Abstract
Increasing age is associated with a poor prognosis following traumatic brain injury (TBI). Central nervous system axons may recover poorly following TBI due to expression of myelin-derived inhibitors to axonal outgrowth such as Nogo-A. To study the role of Nogo-A/B in the pathophysiological response of the elderly to TBI, 1-year old mice deficient in Nogo-A/B (Nogo-A/B homozygous −/− mice), Nogo-A/B heterozygous −/+ mice, and age-matched wild-type (WT) littermate controls were subjected to a controlled cortical impact (CCI) TBI. Sham-injured WT mice (7 months old) and 12 months old naïve Nogo-A/B−/− and Nogo-A/B−/+ served as controls. Neurological motor function was evaluated up to 3 weeks, and cognitive function, hemispheric tissue loss, myelin staining and hippocampal β-amyloid (Aβ) immunohistochemistry was evaluated at 4 weeks post-injury. In WT littermates, TBI significantly impaired learning ability at 4 weeks and neurological motor function up to 2 weeks post-injury and caused a significant loss of hemispheric tissue. Following TBI, Nogo-A/B−/− mice showed significantly less recovery from neurological motor deficits compared to brain-injured WT mice. Naïve Nogo-A/B−/−and Nogo-A/B−/+ mice quickly learned the MWM task in contrast to brain-injured Nogo-A/B−/− mice who failed to learn the MWM task at 4 week post-injury. Hemispheric tissue loss and cortical lesion volume was similar among the brain-injured genotypes. Neither TBI nor the absence of NogoA/B caused an increased Aβ expression. Myelin staining showed a reduced area and density in the corpus callosum in brain-injured Nogo-A/B−/−animals compared to their littermate controls. These novel and unexpected behavioral results demonstrate that the absence of Nogo-A/B may negatively influence outcome, possibly related to hypomyelination, following TBI in mice and suggest a complex role for this myelin-associated axonal growth inhibitor following TBI.
Keywords: Traumatic brain injury, Nogo-A, Nogo-B, Morris Water Maze, neurological motor deficits
Introduction
Prognostic factors related to the clinical outcome following traumatic brain injury (TBI) include lesion type, level of consciousness and the extent of associated systemic injuries. Additionally, age has long been recognized as a critical prognostic factor when predicting outcome (Harris et al., 2003; Hukkelhoven et al., 2003; Brain Trauma Foundation, 2000). Although elderly patients may gradually regain some of their lost abilities following TBI, the functional recovery is usually incomplete and unsatisfactory since the adult brain loses much of the capacity for plasticity and repair as it ages (Hukkelhoven et al., 2003).
In approximately 50% of patients with a severe TBI, there is wide-spread axonal injury believed to contribute to the cognitive and behavioral sequelae observed post-injury (Adams et al., 2001; Maxwell et al., 1997; Graham et al., 2005; Hurley et al., 2004). Although originally described in severely brain-injured patients, damage to axons has also been observed in cases of mild and/or predominately focal TBI. Despite an increase in our understanding of the prevalence of axonal injury following TBI, specific treatment strategies reducing the deficits associated with axonal injury remain unavailable.
A major impediment to the recovery of both young and old TBI patients may be the failure of adult central nervous system (CNS) axon regeneration following injury (Hunt et al., 2002; Teng and Tang, 2005a). Although numerous factors including an insufficient intrinsic growth-promoting capacity of CNS neurons and the presence of a glial scar may contribute to the failure of axon regeneration, numerous studies indicate that myelin-associated inhibitors to axonal outgrowth (MAIs) constitute a major obstacle to axonal regeneration (for review see Buchli and Schwab, 2005). These MAIs include Nogo-A, myelin-associated glycoprotein (MAG) and oligodendrocyte-myelin glycoprotein (OMgp; Hunt et al., 2002). The structurally distinct proteins Nogo-A, MAG and OMgp all bind to the same receptor, the neuronal Nogo-66 receptor 1 (NgR1; Fournier et al., 2001). The Nogo gene was identified encoding a member of the Reticulon family of proteins (Chen et al., 2000; Prinjha et al., 2000; Grandpre et al., 2000). Three major protein isoforms named Nogo-A, B and C are generated from the Nogo gene via alternate splicing and differential promotor usage (Chen et al., 2000; GrandPré et al., 2000). The inhibitory actions of Nogo-A are mediated via at least two active regions including a 66 amino acid extracellular loop (also named Nogo-66), common to all isoforms of Nogo, and an N-terminal region specific to Nogo-A (Oertle et al., 2003; Fournier et al., 2002). Considerable in vitro and in vivo evidence exists implicating the Nogo protein family in axon growth inhibition where Nogo-A is considered, by far, the most important inhibitory isoform in models of CNS injury. Using neutralizing monoclonal antibodies (mAbs) to Nogo-A (Oertle et al., 2003), increased axonal outgrowth and sprouting correlated with enhanced functional recovery following experimental spinal cord injury (SCI; Liebscher et al., 2005) and focal cerebral ischemia in rodents (Markus et al., 2005; Seymour et al., 2005). These mAbs have also been shown to enhance cognitive recovery following experimental TBI in rats (Lenzlinger et al., 2005; Marklund et al., 2007). Furthermore, soluble fragments of NgR1 which block Nogo-A-NgR1 interaction were found to increase axonal growth and improve neurological outcome after rat SCI and stroke (Li and Strittmatter, 2003, Lee et al., 2004, Wang et al., 2006). These reports suggest that pharmacological interventions to overcome myelin inhibition to axonal regeneration may be a promising treatment strategy for acute CNS injury (see Teng and Tang, 2005b).
When mice with genetic modification of Nogo was evaluated following SCI, there was no or moderate axonal regeneration observed in Nogo-A/B−/−, Nogo-A/B/C−/− or Nogo-A−/− mice (Simonen et al., 2003; Zheng et al., 2003). Furthermore, the extent of corticospinal tract (CST) regeneration following SCI in Nogo-A−/− mice was influenced by strain background (Simonen et al., 2003; Dimou et al., 2006). In contrast, marked CST sprouting and functional recovery was observed in young Nogo-A/B−/− mice (Kim et al., 2003). Intercrosses of these alleles and the examination of a pyramidotomy injury model showed that both Nogo alleles allow CNS axonal growth but that the penetrance of the phenotype for CST regeneration is highly variable, depending on age and allele structure (Cafferty and Strittmatter, 2006, Cafferty et al., 2007).
The response to genetic modification of Nogo has not previously been evaluated in experimental models of TBI. We used a well-characterized and clinically relevant model of TBI in aging Nogo-A/B homozygous and heterozygous knockout mice (12 months old) to evaluate the potential role of Nogo-A/B on behavioral and histological outcome following TBI in aging animals. Although Nogo-B has growth-inhibitory properties in vitro, it is a minor isoform in the brain and was deleted from our mice due to the gene constructs used (vide infra). The controlled cortical impact (CCI) injury model used in the present report is a predominately focal TBI model although widespread axonal injury is also observed in both hemispheres (Hall et al., 2005; Hall et al., 2008). We used a NogoA/B−/− mouse strain previously showing marked axonal regeneration following SCI (Kim et al., 2003) and hypothesized that genetic absence of NogoA/B would result in an improved functional outcome following TBI.
MATERIAL AND METHODS
All animals were housed at 24°C in cages with 3–5 mice per cage with food and water ad libitum in a 12-hour light/dark cycle. The animals were kept in the colony for a minimum of one week prior to any surgical procedures. All procedures described herein were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania and conducted in accordance with National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, 1996).
Gene-targeted Mouse Strains
Aged Nogo-A/B−/− Mice
Twelve month-old mice (n=8, 5 male, 3 female) with a targeted deletion of Nogo A/B (Nogo-A/B−/−) were generated by one of us (SS). A nogo-targeted embryonic stem cell clone was identified from the OmniBank Sequence Tag database 8OST 45048, Lexicon Genetics, Inc.) Here, the nogo gene had been “trapped” using retroviral-based gene trap methodology and was identified from sequences of the trapped exons. The gene trap insertion site maps near the 5′ end of the largest nogo-A-selective exon. Based on the gene trap design and the insertion site, both nogo-A and nogo-B, but not nogo-C, expression will be disrupted by this insertion (see Kim et al., 2003). Heterozygous mice harboring the disrupted allele were backcrossed to wild type C57BL/6J mice over a minimum of 5 generations. The heterozygotes were then intercrossed to collect litters with wild type, +/− and −/− genotypes. These littermate-matched mice were subjected to CCI brain injury (vide infra). For comparison, mice heterozygous for Nogo A/B (Nogo-A/B+/− mice, n=8, 4 male and 4 female) and wild-type, littermate controls (n=8, 3 male and 5 female) of the same age were also subjected to CCI brain injury. No obvious phenotypic differences were observed among the Nogo-A/B−/−, NogoA/B+/− WT littermate controls or sham-injured control mice. In all behavioral and histological studies, the investigators were unaware of the mouse genotypes at the time of behavioral or histological assessment. The limited availability of Nogo-A/B−/− mice, NogoA/B−/+ mice and their wild-type littermates at the time for the CCI brain injury experiments did not allow us to use separate sham-injured controls of these genotypes. We have sought to transfer animals from the breeding facility at Yale University, New Haven to Uppsala, Sweden to conduct such control experiments but this transfer cannot be completed without rederiving the line. Instead, sham-injured, wild-type C57BL/6J control mice (seven months old male mice, n=6) were used as internal controls for the injury effects. Later, naïve 12 months old Nogo-A/B−/− (n=7; 3 male and 4 female), NogoA/B−/+ mice (n= 7; 4 male and 3 female) were used as controls in the Morris Water Maze task (vide infra). During surgery and injury, mice from all injury groups were randomized into one of four injury groups based on age, genotype and gender. Surgery and injury was performed on the same day in each group. Due to the limited availability of the genetically modified aging mice and their littermates at the time of CCI brain injury, no sham-injured, age-matched mice for each genotype could be made available for our study. Instead, we separately evaluated the cognitive performance of naive, genetically modified and age-matched mice at a later time point. Second, the limited number of brain-injured animals did not allow a detailed histological analysis at earlier or later post-injury time-points. The group composition of all mice involved in the present study is shown in Table 1.
Table 1.
Animal age and numbers (n) in the evaluated groups.
| Group | Age | n |
|---|---|---|
| Sham WT | 7 months | 6 |
| Nogo-A/B WT | 12 months | 8 |
| Nogo-A/B−/+ | 12 months | 8 |
| Nogo-A/B−/− | 12 months | 8 |
| Nogo-A/B−/+ Naive | 12 months | 6 |
| Nogo-A/B−/− Naive | 12 months | 7 |
Controlled Cortical Impact Traumatic Brain Injury
Mice (n=30) were anaesthetized with sodium pentobarbital (65 mg/kg i.p., Nembutal sodium solution; Abbott Laboratories, North Chicago, IL) and placed in a stereotaxic frame adopted for mice (Kopf CA), on a 37°C-heating pad. An eye lubricant ointment (Duratears naturale; Alcon Laboratories, Fort Worth, TX) protected the cornea during surgery. After exposing the skull using a midline scalp incision, a 5-mm rounded craniectomy was performed over the left parieto-temporal cortex between lambda and bregma, keeping the dura mater intact (Murai et al., 1998). CCI brain injury was performed in 24 mice as described previously (Dixon et al., 1991; Smith et al., 1995) using a 3 mm diameter rounded rigid impounder driven by a pneumatic piston, mounted at a 20° angle from the vertical plane and applied perpendicularly to the exposed dura mater over the left parieto-temporal cortex, at an established velocity and depth of deformation. To obtain the zero point, the impactor tip was lowered until it touched the intact dura mater. A transducer positioned on the device was connected to a linear velocity displacement transducer (model ATC-101; Schaevitz, Pennsauken, NJ, USA), which produces an analog signal recorded by a computer program (R.C. Electronics, Goleta, CA, USA) for the analysis of the biomechanics of the impact. The impact was set at a velocity of 4.8–5.0 m/sec and depth of deformation of 1.0 mm relative to the dura mater. After the impact, the craniectomy was covered with a cranioplasty and the scalp sutured. Sham-injured animals (n=6) received the same anesthesia and all surgical procedures (craniectomy), but were not subjected to CCI brain injury. All animals were then allowed to recover from surgery on a heating pad maintained at 37°C. We have previously shown that the temperature of the temporalis muscle remains at 37°C during the surgical procedure in mice (Nakamura et al., 1999). To minimize the variability, all surgeries and injuries were performed by the same investigator, blinded to the genotype of each animal.
Assessment of Motor Function in mice pre- and post-injury
Prior to surgery and injury, motor function was assessed for baseline values and then again at 48h, 1 week and 2 and 3 weeks post-injury by a trained observer who was blinded to animal status regarding injury or genotype. Forelimb function was evaluated by suspending the animals by the tail and observing how they grasped a grid when lowered towards it and the pattern of toe spread and hind limb extension during the suspension to asses for hind limbs deficits. In addition, animals were tested for both right and left resistance to lateral pulsion. The animals were scored from 4 (normal) to 0 (severely impaired) for each of the following indices: 1) forelimb function during walking on the grid and flexion response during suspension by the tail; 2) hind limb function during walking on the grid and flexion/toe extension during suspension by the tail; 3) resistance to lateral right and left pulsion as previously described (Longhi et al., 2004). The maximum score for each animal was 12.
Assessment of Post-Injury Cognitive Function
Evaluation of learning ability in mice following sham- or CCI brain injury was performed at four weeks post-injury using the Morris water maze (MWM) test of learning ability. The MWM has been previously shown to be a highly sensitive paradigm for measuring post-traumatic visuospatial learning and memory in mice (Longhi et al., 2004; Fox et al., 1998; Smith et al., 1995). Our MWM is a white circular pool (1 m in diameter) that is filled with water (18–20°C) made opaque by adding non-toxic water-soluble white paint. The post-injury visuospatial learning task requires that animals learn how to locate a 10 cm diameter platform, submerged 0.5 cm under the surface of the water and 20 cm from the wall of the pool, using external visual cues. The feature of this task is to evaluate how fast mice learn to escape from the water onto the platform after being randomly placed into the pool at one of four sites. Latencies to reach and climb onto the platform are recorded for each trial, with a maximum of 60 sec per trial. The learning task consists of 8 trials/day, using two trial blocks with four trials per block for three consecutive days for a total of 24 trials. Following each MWM trial, the animals were placed under a heating lamp to maintain normothermia. Using an identical testing paradigm including pool diameter, water temperature and trial block design (experiments performed at Department of Neurology, Yale University, USA) naïve NogoA/B−/− and NogoA/B−/+ were evaluated as uninjured controls in the MWM. Since the breeding strategy used for the generation of NogoA/B−/− mice focused on crossing of NogoA/B−/− with NogoA/B−/+, no one-year old NogoA/B+/+ control mice were available.
Sham- or brain-injured animals were evaluated in the maze for their ability to recall the previously learned task (probe trial) at 48 hours following the last MWM learning trial. In this memory test, each animal was allowed 60 seconds in the maze with the platform removed, while its swim pattern was tracked. The MWM was divided into specified zones, and a memory score was calculated for each animal by multiplying the amount of time spent in these zones by a weighting factor according to its proximity to the platform. These assigned numbers were multiplied by the number of seconds spent in the corresponding zone and totaled according to a paradigm originally described by Smith et al. (1991). The average of the two trial scores or the first score, if that was higher than the second trial score, was used as the numeric score for the probe trial.
Hemispheric Tissue Loss
Following behavioral evaluation, all animals were overanaesthetised with sodium pentobarbital (200 mg/kg, i.p.) and transcardially perfused with 0.9% saline containing 1000 U of heparin/liter followed by 4 % paraformaldehyde (PFA) for a minimum of 5 minutes. The brains were removed and postfixed at 4°C in PFA and the brains were then embedded in paraffin. The blocks were cut from bregma 1 mm to −4.5 mm in 5 μm thick coronal sections on a microtome (Anglia Scientific, Cambridge, U.K.) and six sections evenly distributed between bregma 0.3 mm and −4.0 mm (Paxinos and Franklin, 2001) were selected for the evaluation of loss of hemispheric tissue (Fox et al., 1998). Sections were stained with Mayer’s Heamatoxylin (Histolab, Gothenburg, Sweden) and Eosin (Histolab) and imaged using a digital scanner (Nikon Super CoolScan 4000ED, Nikon Imaging, Japan). The periphery of the contralateral and ipsilateral hemispheres were traced on each image by an evaluator blinded to the injury and treatment status of the animals and the area of each hemisphere was calculated using a calibrated image analysis routine, using the software ImageJ (NIH, Bethesda, USA). Based on previous investigations, which showed negligible contralateral tissue loss following experimental focal TBI, the contralateral hemisphere was used in each section to control for inter-animal variation in brain size (Zhang et al., 1998). Hemispheric tissue loss was calculated as a percentage of the contralateral (uninjured) hemisphere volume (Vc) using the following formula
where Vi represents the volume of the ipsilateral (injured) hemisphere. To calculate hemispheric volume, areas were integrated over the 4.3 mm rostro-caudal distance. Similarly, the ipsilateral cortical lesion volume was evaluated using the formula Σ(An+An+1)*d/2 were d is the distance between sections and A is the hemispheric area (Zheng et al., 1998).
To evaluate potential histological correlates to the unexpected behavioral results, we then performed immunohistochemistry for hippocampal β-amyloid (Aβ) and stained sections for myelin using the Luxol Fast Blue (LFB) technique (Marriott et al., 2008).
Evaluation of β-amyloid Immunohistochemistry
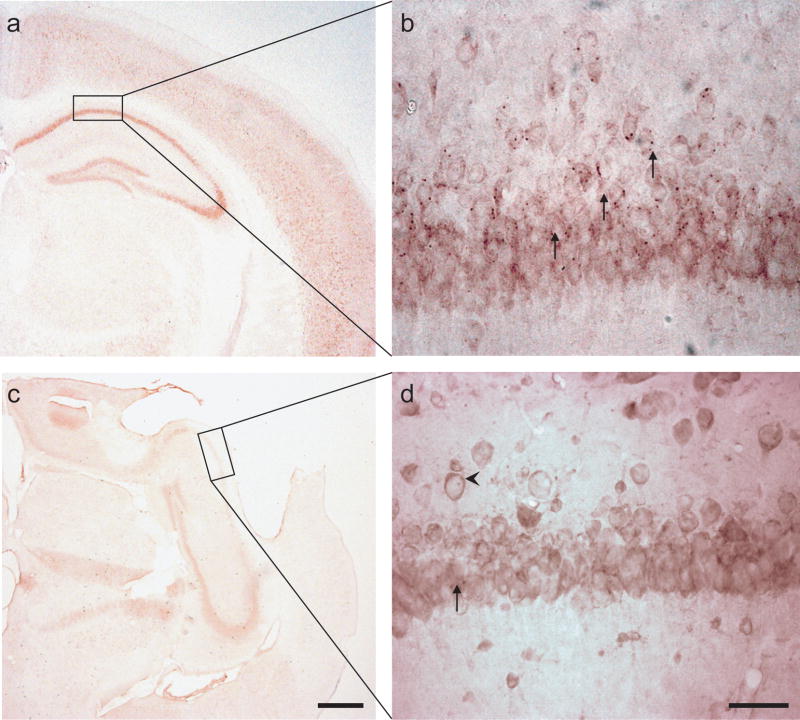
Several studies have demonstrated physical interactions between Reticulon family protein (including Nogo) and BACE1 (β-site amyloid precursor protein (APP)-cleaving enzyme), one of the proteases responsible for β-amyloid (Aβ) production from APP (He et al., 2004). Here, the sections were treated for antigen retrieval (citrate buffer; 25 mM at pH 7.3) rinsed in 1× PBS and immersed in 96% formic acid for 10 min followed by extensive rinse in water. The sections were then incubated in H2O2 (0.3%) in 50% DAKO block/50% 1× PBS for 15 min. MOM kits (Vector Laboratories, Burlingame, CA, USA) were used according to manufacturer’s instructions when using monoclonal murine antibodies (6E10, Signet, Dedham, MA). DAKO block was used to diminish unspecific binding by polyclonal antibodies (Aβ40 specific antibody, Camarillo, CA. USA). Sections were permeabilized using Triton X-100 for 5 min prior to application of primary antibodies. The incubations with primary antibodies were pursued overnight at +4°C. The reactions were developed with NOVA Red or DAB (Vector). The sections were washed, dehydrated, immersed in xylene and mounted in dibutyl phthalate xylene for light microscopy. Separate sections from positive control mice known to form intracellular Aβ deposits (APP-ArcSwe transgenic mice; Lord et al., 2006) were used for comparison (Fig. 4).
Fig. 4. Amyloid-beta (Aβ) immunohistochemistry at 40× (a, c) and 400× (b, d).
(a–b). In positive control mice (APP-ArcSwe transgenic mice: Lord et al., 2006), numerous intracellular deposits of Aβ are shown (arrows). (c–d) In brain-injured mice, only few and scattered, non-distinct inclusions of Aβ (arrows) were observed in the hippocampus ipsilateral to the injury, similar in NogoA/B−/− and wild-type littermate controls. (b,d) The pyramidal cell layer of the hippocampal CA1 field ipsilateral to injury is shown. With the antibody used here, the immunohistochemistry also showed APP immunostaining in the cellular membrane (d; arrowhead).
Evaluation of myelin staining
Nogo and myelin-associated glycoprotein may be involved in oligodendrocyte differentiation and myelination (Pernet et al., 2008). To evaluate myelin staining in our brain-injured animals, sections were stained using LFB. Briefly, sections were deparaffinised, hydrated and incubated in 1% LFB overnight at 60°C. Following the incubation, the sections were rinsed and differentiated in 0.05% lithium carbonate solution and mounted. Images were captured at 40× and 200× magnification. Due to marked TBI-induced damage to ipsilateral white matter tracts such as the external and internal capsule, we focused our evaluation on the corpus callosum (CC; Marriott et al., 2008). The borders of the CC was defined based on morphology and its area and staining density, measured in arbitrary units (pixels/area unit), were determined using Image J software (NIH, Bethesda, USA). All Images were analyzed by an investigator blinded to the injury and treatment status of each animal (O.K for LFB staining, O.P for Aβ immunohistochemistry).
Statistical analysis
Parametric data (lesion volume and loss of hemispheric tissue, swim speed and memory score data) are presented in Figures with means ± Standard Error of the Mean (SEM). These data were analyzed with a two-way analysis of variance (ANOVA) and, if p< 0.05, followed by Neumann-Keuls post-hoc test. Non-parametric data (composite neuroscore, myelin data) were analyzed with Kruskal-Wallis ANOVA, and, if p< 0.05, followed by Mann-Whitney’s U-test for pair-wise comparisons and are presented as medians and 75th percentile. In addition, Morris Water Maze latencies did not follow a Gaussian (normal) distribution and were also analyzed with Kruskal-Wallis ANOVA followed by Mann-Whitney’s U-test for each trial block. For clarity, MWM latencies are presented in Figure 2 with means ± standard error of the mean (SEM). A p-value of < 0.05 was considered significant. Data was analyzed with Statistica® (StatSoft, Tulsa, OK, USA) software.
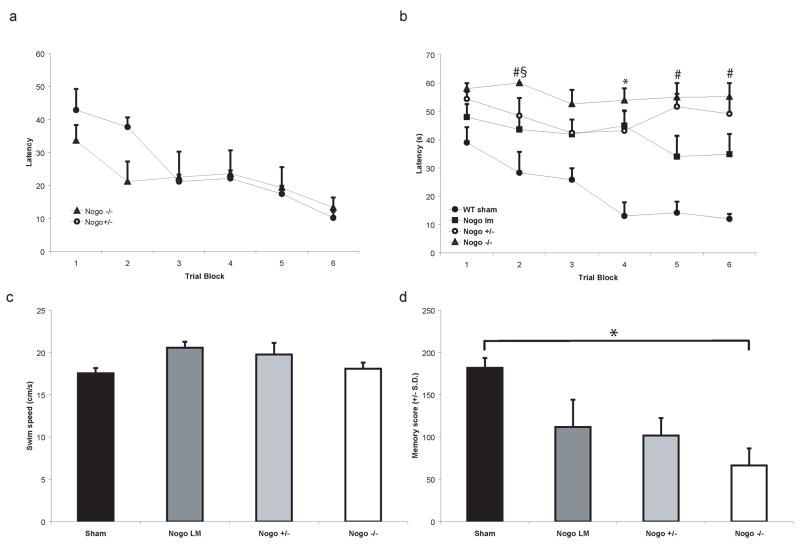
Fig. 2. Cognitive performance of naive (a) and sham-or brain-injured mice (b–d).
(a) Latency (seconds ± SEM) to locate the hidden platform using external visual cues in the Morris Water Maze over three days in naïve, uninjured Nogo-A/B−/− mice and Nogo-A/B+/− mice. There were no significant differences among the groups. (b) Latency (seconds ± SEM) to locate hidden platform in the Morris Water Maze over three days with 4 learning trials per block with 2 trial blocks per day, at 4 weeks post-injury. Sham-injured, wild-type (WT) mice rapidly learnt to locate the platform. Brain-injured, age-matched littermate WT controls had significantly longer latencies when compared to the sham-injured group at trial block 4 (*p<0.05). Brain-injured, Nogo-A/B−/− mice (open triangles) performed significantly worse than sham-injured WT controls at all trial blocks. Nogo-A/B−/− mice had significantly longer latencies than brain-injured, Nogo-A/B+/− mice (§p<0.05) and brain-injured WT littermate control mice (#p<0.05). (c) Average swim speed in the MWM at four weeks post-injury (means ± SEM). There were no significant differences among the sham- or brain-injured groups.
(d) Memory probe data at four weeks post-injury, 48 hours following the last MWM learning trial. In this test, the platform was removed and the probe trial for evaluation of memory was performed. Brain-injured, WT littermates showed a trend towards lower memory scores when compared to the sham-injured, WT controls. NogoA/B−/− mice had significantly lower memory scores when compared to the sham-injured WT controls (p<0.05), but were not statistically different from the corresponding brain-injured WT littermates or Nogo A/B−/+ mice.
RESULTS
Injury/Surgery
One sham-injured animal died on recovery from the surgical procedure, and one female Nogo-A/B−/− mouse developed a circling behavior, possibly related to ischemia (observed by the rotation of the mouse in the opposite direction to the ischemic hemisphere; Modo et al., 2000) and was euthanized before completion of the evaluation. All animals, including sham-injured controls, showed a mild weight-loss during the first week post-injury that gradually recovered to exceed the pre-injury weights (Table 2).
Table 2.
Animal weights (g) pre-injury and at one and three weeks post-injury.
| Weight (g) | Pre-injury | One week post-injury | 3 weeks post-injury |
|---|---|---|---|
| Sham WT | 30.3 ± 2.5 | 30.0 ± 3.4 | 31.2 ± 3.4 |
| Nogo-A/B WT | 35.4 ± 6.6 | 32.2 ± 7.7 | 33.7 ± 7.8 |
| Nogo-A/B−/+ | 35.1 ± 9.5 | 33.7 ± 8.9 | 34.0 ± 8.6 |
| Nogo-A/B−/− | 33.9 ± 5.9 | 33.0 ± 6.6 | 32.3 ± 5.8 |
Neurological motor function
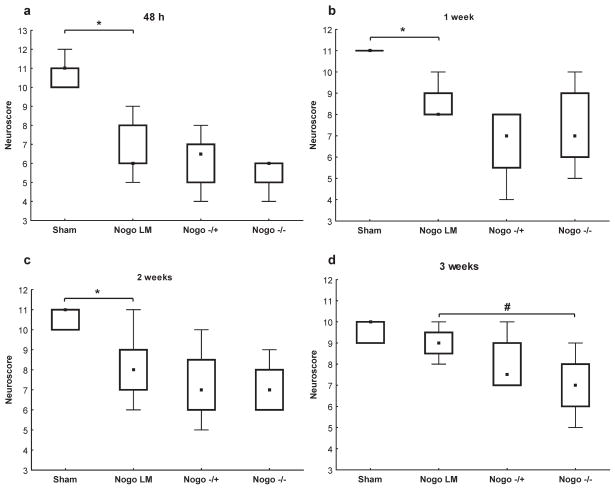
Using the composite neuroscores (NS), there were no pre-injury, baseline deficits among any of the groups (data not shown). All brain-injured mice showed significant neurological deficits from 48h-2 weeks post-injury when compared to sham-injured, aged-matched controls (p<0.05, Figure 1). There were no significant gender differences observed between the brain-injured Nogo-A/B−/−, Nogo-A/B−/+ and WT littermate groups (data not shown). The brain-injured WT littermate and Nogo-A/B−/+ mice showed a significant deficit in neurological motor function up to 2 weeks post-injury (p<0.05) while brain-injured, Nogo-A/B−/− mice showed more marked and prolonged neurological motor deficits (up to 3 weeks post-injury) when compared to sham-injured WT controls (p<0.05). The median scores of all groups of brain-injured mice gradually recovered over the 3-week observation period, although recovery of brain-injured Nogo-A/B−/− mice was delayed when compared with brain-injured WT littermates (Fig. 1).
Fig. 1.
(a–d) Neurological motor function 48h-3 weeks post-injury assessed by the composite neuroscore (boxplots showing medians, interquartile range and min-max values; maximum achievable score 12) among sham-injured wild-type controls and brain-injured, age-matched wild-type littermates (WT), Nogo-A/B+/− and Nogo-A/B−/− mice. When compared to sham-injured animals, brain-injured WT animals had significantly lower neuroscores up to 2 weeks post-injury (a–c; *p<0.05). At 3 weeks post-injury, brain-injured Nogo-A/B−/− animals had significantly lower neuroscores when compared to brain-injured, WT controls (d; #p<0.05).
At both 2 and 3 weeks post-injury, brain-injured Nogo-A/B−/− mice had lower composite NS when compared to the brain-injured WT littermates (p=0.08 at two weeks and p<0.05 at three weeks post-injury, Fig 1). There was no significant difference between the neurological performance of brain-injured, Nogo-A/B−/− and Nogo-A/B−/+ mice, so any impaired recovery phenotype does not fit a simple recessive loss-of-function model.
Cognitive function
Four weeks following CCI brain injury or sham injury, animals were evaluated for their ability to learn the position of a hidden platform in the MWM. All animals were able to swim without alterations in swimming ability, consistent with reports in younger brain-injured animals (Longhi et al., 2004).
Naïve Nogo-A/B−/− and NogoA/B−/+ mice quickly learned the MWM visuospatial task defined by decreasing latencies to find the platform during the 3-day training (Fig. 2a), with similar results when compared to sham-injured, WT animals (Fig. 2b). Brain-injured, Nogo-A/B WT littermates had consistently longer latencies than sham-injured controls (p<0.05 at trial block 4; Fig. 2b). Brain-injured, Nogo-A/B−/− mice also performed worse in the MWM learning task than sham-injured WT controls (trial blocks 1–6 p<0.05; Fig. 2b), and were significantly impaired with respect to their ability to learn when compared to the brain-injured WT littermates (trial blocks 2, 5 and 6; p<0.05). Brain-injured Nogo-A/B−/− mice had significantly longer latencies to locate the platform than the brain-injured Nogo-A/B−/+ mice during only one early phase of the learning paradigm (e.g., trial block 2, p<0.05; Fig. 2b but not later trial blocks. There were no significant differences between the brain-injured WT littermate and Nogo-A/B−/+ groups with respect to learning latencies. Male brain-injured Nogo-A/B WT littermate controls had significantly longer latencies in the MWM task at trial block 3 when compared to the female Nogo-A/B WT counterpart (p<0.05; data not shown). There were no other significant gender differences between the brain-injured groups.
Swim speed (Fig. 2c) was not influenced by injury status or genotype of the animals and there were no significant differences among the treatment groups. This further indicates that the neurological motor deficits (Fig. 1) produce no detectable functional impairment in swimming.
At 48 hours following the last MWM trial, the platform was removed and the probe trial for evaluation of memory was performed. Brain-injured, Nogo-A/B WT littermates had lower memory scores when compared to the sham-injured, wild-type controls. NogoA/B−/− mice had significantly lower memory scores when compared to the sham-injured WT controls (p<0.05; Fig 2d), although not when compared to the corresponding brain-injured WT littermates or Nogo A/B−/+ mice (Fig. 2d).
Total Hemispheric Tissue Loss and Cortical Lesion Volume
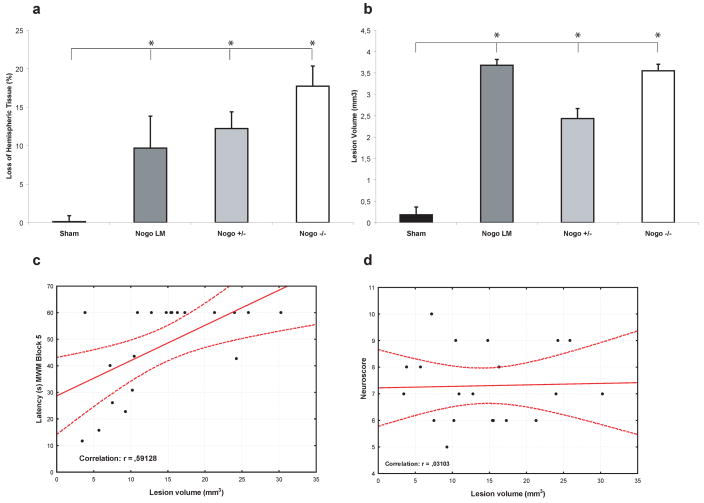
CCI brain injury induced a significant loss of tissue in the injured (ipsilateral) hemisphere in all brain-injured groups at four weeks post-injury when compared to the sham-injured controls (p<0.05; Fig 3). There were no significant differences in the loss of total hemispheric tissue among the brain-injured groups, regardless of genotype (Fig. 3a) or gender (data not shown). Additionally, there were no differences in cortical lesion volume among the brain-injured groups (Fig. 3b). Loss of hemispheric tissue, analyzing all brain-injured animals, was significantly correlated to cognitive performance (MWM trial block 4–6; r= 0.45, 0.59 (Fig. 3c) and 0.56, respectively, and the memory probe trial; r= 0.45, p<0.05) although not to neurological motor function (r= 0.03; Fig.3d).
Fig. 3.
Loss of hemispheric tissue (a) in the cerebral hemisphere ipsilateral to the controlled cortical impact brain injury at 4 weeks post-injury (means ± SEM). Brain injury, regardless of group caused a marked loss of hemispheric tissue (a) and a cortical lesion volume (b) when compared to sham-injured, wild-type (WT) control mice (*p< 0.05) without significant differences among the brain-injured groups. The loss of hemispheric tissue significantly correlated to MWM performance (shown in (c) for trial block 5) but not to neurological motor function (d).
Aβ Immunohistochemistry
We attempted to elucidate potential mechanism responsible for the surprising cognitive results in aging NogoA/B KO mice and evaluated beta-amyloid (Aβ) immunohistochemistry in the hippocampus of the brain-injured animals. In positive control mice (APP-ArcSwe transgenic mice: Lord et al., 2006), numerous intracellular deposits of Aβ are shown (Fig. 4a–b). In contrast, only a few scattered intracellular Aβ immunodeposits were observed in brain-injured animals, similar in NogoA/B−/− and WT littermate controls (Fig. 4c–d). Thus, this TBI model produces little persistent APP/Aβ-related pathology, and any modulating effect of Nogo genotype could not be assessed with high fidelity.
Myelin staining
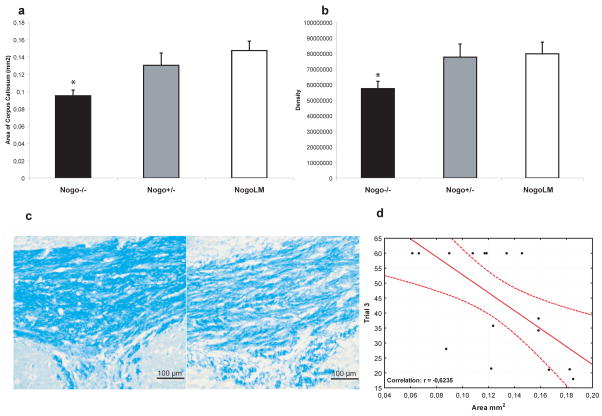
In previous studies, no morphometric differences in the size of the corpus callosum (CC) or other white matter tracts has been detected in adult un-injured NogoA/B−/− or NogoA/B−/+ mice (Kim et al., 2003). To evaluate whether Nogo-A-expressing white matter tracts might be especially sensitive to TBI, we evaluated the area and myelin density using LFB staining in the CC of brain-injured animals (Fig. 5a–c). NogoA/B −/−-animals had significantly smaller area of the CC (p<0.05, Fig. 5a) and reduced myelin density (p<0.05, Fig. 5b) when compared to their brain-injured wild-type littermates. The brain-injured Nogo A/B +/− mice had intermediate values, suggesting a gene-dosage dependent effect.
Fig. 5.
We evaluated myelin staining in the corpus callosum (CC) of brain-injured animals. We observed that brain-injured Nogo-A−/− mice had significantly (*p<0.05) reduced area of the CC (a) and in the CC, reduced myelin density (b) measured in arbitrary units (pixels/area unit) compared to their brain-injured, wild-type littermates. In (c), representative Images of the CC of a littermate, brain-injured control (left) and a NogoA/B−/−, brain-injured animal (right) is shown. Scale bar =100 μm. MWM performance (shown for trial block 3) was negatively correlated with CC area (d).
For all brain-injured groups, the MWM performance (trial block 3 and 4) was significantly correlated to myelin density (r= −0.6 and r= −0.52, respectively; data not shown). In addition, the area of the CC also significantly correlated to MWM performance at trial block 3 and 4 (r=−0.62; Fig. 5d and r= −0.55, p<0.05, respectively) but not to probe trial performance (r=0.38).
DISCUSSION
Contrary to our initial hypothesis, the genetic deletion of Nogo-A/B did not improve functional and histological outcome following TBI in aging mice. Instead, the absence of Nogo-A/B in a mouse strain previously shown to demonstrate enhanced axonal regeneration and improved recovery following spinal cord injury (Kim et al., 2003) was associated with less recovery of cognitive and motor outcome deficits after TBI. The present report is the first to evaluate the response to TBI in mice genetically engineered to be deficient in Nogo-A/B (NogoA/B−/− mice) and provide new insight into the role of myelin inhibitors in the pathogenesis of TBI. Our data suggest that in the aging animal, the absence of NogoA/B has no net benefit on outcome, a finding that may reflect a mixture of beneficial and deleterious effects.
We used a strain of NogoA/B−/− mice previously shown to have normal brain weight and morphology, locomotor activity and neuronal survival similar to wild-type, uninjured control mice. Additionally, there is no failure of early developmental events and the phenotype of these mice is indistinguishble from controls (Kim et al., 2003). Based on the gene trap design and the selected insertion site, both Nogo-A and Nogo-B expression is disrupted in these mice, (Kim et al., 2003). In wild-type animals, there is high expression of Nogo-B in lung tissue but very low in brain (Kim et al., 2003). Although Nogo-B may be involved in vascular remodelling (Acevedo et al., 2004), interact with β–site cleaving enzyme 1(BACE-1; Tang & Liou, 2007) and apoptosis (Li et al., 2001), its physiological function in brain is not clear. In “pure” Nogo-A−/− mice, Nogo-B protein was upregulated (Simonen et al., 2003) but is absent from the NogoA/B−/− mice used in our study (Kim et al., 2003). Based on available in vivo and in vitro evidence, we suggest that the absence of Nogo-A, not Nogo-B, is the most likely factor contributing to our unexpected behavioural results. When using genetically modified mice, the background strain may markedly influence the extent of neurite regeneration in vivo (Dimou et al., 2006). Additionally, developmental aspects, although not readily apparent in the strain used in our report (Kim et al., 2003), may also contribute to behavioural deficits in naïve animals. In a Nogo-A−/− strain, anxiety-related behavior, spatial learning and associative learning was similar in the genetically modified mice when compared to control mice. However, motor coordination, spontaneous locomotion and balance were enhanced in Nogo-A−/− mice (Willi et al., 2009). We submit that a weakness of the present study is that no sham-injured Nogo-A/B−/−, Nogo-A/B−/+ or wild-type littermate controls were available at the time of the TBI experiments. However, naïve, aged Nogo-A/B−/− mice rapidly learnt the Morris Water Maze task at a rate similar to the sham-injured controls used for TBI, suggesting that a different response to TBI in Nogo-A/B−/− mice is responsible for the unexpected cognitive impairment observed in our present report.
In comparison to previous studies of SCI and stroke, there are several features of TBI that might explain the deleterious effect of Nogo-A/B gene inactivation. Here, we used 12-month old mice, substantially older than mice of this strain used in previous reports on spinal cord injury (SCI) and brain ischemia (Kim et al., 2003, Lee et al., 2004, Cafferty et al., 2007). This age is also greater than that of the brain-injured rats exhibiting improved memory function after anti-Nogo-A antibody treatment (Lenzlinger et al., 2005; Marklund et al; 2007). However, age alone is unlikely to account for the lack of Nogo-A/B benefit in the current study. In SCI studies, anatomical and behavioral recovery was reduced with advanced age but a benefit of Nogo-A/B gene inactivation persisted in older animals (Cafferty et al., 2007). Similarly, Nogo-A inhibition in the aged, 2-year old rat improved recovery of a skilled sensorimotor task and was associated with subcortical neuronal reorganization following focal cerebral ischemia (Markus et al., 2005), demonstrating that the aged CNS still responds to pharmacological inhibition of Nogo-A. While age may interact with Nogo-A genotype to modify the extent of neurological recovery, it is doubtful that the impaired performance of the brain-injured Nogo-A/B−/− mice is fully explained by this factor.
Instead, unique features of TBI are likely to be the key issue. Differences between SCI and TBI with regard to Nogo-A have been reported. For example, markedly increased expression of Nogo-A in both oligodendrocytes and neurons in selectively vulnerable brain regions have been reported following TBI in the adult rat (Marklund et al., 2006), in contrast to the minor changes in Nogo-A expression observed following SCI in the adult rat (Huber et al., 2002; Hunt et al., 2003) or mouse (Wang et al., 2002; Meier et al., 2003).
It is striking that pre-TBI constitutive gene deletion and post-TBI anti-Nogo-A antibody therapy have opposite effects on cognitive performance. Assuming that the antibodies are selective for the intended target, the most obvious difference is the timing at which Nogo-A function is lost, prior to injury in this genetic study versus after injury in the pharmacological studies. We considered several potential deleterious factors that might be associated with Nogo-A action, including sprouting-related seizures, Aβ deposition and tissue loss. TBI is known to enhance hippocampal mossy fiber sprouting and to be associated with increased risk of epilepsy (Kharatishvili et al., 2006). In the current study, no convulsive episodes were witnessed so the relatively impaired memory function of the Nogo-A/B−/− mice could be explained only if there were subclinical electroencephalographic alterations that were not monitored here.
Accumulation of amyloid precursor protein (APP) and its metabolite Aβ in the neuropil, the neuropathological hallmark of Alzheimer’s disease (AD), occur after human TBI (e.g. Ikonomovic et al., 2004;Dekosky et al., 2007) and in pig models of TBI (Smith et al., 1999; Chen et al. 2004). Several studies have demonstrated physical interactions between Reticulon family protein (including Nogo) and BACE1, one of the proteases responsible for β-amyloid (Aβ) production from APP (He et al., 2004) and mice deficient in NgR1, bred to a mouse transgenic AD model, showed enhanced Aβ (Park et al., 2006). Thus, we evaluated Aβ immunohistochemistry and found only minor and non-distinct intracellular inclusions of Aβ in brain-injured mice irrespective of mouse genotype. Following TBI in the mouse, no or only minor increases of Aβ have been observed similar to our present results (Abrahamson et al., 2006; Nakagawa 1999; Smith et al.,1998; Murai et al.,1998). Our results suggest that increased Aβ is not the cause for the impaired cognitive function in NogoA/B−/− mice.
We have observed greater susceptibility to tissue loss after CCI in the mice lacking Nogo-A/B, providing a likely explanation for the poorer memory performance. While total hemispheric tissue loss showed an insignificant trend towards being more severe in the KO animals, there was a clear difference in corpus callosum loss after CCI brain injury in mice lacking Nogo-A/B. Moreover, there was a trend towards more callosal damage in the mice heterozygous for Nogo-A/B. While total area was decreased, future studies with cell type specific markers will be required to determine if this enhanced susceptibility is due to axons, oligodendroglia or the axo-glial unit. In the current study, increased loss of white matter volume in the corpus callosum correlated well with poorer memory performance after TBI. Future studies using conditional Nogo gene knockouts induced before and after injury, as well as variable times of anti-Nogo-A antibody administration will be useful to separate early effects of Nogo-A in protecting white matter from later effects in blocking repair.
Conclusion
In our present study, we show that mice deficient in Nogo-A/B had impaired behavioral outcome and greater white matter loss following traumatic brain injury (TBI), contrary to results obtained using pharmacological inhibition of Nogo-A following experimental stroke, spinal cord injury and TBI. We argue that there may be two plausible explanations for these unexpected results. On the one hand, NogoAB−/− mice may have subtle developmental deficit that cannot be discerned by any standard methods prior to injury but which allows greater behavioral deficits and tissue damage to occur post-TBI. More likely, the Nogo-A/B −/− brain has enhanced susceptibility to TBI. Thus, it may be advantageous in treatment paradigms to avoid blocking Nogo-A action too early after injury. However, our previous anti-Nogo-A antibody experiments with beneficial outcomes included short delays between TBI and antibody administration, so this delay is unlikely to complicate the clinical utility of Nogo blockade. Future studies that include anti-Nogo antibody pretreatment prior to TBI and conditional gene deletion after injury will clarify this issue.
Acknowledgments
Supported by NIH NS RO1-40978, a Merit Review Grant from the Veterans Administration, NIH NS P50-08803, Swedish MRC, Uppsala University Hospital and the Swedish Brain Foundation. NIH NS RO1-56485 and NS RO1-39962 to S.M.S. We thank Rishi Puri, David LeBold, Carl T. Fulp, Lena Rundström and Hilaire J. Thompson for excellent technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abrahamson EE, Ikonomovic MD, Ciallella JR, Hope CE, Paljug WR, Isanski BA, Flood DG, Clark RS, DeKosky ST. Caspase inhibition therapy abolishes brain trauma-induced increases in Abeta peptide: implications for clinical outcome. Exp Neurol. 2006;197:437–450. doi: 10.1016/j.expneurol.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Acevedo L, Yu J, Erdjument-Bromage H, Miao RQ, Kim JE, Fulton D, Tempst P, Strittmatter SM, Sessa WC. A new role for Nogo as a regulator of vascular remodeling. Nat Med. 2004;10:382–388. doi: 10.1038/nm1020. [DOI] [PubMed] [Google Scholar]
- Adams JH, Graham DI, Jennett B. The structural basis of moderate disability after traumatic brain damage. J Neurol Neurosurg Psychiatry. 2001;71:521–524. doi: 10.1136/jnnp.71.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SP, Pask T, Jones L, Dunnett SB. Behavioural profiles of inbred mouse strains used as transgenic backgrounds. II: cognitive tests. Genes Brain Behav. 2005;4:307–317. doi: 10.1111/j.1601-183X.2004.00109.x. [DOI] [PubMed] [Google Scholar]
- Buchli AD, Schwab ME. Inhibition of Nogo: a key strategy to increase regeneration, plasticity and functional recovery of the lesioned central nervous system. Ann Med. 2005;37:556–567. doi: 10.1080/07853890500407520. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Kim JE, Lee JK, Strittmatter SM. Response to correspondence: Kim et al. “axon regeneration in young adult mice lacking Nogo-A/B Neuron 38, 187–199”. Neuron. 2007;54(2):195–199. doi: 10.1016/j.neuron.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Strittmatter SM. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J Neurosci. 2006;26:12242–12250. doi: 10.1523/JNEUROSCI.3827-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- Chen XH, Siman R, Iwata A, Meaney DF, Trojanowski JQ, Smith DH. Long-term accumulation of amyloid-beta, beta-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma. Am J Pathol. 2004;165:357–371. doi: 10.1016/s0002-9440(10)63303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colello RJ, Pott U, Schwab ME. The role of oligodendrocytes and myelin on axon maturation in the developing rat retinofugal pathway. J Neurosci. 14:2594–2605. doi: 10.1523/JNEUROSCI.14-05-02594.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Abrahamson EE, Ciallella JR, Paljug WR, Wisniewski SR, Clark RS, Ikonomovic MD. Association of increased cortical soluble abeta42 levels with diffuse plaques after severe brain injury in humans. Arch Neurol. 2007;64:541–544. doi: 10.1001/archneur.64.4.541. [DOI] [PubMed] [Google Scholar]
- Dimou L, Schnell L, Montani L, Duncan C, Simonen M, Schneider R, Liebscher T, Gullo M, Schwab ME. Nogo-A-deficient mice reveal strain-dependent differences in axonal regeneration. J Neurosci. 2006;26:5591–5603. doi: 10.1523/JNEUROSCI.1103-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Grandpre T, Gould G, Wang X, Strittmatter SM. Nogo and the Nogo-66 receptor. Prog Brain Res. 2002;137:361–369. doi: 10.1016/s0079-6123(02)37027-4. [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Fox GB, Fan L, LeVasseur RA, Faden AI. Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J Neurotrauma. 1998;15:599–614. doi: 10.1089/neu.1998.15.599. [DOI] [PubMed] [Google Scholar]
- Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller EM. Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med. 2006;12:790–792. doi: 10.1038/nm1436. [DOI] [PubMed] [Google Scholar]
- Graham DI, Maxwell WL, Adams JH, Jennett B. Novel aspects of the neuropathology of the vegetative state after blunt head injury. Prog Brain Res. 2005;150:445–455. doi: 10.1016/S0079-6123(05)50031-1. [DOI] [PubMed] [Google Scholar]
- Grandpre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Hall ED, Sullivan PG, Gibson TR, Pavel KM, Thompson BM, Scheff SW. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J Neurotrauma. 2005;22:252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- Hall ED, Bryant YD, Cho W, Sullivan PG. Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J Neurotrauma. 2008;25(3):235–247. doi: 10.1089/neu.2007.0383. [DOI] [PubMed] [Google Scholar]
- Harris C, DiRusso S, Sullivan T, Benzil DL. Mortality risk after head injury increases at 30 years. J Am Coll Surg. 2003;197:711–716. doi: 10.1016/S1072-7515(03)00729-4. [DOI] [PubMed] [Google Scholar]
- He W, Lu Y, Qahwash I, Hu XY, Chang A, Yan R. Reticulon family members modulate BACE1 activity and amyloid-beta peptide generation. Nat Med. 2004;10:959–965. doi: 10.1038/nm1088. [DOI] [PubMed] [Google Scholar]
- Huber AB, Weinmann O, Brosamle C, Oertle T, Schwab ME. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci. 2002;22:3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukkelhoven CW, Steyerberg EW, Rampen AJ, Farace E, Habbema JD, Marshall LF, Murray GD, Maas AI. Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J Neurosurg. 2003;99:666–673. doi: 10.3171/jns.2003.99.4.0666. [DOI] [PubMed] [Google Scholar]
- Hunt D, Coffin RS, Anderson PN. The Nogo receptor, its ligands and axonal regeneration in the spinal cord; a review. J Neurocytol. 2002;31:93–120. doi: 10.1023/a:1023941421781. [DOI] [PubMed] [Google Scholar]
- Hunt D, Coffin RS, Prinjha RK, Campbell G, Anderson PN. Nogo-A expression in the intact and injured nervous system. Mol Cell Neurosci. 2003;24:1083–1102. doi: 10.1016/j.mcn.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Hurley RA, McGowan JC, Arfanakis K, Taber KH. Traumatic axonal injury: novel insights into evolution and identification. J Neuropsychiatry Clin Neurosci. 2004;16:1–7. doi: 10.1176/jnp.16.1.1. [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, Uryu K, Abrahamson EE, Ciallella JR, Trojanowski JQ, Lee VM, Clark RS, Marion DW, Wisniewski SR, DeKosky ST. Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol. 2004;190:192–203. doi: 10.1016/j.expneurol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Nissinen JP, McIntosh TK, Pitkänen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience. 2006;30;140(2):685–697. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Kim JE, Li S, GrandPre T, Qiu D, Strittmatter SM. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzlinger PM, Shimizu S, Marklund N, Thompson HJ, Schwab ME, Saatman KE, Hoover RC, Bareyre FM, Motta M, Luginbuhl A, Pape R, Clouse AK, Morganti-Kossmann C, McIntosh TK. Delayed inhibition of Nogo-A does not alter injury-induced axonal sprouting but enhances recovery of cognitive function following experimental traumatic brain injury in rats. Neuroscience. 2005;134:1047–1056. doi: 10.1016/j.neuroscience.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Li Q, Qi B, Oka K, Shimakage M, Yoshioka N, Inoue H, Hakura A, Kodama K, Stanbridge EJ, Yutsudo M. Link of a new type of apoptosis-inducing gene ASY/Nogo-B to human cancer. Oncogene. 2001;5(20):3929–3936. doi: 10.1038/sj.onc.1204536. [DOI] [PubMed] [Google Scholar]
- Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebscher T, Schnell L, Schnell D, Scholl J, Schneider R, Gullo M, Fouad K, Mir A, Rausch M, Kindler D, Hamers FP, Schwab ME. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- Longhi L, Watson DJ, Saatman KE, Thompson HJ, Zhang C, Fujimoto S, Royo N, Castelbuono D, Raghupathi R, Trojanowski JQ, Lee VM, Wolfe JH, Stocchetti N, McIntosh TK. Ex vivo gene therapy using targeted engraftment of NGF-expressing human NT2N neurons attenuates cognitive deficits following traumatic brain injury in mice. J Neurotrauma. 2004;21:1723–1736. doi: 10.1089/neu.2004.21.1723. [DOI] [PubMed] [Google Scholar]
- Lord A, Kalimo H, Eckman C, Zhang XQ, Lannfelt L, Nilsson LN. The Arctic Alzheimer mutation facilitates early intraneuronal Abeta aggregation and senile plaque formation in transgenic mice. Neurobiol Aging. 2006;27:67–77. doi: 10.1016/j.neurobiolaging.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Marklund N, Bareyre FM, Royo NC, Thompson HJ, Mir AK, Grady MS, Schwab ME, McIntosh TK. Cognitive outcome following brain injury and treatment with an inhibitor of Nogo-A in association with an attenuated downregulation of hippocampal growth-associated protein-43 expression. J Neurosurg. 2007;107:844–853. doi: 10.3171/JNS-07/10/0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus TM, Tsai SY, Bollnow MR, Farrer RG, O’Brien TE, Kindler-Baumann DR, Rausch M, Rudin M, Wiessner C, Mir AK, Schwab ME, Kartje GL. Recovery and brain reorganization after stroke in adult and aged rats. Ann Neurol. 2005;58:950–953. doi: 10.1002/ana.20676. [DOI] [PubMed] [Google Scholar]
- Marriott MP, Emery B, Cate HS, Binder MD, Kemper D, Wu Q, Kolbe S, Gordon IR, Wang H, Egan G, Murray S, Butzkueven H, Kilpatrick TJ. Leukemia inhibitory factor signaling modulates both central nervous system demyelination and myelin repair. Glia. 2008;15;56:686–698. doi: 10.1002/glia.20646. [DOI] [PubMed] [Google Scholar]
- Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma. 1997;14:419–440. doi: 10.1089/neu.1997.14.419. [DOI] [PubMed] [Google Scholar]
- Meier S, Brauer AU, Heimrich B, Schwab ME, Nitsch R, Savaskan NE. Molecular analysis of Nogo expression in the hippocampus during development and following lesion and seizure. FASEB J. 2003;17:1153–1155. doi: 10.1096/fj.02-0453fje. [DOI] [PubMed] [Google Scholar]
- Mingorance A, Fontana X, Sole M, Burgaya F, Urena JM, Teng FY, Tang BL, Hunt D, Anderson PN, Bethea JR, Schwab ME, Soriano E, del Rio JA. Regulation of Nogo and Nogo receptor during the development of the entorhino-hippocampal pathway and after adult hippocampal lesions. Mol Cell Neurosci. 2004;26:34–49. doi: 10.1016/j.mcn.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Modo M, Stroemer RP, Tang E, Veizovic T, Sowniski P, Hodges H. Neurological sequelae and long-term behavioural assessment of rats with transient middle cerebral artery occlusion. J Neurosci Methods. 2000;104:99–109. doi: 10.1016/s0165-0270(00)00329-0. [DOI] [PubMed] [Google Scholar]
- Murai H, Pierce JE, Raghupathi R, Smith DH, Saatman KE, Trojanowski JQ, Lee VM, Loring JF, Eckman C, Younkin S, McIntosh TK. Twofold overexpression of human beta-amyloid precursor proteins in transgenic mice does not affect the neuromotor, cognitive, or neurodegenerative sequelae following experimental brain injury. J Comp Neurol. 1998;392:428–438. doi: 10.1002/(sici)1096-9861(19980323)392:4<428::aid-cne2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Nakamura M, McIntosh TK, Rodriguez A, Berlin JA, Smith DH, Saatman KE, Raghupathi R, Clemens J, Saido TC, Schmidt ML, Lee VM, Trojanowski JQ. Traumatic brain injury in young, amyloid-beta peptide overexpressing transgenic mice induces marked ipsilateral hippocampal atrophy and diminished Abeta deposition during aging. J Comp Neurol. 1999;411:390–398. [PubMed] [Google Scholar]
- Nakamura M, Saatman KE, Galvin JE, Scherbel U, Raghupathi R, Trojanowski JQ, McIntosh TK. Increased vulnerability of NFH-LacZ transgenic mouse to traumatic brain injury-induced behavioral deficits and cortical damage. J Cereb Blood Flow Metab. 1999;19:762–770. doi: 10.1097/00004647-199907000-00006. [DOI] [PubMed] [Google Scholar]
- Oertle T, van der Haar ME, Bandtlow CE, Robeva A, Burfeind P, Buss A, Huber AB, Simonen M, Schnell L, Brosamle C, Kaupmann K, Vallon R, Schwab ME. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J Neurosci. 2003;23:5393–5406. doi: 10.1523/JNEUROSCI.23-13-05393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Gimbel DA, GrandPre T, Lee JK, Kim JE, Li W, Lee DH, Strittmatter SM. Alzheimer precursor protein interaction with the Nogo-66 receptor reduces amyloid-beta plaque deposition. J Neurosci. 2006;26:1386–1395. doi: 10.1523/JNEUROSCI.3291-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos CM, Tsai SY, Alsbiei T, O’Brien TE, Schwab ME, Kartje GL. Functional recovery and neuroanatomical plasticity following middle cerebral artery occlusion and IN-1 antibody treatment in the adult rat. Ann Neurol. 2002;51:433–441. doi: 10.1002/ana.10144. [DOI] [PubMed] [Google Scholar]
- Papadopoulos CM, Tsai SY, Cheatwood JL, Bollnow MR, Kolb BE, Schwab ME, Kartje GL. Dendritic plasticity in the adult rat following middle cerebral artery occlusion and Nogo-a neutralization. Cereb Cortex. 2006;16:529–536. doi: 10.1093/cercor/bhi132. [DOI] [PubMed] [Google Scholar]
- Pernet V, Joly S, Christ F, Dimou L, Schwab ME. Nogo-A and myelin-associated glycoprotein differently regulate oligodendrocyte maturation and myelin formation. J Neurosci. 2008;16:7435–7444. doi: 10.1523/JNEUROSCI.0727-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons DL, Walsh FS. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- Seymour AB, Andrews EM, Tsai SY, Markus TM, Bollnow MR, Brenneman MM, O’Brien TE, Castro AJ, Schwab ME, Kartje GL. Delayed treatment with monoclonal antibody IN-1 1 week after stroke results in recovery of function and corticorubral plasticity in adult rats. J Cereb Blood Flow Metab. 2005;25:1366–1375. doi: 10.1038/sj.jcbfm.9600134. [DOI] [PubMed] [Google Scholar]
- Simonen M, Pedersen V, Weinmann O, Schnell L, Buss A, Ledermann B, Christ F, Sansig G, van der PH, Schwab ME. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron. 2003;38:201–211. doi: 10.1016/s0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- Smith DH, Okiyama K, Thomas MJ, Claussen B, McIntosh TK. Evaluation of memory dysfunction following experimental brain injury using the Morris water maze. J Neurotrauma. 1991;8:259–269. doi: 10.1089/neu.1991.8.259. [DOI] [PubMed] [Google Scholar]
- Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995;12:169–178. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- Smith DH, Nakamura M, McIntosh TK, Wang J, Rodríguez A, Chen XH, Raghupathi R, Saatman KE, Clemens J, Schmidt ML, Lee VM, Trojanowski JQ. Brain trauma induces massive hippocampal neuron death linked to a surge in beta-amyloid levels in mice overexpressing mutant amyloid precursor protein. Am J Pathol. 1998;153:1005–1010. doi: 10.1016/s0002-9440(10)65643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Chen XH, Nonaka M, Trojanowski JQ, Lee VM, Saatman KE, Leoni MJ, Xu BN, Wolf JA, Meaney DF. Accumulation of amyloid beta and tau and the formation of neurofilament inclusions following diffuse brain injury in the pig. J Neuropathol Exp Neurol. 1999;58:982–992. doi: 10.1097/00005072-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Steward O, Schauwecker PE, Guth L, Zhang Z, Fujiki M, Inman D, Wrathall J, Kempermann G, Gage FH, Saatman KE, Raghupathi R, McIntosh T. Genetic approaches to neurotrauma research: opportunities and potential pitfalls of murine models. Exp Neurol. 1999;157:19–42. doi: 10.1006/exnr.1999.7040. [DOI] [PubMed] [Google Scholar]
- Tang BL, Liou YC. Novel modulators of amyloid-beta precursor protein processing. J Neurochem. 2007;100(2):314–323. doi: 10.1111/j.1471-4159.2006.04215.x. [DOI] [PubMed] [Google Scholar]
- Teng FY, Tang BL. Nogo signaling and non-physical injury-induced nervous system pathology. J Neurosci Res. 2005a;79:273–278. doi: 10.1002/jnr.20361. [DOI] [PubMed] [Google Scholar]
- Teng FY, Tang BL. Why do Nogo/Nogo-66 receptor gene knockouts result in inferior regeneration compared to treatment with neutralizing agents? J Neurochem. 2005b;94:865–874. doi: 10.1111/j.1471-4159.2005.03238.x. [DOI] [PubMed] [Google Scholar]
- The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Age J Neurotrauma. 2000;17:573–581. doi: 10.1089/neu.2000.17.573. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, LeBold DG, Marklund N, Morales DM, Hagner AP, McIntosh TK. Cognitive evaluation of traumatically brain-injured rats using serial testing in the Morris water maze. Restor Neurol Neurosci. 2006;24:109–114. [PMC free article] [PubMed] [Google Scholar]
- Trifunovski A, Josephson A, Bickford PC, Olson L, Brene S. Selective decline of Nogo mRNA in the aging brain. Neuroreport. 2006;17:913–916. doi: 10.1097/01.wnr.0000221831.95598.a3. [DOI] [PubMed] [Google Scholar]
- Wang E, Wong A, Cortopassi G. The rate of mitochondrial mutagenesis is faster in mice than humans. Mutat Res. 1997;377:157–166. doi: 10.1016/s0027-5107(97)00091-2. [DOI] [PubMed] [Google Scholar]
- Wang X, Chun SJ, Treloar H, Vartanian T, Greer CA, Strittmatter SM. Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J Neurosci. 2002;22:5505–5515. doi: 10.1523/JNEUROSCI.22-13-05505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willi R, Aloy EM, Yee BK, Feldon J, Schwab ME. Behavioral characterization of mice lacking the neurite outgrowth inhibitor Nogo-A. Genes Brain Behav. 2009;8:181–192. doi: 10.1111/j.1601-183X.2008.00460.x. [DOI] [PubMed] [Google Scholar]
- Zhang C, Raghupathi R, Saatman KE, Smith DH, Stutzmann JM, Wahl F, McIntosh TK. Riluzole attenuates cortical lesion size, but not hippocampal neuronal loss, following traumatic brain injury in the rat. J Neurosci Res. 1998;52:342–349. doi: 10.1002/(SICI)1097-4547(19980501)52:3<342::AID-JNR10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Zheng B, Ho C, Li S, Keirstead H, Steward O, Tessier-Lavigne M. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]