Abstract
Background
The present study determined if, akin to cocaine, nicotine self-administration in rats induces adaptations in the expression of glutamate transporters and cystine-glutamate exchangers in brain nuclei implicated in reinforcement, and if treating cigarette smokers with a drug that restores cystine-glutamate exchange affected the number of cigarettes smoked.
Methods
Rats self-administered nicotine intravenously for 12 hours/day or received nicotine through osmotic mini-pumps for 21 days. Somatic signs of withdrawal were measured and immunoblotting performed 12 hours after the last nicotine exposure to determine if the catalytic subunit of the cystine-glutamate exchanger, xCT, or the glial glutamate transporter, GLT-1, were altered in the ventral tegmental area (VTA), nucleus accumbens, prefrontal cortex or amygdala. For the smoking-reduction study in humans, nicotine-dependent smokers were treated for four weeks with N-acetylcysteine (2400 mg/daily) to promote cystine-glutamate exchange or placebo. Participants provided weekly ratings of withdrawal symptoms, craving, CO measurements and logged daily cigarette and alcohol use.
Results
Rats receiving nicotine via self-administration or minipumps displayed somatic signs of withdrawal, but only nicotine self-administering rats showed decreased xCT expression in the nucleus accumbens and VTA, and decreased GLT-1 expression in the nucleus accumbens. Human smokers treated with N-acetylcysteine reported a reduction in cigarettes smoked, and there was no effect of N-acetylcysteine on estimates of CO levels, craving, or withdrawal.
Conclusions
These results indicate that the cystine-glutamate exchanger and the glial glutamate transporter are down-regulated after nicotine self-administration, and augmenting exchanger activity with N-acetylcysteine reduced the number of cigarettes smoked in nicotine-dependent individuals.
Keywords: nicotine, accumbens, cystine-glutamate exchange, self-administration, VTA, cigarette
Chronic treatment with cocaine is associated with decreased basal levels of glutamate in the nucleus accumbens as measured by microdialysis (1-3). System xc-, which exchanges extracellular cystine for intracellular glutamate, is the rate-limiting step in glutathione synthesis and the main source of extracellular glutamate measured by dialysis in the nucleus accumbens (4). Down-regulation of system xc- accounts for the reduction in basal glutamate levels observed after chronic cocaine (1, 2). The nutritional supplement N-acetylcysteine is a cystine prodrug that activates system xc-, thereby restoring glutamate levels to normal (1) and preventing cocaine-and heroin-seeking in the reinstatement animal model of relapse (1, 2, 5), as well as the desire for cocaine in human addicts (6). System xc- is a heterodimer and the downregulation of system xc-by cocaine results at least in part from reduced expression of the catalytic subunit xCT (7). In addition to system xc-, it was recently shown that sodium-dependent glutamate uptake (system XAG) and the membrane level of the primary glial glutamate transporter (GLT-1; EAAT2) are also reduced in the nucleus accumbens after withdrawal from self-administered cocaine (7).
Nicotine self-administration also produces glutamatergic adaptations in brain areas involved in reinforcement (8). For example, nicotine self-administration upregulates N-methyl-D-aspartate receptor subunit expression in the VTA and amygdala (9). Further, brief withdrawal from nicotine self-administration leads to down-regulation of metabotropic glutamate 2/3 receptor (mGluR2/3) function in the nucleus accumbens shell, ventral tegmental area (VTA), amygdala, prefrontal cortex (PFC), hypothalamus and hippocampus (10). Because withdrawal from cocaine is also associated with down-regulated mGluR2/3 (11), we hypothesized that, akin to cocaine self-administration, the expression of xCT and GLT-1 proteins may be reduced in some of these brain areas after nicotine self-administration in rats. Further, given the potential influence of nicotine on xCT expression, combined with the capacity of N-acetylcysteine to restore system xc- activity and reduce aspects of drug-seeking behavior in cocaine-dependent humans (6, 12), we sought to determine whether N-acetylcysteine would decrease nicotine use in cigarette smokers.
Methods
Animals & Surgery
All procedures were conducted in accordance with the guidelines from the National Institutes of Health and the Association for the Assessment and Accreditation of Laboratory Animal Care and were approved by the institute’s Animal Use and Care Committee. Male Wistar rats weighing 250–350 g were assigned to one of four groups: nicotine self-administration (N-SA; n=10), saline (SAL; n=10), nicotine osmotic mini-pump 1.3 mg/kg/day base (N-P1.3; n=9), or nicotine osmotic mini-pump 3.16 mg/kg/day base (N-P3.6; n=9).
Nicotine administration
Rats originally trained to respond for food reinforcement were allowed to intravenously self-administer nicotine (0.03 mg/kg base/infusion) for 21 days on an Fixed Ratio 5 Time-Out 20 s schedule of reinforcement for 12 hr/day 5 d/week (see Supplement). Saline rats (SAL) self-administered saline. The remaining rats were surgically prepared with osmotic mini-pumps (2.5 microliters/hr) containing a nicotine/saline solution. The pumps released 1.3 mg/kg/day or 3.16 mg/kg/day nicotine base; the higher dose produces reliable affective and somatic signs of nicotine withdrawal upon cessation of nicotine administration (13), while the lower dose approximates the dose self-administered in a 12 hr daily session (14). Pumps were removed after 21 days.
Observation of somatic signs of withdrawal
Somatic signs of nicotine withdrawal were assessed 12 hours after the last nicotine self-administration session or removal of osmotic mini-pumps. Each subject was placed under white light conditions in cylindrical Plexiglas chambers (diameter 15 cm) and observed for 10 min by an observer blind to the subjects’ treatments. The standard checklist used was adapted from an opiate withdrawal signs checklist (see (13)). The following somatic signs of withdrawal were recorded: body shakes, chews, cheek tremors, escape attempts, eye blinks, foot licks, gasps, writhes, genital licks, head shakes, ptosis, teeth chattering, and yawns. Ptosis, if present, was recorded only once per minute.
Tissue Preparation and Immunoblotting
Immediately after the measurement of somatic signs, animals underwent rapid decapitation without anesthesia. The brains were removed and the nucleus accumbens shell, VTA, PFC and amygdala were dissected and immediately frozen with liquid nitrogen (10). Immunoblotting of whole cell homogenates was used to measure levels of xCT (1:500; kindly provided by David Baker, Marquette University) and GLT-1 (1:500; Santa Cruz Biotechnologies, Santa Cruz, CA) protein using calnexin as an internal control, and densitometry was conducted with NIH Image J software (15).
Double-blind N-acetylcysteine pilot trial in human smokers
This double-blind study was designed as a preliminary pilot trial. A power analysis was performed to determine adequate sample size for such a trial. With two groups (N-acetylcysteine versus placebo), it was determined that a sample size of 16 per treatment arm would provide sufficient power (see Supplemental Methods for additional details). Thirty-three participants who were seeking to reduce the number of daily cigarettes smoked were recruited via flyers and newspaper advertisements. Participants were in stable mental and physical health and dependent on nicotine [10 or more cigarettes per day for at least one year; Fagerstrom scores indicated equivalent nicotine dependence for the placebo- (6.2±2.2) and N-acetylcysteine-treated (6.8±1.6) groups]. The demographic characteristics of the treatment groups are shown in Table S1, and the only significant difference was that individuals in the N-acetylcysteine group had significantly more years of alcohol use. At baseline and at each study visit for the four subsequent weeks, participants provided ratings for craving using the Questionnaire for Smoking Urges–Brief (QSU-B), ratings for withdrawal symptoms using the Minnesota Nicotine Withdrawal Scale (M-NWS), as well as a carbon monoxide (CO) measurement. Participants also kept a smoking diary in which they recorded daily cigarette and alcohol use. Four participants did not return after baseline testing and were excluded from the study. The remaining 29 participants were randomly assigned to oral self-administration of either 1200 mg of N-acetylcysteine twice per day (n=14) or placebo (n=15). At the end of weeks 1–4 of medication, participants returned for a brief visit during which they provided ratings on the QSU-B, the MNWS, as well as CO measurements, and were assessed for side effects (see Supplement for details).
Analyses of Clinical Data
The primary outcome measure was an independent t-test examining average daily cigarettes smoked. In addition, exploratory analyses were conducted using the Mixed Linear procedure within SPSS 15.0, assuming compound symmetry. In addition, the data were evaluated using the SAS MMRM statistical package that yielded identical estimates of statistical significance. The analysis values shown in the manuscript and supplementary materials are from the analysis employing the SPSS 15.0 software. This procedure essentially emulates a repeated-measures ANOVA, only it allows for inclusion of subjects with missing data (assumes data are missing randomly). For weekly measures of average cigarette use, CO-levels, and ratings on the QSU-B and M-NWS, Time (week) served as the within-subjects variable, with Treatment Group as a between subjects measure, and baseline levels serving as a covariate. In addition, daily smoking was assessed, with Time (day) as the repeated-measures variable. This approach allowed for the possibility of including daily alcohol consumption (whether consumption occurred or not) as a covariate in the model.
Results
Nicotine self-administration and somatic signs of withdrawal
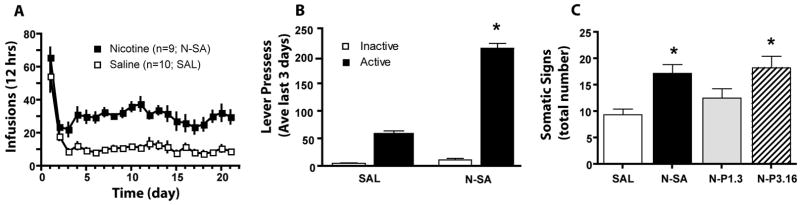
Rats self-administering nicotine for 21 days received an average dose of 0.95±0.01 mg/kg/day nicotine base (Figure 1A). The relatively high rates of responding on the first day resulted from initial food training prior to nicotine self-administration, and in both groups stable baseline responding was achieved within the first 5 days of training. A two-way ANOVA revealed significant main effects of Nicotine exposure (F(1,17)=63.49, p< 0.001) and Day (F(20,340)= 20.66, p< 0.001), but no Nicotine exposure X Day interaction. Both saline and nicotine maintained more responding on the active than the inactive lever, and nicotine maintained significantly more active lever pressing saline (Figure 1B). A two-way ANOVA revealed significant effects of Group (F(1,15)=19.974, p< 0.001), Lever (F(1,15)=50.441, p<0.001) and a Group X Lever interaction (F(1,15)=15.43, p<0.001) on number of infusions earned. Both the nicotine self-administering (N-SA) and the group receiving 3.16 mg/kg/day nicotine via minipumps (N-P3.16) exhibited more somatic signs of withdrawal than the saline (SAL) group (Figure 1C). A one-way ANOVA revealed a significant effect of Group (F(3,32)= 7.03, p< 0.001) on number of somatic signs exhibited. Interestingly, the N-SA animals presented more somatic signs of withdrawal compared with rats exposed to approximately the same nicotine dose via minipumps (N-P1.3).
Figure 1.
Nicotine self-administration and somatic signs of withdrawal in rats. A. Mean (±SEM) number of infusions obtained daily during the 21 day self-administration period. B.Both nicotine self-administration (N-SA) and saline self-administering control (SAL) groups exhibited higher levels of responding on the active, compared with the inactive lever. Data are shown as mean ± SEM of the average lever presses over the last 3 days of training. C. Mean (±SEM) number of somatic signs of nicotine withdrawal. N-P1.3= minipump infusion of 1.3 mg/kg/day; N-P3.16= minipump infusion of 3.16 mg/kg/day.
*p< 0.05, compared to SAL using Newman-Keuls post hoc comparisons
Expression of xCT and GLT-1 is reduced by nicotine self-administration
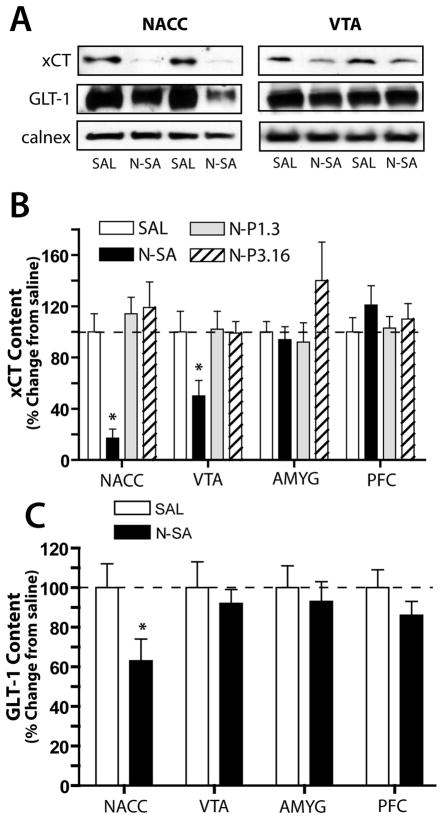
There was a significant decrease in xCT protein expression in the nucleus accumbens shell (F(3,24)= 8.99, p<0.001) and VTA (F(3,25)=2.97, p=0.005) of rats that had self-administered nicotine relative to SAL rats and either group receiving nicotine via minipumps (Figure 2B). The expression xCT was not altered in the PFC or amygdala between any groups. GLT-1 expression was significantly decreased in the nucleus accumbens shell (F(1,9)= 5.181, p<0.05), but not in the other brain regions examined, following nicotine self-administration (Figure 2C).
Figure 2.
xCT protein expression is reduced in the nucleus accumbens and VTA after 12 hours withdrawal from nicotine self-administration in rats. A. Representative immunoblots illustrating the reduction in xCT levels in the nucleus accumbens and VTA and a reduction of GLT-1 in the nucleus accumbens. B. Mean (±SEM) values for xCT in all brain areas and treatment groups investigated. C. Mean (±SEM) values for GLT-1 expression in all brain areas examined in the nicotine and saline self-administering groups. There was a significant decrease in GLT-1 expression in only the nucleus accumbens.
* p< 0.05, compared with SAL group
N-acetylcysteine decreased the number of cigarettes smoked
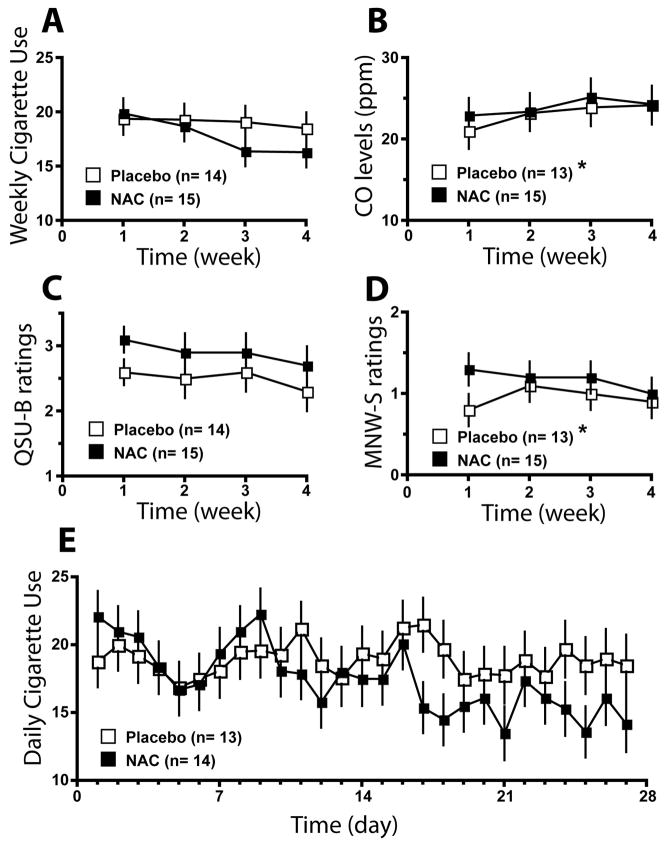
The mean number of daily cigarettes smoked within the N-acetylcysteine group was 17.7 (SD=7.1) and was not significantly different from the placebo group, 19.7 (SD=6.9). The results of the exploratory analyses for the weekly data are presented in Figure 3 (for detailed statistics on the data averaged weekly, see Table S2). There was a trend (p<0.06) for the Time effect, suggesting a reduction in average weekly smoking overall (Figure 3A). No significant effect of N-acetylcysteine (p>0.15) was noted for CO Levels (Figure 3B), QSU-B ratings (Figure 3C), or M-NWS ratings (Figure 3D). Analysis of the daily smoking data revealed a significant effect for Day, F(26,600) = 2.4, p<0.001, and a trend (p<0.11) for the Time x Treatment Group interaction. However, alcohol consumption proved to be a highly significant covariate, F(1,620) = 19.9, p<0.001. Further analysis of the daily smoking behavior revealed that the frequency of the days when participants reported simultaneous alcohol and cigarette use revealed that two individuals had a frequency of co-administration of alcohol and cigarettes that was greater than two standard deviations from the mean (Table S3). Excluding these two individuals from the analysis resulted in a significant effect for Time, F(26,540) =2.5, p<0.001, as well as a significant Time x Treatment Group Interaction, F(26,549) =1.6, p<0.05. Examination of the data revealed that the number of cigarettes smoked tended to be less in the N-acetylcysteine group (Figure 3E).
Figure 3.
Results of Clinical Trial. A. Average daily cigarette use (mean+SEM). B. CO levels (ppm). C. QSU-B Ratings. D. Withdrawal ratings. E. Daily smoking data, controlling for alcohol use.
* Baseline values were not available for subject for CO level and QSU-B rating, and this subject was removed from the data analysis
Discussion
Rats that self-administered nicotine intravenously showed a marked decrease in xCT in the nucleus accumbens and the VTA, and GLT-1 expression in the nucleus accumbens relative to saline controls. As well, xCT levels after nicotine self-administration were lower than in animals administered nicotine passively via continuous subcutaneous osmotic minipump delivery. These findings indicate that phasic administration of nicotine, rather than continuous release via minipumps, is necessary to down-regulate xCT. The reduction in xCT was independent of the development of withdrawal signs since both self-administration and continuous infusion of nicotine led to significant increases in somatic signs of withdrawal. Different adaptations induced by intermittent and continuous nicotine infusion have been observed previously. For example, continuous nicotine upregulates nicotinic acetylcholine receptors to a greater extent than intermittent administration (16). An alternative explanation is that contingent administration of nicotine may be required to affect xCT and GLT-1. While this possibility cannot be ruled out, at least system xc- is downregulated by either repeated contingent or non-contingent cocaine administration (1, 2).
It is interesting that both xCT and GLT-1 are down-regulated in the nucleus accumbens by two different classes of addictive drugs, cocaine (2, 7) and nicotine (present data). These data support not only the potential relevance of these proteins in the development and/or expression of dependence, but also pose the possibility that systems xc- and XAG may be co-regulated. Both system xc- and glutamate transport via GLT-1 occur predominantly in glia (17, 18), and previous in vitro studies provide indications that these proteins may be co-regulated (19, 20). For example, down-regulating system XAG reduces system xc- activity (20). However, in the VTA the regulation of the two proteins was dissociated since only xCT was reduced after nicotine self-administration.
The preclinical data presented here suggest that nicotine self-administration decreased xCT levels in brain regions critical to nicotine reinforcement. Because N-acetylcysteine activates system xc- and thereby increases intracellular glutathione synthesis and releases glutamate into the extracellular space (1, 2, 21), it seems likely that the administration of N-acetylcysteine is also increasing system xc- activity in smokers. Such a restoration of xc- activity may underlie the improved success by the N-acetylcysteine treated subjects in resisting cigarette smoking. In future studies the link between effects of N-acetylcysteine treatment in cigarette smokers and reduced xc- activity will benefit from further experimentation in animal models, akin to what has already been done in animal models of cocaine, and to a lesser extent heroin, relapse. For example, these cocaine and heroin studies have shown that N-acetylcysteine restores xc- activity and that this restoration is associated with reduced drug-seeking (1, 2, 22).
Although N-acetylcysteine reduced the number of cigarettes smoked there was no reduction in CO levels. Previous smoking reduction studies found similar decreases in number of cigarettes smoked without corresponding decreases in CO due to smoking fewer cigarettes but with deeper and longer inhalations (23). Based on the significant decrease in number of cigarettes smoked, N-acetylcysteine may be assisting smokers resist the urge to seek nicotine, but may not reduce nicotine consumption once smoking has begun. Importantly, the daily number of cigarettes smoked co-varied with daily alcohol use, and the effect of N-acetylcysteine was statistically significant only when two individuals were excluded from analysis that had daily alcohol use greater than two standard deviations above the mean of all participants. This highlights the importance of defining populations used in future studies in terms of simultaneous cigarette and alcohol use. Finally, N-acetylcysteine did not affect self-reported withdrawal symptoms, a finding that is in agreement with the lack of relationship between xCT expression and somatic signs of withdrawal in rats trained to self-administer nicotine. Additionally, since the human subjects of this study were not attempting to cease smoking altogether, and continued nicotine consumption throughout the study, there was not an opportunity for them to undergo nicotine withdrawal.
In summary, nicotine self-administration reduces the expression of xCT and GLT-1 in brain structure(s) involved in nicotine reinforcement, suggesting that, akin to cocaine self-administration, the activities of system xc- and GLT-1 are compromised. Moreover, treatment with a pro-cystine drug that increases the activity of system xc- reduced the number of cigarettes smoked on days when alcohol was not consumed. This combination of preclinical and clinical data indicates that N-acetylcysteine may aid in the cessation of cigarette smoking.
Supplementary Material
Acknowledgments
This work was supported by NIDA grants R01DA11946 (AM), P50DA015369 (PWK), RO1DA03906 (PWK) and Tobacco Related Disease Research Program grant 15RT-0022 from the State of California (AM). AM would like to thank Dr. Svetlana Semenova for assistance with data analyses, and Ms. Jessica Benedict and Ms. Gina Finnerman for technical assistance.
Footnotes
Disclosures
Athina Markou declares that she has received contract research support from Intracellular Therapeutics, Inc., and Lundbeck Research USA, Inc., and an honorarium from Abbott GmbH and Co during the past three years. Athina Markou has a patent application on metabotropic glutamate receptors and drug dependence. Peter Kalivas declares an honorarium received from Solvay during the past three years. None of the other authors report any biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 2.Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27:13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McBean GJ. Cerebral cystine uptake: a tale of two transporters. Trends Pharmacol Sci. 2002;23:299–302. doi: 10.1016/s0165-6147(02)02060-6. [DOI] [PubMed] [Google Scholar]
- 5.Zhou W, Kalivas PW. N-Acetylcysteine Reduces Extinction Responding and Induces Enduring Reductions in Cue- and Heroin-Induced Drug-Seeking. Biol Psychiatry. 2007;63:338–340. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, et al. Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- 7.Knackstedt L, Melendez R, Kalivas PW. Cocaine self-administration alters the expression of proteins associated with glutamatergic transmission and homeostasis at cortico-accumbens synapses. Society for Neuroscience Annual Meeting; San Diego, CA. 2007. [Google Scholar]
- 8.Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Jr, Markou A. NMDA Receptors Regulate Nicotine-Enhanced Brain Reward Function and Intravenous Nicotine Self-Administration: Role of the Ventral Tegmental Area and Central Nucleus of the Amygdala. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther. 2002;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- 12.Markidian P, LaRowe SD, Hedden S, Kalivas P, Malcolm R. An open label trial of N-acetylcysteine for the treatment of cocaine dependence: A pilot study. Prog Neuro-Psychoph Biol Psychiat. 2007;31:389–394. doi: 10.1016/j.pnpbp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 14.Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- 15.Toda S, Alguacil LF, Kalivas PW. Repeated cocaine administration changes the function and subcellular distribution of adenosine A1 receptor in the rat nucleus accumbens. J Neurochem. 2003;87:1478–1484. doi: 10.1046/j.1471-4159.2003.02121.x. [DOI] [PubMed] [Google Scholar]
- 16.Ulrich YM, Hargreaves KM, Flores CM. A comparison of multiple injections versus continuous infusion of nicotine for producing up-regulation of neuronal [3H]-epibatidine binding sites. Neuropharmacology. 1997;36:1119–1125. doi: 10.1016/s0028-3908(97)00107-x. [DOI] [PubMed] [Google Scholar]
- 17.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 18.Pow DV. Visualising the activity of the cystine-glutamate antiporter in glial cells using antibodies to aminoadipic acid, a selectively transported substrate. Glia. 2001;34:27–38. doi: 10.1002/glia.1037. [DOI] [PubMed] [Google Scholar]
- 19.Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem. 1986;261:2256–2263. [PubMed] [Google Scholar]
- 20.Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron. 1989;2:1547–1558. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- 21.Flanagan RJ, Meredith TJ. Use of N-acetylcysteine in clinical toxicology. Am J Med. 1991;91:131S–139S. doi: 10.1016/0002-9343(91)90296-a. [DOI] [PubMed] [Google Scholar]
- 22.Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry. 2008;63:338–340. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scherer G. Smoking behaviour and compensation: a review of the literature. Psychopharmacology (Berl) 1999;145:1–20. doi: 10.1007/s002130051027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.