Abstract
OBJECTIVE
Compared with nondiabetic subjects, type 2 diabetic subjects are metabolically inflexible with impaired fasting fat oxidation and impaired carbohydrate oxidation during a hyperinsulinemic clamp. We hypothesized that impaired insulin-stimulated glucose oxidation is a consequence of the lower cellular glucose uptake rate in type 2 diabetes. Therefore, we compared metabolic flexibility to glucose adjusted for glucose disposal rate in nondiabetic versus type 2 diabetic subjects and in the latter group after 1 year of lifestyle intervention (the Look AHEAD [Action For Health in Diabetes] trial).
RESEARCH DESIGN AND METHODS
Macronutrient oxidation rates under fasting and hyperinsulinemic conditions (clamp at 80 mU/m2 per min), body composition (dual-energy X-ray absorptiometry), and relevant hormonal/metabolic blood variables were assessed in 59 type 2 diabetic and 42 nondiabetic individuals matched for obesity, sex, and race. Measures were repeated in diabetic participants after weight loss.
RESULTS
Metabolic flexibility to glucose (change in respiratory quotient [RQ]) was mainly related to insulin-stimulated glucose disposal rate (R2 = 0.46, P < 0.0001) with an additional 3% of variance accounted for by plasma free fatty acid concentration at the end of the clamp (P = 0.03). The impaired metabolic flexibility to glucose observed in type 2 diabetic versus nondiabetic subjects (ΔRQ 0.06 ± 0.01 vs. 0.10 ± 0.01, respectively, P < 0.0001) was no longer observed after adjusting for glucose disposal rate (P = 0.19). Additionally, the increase in metabolic flexibility to glucose after weight loss was accounted for by the concomitant increase in insulin-stimulated glucose disposal rate.
CONCLUSIONS
This study suggests that metabolic inflexibility to glucose in type 2 diabetic subjects is mostly related to defective glucose transport.
Metabolic flexibility is the capacity of the body to match fuel oxidation to fuel availability. It is typically assessed by the increase in respiratory quotient (RQ) from fasting to glucose/insulin-stimulated conditions (1). During the overnight transition from the fed to the fasting state, metabolic inflexibility can also be evident by a higher fasting RQ (2). Finally, the lower capacity to adapt fat oxidation to a fat overload is another feature of metabolic inflexibility (3,4).
Insulin-resistant and type 2 diabetic subjects have shown both higher fasting RQ (2,5,6) and blunted increase in RQ during a hyperinsulinemic clamp compared with insulin-sensitive subjects (2,7). Structural and functional mitochondrial impairments in obesity and type 2 diabetes are proposed to be a cause of insulin resistance and metabolic inflexibility (8,9).
During a euglycemic-hyperinsulinemic clamp, the metabolic flexibility to glucose should be lower in type 2 diabetic versus nondiabetic subjects, since cellular glucose uptake rate and therefore free cellular glucose available for oxidation is reduced. Such phenomenon is analogous to the thermic effect of a meal, which is proportional to the energy content of the meal (10). The high correlation between insulin-stimulated glucose disposal rate and metabolic flexibility to glucose supports this idea (2,11).
We hypothesized that type 2 diabetic and nondiabetic obese individuals have similar metabolic flexibility to glucose after controlling for glucose disposal rate. Furthermore, after 1-year of intensive lifestyle therapy, causing substantial weight loss and improvement in insulin sensitivity, the metabolic flexibility to glucose is not improved in type 2 diabetic volunteers if adjusted for glucose disposal rate.
RESEARCH DESIGN AND METHODS
This study is an ancillary project at 3 of the 16 participating sites of the Look AHEAD (Action For Health in Diabetes) trial (Pennington Biomedical Research Center, Baton Rouge, LA; the University of Pittsburgh, Pittsburgh, PA; and St. Luke's Roosevelt Hospital Center, NY). Inclusion and exclusion criteria are described elsewhere (12). Only participants randomized to the lifestyle intervention arm were enrolled in this study to examine the effects of 1-year intervention on body composition and insulin sensitivity. This report includes pre-intervention and 1-year follow-up data in 59 type 2 diabetic subjects (44 Caucasian, 12 African-American, and 3 Hispanic) and baseline data in 42 nondiabetic volunteers (34 Caucasian and 8 African American) matched for BMI, sex, and race. All participants gave informed written consent, and each institutional review board as well as the Look AHEAD Steering Committee approved the protocol.
The intervention was designed to achieve and maintain weight loss through decreased caloric intake and increased physical activity with an expected 1-year weight loss of ≥7% of the initial value (13). Volunteers were admitted on the evening preceding the metabolic studies and were not allowed any medication until after the metabolic testing. Those on oral antidiabetic medications were put on the same dose at the 1-year repeat testing.
Body composition
Weight (in a pre-weighted gown) and height were obtained, and fat mass (FM) and fat-free mass (FFM) were measured by dual-energy X-ray absorptiometry (Hologic QDR 4500A, Waltham, MA). A single cross-sectional 1 cm–thick computed tomography scan centered at L4-L5 was used to measure abdominal adipose tissue, and images were analyzed for fat content at the University of Pittsburgh with attenuation values >200 Hounsfield units (HU) for bone, −30 to −190 HU for adipose tissue, and 0–100 HU for muscle.
Hyperinsulinemic clamp
Intravenous catheters were inserted in an antecubital vein for infusions and in a vein on the dorsum of the contralateral hand for sampling of arterialized blood. After baseline sampling, a primed-continuous insulin infusion (80 mU/m2 per min) was started for 3–4 h. Insulin was infused for at least 1 h after reaching a glycemia of ~100 mg/dl in type 2 diabetic volunteers. Plasma glucose was measured every 5 min and maintained by a variable 20% glucose infusion. The mean rate of exogenous glucose infusion during steady state (last 30 min) was corrected for changes in glycemia and divided by FFM to assess insulin sensitivity (14).
Resting metabolic, glucose, and lipid oxidation rates were determined by indirect calorimetry using a Deltatrac II instrument (SensorMedics, Anaheim, CA) over 30 min at baseline and at steady state (15). Nonoxidative glucose disposal rate was calculated as the difference between total glucose and glucose oxidative disposal rates. Metabolic flexibility to glucose was calculated as the difference between the steady-state RQ at the end of the clamp and fasting RQ (ΔRQ). Plasma glucose (Synchron CX Delta Systems, Beckman, Brea, CA), free fatty acid (FFA) (NEFA-C kit; WAKO, Denver, CO), glycerol (UniCel DxC 600; Synchron, Brea, CA), insulin (Immulite 2000 analyzer; DPC, Los Angeles, CA), and adiponectin (RIA kit; Linco, St. Charles, MO) were analyzed.
Statistical analysis
Data are presented as means ± SE. Analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, NC). Data distribution was tested with the Shapiro test. Log or root square transformations were applied as necessary. Pearson correlation was used to assess the relationships between ΔRQ and measured variables. Stepwise multiple regression analysis was used to explore the factors best predicting ΔRQ. A regression analysis was then derived to generate a predictive equation with ΔRQ as the dependent variable. “Metabolically flexible” individuals were those with positive residuals (measured ΔRQ higher than predicted ΔRQ), whereas “inflexible” individuals were those with negative residuals. Differences between groups were evaluated by covariance analysis adjusting for age, research center, race, and sex. Energy expenditure was controlled for FFM and FM. Multiple comparisons were adjusted by Tukey analysis, and χ2 analysis was performed to determine differences in frequencies for sex, race, and presence of diabetes.
RESULTS
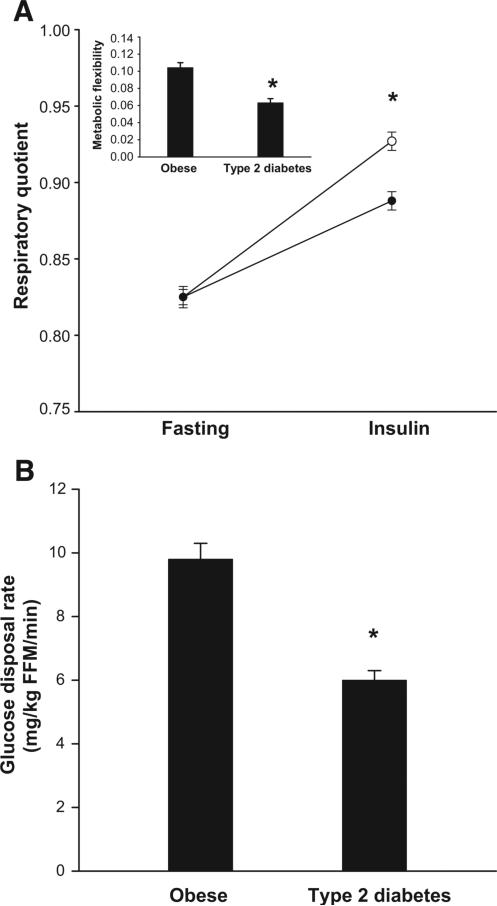
The characteristics of the subjects are shown in Table 1. As expected, type 2 diabetic subjects had elevated fasting plasma glucose and FFA concentrations and impaired insulin sensitivity. Fasting RQ was similar in type 2 diabetic (0.83 ± 0.01) and nondiabetic participants (0.83 ± 0.01). However, under insulin-stimulated conditions, the ΔRQ was lower in type 2 diabetic versus nondiabetic individuals (0.06 ± 0.01 vs. 0.10 ± 0.01, P < 0.0001 adjusted for age, sex, race, and research center; Fig. 2). Similarly, insulin-stimulated oxidative and nonoxidative glucose disposal rate were lower in type 2 diabetic versus nondiabetic subjects (P < 0.05 adjusted for FFM, FM, age, sex, race, and research center).
TABLE 1.
Characteristics of the subjects
| Nondiabetic subjects | Type 2 diabetic subjects |
||
|---|---|---|---|
| Before | After | ||
| Female/male | 25/17 | 33/26 | 33/26 |
| African American/Caucasian/Other | 8/34/0 | 12/44/3 | 12/44/3 |
| Age (years) | 54.9 ± 1.2 | 60.0 ± 1.0* | 61.0 ± 1.0 |
| Body weight (kg) | 94.4 ± 2.2 | 95.9 ± 1.4 | 86.4 ± 1.3† |
| BMI (kg/m2) | 32.7 ± 0.4 | 33.8 ± 0.4 | 30.5 ± 0.5† |
| Body fat (%) | 37.3 ± 1.1 | 35.9 ± 0.9 | 31.9 ± 1.1† |
| FFM (kg) | 59.7 ± 2.1 | 61.5 ± 1.3 | 58.7 ± 1.2† |
| FM (kg) | 34.8 ± 1.0 | 34.4 ± 0.9 | 27.7 ± 1.1† |
| Fasting glucose (mg/dl) | 99.8 ± 1.3 | 143.4 ± 4.3* | 125.9 ± 3.7† |
| Fasting FFA (mmol/l) | 0.58 ± 0.02 | 0.69 ± 0.02* | 0.56 ± 0.02† |
| Fasting insulin (IU/l) | 10.0 ± 0.9 | 13.6 ± 0.8* | 11.3 ± 1.0† |
| Fasting adiponectin (μg/ml) | 8.2 ± 0.8 | 6.2 ± 0.4* | 7.9 ± 0.6† |
| Steady-state glucose (mg/dl) | 104.1 ± 1.1 | 103.2 ± 0.8 | 103.9 ± 0.8 |
| Steady-state FFA (mmol/l) | 0.013 ± 0.002 | 0.042 ± 0.006* | 0.014 ± 0.002† |
| Steady-state insulin (IU/l) | 165.7 ± 7.7 | 152.7 ± 5.7 | 140.3 ± 4.4 |
| Adjusted fasting metabolic rate (Kcal/day)‡ | 1749 ± 42 | 1805 ± 27* | 1665 ± 23 |
| Adjusted steady-state metabolic rate (Kcal/day)‡ | 1,802 ± 41 | 1,846 ± 27 | 1,730 ± 23§ |
| Steady-state GDR (mg · kg FFM−1 · min−1) | 9.8 ± 0.5 | 6.0 ± 0.3* | 8.6 ± 0.4† |
| Steady-state oxidative GDR (mg · kg FFM−1 · min−1) | 3.2 ± 0.2 | 2.2 ± 0.1* | 2.9 ± 0.1† |
| Steady-state nonoxidative GDR (mg · kg FFM−1 · min−1) | 6.6 ± 0.4 | 3.6 ± 0.3* | 5.6 ± 0.3† |
| Metabolic flexibility (ΔRQ) | 0.10 ± 0.01 | 0.06 ± 0.01* | 0.10 ± 0.01† |
Data are means ± SE. Covariance analysis was used to compare means between nondiabetic and type 2 diabetic subjects, controlling for age, sex, race, and research center. Energy expenditure comparisons were additionally controlled for FFM and FM. Multiple comparisons between groups and time periods (fasting and insulin-stimulated metabolic rates) were adjusted by Tukey-Kramer analysis.
P < 0.05 for comparison between nondiabetic and type 2 diabetic subjects
P < 0.05 for comparison before and after weight loss in type 2 diabetics subjects
P < 0.05 for comparison between insulin-stimulated and fasting energy expenditure
adjusted energy expenditure for FM and FFM. GDR, glucose disposal rate.
FIG. 2.
A: Respiratory quotient under fasting and insulin-stimulated conditions in obese with type 2 diabetes (•, n = 59) and obese without type 2 diabetes (○, n = 42); inset: metabolic flexibility in both groups. B: Glucose disposal rate in both groups. Data are means ± SE. *P < 0.05.
Determinants of metabolic flexibility to glucose
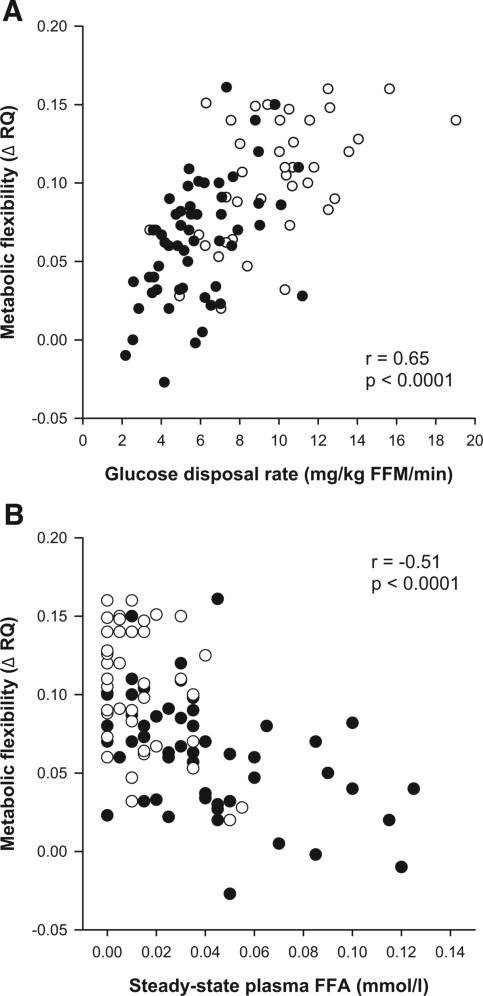
In the whole group, metabolic flexibility to glucose was inversely correlated with fasting plasma glucose (r = −0.45, P < 0.0001) and insulin (r = −0.41, P < 0.0001) concentrations but positively with fasting plasma adiponectin concentration (r = 0.22, P = 0.04). During glucose/insulin infusion, metabolic flexibility to glucose was positively associated with glucose disposal rate (r = 0.65, P < 0.0001; Fig. 1) but inversely to plasma FFA (r = −0.51, P < 0.0001; Fig. 1) and glycerol (r = −0.29, P < 0.005).
FIG. 1.
Correlation analysis in the whole study group between metabolic flexibility (steady-state RQ – fasting RQ) and glucose disposal rate (A) (type 2 diabetes: r = 0.52, P < 0.0001; obese without type 2 diabetes: r = 0.52, P = 0.0007) and steady-state plasma FFA concentration (B) (type 2 diabetes: r = −0.47, P = 0.0002; obese without type 2 diabetes: r = −0.40, P = 0.012). •, obese with type 2 diabetes (n = 59); ○, obese without type 2 diabetes (n = 42).
By stepwise multiple regression analysis, glucose disposal rate (in milligrams per kilogram FFM per minute) was the main determinant of ΔRQ, explaining 46% of variance (slope = 0.04, P < 0.0001), whereas an additional 3% was explained by steady-state plasma FFA concentration (slope = −0.20, P = 0.03). Diabetes status, sex, and race were not significant.
Characteristics of metabolically flexible and inflexible to glucose
A total of 47 subjects were defined as metabolically flexible and 54 as inflexible. Similar proportions of type 2 diabetic individuals (62 vs. 56%, respectively, P = 0.53), women (55 vs. 59%, P = 0.69), and African-American subjects (21 vs. 19%, P = 0.86) were observed in each group. Anthropometric and metabolic characteristics were similar except for a higher BMI in the metabolically inflexible group (34.1 ± 0.4 vs. 32.3 ± 0.4 kg/m2, P = 0.01).
Metabolic flexibility to glucose in type 2 diabetes
The lower ΔRQ observed in type 2 diabetic versus nondiabetic individuals (Fig. 2) was still significant after controlling for steady-state plasma FFA concentration (P = 0.0004); however, the difference was abolished after adjusting for glucose disposal rate (P = 0.19). Accordingly, the slopes and intercepts of the relationships between ΔRQ and glucose disposal rate were similar in both groups. Similar results were found after applying the same analysis to insulin-stimulated fat (P = 0.86) and glucose (P = 0.92) oxidation expressed in absolute rates or adjusted for FFM and FM. Nonoxidative glucose disposal rate between groups was also similar after controlling for glucose disposal rate (P = 0.87). Fasting energy expenditure was higher in type 2 diabetic versus nondiabetic participants after controlling for FFM, FM, age, race, sex, and research center (P = 0.003, Table 1) but not after insulin stimulation (P = 0.10, Table 1).
Effect of weight loss on metabolic flexibility to glucose
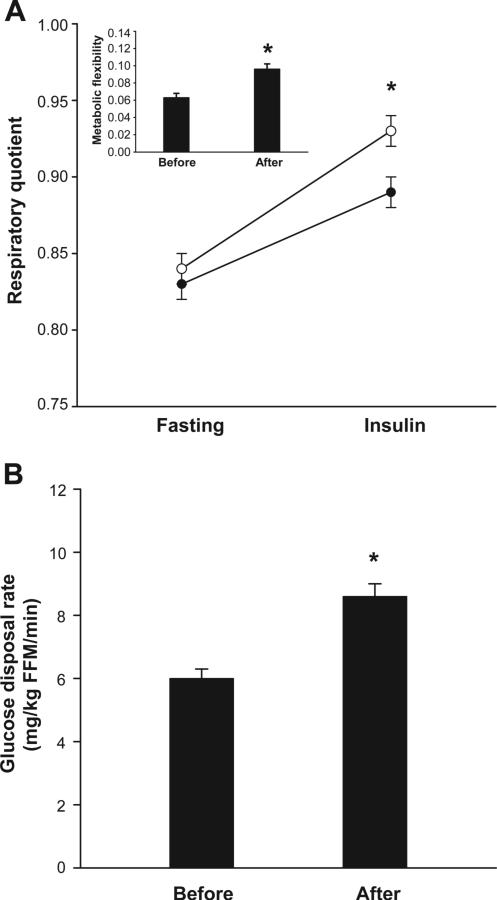
After 1-year of intensive lifestyle intervention in the type 2 diabetic participants, body weight (−9.5 ± 0.9 kg, P < 0.0001), body fat (−3.8 ± 0.4%, P < 0.0001), FM (−6.5 ± 0.7 kg, P < 0.0001), FFM (−3.0 ± 0.4 kg, P < 0.05), fasting plasma glucose (−17.3 ± 4.0 mg/dl, P < 0.0001), FFA (−0.13 ± 0.02 mmol/l, P < 0.0001), fasting insulin concentrations (−2.4 ± 0.8 IU/l, P = 0.004), and steady-state FFA concentration (−0.03 ± 0.01 mmol/l, P < 0.0001) were all reduced. Glucose disposal rate (P < 0.0001) and metabolic flexibility to glucose (P < 0.0001) were increased (Fig. 3) mostly due to larger increases in steady-state RQ, since fasting RQ was unaltered. After controlling for glucose disposal rate, there was no difference in metabolic flexibility to glucose before and after weight loss (P = 0.17). Additional control for steady-state plasma FFA concentration did not modify this finding (P = 0.41).
FIG. 3.
A: Respiratory quotient under fasting and insulin-stimulated conditions in obese type 2 diabetic subjects before (•, n = 59) and after (○, n = 59) 1-year weight loss intervention; inset: metabolic flexibility before and after intervention. B: Glucose disposal rate before and after intervention. Data are means ± SE. *P < 0.05.
DISCUSSION
As previously reported (2,16), we observed that whole-body metabolic flexibility to glucose was lower in type 2 diabetic versus obesity-matched nondiabetic individuals, and this metabolic inflexibility to glucose was improved after weight loss. However, once values were adjusted for glucose disposal rate, the differences in ΔRQ between groups and after weight loss were abolished. This suggests that the metabolic inflexibility to glucose in type 2 diabetes is not explained by a primary impairment in glucose oxidation but instead is the consequence of impaired glucose transport. Similarly, we observed reduced steady-state nonoxidative glucose disposal rate in type 2 diabetic versus nondiabetic subjects, but not after controlling for glucose disposal rate.
Glucose disposal rate is equal to glucose oxidation and nonoxidative glucose disposal rates; therefore, a change in glucose disposal rate will encompass concomitant changes in glucose oxidation and/or glucose storage. Since insulin-resistant subjects receive lower amounts of glucose during a clamp, it is appropriate to compare the ΔRQ after taking rates of glucose disposal into account.
Few studies have compared in vivo fuel oxidation under similar glucose disposal rates in diabetic and nondiabetic subjects (5,17,18). To achieve this goal, Yki-Järvinen et al. (17) matched glucose disposal rates in insulin-resistant type 1 diabetic and nondiabetic subjects by increasing glucose infusion rate in the former group and found similar increases in glucose oxidation. In contrast, Thor-burn et al. (18) reported lower glucose oxidation rates in type 2 diabetic individuals than in nondiabetic subjects under matched glucose disposal rates. By using the leg balance technique, Kelley and Mandarino (5) found similar leg RQ at the end of the glucose and insulin infusion in type 2 diabetic and nondiabetic individuals after matching leg glucose uptake. Recently, it was found that glucose oxidation during a clamp was similar between normal glucose-tolerant and type 2 diabetic Pima Indians after controlling for glucose disposal rate (16).
The present study did not show differences in whole-body fasting RQ between nondiabetic versus type 2 diabetic subjects or in the latter group after weight loss. This finding might suggest a similar inflexibility to lipids in both groups, as previously found when skeletal muscle RQ was assessed (2,5,6). On the other hand, whole-body RQ might not be representative of the skeletal muscle metabolism.
The variance in ΔRQ was also partly explained by the insulin-suppressed FFA concentration. Higher plasma FFA concentration may increase lipid oxidation and in turn the reliance on glucose as a source of energy (19). Additionally, plasma FFA may reduce insulin-stimulated glucose uptake (20). The small influence (3%) of steady-state plasma FFA on metabolic flexibility may be underscored by the high insulin dose infused, which strongly suppresses plasma FFA concentration. No additional factors explained any significant variance in ΔRQ. However, inflexible compared with flexible subjects had a slightly higher BMI. Whether increased body mass is a cause or a consequence of metabolic inflexibility to glucose could not be assessed in the present cross-sectional analysis.
In conclusion, metabolic inflexibility to glucose in type 2 diabetes disappears after taking into account the lower glucose disposal rates observed in these individuals compared with nondiabetic subjects. Additionally, the inability of insulin to suppress plasma FFA may also play a role on metabolic flexibility.
ACKNOWLEDGMENTS
This study was funded by grant DK60412 (to E.R.), with additional support by grant U01 DK056990 (to D.E.K.), from the National Institutes of Diabetes and Digestive and Kidney Diseases. This work was also supported by the University of Pittsburgh Obesity & Nutrition Research Center (P3-DK46204), the University of Pittsburgh General Clinical Research Center (MO1-RR000056), Pennington Biomedical Research Center Clinical Nutrition Research Unit (P30 DK072476), the Columbia Diabetes and Endocrinology Research Center (National Institutes of Health Grant NIH P30 DK63608), and Columbia General Clinical Research Center (NIH MO1-RR00645). J.E.G. is supported by a fellowship from The International Nutrition Foundation/Ellison Medical Foundation.
We acknowledge the other members of the Look AHEAD adipose research group, not included in the writing group, who contributed generously to this research project. We are grateful to the participants of the primary Look AHEAD trial for their enthusiastic willingness to participate in this ancillary study. We are also grateful to the control participants and to the nursing and nutritional staffs of the three investigational sites.
Glossary
- FFA
free fatty acid
- FM
fat mass
- FFM
fat-free mass
- RQ
respiratory quotient
APPENDIX
Members of the Look AHEAD Adipose Research Group
Pennington Biomedical Research Center (also the coordinating center)
George A. Bray, MD; Donna H. Ryan, MD; Donald Williamson, PhD; Frank L. Greenway, MD; Allison Strate, RN; Elizabeth Tucker; Kristi Rau; Brandi Armand, LPN; Mandy Shipp, RD; Kim Landry; Evan S. Berk, PhD; Julia A. Johnson, PhD; Linda Haselman, RN, MS, CDE; and Jennifer Perault.
St. Luke's Roosevelt Hospital Center
Jennifer Patricio, MS; Jennifer Mayer, MS; Stanley Heshka, PhD; Carmen Pal, MD; Mary Anne Holowaty, MS, CN; and Diane Hirsch, RNC, MS, CDE.
University of Pittsburgh
Carol A. Kelley, RN; Jacqueline Wesche-Thobaben, RN, BSN, CDE; Rebecca Danchenko, BS; and Jowand Green, BS.
REFERENCES
- 1.Kelley D, Mandarino L. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 2.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277:E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 3.Ukropcova B, Sereda O, de Jonge L, Bogacka I, Nguyen T, Xie H, Bray G, Smith S. Family history of diabetes links impaired substrate switching and reduced mitochondrial content in skeletal muscle. Diabetes. 2007;56:720–727. doi: 10.2337/db06-0521. [DOI] [PubMed] [Google Scholar]
- 4.Heilbronn LK, Gregersen S, Shirkhedkar D, Hu D, Campbell LV. Impaired fat oxidation after a single high-fat meal in insulin-sensitive nondiabetic individuals with a family history of type 2 diabetes. Diabetes. 2007;56:2046–2053. doi: 10.2337/db06-1687. [DOI] [PubMed] [Google Scholar]
- 5.Kelley DE, Mandarino LJ. Hyperglycemia normalizes insulin-stimulated skeletal muscle glucose oxidation and storage in noninsulin-dependent diabetes mellitus. J Clin Invest. 1990;86:1999–2007. doi: 10.1172/JCI114935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulindependent diabetes mellitus. J Clin Invest. 1994;94:2349–2356. doi: 10.1172/JCI117600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley DE, Mokan M, Mandarino LJ. Intracellular defects in glucose metabolism in obese patients with NIDDM. Diabetes. 1992;41:698–706. doi: 10.2337/diab.41.6.698. [DOI] [PubMed] [Google Scholar]
- 8.Ritov V, Menshikova E, He J, Ferrell R, Goodpaster B, Kelley D. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 9.Mogensen M, Sahlin K, Fernström M, Glintborg D, Vind BF, Beck-Nielsen H, Højlund K. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes. 2007;56:1592–1599. doi: 10.2337/db06-0981. [DOI] [PubMed] [Google Scholar]
- 10.D'Alessio DA, Kavle EC, Mozzoli MA, Smalley KJ, Polansky M, Kendrick ZV, Owen LR, Bushman MC, Boden G, Owen OE. Thermic effect of food in lean and obese men. J Clin Invest. 1988;81:1781–1789. doi: 10.1172/JCI113520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ukropcova B, McNeil M, Sereda O, de Jonge L, Xie H, Bray G, Smith S. Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. J Clin Invest. 2005;115:1934–1941. doi: 10.1172/JCI24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ. Look AHEAD (action for health in diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 13.The LOOK AHEAD Research Group The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 15.Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Annu Rev Nutr. 1987;7:187–208. doi: 10.1146/annurev.nu.07.070187.001155. [DOI] [PubMed] [Google Scholar]
- 16.Koska J, Ortega E, Bogardus C, Krakoff J, Bunt JC. The effect of insulin on net lipid oxidation predicts worsening of insulin resistance and development of type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2007;293:E264–E269. doi: 10.1152/ajpendo.00662.2006. [DOI] [PubMed] [Google Scholar]
- 17.Yki-Järvinen H, Sahlin K, Ren J, Koivisto V. Localization of rate-limiting defect for glucose disposal in skeletal muscle of insulin-resistant type I diabetic patients. Diabetes. 1990;39:157–167. doi: 10.2337/diab.39.2.157. [DOI] [PubMed] [Google Scholar]
- 18.Thorburn A, Gumbiner B, Bulacan F, Wallace P, Henry R. Intracellular glucose oxidation and glycogen synthase activity are reduced in non-insulin-dependent (type II) diabetes independent of impaired glucose uptake. J Clin Invest. 1990;85:522–529. doi: 10.1172/JCI114468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiffelers S, Saris W, van Baak M. The effect of an increased free fatty acid concentration on thermogenesis and substrate oxidation in obese and lean men. Int J Obes. 2001;25:33–38. doi: 10.1038/sj.ijo.0801528. [DOI] [PubMed] [Google Scholar]
- 20.Kelley DE, Williams KV, Price JC, McKolanis TM, Goodpaster BH, Thaete FL. Plasma fatty acids, adiposity, and variance of skeletal muscle insulin resistance in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2001;86:5412–5419. doi: 10.1210/jcem.86.11.8027. [DOI] [PubMed] [Google Scholar]