Abstract
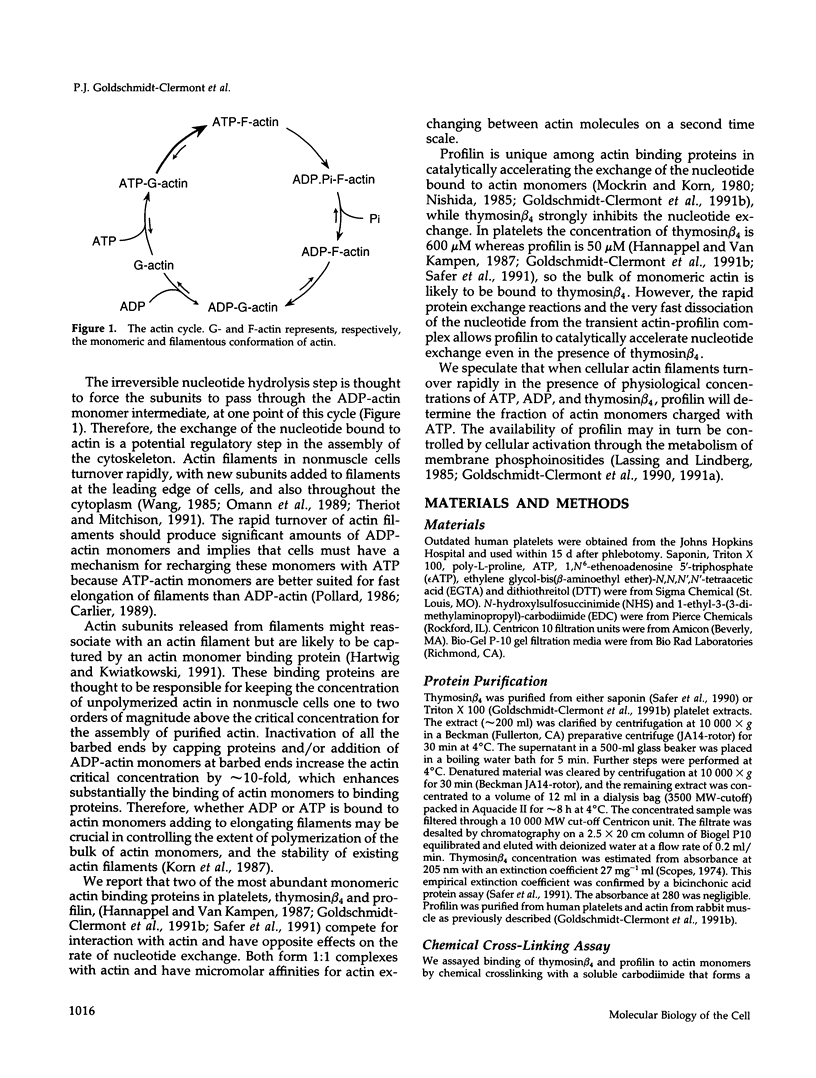
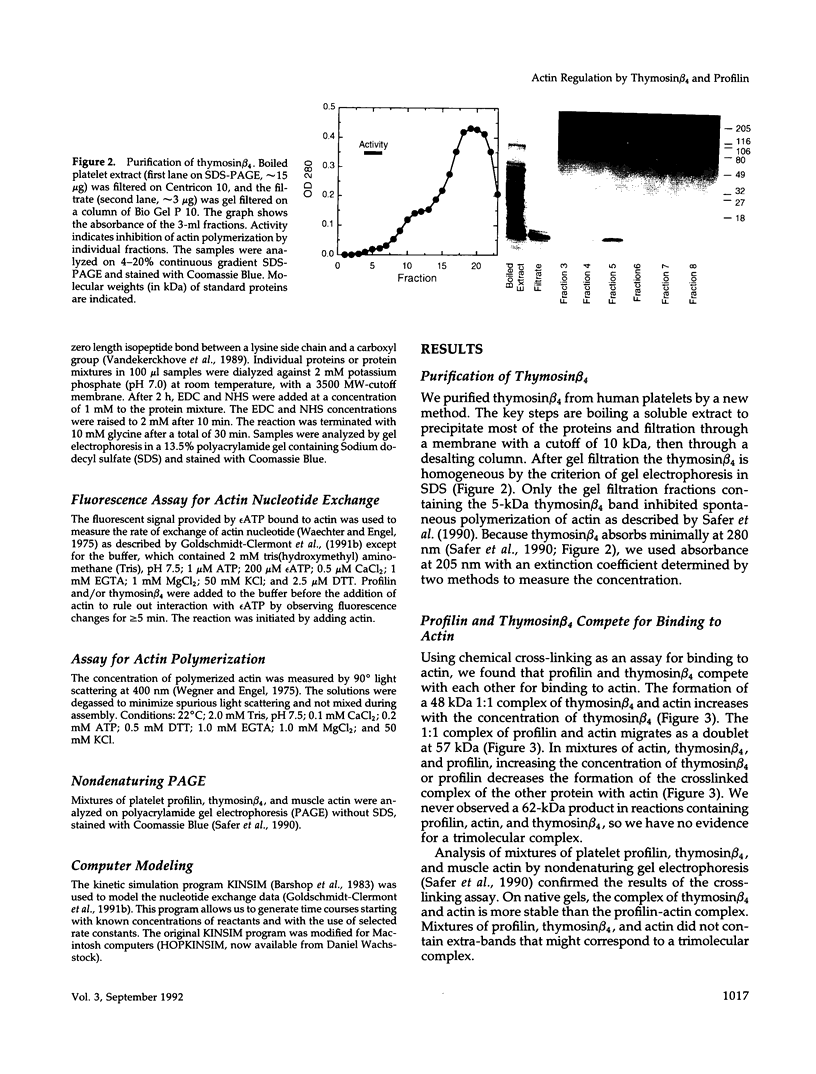
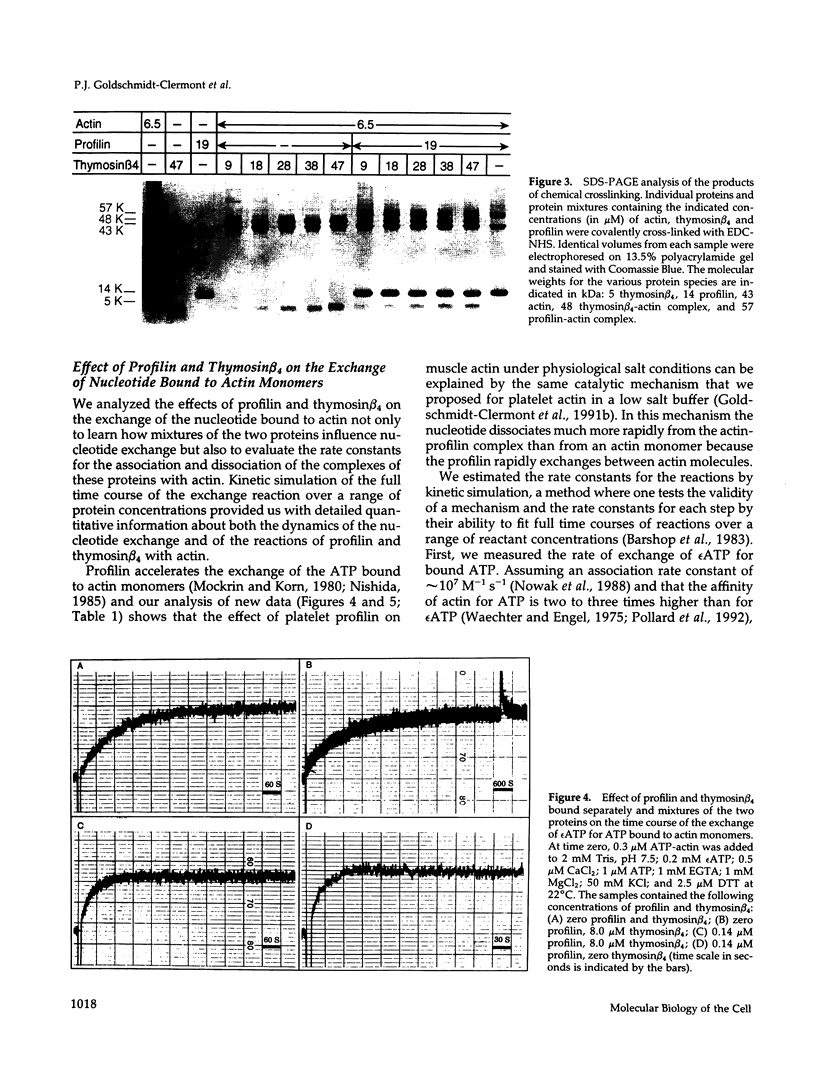
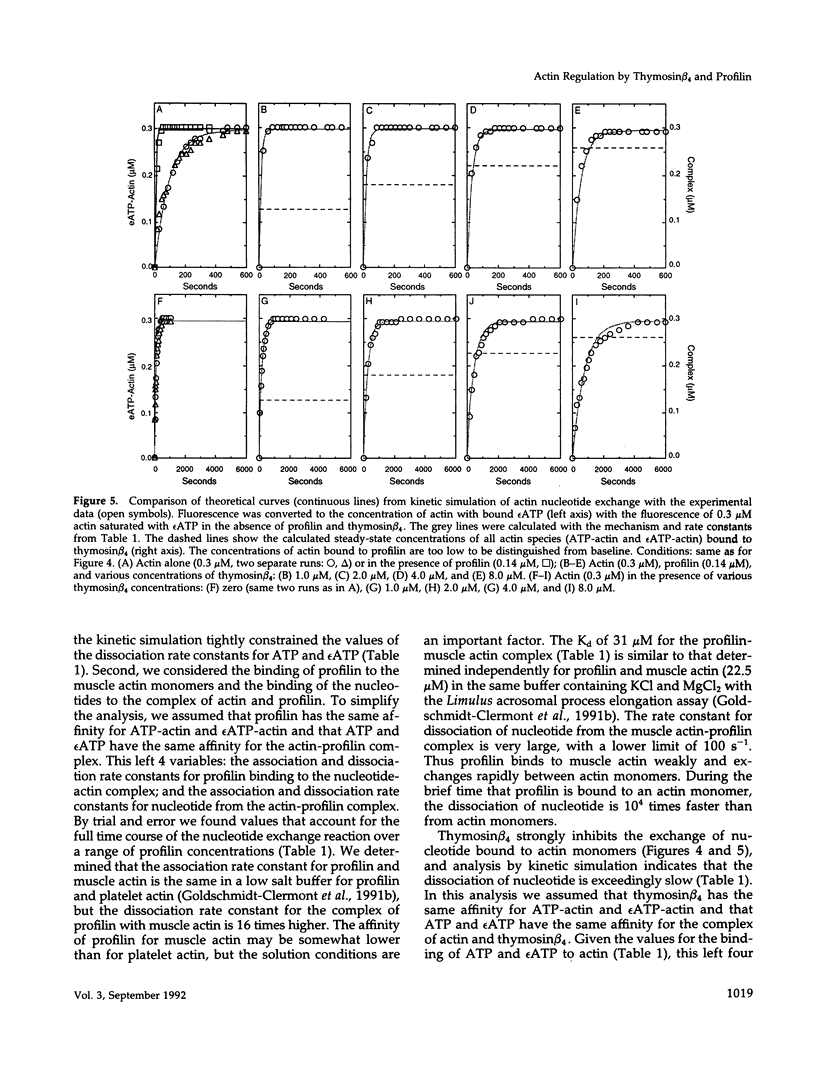
We present evidence for a new mechanism by which two major actin monomer binding proteins, thymosin beta 4 and profilin, may control the rate and the extent of actin polymerization in cells. Both proteins bind actin monomers transiently with a stoichiometry of 1:1. When bound to actin, thymosin beta 4 strongly inhibits the exchange of the nucleotide bound to actin by blocking its dissociation, while profilin catalytically promotes nucleotide exchange. Because both proteins exchange rapidly between actin molecules, low concentrations of profilin can overcome the inhibitory effects of high concentrations of thymosin beta 4 on the nucleotide exchange. These reactions may allow variations in profilin concentration (which may be regulated by membrane polyphosphoinositide metabolism) to control the ratio of ATP-actin to ADP-actin. Because ATP-actin subunits polymerize more readily than ADP-actin subunits, this ratio may play a key regulatory role in the assembly of cellular actin structures, particularly under circumstances of rapid filament turnover.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barshop B. A., Wrenn R. F., Frieden C. Analysis of numerical methods for computer simulation of kinetic processes: development of KINSIM--a flexible, portable system. Anal Biochem. 1983 Apr 1;130(1):134–145. doi: 10.1016/0003-2697(83)90660-7. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Pantaloni D., Evans J. A., Lambooy P. K., Korn E. D., Webb M. R. The hydrolysis of ATP that accompanies actin polymerization is essentially irreversible. FEBS Lett. 1988 Aug 1;235(1-2):211–214. doi: 10.1016/0014-5793(88)81264-x. [DOI] [PubMed] [Google Scholar]
- Carlier M. F. Role of nucleotide hydrolysis in the dynamics of actin filaments and microtubules. Int Rev Cytol. 1989;115:139–170. doi: 10.1016/s0074-7696(08)60629-4. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Nyström L. E., Sundkvist I., Markey F., Lindberg U. Actin polymerizability is influenced by profilin, a low molecular weight protein in non-muscle cells. J Mol Biol. 1977 Sep 25;115(3):465–483. doi: 10.1016/0022-2836(77)90166-8. [DOI] [PubMed] [Google Scholar]
- Cooley L., Verheyen E., Ayers K. chickadee encodes a profilin required for intercellular cytoplasm transport during Drosophila oogenesis. Cell. 1992 Apr 3;69(1):173–184. doi: 10.1016/0092-8674(92)90128-y. [DOI] [PubMed] [Google Scholar]
- Cooper J. A. The role of actin polymerization in cell motility. Annu Rev Physiol. 1991;53:585–605. doi: 10.1146/annurev.ph.53.030191.003101. [DOI] [PubMed] [Google Scholar]
- Daniel J. L., Molish I. R., Robkin L., Holmsen H. Nucleotide exchange between cytosolic ATP and F-actin-bound ADP may be a major energy-utilizing process in unstimulated platelets. Eur J Biochem. 1986 May 2;156(3):677–684. doi: 10.1111/j.1432-1033.1986.tb09631.x. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Parada L. F., Weinberg R. A. Specific growth response of ras-transformed embryo fibroblasts to tumour promoters. Nature. 1985 Dec 5;318(6045):472–475. doi: 10.1038/318472a0. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Janmey P. A. Profilin, a weak CAP for actin and RAS. Cell. 1991 Aug 9;66(3):419–421. doi: 10.1016/0092-8674(81)90002-7. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Kim J. W., Machesky L. M., Rhee S. G., Pollard T. D. Regulation of phospholipase C-gamma 1 by profilin and tyrosine phosphorylation. Science. 1991 Mar 8;251(4998):1231–1233. doi: 10.1126/science.1848725. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Machesky L. M., Baldassare J. J., Pollard T. D. The actin-binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science. 1990 Mar 30;247(4950):1575–1578. doi: 10.1126/science.2157283. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Machesky L. M., Doberstein S. K., Pollard T. D. Mechanism of the interaction of human platelet profilin with actin. J Cell Biol. 1991 Jun;113(5):1081–1089. doi: 10.1083/jcb.113.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondo H., Kudo J., White J. W., Barr C., Selvanayagam P., Saunders G. F. Differential expression of the human thymosin-beta 4 gene in lymphocytes, macrophages, and granulocytes. J Immunol. 1987 Dec 1;139(11):3840–3848. [PubMed] [Google Scholar]
- Haarer B. K., Lillie S. H., Adams A. E., Magdolen V., Bandlow W., Brown S. S. Purification of profilin from Saccharomyces cerevisiae and analysis of profilin-deficient cells. J Cell Biol. 1990 Jan;110(1):105–114. doi: 10.1083/jcb.110.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannappel E., van Kampen M. Determination of thymosin beta 4 in human blood cells and serum. J Chromatogr. 1987 Jun 26;397:279–285. doi: 10.1016/s0021-9673(01)85010-x. [DOI] [PubMed] [Google Scholar]
- Hartwig J. H., Chambers K. A., Hopcia K. L., Kwiatkowski D. J. Association of profilin with filament-free regions of human leukocyte and platelet membranes and reversible membrane binding during platelet activation. J Cell Biol. 1989 Oct;109(4 Pt 1):1571–1579. doi: 10.1083/jcb.109.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H., Kwiatkowski D. J. Actin-binding proteins. Curr Opin Cell Biol. 1991 Feb;3(1):87–97. doi: 10.1016/0955-0674(91)90170-4. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Hvidt S., Lamb J., Stossel T. P. Resemblance of actin-binding protein/actin gels to covalently crosslinked networks. Nature. 1990 May 3;345(6270):89–92. doi: 10.1038/345089a0. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Korn E. D., Carlier M. F., Pantaloni D. Actin polymerization and ATP hydrolysis. Science. 1987 Oct 30;238(4827):638–644. doi: 10.1126/science.3672117. [DOI] [PubMed] [Google Scholar]
- Machesky L. M., Goldschmidt-Clermont P. J., Pollard T. D. The affinities of human platelet and Acanthamoeba profilin isoforms for polyphosphoinositides account for their relative abilities to inhibit phospholipase C. Cell Regul. 1990 Nov;1(12):937–950. doi: 10.1091/mbc.1.12.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciver S. K., Wachsstock D. H., Schwarz W. H., Pollard T. D. The actin filament severing protein actophorin promotes the formation of rigid bundles of actin filaments crosslinked with alpha-actinin. J Cell Biol. 1991 Dec;115(6):1621–1628. doi: 10.1083/jcb.115.6.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannherz H. G., Goody R. S., Konrad M., Nowak E. The interaction of bovine pancreatic deoxyribonuclease I and skeletal muscle actin. Eur J Biochem. 1980 Mar;104(2):367–379. doi: 10.1111/j.1432-1033.1980.tb04437.x. [DOI] [PubMed] [Google Scholar]
- Mockrin S. C., Korn E. D. Acanthamoeba profilin interacts with G-actin to increase the rate of exchange of actin-bound adenosine 5'-triphosphate. Biochemistry. 1980 Nov 11;19(23):5359–5362. doi: 10.1021/bi00564a033. [DOI] [PubMed] [Google Scholar]
- Neidl C., Engel J. Exchange of ADP, ATP and 1: N6-ethenoadenosine 5'-triphosphate at G-actin. Equilibrium and kinetics. Eur J Biochem. 1979 Nov 1;101(1):163–169. doi: 10.1111/j.1432-1033.1979.tb04228.x. [DOI] [PubMed] [Google Scholar]
- Nishida E. Opposite effects of cofilin and profilin from porcine brain on rate of exchange of actin-bound adenosine 5'-triphosphate. Biochemistry. 1985 Feb 26;24(5):1160–1164. doi: 10.1021/bi00326a015. [DOI] [PubMed] [Google Scholar]
- Nowak E., Strzelecka-Golaszewska H., Goody R. S. Kinetics of nucleotide and metal ion interaction with G-actin. Biochemistry. 1988 Mar 8;27(5):1785–1792. doi: 10.1021/bi00405a060. [DOI] [PubMed] [Google Scholar]
- Omann G. M., Porasik M. M., Sklar L. A. Oscillating actin polymerization/depolymerization responses in human polymorphonuclear leukocytes. J Biol Chem. 1989 Oct 5;264(28):16355–16358. [PubMed] [Google Scholar]
- Pantaloni D., Carlier M. F., Coué M., Lal A. A., Brenner S. L., Korn E. D. The critical concentration of actin in the presence of ATP increases with the number concentration of filaments and approaches the critical concentration of actin.ADP. J Biol Chem. 1984 May 25;259(10):6274–6283. [PubMed] [Google Scholar]
- Pollard T. D. Actin. Curr Opin Cell Biol. 1990 Feb;2(1):33–40. doi: 10.1016/s0955-0674(05)80028-6. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Quantitative analysis of the effect of Acanthamoeba profilin on actin filament nucleation and elongation. Biochemistry. 1984 Dec 18;23(26):6631–6641. doi: 10.1021/bi00321a054. [DOI] [PubMed] [Google Scholar]
- Pollard T. D. Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J Cell Biol. 1986 Dec;103(6 Pt 2):2747–2754. doi: 10.1083/jcb.103.6.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pring M., Weber A., Bubb M. R. Profilin-actin complexes directly elongate actin filaments at the barbed end. Biochemistry. 1992 Feb 18;31(6):1827–1836. doi: 10.1021/bi00121a035. [DOI] [PubMed] [Google Scholar]
- Safer D., Elzinga M., Nachmias V. T. Thymosin beta 4 and Fx, an actin-sequestering peptide, are indistinguishable. J Biol Chem. 1991 Mar 5;266(7):4029–4032. [PubMed] [Google Scholar]
- Safer D., Golla R., Nachmias V. T. Isolation of a 5-kilodalton actin-sequestering peptide from human blood platelets. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2536–2540. doi: 10.1073/pnas.87.7.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöbitz B., Netzker R., Hannappel E., Brand K. Rapid induction of thymosin beta 4 in concanavalin A-stimulated thymocytes by translational control. J Biol Chem. 1990 Sep 15;265(26):15387–15391. [PubMed] [Google Scholar]
- Scopes R. K. Measurement of protein by spectrophotometry at 205 nm. Anal Biochem. 1974 May;59(1):277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]
- Shariff A., Luna E. J. Diacylglycerol-stimulated formation of actin nucleation sites at plasma membranes. Science. 1992 Apr 10;256(5054):245–247. doi: 10.1126/science.1373523. [DOI] [PubMed] [Google Scholar]
- Shimamura R., Kudo J., Kondo H., Dohmen K., Gondo H., Okamura S., Ishibashi H., Niho Y. Expression of the thymosin beta 4 gene during differentiation of hematopoietic cells. Blood. 1990 Sep 1;76(5):977–984. [PubMed] [Google Scholar]
- Simon M. A., Bowtell D. D., Dodson G. S., Laverty T. R., Rubin G. M. Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell. 1991 Nov 15;67(4):701–716. doi: 10.1016/0092-8674(91)90065-7. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. From signal to pseudopod. How cells control cytoplasmic actin assembly. J Biol Chem. 1989 Nov 5;264(31):18261–18264. [PubMed] [Google Scholar]
- Theriot J. A., Mitchison T. J. Actin microfilament dynamics in locomoting cells. Nature. 1991 Jul 11;352(6331):126–131. doi: 10.1038/352126a0. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Bonder E. M., Coluccio L. M., Mooseker M. S. Actin from Thyone sperm assembles on only one end of an actin filament: a behavior regulated by profilin. J Cell Biol. 1983 Jul;97(1):112–124. doi: 10.1083/jcb.97.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J. S., Kaiser D. A., Pollard T. D. Acanthamoeba actin and profilin can be cross-linked between glutamic acid 364 of actin and lysine 115 of profilin. J Cell Biol. 1989 Aug;109(2):619–626. doi: 10.1083/jcb.109.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter F., Engel J. The kinetics of the exchange of G-actin-bound 1: N6-ethenoadenosine 5'-triphosphate with ATP as followed by fluorescence. Eur J Biochem. 1975 Sep 15;57(2):453–459. doi: 10.1111/j.1432-1033.1975.tb02320.x. [DOI] [PubMed] [Google Scholar]
- Wang Y. L. Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling. J Cell Biol. 1985 Aug;101(2):597–602. doi: 10.1083/jcb.101.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner A., Engel J. Kinetics of the cooperative association of actin to actin filaments. Biophys Chem. 1975 Jul;3(3):215–225. doi: 10.1016/0301-4622(75)80013-5. [DOI] [PubMed] [Google Scholar]
- Wegner A. Head to tail polymerization of actin. J Mol Biol. 1976 Nov;108(1):139–150. doi: 10.1016/s0022-2836(76)80100-3. [DOI] [PubMed] [Google Scholar]
- Wegner A., Isenberg G. 12-fold difference between the critical monomer concentrations of the two ends of actin filaments in physiological salt conditions. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4922–4925. doi: 10.1073/pnas.80.16.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]




