Abstract
There is controversy within the literature regarding the influence of task instruction on the size of the long-latency stretch reflex (M2) elicited by a joint displacement. The aim of this study was to investigate if the previously reported task-dependent modulation of the M2 is specific to the M2 or can be explained by an early release of the intended voluntary response. We took advantage of the fact that the M2 is absent when the duration of the applied perturbation is less than a critical time period. This allowed us to examine modulation of muscle activity with and without the contribution of the M2. In addition, we applied transcranial magnetic stimulation (TMS) over the primary motor cortex to examine the modulation of corticomotor excitability with task instruction. Elbow joint extension displacements were used to elicit a stretch reflex in the biceps muscle. Subjects were instructed to “do not intervene” (DNI) with the applied perturbation, or to oppose the perturbation by activating the elbow flexors in response to the perturbation (FLEX). Electromyographic (EMG) activity in the time period corresponding to the M2 was significantly facilitated in the FLEX task instruction both with and without the presence of the M2. Motor evoked potentials (MEPs) elicited by TMS were also facilitated during the FLEX condition in the absence of the M2. EMG and MEP responses were not facilitated until immediately prior to the onset of the M2. Paired-pulse TMS revealed a significant reduction in short-interval intracortical inhibition (SICI) during the M2 response, but the level of SICI was not altered by the task instruction. We conclude that the task-dependent modulation of the biceps M2 results, at least in part, from an early release of the prepared movement and is accompanied by an increase in corticospinal excitability that is not specific to the M2 pathway. Task-dependent modulation of the response cannot be explained by an alteration in the excitability of intracortical inhibitory circuits.
Keywords: Stretch reflex, Upper limb, Transcranial magnetic stimulation, Voluntary movement
Introduction
The term “reflex” often conjures notions of a stereo-typed, involuntary response, but many investigators have demonstrated that reflex responses can be modulated in a task-dependent manner (Hammond 1955; Lee and Tatton 1975; Colebatch et al. 1979; Jaeger et al. 1982; Calancie and Bawa 1985; MacKinnon et al. 2000). Such modulation is evident in the human stretch reflex. When an upper limb muscle is stretched, the electromyographic (EMG) response can be categorized in terms of a short latency (M1) and a longer latency (M2) response. While the magnitude of the M1 response depends primarily on the level of the background muscle activation and the characteristics of the imposed muscle stretch, the M2 response can vary with current task requirements. However, there remains controversy regarding the specificity of its modulation and the pathways that regulate this modulation. In particular, it has been suggested that the apparent facilitation of response amplitude when subjects are preparing to oppose a perturbation arises through an increase in the level of activation of the target muscle prior to the stretch (Capaday et al. 1994), or that it represents the initial components of a triggered (Crago et al. 1976) or voluntary (Rothwell et al. 1980; Hallett et al. 1981) reaction in the M2 time period.
The main goal of this study was to examine if the task-dependent modulation of the biceps M2 is selective to the pathways mediating this component of the stretch reflex response or if the modulation reflects the superposition of an early voluntary response. Specifically, we were interested in reaction tasks, or those that differ in terms of how the subject is instructed to react to the imposed movement (e.g. Hammond 1955). Under this paradigm, modulation is observed as a facilitation of the M2 when subjects are instructed to react to the perturbation by activating the muscle that is stretched and a reduction in response size when they are asked to assist the joint perturbation by activating the antagonist muscle. Moreover, the observed modulation is selective to the M2 since no change in the size of the M1 response is seen. When subjects are asked to oppose a perturbation it is difficult to distinguish voluntary, triggered, and reflex EMG activity in the M2 time period. Because of this, previous studies relying on muscle stretch alone have failed to clearly discern whether the EMG facilitation represents a true modulation of the reflex response. We took advantage of the fact the M2 response is absent when the duration of the applied perturbation is less than a critical value (29–41 ms for biceps brachii) (Lee and Tatton 1982; Lewis et al. 2005). This permitted us to examine changes in response magnitude over the time period of the M2 both with, and without, the presence of the M2 response. The presence of modulation in the absence of an M2 response would suggest that pathways separate from those that generate the M2 contribute to the observed response. Conversely, if response modulation is only seen when an M2 is present, then this would provide evidence that the modulation is specific to the M2 pathway.
In addition to examining task-dependent modulation of EMG activation, we also used transcranial magnetic stimulation (TMS) to examine the contribution of changes in corticospinal excitability and intracortical inhibition (Kujirai et al. 1993) to the modulation of EMG activity during the M2 time period. Previous studies have shown that the excitability of corticospinal pathways are increased during the time period approximately corresponding to the passage of the afferent volley through the motor cortex (Day et al. 1991; Deuschl et al. 1991; Palmer and Ashby 1992; Petersen et al. 1998; Lewis et al. 2004). To date, no study has used TMS to investigate the effects of task instruction on corticospinal excitability following imposed perturbations of joint posture. If a task-dependent increase in the M2 is mediated by a increase in the excitability of a transcortical pathway via the primary motor cortex, then the amplitude of the motor evoked potentials (MEPs) elicited by TMS should be increased when an M2 response is present, but not when the M2 is absent. Alternatively, if the modulation is mediated by the superposition of an early voluntary response (Rothwell et al. 1980), then corticospinal excitability should increase immediately prior to the onset of agonist EMG activity (Hoshiyama et al. 1996; Chen et al. 1998; MacKinnon and Rothwell 2000) and intracortical inhibition should decrease (Reynolds and Ashby 1999) independent of the presence or absence of the M2.
Two specific hypotheses were tested in the current study. The first was that the task-dependent modulation of the M2 is not specific to the pathways mediating this response. The second was that the observed modulation can be attributed to changes in corticospinal excitability and intracortical inhibition that arise prior to the onset of voluntary intervention. Our results provide evidence of a non-specific modulation of EMG activity in the M2 time period that may arise through the early release of the voluntary response.
Materials and methods
Subjects
Twenty four individuals (age 30 ± 11 years, ten female) volunteered to participate in the study. All subjects were neurologically healthy and had no muscular or orthopedic limitations of the upper limb. Subjects participating in experiments involving TMS were required to have no contraindications to cortical magnetic stimulation. Informed consent was obtained from all subjects prior to testing. Ethical approval for the study was received from the Northwestern University Institutional Review Board.
Equipment
Elbow joint manipulandum
Subjects were seated comfortably with their trunk secured to an adjustable chair (Biodex, Shirley, NY, USA) using padded straps. The subject’s right arm was positioned in the horizontal plane with the shoulder at 45° flexion and 90° abduction, the elbow joint at 90°, and the forearm fully pronated (Fig. 1a). The upper arm was placed in an adjustable trough support to ensure a constant position of the shoulder joint. A fitted fiberglass cast extending from the fingers to the middle of the forearm was used to maintain the wrist joint in a neutral position and to attach the forearm to a linear actuator (Copley ThrustTube TB3806; Copley Controls, Canton, MA, USA). A 10 cm steel plate located on the underside of the cast, centered at the wrist joint, was secured to the top surface of the actuator via a precision bearing that allowed rotation in the horizontal plane. The actuator was mounted at shoulder height on an adjustable aluminum frame and was oriented 45° from the midline, such that perturbations were applied in the horizontal plane in a direction orthogonal to forearm orientation. Displacement of the linear actuator resulted in rotation at the elbow joint in the flexion/extension axis while the upper arm remained stationary. The actuator was instrumented with a linear encoder (RGH24; Renishaw, Gloucestershire, UK) to provide position information (resolution 1 µm) and was configured as a stiff position servo (250 kN/m) using custom software developed in Matlab xPC (The Mathworks Co, Natick, MA, USA).
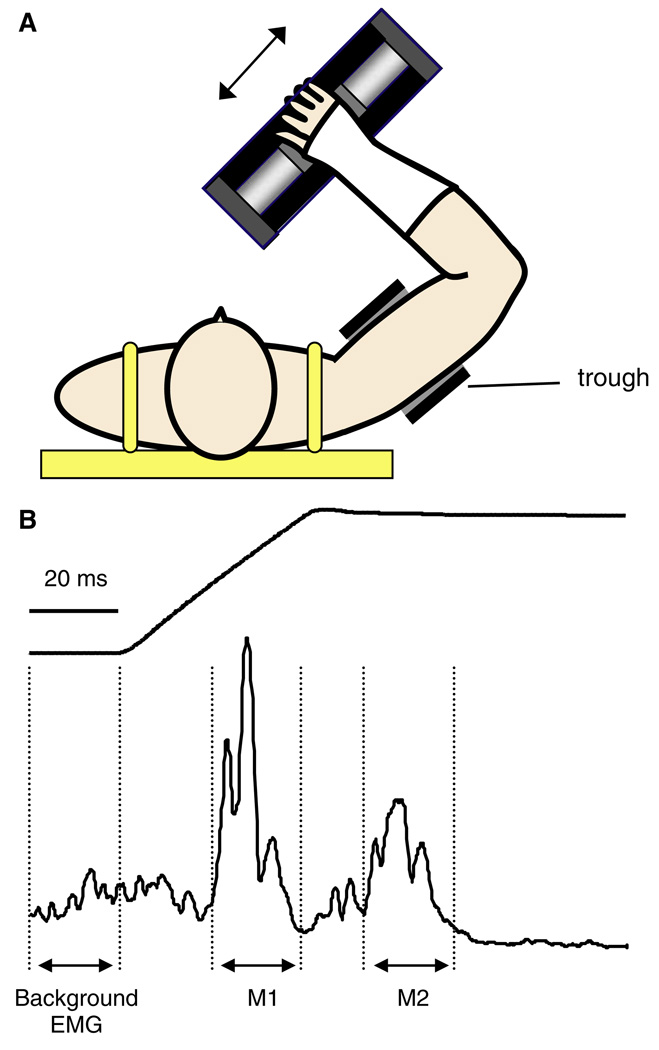
Fig. 1.
a Task set-up. The upper arm and forearm were positioned in the horizontal plane at shoulder height. The position of the elbow was adjusted so that the forearm was perpendicular to the movement axis of the actuator. Motion of the actuator (indicated by arrows) resulted in elbow joint rotation in the flexion/extension axis. The upper arm remained stationary during the displacements. b Example actuator displacement (top) and electromyographic recordings from the biceps muscle (bottom) demonstrating the calculation of M1 and M2 response size. Response area was expressed relative to an equivalent 20 ms window of muscle activation prior to the perturbation
Transcranial magnetic stimulation
Transcranial magnetic stimulation was applied using a MagStim 200 (Magstim Co, Dyfed, UK) or Magstim BiStim (Magstim Co) via a figure-of-eight coil (diameter 70 mm each). The coil was positioned over the subject’s head with the handle pointing backwards and oriented 45° from the mid-line. The optimal site for stimulation (“hot spot”) was located by moving the coil around the head until the site eliciting the largest responses in the biceps muscle was located. Active motor threshold (ATh) was then determined with the coil positioned over the hot spot. ATh was defined as the lowest stimulus intensity at which discernable MEPs were present in at least four of eight consecutive stimuli while the target muscle was active at 5% of maximum voluntary contraction (MVC).
Electromyography
Electromyographic activity was recorded from the biceps brachii (all experiments) and triceps brachii (Experiment 1 only) muscles in the right arm. Standard skin preparation techniques were completed prior to the application of disposable dual electrodes (Noraxon USA Inc, AZ, USA). Surface EMGs were amplified and conditioned using a Bortec AMT-8 (Bortec Biomedical Ltd, Canada) with high- and low-pass cut-off frequencies of 10 and 1,000 Hz, respectively. The resulting signals were anti-aliased filtered using 4th order Bessel filters with a cut-off frequency of 500 Hz and then sampled at 5 kHz for subsequent analysis.
Protocols
At the start of each experimental session, an MVC of the biceps muscle was recorded by having subjects perform a maximum isometric contraction while positioned in the manipulandum. In all subsequent trials, the target background EMG level was set to 5 ± 1% MVC. Subjects were provided with a visual display of EMG activity along with the target range (4–6% MVC) of muscle activation. Perturbations and magnetic stimuli were delivered when EMG activity had been maintained within the target range for at least 500 ms. Elbow joint perturbations and cortical stimulation were always presented at random intervals ranging from 4 to 6 s. Responses to joint perturbations and to TMS were collected in a series of five experiments, as outlined below. Not all subjects participated in all experiments, and most experiments were conducted on separate days.
Experiment 1. Effect of task instruction on the response characteristics of the stretch reflex (n = 11)
In this experiment, we evaluated the effects of perturbation velocity, perturbation duration, and task instruction on the M1 and M2 responses in the biceps muscle. We manipulated perturbation duration to control the presence of the M2, while manipulations of velocity were used to modulate the size of the reflex responses. Perturbations of either a short (20 ms; SHORT) or a long (60 ms; LONG) time duration were applied. In all individuals, the SHORT and LONG duration perturbations were clearly below and above, respectively, the critical time period for eliciting an M2 response (Lee and Tatton 1982; Lewis et al. 2005). At each of the two time durations, perturbations of both fast (500 mm/s, approximately 90°/s elbow rotation; FAST) and slow (250 mm/s, approximately 45°/s; SLOW) velocities were delivered. At the start of the random delay between perturbations, a red or a yellow light was presented to the subjects that indicated the task instruction for that trial. Subjects were instructed not to intervene with the perturbation if the yellow light was displayed (‘do not intervene’; DNI). If the red light was displayed, subjects were instructed to oppose the perturbation by activating their elbow flexor muscles as fast as possible in response to the perturbation (FLEX). Forty reflex responses were collected in each of the four perturbation velocity and time duration combinations. In each set of 40 trials, 20 DNI and 20 FLEX task instruction lights were presented in a randomized order. EMG from the triceps muscle was recorded in all conditions to monitor the level of antagonist muscle activation prior to and during the applied perturbations.
Experiment 2. Modulation of EMG in the M2 time period (n = 9)
In this set of experiments we investigated whether alterations in EMG activity induced by the subject’s preparatory set reflect a selective modulation of the M2 reflex pathway. This was accomplished by evoking an M1 response in the M2 time period. Previous studies have shown that the M1 response evoked by an imposed joint displacement is not modulated by task instruction (e.g. Colebatch et al. 1979; Rothwell et al. 1980). If the effects of task instruction on the M2 arise through a specific facilitation of the M2 reflex pathway, there should be significantly less modulation of the M1 when it is placed in the equivalent time period as the M2.
Two perturbation conditions were compared: (1) a long duration (60 ms) elbow joint extension perturbation (15 mm actuator displacement, 250 mm/s) was imposed at the start of the trial; (2) a brief duration, small amplitude perturbation (1 mm, 250 mm/s) in the elbow flexion direction was imposed at the start of the trial, followed by a long duration (60 ms, 15 mm, 250 mm/s) elbow extension perturbation. In the latter condition, the initial perturbation did not produce a reflex response in the biceps muscle, but served as the imperative stimulus to respond to task instruction. The time between the initial perturbation and the longer stretch perturbation used to elicit an M1 response in the biceps was individually adjusted so that the M1 was evoked in the same time period that the M2 occurred following a single lengthening perturbation (relative to the start of the perturbation).
Prior to each trial, a red or a yellow light was presented to the subjects that indicated the task instruction for that trial. For each perturbation condition, 20 trials with the DNI instruction and 20 trials with the FLEX instruction were presented in a randomized order. In all conditions, subjects were asked to respond in the FLEX task instruction by flexing against the actuator as soon as they detected movement in either direction.
Experiment 3. Effect of task instruction on corticospinal excitability (n = 11)
To examine changes in corticospinal excitability induced by the different perturbations and task instructions, we repeated the joint perturbations from Experiment 1 while applying single-pulse TMS over the contralateral motor cortex. The same subjects completed both Experiments 1 and 3 in the same session. The same perturbation velocity (SLOW, FAST), perturbation duration (SHORT, LONG) and task instruction (DNI, FLEX) conditions as presented in Experiment 1 were applied. The MEP produced in response to TMS was timed to arrive at the onset of the M2 response for each individual. This was achieved by delaying the cortical stimulation after the onset of the joint perturbation (delay = M2latency – MEPlatency). The same delay was employed for perturbations of both SHORT and LONG duration for each subject, even though an M2 was not present following SHORT perturbations. This enabled us to examine corticospinal excitability at a time period approximately corresponding to the passage of the stretch-induced afferent volley through the motor cortex when an M2 was present as well as absent. Twenty responses to TMS were collected in each perturbation velocity and time duration combination; ten in the DNI task instruction and ten in the FLEX task instruction. Stimulation intensity was set to 120% ATh.
Transcranial magnetic stimulation was applied in two further conditions: (1) without a perturbation while the subject maintained a 5% biceps MVC (TMS alone); and (2) with a perturbation (LONG, SLOW, DNI) but the MEP was timed to arrive at the onset of the M1 response. Timing the MEP to arrive at the M1 response provided a control response to evaluate the specificity of MEP facilitation during the M2 response.
Experiment 4. Time course of corticospinal excitability (n = 11)
The time course of changes in corticospinal excitability following a joint perturbation was examined in 11 subjects. If the FLEX task instruction has an effect on corticospinal excitability similar to a voluntary reaction time task, then we expected to see an increase in MEP amplitude following the onset of the joint perturbation compared to MEPs elicited in the DNI task instruction. TMS was applied over the contralateral motor cortex at four time intervals following the onset of a joint perturbation (15 mm, 250 mm/s): (1) prior to onset of M1 (delay = 0 ms), (2) at onset of M1 (delay = 8 ± 2 ms), (3) between M1 and M2 (delay = 30 ms), (4) at onset of M2 (delay = 44 ± 4 ms). Twenty trials were collected at each of the four stimulus delays; ten in the DNI task instruction and ten in the FLEX task instruction. Stimulation intensity was set to 120% ATh for all conditions. Subjects also completed 40 trials (20 DNI; 20 FLEX) in which the joint perturbation was applied without cortical stimulation.
Experiment 5. Effect of task instruction on intracortical excitability (n = 10)
A paired-pulse TMS paradigm was used to examine the effect of task instruction on short-interval intracortical inhibition (SICI) (Kujirai et al. 1993). If modulation of the M2 reflex response arose through alterations in intracortical inhibitory pathways that impose upon corticospinal output, then we would expect to see a difference in the level of SICI elicited during paired-pulse stimulation between the DNI and FLEX task instructions. The responses to TMS were timed to arrive at the onset of the M2 elicited by a joint perturbation (15 mm, 250 mm/s). Ten perturbations were delivered with the DNI task instruction and ten with the FLEX task instruction. In each of these two conditions, the test stimulus intensity was adjusted to produce a MEP of 0.5–1.0 mV amplitude (test MEP amplitude). As the size of the test MEP influences the amount of SICI that is generated, the test MEP amplitude was matched between the two task instruction conditions for each subject. The conditioning stimulus (first pulse of pair) intensity was set to 90% of motor threshold determined when the MEP was timed to arrive at the onset of the M2 in the FLEX task instruction. The inter-stimulus interval between conditioning and test stimuli was set at 2 ms. In both conditioned and non-conditioned trials, the test stimulus was delivered at the same time relative to the onset of the joint perturbation.
In a further control condition, single and paired-pulse TMS were delivered with the arm held stationary in the manipulandum and the biceps muscle pre-activated at 5% MVC. The test stimulus intensity in this condition was set to elicit a response at the previously determined test MEP amplitude. The same conditioning stimulus intensity was used for the joint perturbation and control conditions. The extent of SICI was determined by normalizing conditioned MEP amplitude to the size of the non-conditioned (test) MEP amplitude.
Data processing and analysis
In all experiments, trials in which the subjects did not respond appropriately according to the task instruction lights were discarded (approximately 5% of total trials). All remaining EMG recordings were rectified and averaged before further processing. The onset of the M1 response was determined as the first point following the onset of the perturbation at which biceps EMG activity exceeded 3 SD of the background muscle activation. The onset of the M2 response was determined visually as EMG activity often did not return to baseline following the M1. M1 and M2 response size were quantified as the area under the rectified EMG signal over a 20 ms time window following response onset. A further 20 ms period of EMG activity was evaluated immediately prior to perturbation onset to quantify background muscle activation levels (see Fig. 1b for example). Reflex response size was expressed relative to the level of muscle activation in this background EMG window. Similarly, MEP onset was determined as the first point following the stimulus artifact that exceeded 3 SD of the background muscle activation. MEP area was measured in a 20 ms window following this response onset.
In Experiment 1, a three-way task instruction (DNI, FLEX) × perturbation velocity (SLOW, FAST) × perturbation duration (SHORT, LONG) repeated measures (RM) analysis of variance (ANOVA) was used to investigate M1 reflex area, M2 reflex area, and the level of background EMG activity in the biceps and triceps muscles. In Experiment 2, reflex response size in the M2 time period was compared using a two-way task instruction (DNI, FLEX) · response (M1, M2) RM ANOVA. To examine changes in corticospinal excitability during the stretch reflex response in Experiment 3, the response area in the combined TMS-joint perturbation condition (S12) was expressed relative to the algebraic sum of the joint perturbation alone (Experiment 1, S1) and TMS alone (S2) responses:
| (1) |
This method has been used previously to examine the interaction of cortical stimuli and reflex responses (Petersen et al. 1998; Lewis et al. 2004). While we acknowledge that the interaction of these two responses is not likely to result in an algebraic summation of muscle activity, this method of analysis enabled us to compare the modulation of corticospinal excitability between the different perturbation and task instruction conditions across subjects. A three-way task instruction (DNI, FLEX) × perturbation velocity (SLOW, FAST) × perturbation duration (SHORT, LONG) RM ANOVA was used to investigate changes in relative MEP area. In Experiment 4, the time-course of changes in M2 and MEP area was analyzed by comparing MEP area between the DNI and FLEX task instructions at each of the four stimulus delays. MEPs were compared using one-tailed paired t-tests adjusted using a Bonferroni correction factor. In Experiment 5, a one-way ANOVA was used to compare the level of SICI between conditions (DNI, FLEX, control).
In all analyses, significant main effects and interactions were further examined using t-tests corrected for multiple comparisons. Differences at an α level ≤ 0.05 were considered to be significant. Results are reported as mean ± standard deviation.
Results
Effect of task instruction and perturbation characteristics on the stretch reflex
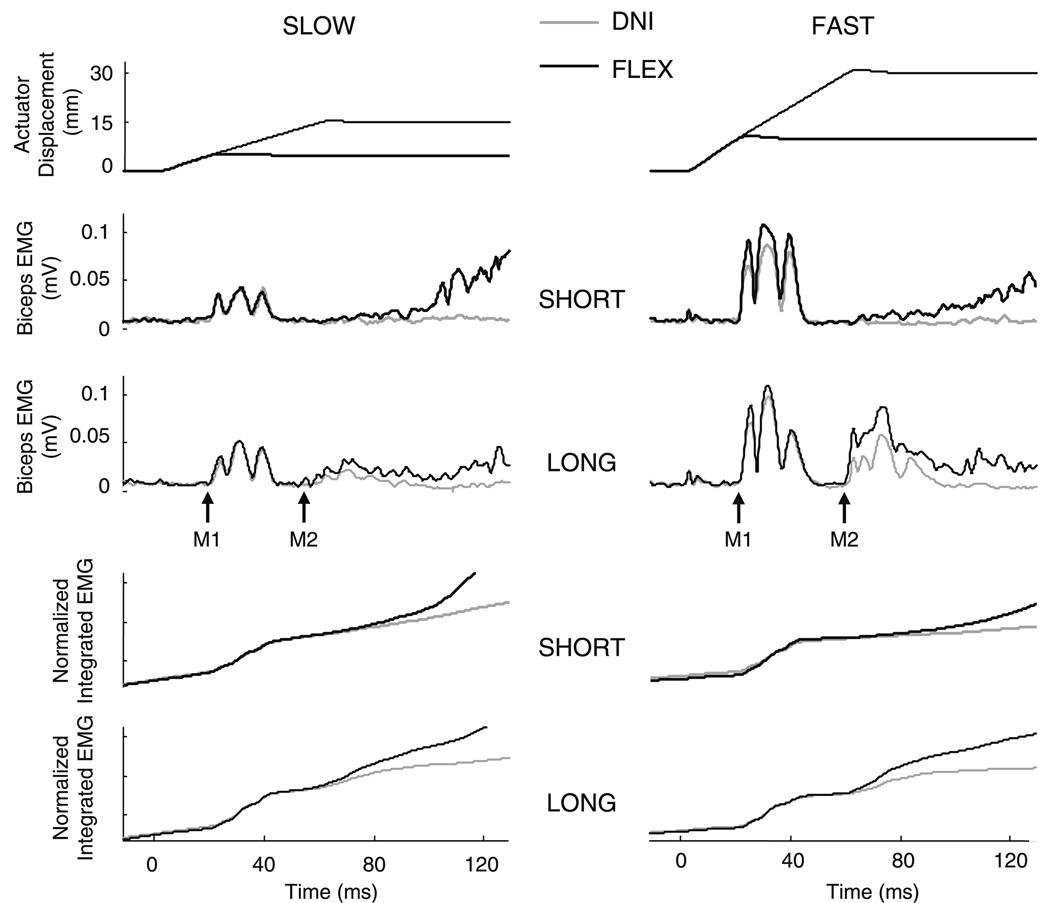
We observed a task-dependent modulation of the EMG in the M2 time period across all conditions, including when an M2 was not present. Both the M1 and M2 also were influenced by the velocity of the perturbation; however, perturbation duration affected only the M2. Example reflex responses from an individual subject are shown in Fig. 2. In this subject, an M2 was not elicited following the two perturbations of SHORT duration. Following perturbations of LONG duration, the M2 response was facilitated in the FLEX task instruction compared to the DNI task instruction. In contrast, the M1 was not affected by task instruction or perturbation duration.
Fig. 2.
Representative stretch reflex responses from an individual subject following SLOW (left, 250 mm/s) and FAST (right, 500 mm/s) velocity perturbations. Thick lines indicate responses at the SHORT (20 ms) duration and thin lines indicate responses at the LONG (60 ms) duration. Responses in the “oppose the displacement” (FLEX) task instruction are shown in black and responses in the “do not intervene” (DNI) instruction are shown in gray. Reflex responses are an average of 20 trials. The onsets of the M1 and M2 responses are indicated by the arrows. The top trace shows the perturbation used to extend the elbow. The second two traces show biceps EMG following perturbations of SHORT and LONG duration. The lower two graphs are a plot of biceps EMG integrated over the reflex time period. In the graphs of integrated EMG it is possible to clearly see the onset of the change in response size between the two task instructions
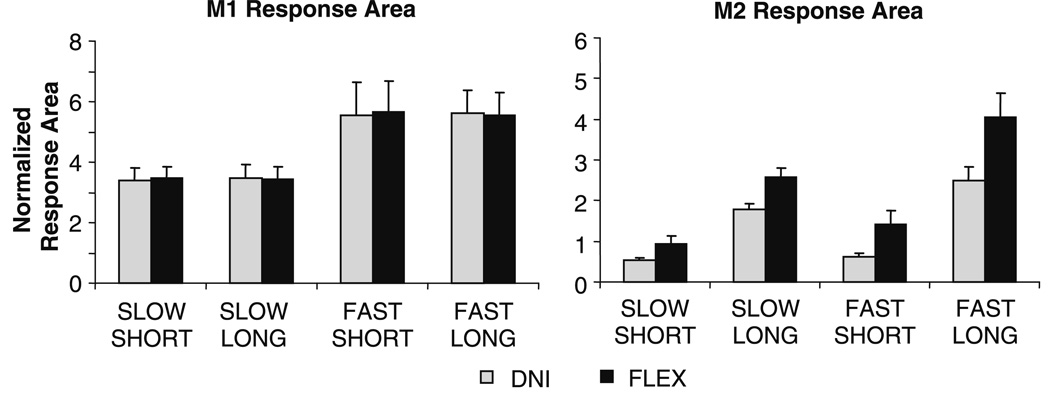
Group results showing M1 and M2 area in all perturbation conditions are displayed in Fig. 3. Overall, the average onset latency of the M2 response was 61 ± 5 ms. By design, an M2 was not elicited following the SHORT perturbation, resulting in a significant effect of perturbation duration for this response (F1,10 = 70, P < 0.001). In fact, EMG activity over the M2 time period following SHORT perturbations in the DNI task instruction was suppressed relative to baseline EMG activity (paired t-test SLOW, corrected P < 0.001; FAST, P = 0.004). Despite the absence of an M2 response for the SHORT perturbation condition, EMG activity over the M2 time period was greater during the FLEX task instruction at all perturbation velocity and duration combinations (F1,10 = 9.6, P = 0.01). The interaction between perturbation duration and task instruction was also significant for the M2 (F1,10 = 5.1, P = 0.05). This interaction indicated that the level of EMG activity was facilitated in the FLEX task instruction to a greater extent following perturbations of a LONG duration compared to SHORT. A main effect of velocity showed that M2 area was larger at the FAST velocity compared to SLOW (F1,10 = 10.7, P = 0.008), irrespective of duration. A significant interaction between perturbation velocity and task instruction also was detected (F1,10 = 7.5, P = 0.02), indicating that responses were facilitated to a greater extent in the FLEX task instruction following perturbations of a FAST velocity.
Fig. 3.
Group averages of M1 (a) and M2 (b) response area for the “do not intervene” (DNI) and “oppose the displacement” (FLEX) task instructions. Response area has been normalized to the level of background muscle activation. The effect of perturbation velocity was significant for the M1 response. For the M2 response, there were significant main effects of perturbation velocity, perturbation duration, and task instruction. Note that the normalized values are less than one for the M2 following SHORT perturbations in the DNI task instruction, i.e. an M2 response is not elicited. Error bars represent one standard error of the mean
The M1 response had an average onset latency of 22 ± 2 ms. The M1 displayed a larger response area following perturbations at the FAST velocity compared to the SLOW (F1,10 = 15.8, P = 0.003). There were no other significant main effects or interactions for M1 response area (all P ≥ 0.2).
The background activation levels of the biceps and triceps muscles were analyzed in all joint perturbation conditions. No significant main effects or interactions with task instruction were detected for either muscle (all P > 0.1). This indicates that the pre-activation levels of the biceps and triceps muscles were comparable in all perturbation conditions.
Modulation of EMG in the M2 time period
The M1 response demonstrated task-dependent modulation when evoked in the M2 time period. Figure 4 shows examples of reflex responses in an individual subject following a LONG elbow extension perturbation (Fig. 4a) and following a LONG elbow extension perturbation that was preceded by a brief flexion displacement (Fig. 4b). Note that, in Fig. 4b, the timing of onset of the M1 corresponded to the normal timing of the M2 for the LONG perturbation condition shown in Fig. 4a. Both of the responses are facilitated in the M2 time period in the FLEX task instruction.
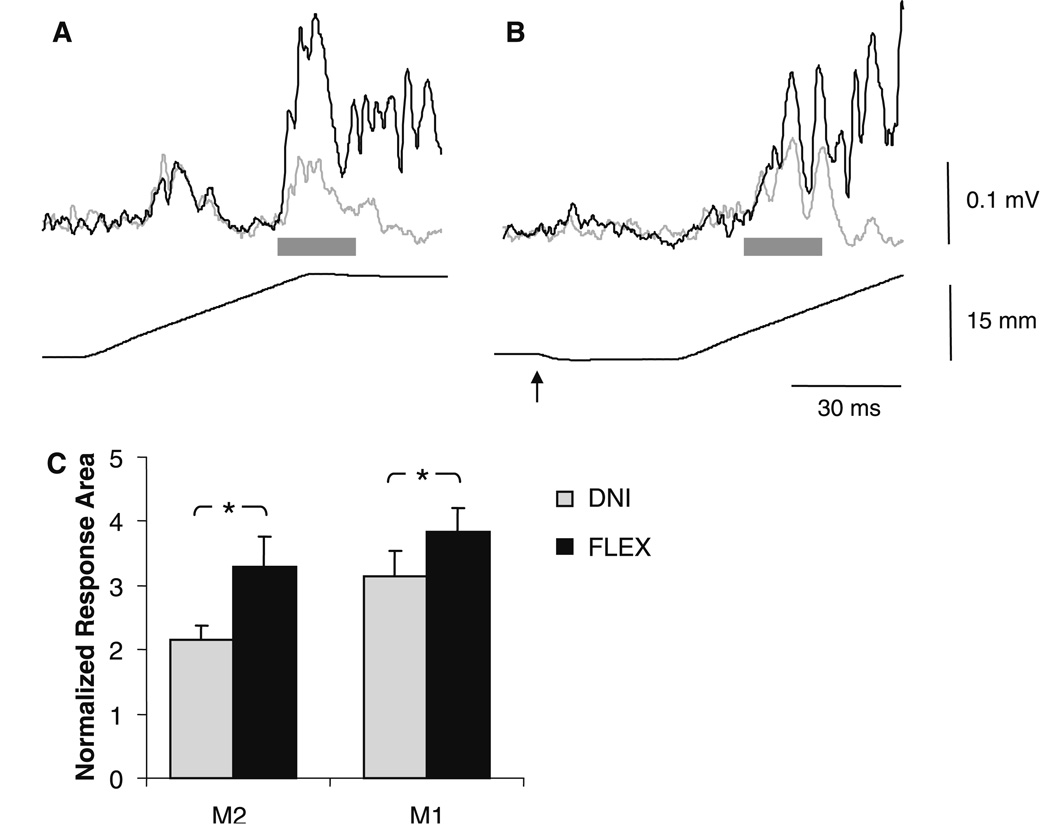
Fig. 4.
Example stretch reflex responses in an individual subject following a long extension perturbation (a), and following a long extension perturbation when preceded by a brief flexion movement (b). The actuator displacement for each condition is shown below the reflex response. The delay between the brief flexion movement (indicated by arrow) and the long perturbation was timed so that the M1 would occur in the normal M2 time period (gray bar). The light traces indicate the response in the “do not intervene” (DNI) task instruction and the dark traces indicate the response in the FLEX task instruction. All traces are an average of 20 responses. c Group results showing the M2 and M1 (in the M2 time period) response size for both DNI (light bars) and FLEX (dark bars) task instructions. Both responses are facilitated in the FLEX task instruction. *P < 0.05. Error bars are one standard error of the mean
Group results comparing response size between the two task instructions are shown in Fig. 4c. Across both joint perturbation conditions, the reflex response over the M2 time period was significantly larger in the FLEX task instruction compared to DNI (F1,8 = 14.2, P = 0.005). The main effect of reflex response (F1,8 = 1.7, P = 0.2) and the interaction between task instruction and reflex response were not significant (F1,8 = 1.6, P = 0.3). This indicates that the M1 and M2 responses were facilitated to a comparable extent when they were in the same time period relative to the initial proprioceptive cue.
An ANOVA comparing the level of background biceps muscle activation prior to the perturbations did not reveal any significant differences between conditions (all P > 0.2). Also, there was no difference in the size of the M1 elicited following the single LONG perturbation and the M1 that was evoked in the M2 time period (paired t-test; P = 0.4).
Effect of task instruction on corticospinal excitability
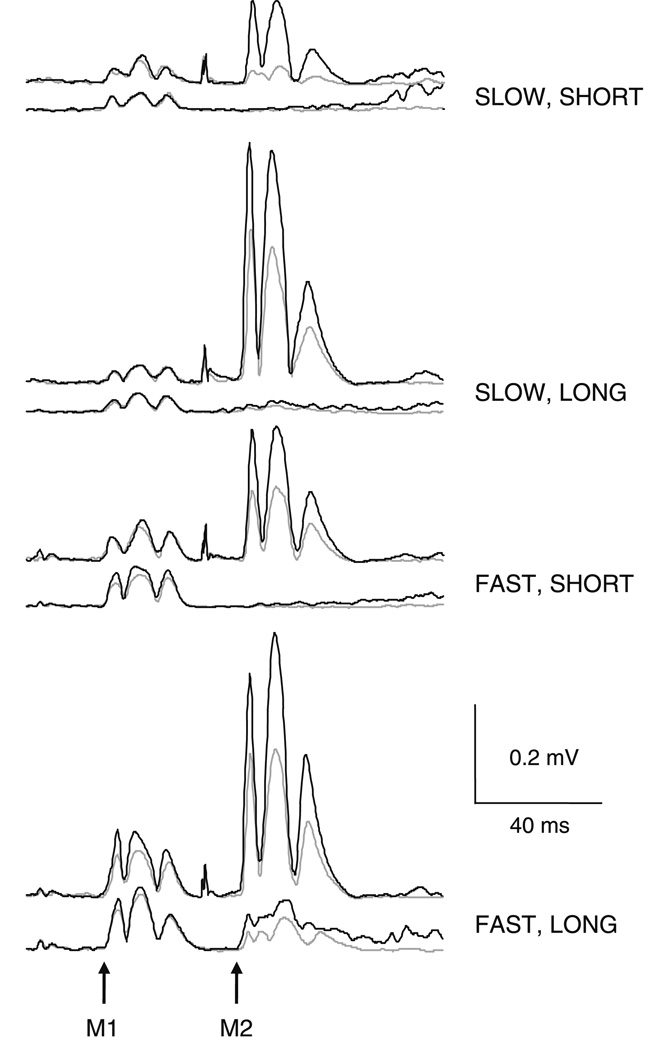
Corticospinal excitability during the M2 time period was influenced both by the duration of the perturbation and the task instruction. The average ATh and test stimulus intensities used in this experiment were 41 ± 10 and 49 ± 12% of maximum stimulator output, respectively. Figure 5 displays representative EMG traces depicting the response to TMS during the stretch reflex response in an individual subject. Shown below each MEP is the stretch reflex response in the same individual when the joint perturbation was presented in isolation. Group results of relative MEP area in the various perturbation conditions are shown in Fig. 6.
Fig. 5.
Biceps EMG recordings from an individual subject when the response to transcranial magnetic stimulation (TMS) was timed to arrive at the onset of the M2. Responses during the “do not intervene” (DNI) task instruction are shown in gray, while those during the “oppose the displacement” (FLEX) task instruction are shown in black. Below each trace are the equivalent responses elicited when the joint perturbation was given without cortical stimulation. The traces are an average of 10 responses for the combined TMS and stretch reflex conditions and an average of 20 responses for the joint perturbation in isolation. The onsets of the M1 and M2 are indicated by the arrows
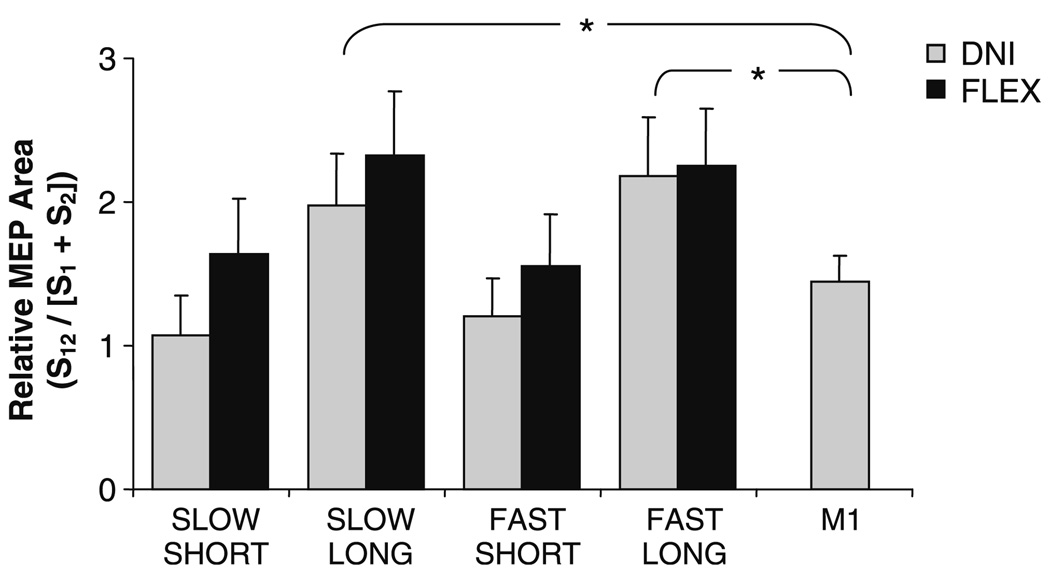
Fig. 6.
Group results showing relative motor evoked potential (MEP) area when the response was timed to arrive at the onset of the M2. MEP area is expressed relative to the sum of the stretch reflex (S1) and MEP (S2) areas when presented alone. Also shown is the normalized MEP size when the MEP was timed to arrive at the onset of the M1 (250 mm/s, 60 ms perturbation). *P < 0.05. Error bars represent one standard error of the mean
The group results showed that the combined MEP and stretch reflex response was larger in the FLEX task instruction compared to DNI (F1,10 = 6.0, P = 0.04). Relative MEP area was also consistently larger following perturbations of a LONG duration compared to SHORT (F1,10 = 5.2, P = 0.05). To confirm a facilitation of MEP amplitude when delivered during the M2 time period, we compared relative MEP amplitude in the two LONG duration DNI task instruction conditions to relative MEP amplitude when timed to arrive at the M1. At both velocities, the relative MEP area at the M2 was larger than the relative MEP area in the M1 (both P = 0.03). There were no further significant effects or interactions for these data (all P ≥ 0.08).
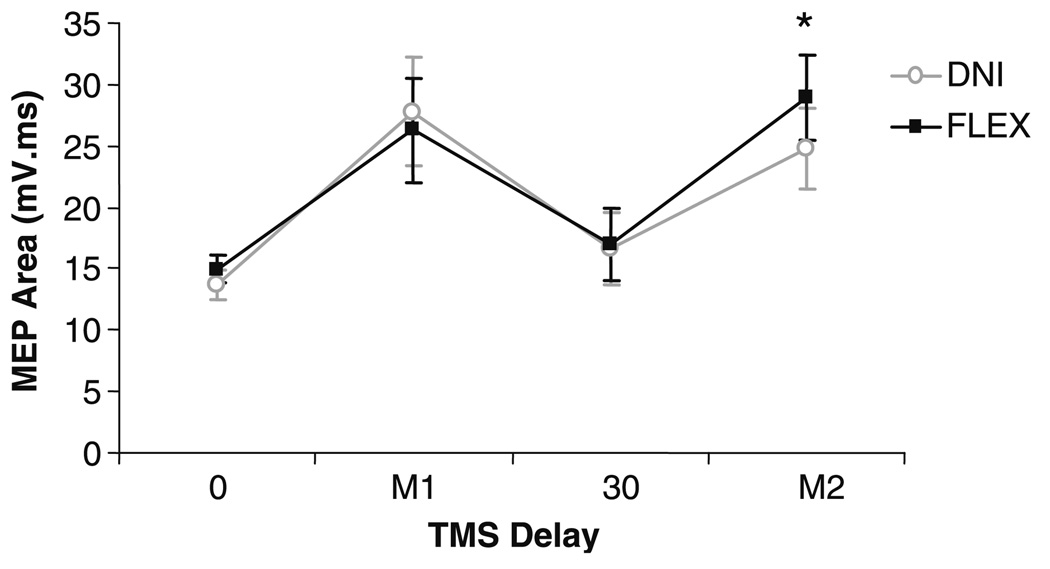
Time course of changes in corticospinal excitability
Significant changes in corticospinal excitability with task instruction were not observed until immediately prior to the onset of the M2. Group results showing MEP area at the four tested stimulus delays are shown in Fig. 7. MEP area in the FLEX task instruction was significantly larger than in the DNI task instruction when TMS was timed to elicit a MEP at the onset of the M2 (delay 44 ± 4 ms; corrected P = 0.04), but not when TMS was applied at 30 ms or earlier (all P > 0.1).
Fig. 7.
Group results showing motor evoked potential (MEP) area when the cortical stimulus was delivered at four time delays following a joint perturbation. Responses are shown for the “do not intervene” (DNI; light) and “oppose the displacement” (FLEX; dark) task instructions. Asterisks indicates a significant difference in MEP area between the two task instructions. Error bars are one standard error of the mean
Effect of task instruction on intracortical excitability
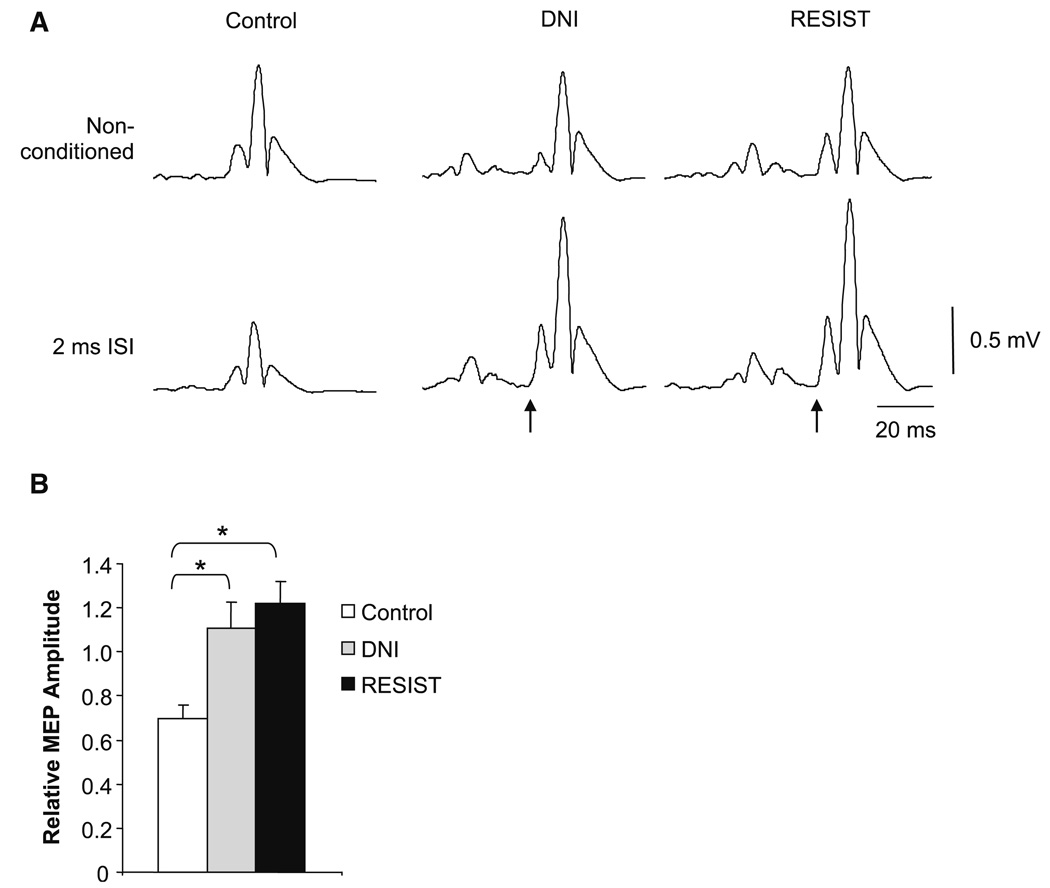
Significant differences in the excitability of intracortical inhibitory circuits were not observed between the DNI and FLEX tasks. Figure 8a shows TMS-elicited responses in the biceps muscle of an individual subject following single- and paired-pulse stimulation given in control (no perturbation) and the two joint perturbation conditions. The top row of Fig. 8a illustrates how the stimulator output was adjusted to match the non-conditioned MEP amplitudes across conditions. The average test stimulus intensity to elicit a MEP of the required amplitude was 56 ± 11% in the FLEX task instruction, 58 ± 11% in the DNI task instruction, and 66 ± 11% in the control condition. In the control condition (left column), the MEP response was inhibited when preceded by a subthreshold conditioning stimulus. When delivered during a reflex response, inhibition following dual stimulation no longer occurred. These findings are reflected in the group results (Fig. 8b).
Fig. 8.
a Example motor evoked potentials (MEPs) in an individual subject following paired-pulse stimulation. Responses on the left were elicited in a control condition with the biceps pre-activated at 5% of maximum voluntary contraction and held stationary. The middle column shows responses when the MEP was timed to arrive at the onset of the M2 during the “do not intervene” (DNI) task instruction. The right column shows responses when the MEP was timed to arrive at the onset of the M2 during the “oppose the displacement” (FLEX) task instruction. The top row shows the non-conditioned response, which was matched between stimulation conditions. Responses in the bottom row were conditioned with a subthreshold stimulus at an inter-stimulus interval (ISI) of 2 ms. The arrows indicate the onset of the MEP in the two conditions involving a joint perturbation. b Group results showing conditioned MEP amplitude (relative to non-conditioned) for the two task instructions and the control condition. *P < 0.05. Error bars are one standard error of the mean
There were no significant differences in non-conditioned MEP amplitude among the three conditions (control = 0.58 ± 0.19 mV; DNI = 0.58 ± 0.14 mV; FLEX = 0.56 ± 0.17 mV; P > 0.8), indicating that test MEP amplitude was matched sufficiently. In the control condition, paired-pulse stimulation resulted in an inhibition of MEP amplitude to 70 ± 19% of the non-conditioned mean. When the MEP was timed to arrive at the onset of the M2, conditioned MEP amplitudes were 111 ± 36 and 122 ± 31% of non-conditioned values in the DNI and FLEX task instructions, respectively. Post-hoc analysis of a main effect of condition (F1,9 = 12.9; P = 0.001) confirmed that the level of SICI was significantly greater in the control condition compared to the DNI (P = 0.02) and FLEX (P = 0.001) task instructions. The difference in the level of SICI between the two task instructions was not significant (P = 0.5).
Discussion
We investigated the effect of task instruction on the biceps stretch reflex and the contribution of the M2 response to the modulation of EMG activity observed over the M2 time period. The results of these experiments showed that task-dependent modulation of EMG activity and MEPs during the M2 time period occurred independent of the presence of a stretch-induced M2 response. SICI was markedly reduced when paired-pulse TMS was timed to coincide with the onset of the M2 time period, but these effects were not influenced by task instruction. These results demonstrate that the biceps M2 response can be modulated by voluntary intent, but that this modulation is not specific to the M2 pathway and does not appear to involve modulation of GABAA-mediated intracortical inhibitory circuits. These findings are discussed below in relation to the possible pathways mediating the task-dependent modulation of EMG activity during the M2 time period.
Modulation of the M2 with task instruction
The results of Experiment 1 showed that M2 response area was clearly modulated by task instruction. This modulation was unlikely to be the result of changes in the level of spinal motorneuron excitability prior to the perturbation (Capaday et al. 1994) since biceps and triceps background activation levels were the same across the various perturbation conditions. Most importantly, we observed that EMG activity over the M2 time period was facilitated during the FLEX condition even when a short duration perturbation was applied. We and others have shown that the M2 response is absent when the perturbation is applied for less than a critical time duration (Lee and Tatton 1982; Lewis et al. 2005). Moreover, there is a marked suppression of EMG activity during this time period below pre-activation baseline levels. This suppression has been attributed, in part, to a disfacilitation of synchronous input to the motor neuron pool (Poliakov and Miles 1994; Lewis et al. 2005). In addition, we showed that the M1 response was modulated by task instruction when its onset was timed to coincide with the usual onset of the M2 response (Experiment 2). Thus, our findings provide support for the hypothesis that the task-dependent modulation of the M2 is mediated by the early release of the prepared voluntary response (Crago et al. 1976; Rothwell et al. 1980).
In the FLEX task instruction, EMG activity in the M2 time period was facilitated to a greater extent following LONG perturbations compared to SHORT. This interaction effect likely reflects the difference in activation threshold of the motoneuron pool in a disfacilitated (SHORT) versus facilitated (LONG) state. Alternatively, we cannot rule out the possibility that some portion of the M2 reflex pathway mediating the LONG duration response is modulated and contributes to the task-dependent effects for the FLEX condition in addition to the non-specific facilitation described above.
Modulation of corticospinal excitability with task instruction
Previous studies using TMS have shown that corticospinal excitability increases following an imposed joint displacement at a time consistent with the passage of the afferent volley through the motor cortex (Day et al. 1991; Deuschl et al. 1991; Palmer and Ashby 1992; Petersen et al. 1998; Lewis et al. 2004). This was also evident in our results. The lower relative amplitude of the MEPs during the SHORT duration perturbations was probably due to the marked suppression of EMG activity below tonic pre-perturbation levels in the M2 time period. It is well known that there is a non-linear relationship between the level of ongoing muscle activity and MEP amplitude (Turton and Lemon 1999). Following the SHORT perturbations, a relative MEP area of less than one would be expected due to the low motor unit activity at the time of the arrival of the TMS-induced volley. Instead, the relative MEP area had an average value that approximated one, suggesting that the MEP had been facilitated upstream of the motoneuron pool.
Our results also indicated that MEPs were enhanced in the FLEX task instruction regardless of perturbation duration. This ubiquitous facilitation suggests that the excitability of synaptic elements somewhere along the corticospinal pathway is increased independently of the pathway that generates the M2 response. Studies of movement-related cortical potentials evoked by imposed displacements of the wrist have shown that activity over the motor cortex begins approximately 35 ms after perturbation onset (MacKinnon and Rothwell 2000). These potentials vary with perturbation velocity (Abbruzzese et al. 1985) but are unaffected by task instruction (MacKinnon et al. 2000). These findings are in agreement with our suggestion that modulation of EMG activity over the M2 time period occurs downstream from the motor cortex.
An alternative explanation for the main effect of task instruction on the size of the MEPs is that the level of motor unit activity at the time of the arrival of the TMS-induced corticospinal volley was different across conditions (Fig. 3). Our results from Experiment 1 showing that M2 size varied with perturbation velocity as well as task instruction provide some evidence against this explanation. Even though there were differences in the level of EMG in the M2 time period between the different perturbation velocities, there was no effect of velocity on the combined MEP/stretch reflex response. This finding, together with the smaller relative MEP area during the M1 response, indicates that the level of motor unit activity has a limited influence on combined response size when comparing responses generated during similar levels of motoneuron activation. Therefore, the facilitation of MEP responses in the FLEX task instruction most likely reflects the presence of an additional input to the corticospinal pathway.
One possibility is that the increase in MEP amplitude across task conditions arises through a potentiation of corticospinal pathway excitability preceding the voluntary initiation of the instructed task. It has been previously reported that H-reflex amplitude (Eichenberger and Ruegg 1984; Hasbroucq et al. 2000) and TMS-evoked MEPs (Pascual-Leone et al. 1992; Hoshiyama et al. 1996; Chen et al. 1998) are facilitated prior to the onset of movement in a reaction time task. A recent study suggested that, when background EMG is carefully controlled, this facilitation occurs not more than 23 ms prior to reaction time (MacKinnon and Rothwell 2000). Based on MacKinnon and Rothwell’s (2000) estimate and the time period of EMG modulation in our data, corticospinal excitability would be expected to increase at approximately the onset of the M2. This could explain the facilitation of MEP responses at this time. Our findings relating to the time course of changes in corticospinal excitability are also consistent with this estimate since task-dependent modulation of the corticospinal excitability was not observed until more than 30 ms after onset of the perturbation. As reaction times in the vicinity of 80 ms are considered to be too fast for a voluntary response, we suggest that the increase in EMG in this time period reflects activity arising downstream from the motor cortex.
Short-interval intracortical inhibition and task instruction
Similarly, if the modulation during the M2 time period is mediated by a voluntary response, then SICI should be selectively decreased in pathways targeting the initial agonist muscle during the FLEX task only (Reynolds and Ashby 1999). We observed a significant reduction in SICI when the test stimulus was delivered during a reflex response compared to when the limb was held stationary. However, we did not find a difference in the level of SICI between the two task instructions. In all conditions, the background level of muscle activation prior to the perturbation and test MEP amplitudes were matched. This finding suggests that the observed task-dependent modulation in the M2 time period is not mediated via GABAA intracortical inhibitory circuits and that changes in EMG activity during the M2 time period are mediated below the level of the motor cortex.
Early triggering of a subcortical response
We propose that the facilitation of the EMG activity observed during theM2 interval when subjects attempted to FLEX the perturbation was generated by an early release of the prepared and intended voluntary response (Houk 1978). Crago et al. (1976) had noted that task-dependent modulation of stretch reflexes in the biceps muscle could occur as early as 70 ms following perturbation onset. They proposed that this response reflected a “triggered reaction” to the perturbation. Similarly, Rothwell et al. (1980) were able to show that modulation of biceps EMG activity over the M2 time period was markedly affected by the predictability of the timing of the perturbation, suggesting that the modulation was due to the superposition of a “very rapid voluntary event” on the M2. Consistent with this idea is the observation that a joint displacement imposed prior to a prepared movement results in the early release of the initial agonist burst associated with that movement, irrespective of whether or not the agonist had been stretched by the perturbation (Koshland and Hasan 2000). It was speculated that these early responses might be mediated by pathways similar to those that generate the startle reflex. It has been shown that a prepared movement sequence can be released by an auditory startle stimulus at latencies in the range of 60–80 ms (Valls-Sole et al. 1999; Carlsen et al. 2004). Due to the very short latency of these reactions to startle, it was hypothesized that these prepared movements are released from subcortical structures, such the reticulospinal pathway, similar to those that mediate the generalized startle reflex. Following LONG duration perturbations in the FLEX task instruction, the afferent volley evoked by the stretch may generate the M2 response and also may release the prepared program from subcortical structures resulting in an apparent facilitation of the M2 response. Following SHORT perturbations, there is no M2 response, however the general facilitation we observed during the M2 time period suggests that the prepared program is still released.
Generalizability across joints and motor tasks
In this study, we examined the effect of task instruction of stretch-evoked EMG responses in the biceps muscle. Extrapolation of these findings to other muscles, particularly muscles that control movement of the distal upper limb, should be exercised with caution. There is compelling evidence that the M2 response in thenar, finger and wrist muscles is mediated, in part, by a transcortical pathway that traverses the primary motor cortex (Marsden et al. 1973, 1977; Capaday et al. 1991; Day et al. 1991; Palmer and Ashby 1992; Tsuji and Rothwell 2002). There has been no evidence to date for such a transcortical loop in more proximal upper limb muscles, including the biceps (Lenz et al. 1983; Cohen et al. 1991; Thilmann et al. 1991; Fellows et al. 1996). Task-dependent modulation of the M2 in distal muscles has been shown to be smaller than the effect seen in proximal muscles (Rothwell et al. 1980). This might be explained by the fact that distal muscles have larger M2 responses than proximal muscles, which may indicate that there is little room for increasing the magnitude of this response. Comparable experiments in distal muscles to those reported in this paper are needed to answer these questions.
Our findings should also be considered separately from studies that have analyzed reflex responses during voluntary tasks requiring greater levels of motor precision. For example, Evarts and Fromm (1978) reported enhanced responses to joint perturbations in corticospinal tract neurons of monkeys during a fine motor control task compared to static holding. Alterations in reflex responses that occur during these types of high precision voluntary tasks may arise through a neural mechanism distinct from that mediating reflex modulation during the more simple and predictable reaction time task used in our study.
Conclusions
We have demonstrated that the modulation of the M2 reflex in the biceps muscle across different reaction tasks is likely to arise from the superposition of a triggered response and a long-latency reflex. In contrast to the stereotypical M1, the ability to modulate EMG activity over the M2 time period provides a means to adapt the overall response to the current task requirements at short latency. Corticospinal excitability increased prior to the M2, but it remains to be seen if this change was due to sensory afferent facilitation of motor cortical excitability or reflected a change in corticospinal excitability preceding the voluntary response to the perturbation. It does not appear that the modulation of the EMG activity over the M2 time period is mediated by a change in activity of GABAA-mediated intracortical inhibitory networks at the level of the motor cortex.
Acknowledgements
The authors would like to thank Tiffany Viant for her technical assistance in the laboratory. This project was supported by NIH grant 1-K25-HD044720 and a grant from the Whitaker Foundation (EP). GL was supported by a fellowship from the Brinson Foundation.
Contributor Information
Gwyn N. Lewis, Sensory Motor Performance Program, Rehabilitation Institute of Chicago, 345 E Superior St, Chicago, IL 60611, USA, E-mail: g-lewis3@northwestern.edu, Tel.: + 1-312-2381235, Fax: + 1-312-2382208
Colum D. MacKinnon, Department of Physical Therapy and Human Movement Sciences, Northwestern University, Chicago, IL 60611, USA Department of Neurology, Northwestern University, Chicago, IL 60611, USA.
Eric J. Perreault, Sensory Motor Performance Program, Rehabilitation Institute of Chicago, 345 E Superior St, Chicago, IL 60611, USA Department of Physical Medicine and Rehabilitation, Northwestern University, Chicago, IL 60611, USA; Department of Biomedical Engineering, Northwestern University, Evanston, IL 60208, USA.
References
- Abbruzzese G, Berardelli A, Rothwell JC, Day BJ, Marsden CD. Cerebral potentials and electromyographic responses evoked by stretch of wrist muscles in man. Exp Brain Res. 1985;58:544–551. doi: 10.1007/BF00235870. [DOI] [PubMed] [Google Scholar]
- Calancie B, Bawa P. Firing patterns of human flexor carpi radialis motor units during the stretch reflex. J Neurophysiol. 1985;53:1179–1193. doi: 10.1152/jn.1985.53.5.1179. [DOI] [PubMed] [Google Scholar]
- Capaday C, Forget R, Fraser R, Lamarre Y. Evidence for a contribution of the motor cortex to the long-latency stretch reflex of the human thumb. J Physiol. 1991;440:243–255. doi: 10.1113/jphysiol.1991.sp018706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C, Forget R, Milner T. A re-examination of the effects of instruction on the long-latency stretch reflex response of the flexor pollicis longus muscle. Exp Brain Res. 1994;100:515–521. doi: 10.1007/BF02738411. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Prepared movements are elicited early by startle. J Motor Behav. 2004;36:253–264. doi: 10.3200/JMBR.36.3.253-264. [DOI] [PubMed] [Google Scholar]
- Chen R, Yaseen Z, Cohen LG, Hallett M. Time course of corticospinal excitability in reaction time and self-paced movements. Ann Neurol. 1998;44:317–325. doi: 10.1002/ana.410440306. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Meer J, Takka I, Biemer S, Leidermann DB, Dubinsky RM, Sanes JM, Jabbari B, Bransum B, Hallet M. Congenital mirror movements: abnormal organisation of motor pathways in two patients. Brain. 1991;114:381–403. doi: 10.1093/brain/114.1.381. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Gandevia SC, McCloskey DI, Potter EK. Subject instruction and long-latency reflex responses to muscle stretch. J Physiol. 1979;292:527–534. doi: 10.1113/jphysiol.1979.sp012869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crago JE, Houk JC, Hasan Z. Regulatory actions of human stretch reflex. J Neurophysiol. 1976;39:925–935. doi: 10.1152/jn.1976.39.5.925. [DOI] [PubMed] [Google Scholar]
- Day BL, Riescher H, Struppler A, Rothwell JC, Marsden CD. Changes in the response to magnetic and electrical stimulation of the motor cortex following muscle stretch in man. J Physiol. 1991;433:41–57. doi: 10.1113/jphysiol.1991.sp018413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G, Michels R, Berardelli A, Schenck E, Inghilleri M, Lucking CH. Effects of electric and magnetic transcranial stimulation on long latency reflexes. Exp Brain Res. 1991;83:403–410. doi: 10.1007/BF00231165. [DOI] [PubMed] [Google Scholar]
- Eichenberger A, Ruegg DG. Relation between the specific H reflex activation preceding a voluntary movement and movement parameters in man. J Physiol. 1984;347:545–559. doi: 10.1113/jphysiol.1984.sp015082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV, Fromm C. The pyramidal tract neuron as summing point in a closed-loop control system in the monkey. In: Desmedt JE, editor. Cerebral cortex in motor control: long loop mechanisms. New York: Karger; 1978. pp. 56–69. [Google Scholar]
- Fellows SJ, Topper R, Schwarz M, Thilmann AF, Noth J. Stretch reflexes of the proximal arm in a patient with mirror movements: absence of bilateral long-latency components. Electroencephalogr Clin Neurophysiol. 1996;101:79–83. doi: 10.1016/0924-980x(95)00247-i. [DOI] [PubMed] [Google Scholar]
- Hallett M, Bielawski M, Marsden CD. Behavior of the long-latency stretch reflex prior to voluntary movement. Brain Res. 1981;219:178–185. doi: 10.1016/0006-8993(81)90279-1. [DOI] [PubMed] [Google Scholar]
- Hammond PH. Involuntary activity in biceps following the sudden application of velocity to the abducted forearm. J Physiol. 1955;127:23P–25P. [PMC free article] [PubMed] [Google Scholar]
- Hasbroucq T, Akamatsu M, Burle B, Bonnet M, Possamai C-A. Changes in spinal excitability during choice reaction time: the H reflex as a probe of information transmission. Psychophysiology. 2000;37:385–393. [PubMed] [Google Scholar]
- Hoshiyama M, Kitamura Y, Koyama S, Watanabe S, Shimojo M, Kakigi R. Reciprocal change of motor-evoked potentials preceding voluntary movement in humans. Muscle Nerve. 1996;19:125–131. doi: 10.1002/(SICI)1097-4598(199602)19:2<125::AID-MUS1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Houk JC. Participation of reflex mechanisms and reaction-time processes in the compensatory adjustments to mechanical disturbances. In: Desmedt JE, editor. Cerebral motor control in man: long loop mechanisms. New York: Karger; 1978. pp. 193–215. [Google Scholar]
- Jaeger RJ, Gottlieb GL, Agarwal GC. Myoelectric responses at flexors and extensors of human wrist to step torque perturbations. J Neurophysiol. 1982;48:388–402. doi: 10.1152/jn.1982.48.2.388. [DOI] [PubMed] [Google Scholar]
- Koshland GF, Hasan Z. Electromyographic responses to a mechanical perturbation applied during impending arm movements in different directions: one-joint and two-joint conditions. Exp Brain Res. 2000;132:485–499. doi: 10.1007/s002210000356. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day DL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RG, Tatton WG. Motor responses to sudden limb displacements in primates with specific CNS lesions and in human patients with motor system disorders. Can J Neurol Sci. 1975;2:285–293. doi: 10.1017/s0317167100020382. [DOI] [PubMed] [Google Scholar]
- Lee RG, Tatton WG. Long latency reflexes to imposed displacements of the human wrist:dependence on duration of movement. Exp Brain Res. 1982;45:207–216. doi: 10.1007/BF00235780. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Tatton WG, Tasker RR. The effect of cortical lesions on the electromyographic response to joint displacement in the squirrel monkey forelimb. J Neurosci. 1983;3:795–805. doi: 10.1523/JNEUROSCI.03-04-00795.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GN, Polych MA, Byblow WD. Proposed cortical and sub-cortical contributions to the long-latency stretch reflex in the forearm. Exp Brain Res. 2004;156:72–79. doi: 10.1007/s00221-003-1767-z. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Perreault EJ, MacKinnon CD. The influence of perturbation duration and velocity on the long-latency response to stretch in the biceps muscle. Exp Brain Res. 2005;163:361–369. doi: 10.1007/s00221-004-2182-9. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Rothwell JC. Time-varying changes in corticospinal excitability accompanying the triphasic EMG pattern in humans. J Physiol. 2000;528:633–645. doi: 10.1111/j.1469-7793.2000.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon CD, Verrier MC, Tatton WG. Motor cortical potentials precede long-latency EMG activity evoked by imposed displacements of the human wrist. Exp Brain Res. 2000;131:477–490. doi: 10.1007/s002219900317. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Is the human stretch reflex cortical rather than spinal? Lancet. 1973;i:759–761. doi: 10.1016/s0140-6736(73)92141-7. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB, Adam J. The effect of lesions in of the sensory motor cortex and capsular pathways on servo-responses from the human long thumb flexor. Brain. 1977;100:503–526. doi: 10.1093/brain/100.3.503. [DOI] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Evidence that a long latency stretch reflex in humans is transcortical. J Physiol. 1992;449:429–440. doi: 10.1113/jphysiol.1992.sp019094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Brasil-Neto JP, Cohen LG, Hallett M. Effects of focal transcranial magnetic stimulation on simple reaction time to acoustic, visual and somatosensory stimuli. Brain. 1992;115:1045–1059. doi: 10.1093/brain/115.4.1045. [DOI] [PubMed] [Google Scholar]
- Petersen N, Christensen LOD, Morita H, Sinkjaer T, Nielsen J. Evidence that a transcortical pathway contributes to stretch reflexes in the tibialis anterior muscle in man. J Physiol. 1998;512:267–276. doi: 10.1111/j.1469-7793.1998.267bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliakov AV, Miles TS. Stretch reflexes in human masseter. J Physiol. 1994;476:323–331. doi: 10.1113/jphysiol.1994.sp020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C, Ashby P. Inhibition in the human motor cortex is reduced just before a voluntary contraction. Neurology. 1999;53:730–735. doi: 10.1212/wnl.53.4.730. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Marsden CD. Influence of voluntary intent on the human long-latency stretch reflex. Nature. 1980;286:496–498. doi: 10.1038/286496a0. [DOI] [PubMed] [Google Scholar]
- Thilmann AF, Schwarz M, Topper R, Fellows SJ, Noth J. Different mechanisms underlie the long-latency stretch reflex response of active human muscle at different joints. J Physiol. 1991;444:631–643. doi: 10.1113/jphysiol.1991.sp018898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Rothwell JC. Long lasting effects of rTMS and associated peripheral sensory input on MEPs, SEPs and transcortical reflex excitability in humans. J Physiol. 2002;540:367–376. doi: 10.1113/jphysiol.2001.013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton A, Lemon RN. The contribution of fast corticospinal input to the voluntary activation of proximal muscles in normal subjects and in stroke patients. Exp Brain Res. 1999;129:559–572. doi: 10.1007/s002210050926. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Rothwell JC, Goulart F, Cossu G, Munoz E. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol. 1999;516:931–938. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]