Abstract
Total pancreatectomy (TP) was abandoned by many surgeons because of its lack of benefits and other major drawbacks. The potential benefits of TP, including its oncological as well as its technical advantages, did not prove to be valid. Problems associated with insulin-deprived diabetes mellitus and high perioperative morbidity and mortality rates were not easily manageable. However, in the new era of pancreatic surgery, new indications for TP have been defined. These have been paralleled by improvements in surgical technique, multidisciplinary management and postoperative intensive care. These factors have transformed TP into a safe and reasonable surgical procedure with excellent perioperative morbidity and mortality, as well as good longterm outcome. We review the indications for TP and describe our operative technique in detail.
Keywords: total pancreatectomy, pancreatic cancer, mortality, morbidity, operative technique
Introduction
Pancreatic surgery remains a challenging procedure which may involve several organs and can require vascular reconstruction. Its complexity is underlined by the value of centralizing pancreatic surgery to high-volume hospitals.1–7 Total pancreatectomy (TP) for pancreatic cancer was first reported by Rockey in 1943.8 Subsequently, it was considered that partial pancreatectomy (PP) would help to circumvent pancreatic fistula.9 Because of high tumour recurrence rates after Kausch–Whipple procedures, any suggestion of possible tumour multicentricity supported a role for TP as a means of achieving R0 resection. Subsequent studies demonstrated no improvement in postoperative outcome and major metabolic problems were found to occur.10–12 These were difficult to address and the procedure fell out of favour. However, recent studies have demonstrated progress regarding postoperative outcomes of TP.13,14 In addition, new pancreatic tumour entities have been identified in the past decade and these require total rather than partial pancreatectomy.
Indications
As operative techniques and peri- and postoperative management strategies have evolved, the indications for TP in pancreatic cancer changed. Indications for TP, as perceived by ourselves and others,15–19 are described below.
The R0 resection
As many patients suffer from local recurrence, tumour resection with negative margins constitutes an important prognostic indicator.20–23 In patients undergoing PP for pancreatic cancer, TP improves survival in isolated neck margin-positive patients and is associated with survival benefit. Because peri- and postoperative complication rates are comparable with those for PP, TP is recommended in cases in which cancer has spread to the left part of the pancreas.24
Pancreatic anastomosis that is likely to leak
Pancreatic fistula (PF) is a major complication of PP; however, its incidence in high-volume centres is reported to be very low, ranging between 2% and 5%.18,25–27 Even if PF becomes evident, up to 85% of patients with PF can be managed by conservative or interventional methods.28 However, PF can lead to sepsis and, via the erosion of vessels, to life-threatening haemorrhage. Such complications and their accompanying sequelae may not respond to conservative treatment and surgical intervention (completion pancreatectomy) may be necessary. Completion pancreatectomy in this critically ill patient population is accompanied by an unfavourable mortality rate of up to 39%.14
Although there is no consensus on the technique for safe pancreatic anastomosis,25,29 the prognostic factors for pancreatic anastomosis failure are well known30 and include dilatation of the pancreatic duct,31,32 texture of the pancreatic tissue,33 surgical technique (traumatic [high blood loss] vs. meticulous and tissue-sparing),34 and extent of resection (multivisceral vs. standard radical).32 In technically challenging intraoperative situations, these prognostic factors may help the surgical team to decide whether a TP should be performed to avoid a likely PF. In certain clinical situations it is desirable to prevent any need for emergency completion pancreatectomy and, instead, to perform an elective TP when multiple unfavourable prognostic factors for pancreatic anastomosis exist.
Hereditary pancreatic cancer
Hereditary pancreatic cancer (HPC) is a rare genetic disease caused by mutations of the trypsinogen cationic gene on chromosome 7.35 In a recent report, Rebours et al. evaluated 78 families (200 patients) with HPC in an exhaustive national series and found their relative risk for pancreatic cancer to be 87 times higher than that in the general population.36 It has been suggested that surveillance be performed for the early detection of cancer or intraepithelial neoplasia in these high-risk individuals. Patients with two or more first-degree relatives with pancreatic cancer, one first-degree relative with cancer diagnosed before the age of 50 years, or two or more second-degree relatives with cancer, one of whom was diagnosed before the age of 50 years, are considered to be at high-risk.33 Patients for resection should be carefully selected. In high-risk patients, evidence of lesions suspected of being dysplastic, diagnosed by contrast-enhanced computed tomography, endoscopic retrograde cholangiopancreatograpy (ERCP), ultrasonography or endoscopic ultrasonography, may help to determine the timing of surgery.37 The key is to diagnose dysplastic lesions before they develop into invasive cancer. It may be reasonable to initiate surveillance when the subject is 5–10 years younger than the age at which his or her youngest affected relative was diagnosed with the onset of pancreatic cancer, or at the onset of symptoms such as weight loss, pain or development of diabetes. In patients with widespread and multifocal pancreatic intraepithelial neoplasia (PanIN) changes throughout the pancreas, prophylactic TP should be considered to avert the development of pancreatic carcinoma.38
Intraductal papillary mucinous neoplasm (IPMN)
The definition of intraductal papillary mucinous neoplasm (IPMN) was introduced by the World Health Organization in 1996 to describe a potentially malignant disease. These neoplasms are connected to the ductal system and can be subclassified into main and branch duct types and a mixed type that contains elements of both. Classification can be made based on ERCP, imaging studies or histology.39 The natural history of IPMNs and their current management have been reviewed in detail recently by Bassi et al.40 and Wente et al.41 Early changes are considered to be premalignant. The recurrence rate of invasive IPMNs in the published literature is 25–100%.42–45 However, recurrences of non-invasive IPMNs are rare. Based on the available literature, main duct IPMNs should be resected whenever possible because of the high prevalence of malignancy. By contrast, asymptomatic branch duct IPMNs with no mural nodules and which are <3 cm in diameter may be followed by cross-sectional imaging,46 although this strategy is under dispute because up to 20% of IPMNs become malignant within 8 years. During PP for main duct disease, TP should be performed to ensure R0 resection if severe dysplasia or invasive cancer is evident at the resection margin. In the surgical treatment of multicentric branch duct disease, TP should be performed in the first place.
Surgical technique
A single administration of antibiotics is given just before the onset of the surgical procedure and repeated if the duration of surgery exceeds 4 h. The abdomen can be opened by a midline or bilateral subcostal incision. Exposure must be adequate and laparotomy includes inspection of the liver and peritoneum. A careful search for lymph nodes is made in the mesenteric root, hepatoduodenal ligament and common hepatic artery. Palpation of the head and the uncinate process of the pancreas in relation to the mesenteric vessels and, particularly, the region of duodenojejunal flexure can demonstrate signs of non-resectability. A biopsy of suspicious areas is taken for intraoperative frozen section histology.
If no contraindication for resection is found, the hepatic and splenic flexures of the colon are mobilized. A wide ‘Kocher manoeuvre’ of the duodenum and head of the pancreas is undertaken to provide exposure. To allow complete access to the anterior surface of the pancreas, the lesser sac is opened, either by dividing the gastrocolic omentum or by separating the greater omentum from the transverse colon. After visualization of the pancreas, the lower border is mobilized by separating the adhesions to the transverse colon mesentery. The superior mesenteric vein is identified, dissected and followed to its passage behind the pancreas. Here the surgeon is able to evaluate for vessel infiltration. In cases of portal or superior mesenteric vein involvement, it has been demonstrated that in resection with curative intent, vascular resection can be performed without increased morbidity and mortality.47–51 We have previously described in detail the technique of portal vein resection in locally advanced pancreatic cancer.52 Above the pancreas, the dissection of the vessels supplying the liver is performed with critical assessment of vascular involvement. Care should be taken if a displaced or accessory right hepatic artery is present. If resectability is questionable, complete dissection and exploration of the common and proper hepatic artery (PHA) is necessary.
If no contraindication for resection is evident, a cholecystectomy is performed, and the cystic artery divided. A complete lymphadenectomy of the hepatoduodenal ligament is performed at the time when the common bile duct is divided proximal to the cystic duct. During dissection of the hepatoduodenal ligament, the left, right and proper hepatic arteries are identified and exposed. Even in pylorus-preserving pancreatectomy, we divide the right gastric artery and complete the lymphadenectomy until the gastroduodenal artery is reached. To assess whether a further reconstruction is required for a coeliac trunk stenosis, we occlude the gastroduodenal artery before dividing it and check the pulse on the PHA. If a sufficient flow is present, the gastroduodenal artery is divided and the lymphadenectomy of the common hepatic artery and coeliac trunk towards the right is completed.
The complete dissection and lymphadenectomy of the hepatoduodenal ligament lead to clear exposure of the portal vein, which can be followed until it disappears behind the upper border of the pancreas, as indicated in Fig. 1. The posterior superior pancreaticoduodenal vein, which ends on the upper border of the pancreas from the right side into the portal vein, is divided securely. In the case of a negative proximal duodenal resection margin, and no evidence of tumour infiltration of the peripyloric lymph nodes, a pylorus-preserving procedure is performed. Therefore, the right gastroepiploic artery is divided. We try to preserve the coronary vein (left gastric vein) if it is a branch of the main portal vein. After resection of the splenic vein, the coronary vein may ensure sufficient venous drainage of the stomach. If venous congestion of the stomach occurs, a subtotal gastrectomy may be necessary. Approximately 4 cm beyond the pylorus, the duodenum is divided by a linear stapling device. To improve access to the pancreas, the stomach is displaced towards the left upper abdomen. Then, the ascending part of the duodenum is divided by a linear stapler. Preservation of the spleen is considered whenever possible. However, if splenectomy is necessary to ensure radicality, the splenorenal ligament is divided and the splenic vein and artery are ligated. Whereas the splenic artery can be located along the upper border of the pancreas, the splenic vein can easily be identified behind the pancreas (Figs 2, 3A, B). After division of the short gastric vessels and the inferior mesenteric vein, en bloc resection is performed together with the pancreas, duodenum and the peripancreatic lymph nodes (Fig. 4). For this purpose, the plane of dissection lies first towards the superior mesenteric artery (SMA). The artery is followed cranially until its origin at the aorta, resecting lymph nodes anterior to the aorta, between the coeliac artery and SMA, as well as those to the left of the SMA. As the dissection continues to the left, the adrenal vein and the adrenal gland become part of the posterior plane, and the renal vein becomes the inferior border of dissection. Further laterally, dissection is continued to the Gerota's fascia of the superior half of the kidney (Fig. 5).
Figure 1.
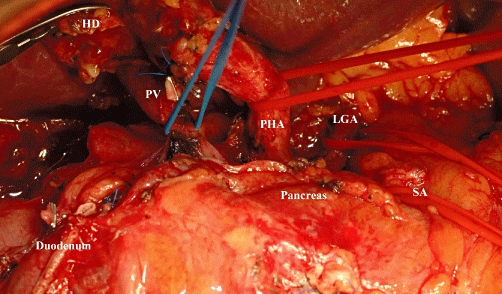
Intraoperative image after complete dissection of the hepatoduodenal ligament and coeliac trunk. The duodenum is divided and the stomach is displaced cranially to the left. HD, hepatic duct; PV, portal vein; PHA, proper hepatic artery; LGA, left gastric artery; SA, splenic artery
Figure 2.
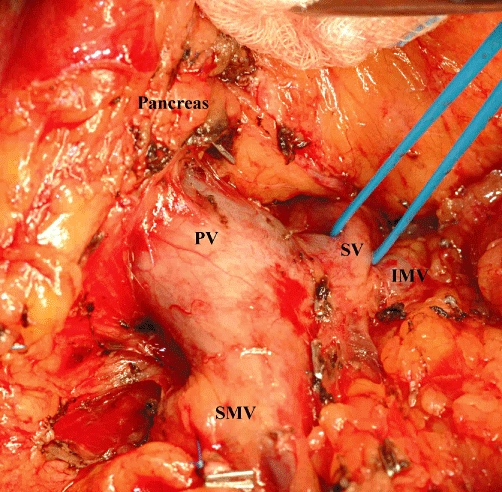
Intraoperative image demonstrating the mobilization of the body of the pancreas. PV, portal vein; SMV, superior mesenteric vein; SV, splenic vein; IMV, inferior mesenteric vein
Figure 3.
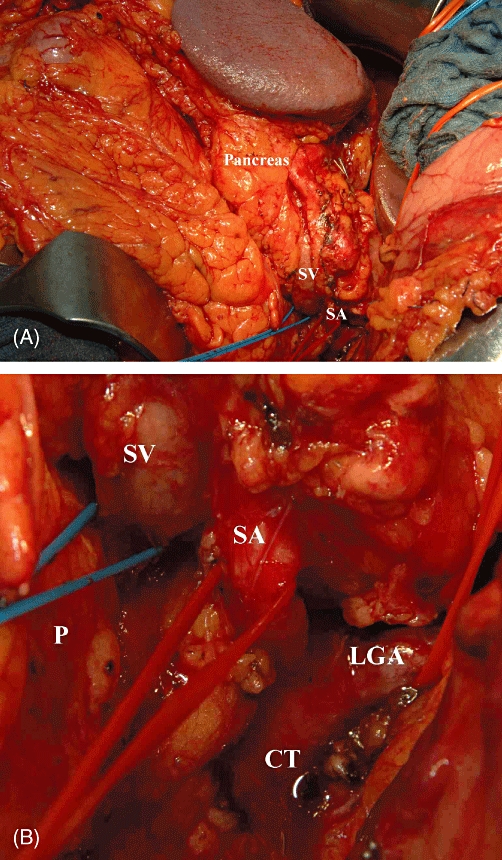
(A) Intraoperative image demonstrating the complete mobilization of the pancreas and spleen. Note that both are displaced to the right, exposing the splenic vessels and the coeliac trunk. (B) Magnification of (A). SV, splenic vein; SA, splenic artery; CT, coeliac trunk; P, pancreas; LGA, left gastric artery
Figure 4.
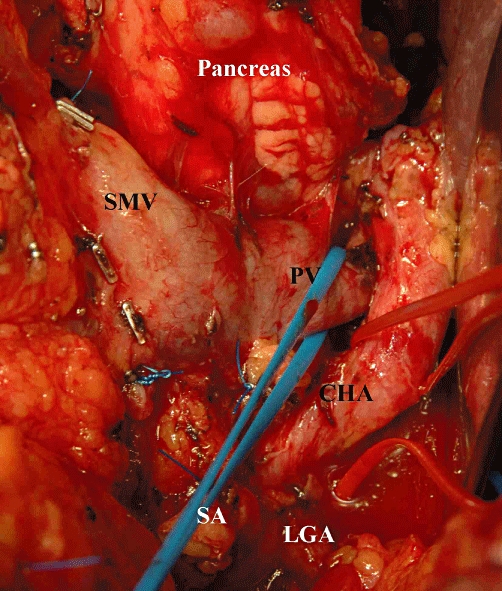
Intraoperative image demonstrating the dissection along the portal vein. Note that the splenic artery is divided and the pancreas is displaced to the left. PV, portal vein; CHA, common hepatic artery; LGA, left gastric artery; SA, splenic artery; SMV, superior mesenteric artery
Figure 5.
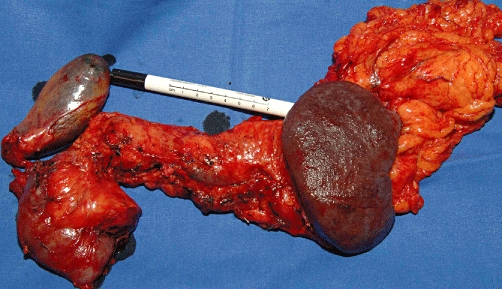
En bloc resection including gall bladder, pancreas, duodenum, spleen and part of the greater omentum
For reconstruction after TP a suitable jejunal loop is prepared. Small bowel is displaced to the right and the transverse colon cranially, leading to exposure of the duodenojejunal flexure. The ligament of Treitz is divided and the proximal 10–15 cm of the jejunum is dissected and resected. The second jejunal loop is carefully dissected and left with a suitable vascular arcade. To prevent an internal hernia, the space behind the superior mesenteric vessels is closed by suture. The prepared jejunal loop is then transposed retrocolically through the transverse mesocolon to perform the end-to-side hepaticojejunostomy at a minimal opening on the antimesenteric side from the jejunum by a single-layer technique (Fig. 6). We fix the mucosa of the jejunum to the circular opening with 6-0 PDS® single stitches and perform the anastomosis with 5–0 PDS® (with a c1 needle). Although adequate mobilization of the bile duct is necessary to ensure a tension-free anastomosis, pericholangic tissue must be preserved as much as possible to maintain optimal blood supply. To finalize the reconstruction, 50 cm distal to the hepatojejunostomy, we perform an antecolic,53 end-to-side duodenojejunal anastomosis in a two-layer continuous fashion. The opening in the transverse mesocolon is closed by sutures and a drain is placed close to the hepaticojejunostomy.
Figure 6.
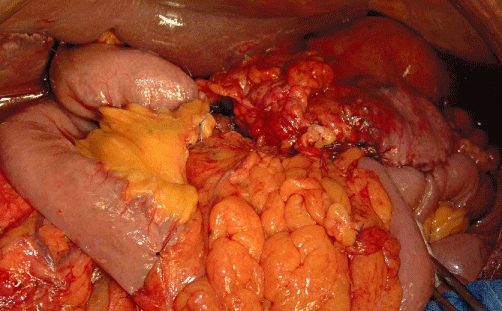
Intraoperative image demonstrating the reconstruction after total pancreatectomy
Results
We performed 147 TP procedures between October 2001 and November 2006.14 Of the subjects, 114 suffered from malignant pancreatic disease. In patients undergoing elective TP, hospital mortality was < 5% and morbidity < 40%. Median survival was 21.9 months, and 1- and 3-year survival rates were 64.3% and 36.6%, respectively. A matched-pairs analysis was performed between TP and PP patients to assess quality of life (QoL). Total pancreatectomy patients had worse values in some parameters concerning function and symptom scales compared with PP patients. However, their global health status was no different and overall QoL was acceptable. This is supported by a recent QoL analysis which concluded that TP patients have a QoL similar to that of patients with diabetes mellitus from other causes.54
Conclusions
Recent reports have demonstrated that modern treatment regimens, interdisciplinary management and improved postoperative care in TP patients result in longterm survival, QoL, mortality and morbidity rates comparable with those of PP patients.13,14,24 Total resection of the pancreas can lead to endocrine and exocrine insufficiency with severe metabolic consequences, such as difficult glycaemic control, malabsorption, steatohepatitis and liver failure. However, these issues, like the term ‘brittle diabetes’, which describes the unfavourable combination of insulin sensitivity and hypoglycaemic unawareness and dates back to the 1930s,55 are outdated and have been challenged. As recent data emphasize that the longterm survival of patients undergoing TP is dependent on the biology of the underlying cancer rather than on physiological changes caused by the apancreatic state, this procedure should no longer be avoided in stringently selected patients with appropriate indications.
Conflicts of interest
None declared.
References
- 1.Gordon TA, Bowman HM, Tielsch JM, Bass EB, Burleyson GP, Cameron JL. Statewide regionalization of pancreaticoduodenectomy and its effect on in-hospital mortality. Ann Surg. 1998;228:71–78. doi: 10.1097/00000658-199807000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gouma DJ, van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, Busch OR, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232:786–795. doi: 10.1097/00000658-200012000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neoptolemos JP, Russell RC, Bramhall S, Theis B. Low mortality following resection for pancreatic and periampullary tumours in 1026 patients: UK survey of specialist pancreatic units. UK Pancreatic Cancer Group. Br J Surg. 1997;84:1370–1376. [PubMed] [Google Scholar]
- 4.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 5.Weitz J, Koch M, Friess H, Buchler MW. Impact of volume and specialization for cancer surgery. Dig Surg. 2004;21:253–261. doi: 10.1159/000080198. [DOI] [PubMed] [Google Scholar]
- 6.van Heek NT, Kuhlmann KF, Scholten RJ, de Castro SM, Busch OR, van Gulik TM, et al. Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005;242:781–788. doi: 10.1097/01.sla.0000188462.00249.36. discussion 788–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fong Y, Gonen M, Rubin D, Radzyner M, Brennan MF. Longterm survival is superior after resection for cancer in high-volume centres. Ann Surg. 2005;242:540–544. doi: 10.1097/01.sla.0000184190.20289.4b. discussion 544–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockey EW. Total pancreatectomy for carcinoma: case report. Ann Surg. 1943;118:603–611. doi: 10.1097/00000658-194310000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross DE. Cancer of the pancreas; a plea for total pancreatectomy. Am J Surg. 1954;87:20–33. doi: 10.1016/0002-9610(54)90038-0. [DOI] [PubMed] [Google Scholar]
- 10.Dresler CM, Fortner JG, McDermott K, Bajorunas DR. Metabolic consequences of (regional) total pancreatectomy. Ann Surg. 1991;214:131–140. doi: 10.1097/00000658-199108000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAfee MK, van Heerden JA, Adson MA. Is proximal pancreatoduodenectomy with pyloric preservation superior to total pancreatectomy? Surgery. 1989;105:347–351. [PubMed] [Google Scholar]
- 12.Grace PA, Pitt HA, Tompkins RK, DenBesten L, Longmire WP., Jr. Decreased morbidity and mortality after pancreatoduodenectomy. Am J Surg. 1986;151:141–149. doi: 10.1016/0002-9610(86)90024-3. [DOI] [PubMed] [Google Scholar]
- 13.Nathan H, Wolfgang CL, Edil BH, Choti MA, Herman JM, Schulick RD, et al. Perioperative mortality and longterm survival after total pancreatectomy for pancreatic adenocarcinoma: a population-based perspective. J Surg Oncol. 2009;99:87–92. doi: 10.1002/jso.21189. [DOI] [PubMed] [Google Scholar]
- 14.Muller MW, Friess H, Kleeff J, Dahmen R, Wagner M, Hinz U, et al. Is there still a role for total pancreatectomy? Ann Surg. 2007;246:966–974. doi: 10.1097/SLA.0b013e31815c2ca3. discussion 974–975. [DOI] [PubMed] [Google Scholar]
- 15.Reddy SK, Tyler DS, Pappas TN, Clary BM. Extended resection for pancreatic adenocarcinoma. Oncologist. 2007;12:654–663. doi: 10.1634/theoncologist.12-6-654. [DOI] [PubMed] [Google Scholar]
- 16.Liu SL, Friess H, Kleeff J, Ji ZL, Buchler MW. Surgical approaches for resection of pancreatic cancer: an overview. Hepatobiliary Pancreat Dis Int. 2002;1:118–125. [PubMed] [Google Scholar]
- 17.Wagner M, Z’Graggen K, Vagianos CE, Redaelli CA, Holzinger F, Sadowski C, et al. Pylorus-preserving total pancreatectomy. Early and late results. Dig Surg. 2001;18:188–195. doi: 10.1159/000050128. [DOI] [PubMed] [Google Scholar]
- 18.Buchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z’Graggen K. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. 2003;138:1310–1314. doi: 10.1001/archsurg.138.12.1310. discussion 1315. [DOI] [PubMed] [Google Scholar]
- 19.Heidt DG, Burant C, Simeone DM. Total pancreatectomy: indications, operative technique, and postoperative sequelae. J Gastrointest Surg. 2007;11:209–216. doi: 10.1007/s11605-006-0025-7. [DOI] [PubMed] [Google Scholar]
- 20.Hishinuma S, Ogata Y, Tomikawa M, Ozawa I, Hirabayashi K, Igarashi S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg. 2006;10:511–518. doi: 10.1016/j.gassur.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Hartel M, Wente MN, Di Sebastiano P, Friess H, Buchler MW. The role of extended resection in pancreatic adenocarcinoma: is there good evidence-based justification? Pancreatology. 2004;4:561–566. doi: 10.1159/000082181. [DOI] [PubMed] [Google Scholar]
- 22.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Buchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–594. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 23.Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt CM, Glant J, Winter JM, Kennard J, Dixon J, Zhao Q, et al. Total pancreatectomy (R0 resection) improves survival over subtotal pancreatectomy in isolated neck margin positive pancreatic adenocarcinoma. Surgery. 2007;142:572–578. doi: 10.1016/j.surg.2007.07.016. discussion 578–580. [DOI] [PubMed] [Google Scholar]
- 25.Z’Graggen K, Uhl W, Friess H, Buchler MW. How to do a safe pancreatic anastomosis. J Hepatobiliary Pancreat Surg. 2002;9:733–737. doi: 10.1007/s005340200101. [DOI] [PubMed] [Google Scholar]
- 26.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210–1211. [DOI] [PubMed] [Google Scholar]
- 27.Welsch T, Frommhold K, Hinz U, Weigand MA, Kleeff J, Friess H, et al. Persisting elevation of C-reactive protein after pancreatic resections can indicate developing inflammatory complications. Surgery. 2008;143:20–28. doi: 10.1016/j.surg.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Munoz-Bongrand N, Sauvanet A, Denys A, Sibert A, Vilgrain V, Belghiti J. Conservative management of pancreatic fistula after pancreaticoduodenectomy with pancreaticogastrostomy. J Am Coll Surg. 2004;199:198–203. doi: 10.1016/j.jamcollsurg.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Wente MN, Shrikhande SV, Muller MW, Diener MK, Seiler CM, Friess H, et al. Pancreaticojejunostomy versus pancreaticogastrostomy: systematic review and meta-analysis. Am J Surg. 2007;193:171–183. doi: 10.1016/j.amjsurg.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Buchler MW, Kleeff J, Friess H. Surgical treatment of pancreatic cancer. J Am Coll Surg. 2007;205(Suppl 81–86) doi: 10.1016/j.jamcollsurg.2007.06.332. [DOI] [PubMed] [Google Scholar]
- 31.van Berge Henegouwen MI, De Wit LT, Van Gulik TM, Obertop H, Gouma DJ. Incidence, risk factors, and treatment of pancreatic leakage after pancreaticoduodenectomy: drainage versus resection of the pancreatic remnant. J Am Coll Surg. 1997;185:18–24. doi: 10.1016/s1072-7515(97)00007-0. [DOI] [PubMed] [Google Scholar]
- 32.Muscari F, Suc B, Kirzin S, Hay JM, Fourtanier G, Fingerhut A, et al. Risk factors for mortality and intra-abdominal complications after pancreatoduodenectomy: multivariate analysis in 300 patients. Surgery. 2006;139:591–598. doi: 10.1016/j.surg.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Bartoli FG, Arnone GB, Ravera G, Bachi V. Pancreatic fistula and relative mortality in malignant disease after pancreaticoduodenectomy. Review and statistical meta-analysis regarding 15 years of literature. Anticancer Res. 1991;11:1831–1848. [PubMed] [Google Scholar]
- 34.Lin JW, Cameron JL, Yeo CJ, Riall TS, Lillemoe KD. Risk factors and outcomes in postpancreaticoduodenectomy pancreaticocutaneous fistula. J Gastrointest Surg. 2004;8:951–959. doi: 10.1016/j.gassur.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 35.Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 36.Rebours V, Boutron-Ruault MC, Schnee M, Ferec C, Maire F, Hammel P, et al. Risk of pancreatic adenocarcinoma in patients with hereditary pancreatitis: a national exhaustive series. Am J Gastroenterol. 2008;103:111–119. doi: 10.1111/j.1572-0241.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 37.Kekis PB, Friess H, Kleeff J, Buchler MW. Timing and extent of surgical intervention in patients from hereditary pancreatic cancer kindreds. Pancreatology. 2001;1:525–530. doi: 10.1159/000055855. [DOI] [PubMed] [Google Scholar]
- 38.Brentnall TA. Management strategies for patients with hereditary pancreatic cancer. Curr Treat Options Oncol. 2005;6:437–445. doi: 10.1007/s11864-005-0046-6. [DOI] [PubMed] [Google Scholar]
- 39.Furukawa T, Takahashi T, Kobari M, Matsuno S. The mucus-hypersecreting tumour of the pancreas. Development and extension visualized by three-dimensional computerized mapping. Cancer. 1992;70:1505–1513. doi: 10.1002/1097-0142(19920915)70:6<1505::aid-cncr2820700611>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 40.Bassi C, Sarr MG, Lillemoe KD, Reber HA. Natural history of intraductal papillary mucinous neoplasms (IPMN): current evidence and implications for management. J Gastrointest Surg. 2008;12:645–650. doi: 10.1007/s11605-007-0447-x. [DOI] [PubMed] [Google Scholar]
- 41.Wente MN, Schmied BM, Schmidt J, Buchler MW. Differentiated therapy for intraductal papillary mucinous neoplasms. Chirurg. 2009;80:7–13. doi: 10.1007/s00104-008-1579-6. [DOI] [PubMed] [Google Scholar]
- 42.Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ, Raimondo M, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–1507. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- 43.Paye F, Sauvanet A, Terris B, Ponsot P, Vilgrain V, Hammel P, et al. Intraductal papillary mucinous tumours of the pancreas: pancreatic resections guided by preoperative morphological assessment and intraoperative frozen section examination. Surgery. 2000;127:536–544. doi: 10.1067/msy.2000.106126. [DOI] [PubMed] [Google Scholar]
- 44.Sho M, Nakajima Y, Kanehiro H, Hisanaga M, Nishio K, Nagao M, et al. Pattern of recurrence after resection for intraductal papillary mucinous tumours of the pancreas. World J Surg. 1998;22:874–878. doi: 10.1007/s002689900485. [DOI] [PubMed] [Google Scholar]
- 45.Terris B, Ponsot P, Paye F, Hammel P, Sauvanet A, Molas G, et al. Intraductal papillary mucinous tumours of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372–1377. doi: 10.1097/00000478-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 47.Al-Haddad M, Martin JK, Nguyen J, Pungpapong S, Raimondo M, Woodward T, et al. Vascular resection and reconstruction for pancreatic malignancy: a single-centre survival study. J Gastrointest Surg. 2007;11:1168–1174. doi: 10.1007/s11605-007-0216-x. [DOI] [PubMed] [Google Scholar]
- 48.Siriwardana HPP, Siriwardena AK. Systematic review of outcome of synchronous portal-superior mesenteric vein resection during pancreatectomy for cancer. Br J Surg. 2006;93:662–673. doi: 10.1002/bjs.5368. [DOI] [PubMed] [Google Scholar]
- 49.Tseng JF, Raut CP, Lee JE, Pisters PW, Vauthey JN, Abdalla EK, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935–949. doi: 10.1016/j.gassur.2004.09.046. discussion 949–950. [DOI] [PubMed] [Google Scholar]
- 50.Yekebas EF, Bogoevski D, Cataldegirmen G, Kunze C, Marx A, Vashist YK, et al. En bloc vascular resection for locally advanced pancreatic malignancies infiltrating major blood vessels: perioperative outcome and longterm survival in 136 patients. Ann Surg. 2008;247:300–309. doi: 10.1097/SLA.0b013e31815aab22. [DOI] [PubMed] [Google Scholar]
- 51.Hartel M, Niedergethmann M, Farag-Soliman M, Sturm JW, Richter A, Trede M, et al. Benefit of venous resection for ductal adenocarcinoma of the pancreatic head. Eur J Surg. 2002;168:707–712. doi: 10.1080/00000000000000007. [DOI] [PubMed] [Google Scholar]
- 52.Weitz J, Kienle P, Schmidt J, Friess H, Buchler MW. Portal vein resection for advanced pancreatic head cancer. J Am Coll Surg. 2007;204:712–716. doi: 10.1016/j.jamcollsurg.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Hartel M, Wente MN, Hinz U, Kleeff J, Wagner M, Muller MW, et al. Effect of antecolic reconstruction on delayed gastric emptying after the pylorus-preserving Whipple procedure. Arch Surg. 2005;140:1094–1099. doi: 10.1001/archsurg.140.11.1094. [DOI] [PubMed] [Google Scholar]
- 54.Billings BJ, Christein JD, Harmsen WS, Harrington JR, Chari ST, Que FG, et al. Quality of life after total pancreatectomy: is it really that bad on longterm follow-up? J Gastrointest Surg. 2005;9:1059–1066. doi: 10.1016/j.gassur.2005.05.014. discussion 1066–1067. [DOI] [PubMed] [Google Scholar]
- 55.Tattersall RB. Brittle diabetes revisited: the Third Arnold Bloom Memorial Lecture. Diabet Med. 1997;14:99–110. doi: 10.1002/(SICI)1096-9136(199702)14:2<99::AID-DIA320>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]


