Abstract
Introduction:
Total pancreatectomy (TP) is associated with significant metabolic abnormalities leading to considerable morbidity. With the availability of modern pancreatic enzyme formulations and improvements in control of diabetes mellitus, the metabolic drawbacks of TP have diminished. As indications for TP have expanded, we examine our results in patients undergoing TP.
Materials and methods:
Retrospective study of 47 patients undergoing TP from January 2002 to January 2008 was performed. Patient data and clinical outcomes were collected and entered into a database. Disease-free survival and overall survival were estimated using the Kaplan–Meier method.
Results:
Fifteen males and 32 females with a median age of 70 years underwent TP for non-invasive intraductal papillary mucinous neoplasms (IPMN) (21), pancreatic adenocarcinoma (20), other neoplasm (3), chronic pancreatitis (2) and trauma (1). Median hospital stay and intensive care stay were 11 days and 1 day, respectively. Thirty-day major morbidity and mortality was 19% and 2%, respectively. With a median follow-up length of 23 months, 33 patients were alive at last follow-up. Estimated overall survival at 1, 2 and 3 years for the entire cohort was 80%, 72% and 65%, and for those with pancreatic adenocarcinoma was 63%, 43% and 34%, respectively. Median weight loss at 3, 6 and 12 months after surgery was 6.8 kg, 8.5 kg and 8.8 kg, respectively. Median HbA1c values at 6, 12 and 24 months after surgery were 7.3, 7.5 and 7.7, respectively. Over one-half of the patients required re-hospitalization within 12 months post-operatively.
Conclusion:
TP results in significant metabolic derangements and exocrine insufficiency, diabetic control and weight maintenance remain a challenge and readmission rates are high. Survival in those with malignant disease remains poor. However, the mortality appears to be decreasing and the morbidities associated with TP appear acceptable compared with the benefits of resection in selected patients.
Keywords: Total pancreatectomy, pancreatic cancer, survival, diabetes mellitus
Introduction
Total pancreatectomy (TP) was first successfully performed by Priestley in 19441 for a patient with a non-localizable symptomatic insulinoma. In the ensuing decades, the enthusiasm for TP for malignant disease varied and was mostly performed in those with pancreatic adenocarcinoma. In the 1970s, as a result of the significant morbidity of a subsequent pancreatic fistula, concerns about multicentric pancreatic cancer and theoretical survival benefits with an extended lymphadenectomy, TP became a more accepted option over partial pancreatic resection in selected patients.2–4 However, more recent data from polymerase chain reaction (PCR) studies of pancreatic cancer specimens have indicated that multicentric pancreatic cancer may not be as common as once thought,5 and that survival is not improved by TP over partial pancreatectomy.6 Furthermore, recent experience indicates improved outcomes and decreased surgical morbidity with partial pancreatectomy over TP for malignancy.7
Most recently, there has been a renewed interest in TP as an option in benign pancreatic disease8 and again in select malignant disease. Since the description of intraductal papillary mucinous neoplasms (IPMN) in 1982,9 there has been an increased use of TP as a treatment for diffuse pancreatic involvement of this premalignant lesion.10–15 Furthermore, recognition of familial pancreatic cancer syndromes has only recently been appreciated, leading to more aggressive approaches including TP in those patients with genetic abnormalities.16 For these reasons, there has been an increased utilization of TP. According to a surveillance epidemiology and end results (SEER) database review, the percentage of TP performed compared with partial pancreatectomy for pancreatic adenocarcinoma has risen from 9.3% of patients in 1998 to 14.3% of patients in 2004.17
TP remains a significant undertaking and is associated with major metabolic abnormalities such as difficulties in glucose control and malabsorption leading to multiple medical problems and decreased quality of life.18 However, with the availability of modern pancreatic enzyme formulations and improvements in control of diabetes mellitus, the metabolic drawbacks of TP may have diminished from historic controls. Recognizing the recent increased utilization of TP as well as improved diabetic management tools in the recent decade, the aim of this study was to review our experience with TP and report the outcomes.
Materials and methods
This retrospective study was conducted in accordance with the Helsinki Declaration. After the study had been approved by the Mayo Clinic Institutional Review Board (registration 08000743; 4 March 2008), all patients undergoing pancreatic resection between January 2002 and January 2008 were identified and the subgroup undergoing TP was reviewed in detail.
Data collected included: patient demographics, presenting clinical features, operative and pathological details, peri-operative outcomes and long-term outcomes. Follow-up was obtained through internal and external medical records and patient interview. If no follow-up could be attained, the national social security index was searched for date of death.
Pathology data on pancreatic tumour staging were collected according to the tumor, node, metastases (TNM) staging system. IPMN pathology was described as per WHO criteria. Pancreatic adenocarcinoma arising in the background of IPMN was considered to have arisen from premalignant IPMN, whereas pancreatic adenocarcinoma specimens without any evidence for IPMN were considered as pancreatic adenocarcinoma de novo.
Operative strategy
In this series, TP was performed in a standard open fashion, adhering to oncological principles when appropriate.19 The abdomen was explored for occult metastatic disease and the tumour assessed for resectability. TP was performed with both pylorus preserving and standard technique as well as with and without the spleen sparing technique. If a partial pancreatic resection was attempted, the pancreatic margin was analysed by frozen section. If found to be involved with invasive carcinoma, further margins were analysed and completion TP was performed if multiple margins were involved and the patient was felt to be able to tolerate a TP. Generally, further margin analysis with conversion to completion TP was performed for pancreatic margins found to have carcinoma in situ or severe dysplasia, especially in a young fit patient. Commonly, further margin analysis or completion TP were not performed for findings of moderate dysplasia or adenomatous changes for patients undergoing pancreatic resection.
Post-operative complications were graded according to methods previously described20 and limited to complications seen within 30 days of surgery or within the index hospitalization. A score of 0–5 was assigned based on the most severe post-operative complication experienced by each patient. Grade 0 indicates no complications were recorded. Grade 1 indicates minor complications requiring observation or minor outpatient intervention (e.g. oral antibiotics). Grade 2 indicates complications requiring moderate intervention (e.g. wound debridement, intravenous antibiotics). Grade 3 indicates major intervention (e.g. CT-guided drainage of intra-abdominal fluid collection, surgery). Grade 4 indicates permanent disability (e.g. stroke with residual paresis or paralysis, chronic ventilator dependence) or discharge to long-term care facility. Grade 5 indicates death. Grade 1 and 2 complications were considered minor and Grade 3, 4 and 5 complications were considered major. Complications were further differentiated into ‘medical’ and ‘surgical’ complications and recorded in addition to graded complications to fully characterize their nature. International consensus guidelines were used to evaluate complications when available.21,22
Numerical variables were summarized with the sample median, minimum and maximum. Categorical variables were summarized with number and percentage. The Kaplan–Meier method was used to estimate overall survival and disease-free survival after surgery. Weight loss after surgery was compared according to presence of pylorus preserving using a Wilcoxon rank sum test. P-values ≤ 0.05 were considered statistically significant. Statistical analyses were performed using the SAS software package (SAS Institute; Cary, NC, USA).
Results
From January 2002 to January 2008, a total of 397 pancreatic resections were performed. These included: 234 (59%) proximal pancreatectomies (pancreaticoduodenectomies), 116 (29%) distal pancreatectomies and 47 (12%) TPs. The 47 patients undergoing TP [32 females, median age of 70 (range 32–83)] were included in the study.
Patient characteristics
Pre-operative patient demographics and clinical characteristics are presented in Table 1a. Table 1b shows pre-operative symptoms. Abdominal pain was the most common presenting symptom and nearly half of the patients experienced a median weight loss of 9 kg.
Table 1a.
Patient characteristics
| Variable | All patients (n= 47) | Pancreatic adenocarcinoma de novo (n= 10) | Pancreatic adenocarcinoma from IPMN (n= 10) | Non-invasive IPMN (n= 21) |
|---|---|---|---|---|
| Age | 70 (32, 83) | 70 (58, 83) | 71 (57, 78) | 71 (43, 78) |
| Gender (male) | 15 (32%) | 5 (50%) | 4 (40%) | 4 (19%) |
| BMI | 26 (18, 43) | 25 (18, 33) | 27 (21, 33) | 27 (19, 43) |
| CA 19-9 (U/ml) | 40 (1, 11841) | 408 (1, 6284) | 203 (1, 11841) | 21 (1, 60) |
| ASA class | ||||
| 2 | 15 (32%) | 3 (30%) | 1 (10%) | 10 (48%) |
| 3 | 27 (57%) | 6 (60%) | 6 (60%) | 10 (48%) |
| 4 | 5 (11%) | 1 (10%) | 3 (30%) | 1 (5%) |
| Diabetes | ||||
| Long standing | 11 (23%) | 2 (20%) | 4 (40%) | 4 (19%) |
| Recent onset | 6 (13%) | 2 (20%) | 1 (10%) | 2 (10%) |
| Insulin-dependant | 4 (9%) | 0 (0%) | 2 (20%) | 1 (5%) |
| Hypertension | 29 (62%) | 6 (60%) | 8 (80%) | 11 (52%) |
| CAD | 13 (28%) | 5 (50%) | 3 (30%) | 5 (24%) |
| COPD | 5 (11%) | 0 (0%) | 1 (10%) | 4 (19%) |
| ETOH | 26 (55%) | 4 (40%) | 6 (60%) | 12 (57%) |
| Pancreatitis | 11 (23%) | 1 (10%) | 2 (20%) | 7 (33%) |
| Length of follow up (y) | 1.9 (0.0, 4.9) | 0.8 (0.0, 2.8) | 1.3 (0.2, 3.9) | 2.5 (0.2, 4.9) |
The sample median (minimum, maximum) is given for numerical variables. Information was unavailable for the following variables: weight loss at surgery (n= 2), and CA 19-9 (n= 8).
IPMN, intraductal papillary mucinous neoplasms; BMI, body mass index; ASA, American Society of Anesthesia; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; ETOH, alcohol use.
Table 1b.
Patient pre-operative symptoms
| Variable | Summary (n= 47) |
|---|---|
| Abdominal pain | 33 (70%) |
| Weight loss | 21 (47%) |
| Median (minimum, maximum) weight loss (kg) | 9 (3–34) |
| Jaundice | 14 (30%) |
| Nausea/vomiting | 7 (15%) |
| Diarrhoea | 7 (15%) |
| Early satiety | 4 (9%) |
| Asymptomatic | 3 (6%) |
| Other | 3 (6%) |
Indications for surgery and operative data
Portal vein resection was carried out in 10 (21%) patients and pylorus preservation in 23 (49%). Median estimated blood loss was 800 ml (range 195–7800), median operative time was 396 min (range 232–553) and median number of intra-operative packed red blood cell transfusions was 3 units (range 0–22). Spleen preservation technique was carried out in 13 (28%). Three patients had undergone a previous partial pancreatic resection and were found to have indications for an elective completion TP at 1, 4, and 6 years from previous distal pancreatectomy for neuroendocrine tumour, pancreaticoduodenctomy for pancreatic adenocarcinoma and distal pancreatectomy for chronic pancreatitis, respectively.
Pathology
Pathology finding are shown in Table 2a. Twenty-three patients (49%) had malignant findings with the majority (n= 20, 87%) having pancreatic adenocarcinoma. Among patients with pancreatic adenocarcinoma, 10 (50%) were found to arise de novo, whereas the remaining 10 (50%) were found to arise in the background of IPMN. Table 2b displays tumour staging information in the pancreatic adenocarcinoma patients. Those patients with pancreatic adenocarcinoma arising de novo were generally seen at a higher stage with larger tumour size. Five patients with R1 positive margins included an uncinate process/superior mesenteric artery positive margin in two, superior mesenteric vein positive margin in two and radial positive margin in one. One patient underwent R2 resection with gross residual disease involving the superior mesenteric artery.
Table 2a.
Indications for total pancreatectomy (TP)
| Summary (n= 47) | |
|---|---|
| Malignant | 23 (49%) |
| Pancreatic adenocarcinoma | 20 (43%) |
| Pancreatic adenocarcinoma de novo | 10 (21%) |
| Pancreatic adenocarcinoma with underlying IPMN | 10 (21%) |
| Cholangiocarcinoma with IPMN | 1 (2%) |
| Neuroendocrine tumour | 1 (2%) |
| Renal cell carcinoma | 1 (2%) |
| Non-malignant | 24 (51%) |
| Non-invasive IPMN | 21 (45%) |
| Chronic pancreatitis | 2 (4%) |
| Trauma | 1 (2%) |
IPMN, intraductal papillary mucinous neoplasms.
Table 2b.
Tumour information in pancreatic adenocarcinoma patients
| Variable | Pancreatic adenocarcinoma (n= 20) | Pancreatic adenocarcinoma de novo (n= 10) | Pancreatic adenocarcinoma from IPMN (n= 10) |
|---|---|---|---|
| Tumour grade | |||
| 1 | 3 (15%) | 0 (0%) | 3 (30%) |
| 2 | 10 (50%) | 6 (60%) | 4 (40%) |
| 3 | 7 (45%) | 4 (40%) | 3 (30%) |
| Tumour stage | |||
| IA | 1 (5%) | 0 (0%) | 1 (10%) |
| IIB | 2 (10%) | 1 (10%) | 1 (10%) |
| IIA | 4 (20%) | 0 (0%) | 4 (40%) |
| IIB | 11 (55%) | 7 (70%) | 4 (40%) |
| III | 2 (10%) | 2 (20%) | 0 (0%) |
| Positive lymph nodes | 13 (65%) | 9 (90%) | 4 (40%) |
| Lymphatic invasion | 17 (85%) | 9 (90%) | 8 (80%) |
| Positive margins | 6 (30%) | 4 (40%) | 2 (20%) |
| Tumour size (cm) | 3.5 (0.8, 7.0) | 3.9 (2.5, 7.0) | 2.2 (1.0, 7.0) |
| Reason for total pancreatectomy | |||
| Diffuse involvement | 9 (45%) | 4 (40%) | 5 (50%) |
| Positive neck margins | 9 (45%) | 5 (50%) | 4 (40%) |
| Familial syndrome | 1 (5%) | 0 (0%) | 1 (10%) |
| Completion | 1 (5%) | 1 (10%) | 0 (0%) |
The sample median (minimum, maximum) is given for tumour size. Information was unavailable for positive lymph nodes (n= 1) and lymphatic invasion (n= 2).
IPMN, intraductal papillary mucinous neoplasms.
Among the 24 (51%) patients with benign pathology, 21 (88%) had non-invasive IPMN (Table 2c). The median time from diagnosis of IPMN to surgery was 5 months (range 2–120), and the most common reason for TP was planned procedure for diffuse disease (n= 15, 71%). When considering the entire cohort of 47 TPs, IPMN was seen in 32 (68%) of the resected specimens. Of these, according to the WHO classification system, 11 patients (34%) had simple adenoma, 1 patient (3%) had low-moderate dysplasia, 10 patients (31%) had high-grade dysplasia and 10 patients had invasive adenocarcinoma (31%).
Table 2c.
Characteristics of intraductal papillary mucinous neoplasm (IPMN) lesions
| Summary (n= 21) | |
|---|---|
| WHO classification | |
| High grade (CISa) | 10 (48%) |
| Low-moderate (Borderine) | 1 (4%) |
| None (adenoma) | 10 (48%) |
| Reason for total pancreatectomy | |
| Diffuse involvement | 15 (71%) |
| Positive margins | 5 (24%) |
| Completion | 1 (5%) |
| Disease duration (months) | 5 (2, 120) |
Carcinoma in situ.
The sample median (minimum, maximum) is given for disease duration.
Survival and disease recurrence
With a median length of follow-up of 23 months (range 0.5–59) after pancreatectomy, 14 patients (30%) had died. Estimated patient survival after TP according to disease pathology is displayed in Table 3 and Fig. 1. Overall, 65% (95% CI: 18–51%) of patients were still alive at 3 years after TP; this estimated rate was 95% (95% CI: 85–100%) in non-invasive IPMN patients, 34% (95% CI: 17–70%) for all pancreatic adenocarcinoma, 49% (95% CI: 20–100%) in patients with pancreatic adenocarcinoma from IPMN and 0% (95% CI: 0–37%) in patients with pancreatic adenocarcinoma de novo. There were nine deaths within 1 year of pancreatectomy, and five were related to cancer recurrence. Of the four deaths not related to cancer, two deaths were caused by medical deconditioning related to total pancreatectomy at 0.5 and 11.4 months, and two were not related to total pancreatectomy or cancer. One patient expired from a mycotic abdominal aortic aneurysm at 5.7 months and another developed metastatic Merkel cell cancer and died at 8.9 months. Of the five remaining deaths occurring after 1 year of surgery, four were related to cancer recurrence and one was related to medical deconditioning related to TP at 25 months.
Table 3.
Estimated survival after total pancreatectomy (TP) according to underlying disease
| Time after surgery |
Estimated survival (95% CI) |
||||
|---|---|---|---|---|---|
| All patients (n= 47) | Pancreatic adenocarcioma (n= 20) | Pancreatic adenocarcinoma de novo (n= 10) | Pancreatic adenocarcinoma from IPMN (n= 10) | Non-invasive IPMN (n= 21) | |
| 6 months | 93% (86–100%) | 84% (70–100%) | 80% (59–100%) | 89% (71–100%) | 100% (83–100%) |
| 1 year | 80% (68–92%) | 63% (55–89%) | 40% (19–85%) | 89% (71–100%) | 95% (85–100%) |
| 2 years | 72% (59–87%) | 43% (24–76%) | 30% (12–77%) | 49% (20–100%) | 95% (85–100%) |
| 3 years | 65% (49–82%) | 34% (17–70%) | 0% (0–37%) | 49% (20–100%) | 95% (85–100%) |
IPMN, intraductal papillary mucinous neoplasms.
Figure 1.
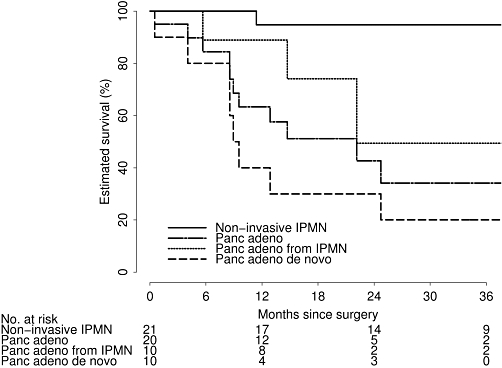
Estimated survival after total pancreatectomy according to underlying disease
Estimated disease-free survival after TP for pancreatic adenocarcinoma is shown in Table 4 and Fig. 2. At a median time follow-up of 30 months (range 2–59), none of the 21 non-invasive IPMN patients had experienced disease recurrence. For all pancreatic adenocarcinoma patients, estimated disease-free survival at 3 years after total pancreatectomy was 16% (95% CI: 3–85%), but was slightly higher in patients with pancreatic adenocarcinoma from IPMN (35%, 95% CI: 8–100%) than in patients with pancreatic adenocarcinoma de novo (0%, 95% CI: 59–100%).
Table 4.
Estimated cumulative rate of disease recurrence after pancreatectomy in patients with pancreatic adencarcinoma
| Time after surgery |
Estimated disease-free survival (95% CI) |
||
|---|---|---|---|
| Pancreatic adenocarcinoma (n= 19)a | Pancreatic adenocarcinoma de novo (n= 9)a | Pancreatic adenocarcinoma from IPMN (n= 10) | |
| 6 months | 77% (59–100%) | 47% (21–100%) | 100% (63–100%) |
| 1 year | 56% (26–87%) | 16% (3–93%) | 87% (67–100%) |
| 2 years | 31% (12–83%) | 0% (0–41%) | 70% (40–100%) |
| 3 years | 16% (3–85%) | 0% (0–41%) | 35% (8–100%) |
Information regarding disease recurrence was unknown for one pancreatic adenocarcinoma de novo patient.
IPMN, intraductal papillary mucinous neoplasms.
Figure 2.
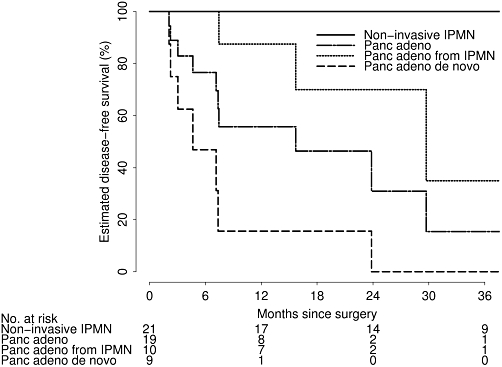
Estimated disease-free survival after total pancreatectomy according to underlying disease
Other patient outcomes
Table 5a summarizes other patient outcomes after TP. The median length of intensive care unit stay was 1 day, whereas the median length of hospital stay was 11 days. During their hospital stay, 8 patients required supplemental parenteral nutrition for a median of 7 days (range 3–37). No complications were seen in 25 patients (53%), minor complications in 13 (28%) and major complications in 9 (19%). There were 12 (26%) medical complications and 17 (36%) surgical complications in 22 patients, with 7 patients (15%) experiencing both. Four patients (9%) required reoperation and there was one mortality. All reoperations were performed for post-operation intra-abdominal abscess with findings of colonic fistula in two (one patient underwent concomitant colon resection and TP), gastrojejunostomy breakdown in one and biliary peritonitis in one. The mortality was in a 68-year-old morbidly obese, malnourished woman with chronic deep vein thrombosis who had locally advanced pancreatic cancer and underwent TP with portal vein resection. After surgery, she had continued malnourishment and deconditioning and discharged home after a 9-day hospital stay. Eight days later, she presented to an outside institution with severe dehydration and hyperglycaemia and expired.
Table 5a.
Patient outcomes
| Variable | Summary (n= 47) |
|---|---|
| Length of hospital stay (days) | 11 (7, 72) |
| Length of ICU stay (days) | 1 (0, 12) |
| Supplemental parental nutrition | 8 (17%) |
| Post-operative insulin drip | 43 (47%) |
| Complication grade | |
| 0 | 25 (53%) |
| 1 | 5 (11%) |
| 2 | 8 (17%) |
| 3 | 8 (17%) |
| 4 | 0 (0%) |
| 5 | 1 (2%) |
| Surgical complication | 17 (36%) |
| Intra-abdominal abscess/sepsis | 5 (11%) |
| Biliary/GI fistula | 4 (9%) |
| Delayed gastric emptying | 5 (11%) |
| Wound infection | 7 (15%) |
| Reoperation | 4 (9%) |
| Medical complication | 12 (26%) |
| Gastritis/colitis | 1 (2%) |
| Pulmonary | 3 (6%) |
| Cardiac | 2 (4%) |
| Thrombosis | 1 (2%) |
| Glycaemic events (major) | 2 (4%) |
| Renal insufficiency/urinary tract infection | 3 (6%) |
| Weight loss (kg) | |
| 3 months after surgery | −6.8 (−25.0, 12.1) |
| 6 months after surgery | −8.5 (−28.6, 2.4) |
| 12 months after surgery | −8.8 (−39.5, 1.2) |
| HbA1c | |
| 3 months after surgery | 6.5 (4.6, 7.5) |
| 6 months after surgery | 7.3 (5.0, 9.6) |
| 12 months after surgery | 7.5 (5.2, 8.6) |
| 24 months after surgery | 7.7 (6.6, 8.8) |
The sample median (minimum, maximum) is given for numerical variables. Information was unavailable for the following variables: weight loss at 3 months after surgery (n= 4), weight loss at 6 months after surgery (n= 15), weight loss at 1 year after surgery (n= 19), HbA1c at 3 months after surgery (n= 34), HbA1c at 6 months after surgery (n= 31), HbA1c at 1 year after surgery (n= 28), and HbA1c at 2 years after surgery (n= 32).
Forty-three patients (92%) were placed on an insulin drip post-operatively for a median of 6 days (range 0–18). Most patients were maintained on an insulin drip until tolerating an oral diet, which was then transitioned to a long-acting injectable insulin glargine in addition to a sliding scale of short-acting injectable insulin. All patients were seen by our inpatient diabetic education and nutrition management team. The median dose of long-acting insulin glargine at discharge was 10 units (range 0–80).
Median weight loss at 3, 6 and 12 months after surgery was 6.8, 8.5 and 8.8 kg, respectively, and did not differ dramatically between patients with and without pylorus preserving at any post-operative time point considered (all P > 0.35).
From the date of discharge, 12 out of the 46 patients (26%) available for follow-up required rehospitalization within 1 month of discharge. Of the 42 patients available for follow-up at 1 year, 23 (55%) required hospitalization within 12 months of discharge. The 23 patients hospitalized within 12 months experienced a total of 45 admissions, and 42 (93%) of these rehospitalizations were successfully treated and discharged without complications. The median length of stay for rehospitalization was 6 days (range 1–56). This and other rehospitalization information is displayed in Table 5b.
Table 5b.
Rehospitalization information
| Variable | Summary |
|---|---|
| Rehospitalization by 1 month after discharge (n= 46) | 12 (26%) |
| Rehospitalization by 4 months after discharge (n= 43) | 20 (47%) |
| Rehospitalization by 12 months after discharge (n= 42) | 23 (55%) |
| Reasons for re-hospitalization (n= 45 total rehospitalizations) | |
| Malnutrition/FTT/N/V/D/weakness | 20 (44%) |
| Gastrointestinal even (e.g. GI bleed) | 9 (20%) |
| Glycaemic events | 5 (11%) |
| Infection | 5 (11%) |
| Other | 6 (13%) |
The sample sizes given associated with rehospitalization indicate the number of patients with a length of follow-up necessary to calculate the given rehospitalization rate.
FTT, Failure to thrive; N, Nausea; V, Vomiting; D, Diarrhoea.
Discussion
TP appears to be increasingly accepted for selected pancreatic pathologies. In the first half of our study period, 8.3% (n= 12) of all pancreatic resections performed at our institution were TP, whereas in the second half, the rate more than doubled to 17.4% (n= 35), even without any variation in the underlying disease pathology between the two time periods. In fact, there were no TPs performed from the years 2000 to 2002 at our institution. While the reason for the increased frequency of TP is not entirely clear based on the retrospective nature of our review, there are several reasons found in the literature and confirmed in our cohort which may account for this finding.
The first reason may be that surgeons, gastroenterologists and endocrinologists alike may be more comfortable with the management of the apancreatic state that was so feared in the past. In a study very similar to ours, Jethwa et al.23 reported that there were no observed mortalities as a result of diabetic complications or metabolic consequences of pancreatic resection during long-term follow-up of 47 patients undergoing TP. They concluded that diabetic control after TP is less difficult and associated with fewer glycaemic complications than generally assumed. Similarly, in the largest single institution review of 124 elective TPs, a group from Germany found TP was performed with the same morbidity rate as partial pancreatectomy, and quality of life was not significantly different in the two groups.24 This confirmed a previous report from 2005 where quality of life in 34 TP patients was compared with matched type I diabetic controls, and no difference was found.25 The improved overall results seen in the past decade over historic reports may also be multifactorial. Improvements in glucose monitoring systems, insulin delivery systems and insulin formulations may contribute to superior glycaemic control for these patients.26 The HbA1c levels seen in our patient series is very reasonable for insulin-dependant diabetes and is comparable to that seen in other recent reports.23–25,27 Our conclusion in this study may be limited by the fact that the 29 patients for whom HbA1c data were available were generally the healthiest patients with benign disease. This introduces a bias by excluding those with significant morbidities or those with a poor prognosis who have a limited need for tight long-term glucose control for long-term diabetic complication prevention.
In addition to improved endocrine control, exocrine insufficiency may be improved by modern pancreatic enzyme formulations, proton pump inhibitor therapy and the use of the duodenum-preserving pancreatic head resections. In a report from Japan, the pylorus-preserving technique was noted to be associated with significantly improved albumin levels and weight loss over the standard TP.28 However, we did not find a significant difference in weight loss between these two patient groups at any point of follow-up. Weight loss continues to be problematic for these patients as we found that nearly all patients experienced continued steady weight loss in the months after TP, generally plateauing at 12 months. This trend was true for all patient subgroups, and there were no differences between the patients with malignant and benign disease. The reasons for this weight loss were multifactorial and mostly attributed to failure to thrive as a result of poor oral intake, malabsorption and diarrhoea, chronic postprandial abdominal pain, cancer recurrence, or a combination of the prior for all patients despite pancreatic enzyme replacement.
In addition to the improved long-term morbidity, another explanation for the increased use of TP may be the improved peri-operative mortality seen in recent years. Although historically reported to be a high-risk procedure, our experience and recent literature are reporting a mortality rate of less than 5%,6,8,14,24,25,28 confirming that TP can be performed safely and that overall survival is generally based on the underlying disease process.
Another rationale for the increased rate of TP may be an increased diagnosis of diffuse ductal disease in all patients undergoing pancreatic resection. Improved radiographic and endoscopic surveillance during preoperative testing as well as close attention to intra-operative frozen section pathologic analysis of the pancreatic duct margin may contribute to this. At our institution, magnetic resonance cholangiopancreatography and endoscopic ultrasound evaluation are common for evaluation of all pancreatic masses and may increase the sensitivity for finding ductal abnormalities at more diffuse locations than the primary tumour. Intra-operative microscopic analysis of the pancreatic margin is important as margin clearance and the risk of leaving dysplastic or cancerous cells has been emphasized as important predictors of recurrence and survival. A retrospective analysis of 33 patients with pancreatic adenocarcinoma undergoing conversion to TP after findings of a positive margin during pancreaticoduodenectomy conferred a significant survival benefit over patients with an R1 resection.29 Recurrence after partial pancreatic resection for non-invasive IPMN is low but has been reported to be as high as 8%.10,11,30 These recurrences may be the result of a failure to diagnose diffuse main duct disease either by pre-operative radiographic studies or by intra-operative microscopical analysis. In our series, conversion to TP as a result of positive margins was seen in 45% of all patients with pancreatic adenocarcinoma and 24% of those with non-invasive IPMN. However, because of the retrospective nature of this study and the lack of pertinent data, we are unable to fully define or standardize each particular surgeon's decision based on frozen section analysis for each specific patient. Generally, however, in all patients undergoing pancreatic resection, pancreatic margins found to have frank carcinoma or high grade dysplasia of the duct were considered for additional margin analysis or conversion to TP.
Survival of patients undergoing TP varied tremendously in our cohort between different patient groups and was generally as a result of the underlying disease process, as demonstrated by the similarity of the patient disease-free survival rates and the overall survival rates. As expected, those with benign disease had very good survival whereas those with invasive cancer had poor survival. This finding confirms multiple previous reports concerning the difference in survival between those with invasive versus non-invasive IPMN,10–12,14,31 as well as the difference in survival for those with pancreatic cancer arising in the background of IPMN versus sporadic pancreatic adenocarcinoma.32 Survival after TP for pancreatic adenocarcinoma has in the past been reported to be poor and some authors have questioned the value of the operation in light of the significant metabolic consequences conferred to a patient with a limited life span.4,6 However, it appears that the 3-year survival of those with pancreatic cancer from IPMN after total pancreatectomy seen in our series compares to 3-year survivals seen in those reports for all of invasive IPMN's undergoing pancreatic resection.12,14,31 It remains to be seen whether TP in this patient subgroup may improve survival over partial pancreatectomy. Survival differences, specifically between those undergoing partial pancreatic resection for pancreatic adenocarcinoma with IPMN versus TP, remains unknown.
The poor survival experienced by those with pancreatic adenocarcinoma de novo can also be explained by the high rate of positive lymph nodes (9 of 10), high positive margin rate (4 of 10), need for portal vein resection (4 of 10) and large tumour size (median 3.9 cm) which indicates that most of these tumours were locally advanced at the time of surgery and required complete pancreas removal in an attempt to obtain an R0 resection. Recurrence rates were also very high for all pancreatic adenocarcinoma for the same reasons as above. Site of recurrence was difficult to determine for most patients as they were generally found to have abdominal adenopathy as well as distant lesions found at the same time on follow-up imaging. The value of conversion to TP after attempted partial pancreatic resection with intra-operative findings of a positive margin at a location other than the pancreatic margin is debatable. In some cases, margins originally reported as negative may turn out to be involved by permanent section analysis, theoretically reducing the benefit of a completion pancreatectomy. However, with the use of adjuvant local radiation therapy, the extended survival advantage of an R1 over R2 resection may outweigh the metabolic consequences of TP for selected patients.
The rehospitalization rate observed in this patient population is very high and is even likely underestimated. The majority of these patients live some distance from our institution and may present to outside facilities for medical problems arising within a year after discharge. Retrospective review of the available admission data indicates that the majority could have been avoided with improved preventive management. The significant metabolic derangements of the apancreatic state may not become immediately apparent in the post-operative inpatient recovery phase. Most of these elderly patients do not have baseline endocrine or exocrine insufficiency before surgery and have difficulty adjusting to complex diabetic and nutritional changes seen after TP. Multidisciplinary management is mandatory for improved outcomes and intensive diabetic and nutritional counselling are essential. Providing extended care by discharge to a skilled nursing facility with diabetic education and nutrition capabilities appears appropriate. Additionally, consideration should be given for providing all patients with supplemental enteral feeding for several weeks post-operatively. A strict follow-up protocol should be available for these patients which includes close endocrine and nutrition supervision, strict adherence to proton pump inhibitor (PPI) and pancreatic enzyme replacement therapy and adequate hydration and protein intake. These measures may avoid readmission in the majority of the patients, particularly for the diagnosis of dehydration, diabetic complications and failure to thrive.
All patients who have undergone open or laparoscopic TP (n= 5) since the end of this study period have had enteral feeding tubes placed during their index procedure. A patient pathway has been developed and all patients will be followed. Those patients identified as likely to undergo TP are now enrolled into a series of pre-operative education appointments which allow for diabetic teaching to be performed in a setting more conducive to long-term retention rather than that of the recovery phase in the hospital. A primary coordinator is made available to manage insulin adjustment difficulties with routine measurements of HbA1c at intervals during the post-operative period. After surgery, frequent weight measurements, serum protein levels, daily caloric intake and the number of stools per day are also recorded at intervals in order to determine nutritional adequacy.
Limitations in this study were related to the small sample size as we were unable to find significant associations concerning patient characteristics and pathology information with survival, recurrence or post-operative outcomes other than those expected to be associated with poorer survival (higher tumour stage, positive lymph nodes, lymphovascular invasion and more than three units of blood transfused during the procedure). Other limitations of this study included inadequate follow-up, as three patients were lost within 2 months of the surgery, and short follow-up in others, as well as incomplete data on the HbA1c values. Additionally, the overall cohort was a heterogenous population undergoing total pancreatectomy for a variety of disease states and was performed by four different surgeons, introducing surgeon-specific bias and perspective.
Conclusion
TP for premalignant and malignant lesions will continue to have a significant role in the treatment of pancreatic pathology. However, TP results in significant metabolic derangements which require multidisciplinary management for improved outcomes. Diabetic control and weight maintenance remain a challenge. Intensive diabetic and nutritional counselling combined with advanced insulin formulations and delivery systems, pancreatic exocrine formulations and vitamin supplementations are essential. Readmission rates and weight losses are significant and indicate that these patients need rigorous outpatient observations and provision of additional nutrition for an extended period of time.
Mortality and long-term morbidity associated with TP appear to be lessening over the past decade, indicating that the risks of TP appear acceptable compared with the benefits of resection, especially for those patients with premalignant disease. Overall, survival is generally based on the underlying disease process, not a consequence of the surgery. However, because of our past results we would hesitate in performing a TP on an elderly patient with evidence of a locally advanced pancreatic tumour, as occurred in our single mortality. Instead, TP may be a more practical procedure for a young, motivated and educated patient who has diffuse disease of the whole pancreas with early malignancy and main duct IPMN or those with familial pancreatic cancer.
Acknowledgments
The authors would like to acknowledge Britney Langley for data collection and Kathleen Norton for editorial services throughout the study.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Priestley JT, Comfort MW, Radcliffe J., Jr Total pancreatectomy for hyperinsulinism due to an islet-cell adenoma: survival and cure at sixteen months after operation presentation of metabolic studies. Ann Surg. 1944;119:211–221. doi: 10.1097/00000658-194402000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ihse I, Anderson H, Andren S. Total pancreatectomy for cancer of the pancreas: is it appropriate? World J Surg. 1996;20:288–293. doi: 10.1007/s002689900046. discussion 294. [DOI] [PubMed] [Google Scholar]
- 3.Fortner JG. Regional pancreatectomy for cancer of the pancreas, ampulla and other related sites. Jpn J Surg. 1983;13:385–394. doi: 10.1007/BF02469723. [DOI] [PubMed] [Google Scholar]
- 4.van Heerden JA, ReMine WH, Weiland LH, McIlrath DC, Ilstrup DM. Total pancreatectomy for ductal adenocarcinoma of the pancreas. Mayo Clinic experience. Am J Surg. 1981;142:308–311. doi: 10.1016/0002-9610(81)90336-6. [DOI] [PubMed] [Google Scholar]
- 5.Motojima K, Urano T, Nagata Y, Shiku H, Tsurifune T, Kanematsu T. Detection of point mutations in the Kirsten-ras oncogene provides evidence for the multicentricity of pancreatic carcinoma. Ann Surg. 1993;217:138–143. doi: 10.1097/00000658-199302000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karpoff HM, Klimstra DS, Brennan MF, Conlon KC. Results of total pancreatectomy for adenocarcinoma of the pancreas. Arch Surg. 2001;136:44–47. doi: 10.1001/archsurg.136.1.44. discussion 48. [DOI] [PubMed] [Google Scholar]
- 7.Buchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z'Graggen K. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. 2003;138:1310–1314. doi: 10.1001/archsurg.138.12.1310. discussion 1315. [DOI] [PubMed] [Google Scholar]
- 8.Garcea G, Weaver J, Phillips J, Pollard CA, Ilouz SC, Webb MA, et al. Total pancreatectomy with and without islet cell transplantation for chronic pancreatitis: a series of 85 consecutive patients. Pancreas. 2009;38:1–7. doi: 10.1097/MPA.0b013e3181825c00. [DOI] [PubMed] [Google Scholar]
- 9.Ohhashi K, Murakami Y, Takekoshi T, Ohta H, Ohhashi I. Four cases of ‘mucin-producing’ cancer of the pancreas on specific findings of the papilla of Vater. Prog Dig Endosc. 1982;20:348–251. [Google Scholar]
- 10.Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ, Raimondo M, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–1507. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- 11.Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang AD, Melstrom LG, Bentrem DJ, Ujiki MB, Wayne JD, Strouch M, et al. Outcomes after pancreatectomy for intraductal papillary mucinous neoplasms of the pancreas: an institutional experience. Surgery. 2007;142:529–534. doi: 10.1016/j.surg.2007.07.007. discussion 534–537. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi K, Konomi H, Kobayashi K, Ogura Y, Sonoda Y, Kawamoto M, et al. Total pancreatectomy for intraductal papillary-mucinous tumor of the pancreas: reappraisal of total pancreatectomy. Hepatogastroenterology. 2005;52:1585–1590. [PubMed] [Google Scholar]
- 14.Kim SC, Park KT, Lee YJ, Lee SS, Seo DW, Lee SK, et al. Intraductal papillary mucinous neoplasm of the pancreas: clinical characteristics and treatment outcomes of 118 consecutive patients from a single center. J Hepatobiliary Pancreat Surg. 2008;15:183–188. doi: 10.1007/s00534-007-1231-8. [DOI] [PubMed] [Google Scholar]
- 15.Bendix Holme J, Jacobsen N, Rokkjaer M, Kruse A. Total pancreatectomy in six patients with intraductal papillary mucinous tumour of the pancreas: the treatment of choice. HPB (Oxford) 2001;3:257–262. doi: 10.1080/136518201753335539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartsch DK. Familial pancreatic cancer. Br J Surg. 2003;90:386–387. doi: 10.1002/bjs.4127. [DOI] [PubMed] [Google Scholar]
- 17.Nathan H, Wolfgang CL, Edil BH, Choti MA, Herman JM, Schulick RD, et al. Peri-operative mortality and long-term survival after total pancreatectomy for pancreatic adenocarcinoma: a population-based perspective. J Surg Oncol. 2009;99:87–92. doi: 10.1002/jso.21189. [DOI] [PubMed] [Google Scholar]
- 18.Dresler CM, Fortner JG, McDermott K, Bajorunas DR. Metabolic consequences of (regional) total pancreatectomy. Ann Surg. 1991;214:131–140. doi: 10.1097/00000658-199108000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans DB, Lee JE, Tamm EP, Pister PW. Pancreaticoduodenectomy (Whipple Operation) and total pancreatectomy for cancer. In: Fischer JE, editor. Mastery of Surgery. Philadelphia, PA: Lippincott Williams and Wilkins; 2007. pp. 1299–1317. [Google Scholar]
- 20.Martin RC, 2nd, Brennan MF, Jaques DP. Quality of complication reporting in the surgical literature. Ann Surg. 2002;235:803–813. doi: 10.1097/00000658-200206000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Jethwa P, Sodergren M, Lala A, Webber J, Buckels JA, Bramhall SR, et al. Diabetic control after total pancreatectomy. Dig Liver Dis. 2006;38:415–419. doi: 10.1016/j.dld.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Muller MW, Friess H, Kleeff J, Dahmen R, Wagner M, Hinz U, et al. Is there still a role for total pancreatectomy? Ann Surg. 2007;246:966–974. doi: 10.1097/SLA.0b013e31815c2ca3. discussion 974–975. [DOI] [PubMed] [Google Scholar]
- 25.Billings BJ, Christein JD, Harmsen WS, Harrington JR, Chari ST, Que FG, et al. Quality-of-life after total pancreatectomy: is it really that bad on long-term follow-up? J Gastrointest Surg. 2005;9:1059–1066. doi: 10.1016/j.gassur.2005.05.014. discussion 1066–1067. [DOI] [PubMed] [Google Scholar]
- 26.Heidt DG, Burant C, Simeone DM. Total pancreatectomy: indications, operative technique, and postoperative sequelae. J Gastrointest Surg. 2007;11:209–216. doi: 10.1007/s11605-006-0025-7. [DOI] [PubMed] [Google Scholar]
- 27.Landoni L, Salvia R, Festa L, Muselli P, Giardino A, Butturini G, et al. Quality of life after total pancreatectomy. Ten-year experience. J Pancreas (Online) 2004;5:441. [Google Scholar]
- 28.Sugiyama M, Atomi Y. Pylorus-preserving total pancreatectomy for pancreatic cancer. World J Surg. 2000;24:66–70. doi: 10.1007/s002689910013. discussion 70–71. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt CM, Glant J, Winter JM, Kennard J, Dixon J, Zhao Q, et al. Total pancreatectomy (R0 resection) improves survival over subtotal pancreatectomy in isolated neck margin positive pancreatic adenocarcinoma. Surgery. 2007;142:572–578. doi: 10.1016/j.surg.2007.07.016. discussion 578–580. [DOI] [PubMed] [Google Scholar]
- 30.White R, D'Angelica M, Katabi N, Tang L, Klimstra D, Fong Y, et al. Fate of the remnant pancreas after resection of noninvasive intraductal papillary mucinous neoplasm. J Am Coll Surg. 2007;204:987–993. doi: 10.1016/j.jamcollsurg.2006.12.040. discussion 993–995. [DOI] [PubMed] [Google Scholar]
- 31.Raut CP, Cleary KR, Staerkel GA, Abbruzzese JL, Wolff RA, Lee JH, et al. Intraductal papillary mucinous neoplasms of the pancreas: effect of invasion and pancreatic margin status on recurrence and survival. Ann Surg Oncol. 2006;13:582–594. doi: 10.1245/ASO.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Wasif N, Ko CY, Farrell J, Hines OJ, Reber H, Tomlinson JS. Is the Natural History of Invasive IPMN More Indolent than Sporadic Pancreatic Adenocarcinoma? San Francisco, CA: Gastrointestinal Cancers Symposium; 2009. [Google Scholar]


