (tcMMEP and SSEP; 0.2–0.4 mm [p < 0.05], 0.4–0.6 mm [p < 0.01], and 0.6–0.8 mm [p < 0.005]) or (2) the combination of both tcMMEP and SSEP onset latencies and amplitudes (all pairs from 0.2–0.8 mm [p < 0.005]) were able to discern significant differences between these injury levels.
Discussion
Animal models are generated to reproduce the type and magnitude of SCI in humans. Moreover, SCI devices have evolved to better reflect the human injury and provide additional precision in both creating the injury and analyzing its biomechanical parameters. As indicated in Table 1, there has been a remarkable progression since the pioneering work of Allen (1911). Previous weight-drop devices used to create SCI (Ducker et al., 1971; Sivasankaran et al., 2004; Wrathall et al., 1985) allowed bouncing of the impactor against the spinal cord, making it difficult to precisely control injury severity. The IH (Scheff et al., 2003), ESCID (Jakeman et al., 2000; Stokes, 1992), and LISA avoid the effects of bouncing by immediate removal of the impactor once contact with the spinal cord has occurred. The initial computer-monitored device was the NYU impactor (Gruner, 1992), which remains in widespread use. The NYU system addressed the importance of spine stability during impact but did not recommend methods to improve it. The use of velocity and force as the controlling parameters has the potential to create difficulties in data sampling. Both velocity and force are variables that change during injury, and accuracy of measurement is limited by the sampling frequency (Fig. 9). In the NYU system, the spinal cord surface is identified by an electrical conduction that triggers an auditory signal when contact is made (Constantini et al., 1994). However, error may occur when false dural contact is made between dural and impactor surfaces due to capillary action drawing blood and/or fluid into the space between the surfaces (personal observation).
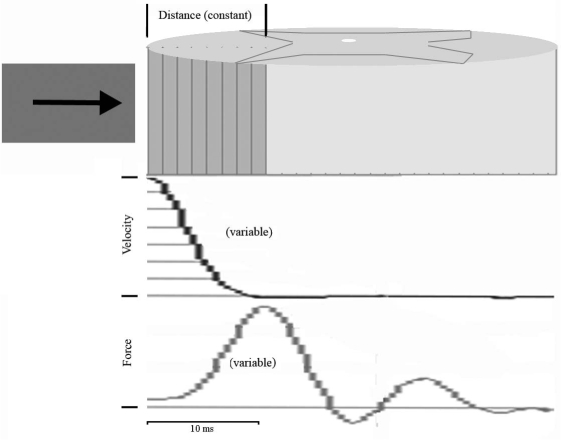
FIG. 9.
Representation of tissue displacement, velocity, and force during impact. Tissue displacement is constant and therefore can be precisely controlled (arrow, upper panel). Velocity decelerates throughout the contact period (middle panel). Force increased and peaked before the impactor stopped (lower panel). Controllable parameters (displacement) create a more reproducible injury than variable parameters (velocity, force).
Both the ESCID (Stokes, 1992) and IH devices (Scheff et al., 2003) represent further refinements in computer-controlled devices with slightly different approaches. The ESCID uses tissue displacement as the only controlling parameter. Tissue displacement is a constant measurement that can be accurately controlled in creating SCI (Fig. 9). Stabilization of the target spine used a similar principle as that of the NYU system and uses a Ling vibrator in contact with the surface of the spinal cord to identify the zero point on the spinal cord surface.
The IH system utilizes force as a user-defined parameter in an attempt to minimize potential errors that may occur from the spinal cord yielding during impact. However, measurements of force are vulnerable to “noise” generated from the sudden changes in the impactor tip velocity. The force required to deform the thin and soft spinal cord is small, but the “noise” generated during injury is not. The noise generated throughout this process greatly distorts measurement of force (Table 2). Mechanical inertia is another problem in using force as the injury parameter as it requires a feedback mechanism to control injury magnitude once the desired force is attained. The greater mechanical inertia associated with the high velocity of the impactor will prevent the impactor stopping at the desired position. To minimize the influence of noise and reduce the mechanical inertia, the IH device has a 0.13 m/sec injury velocity. At mild-moderate injury severities, IH injuries spare the ventral spinal cord as assessed by tcMMEP responses which are transmitted through the ventrolateral funiculus (VLF) (Loy et al., 2002) with preservation of vessels and minimal hemorrhage (Cao et al., 2005). The histopathology of this moderate injury is very different than that which is seen at higher injury velocities (Loy et al., 2002; Magnuson et al., 1999).
The LISA combines the strengths of those devices with additional components that should enhance the reliability of SCI research. It addresses the importance of complete vertebral column stabilization at the site of impact, a variable that has been underappreciated and not well-controlled in many earlier systems. Failure to do so can be a major source of error in creating experimental SCI. If the fixation clamps on the spinous processes are not secure, they may fracture or slip. Moreover, fixation points that are separated by at least two mobile joints may cause motion at those segments. We demonstrated that clamping vertebral facets at the level of injury would prevent all movement.
The LISA uses tissue displacement as the independent parameter, similar to the ESCID. It provides several features that were unavailable in earlier models, including 1) a noncontact laser guided technique to identify the surface of the spinal cord and determine the depth of cord displacement, 2) being multi-functional as it is capable of creating precise laceration injuries and contusion SCI in the rat and mouse of equal precision with a simple exchange of attachments (Iannotti et al., 2006; Sivasankaran et al., 2004; Zhang et al., 2007), 3) a variable and controllable impactor velocity and contact time between the impactor and the spinal cord, 4) precision in creating lesions to a depth of ± 0.007 mm, and 5) the ability to deliver a wide range of injury velocities. Comparing present and published data, the NYU (Basso et al., 1996b), ESCID (Basso et al., 1995), IH (Scheff et al., 2003), and LISA devices showed variability in reported BBB scores 6 weeks post-SCI at moderate injury levels to be 10.3 ± 1.6, 11.5 ± 5.2, 9.9 ± 2.5, and 11.4 ± 1.3, respectively. These mean BBB scores cannot be compared without the raw data, but an estimate of statistical significance between means (Cumming et al., 2007) did not detect a significant difference among these averaged BBBs. Similarly, the correlation between BBB scores and white matter sparing for these devices is 0.66, 0.77, 0.60, and 0.90, respectively.
Present data demonstrate that tissue displacement is a reliable parameter in generating reproducible experimental contusion SCI at multiple injury velocities. The effect of velocity was minor in altering the pathological appearance of the SCI when lesion depth was controlled, based on the slight decrease in function and similar pathophysiology of injured spinal cords caused by impactor velocities of 1 or 2 m/s. However, SSEP responses were markedly reduced at the higher injury velocity. These data underscore the fact that gross histology and behavioral evaluation may not be sensitive enough to detect physiologically relevant and important differences in lesion severity. Increasing the dwell time of the impactor compression against the spinal cord from 0.2 to 5 sec did not alter behavior or histology. This temporal change likely was not long enough to discriminate functional changes. Prolonging spinal cord compression for 30 minutes to 24 hours before decompression does cause more severe anatomical and behavioral outcomes (Dimar et al., 1999; Shields et al., 2005), but determining the functional consequences of the specific temporal profile of duration of compression was outside the scope of this study.
The LISA was designed to create multiple level injuries in the mild-moderate range. However, such precision in graded lesion severity is of little use if the outcome measures used cannot distinguish between these small lesion severities. Neither the BBB nor the LSS scales when used alone were sensitive enough to detect 0.2 mm displacement differences. The average change in BBB scores for each 0.2 mm increment in displacement was 2.2 ± 1.3 at these mild to moderate injury levels. Power analysis indicated that a group size of 20 would be necessary to discriminate with 95% confidence differences in BBB scores between successive 0.2 mm displacement groups. This group size is obviously unfeasible. Electro-physiological evaluation provides an objective assessment of cord conduction following SCI, but tcMMEP or SSEP responses also could not detect significant differences between 0.2 mm lesion severities. However, 0.2 mm differences in displacement injuries could be significantly differentiated when tcMMEP, SSEP, and BBB scores were combined.
TcMMEP and SSEP responses reflect conduction through descending and ascending fiber tracts in the medial aspect of the VLF (Cao et al., 2005; Loy et al., 2002) and the dorsal columns, respectively. Both walking and swimming require multiple motor pathways that are distributed throughout the lateral and ventral white matter. Sensory input comes primarily from spared dorsal column axons at low and moderate stimulus intensities, and will involve local and pro-priospinal circuitry at higher stimulus intensities, as used here. Moreover, these pathways are mixtures of large myelinated as well as unmyelinated axons which are differentially sensitive to contusive axonal damage. When one area of white matter is lost, other pathways can take over control of locomotor function (Loy et al., 2002; Schucht et al., 2002). There is no definitive explanation as to why these electro-physiological and behavioral outcome measures are lost at different injury severities. Conclusive explanations will require a much better understanding of spinal cord circuitry and the vulnerability of individual pathways to answer this question.
Present data point out the need to utilize multiple modes of assessment that when combined, provide the ability to distinguish between very slight differences in injury severity. This concept was first addressed by Wrathall et al. in the development of the Combined Behavioral Score (CBS) (Gale, 1985). This strategy is of crucial importance when trying to evaluate subtle effects of a given therapeutic approach to treat SCI as most open-field behavioral tests such as the BBB (Basso et al., 1995, 1996a) were initially developed only to be an initial rapid screen for behavioral changes, not the single test of recovered function.1 Moreover, the reported beneficial therapies have only provided modest functional recovery (Reier, 2004; Schwab et al., 2006; Thuret et al., 2006) and will be best discriminated with multiple outcome measures that target distinct aspects of recovery.
Footnotes
A Medline search of ‘spinal cord injury’, ‘BBB’, and ‘rat’ gave 106 references that utilized the BBB to assess hindlime locomotor function and 76% of those used the BBB as the single analysis of functional recovery.
Acknowledgments
We thank Kim Fentress for her expert technical assistance and Aaron Puckett and the Research Resources Center staff at the University of Louisville for their excellent veterinary care. This work was supported by the Kentucky Spinal Cord and Head Injury Research Trust (grant RR015576 to S.R.W., C.B.S., D.S.K.M.), Neurosurgical Institute of Kentucky, and Norton Healthcare and the Commonwealth of Kentucky Challenge for Excellence (to S.R.W., C.B.S.).
Author Disclosure Statement
Dr. C.B. Shields holds an ownership position in LIS, Inc.
References
- Allen A.R. Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column. A preliminary report. J.A.M.A. 1911;57:878–880. [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996a;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. Anderson D.K. Faden A.I. Gruner J.A. Holford T.R. Hsu C.Y. Noble L.J. Nockels R. Perot P.L. Salzman S.K. Young W. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J. Neurotrauma. 1996b;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- Behrmann D.L. Bresnahan J.C. Beattie M.S. Shah B.R. Spinal cord injury produced by consistent mechanical displacement of the cord in rats: behavioral and histologic analysis. J. Neurotrauma. 1992;9:197–217. doi: 10.1089/neu.1992.9.197. [DOI] [PubMed] [Google Scholar]
- Cao Q. Xu X.M. Devries W.H. Enzmann G.U. Ping P. Tsoulfas P. Wood P.M. Bunge M.B. Whittemore S.R. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J. Neurosci. 2005;25:6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantini S. Young W. The effects of methyl-prednisolone and the ganglioside GM1 on acute spinal cord injury in rats. J. Neurosurg. 1994;80:97–111. doi: 10.3171/jns.1994.80.1.0097. [DOI] [PubMed] [Google Scholar]
- Cumming G. Fidler F. Vaux D.L. Error bars in experimental biology. J. Cell Biol. 2007;177:7–11. doi: 10.1083/jcb.200611141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimar J.R. Glassman S.D. Raque G.H. Zhang Y.P. Shields C.B. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine. 1999;24:1623–1633. doi: 10.1097/00007632-199908150-00002. [DOI] [PubMed] [Google Scholar]
- Ducker T.B. Perot P.L., Jr. Local tissue oxygen and blood flow in the acutely injured spinal cord. Proc. Veterans Adm. Spinal Cord. Inj. Conf. 1971;18:29–32. [PubMed] [Google Scholar]
- Falconer J.C. Narayana P.A. Bhattacharjee M. Liu S.J. Characterization of an experimental spinal cord injury model using waveform and morphometric analysis. Spine. 1996;21:104–112. doi: 10.1097/00007632-199601010-00025. [DOI] [PubMed] [Google Scholar]
- Gale K. Kerasidis H. Wrathall J.R. Spinal cord contusion in the rat: behavioral analysis of functional neurologic impairment. Exp. Neurol. 1985;88:123–134. doi: 10.1016/0014-4886(85)90118-9. [DOI] [PubMed] [Google Scholar]
- Ghasemlou N. Kerr B.J. David S. Tissue displacement and impact force are important contributors to outcome after spinal cord contusion injury. Exp. Neurol. 2005;196:9–17. doi: 10.1016/j.expneurol.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Glassman S.D. Zhang Y.P. Shields C.B. Linden R.D. Johnson J.R. An evaluation of motor-evoked potentials for detection of neurologic injury with correction of an experimental scoliosis. Spine. 1995;20:1765–1775. doi: 10.1097/00007632-199508150-00004. [DOI] [PubMed] [Google Scholar]
- Grill R.J. User-defined variables that affect outcome in spinal cord contusion/compression models. Exp. Neurol. 2005;196:1–5. doi: 10.1016/j.expneurol.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Gruner J.A. A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma. 1992;9:123–126. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- Hadi B. Zhang Y.P. Burke D.A. Shields C.B. Magnuson D.S. Lasting paraplegia caused by loss of lumbar spinal cord interneurons in rats: no direct correlation with motor neuron loss. J. Neurosurg. 2000;93:266–275. doi: 10.3171/spi.2000.93.2.0266. [DOI] [PubMed] [Google Scholar]
- Iannotti C. Zhang Y.P. Shields L.B.E. Han Y. Burke D.A. Xu X.M. Shields C.B. Dural repair reduces connective tissue scar invasion and cystic cavity formation after acute spinal cord laceration injury in adult rats. J. Neurotrauma. 2006;23:853–865. doi: 10.1089/neu.2006.23.853. [DOI] [PubMed] [Google Scholar]
- Jakeman L.B. Guan Z. Wei P. Ponnappan R. Dzwonczyk R. Popovich P.G. Stokes B.T. Traumatic spinal cord injury produced by controlled contusion in mouse. J. Neurotrauma. 2000;17:299–319. doi: 10.1089/neu.2000.17.299. [DOI] [PubMed] [Google Scholar]
- Loy D.N. Magnuson D.S. Zhang Y.P. Onifer S.M. Mills M.D. Cao Q.L. Darnall J.B. Fajardo L.C. Burke D.A. Whittemore S.R. Functional redundancy of ventral spinal locomotor pathways. J. Neurosci. 2002;22:315–323. doi: 10.1523/JNEUROSCI.22-01-00315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson D.S. Lovett R. Coffee C. Gray R. Han Y. Zhang Y.P. Burke D.A. Functional consequences of lumbar spinal cord contusion injuries in the adult rat. J. Neurotrauma. 2005;22:529–543. doi: 10.1089/neu.2005.22.529. [DOI] [PubMed] [Google Scholar]
- Magnuson D.S. Trinder T.C. Zhang Y.P. Burke D. Morassutti D.J. Shields C.B. Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp. Neurol. 1999;156:191–204. doi: 10.1006/exnr.1999.7016. [DOI] [PubMed] [Google Scholar]
- Panjabi M.M. Wrathall J.R. Biomechanical analysis of experimental spinal cord injury and functional loss. Spine. 1988;13:1365–1370. doi: 10.1097/00007632-198812000-00007. [DOI] [PubMed] [Google Scholar]
- Ramer M.S. Harper G.P. Bradbury E.J. Progress in spinal cord research—a refined strategy for the International Spinal Research Trust. Spinal Cord. 2000;38:449–472. doi: 10.1038/sj.sc.3101055. [DOI] [PubMed] [Google Scholar]
- Reier P.J. Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx. 2004;1:424–451. doi: 10.1602/neurorx.1.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sances A., Jr. Myklebust J.B. Maiman D.J. Larson S.J. Cusick J.F. Jodat R.W. The biomechanics of spinal injuries. Crit. Rev. Biomed. Eng. 1984;11:1–76. [PubMed] [Google Scholar]
- Scheff S.W. Rabchevsky A.G. Fugaccia I. Main J.A. Lumpp J.E., Jr. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- Schucht P. Raineteau O. Schwab M.E. Fouad K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp. Neurol. 2002;176:143–153. doi: 10.1006/exnr.2002.7909. [DOI] [PubMed] [Google Scholar]
- Schwab J.M. Brechtel K. Mueller C.A. Failli V. Kaps H.P. Tuli S.K. Schluesener H.J. Experimental strategies to promote spinal cord regeneration–an integrative perspective. Prog. Neurobiol. 2006;78:91–116. doi: 10.1016/j.pneurobio.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Seki T. Hida K. Tada M. Koyanagi I. Iwasaki Y. Graded contusion model of the mouse spinal cord using a pneumatic impact device. Neurosurgery. 2002;50:1075–1081. doi: 10.1097/00006123-200205000-00024. [DOI] [PubMed] [Google Scholar]
- Shields C.B. Zhang Y.P. Shields L.B.E. Han Y. Burke D.A. Mayer N.W. The therapeutic window for spinal cord decompression in a rat spinal cord injury model. J. Neurosurg. Spine. 2005;3:302–307. doi: 10.3171/spi.2005.3.4.0302. [DOI] [PubMed] [Google Scholar]
- Sivasankaran R. Pei J. Wang K.C. Zhang Y.P. Shields C.B. Xu X.M. He Z. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat. Neurosci. 2004;7:261–268. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- Smith R.R. Burke D.A. Baldini A.D. Shum-Siu A. Baltzley R. Bunger M. Magnuson D.S. The Louisville Swim Scale: a novel assessment of hindlimb function following spinal cord injury in adult rats. J. Neurotrauma. 2006a;23:1654–1670. doi: 10.1089/neu.2006.23.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.R. Shum-Siu A. Baltzley R. Bunger M. Baldini A. Burke D.A. Magnuson D.S. Effects of swimming on functional recovery after incomplete spinal cord injury in rats. J. Neurotrauma. 2006b;23:908–919. doi: 10.1089/neu.2006.23.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soblosky J.S. Song J.H. Dinh D.H. Graded unilateral cervical spinal cord injury in the rat: evaluation of forelimb recovery and histological effects. Behav. Brain Res. 2001;119:1–13. doi: 10.1016/s0166-4328(00)00328-4. [DOI] [PubMed] [Google Scholar]
- Stokes B.T. Experimental spinal cord injury: a dynamic and verifiable injury device. J. Neurotrauma. 1992;9:129–131. doi: 10.1089/neu.1992.9.129. [DOI] [PubMed] [Google Scholar]
- Thuret S. Moon L.D. Gage F.H. Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- Wrathall J.R. Pettegrew R.K. Harvey F. Spinal cord contusion in the rat: production of graded, reproducible, injury groups. Exp. Neurol. 1985;88:108–122. doi: 10.1016/0014-4886(85)90117-7. [DOI] [PubMed] [Google Scholar]
- Yeo J.D. Importance of specialised spinal units. Spinal Cord. 2004;42:488. doi: 10.1038/sj.sc.3101626. [DOI] [PubMed] [Google Scholar]
- Zhang Y.P. Shields L.B.E. Zhang Y. Pei J. Xu X.M. Hoskins R. Cai J. Qui M.S. Magnuson D.S.K. Burke D.A. Shields C.B. Use of magnetic stimulation to elicit motor evoked potentials, somatosensory evoked potentials, and H-reflexes in non-sedated rodents. J. Neurosci. Methods. 2007;165:9–17. doi: 10.1016/j.jneumeth.2007.05.021. [DOI] [PubMed] [Google Scholar]