Abstract
The acid sensing ion channel 3 (ASIC3) is critical for the development of secondary hyperalgesia as measured by mechanical stimulation of the paw following muscle insult. We designed experiments to test whether ASIC3 was necessary for the development of both primary and secondary mechanical hyperalgesia that develops after joint inflammation. We used ASIC3 -/- mice and examined the primary (response to tweezers) and secondary hyperalgesia (von Frey filaments) that develops after joint inflammation comparing to ASIC3 +/+ mice. We also examined the localization of ASIC3 to the knee joint afferents innervating the joint capsule using immunohistochemical techniques before and after joint inflammation. We show that secondary mechanical hyperalgesia does not develop in ASIC3 -/- mice. However, the primary mechanical hyperalgesia of the inflamed knee joint still develops in ASIC3 -/- mice and is similar to ASIC3 +/+ mice. In knee joint synovium from ASIC3 +/+ mice without joint inflammation, ASIC3 was not localized to joint afferents that were stained with an antibodies to protein gene product (PGP) 9.5 or calcitonin gene-related peptide (CGRP). ASIC3 was found, however, in synoviocytes of the knee joint of uninflamed mice. In ASIC3 +/+ mice with joint inflammation, ASIC3 co-localized with PGP 9.5 or CGRP in joint afferents innervating the synovium. We conclude that the decreased pH that occurs after inflammation would activate ASIC3 on primary afferent fibers innervating joint, increasing the input to the spinal cord resulting in central sensitization manifested behaviorally as secondary hyperalgesia of the paw.
1. Introduction
The acid sensing ion channel 3 (ASIC3) is critical for the development of hyperalgesia to mechanical stimulation of the paw following muscle insult. Prior studies from our laboratory show that in ASIC3-/- mice, mechanical hyperalgesia of the paw does not develop after repeated intramuscular injections of acid or after intramuscular inflammation induced by carrageenan [22,23]. Heat hyperalgesia of the paw still develops similarly between ASIC3-/- and ASIC3+/+ mice after carrageenan inflammation of the muscle [23]. These studies all measured an increased nociceptive response to noxious stimuli outside the site of injury, termed secondary hyperalgesia. Prior studies measuring mechanical and heat sensitivity of the paw after inflammation of the paw, i.e. primary hyperalgesia, show that hyperalgesia still develops in ASIC3-/- mice and is either similar or increased to that of ASIC3+/+ mice [1,7,13]. We previously interpreted this as a difference between cutaneous and muscle pain models. However, one could alternatively suggest that ASIC3 plays a different role in primary and secondary hyperalgesia.
Joint inflammation is associated with both primary and secondary hyperalgesia. In an animal model of joint inflammation, induced by injection of kaolin and/or carrageenan into the knee joint, both primary and secondary hyperalgesia to mechanical stimuli develop [14,21,28]. Secondary hyperalgesia develops at the paw to heat and mechanical stimuli [14,24], and primary hyperalgesia is present over the inflamed knee joint [21]. This carrageenan-induced knee joint inflammation model produces sensitization of nociceptors innervating the knee joint, a substrate for primary hyperalgesia; and sensitization of neurons in the dorsal horn, a substrate for secondary hyepralgesia [2,11,16-18]. Well characterized biochemical changes occur in the inflamed knee joint, the primary afferent fibers innervating the knee joint, and in the central nervous system including the spinal cord [19,20]. These biochemical changes may underlie the development and maintenance of inflammatory hyperalgesia, both primary and secondary.
ASIC3 is located in primary afferent fibers innervating the skin and the muscle [8,13,22]. It is unclear if ASIC3 is also located in primary afferent fibers innervating the joint. The localization of ASIC3 to primary afferent fibers innervating the muscle is critical to the development of secondary mechanical hyperalgesia after muscle insult [23]. We show that re-expression of ASIC3 in primary afferent fibers innervating muscle in ASIC3-/- mice restores the secondary mechanical hyperalgesia of the paw that occurs after muscle inflammation [23]. Re-expression of ASIC3 in primary afferent fibers innervating the skin of ASIC3-/- mice, however, has no effect on the mechanical hyperalgesia of the paw after muscle inflammation [23].
The purpose of this study was, therefore, to test the role of ASIC3 in the mechanical hyperalgesia associated with joint inflammation; and to assess if ASIC3 is located in primary afferent fibers innervating the knee joint. We hypothesized that ASIC3 was necessary for the development of both primary and secondary mechanical hyperalgesia that develops after joint inflammation. We also hypothesized that ASIC3 was located in primary afferent fibers innervating the knee joint.
2. Methods
2.1. Mice
All experiments were approved by the University of Iowa Animal Care and Use Committee and were conducted in accordance with National Institutes of Health guidelines. Congenic ASIC3 -/- and ASIC3 +/+ mice on a C57Bl/6J background were bred at the University of Iowa Animal Care Facility [13]. Both male and female mice, 4-8 months of age, were used in these studies. For behavior testing, mice were divided up into ASIC3 +/+ (n=23) and ASIC3 -/- (n=24) groups. For immunohistochemical testing mice we tested tissue sections obtained from both ASIC3 +/+ (n=6) and ASIC3 -/- mice (n=6)
2.2. Behavior Experiment with Mechanical, Thermal and Joint Sensitivity
2.2.1. Acclimation Training
Mice were trained for two days on the mechanical plate, thermal pate and tweezer restraining glove. Separate groups of mice were tested for mechanical and thermal sensitivity of the paw; and for mechanical joint sensitivity. On day 1, training consisted of placing the mice individually into ventilated plexiglass cubicles (9 × 3 × 5) on top of a wire mesh table for 30 minutes. This allowed for the mice to acclimate to the confined area and to standing on the wire mesh surface. Mice were then acclimated to thermal testing by placing them onto a flat glass surface in the same individual plexiglass cubicles, allowing for the mice to get acclimated to the confined area and standing on a glass surface. In order to acclimate the mice to the tweezer regimen mice were individually placed in a glove with their hind quarters outside of the glove and allowed to remain in this position for 5 minutes per session. The mice were stroked to relax and had their hind paws gently pulled in order to get them acclimated to being handled for tweezer testing. This was done twice a day for 2 days.
2.2.2. Mechanical Testing of the Paw
Mechanical sensitivity was tested using the von-Frey filaments for measuring cutaneous sensitivity. A von-Frey filament of bending force 0.04 g was applied to the hind paws 5 times per trial and was repeated 10 times. An average of the 10 trials was used to generate one number at each testing time. A withdrawal was defined as positive if the mouse lifted its paw after the filament was pressed against the paw. Mechanical sensitivity was tested as follows: before injection of the knee joint (see 2.3), 24 h after the injection, 1 week after the injection and 2 weeks after the injection. An increased number of responses were interpreted as mechanical hyperalgesia.
2.2.3. Thermal Testing of the Paw
Thermal sensitivity was tested using a radiant heat source shone on the hindpaw of the mice until a withdrawal was produced. The latency to withdraw was measured as the paw withdrawal latency. The light source was applied to the hindpaws 1 time per trial and was repeated 3 times. An average of the 3 trials was used to generate one number per testing time. There were five testing periods: before injection of the knee joint (see 2.3), 24 hours after injection, 72 hours after injection, 1 week after injection and 2 weeks after injection. A decrease in latency was interpreted as increased heat hyperaglesia.
2.2.4. Mechanical Testing of the Joint
To measure joint sensitivity, the mouse was placed in a glove, each hindlimb extended and a pair of forceps applied to the knee joint until the animal withdrew from the stimulus. Three trials consisting of squeezing the knee were applied at each testing period. The computer program TestPoint was used to measure the magnitude of each squeeze in millinewtons (mN). There were five testing periods: before injection of the knee joint (see 2.3), 24 hours after injection, 72 hours after injection, 1 week after injection and 2 weeks after injection. A decrease in threshold was interpreted as higher joint sensitivity.
2.3. Induction of inflammation
To induce knee joint inflammation, an injection of 20 ul of 3% carrageenan was injected into the left knee joint cavity while the mice were briefly anaesthetized with isoflorane (3%).
2.4. Histology
For immunohistochemical staining two different protocols for tissue collection were used. All inflamed tissues were removed 24h after injection of 3% carrageenan into the knee joint. Figures 2A,B,D,E, Figure 3, and Figure 4 were obtained by transcardially perfusing the mice with heparinized saline followed by 4% paraformaldehye and removing the whole knee joint, including muscle and bone. Whole specimens were then decalcified for 4 weeks with 5% EDTA (ethylenediaminetetraacetic acid) with five changes of fresh solution per week. After complete demineralization, the tissues were rinsed thoroughly in phosphate buffered saline (PBS) and placed in 30% sucrose overnight. All tissues were rapidly frozen and stored at -80°C until cut on a cryostat. Sections were then cryosectioned at 14 μm and placed on slides slides using CryoJane Tape Transfer System (Instrumedics Inc., St Louis, MO) for subsequent processing. The 20 μm cryosection in Figure 2C was obtained by removal of the anterior synovial tissue, snap freezing at -160°C, and postfixing on the slide with 2% paraformaldehyde.
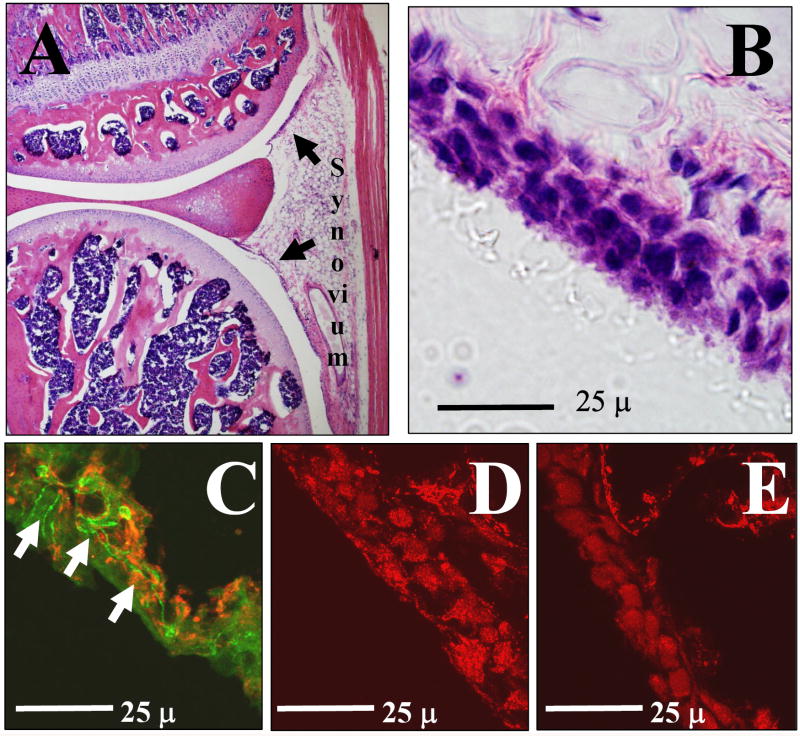
Figure 2.
A. Cross section through the knee joint of a mouse without joint inflammation stained with H & E depicting the location of the synovium. B. High power magnification of the synovium from the knee joint showing synoviocytes lining the joint cavity and fat cells below. C. Double label for PGP 9.5 (green, arrows) showing the nerve fibers innervating the synovium. Red is labeled for ASIC3 and shows localization in the synovium. D. Shows ASIC3 localization in synovioctyes lining the joint cavity in ASIC3 +/+ mice. E. Shows lack of ASIC3 staining in synoviocytes from ASIC3 -/- mice.
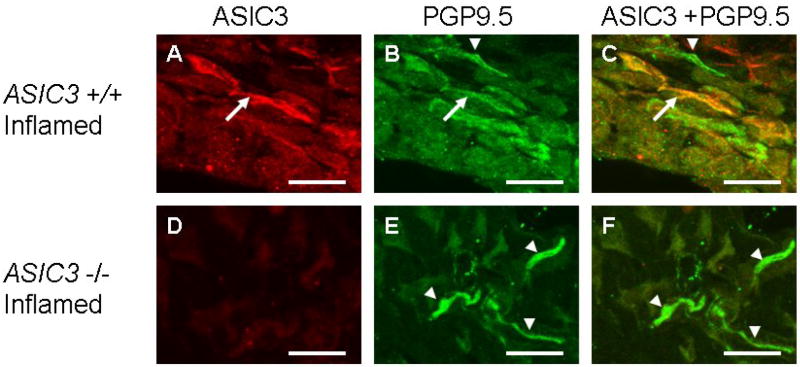
Figure 3.
Immunohistochemistry staining of the synovium from ASIC3 +/+ mice with joint inflammation stained for ASIC3 (A), PGP 9.5 (B). The merged image shows co-localization of ASIC3 and PGP 9.5 (C) depicted by arrows. Some PGP 9.5 labeled nerve fibers in the synovium do not localize with ASIC3 (arrowhead). Immunohistochemistry staining from ASIC3 -/- mice with joint inflammation stained for ASIC3 (D), PGP 9.5 (E). There is no staining for ASIC3 the ASIC3 -/- mice. The merged image shows no co-localization of ASIC3 and PGP 9.5 (E; arrowheads). Bar = 25 μm
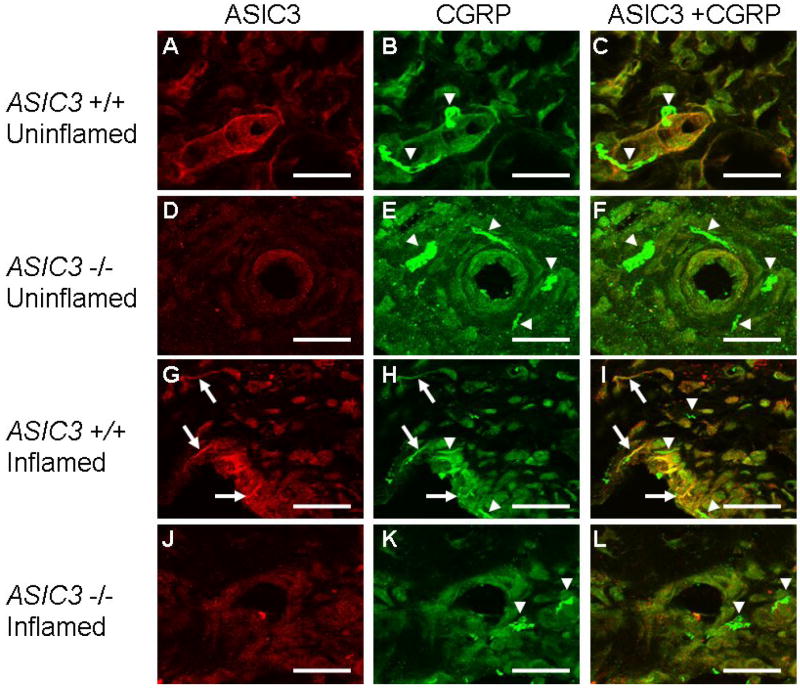
Figure 4.
Immunohistochemsitry staining for ASIC3 and CCRP from sections in whole specimens showing the synovium from ASIC3 +/+ and ASIC3 -/- mice without joint inflammation and those 24 h after joint inflammation. A-F shows immunoreactivity in animals without joint inflammation and G-L shows immunoreactivity in animals with joint inflammation. ASIC3 staining is shown in A,D,G and J; CGRP staining is shown in B,E,H and K; and the merged images of ASIC3 and CGRP are shown in C,F,I and L. CGRP staining occurs in all groups, both ASIC3 +/+ and ASIC3 -/-. No immunoreactivity for ASIC3 was observed in ASIC3 -/- mice; and nerve fibers did not stain for ASIC3 in ASIC3 +/+ mice. After joint inflammation, ASIC3 was located in nerve fibers innervating the synovium in ASIC3 +/+ mice. Some of these fibers co-localize with CGRP (arrows). However some fibers stain positive for CGRP that do not localize with ASIC3 (arrowheads), and some fibers stain positive for ASIC3 that do not localize with CGRP (not shown).
For simultaneous visualization of ASIC3 with CGRP or PGP9.5, a double immunofluorescence method was used. Preliminary experiments were carried out to determine the optimal dilution and the order of application of the primary antisera. The primary antisera used in this study were rabbit anti-ASIC3 serum (1:500; Alomone Labs, Jerusalem, Israel), rabbit anti-CGRP serum (1:1000; Peninsula Laboratories, SanCarlos, CA), and guinea pig anti-PGP9.5 serum (1:1000; Neuromics, Minneapolis, MN). Because anti-ASIC3 and anti-CGRP serum were raised in a same species (rabbit), nonspecific rabbit IgG and monovalent Fab fragment of goat anti-rabbit were used between the ASIC3 and CGRP staining in order to avoid crossreactivity between the antibodies. The avoidance of cross-reactivity was confirmed by omitting either of the primary antibodies.
The sections were blocked in 3% normal goat serum for 30 minutes, then incubated in rabbit anti-ASIC3 serum overnight in a humid atmosphere. The next day, the sections were incubated with biotinylated goat anti-rabbit IgG (1:250; Inivtrogen) for 1 hour followed by strepavidin Alexa 568 (1:500; Invitrogen, Carlsbad, CA) for 1 hour. Subsequently, the sections for PGP 9.5 labeling were incubated with guinea pig anti-PGP 9.5 serum overnight in a humid atmosphere. The sections for CGRP labeling were incubated with nonspecific rabbit IgG (1:1000; Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hour to saturate any open antigen binding sites on the anti-rabbit biotinylated IgG, followed by unconjugated monovalent Fab fragment of goat anti-rabbit (final concentration 0.025 mg/ml; Jackson ImmunoResearch Laboratories, West Grove, PA) to prevent binding from future secondary IgG. Next, sections for CGRP labeling were incubated with rabbit anti-CGRP serum overnight in a humid atmosphere. On the 3rd day, the sections for PGP 9.5 labeling were incubated with goat anti-guinea pig Alexa 488 fluorescent antibody conjugate (1:500; Invitrogen, Carlsbad, CA) for 1 hour, while those for CGRP were with goat anti-rabbit Alexa 647 (1:500; Invitrogen, Carlsbad, CA) for 1 hour. All antisera used were diluted in PBS containing 1% normal goat serum and 0.5% Triton X-100. Before, between, and after each incubation step, the sections were washed with 5 times for 5 minutes in PBS. Finally, all sections were dehydrated through an alcohol gradient (70, 80, 90, and 100%), mounted with a 2:1 preparation of benzyl-alcohol-benzyl-bensoate (BABB), pH=7, and viewed with an MRC-1024 Confocal Imaging system (Bio-Rad, Richmond, CA, USA).
3. Results
3.1. Behavior Experiment
3.1.1. Mechanical Testing of the Paw
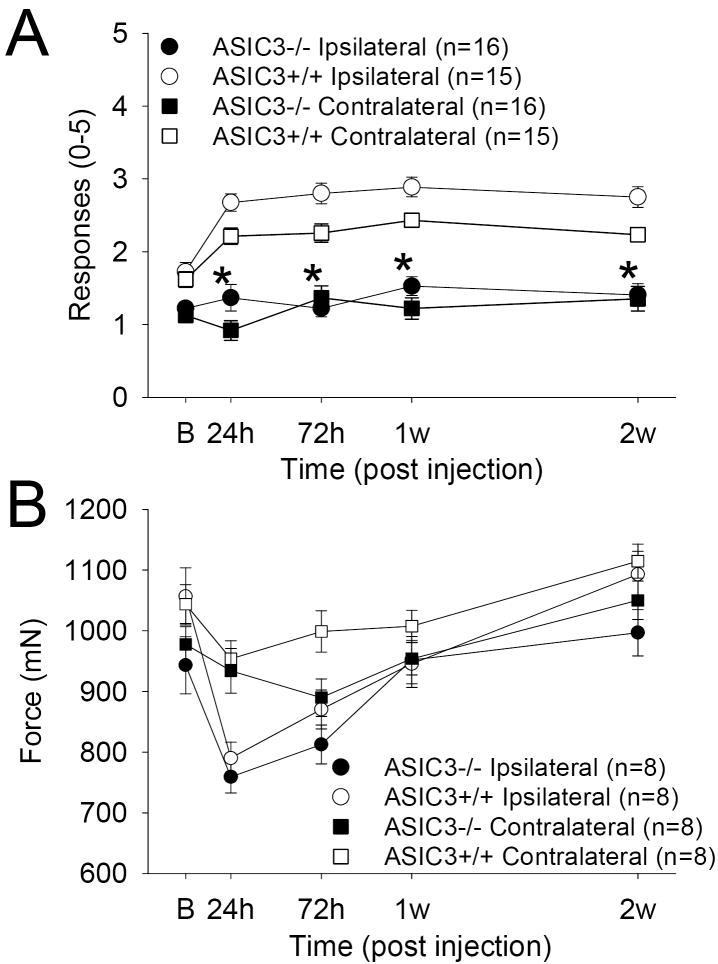
In ASIC3 +/+ mice, a significant increase in the number of withdrawals to an 0.4 mN force occurs 24 h after injection of 3% carrageenan into the knee joint and lasts 2 weeks both ipsilaterally (F1,29=30.1, p=0.0001) and contralaterally (F1,29=11.1. P=0.002) (Figure 1A). There was also a difference between the ASIC3 +/+ and the ASIC3 -/- mice both ipsilaterally (F1,29=69.5, p=0.0001) and contralaterally (F1,29=54.9 P=0.0001). In ASIC3-/- mice there was a significant (p<0.05) reduction in the number of responses to mechanical stimuli at all time periods after induction of inflammation for both the ipsilateral and the contralateral hindpaws. There were no differences for sex of the animal in their responses to mechanical stimuli before and after the induction of inflammation.
Figure 1.
A. The mechanical withdrawal thresholds of the paw are shown before and after joint inflammation in ASIC3 +/+ and ASIC3 -/- mice for the ipsilateral (circles) and the contralateral (squares) hindpaws. The withdrawal thresholds were significantly less in the ASIC3 -/- mice after inflammation when compared to the withdrawal thresholds from ASIC3 +/+ mice for both the ipsilateral and the contralateral hindpaws. *, P<0.05. B. The mechanical withdrawal threshold of the knee joint before and after joint inflammation in ASIC3 +/+ and ASIC3 -/- mice for the ipsilateral (circles) and the contralateral (squares) knees. There was no difference between ASIC 3 +/+ and ASIC3 -/- mice with decreases in withdrawal thresholds occurring 24 h after joint inflammation in both groups.
3.1.2. Thermal Testing of the Paw
In both ASIC3 +/+ and ASIC3 -/- mice, there was a significant difference across time for the changes in the withdrawal thresholds to radiant heat ipsilaterally (F1, 29=7.9, p=0.009) but no difference between ASIC3 +/+ and ASIC3 -/- mice. The paw withdrawal latency to heat decreased ipsilaterally 24 h after induction of inflammation and remained decreases for two weeks after induction of knee joint inflammation (data not shown). In ASIC3 +/+ mice, baseline withdrawal thresholds averaged 8.24 s +/- 0.58 and decreased to a maximum of 6.93 s +/- 0.64 72 h after induction of inflammation. There were no differences between male and female mice, and no differences on the contralateral limb.
3.1.3. Mechanical Testing of the Joint
In both ASIC3 +/+ and ASIC3-/- mice, there was a significant difference across time for the changes in the compression withdrawal threshold of the knee joint ipsilaterally (F1,14=9.2, p=0.009) but no difference between ASIC3 +/+ and ASIC3 -/- mice (Figure 1B). The time course for the decrease in withdrawal thresholds of the knee joint was different from the other measures. There were maximal decreases 24 h after induction of inflammation and were significantly decreased from baseline at both 24 h (ASIC3 +/+ p=0.004; ASIC3 -/- p=0.0001) and 72 h (ASIC3 +/+ p=0.005; ASIC3 -/- p=0.002) for ASIC3 +/+ and ASIC3 -/- mice. By 1 week the compression withdrawal thresholds were similar to baseline numbers and were fully resolved by two weeks. The decrease in compression withdrawal threshold of the knee joint was also only found for the ipsilateral side of the ASIC3 +/+ or ASIC3 -/- mice and did not extend to the contralateral hindlimb.
3.2. Histology
The synovium of the knee joint of the uninflamed knee joint (Figure 2A,B) was examined for ASIC3 innervation (Figure 2C) by examining co-localization with the neural marker PGP 9.5. Surprisingly, there was no co-localization of ASIC3 with PGP 9.5 in the synovium of the knee joint in animals without inflammation. There was, however, ASIC3 localization to the synoviocytes (Figure 2D) in ASIC3 +/+ mice that did not occur in ASIC3 -/- mice (Figure 2E). Similarly, there was no co-localization of ASIC3 with either PGP 9.5 or CGRP in the synovium of the knee joint in whole specimens of the knee joint in animals without inflammation. (Figure 3, 4). In contrast, ASIC3 was located in afferent fibers innervating the knee joint 24h after induction of joint inflammation. In some fibers, ASIC3 co-localized with either PGP 9.5 or with CGRP afferent fibers in the knee joint (Figure 3, 4).
4. Discussion
The current study shows that mechanical hyperalgesia of the paw after induction of knee joint inflammation does not develop in ASIC3 -/- mice when compared to ASIC3 +/+ mice. These data agree with prior studies that show mechanical hyperalgesia of the paw after muscle insult, by acid or inflammation, does not develop in ASIC3 -/- mice (Sluka et al. 2003;Sluka et al. 2007). Further, the current study also shows that there was no difference between ASIC3 +/+ mice and ASIC3 -/- mice in their responses to heat stimuli; similar to that observed after muscle inflammation [23]. These data therefore suggest that secondary mechanical hyperalgesia, i.e. increased response to noxious stimuli outside the site of injury, depends on activation of ASIC3 in primary afferent fibers.
The current study showed no differences between ASIC3 -/- and ASIC3 +/+ mice in the mechanical hyperalgesia of the inflamed knee joint, i.e primary hyperalgesia. Previous studies show that inflammatory hyperalgesia of the paw, both mechanical and heat, is also similar between ASIC3 -/- and ASIC3 +/+ mice [1,7,13]. Thus, although we previously interpreted these data as a difference between cutaneous and muscle insult [22,23], the current data support the conclusion that primary hyperalgesia is unaffected by loss of ASIC3. Further, the data together suggest that secondary hyperalgesia develops in response to peripheral activation of ASIC3. In support, central sensitization does not develop in ASIC3 -/- mice [22]. Specifically, receptive fields of dorsal horn neurons do not expand after muscle insult; and the responses of dorsal horn neurons to mechanical stimuli are reduced in ASIC3 -/- mice when compared to ASIC3+/+ after muscle insult [22]. Further re-expression of ASIC3 into primary afferent fibers innervating muscle of ASIC3 -/- mice restores the development of secondary hyperalgesia [23]. Together these data suggest that activation of ASIC3 at peripheral sites send nociceptive information to the central nervous system to result in sensitization of dorsal horn neurons, and the consequent secondary hyperalgesia. It further suggests that, ASIC3 at the peripheral site does not result in direct nociceptor sensitization that contributes to primary hyperalgesia.
The current data also show that there was no ASIC3 in primary afferent fibers innervating the synovium in animals without joint inflammation. This was surprising but supports the notion that low levels of ASIC3 are found in peripheral tissues when compared to brain. Previously, Gitterman et al. [4], show that muscle expresses 16% of the amount of ASIC3 mRNA in comparison with expression levels of the brain. However, approximately 50% of DRG express ASIC3 and there is clear localization of ASIC3 in primary afferents innervating skin and muscle in animals without tissue injury. It is possible that under normal conditions, ASIC3 serves a functional role in cutaneous and muscle receptors as a mechanosensor and/or a pH sensing ion channel. There are clear differences in responses of cutaneous rapidly adapting mechanosensors and in Aδ nociceptors in ASIC3 -/- mice when compared to ASIC3 +/+ mice [13]. Specifically, Aδ mechanonociceptors show decreased responsiveness and increased threshold to mechanical3 stimuli [13] in ASIC3-/- mice. Further, 80% of ASIC3 positive DRG neurons innervating muscle also express CGRP, and nerve terminals co-expressing ASIC3 and CGRP are found in primary afferent fibers innervating muscle arterioles in animals without tissue injury [8]. The ASIC3 co-localization in muscle arterioles is found mostly in large diameter afferent fibers, and not in thin diameter nociceptors [8]. In contrast, few proprioceptors express ASIC3 [8]. It is yet unknown if primary afferent fibers innervating deep tissue afferents, i.e. muscle and joint, show similar changes in mechanosensors and nociceptors in ASIC3 -/- mice electrophysiologically; or if expression patterns are similar in muscle and joint. Our data suggests that before inflammation ASIC3 is not expressed at detectable levels in primary afferent fibers innervating the knee joint. After inflammation, however, there is expression of ASIC3, and this expression partially co-localizes with CGRP.
This increased expression of ASIC3 after inflammation suggests there is an upregulation of ASIC3 in DRG in response to tissue inflammation. In support, prior studies show ASIC3 mRNA is upregulated in DRG after complete Freund’s adjuvant inflammation of the paw or after placing nucleus pulposis on the nerve root [10,12,26] suggesting there may be an increase in ASIC3 protein after inflammation. Application of inflammatory mediators to DRG neurons (nerve growth factor, serotonin, interleukin-1, arachidonic acid, and bradykinin), mimics this increased expression of ASIC3 mRNA in DRG after inflammation [6,25]. Further, application of these inflammatory mediators to DRG neurons in culture results in an increase in the number of neurons expressing ASIC currents, including ASIC3-like currents, and increased co-expression of ASIC3 and TRPV1 [6]. For the ASIC3-like currents there is an increase in current density and an increased in the number of neurons expressing ASIC3 [6]. These changes in ASIC3 current should increase acid-induced excitation of ASIC3 containing neurons and increase the number of neurons capable of responding to acid.
Surprisingly, in animals without inflammation, there was detectable ASIC3 in synoviocytes of the knee joint. This role of ASIC3 in synoviocytes is unclear and at present speculative. However, other ion channels, i.e.TRPV1, thought to be neuronal in nature, are also found in synoviocytes [3,5,27]. Application of capsaicin to human synoviocytes increases intracellular calcium and application of the TRPV1 receptor antagonist, capsazepine, blocks the increase in intracellular calcium [5]. The role of ion channels in synoviocytes may be to regulate expression of the extracellular matrix substances such as hyaluronan. Indeed hyaluronan is released from synoviocytes in response to stretch [9] and the inflammatory cytokine interleukin-1 decreases synthesis of glycosaminoglycans from synoviocytes [15]. Thus, inflammation and activation of ion channels could modulate the extracellular matrix to maintain adequate lubrication. There may also be direct influences on the levels of ASIC3 by cell-cell contact between synoviocytes and the DRG neurons. Recently, von Banchet et al. [27] show an increased expression of neurokinin-1, bradykinin 2 receptors, and TRPV1 in DRG in co-cultures of synoviocytes and DRG when compared to DRG alone. In this study, the increased NKI expression in co-cultures was mimicked by application of the supernatant from synoviocytes. However, the increased expression of bradykinin-2 receptors and TRPV1 was not mimicked by application of the supernatant from synovioctyes [27]. Taken together these studies suggest that synoviocytes not only secrete substances that interact with DRG but that there also may be cell-cell interactions between synoviocytes and DRG. ASIC3 expression might similarly be affected by secretions from the synoviocytes into the extracellular matrix and/or by cell-cell interactions between synoviocytes and sensory neurons.
In summary, ASIC3 plays a role in the development of secondary, but not primary, hyperalgesia. ASIC3 -/- mice show differences in the development of secondary, but not primary mechanical hyperalgesia as compared to ASIC3 +/+ mice. Further ASIC3 is located in primary afferent fibers after joint inflammation, but not before joint inflammation. Thus, joint inflammation should result in release of inflammatory mediators that would sensitize primary afferent fibers, increasing expression of ASIC3 in primary afferent fibers innervating synovium. The decreased pH that occurs after inflammation would activate ASIC3 on primary afferent fibers innervating joint, increasing the input to the spinal cord resulting in central sensitization manifested behaviorally as secondary hyperalgesia of the paw.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ, Zimmer A. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci U S A. 2002;99:8992–8997. doi: 10.1073/pnas.122245999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dougherty PM, Sluka KA, Sorkin LS, Westlund KN, Willis WD. Neural changes in acute arthritis in monkeys. I. Parallel enhancement of responses of spinothalamic tract neurons to mechanical stimulation and excitatory amino acids. Brain Res Rev. 1992;17:1–13. doi: 10.1016/0165-0173(92)90002-4. [DOI] [PubMed] [Google Scholar]
- 3.Engler A, Aeschlimann A, Simmen BR, Michel BA, Gay RE, Gay S, Sprott H. Expression of transient receptor potential vanilloid 1 (TRPV1) in synovial fibroblasts from patients with osteoarthritis and rheumatoid arthritis. Biochem Biophys Res Commun. 2007;359:884–888. doi: 10.1016/j.bbrc.2007.05.178. [DOI] [PubMed] [Google Scholar]
- 4.Gitterman DP, Wilson J, Randall AD. Functional properties and pharmacological inhibition of ASIC channels in the human SJ-RH30 skeletal muscle cell line. Journal of Physiology-London. 2005;562:759–769. doi: 10.1113/jphysiol.2004.075069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochukov MY, McNearney TA, Fu Y, Westlund KN. Thermosensitive TRP ion channels mediate cytosolic calcium response in human synoviocytes. Am J Physiol Cell Physiol. 2006;291:C424–C432. doi: 10.1152/ajpcell.00553.2005. [DOI] [PubMed] [Google Scholar]
- 6.Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci. 2002;22:10662–10670. doi: 10.1523/JNEUROSCI.22-24-10662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mogil JS, Breese NM, Witty MF, Ritchie J, Rainville ML, Ase A, Abbadi N, Stucky CL, Seguela P. Transgenic expression of a dominant-negative ASIC3 subunit leads to increased sensitivity to mechanical and inflammatory stimuli. J Neurosci. 2005;25:9893–9901. doi: 10.1523/JNEUROSCI.2019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Molecular Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Momberger TS, Levick JR, Mason RM. Hyaluronan secretion by synoviocytes is mechanosensitive. Matrix Biol. 2005;24:510–519. doi: 10.1016/j.matbio.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagae M, Hiraga T, Wakabayashi H, Wang L, Iwata K, Yoneda T. Osteoclasts play a part in pain due to the inflammation adjacent to bone. Bone. 2006;39:1107–1115. doi: 10.1016/j.bone.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 11.Neugebauer V, Schaible H-G. Evidence for a central component in the sensitization of spinal neurons with joint input during development of acute arthritis in cat’s knee. J Neurophysiol. 1990;64:299–311. doi: 10.1152/jn.1990.64.1.299. [DOI] [PubMed] [Google Scholar]
- 12.Ohtori S, Inoue G, Koshi T, Ito T, Doya H, Saito T, Moriya H, Takahashi K. Up-regulation of acid-sensing ion channel 3 in dorsal root ganglion neurons following application of nucleus pulposus on nerve root in rats. Spine. 2006;31:2048–2052. doi: 10.1097/01.brs.0000231756.56230.13. [DOI] [PubMed] [Google Scholar]
- 13.Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC Cation Channel Contributes to the Detection of Cutaneous Touch and Acid Stimuli in Mice. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 14.Radhakrishnan R, Moore SA, Sluka KA. Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. Pain. 2003;104:567–577. doi: 10.1016/s0304-3959(03)00114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redini F, Mauviel A, Loyau G, Pujol JP. Modulation of extracellular matrix metabolism in rabbit articular chondrocytes and human rheumatoid synovial cells by the non-steroidal anti-inflammatory drug etodolac. II: Glycosaminoglycan synthesis. Agents Actions. 1990;31:358–367. doi: 10.1007/BF01997632. [DOI] [PubMed] [Google Scholar]
- 16.Schaible H-G, Schmidt RF. Effects of an experimental arthritis on the sensory properties of fine articular afferent units. J Neurophysiol. 1985;54:1109–1122. doi: 10.1152/jn.1985.54.5.1109. [DOI] [PubMed] [Google Scholar]
- 17.Schaible H-G, Schmidt RF. Time course of mechanosensitivity changes in articular afferents during a developing experimental arthritis. J Neurophysiol. 1988;60:2180–2194. doi: 10.1152/jn.1988.60.6.2180. [DOI] [PubMed] [Google Scholar]
- 18.Schaible H-G, Schmidt RF, Willis WD. Enhancement of the responses of ascending tract cells in the cat spinal cord by acute inflammation of the knee joint. Exp Brain Res. 1987;66:489–499. doi: 10.1007/BF00270681. [DOI] [PubMed] [Google Scholar]
- 19.Schaible HG, Ebersberger A, Von Banchet GS. Mechanisms of pain in arthritis. Ann N Y Acad Sci. 2002;966:343–354. doi: 10.1111/j.1749-6632.2002.tb04234.x. [DOI] [PubMed] [Google Scholar]
- 20.Schaible HG, Grubb BD. Afferent and spinal mechanisms of joint pain. Pain. 1993;55:5–54. doi: 10.1016/0304-3959(93)90183-P. [DOI] [PubMed] [Google Scholar]
- 21.Skyba DA, Radhakrishnan R, Sluka KA. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. Journal of Pain. 2005;6:41–47. doi: 10.1016/j.jpain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- 23.Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102–112. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sluka KA, Westlund KN. Behavioral and immunohistochemical changes in an experimental arthritis model in rats. Pain. 1993;55:367–377. doi: 10.1016/0304-3959(93)90013-F. [DOI] [PubMed] [Google Scholar]
- 25.Smith ES, Cadiou H, McNaughton PA. Arachidonic acid potentiates acid-sensing ion channels in rat sensory neurons by a direct action. N S. 2007;145:686–698. doi: 10.1016/j.neuroscience.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 26.Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001;21:8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Von Banchet GS, Richter J, Huckel M, Rose C, Brauer R, Schaible HG. Fibroblast-like synovial cells from normal and inflamed knee joints differently affect the expression of pain-related receptors in sensory neurones: a co-culture study. Arthritis Res Ther. 2007;9:R6. doi: 10.1186/ar2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokoyama T, Audette KM, Sluka KA. Pregabalin reduces muscle and cutaneous hyperalgesia in two models of chronic muscle pain in rats. J Pain. 2007;8:422–429. doi: 10.1016/j.jpain.2006.11.007. [DOI] [PubMed] [Google Scholar]