Abstract
Background:
Traditionally a 1-cm margin has been accepted as the gold standard for resection of colorectal liver metastases. Evidence is emerging that a lesser margin may provide equally acceptable outcomes, but a critical margin, below which recurrence is higher and survival poorer, has not been universally agreed. In a recent publication, we reported peri-operative morbidity and clear margin as the two independent prognostic factors. The aim of the current study was to further analyse the effect of the width of the surgical margin on patient survival to determine whether a margin of 1 mm is adequate.
Methods:
Two hundred and sixty-one consecutive primary liver resections for colorectal metastases were analysed from 1992 to 2007. The resection margins were assessed by microscopic examination of paraffin sections. The initial analysis was performed on five groups according to the resection margins: involved margin, 0–1 mm, >1–<4 mm, 4–<10 mm and ≥ 10 mm. Subsequent analysis was based on two groups: margin <1 mm and >1 mm.
Results:
With a median follow-up of 4.7 years, the overall 5-year patient and disease-free survival were 38% and 22%, respectively. There was no significant difference in patient- or disease-free survival between the three groups with resection margins >1 mm. When a comparison was made between patients with resection margins ≤1 mm and patients with resection margins >1 mm, there was a significant 5-year patient survival difference of 25% versus 43% (P < 0.04). However, the disease-free survival difference did not reach statistical significance (P= 0.14).
Conclusions:
In this cohort of patients, we have demonstrated that a resection margin of greater than 1 mm is associated with significantly improved 5-year overall survival, compared with involved margins or margins less than or equal to 1 mm. The possible beneficial effect of greater margins beyond 1 mm could not be demonstrated.
Keywords: colorectal cancer, liver metastases, hepatic resection, resection margins
Introduction
Colorectal cancer is one of the most common malignant tumours accounting for at least 1 million new cases worldwide each year.1 Haematogenous spread to the liver occurs in 40–60% of these patients.2 Hepatic resection is still the only potentially curative therapeutic option in patients with colorectal liver metastases.3–5
Surgical resection margin status is one of the factors that has been evaluated for its influence on long-term survival. Most authors have reported that resection margin involvement is a significant, and consistently the most important factor influencing overall patient- and disease-free survival. For decades, the accepted gold standard was the ‘1-cm rule’, which stated that resection for colorectal liver metastases should only be performed if a margin of 10 mm or more could be achieved.6,7
However, recent studies have indicated that margins of less than 1 cm are not a contraindication to resection of colorectal liver metastasis; and in fact have a favourable outcome. This implies that patients, in whom only a sub-centimetre resection margin is possible, should not be precluded from undergoing resection.8,9
We had previously reported our experience with prognostic factors after resection of colorectal liver metastases.10 Peri-operative morbidity and a clear margin were the only independent prognostic factors in the multivariate analysis. We defined a clear margin as 1 mm or greater and in this study aimed to validate the selection of 1 mm as the appropriate cut off margin.
Material and methods
Patients, data collection and surgical therapy
All patients who underwent liver resection for colorectal metastasis with curative intent were included in the study (staged resections and re-do resections were excluded from the study). The total number of patients included in this study was 261. The operations were performed between February 1992 and December 2007 at Flinders Medical Centre, Ashford Community Hospital and The Queen Elizabeth Hospital in Adelaide, South Australia, Australia.
Data about the operation, type of resection and complications were recorded prospectively by the responsible surgeons (R.T.A.P., J.W.C.C. and G.J.M.). Data on resection margins, number of lesions, stage and location of the primary tumour, time between the bowel resection and liver surgery, and recurrence were obtained from a database. Mortality was confirmed with the South Australian cancer registry.
Patients were staged preoperatively using computed tomography (CT) or magnetic resonance imaging (MRI) of the abdomen, as well as chest X-rays or CT chest. In the latter component of the series, selected cases underwent positron emission tomography (PET) scanning to rule out extra-hepatic disease. Colonoscopies were also performed where appropriate as part of staging.
All patients underwent exploratory laparotomy and intra-operative evaluation using ultrasound to confirm intra-hepatic lesions. The types of resections were classified as wedge resection, segmentectomy, sectionectomy, hemi-hepatectomy or extended hemi-hepatectomy. The resections were performed or supervised by three hepato-pancreatico-biliary surgeons (R.T.A.P., J.W.C.C., G.J.M.). The operations were performed using a combination of clip dissection, cavitron ultrasonic aspirator, argon plasma coagulation and ultrasonic shears. The Pringle manoeuvre was applied at the discretion of the operating surgeon. Fourteen patients underwent synchronous colorectal and liver resections.
Histopathological analysis
The resection margins were measured by microscopic examination of paraffin sections by a pathologist. The resections were defined as microscopically involved (R1 resection) by the presence of tumour cells at the line of transection detected by histological examination. In specimens with uninvolved resection margins (R0 resection), the distance from the tumour to the closest resection margin was measured in millimetres. The specimens in which no measurements were recorded in the original pathology report, were re-reviewed by a single pathologist and the margins measured.
Statistics
Overall survival and disease-free survival rates were analysed using Kaplan–Meier survival statistics. All patients were included in the analysis (including the patients who died within 30 days post-operatively or in hospital). Statistical analyses were carried out using Stata Statistical Software: Release 10 (StataCorp. 2007).
Results
Demographics
The clinical features of the patients included in this study are presented in Table 1. Two hundred and sixty-one patients were included in the study, 163 (62.5%) were male and 98 (37.5%) female. Median age was 64 years (range 21.7 to 92.0 years). Eighty-two (31.4%) were older than 70 years, 148 (56.7%) between 50 and 70 years and 31 (11.9%) were younger than 50 years of age.
Table 1.
Clinical features of patients (n= 261)
| Patient factors | No. of patients (%) |
|---|---|
| Age | |
| Median | 64 years |
| >70 years | 82 (31.4) |
| 50–70 years | 148 (56.7) |
| <50 years | 31 (11.9) |
| Gender | |
| Female | 98 (37.5) |
| Male | 163 (62.5) |
| Site of primary CRC | |
| Colon | 158 (60.5) |
| Rectum | 103 (39.5) |
| Dukes staging | |
| A | 11 (4) |
| B | 70 (27) |
| C | 110 (42) |
| Synchronous liver metastases | 70 (27) |
| Type of hepatic resection | |
| Wedge resection/segmentectomy | 51 (19.5) |
| ≥2 segmentectomies | 52 (20) |
| Hemi-hepatectomy | 101 (39) |
| Extended hemi-hepatectomy | 57 (21.5) |
CRC, colorectal carcinoma.
The primary colorectal carcinoma (CRC) was located in the colon in 158 patients and in the rectum in 103 patients. Eleven patients had CRC stage Dukes' A (4%), 70 were Dukes' B (27%), 110 were Dukes' C (42%) and 70 patients had synchronous liver metastases (27%).
Operative details
Fifty-one (19.5%) patients were treated with wedge resection or segmentectomy. Fifty-two (20%) patients underwent at least two segmentectomies. One hundred and one (39%) were subjected to a hemi-hepatectomy and 57 (21.5%) were treated with at least an extended hemi-hepatectomy. Fourteen patients underwent synchronous colorectal and hepatic resections. One patient underwent a hepatic resection prior to a colorectal resection.
Two hundred and forty-six patients were first treated with a colonic or rectal resection followed by hepatic resection, with a mean time between first and second operations of 19.1 months (range 1.2 to 144.8 months). Of these patients, 118 (48%) patients underwent hepatic resection within a year after their colorectal resection. The post-operative mortality rate (in-hospital and 30-day post-operative) was 1.9% (5 out of 261 patients).
Resection margins and survival
The overall 1-, 3- and 5-year patient survival for this cohort was 88.1%, 61.4% and 37.9%, respectively, with a median patient survival of 46.7 months. The 1-, 3- and 5-year disease-free survival for this group was 58.5%, 32.5% and 22.4%, respectively, with a median disease-free survival of 17.5 months. The median follow-up was 4.7 years (range 0.3 to 192 months).
In our study, we first divided all patients into five categories according to their resection margins; microscopically involved or R1 resection (n= 28), uninvolved resection margins up to 1 mm (n= 39), more than 1 mm to less than 4 mm (n= 44), 4 mm to less than 10 mm (n= 63), 10 mm or more (n= 87).
The 1-, 3- and 5-year patient survival data for the separate groups are tabulated in Table 2. In this analysis, there were statistically significant differences between the group with involved margin and the group with margins ≥ 10 mm only (P < 0.03).
Table 2.
Resection margins and survival
| Resection margin | n |
Patient survival |
P | |||
|---|---|---|---|---|---|---|
| Median (months) | 1 year | 3 years | 5 years | |||
| Involved | 28 | 25.8 | 81% | 45% | 19% | reference |
| 0–1 mm | 39 | 45.4 | 79% | 63% | 29% | ns |
| >1–<4 mm | 44 | 54.5 | 91% | 71% | 47% | ns |
| 4–<10 mm | 63 | 51.7 | 86% | 56% | 38% | ns |
| ≥10 mm | 87 | 54.7 | 95% | 65% | 44% | <0.03 |
| Overall | 261 | 46.7 | 88% | 61% | 38% | |
In order to compare our results with other studies, we performed a second analysis where the patients were divided into three groups according to their resection margins; margin involved and up to 1 mm (n= 67), margin > 1 mm to less than 10 mm (n= 107), and margin at least 10 mm (n= 87).
The disease-free survival and overall survival analyses of these three groups are shown in Tables 3 and 4. The results show a statistically significant difference between the first and third groups in overall survival as well as in disease-free survival. However, there was no significant survival difference between the first and second, or the second and third groups.
Table 3.
Analysis of impact of margin on disease-free survival
| Resection margin | n |
Disease free survival |
P | |||
|---|---|---|---|---|---|---|
| Median (months) | 1 year | 3 years | 5 years | |||
| Involved – 1 mm | 67 | 10.1 | 41% | 21% | 17% | reference |
| >1 mm to <10 mm | 107 | 16.8 | 58% | 28% | 20% | 0.15 |
| ≥10 mm | 87 | 24.1 | 64% | 41% | 29% | <0.02 |
| Overall | 261 | 17.5 | 58% | 33% | 22% | |
Table 4.
Analysis of impact of margin on overall survival
| Resection margin | n |
Patient survival |
P | |||
|---|---|---|---|---|---|---|
| Median (months) | 1 year | 3 years | 5 years | |||
| Involved – 1 mm | 67 | 39 | 81% | 45% | 19% | reference |
| >1 mm to <10 mm | 107 | 52 | 85% | 63% | 38% | 0.081 |
| ≥10 mm | 87 | 54 | 95% | 65% | 43% | <0.03 |
| Overall | 261 | 46 | 88% | 61% | 38% | |
Further analyses where performed to ascertain the minimum resection margin that was significant in terms of overall survival and disease-free survival. The resection margins were sequentially analysed until statistical significance was reached. We found that a 1-mm margin was the statistically significant cut-off point.
Comparing patients with resection margins ≤ 1 mm and resection margins > 1 mm, there was a statistically significant difference in overall survival (P= 0.04). However, no significant difference in disease-free survival was found (P= 0.14). (Tables 5,6, Fig. 1).
Table 5.
Comparison of impact of resection margins ≤ 1 mm and >1 mm on overall survival
| Resection margin | n |
Patient survival |
P | |
|---|---|---|---|---|
| 3 years | 5 years | |||
| ≤1 mm | 67 | 55% | 25% | reference |
| >1 mm | 194 | 63.7% | 42.7% | 0.04 |
Table 6.
Comparison of impact of resection margins ≤1 mm and >1 mm on disease-free survival
| Resection margin | n |
Disease-free survival |
P | |
|---|---|---|---|---|
| 3 years | 5 years | |||
| ≤1 mm | 67 | 27.5% | 18.6% | reference |
| >1 mm | 194 | 35% | 23.6% | 0.14 |
Figure 1.
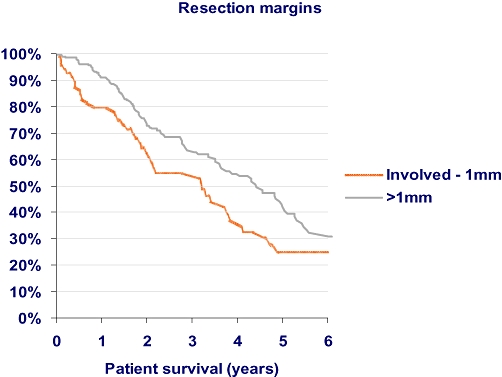
Graph of overall survival according to resection margin
Discussion
A number of variables have been described as prognostic factors in hepatic resection for colorectal metastasis to the liver, including nodal status of the primary tumour, disease-free interval, carcinoembryonic antigen (CEA) level, number and distribution of metastatic lesions, size of largest tumour and extent of resection.3–5,7 It is generally accepted that the surgical resection margin is a significant prognostic factor, however, there is currently no clear-cut consensus regarding the accepted minimum margin of clearance associated with favourable survival and disease-free outcomes. Since the 1980s, there has been a general consensus that a 10 mm or more tumour-free resection margin is required, and if it is not possible then resection should not be attempted. However, recent studies suggest that similar outcomes can be obtained with sub-centimetre resection margins.7–9,11
It is essential to note the importance of avoiding an incomplete resection when performing a hepatic resection for colorectal metastasis. It is our routine practice to utilize radiological investigations including CT scans and MRI scans of the liver pre-operatively. In addition, intra-operative ultrasound is an essential tool during these operations.12 Most surgeons would agree that the resection margin of the post-operative specimen is always narrower than suggested on the pre-operative imaging studies. Elias et al.13 investigated the amount of liver lost during hemi-hepatectomies using the clamp transection technique and indicated that between 5 and 8 mm of tumour-free margin will be lost during a hemi-hepatectomy because of this surgical technique, compared with the pre-operative measurements on imaging. We do not know the width of parenchyma lost during Cavitron ultrasonic surgical aspirator (CUSA) dissection.
There have been several recent publications evaluating outcomes in relation to liver resection margins. Table 7 summarizes the outcomes from studies which have been noted to have significant patient numbers. Earlier studies by Wray et al.14 and Cady et al.15 demonstrated the need for 10-mm resection margins in order to obtain significant survival benefits and low recurrence rates. In fact, Wray et al. recommended that a formal anatomical resection may confer an oncological benefit compared with a limited resection. Current trends for the approach to hepatic resections for colorectal metastases are more aggressive, and our data would support that of others that a significant survival benefit is obtained with a smaller margin.
Table 7.
Summary of publications on hepatic resection margins for colorectal liver metastases
| Paper | Year | No. of patients | Median follow-up (months) | Significant resection margin | 1 year (%) | 3 years (%) | 5 years (%) |
|---|---|---|---|---|---|---|---|
| de Haas et al.17 | 2008 | 436 | 40 | Higher recurrence in R1 vs. R0 | 61 (R0 group) 57 (R1 group) | ||
| Konopke et al.18 | 2008 | 333 | 28 | ≥3 mm | 58.9 | 32.5 | |
| Are et al.9 | 2007 | 1019 | 42 | >1 cm | 90 | 59 | 37 |
| Bodingbauer et al.16 | 2007 | 176 | 33 | No significant resection margin | 82.2 | 60.1 | |
| Pawlik et al.8 | 2005 | 557 | 29 | Negative (margin > 1 mm) |
97 | 74 | 58 |
| 17.1 (−ve margin) 63.8 (+ve margin) | |||||||
| Wray et al.a14 | 2004 | 112 | 25 | >1 cm | 43 (margin > 1 cm) |
||
| 37 (margin < 1 cm) | |||||||
| Cady et al.15 | 1998 | 244 | 37 | Negative margin ≥ 1 cm |
disease-free survival – 36.0 months (margin < 5 mm), 19.3 months (margin 5–10 mm), 15.7 months (margin > 10 mm); P= 0.0015.
The study published by the Memorial Sloan Kettering group9 was impressively large, but could only prove that a margin greater than 10 mm is an independent predictor of survival after hepatic resection for colorectal metastases. However, the authors did note that sub-centimetre resections are associated with favourable outcomes when compared with other modalities of treatment, and therefore recommended that the inability to achieve a 10-mm resection margin should not preclude the patient from undergoing a hepatic resection. The authors also indicated that their exclusive use of the Kelly clamp crush technique may have created a margin of different character than other techniques.
In contrast to the above studies, Bodingbauer et al.16 described recurrence-free survival as well as overall survival curves that were not influenced by the pathological margin status in their study of 176 patients. This conclusion was supported by de Haas et al.17 who looked at R0 compared with R1 resection margins of colorectal liver metastases and found no significant difference in 5-year overall survival or disease-free recurrence between these two groups. However, this study did not define the exact nature of an R1 resection margin, nor did it quantify the exact margins widths for R0 resections.
In our study, a resection margin of 10 mm or greater was achieved in 33.3% (87 out of 261) of resected specimens. This is comparable to other reported series with rates between 33% and 40%.9,16 The initial conclusions of significant overall survival and disease-free survival were drawn from data comparing involved resection margins with margins of at least 10 mm. Further analyses found that a resection margin of greater than 1 mm is associated with a favourable outcome and good long-term survival. The possible beneficial effect of greater margins beyond 1 mm could not be clearly demonstrated. The results of our study are consistent with those reported by the MD Anderson study.8 It is worth noting that in this study, the authors defined a positive margin as the presence of tumour cells at the line of transection or a margin less than 1 mm.
It is possible that a margin of less than 1 mm with at least some hepatic parenchyma may be adequate. We could not definitely determine this in our study. While the survival with a lesser margin is inferior, worthwhile 5-year survivals are still reported, in concordance with our study. Our results show that patients who had involved margins had a 5-year survival of 19%; still a much better outcome when compared with chemotherapy alone as initial treatment. Four out of the 28 patients with involved resection margins were R2 resections; however, it is likely that a possible margin had been achieved using our transection technique.
We also found no significant difference in the rate of resection margins ≤ 1 mm between patients who underwent anatomical liver resections (hemi-hepatectomies and extended hemi-hepatectomies) (21.5%) and patients with lesser resections (32.0%). Our study did not define the pattern of liver recurrence; however, previous studies have shown no statistical significance between the rate of surgical margin recurrence and the resection margin width.8
Our findings are very relevant as hepatic resection is considered the only potential curative treatment option for colorectal liver metastasis associated with long-term survival. The goal of hepatic resection is to achieve a complete resection while leaving sufficient remnant functioning liver in patients who are fit for surgery. Recent advances in surgical technique, anaesthesia and oncology have enabled more patients to undergo hepatic resection. This study demonstrates that a histological margin of greater than 1 mm is associated with significantly better patient survival. Although we aim to achieve a margin larger than 1 mm, and for safety 10 mm where possible, it is appropriate to embark on potentially curative resections in patients where at least a 1-mm margin can be obtained.
Acknowledgments
Special thanks to Carol Beeke, research officer of the South Australian metastatic colorectal cancer registry, for helping to collect the necessary data, and Paul Hakendorf, epidemiologist at Flinders Medical Centre, for the statistical analysis of the data.
Conflicts of interest
None declared.
References
- 1.Punt CJA. New options and old dilemmas in the treatment of patients with advanced colorectal cancer. Ann Oncol. 2004;15:1453–1459. doi: 10.1093/annonc/mdh383. [DOI] [PubMed] [Google Scholar]
- 2.Scheele J, Stang R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- 3.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival sollowing liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes KS, Rosenstein RB, Songhorabodi S, Adson MA, Ilstrup DM, Fortner JG, et al. Resection of the liver for colorectal carcinoma metastases. A multi-institutional study of long-term survivors. Dis Colon Rectum. 1988;31:1–4. doi: 10.1007/bf02552560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 6.Ekberg H, Tranberg KG, Andersson R, Jeppsson B, Bengmark S. Determinants of survival in liver resection for colorectal secondaries. Br J Surg. 1986;73:727–731. doi: 10.1002/bjs.1800730917. [DOI] [PubMed] [Google Scholar]
- 7.Registry of Hepatic Metastases. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of indications for resection. Surgery. 1988;103:278–288. [PMC free article] [PubMed] [Google Scholar]
- 8.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–724. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Are C, Gonen M, Zazzali K, Dematteo RP, Jarnaqin WR, Fong Y, et al. The impact of margins on outsome after hepatic resection for colorectal metastasis. Ann Surg. 2007;246:295–300. doi: 10.1097/SLA.0b013e31811ea962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiesser M, Chen JW, Maddern GJ, Padbury RT. Perioperative morbidity affects long-term survival in patients following liver resection for colorectal metastases. J Gastrointest Surg. 2008;12:1054–1060. doi: 10.1007/s11605-007-0438-y. [DOI] [PubMed] [Google Scholar]
- 11.Figueras J, Burdio F, Ramos E, Torras J, Llado L, Lopez-Ben S, et al. Effect of subcentimeter nonpositive resection margin on hepatic recurrence in patients undergoing hepatectomy for colorectal liver metastases. Evidence from 663 liver resections. Ann Oncol. 2007;18:1190–1195. doi: 10.1093/annonc/mdm106. [DOI] [PubMed] [Google Scholar]
- 12.Pawlik TM, Vauthey J-N. Surgical margins during hepatic surgery for colorectal liver metastases: complete resection not milimeters defines outcome. Ann Surg Oncol. 2008;15:677–679. doi: 10.1245/s10434-007-9703-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias D, Bonnet S, Honore C, Kohneh-Shahri N, Tomasic G, Lassau N, et al. Comparison between the minimum margin defined on preoperative imaging and the final surgical margin after hepatectomy for cancer: how to manage it? Ann Surg Oncol. 2008;15:777–781. doi: 10.1245/s10434-007-9697-9. [DOI] [PubMed] [Google Scholar]
- 14.Wray CJ, Lowy AM, Mathews JB, Park S, Choe KA, Hanto DW, et al. The significance and clinical factors associated with a subcentimeter resection of colorectal liver metastases. Ann Surg Oncol. 2004;12:1–7. doi: 10.1245/ASO.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 15.Cady B, Jenkins RL, Steele GD, Lewis WD, Stone MD, McDermott WV, et al. Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome. Ann Surg. 1998;227:566–571. doi: 10.1097/00000658-199804000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodingbauer M, Tamandl D, Schmid K, Plank C, Schima W, Gruenberger T. Size of surgical margin does not influence recurrence rates after curative liver resection for colorectal cancer liver metastases. Br J Surg. 2007;94:1133–1138. doi: 10.1002/bjs.5762. [DOI] [PubMed] [Google Scholar]
- 17.de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008;248:626–637. doi: 10.1097/SLA.0b013e31818a07f1. [DOI] [PubMed] [Google Scholar]
- 18.Konopke R, Kersting S, Makowiec F, Gassmann P, Kuhlisch E, Senninger N, et al. Resection of colorectal liver metastases: is a resection margin of 3 mm enough? World J Surg. 2008;32:2047–2056. doi: 10.1007/s00268-008-9629-2. [DOI] [PubMed] [Google Scholar]


