Abstract
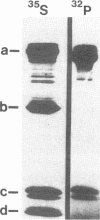
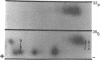
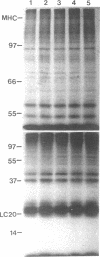
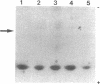
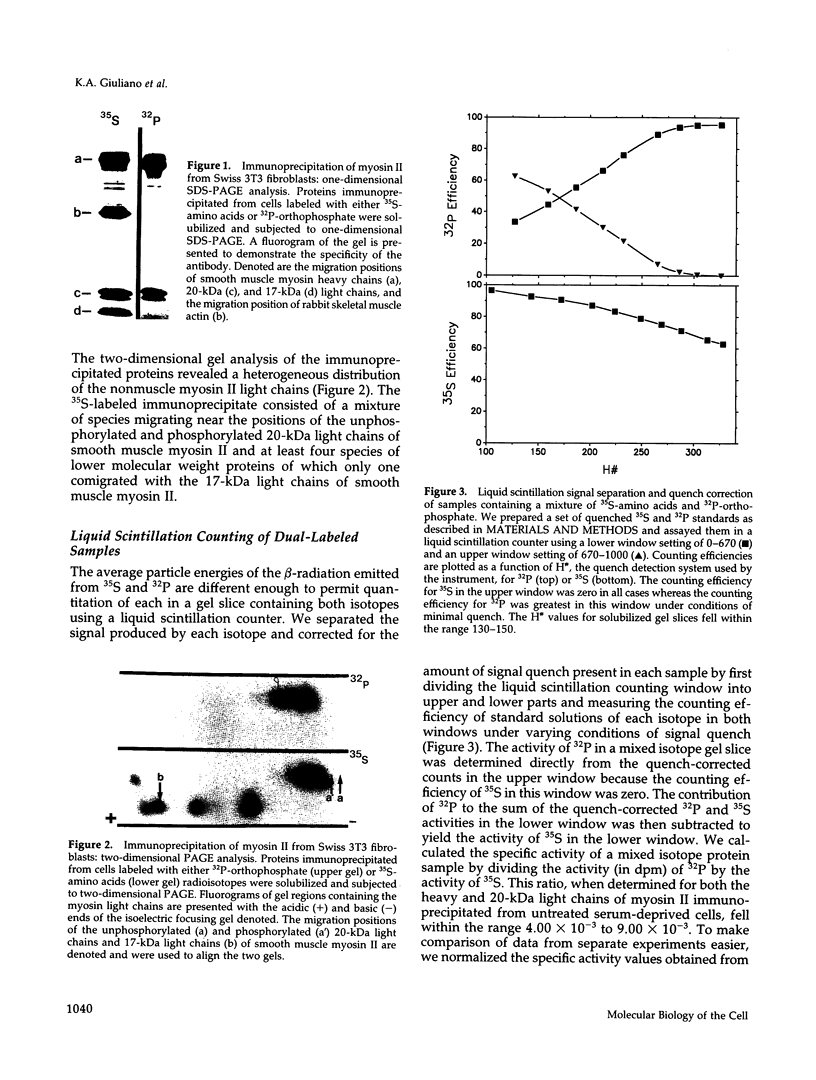
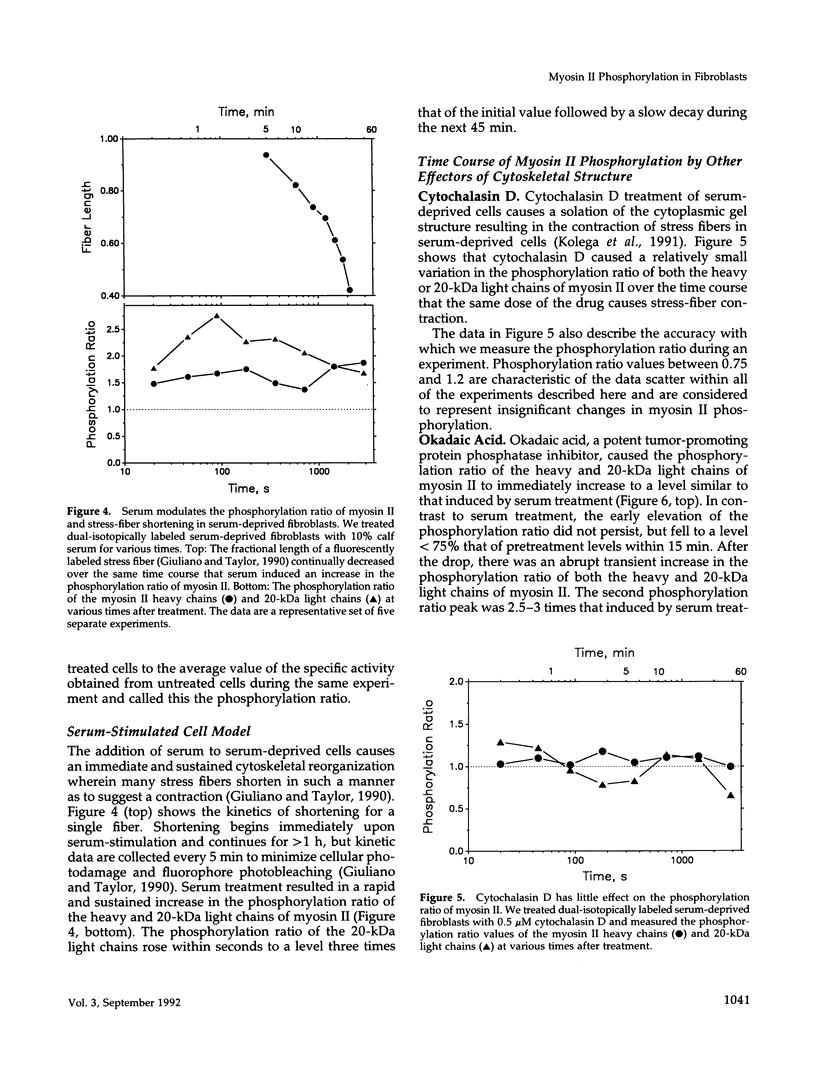
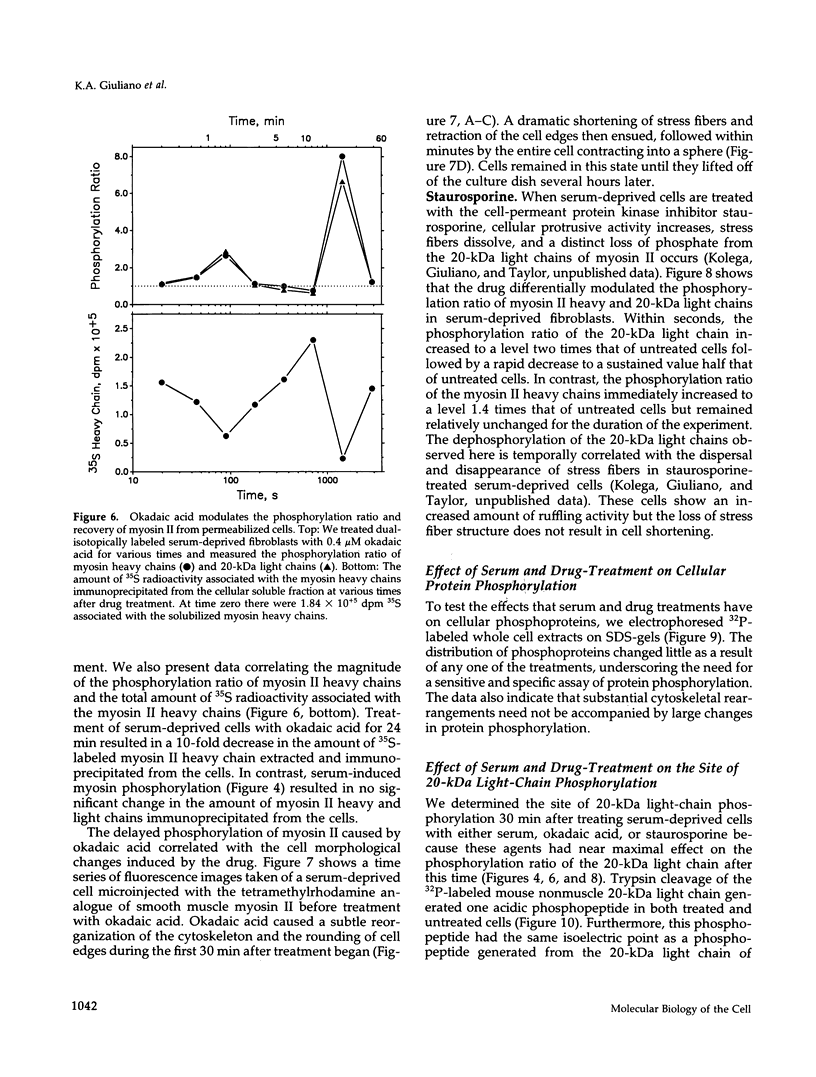
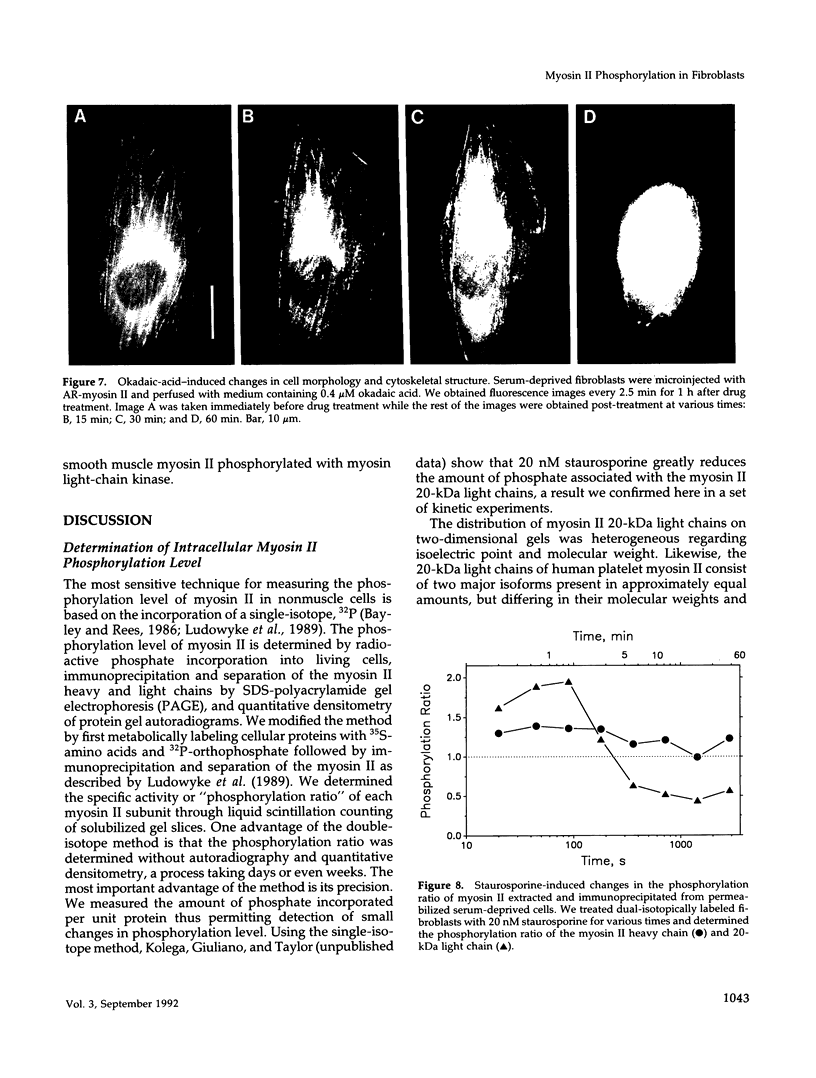
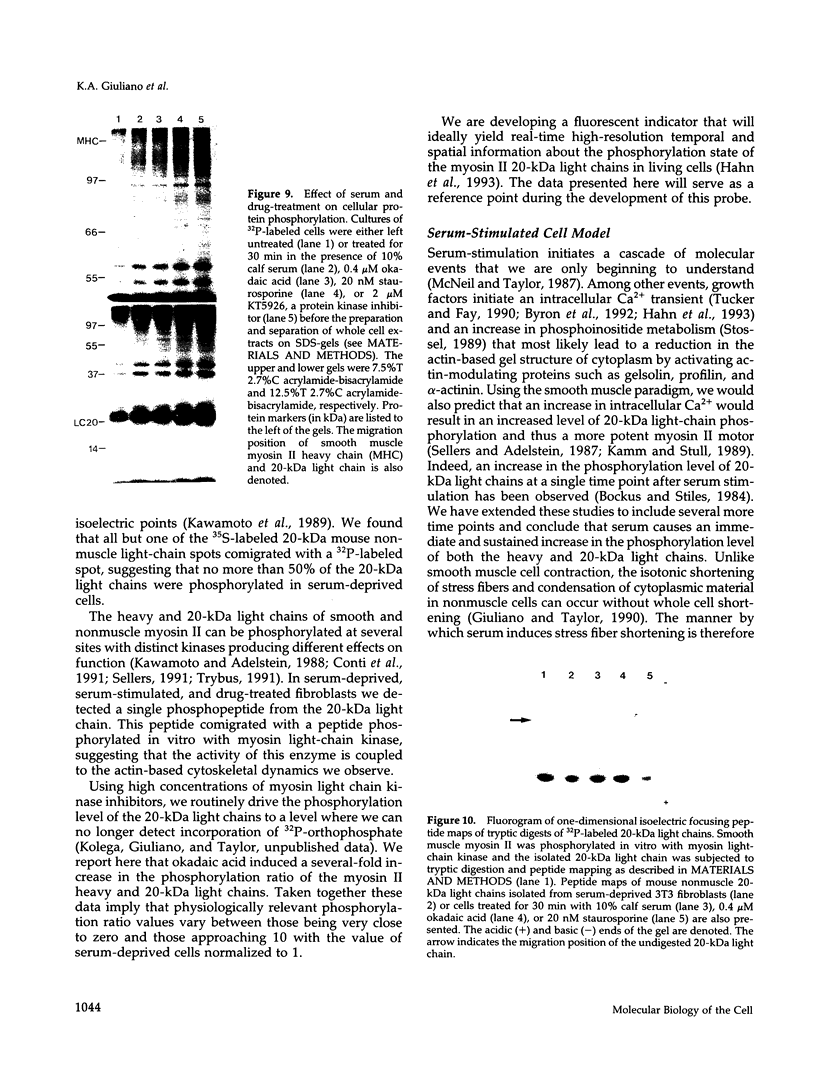
The actin-based cytomatrix generates stress fibers containing a host of proteins including actin and myosin II and whose dynamics are easily observable in living cells. We developed a dual-radioisotope-based assay of myosin II phosphorylation and applied it to serum-deprived fibroblasts treated with agents that modified the dynamic distribution of stress fibers and/or altered the phosphorylation state of myosin II. Serum-stimulation induced an immediate and sustained increase in the level of myosin II heavy chain (MHC) and 20-kDa light chain (LC20) phosphorylation over the same time course that it caused stress fiber contraction. Cytochalasin D, shown to cause stress fiber fragmentation and contraction, had little effect on myosin II phosphorylation. Okadaic acid, a protein phosphatase inhibitor, induced a delayed but massive cell shortening preceded by a large increase in MHC and LC20 phosphorylation. Staurosporine, a kinase inhibitor known to effect dissolution but not contraction of stress fibers, immediately caused an increase in MHC and LC20 phosphorylation followed within minutes by the dephosphorylation of LC20 to a level below that of untreated cells. We therefore propose that the contractility of the actin-based cytomatrix is regulated by both modulating the activity of molecular motors such as myosin II and by altering the gel structure in such a manner as to either resist or yield to the tension applied by the motors.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amato P. A., Taylor D. L. Probing the mechanism of incorporation of fluorescently labeled actin into stress fibers. J Cell Biol. 1986 Mar;102(3):1074–1084. doi: 10.1083/jcb.102.3.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley S. A., Rees D. A. Myosin light chain phosphorylation in fibroblast shape change, detachment and patching. Eur J Cell Biol. 1986 Oct;42(1):10–16. [PubMed] [Google Scholar]
- Bockus B. J., Stiles C. D. Regulation of cytoskeletal architecture by platelet-derived growth factor, insulin and epidermal growth factor. Exp Cell Res. 1984 Jul;153(1):186–197. doi: 10.1016/0014-4827(84)90460-9. [DOI] [PubMed] [Google Scholar]
- Buckley I. K., Porter K. R. Cytoplasmic fibrils in living cultured cells. A light and electron microscope study. Protoplasma. 1967;64(4):349–380. doi: 10.1007/BF01666538. [DOI] [PubMed] [Google Scholar]
- Burridge K. Are stress fibres contractile? Nature. 1981 Dec 24;294(5843):691–692. doi: 10.1038/294691a0. [DOI] [PubMed] [Google Scholar]
- Byron K. L., Babnigg G., Villereal M. L. Bradykinin-induced Ca2+ entry, release, and refilling of intracellular Ca2+ stores. Relationships revealed by image analysis of individual human fibroblasts. J Biol Chem. 1992 Jan 5;267(1):108–118. [PubMed] [Google Scholar]
- Cande W. Z., Ezzell R. M. Evidence for regulation of lamellipodial and tail contraction of glycerinated chicken embryonic fibroblasts by myosin light chain kinase. Cell Motil Cytoskeleton. 1986;6(6):640–648. doi: 10.1002/cm.970060612. [DOI] [PubMed] [Google Scholar]
- Conti M. A., Sellers J. R., Adelstein R. S., Elzinga M. Identification of the serine residue phosphorylated by protein kinase C in vertebrate nonmuscle myosin heavy chains. Biochemistry. 1991 Jan 29;30(4):966–970. doi: 10.1021/bi00218a012. [DOI] [PubMed] [Google Scholar]
- DeBiasio R. L., Wang L. L., Fisher G. W., Taylor D. L. The dynamic distribution of fluorescent analogues of actin and myosin in protrusions at the leading edge of migrating Swiss 3T3 fibroblasts. J Cell Biol. 1988 Dec;107(6 Pt 2):2631–2645. doi: 10.1083/jcb.107.6.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A., Brautigan D. L., Mumby M., Lamb N. J. Protein phosphatase type-1, not type-2A, modulates actin microfilament integrity and myosin light chain phosphorylation in living nonmuscle cells. J Cell Biol. 1990 Jul;111(1):103–112. doi: 10.1083/jcb.111.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano K. A. Aqueous two-phase protein partitioning using textile dyes as affinity ligands. Anal Biochem. 1991 Sep 2;197(2):333–339. doi: 10.1016/0003-2697(91)90401-e. [DOI] [PubMed] [Google Scholar]
- Giuliano K. A., Gillies R. J. Determination of intracellular pH of BALB/c-3T3 cells using the fluorescence of pyranine. Anal Biochem. 1987 Dec;167(2):362–371. doi: 10.1016/0003-2697(87)90178-3. [DOI] [PubMed] [Google Scholar]
- Giuliano K. A., Khatib F. A., Hayden S. M., Daoud E. W., Adams M. E., Amorese D. A., Bernstein B. W., Bamburg J. R. Properties of purified actin depolymerizing factor from chick brain. Biochemistry. 1988 Dec 13;27(25):8931–8938. doi: 10.1021/bi00425a009. [DOI] [PubMed] [Google Scholar]
- Giuliano K. A., Taylor D. L. Formation, transport, contraction, and disassembly of stress fibers in fibroblasts. Cell Motil Cytoskeleton. 1990;16(1):14–21. doi: 10.1002/cm.970160104. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Machesky L. M., Doberstein S. K., Pollard T. D. Mechanism of the interaction of human platelet profilin with actin. J Cell Biol. 1991 Jun;113(5):1081–1089. doi: 10.1083/jcb.113.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy G. R., Cao X., Chua S. P., Tan Y. H. Okadaic acid mimics multiple changes in early protein phosphorylation and gene expression induced by tumor necrosis factor or interleukin-1. J Biol Chem. 1992 Jan 25;267(3):1846–1852. [PubMed] [Google Scholar]
- Hartwig J. H., Kwiatkowski D. J. Actin-binding proteins. Curr Opin Cell Biol. 1991 Feb;3(1):87–97. doi: 10.1016/0955-0674(91)90170-4. [DOI] [PubMed] [Google Scholar]
- Hartwig J. H., Shevlin P. The architecture of actin filaments and the ultrastructural location of actin-binding protein in the periphery of lung macrophages. J Cell Biol. 1986 Sep;103(3):1007–1020. doi: 10.1083/jcb.103.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel G., Wehland J., Weber K. Calcium control of actin-myosin based contraction in triton models of mouse 3T3 fibroblasts is mediated by the myosin light chain kinase (MLCK)-calmodulin complex. Exp Cell Res. 1983 Oct;148(1):117–126. doi: 10.1016/0014-4827(83)90192-1. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Formation of arrowhead complexes with heavy meromyosin in a variety of cell types. J Cell Biol. 1969 Nov;43(2):312–328. [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Ikebe M., Kargacin G. J., Hartshorne D. J., Kemp B. E., Fay F. S. Effects of modulators of myosin light-chain kinase activity in single smooth muscle cells. Nature. 1989 Mar 9;338(6211):164–167. doi: 10.1038/338164a0. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Stossel T. P. Gelsolin-polyphosphoinositide interaction. Full expression of gelsolin-inhibiting function by polyphosphoinositides in vesicular form and inactivation by dilution, aggregation, or masking of the inositol head group. J Biol Chem. 1989 Mar 25;264(9):4825–4831. [PubMed] [Google Scholar]
- Janson L. W., Kolega J., Taylor D. L. Modulation of contraction by gelation/solation in a reconstituted motile model. J Cell Biol. 1991 Sep;114(5):1005–1015. doi: 10.1083/jcb.114.5.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm K. E., Hsu L. C., Kubota Y., Stull J. T. Phosphorylation of smooth muscle myosin heavy and light chains. Effects of phorbol dibutyrate and agonists. J Biol Chem. 1989 Dec 15;264(35):21223–21229. [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. Regulation of smooth muscle contractile elements by second messengers. Annu Rev Physiol. 1989;51:299–313. doi: 10.1146/annurev.ph.51.030189.001503. [DOI] [PubMed] [Google Scholar]
- Kawamoto S., Adelstein R. S. The heavy chain of smooth muscle myosin is phosphorylated in aorta cells. J Biol Chem. 1988 Jan 25;263(3):1099–1102. [PubMed] [Google Scholar]
- Kawamoto S., Bengur A. R., Sellers J. R., Adelstein R. S. In situ phosphorylation of human platelet myosin heavy and light chains by protein kinase C. J Biol Chem. 1989 Feb 5;264(4):2258–2265. [PubMed] [Google Scholar]
- Kolega J., Janson L. W., Taylor D. L. The role of solation-contraction coupling in regulating stress fiber dynamics in nonmuscle cells. J Cell Biol. 1991 Sep;114(5):993–1003. doi: 10.1083/jcb.114.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Conti M. A., Adelstein R., Glass D. B., Welch W. J., Feramisco J. R. Regulation of actin microfilament integrity in living nonmuscle cells by the cAMP-dependent protein kinase and the myosin light chain kinase. J Cell Biol. 1988 Jun;106(6):1955–1971. doi: 10.1083/jcb.106.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langanger G., Moeremans M., Daneels G., Sobieszek A., De Brabander M., De Mey J. The molecular organization of myosin in stress fibers of cultured cells. J Cell Biol. 1986 Jan;102(1):200–209. doi: 10.1083/jcb.102.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specificity of the interaction between phosphatidylinositol 4,5-bisphosphate and the profilin:actin complex. J Cell Biochem. 1988 Jul;37(3):255–267. doi: 10.1002/jcb.240370302. [DOI] [PubMed] [Google Scholar]
- Ludowyke R. I., Peleg I., Beaven M. A., Adelstein R. S. Antigen-induced secretion of histamine and the phosphorylation of myosin by protein kinase C in rat basophilic leukemia cells. J Biol Chem. 1989 Jul 25;264(21):12492–12501. [PubMed] [Google Scholar]
- Masuda H., Owaribe K., Hayashi H., Hatano S. Ca2+-dependent contraction of human lung fibroblasts treated with Triton X-100: a role of Ca2+-calmodulin-dependent phosphorylation of myosin 20,000-dalton light chain. Cell Motil. 1984;4(5):315–331. doi: 10.1002/cm.970040503. [DOI] [PubMed] [Google Scholar]
- McNeil P. L., McKenna M. P., Taylor D. L. A transient rise in cytosolic calcium follows stimulation of quiescent cells with growth factors and is inhibitable with phorbol myristate acetate. J Cell Biol. 1985 Aug;101(2):372–379. doi: 10.1083/jcb.101.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal B., Sanger J. M., Sanger J. W. Visualization of myosin in living cells. J Cell Biol. 1987 Oct;105(4):1753–1760. doi: 10.1083/jcb.105.4.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabayashi H., Sellers J. R., Huang K. P. Catalytic fragment of protein kinase C exhibits altered substrate specificity toward smooth muscle myosin light chain. FEBS Lett. 1991 Dec 2;294(1-2):144–148. doi: 10.1016/0014-5793(91)81362-c. [DOI] [PubMed] [Google Scholar]
- Pollard T. D. Assays for myosin. Methods Enzymol. 1982;85(Pt B):123–130. doi: 10.1016/0076-6879(82)85015-5. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Sanger J. M., Sanger J. W. Banding and polarity of actin filaments in interphase and cleaving cells. J Cell Biol. 1980 Aug;86(2):568–575. doi: 10.1083/jcb.86.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. W., Mittal B., Sanger J. M. Interaction of fluorescently-labeled contractile proteins with the cytoskeleton in cell models. J Cell Biol. 1984 Sep;99(3):918–928. doi: 10.1083/jcb.99.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul A., Don M. A rapid method of concentrating proteins in small volumes with high recovery using Sephadex G-25. Anal Biochem. 1984 May 1;138(2):451–453. doi: 10.1016/0003-2697(84)90838-8. [DOI] [PubMed] [Google Scholar]
- Sellers J. R. Regulation of cytoplasmic and smooth muscle myosin. Curr Opin Cell Biol. 1991 Feb;3(1):98–104. doi: 10.1016/0955-0674(91)90171-t. [DOI] [PubMed] [Google Scholar]
- Springer W. R. A method for quantifying radioactivity associated with protein in silver-stained polyacrylamide gels. Anal Biochem. 1991 May 15;195(1):172–176. doi: 10.1016/0003-2697(91)90314-j. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Chaponnier C., Ezzell R. M., Hartwig J. H., Janmey P. A., Kwiatkowski D. J., Lind S. E., Smith D. B., Southwick F. S., Yin H. L. Nonmuscle actin-binding proteins. Annu Rev Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. From signal to pseudopod. How cells control cytoplasmic actin assembly. J Biol Chem. 1989 Nov 5;264(31):18261–18264. [PubMed] [Google Scholar]
- Tansey M. G., Hori M., Karaki H., Kamm K. E., Stull J. T. Okadaic acid uncouples myosin light chain phosphorylation and tension in smooth muscle. FEBS Lett. 1990 Sep 17;270(1-2):219–221. doi: 10.1016/0014-5793(90)81272-p. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Fechheimer M. Cytoplasmic structure and contractility: the solation--contraction coupling hypothesis. Philos Trans R Soc Lond B Biol Sci. 1982 Nov 4;299(1095):185–197. doi: 10.1098/rstb.1982.0125. [DOI] [PubMed] [Google Scholar]
- Tindall S. H. Selection of chemical spacers to improve isoelectric focusing resolving power: implications for use in two-dimensional electrophoresis. Anal Biochem. 1986 Dec;159(2):287–294. doi: 10.1016/0003-2697(86)90345-3. [DOI] [PubMed] [Google Scholar]
- Trybus K. M. Assembly of cytoplasmic and smooth muscle myosins. Curr Opin Cell Biol. 1991 Feb;3(1):105–111. doi: 10.1016/0955-0674(91)90172-u. [DOI] [PubMed] [Google Scholar]
- Tucker R. W., Fay F. S. Distribution of intracellular free calcium in quiescent BALB/c 3T3 cells stimulated by platelet-derived growth factor. Eur J Cell Biol. 1990 Feb;51(1):120–127. [PubMed] [Google Scholar]
- Wysolmerski R. B., Lagunoff D. Regulation of permeabilized endothelial cell retraction by myosin phosphorylation. Am J Physiol. 1991 Jul;261(1 Pt 1):C32–C40. doi: 10.1152/ajpcell.1991.261.1.C32. [DOI] [PubMed] [Google Scholar]
- Yonezawa N., Nishida E., Iida K., Yahara I., Sakai H. Inhibition of the interactions of cofilin, destrin, and deoxyribonuclease I with actin by phosphoinositides. J Biol Chem. 1990 May 25;265(15):8382–8386. [PubMed] [Google Scholar]