Abstract
Background
Kidney disease alters the pharmacokinetic disposition of many medications, requiring dosage adjustment to maintain therapeutic serum concentrations. The Cockcroft-Gault equation is used for pharmacokinetic studies and drug dosage adjustments, but the MDRD Study equation is more accurate and more often reported by clinical laboratories than the Cockcroft-Gault equation.
Study Design
Diagnostic test study.
Settings and Participants
Pooled dataset in 5,504 participants from 6 research studies and 4 clinical populations with measured GFR.
Index Test
Estimated kidney function using the MDRD Study and Cockcroft-Gault equations incorporating actual (CG) or ideal body weight (CGIBW) and standardized serum creatinine concentrations.
Reference test
Measured GFR (mGFR) assessed by 125I-iothalamate urinary clearance.
Outcome
Concordance of assigned kidney function categories designated by the FDA Guidance for Industry for pharmacokinetic studies, and recommended dosages of 15 medications cleared by the kidneys.
Results
Concordance of kidney function estimates with mGFR for FDA assigned kidney function categories was 78% for the MDRD Study equation compared to 73% for the CG equation (p<0.001) and 66% for the CGIBW equation (p<0.001). Concordance between the MDRD Study equation and the CG and CGIBW equations was 78% and 75%, respectively (p<0.001). Concordance of kidney function estimates with mGFR for recommended drug dosages was 88% for MDRD Study equation compared to 85% for CG equation (p<0.001) and 82% for the CGIBW equations (p<0.001), with lower concordance when dosing recommendations for drugs included narrow GFR ranges. Concordance rates between the CG and CGIBW equations and the MDRD Study equation were 89% and 88%, respectively (p<0.05).
Limitations
Results based on simulation rather than pharmacokinetic studies. Outcome was drug dosage recommendations, rather than observed drug efficacy and safety.
Conclusions
The MDRD Study equation can also be used for pharmacokinetic studies and drug dosage adjustments. As more accurate GFR estimating equations are developed, they should be used for these purposes.
Introduction
Impairment of kidney function alters the pharmacokinetics of many medications prescribed in both the acute and chronic settings. The Guidance for Industry: Pharmacokinetics in Patients with Impaired Renal Function — Study Design, Data Analysis, and Impact on Dosing and Labeling from the US Food and Drug Administration (FDA), published in 1998 and herein referred to as the FDA Guidance for Industry, recommends that pharmaceutical companies use the Cockcroft-Gault (CG) equation to estimate kidney function, which is incorporated in the design of pharmacokinetics studies and the development of drug dosing guidelines.1 The rationale for the use of the CG equation is that it was the most commonly used method for assessment of kidney function in clinical practice at the time.
The Modification of Diet in Renal Disease (MDRD) Study equation is now widely recognized as providing more accurate estimates of glomerular filtration rate (GFR) than the CG equation, and has been re-expressed for use with standardized serum creatinine, enabling consistent performance across clinical laboratories after standardization of serum creatinine assays, anticipated to be implemented in all U.S. clinical laboratories by the end of 2009.2–12 International and national organizations now recommend that clinical laboratories report estimated GFR when serum creatinine is ordered and the latest surveys from College of American Pathologists suggest that 70% of clinical laboratories in the United States are now reporting eGFR using the MDRD Study equation.13–19 Using these readily available GFR estimates would likely facilitate drug dosing decisions. However, many clinicians are reluctant to use them for this purpose because the FDA Guidance for Industry, and consequently dosing adjustments listed in product labels for most medications, recommends using the CG equation.
Many studies have compared drug dosing recommendations based on CG equation to those based on the MDRD Study equation20–24, but none have compared these recommendations to those based on measured GFR in a large, clinically diverse population. The two objectives of this study were: 1) to compare kidney function categories as defined by the FDA Guidance for Industry using kidney function estimates based on the MDRD Study equation and CG equation using actual and ideal body weight to measured GFR and, 2) to compare differences in hypothetical recommended dosing of 15 medications that are cleared by the kidneys among 5,504 patients from 6 research and 4 clinical populations with diverse clinical characteristics.
Methods
Sources of Data
The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) is a research group that was formed to develop and validate improved estimating equations for GFR by pooling data from research studies and clinical populations (hereafter referred to as “studies”), which include individuals with diverse clinical characteristics, with and without kidney disease, across a wide range of GFR. Methods for identification of and inclusion criteria for these studies have been previously described.2 The population described in this study includes people whose measurements were used for equation development.
Measurements
All studies measured GFR using urinary clearance of iothalamate. Serum creatinine assays were calibrated to the creatinine reference standard using Roche enzymatic method (Roche-Hitachi Module-P instrument with Roche Creatinine Plus assay; Roche Diagnostics, Indianapolis, IN) at the Cleveland Clinic Research Laboratory.2.
GFR and creatinine clearance estimation
Kidney function was estimated using the equations listed in Box 1. The MDRD Study equation was expressed for use with creatinine values standardized by isotope dilution mass spectrometry (IDMS). The CG equation cannot be re-expressed for use with IDMS-standardized creatinine values because to our knowledge the original serum creatinine samples are not available for calibration. Measured GFR and the MDRD Study equations are adjusted for body surface area (BSA) and are generally reported in ml/min/1.73 m2.27 We converted these BSA-adjusted values by multiplying by each individual’s body surface area and dividing by 1.73 m^2 so that all were expressed in units of ml/min, the units of GFR that are expressed in the majority of FDA-approved drug dosing labels. Values for estimated GFR were rounded to the nearest whole number.
Box 1. Equations used in this study
The IDMS-traceable 4-variable MDRD Study equation11:
The Cockcroft-Gault equation (CG)25:
The Cockcroft-Gault equation using ideal body weight (CGIBW):
In CGIBW, IBW was calculated as 50 kg + [2.3 kg × (Height in inches − 60)] for men and 45.5 kg + [2.3 kg × (Height in inches − 60)] for women. If actual body weight (ACT) was less then IBW, then ACT was used or if ACT exceeded IBW by more than 30%, then adjusted body weight (ABW) was used according to the following formula26: ABW = IBW + [0.4 × (ACT − IBW)].
Abbreviations: ABW, adjusted body weight; ACT, actual body weight; CG, Cockcroft-Gault; CGIBW, Cockcroft-Gault equation using ideal body weight; IDMS, isotope-dilution mass spectrometry; MDRD, Modification of Diet in Renal Disease; SCr, serum creatinine.
Variables
To assess the consistency of the results among clinically relevant subgroups, comparisons were also performed according to subgroups. Clinical characteristics were categorized as follows: age (less than 40, 40–65, or greater than 65 years); sex; race (African American or other); diabetes (yes or no), prior organ transplant (yes or no); weight (less than 60, 60 to 90, or greater than 90 kg). Classification of race, diabetes status, and transplant status were based on the definitions used in each study.
Statistical analyses
Data were expressed using standard descriptive statistics (means and standard deviations or medians and interquartile ranges, as appropriate. Analyses were computed using Excel (Microsoft Office Excel 2003; Microsoft Corp, Redmond, WA) and SAS software (version, 9.1, Cary, NC).
Assignment to FDA Guidance to Industry Kidney Function Category
The percentages of participants assigned to the kidney function categories recommended by the FDA Guidance to Industry (>80, 50–80, 30–49, or less than 30 ml/min), were calculated based on measured GFR and kidney function estimates from the three equations1. Concordance and discordance for assignment of categories between measured GFR and each of the estimates were calculated as was concordance and discordance between the MDRD Study equation-derived estimates with the other two. Significance of the differences in concordance for the kidney function categories was tested using the McNemar’s and Jonckheere-Terpstra tests for binary and categorical data with more than two categories, respectively.
Drug simulation study
A simulation study was used to compare drug dosage recommendations for fifteen medications based on measured GFR and estimated kidney function. We did not include medications that are dosed primarily by serum levels or evidence of toxicity. The fifteen medications were selected for inclusion in the simulation study because they are commonly used in clinical practice, are cleared by the kidneys, and either have narrow therapeutic windows or are commonly associated with dosing errors or adverse drug events. The recommended doses of each of the fifteen medications were determined from the dosing recommendations in the package insert for measured GFR and the three estimates (table 1). The percentage that fell into the recommended drug dosing categories for each drug were calculated. Significance of the differences in recommended drug dosing was tested using the sign test.
Table 1.
Individual drug dosing recommendations by kidney function
| Drug | Levels of Dosing | CCr Range | Recommended Dose (Route) |
|---|---|---|---|
| Enoxaparin 28 | 2 | ≥30 | 40 mg every day (subcutaneous) |
| <30 | 30 mg every day | ||
| Eptifibatide 28 | 2 | ≥50 | 2.0 mcg/kg/min (intravenous) |
| <50 | 1.0 mcg/kg/min | ||
| Ranitidine 28 | 2 | ≥50 | 150 mg twice a day (by mouth) |
| <50 | 150 mg every day | ||
| Acyclovir 28 | 3 | >25 | 800 mg every 4 hrs (by mouth) |
| 10–25 | 800 mg every 8 hrs | ||
| <10 | 800 mg every 12 hrs | ||
| Atenolol 29 | 3 | >35 | 50–100 mg every day (by mouth) |
| 15 to 35 | 50 mg every day | ||
| <15 | 25 mg every day | ||
| Cefazolin 30 | 3 | >35 | 1 g every 8 hrs (intravenous) |
| 11 to 35 | 1 gm every 12 hrs | ||
| ≤10 | 500 mg every day | ||
| Digoxin 31 | 3 | >50 | every 24 hrs (by mouth) |
| 10 to 50 | 25% to 75% of dose or every 36 hrs | ||
| <10 | 10% to 25% of dose or every 48 hrs | ||
| Levofloxacin 28 | 3 | ≥50 | 500 mg every day (by mouth) |
| 20 to 49 | 250 mg every day | ||
| <20 | 250 mg every 2 days | ||
| Tenofovir 28 | 3 | ≥50 | 300 mg every day (by mouth) |
| 30 to 49 | 300 mg every 48 hrs | ||
| <30 | 300 mg twice weekly | ||
| Tramadol 32 | 3 | >30 | 50–100 mg every 6 hrs (by mouth) |
| 10 to 30 | 50–100 mg every 12 hrs | ||
| <10 | 50 mg every day | ||
| Allopurinol 31 | 4 | >90 | 300 mg every day (by mouth) |
| 50 to 90 | 75% of dose | ||
| 10 to 49 | 50% of dose | ||
| <10 | 25% of dose | ||
| Gabapentin 33 | 4 | >60 | 300–1200 mg 3 times a day (by mouth) |
| 30 to 60 | 200–700 mg twice a day | ||
| 15 to 29 | 200–700 mg every day | ||
| <15 | 100–300 mg every day | ||
| Sotalol 34 | 4 | ≥60 | 80–160 mg every 12 hrs (by mouth) |
| 30–59 | 80–160 mg every day | ||
| 10 to 29 | 80–160 mg every 36–48 hrs | ||
| <10 | ‘Not recommended' | ||
| Disopyramide 35 | 5 | >90 | 150 mg every 6 hrs (by mouth) |
| 41 to 90 | 100 mg every 6 hrs | ||
| 31 to 40 | 100 mg every 8 hrs | ||
| 15 to 30 | 100 mg every 12 hrs | ||
| <15 | 100 mg every day | ||
| Lamivudine 28 | 5 | >50 | 150 mg twice a day (by mouth) |
| 30 to 50 | 150 mg every day | ||
| 15 to 29 | 100 mg every day | ||
| 5 to 14 | 50 mg every day | ||
| <5 | 25 mg every day | ||
Abbreviation: CCr, creatinine clearance
The institutional review boards of all participating institutions approved inclusion of the data into the pooled dataset for these analyses.
Results
Study Population
The clinical characteristics of the 5504 participants included in the study population are shown in Table 2. The mean (SD) age of the cohort is 47 (15) years. Approximately a third of the cohort was African American, a similar number had diabetes and 5% were kidney transplant recipients. The mean (SD) measured GFR was 75 (44) ml/min; eGFR from the MDRD Study equation and estimated creatinine clearance from the CG and CGIBW equations were 69 (38), 75 (42) and 62 (36) ml/min, respectively. All pair-wise comparisons between the values for estimated and measured GFR were significantly different from each other (p < 0.001). Table 2 shows the values for measured GFR as well as estimated kidney function using the three equations across subgroups.
Table 2.
Clinical characteristics
| Name | Total | Measured and Estimated Kidney function (ml/min) mean (SD) | |||
|---|---|---|---|---|---|
| No. (%) | mGFR | MDRD Study | CG | CGIBW | |
| All | 5504 (100) | 75 (44) | 69 (38) | 75 (42) | 62 (36) |
| Age, mean (SD) years | 47 (15) | - | - | - | - |
| < 40 | 2058 (37) | 100 (46) | 91 (41) | 102 (43) | 87 (37) |
| 40–65 | 2751 (50) | 65 (36) | 60 (31) | 64 (33) | 51 (25) |
| > 65 | 695 (13) | 45 (26) | 45 (23) | 42 (20) | 33 (15) |
| Sex | |||||
| Women | 2391 (43) | 72 (42) | 65 (36) | 74 (41) | 60 (34) |
| Men | 3113 (57) | 77 (45) | 73 (39) | 76 (42) | 65 (37) |
| Race | |||||
| African American | 1740 (32) | 65 (32) | 62 (30) | 62 (30) | 49 (30) |
| White or other | 3764 (68) | 80 (47) | 73 (41) | 82 (45) | 69 (38) |
| Weight, mean (SD), kg | 82 (20) | - | - | - | - |
| < 60 | 590 (11) | 64 (40) | 58 (35) | 58 (34) | 54 (33) |
| 60–90 | 3296 (60) | 76 (44) | 70 (39) | 74 (41) | 63 (36) |
| > 90 | 1618 (29) | 78 (43) | 72 (37) | 84 (43) | 64 (34) |
| Diabetes | |||||
| Yes | 1581 (29) | 100 (48) | 91 (44) | 100 (46) | 86 (41) |
| No | 3923 (71) | 65 (37) | 61 (32) | 65 (35) | 53 (28) |
| Transplant | |||||
| Yes | 251 (5) | 51 (27) | 52 (27) | 59 (31) | 48 (24) |
| No | 5253 (95) | 76 (44) | 70 (38) | 76 (42) | 63 (36) |
| Body surface area, mean (SD), 1.73 m2 | 1.93 (0.24) | - | - | - | - |
| Serum creatinine, mean (SD), mg/dL | 1.65 (1.15) | - | - | - | - |
| Body mass index, kg/m2 | 28 (6) | - | - | - | - |
Abbreviations: mGFR, measured glomerular filtration rate; MDRD Study, Modification of Diet in Renal Disease Study equation; CG, Cockcroft-Gault equation using actual body weight; CGIBW, Cockcroft-Gault equation using ideal body weight.
Conversion factors for units: GFR from mL/min to mL/s, x0.0167; serum creatinine in mg/dL to μmol/L, x88.4.
Assignment to FDA Guidance to Industry Kidney Function Category
Comparison to measured GFR
Table 3 shows the concordance and discordance between each of the estimating equations and measured GFR with respect to the assigned kidney function categories defined in the FDA Guidance to Industry. The MDRD Study equation demonstrated the highest (78%), and the CGIBW (66%) the lowest, concordance with measured GFR (p<0.001). The direction of discordance was different for the three equations. The CG equation assigned a higher kidney function category compared to measured GFR for 16% of people compared to 5% for CGIBW equation, and 8% for the MDRD Study equation. Conversely, CGIBW assigned a lower kidney function category in 29% of people compared to 12% for CG, and 14% for MDRD Study equation.
Table 3.
Concordance between kidney function categories assigned using measured GFR vs. estimated kidney function
| Equation | Concordant (%)* | Discordant (%) | |
|---|---|---|---|
| Lower than mGFR | Higher than mGFR | ||
| MDRD Study | 78 | 14 | 8 |
| CG | 73 | 12 | 16 |
| CGIBW | 66 | 29 | 5 |
p-value <0.001 for the difference in concordance among all equations
Abbreviations: mGFF, measured glomerular filtration rate; MDRD, Modification of Diet in Renal Disease Study equation; CG, Cockcroft-Gault equation using actual body weight; CGIBW, Cockcroft-Gault equation using ideal body weight.
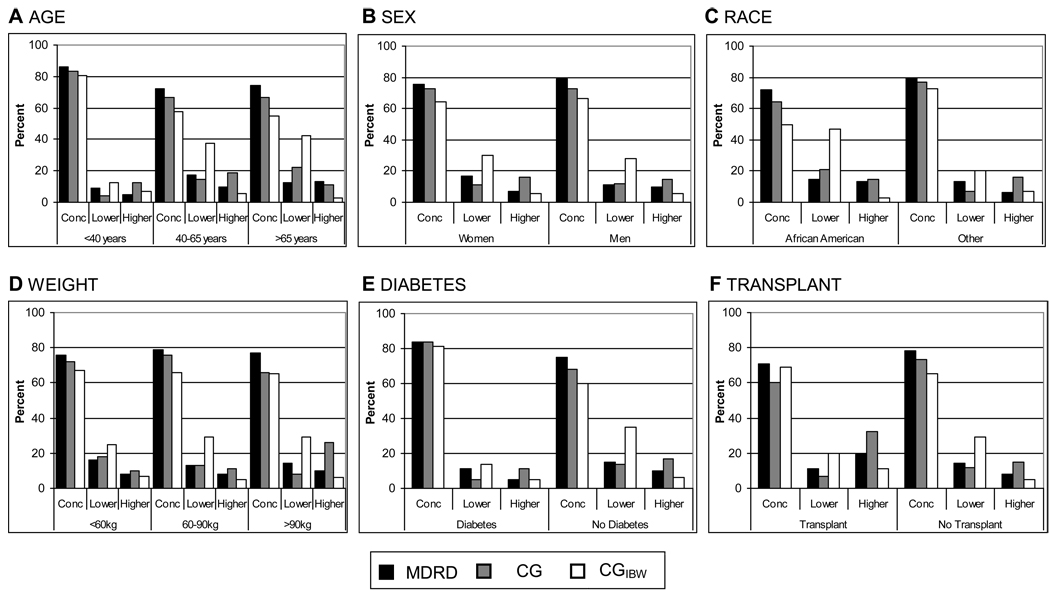
The MDRD Study equation has the higher rate of concordance with measured GFR for all subgroups tested (Figure 1). Other than for transplant recipients, the CG equation had a higher rate of concordance with measured GFR than the CGIBW equation. Large differences in concordance rates among equations were observed in many of the subgroups.
Figure 1. Concordance of Modification of Diet in Renal Disease (MDRD) Study, Cockcroft-Gault (CG), and the Cockcroft-Gault adjusted for ideal body weight (CGIBW) equations with measured GFR for assignment of kidney function categories by patient subgroup.
Each bar indicates percentage concordance to measured glomerular filtration rate (GFR) for each of the 43 different equations. A) Age (< 40, 40–65, > 65 years). (B) Sex. (C) Race (African American or other; White; Asian; Native American, Hispanic, or Pacific Islander). (D) Weight (< 60, 60–90, > 90 kg); (E) Presence or absence of diabetes. (F) Presence or absence of kidney transplant. Rate of concordance to measured GFR for CG and CGIBW was significantly different (p-value < 0.001) from the concordance to measured GFR for the MDRD Study equation for all subgroups except weight 60–90 kg (CG), weight >90 kg (CGIBW), diabetes (CG), and transplant recipients (CGIBW).
Comparison to the MDRD Study equation
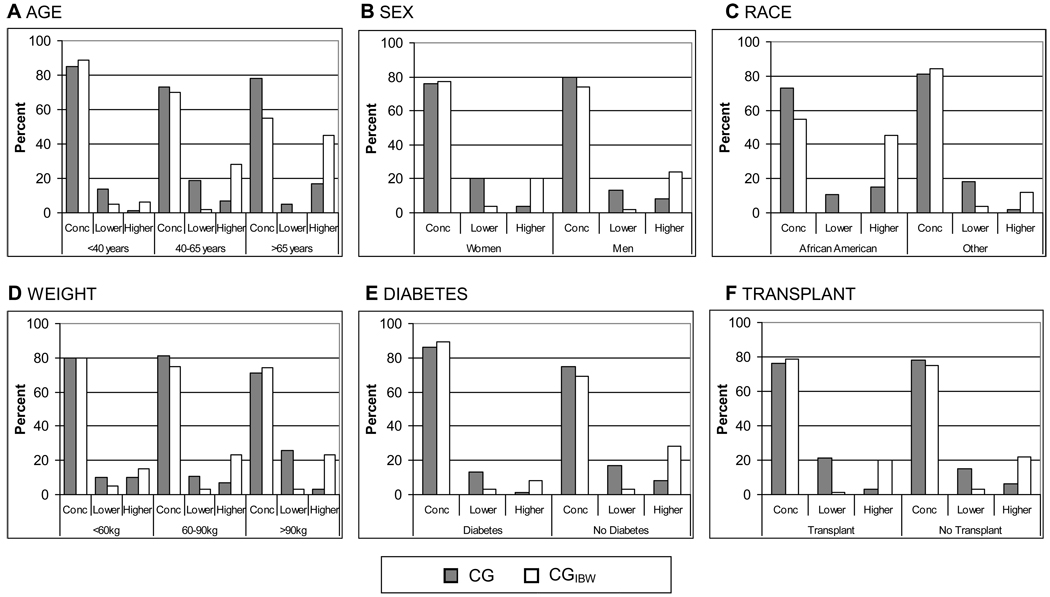
The CG equation was concordant with the MDRD Study equation in 78% of cases, while the CGIBW had a slightly lower rate of concordance at 75% (p<0.001). When discordance was observed, the CG equation was more likely to predict assignment to a higher kidney function categories than the MDRD Study equation (16% higher kidney function category vs. 6% prediction of lower kidney function categories), and the CGIBW equation was more likely to predict assignment to a lower kidney function category (22% lower kidney function category vs. 3% prediction of higher kidney function category). Among subgroups, the CG and CGIBW equations both demonstrated variable rates of concordance with the MDRD Study equation (71 to 86% and 55 to 89%, respectively) (Figure 2).
Figure 2. Concordance of Cockcroft-Gault (CG) and Cockcroft-Gault adjusted for ideal body weight (CGIBW) with the Modification of Diet in Renal Disease (MDRD) Study equation for assignment of kidney function categories by patient subgroups.
Each bar indicates percentage concordance to MDRD Study equation for the two equations. (A) Age (<40, 40–65, > 65 years). (B) Sex. (C) Race (African American or other; White; Asian; Native American, Hispanic, or Pacific Islander). (D) Weight (< 60, 60–90, > 90 kg); (E) Presence or absence of diabetes. (F) Presence or absence of kidney transplant.
*p-value < 0.0001 for comparisons of each equation to MDRD study equation for each subgroup
Recommended Drug Dosages
Comparison to measured GFR
Compared to measured GFR, the average concordance rate for the specific drug dosing recommendations was 88% for the MDRD Study equation compared to 85% for the CG equation (p<0.001), and 82% for the CGIBW equation (p<0.001) (Table 4). Concordance for all equations was lower for drugs that have a greater number of kidney function categories for dose adjustment. As observed with the FDA-assigned kidney function categories, use of the CG equation was most likely to translate into higher recommended drug dosages, while the CGIBW was most likely to translate into lower recommended drug dosages.
Table 4.
Concordance between drug dosing recommendations using measured vs. estimated kidney function
| Drug | MDRD Study | Cockcroft-Gault | Cockcroft-GaultIBW | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Con | Discordant | Con | Discordant | Con | Discordant | ||||
| Lower than mGFR | Higher than mGFR | Lower than mGFR | Higher than mGFR | Lower than mGFR | Higher than mGFR | ||||
| 2 Dosing Levels | |||||||||
| Enoxaparin | 95 | 2 | 3 | 93 | 1 | 5 | 93 | 4 | 3 |
| Eptifibatide | 93 | 4 | 3 | 90 | 4 | 7 | 87 | 11 | 2 |
| Ranitidine | 93 | 4 | 3 | 90 | 4 | 7 | 87 | 11 | 2 |
| Average | 94 | 3 | 3 | 91 | 3 | 6 | 89 | 9 | 2 |
| 3 Dosing Levels | |||||||||
| Acylovir | 95 | 2 | 3 | 93 | 1 | 6 | 94 | 3 | 3 |
| Atenolol | 91 | 4 | 6 | 88 | 2 | 10 | 85 | 12 | 3 |
| Cefazolin | 92 | 3 | 5 | 90 | 2 | 8 | 90 | 6 | 4 |
| Digoxin | 91 | 5 | 4 | 88 | 4 | 8 | 85 | 12 | 3 |
| Levofloxacin | 88 | 5 | 7 | 84 | 4 | 12 | 82 | 12 | 6 |
| Tenofavir | 86 | 5 | 9 | 82 | 4 | 14 | 79 | 14 | 8 |
| Tramadol | 93 | 2 | 4 | 92 | 2 | 7 | 92 | 4 | 4 |
| Average | 91 | 4 | 5 | 88 | 3 | 9 | 87 | 9 | 4 |
| 4 Dosing Levels | |||||||||
| Allopurinol | 79 | 14 | 6 | 77 | 11 | 12 | 69 | 27 | 4 |
| Gabapentin | 84 | 9 | 8 | 79 | 7 | 14 | 74 | 19 | 6 |
| Sotolol | 85 | 8 | 7 | 81 | 7 | 12 | 77 | 18 | 5 |
| Average | 83 | 10 | 7 | 79 | 8 | 13 | 73 | 21 | 5 |
| 5 Dosing Levels | |||||||||
| Disopyramide | 75 | 15 | 10 | 72 | 11 | 17 | 65 | 27 | 8 |
| Lamivudine | 85 | 7 | 8 | 80 | 5 | 15 | 77 | 16 | 7 |
| Average | 80 | 11 | 9 | 76 | 8 | 16 | 71 | 22 | 8 |
| Overall Average | 88 | 6 | 6 | 85 | 5 | 10 | 82 | 13 | 4 |
Units are ml/min; conversion factor from mL/min to mL/s, x0.0167.
Abbreviations: MDRD, Modification of Diet in Renal Disease Study equation; CG, Cockcroft-Gault equation using actual body weight; CGIBW, Cockcroft-Gault equation using ideal body weight; Con, concordant; eGFR, estimated glomerular filtration rate; mGFR, measured glomerular filtration rate.
Comparison to the MDRD Study equation
The concordance rate between the MDRD Study and CG equations was 89%, with the MDRD Study equation recommending lower drug dosages in 9% of the study population. The concordance rate between the MDRD Study and CGIBW equations was 88%, the MDRD Study equation recommending higher drug dosages in 10% of the study population. Concordance was lower for drugs with a greater number of kidney function categories (ranging from 90% for drugs with two dosing levels to 81% with five dosing levels).
Discussion
Accurate estimates of kidney function are essential for optimal dosing of drugs cleared by the kidney. Overestimates of kidney function may lead to administration of inappropriately large doses and possible toxicity, and conversely underestimates may lead to sub-therapeutic dosing, treatment failures, and prolonged illness. In this study, we demonstrated that the MDRD Study equation had the highest rate of concordance with measured GFR for both assignment of kidney function categories recommended by the FDA Guidance for Industry and adjustment of specific drug dosing. For specific drug dosing concordance rates among equations was high, with a lower concordance for drugs with greater number of dosing levels.
The CG equation, published in 1976, estimates creatinine clearance and therefore overestimates measured GFR due to creatinine secretion. Even after correcting for this overestimation, substantial imprecision remains.36 Modifications of the CG equation, such as the use of ideal body weight, were developed in an attempt to overcome the imprecision with the use of measured body weight. However, as shown here and previously, this modification results in substantially worse performance compared to measured GFR.37–39 Use of standardized serum creatinine leads to another source of error for the CG equation. In previous analyses of these same data, we demonstrated that the CG equation kidney function estimates were 11.4% higher than measured GFR with standardized creatinine compared to 2% higher with non-standardized values.2 Serum samples are not available to enable re-expression of the CG equation for standardized serum creatinine. Altogether, these considerations do not support continued sole reliance on the CG equation for estimating kidney function for drug dosing adjustments.
Our finding of 11 to 29% discordance between the MDRD Study and CG equations overall and in subgroups is consistent with some previous studies which have showed discordance rates between approximately 20 to 40% between the equations.20–24 Possible explanations for the variation in reported discordance rates may be related to true differences in accuracy of equations among study populations included in the different reports or to variations in the methods utilized to estimate kidney function (e.g. actual body weight vs. ideal body weight for the CG equation), units of kidney function (i.e. adjustment vs. no adjustment for body surface area), or presence or absence of calibration of the creatinine assay. Our results are also consistent with one study which compared carboplatin doses determined by nuclear imaging of the kidneys to doses calculated using estimates based on the MDRD Study and CG equations, and demonstrated that the MDRD Study equation resulted in more accurate dosing.40
Strengths of our approach include a large and diverse population; inclusion of measured GFR determined by urinary clearance of 125I-iothalamate as the gold standard; calibrated serum creatinine in all studies; standard units for all equations; and inclusion of medications representative of those commonly used in both inpatient and outpatient settings, and which have narrow therapeutic windows or are commonly associated with dosing errors or adverse drug events.
There are several limitations. First, the results presented here may not be fully applicable to other populations whose characteristics are different than the current study population. For example, in populations with a lower prevalence of CKD we would expect higher concordance rates since drug dosing adjustment is only relevant to patients with kidney disease. As such, our findings of differences among patients of different characteristics may reflect differences in level of GFR of the study participants, rather than patient characteristics, per se. However, this dataset is more diverse than prior studies, and the performance of the equations here may be more representative of their performance when applied in clinical practice than prior studies. Second, we have not considered the contribution of tubular reabsorption or secretion to renal clearance of drugs. However, pharmacokinetic studies do not measure tubular reabsorption or secretion directly, and tubular handling of creatinine is not likely to reflect tubular handling of many drugs, which are actively secreted by a number of transporters primarily along the renal proximal tubule.41 Third, our results are based on simulation rather than pharmacokinetic studies and we included only a sample of commonly used drugs. Finally, we used drug dosage recommendations as an outcome, rather than observed drug efficacy and safety.
All three equations are based on serum creatinine and, therefore, all suffer the same irremediable limitations of creatinine as a filtration marker. The serum level of creatinine is determined by factors other than the GFR, and in particular tubular secretion, muscle mass and diet, leading to bias in some populations and imprecision for all.42 This is particularly relevant for populations with reduced muscle mass, including the frail elderly, critically ill, or cancer patients,43 Finally, kidney function must be at a steady state to use any endogenous filtration markers, so estimates must be used cautiously in hospitalized patients.
Adjustment of drug dosages is the most common use of kidney function estimates, and these results have implications for prescriptions of both new and existing drugs. The MDRD Study equation is commonly used as a clinical tool for detection and stratification of kidney disease, is widely available to most clinicians, and currently provides the best approximation of measured GFR.16 Using the same estimate for drug dosing, as well as detection and evaluation of kidney disease, would likely facilitate clinical decision making and improve care. The stated intent of the FDA Guidance for Industry is to use measures of kidney function that are “used widely in patient care settings”, as such measures are “more practical than other alternatives” (Guidance page 6).1 For new drug development, we propose that pharmaceutical manufacturers use the MDRD Study equation for pharmacokinetic studies and in dosing recommendations listed in the product label. For drugs that are currently in use, it is neither practical nor feasible for pharmacokinetic studies to be repeated using the MDRD Study equation. We propose that either the MDRD Study equation or CG equation using actual body weight can be used for determination of drug dosage. If more accurate equations replace the MDRD Study equation for GFR estimation by clinical laboratories, then these equations should be used instead, and the FDA Guidance for Industry should incorporate this flexibility in its recommendations.
Currently, no equation provides accurate estimates for all patients. Clinicians must use available estimates together with their best judgment to determine drug dosing for individual patients, particularly for medications with a narrow therapeutic index or high toxicity. For individual patients in whom kidney function estimates from different equations vary substantially, the dose should be determined by weighing the risk of toxicity with a higher dose versus the risk of sub-therapeutic dose and treatment failure with a lower dose. If both risks are high, then it may be prudent to measure the GFR or creatinine clearance prior to administration of the medication. Indeed for such medications, it may be prudent to measure GFR or creatinine clearance in all patients at the extremes of muscle mass in whom all creatinine-based estimates are suspected to be inaccurate. For some drugs, monitoring of serum concentrations can minimize errors due to inaccurate dosage adjustment based on kidney function estimates (e.g., aminoglycosides, phenytoin, lithium). Implementation of computerized clinical decision support systems including automated drug dosing is becoming more common, making such individual assessments of kidney function feasible. Such systems will also easily incorporate more accurate equations as they become used in clinical practice, and would facilitate conversion of the MDRD Study equation derived estimates from units of ml/min per 1.73 m2 to units if ml/min as is recommended for drug dosing.
In conclusion, the MDRD Study equation had the higher concordance with measured GFR for recommended drug dosage than the CG equation. Concordance among equations was higher in the context of specific medications. Either the MDRD Study or CG equation could be used for drug dosage adjustments in most circumstances. Patients for whom estimated GFR from creatinine is likely to be inaccurate require careful consideration. Greater education is needed for physicians, pharmacists, industry and the public about CKD, and the interpretation of GFR estimates for use in drug dosing.
Acknowledgments
A list of the Investigators of CKD-EPI Aims 1 and 2, organized by institution, follows. Tufts Medical Center, Boston, MA: Andrew S. Levey, MD; Lesley A. Stevens, MD, MS; Christopher H. Schmid, PhD; and Yaping (Lucy) Zhang, MS. Cleveland Clinic Foundation, Cleveland, OH: Frederick Van Lente, PhD and Liang Li, PhD. University of Utah, Salt Lake City, UT: Tom Greene, PhD. John Hopkins University, Baltimore, MD: Josef Coresh, MD, PhD, MHS; Jane Manzi, PhD; Brad Astor PhD, MPH; and Elizabeth Selvin, PhD, MPH. University of Pennsylvania, Philadelphia, PA: Harold I. Feldman, MD, MSCE; J. Richard Landis, PhD and Marshall Joffe, MD, MPH, PhD. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK): John W. Kusek, PhD and Paul W. Eggers, PhD.
A list of the Collaborators of CKD-EPI Aim 1, organized by study, follows. African American Study of Kidney Disease and Hypertension (AASK): Gabriel Contreras, MD, MPH and Julia B. Lewis, MD. Captopril in Diabetic Nephropathy Study (CSG): Roger A. Rodby, MD and Richard D. Rohde, BS. Chronic Renal Insufficiency Cohort (CRIC): Harold I. Feldman, MD, MSCE; Lawrence J. Appel, MD, MPH; Jing Chen, MD, MS; Alan S. Go, MD; Lee Hamm, MD; Chi-yuan Hsu, MD; James P. Lash, MD; Akinlolu O. Ojo, MD; Mahboob Rahman, MD; Raymond R. Townsend, MD; Matthew R. Weir, MD; and Jackson T. Wright, MD. Cleveland Clinic Foundation (CCF): Phillip Hall, MD and Emilio Poggio, MD. Diabetes Control and Complications Trial (DCCT): Saul Genuth, MD and Michael W. Steffes, MD, PhD. Diabetic Renal Disease Study Group (DRDS): Robert G. Nelson, MD, PhD. Mayo Clinic: Andrew D. Rule, MD, MS; Timothy Larson, MD; and Fernando Cosio, MD. Modification of Diet in Renal Disease (MDRD) Study: Gerald Beck, PhD.
Support: CKD-EPI is funded by grants from the NIDDK as part of a cooperative agreement in which the NIDDK has substantial involvement in the design of the study and the collection, analysis, and interpretation of the data. The NIDDK was not required to approve publication of the finished manuscript. Dr Stevens receives research support from the NIDDK, the Paul Teschan Fund, Gilead, and the National Kidney Foundation (NKF). Dr Feldman receives research support from the NIDDK and Amgen. Dr Lewis receives research support from Hoffmann-LaRoche, Theravance Inc, Sanofi, and Bristol-Myers Squibb. Dr Townsend receives research support from the NIDDK. Dr Schmid receives research support from the NIDDK, Agency for Healthcare Research and Quality (AHRQ), the National Center for Research Resources (NCRR), the National Heart, Lung and Blood Institute (NHLBI), the Centers for Disease Control and Prevention (CDC), and Pfizer. Dr Levey receives research support from the NIDDK, Amgen, and the NKF.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: None.
References
- 1.Food and Drug Administration. Guidance for Industry: Pharmacokinetics in Patients with Impaired Renal Function — Study Design, Data Analysis, and Impact on Dosing and Labeling. Rockville: U.S. Department of Health and Human Services; 1998. May, [Google Scholar]
- 2.Stevens LA, Manzi J, Levey AS, et al. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis. 2007;50(1):21–35. doi: 10.1053/j.ajkd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Stevens LA. Kidney function estimating equations: Where do we stand? Curr Opin Nephrol Hypertens. 2006;15(3):276–284. doi: 10.1097/01.mnh.0000222695.84464.61. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JB, Agodoa L, Cheek D, et al. Comparison of cross-sectional renal function measurements in African-Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis. 2001 Oct;38(4):744–753. doi: 10.1053/ajkd.2001.27691. [DOI] [PubMed] [Google Scholar]
- 5.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005 Mar;16(3):763–773. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- 6.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: Accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004 Dec 21;141(12):929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 7.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol. 2005 Feb;16(2):459–466. doi: 10.1681/ASN.2004060447. [DOI] [PubMed] [Google Scholar]
- 8.Cirillo M, Anastasio P, De Santo N. Relationship of gender, age, and body mass index to errors in predicted kidney function. Nephrol Dial Transplant. 2005;20:1791–1798. doi: 10.1093/ndt/gfh962. [DOI] [PubMed] [Google Scholar]
- 9.Verhave JC, Fesler P, Ribstein J, du Cailar G, Mimran A. Estimation of renal function in subjects with normal serum creatinine levels: Influence of age and body mass index. Am J Kidney Dis. 2005;46(2):233–241. doi: 10.1053/j.ajkd.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Coresh J, Greene T, et al. Expressing the MDRD study equation for estimating GFR with standardized serum creatinine values. Clin Chem. 2007;53(4):766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006 Aug 15;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 12.National Kidney Disease Education Program. Laboratory Professionals. [Accessed December 19, 2008]; http://nkdep.nih.gov/labprofessionals/index.htm.
- 13.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006 Jan;52(1):5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 14.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2):S1–S266. [PubMed] [Google Scholar]
- 15.Australian and New Zealand Society of Nephrology (ANZSN) Website. [Accessed December 19, 2008]; www.nephrology.edu.au.
- 16.Miller WG. Reporting estimated GFR: a laboratory perspective. Am J Kidney Dis. 2008 Oct;52(4):645–648. doi: 10.1053/j.ajkd.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 17.La Caisse nationale d'assurance maladie des professions indépendantes. Avenant à la convention nationale des directeurs de laboratoire privé d'analyses médicales. [Accessed December 22, 2008]; http://www.admi.net/jo/20030227/SANS0320604X.html.
- 18.Mathew TH. Chronic kidney disease and automatic reporting of estimated glomerular filtration rate: a position statement. Med J Aust. 2005 Aug 1;183(3):138–141. doi: 10.5694/j.1326-5377.2005.tb06958.x. [DOI] [PubMed] [Google Scholar]
- 19.Siegel NJ. Renal Express. [Accessed October, 2003]; http://www.asn-online.org/newsletter/renal_express/2003/03-10_Rxpress.aspx.
- 20.Rosborough TK, Shepherd MF, Couch PL. Selecting an equation to estimate glomerular filtration rate for use in renal dosage adjustment of drugs in electronic patient record systems. Pharmacotherapy. 2005 Jun;25(6):823–830. doi: 10.1592/phco.2005.25.6.823. [DOI] [PubMed] [Google Scholar]
- 21.Wargo KA, Eiland EH, 3rd, Hamm W, English TM, Phillippe HM. Comparison of the modification of diet in renal disease and Cockcroft-Gault equations for antimicrobial dosage adjustments. Ann Pharmacother. 2006 Jul–Aug;40(7–8):1248–1253. doi: 10.1345/aph.1G635. [DOI] [PubMed] [Google Scholar]
- 22.Melloni C, Peterson ED, Chen AY, et al. Cockcroft-Gault versus modification of diet in renal disease: importance of glomerular filtration rate formula for classification of chronic kidney disease in patients with non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol. 2008 Mar 11;51(10):991–996. doi: 10.1016/j.jacc.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 23.Gill J, Malyuk R, Djurdjev O, Levin A. Use of GFR equations to adjust drug doses in an elderly multi-ethnic group--a cautionary tale. Nephrol Dial Transplant. 2007 Oct;22(10):2894–2899. doi: 10.1093/ndt/gfm289. [DOI] [PubMed] [Google Scholar]
- 24.Golik M, Lawrence K. Comparison of dosing recommendations for antimicrobial drugs based on two methods for assessing kidney function: cockcroft-gault and modification of diet in renal disease. Pharmacotherapy. 2008;28:1125–1132. doi: 10.1592/phco.28.9.1125. [DOI] [PubMed] [Google Scholar]
- 25.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 26.Spinler SA, Awarskas JJ, Boyce EG, et al. Predictive Performance of Ten Equations for Estimating Creatinine Clearance in Cardiac Patients. Ann Pharmacotherapy. 1998;32:1275–1283. doi: 10.1345/aph.18122. [DOI] [PubMed] [Google Scholar]
- 27.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987 Oct 22;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 28.Physicians’ Desk Reference. 63. Montvale, NJ: Physicians’ Desk Reference Inc.; 2008. [Google Scholar]
- 29.Product Information: Tenormin®, atenolol. Wilmington, DE: AstraZeneca Pharmaceuticals; 2005. [Google Scholar]
- 30.Product Information: Ancef®, cefazolin. Philadelphia, PA: SmithKline Beecham; 2004. [Google Scholar]
- 31.Aronoff GR, Berns JS, Brier ME, et al. Dosing Guidelines for Adults. 4. Philadelphia, PA: American College of Physicians; 1999. Drug Prescribing in Renal Failure. [DOI] [PubMed] [Google Scholar]
- 32.Product Information: Ultram®, tramadol hydrochloride. Raritan, NJ: Ortho-McNeil Pharmaceutical, Inc.; 2007. [Google Scholar]
- 33.Product Information: Neurontin®, gabapentin. New York, NY: Pfizer Inc.; 2007. [Google Scholar]
- 34.Product Information: Betapace®, sotalol HCl. Wayne, NJ: Berlex Laboratories; 2001. [Google Scholar]
- 35.Product Information: Norpace®, disopyramide. Chicago, IL: Searle Pharmaceuticals; 1995. [Google Scholar]
- 36.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 37.Gonwa TA, Jennings L, Mai ML, Stark PC, Levey AS, Klintmalm GB. Estimation of glomerular filtration rates before and after orthotopic liver transplantation: evaluation of current equations. Liver Transpl. 2004 Feb;10(2):301–309. doi: 10.1002/lt.20017. [DOI] [PubMed] [Google Scholar]
- 38.O'Meara E, Chong KS, Gardner RS, Jardine AG, Neilly JB, McDonagh TA. The Modification of Diet in Renal Disease (MDRD) equations provide valid estimations of glomerular filtration rates in patients with advanced heart failure. Eur J Heart Fail. 2006 Jan;8(1):63–67. doi: 10.1016/j.ejheart.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 39.Rigalleau V, Lasseur C, Perlemoine C, et al. Cockcroft-Gault formula is biased by body weight in diabetic patients with renal impairment. Metabolism. 2006 Jan;55(1):108–112. doi: 10.1016/j.metabol.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 40.de Lemos ML, Hsieh T, Hamata L, et al. Evaluation of predictive formulae for glomerular filtration rate for carboplatin dosing in gynecological malignancies. Gynecol Oncol. 2006 Dec;103(3):1063–1069. doi: 10.1016/j.ygyno.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 41.Launay-Vacher V, Izzedine H, Karie SHJ, Baumelou A, Deray G. Renal tubular drug transporters. Nephron Physiol. 2006;103(3):97–106. doi: 10.1159/000092212. [DOI] [PubMed] [Google Scholar]
- 42.Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007 Oct;18(10):2749–2757. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 43.Poggio ED, Nef PC, Wang X, et al. Performance of the Cockcroft-Gault and modification of diet in renal disease equations in estimating GFR in ill hospitalized patients. Am J Kidney Dis. 2005 Aug;46(2):242–252. doi: 10.1053/j.ajkd.2005.04.023. [DOI] [PubMed] [Google Scholar]