Abstract
Deficiency in docosahexaenoic acid (DHA) is associated with impaired visual and neurological postnatal development, cognitive decline, macular degeneration, and other neurodegenerative diseases. DHA is an omega-3 polyunsaturated fatty acyl chain concentrated in phospholipids of brain and retina, with photoreceptor cells displaying the highest content of DHA of all cell membranes. The identification and characterization of neuroprotectin D1 (NPD1, 10R, 17S-dihydroxy-docosa-4Z, 7Z, 11E, 13E, 15Z, 19Z-hexaenoic acid) contributes to understanding the biological significance of DHA. In oxidative stress-challenged human retinal pigment epithelial (RPE) cells, human brain cells, or rat brains undergoing ischemia-reperfusion, NPD1 synthesis is enhanced as a response for sustaining homeostasis. Thus, neurotrophins, Aβ peptide 42 (Aβ42), calcium ionophore A23187, interleukin (IL)-1 β, or DHA supply enhances NPD1 synthesis. NPD1, in turn, up-regulates the anti-apoptotic proteins of the Bcl-2 family and decreases the expression of pro-apoptotic Bcl-2 family members. Moreover, NPD1 inhibits IL-1 β-stimulated expression of cyclooxygenase-2 (COX-2). Because both RPE and photoreceptors are damaged and then die in retinal degenerations, elucidating how NPD1 signaling contributes to retinal cell survival may lead to a new understanding of disease mechanisms. In human neural cells, DHA attenuates amyloid-β (Aβ) secretion, resulting in concomitant formation of NPD1. NPD1 was found to be reduced in the Alzheimer’s disease (AD) CA1 hippocampal region, but not in other areas of the brain. The expression of key enzymes for NPD1 biosynthesis, cytosolic phospholipase A2 (cPLA2), and 15-lipoxygenase (15-LOX) was found altered in the AD hippocampal CA1 region. NPD1 repressed Aβ42-triggered activation of pro-inflammatory genes and upregulated the anti-apoptotic genes encoding Bcl-2, Bcl-xl, and Bfl-1(A1) in human brain cells in culture. Overall, these results support the concept that NPD1 promotes brain and retina cell survival via the induction of anti-apoptotic and neuroprotective gene-expression programs that suppress Aβ42-induced neurotoxicity and other forms of cell injury, which in turn fosters homeostasis during development in aging, as well as during the initiation and progression of neurodegenerative diseases.
Keywords: n-3 (omega-3) fatty acid, n-6 (omega-6) fatty acid, retinal pigment epithelial cell, Aβ42, Bcl-2 proteins, eicosanoids, docosanoids, inflammation, photoreceptor renewal, liver, neurotrophins, aging, Alzheimer’s disease, macular degeneration
Introduction
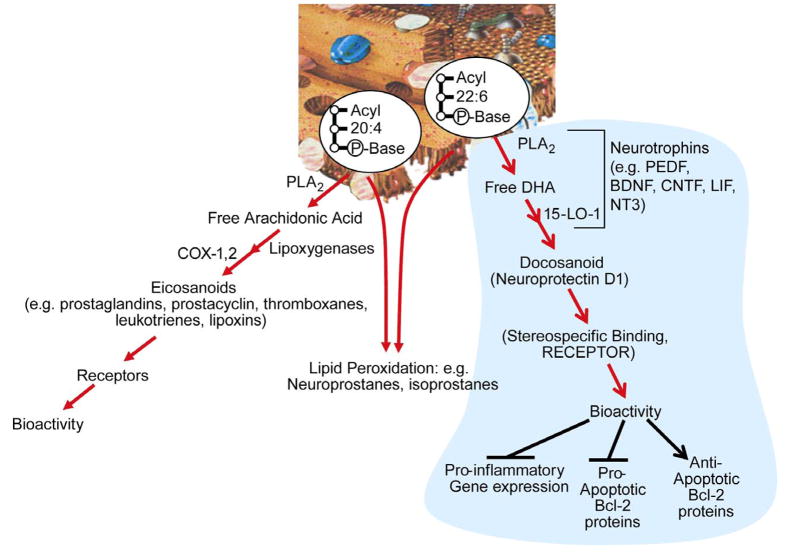
Omega-3 essential fatty acids maintain cellular membrane structural and functional integrity and are necessary to human health (1). Docosahexaenoic acid (22:6, n-3, DHA), a major component of fish oil and marine algae, is most highly concentrated in photoreceptors, the nervous system, and testes, in descending order of concentration (2, 3). Both neurons and glia are richly endowed with this fatty acid. The outer segments of photoreceptors display the highest content of DHA in the human body. Moreover, DHA is present in much smaller quantities in non-nervous system cells. DHA is esterified in C2 of the glycerol backbone of phospholipids. On the other hand, the other major polyunsaturated fatty acyl group of cell phospholipids, the omega-6 family member arachidonic acid (AA), is distributed throughout the human body. Arachidonoyl chains of phospholipids are the reservoir of biologically active eicosanoids, and docosahexaenoyl chains of phospholipids are a reservoir for biologically active docosanoids. Both polyunsaturated fatty acids are also a target for free radical-mediated lipid peroxidation.
Free (unesterified) AA and DHA are released from membrane phospholipids through the action of phospholipases in response to stimulation (e.g., neurotransmitters, cytokines, seizures, ischemia, neurotrauma, etc.) (3–5). This response tells us that phospholipases are a regulatory gatekeeper in the initiation of the eicosanoid and docosanoid pathways under both physiologic and pathologic conditions. It remains to be defined whether any of the docosanoids are esterified back into phospholipids that might, in turn, serve as reservoirs for readily-made bioactive mediators. In connection with this, there are examples of AA-derived lipoxygenation products incorporated into phospholipids of the nervous tissue (6). During basal cell function, active ATP- dependent reacylation of A A and DHA take place in membrane phospholipids (7, 8).
Oxidative stress in the brain generates neuroprostanes from DHA through enzyme- independent reactions (9). It has recently been shown that electrophilic cyclopentenone neuroprostanes elicit anti-inflammatory activity (10). These compounds are formed from the peroxidation of DHA; therefore, it remains to be determined how the production of these compounds might be regulated and how they might exert specific actions such as anti- inflammatory activity.
DHA is required for brain and retina development (11–14) and has been implicated in several functions, including those of excitable membranes (15, 16), memory (17–19), photoreceptor biogenesis and function (20–25), and neuroprotection (26). One property the retina and brain share (insofar as omega-3 fatty acids are concerned) is their unusual ability to retain DHA, even during prolonged dietary deprivation of essential fatty acids of the omega-3 family. To effectively reduce DHA content in retinas and brains of rodents and non-human primates, dietary deprivation for more than one generation has been required (27, 28). This in turn produces impairments of retinal and brain function (25, 29).
Studies on DHA-mediated neuroprotection have prompted the following questions: Is the pro-survival action of DHA the result of replenishing the fatty acid into membrane phospholipids? Is it due to a more selective signaling by a DHA-derived mediator? Or is there a combination of mechanisms? This article highlights the elucidation of a specific DHA mediator that promotes homeostatic regulation of cell integrity and retina and brain protection. Thus, examples of the bioactivity of neuroprotectin D1 (NPD1, 10R, 17S-dihydroxy-docosa- 4Z, 7Z, 11E, 13E, 15Z, 19Z-hexaenoic acid) are provided. These include retinal pigment epithelial (RPE) cells being subjected to oxidative stress, studies using human brain cells in culture exposed to A β peptide 42 (Aβ42), DHA trafficking through the blood stream, DHA uptake by the nervous system, and intercellular shuttling of DHA.
Neuroprotectin D1
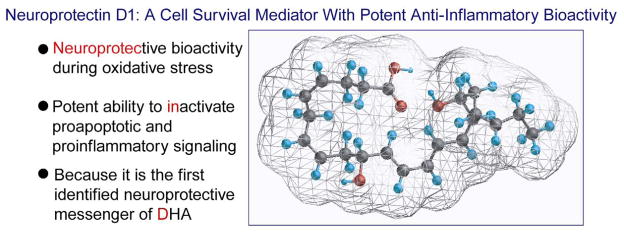
The retina forms mono-, di- and trihydroxy derivatives of DHA. Since lipoxygenase inhibitors block the formation of these derivatives, it was suggested that a lipoxygenase enzyme catalyzes their synthesis, and the name “docosanoids” was introduced for the family of enzyme-derived products of DHA (30). At the time of that study, the stereochemistry and bioactivity of these DHA-oxygenated derivatives were not defined. It was suggested, however, that these lipoxygenase-reaction products might be neuroprotective (30, 31). Upon the advent of mediator lipidomic analysis based on liquid chromatography, photodiode array, electrospray ionization, and tandem mass spectrometry (LC-MS/MS), we identified oxygenation pathways for the synthesis of the stereospecific docosanoid NPD1 during brain ischemia-reperfusion (32, 33), in human RPE cells (34–36), in human brain cells (37), and in the human brain (37). NPD1 synthesis is through DHA oxygenation by 15-lipoxygenase-1 (15-LOX-1) (Figures 1 and 2). NPD1 then works through a stereospecific site (unpublished data), implying that this mediator acts in an autocrine fashion, and/or diffuses through the intercellular space (e.g., inter-photoreceptor matrix to act in paracrine mode on nearby cells). One paracrine target in the retina could be photoreceptor cells and/or Müller cells (36). In addition, interleukin (IL)-1β, oxidative stress, or the Ca2+ ionophore A23187 activate the synthesis of NPD1 in ARPE-19 cells (spontaneously transformed human RPE cells) (34, 35). NPD1 in turn is a potent inhibitor of oxidative-stress-induced apoptosis and of cytokine-mediated pro-inflammatory induction of cyclooxygenase 2 (COX-2). The name “neuroprotectin D1” (34) was proposed, based on its neuroprotective bioactivity in oxidatively stressed RPE cells or brain and its potent ability to inactivate pro-apoptotic and pro-inflammatory signaling. “D1” refers to this being the first identified neuroprotective mediator derived from DHA (Figure 1).
Figure 1.
Neuroprotectin D1 (NPD1, 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid).
Figure 2.
Eicosanoids (from arachidonic acid, AA) and docosanoids (from docosahexaenoic acid, DHA). Lipid peroxidation is depicted to arise from either AA or DHA containing membrane phospholipids.
Photoreceptor Cells and Retinal Pigment Epithelial Cells
The photoreceptor cells and RPE cells are intermittently exposed to potentially adverse conditions such as light, high oxygen consumption, active fluxes of polyunsaturated fatty acids, and the overall active metabolism of these cells. These conditions trigger reactive oxygen species formation in abnormal quantities, as well as lipid peroxidation. It is remarkable that cellular integrity can be maintained in photoreceptor and RPE cells for several decades, so long as homeostasis is not broken (38, 39). Among the factors sustaining homeostasis are antioxidants, including the carotenoids zeaxanthin and lutein, which accumulate in the macula. Similar risks of cellular damage occur in the brain, where the relationship of neurons to astrocytes is extensive. This is particularly evident in the synapses, which are literally “wrapped up” by astrocytes. In addition, astrocytes are part of the neurovascular unit and participate in the retrieval of DHA from the bloodstream.
We designed experiments to test the potential significance of NPD1 in the photoreceptor/RPE cell relationship. Using an in vitro cellular model, we mimicked what may occur in the eye when homeostasis is challenged by exposure to oxidative stress.
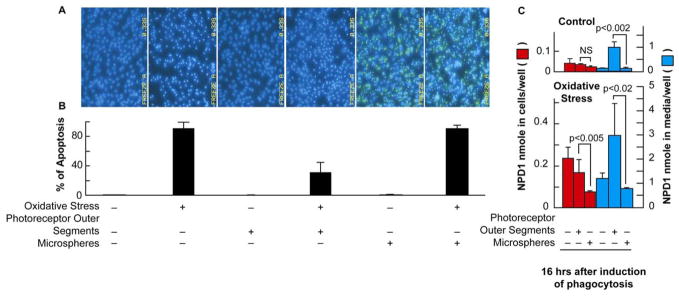
RPE cells fed with bovine outer segments become more resistant to oxidative stress than cells that do not phagocytize rod outer segments (Figure 3, A and B). Neither outer segments nor microspheres alone trigger Hoechst positive cells; outer segments combined with oxidative stress markedly decrease oxidative-stress-induced apoptosis, unlike microspheres.
Figure 3.
Photoreceptor outer segment phagocytosis attenuates oxidative stress-induced apoptosis with concomitant synthesis of NPD1. ARPE-19 cells were plated and maintained in culture for 72 h followed by 8 h of serum starvation before the addition of photoreceptor outer segment or polystyrene microspheres. (A) Hoechst staining shows an increase in cell death after 16 h of H2O2/TNF-α (oxidative stress) exposure, which is ameliorated by photoreceptor outer segment phagocytosis but not by polystyrene microspheres. (B) Quantitative analysis of Hoechst-stained cells indicates that photoreceptor outer segment phagocytosis significantly decreases apoptosis observed during oxidative stress. Phagocytosis of polystyrene microspheres during oxidative stress does not alter apoptosis observed during oxidative stress alone. Results represent averages ± SEM of repeats of two independent experiments. (C) NPD1 changes as a function of time after photoreceptor outer segment phagocytosis or microspheres: effect of oxidative stress. NPD1 has been quantified in cells as well as in incubation media. NS = not statistically significant. (Modified and published with permission from Mukherjee, et al. Photoreceptor outer segment phagocytosis attenuates oxidative stress-induced apoptosis with concomitant neuroprotectin D1 synthesis. Proc Natl Acad Sci USA. 2007;104:13158–63. Copyright 2007 National Academy of Sciences, USA).
The RPE cell recycles DHA from phagocytized disc membranes back to the inner segment of the photoreceptor cell through the interphotoreceptor matrix (31, 40–42). Thus, the bulk of DHA in RPE cells is a component of photoreceptor disc membrane phospholipids that, after phagocytosis, is recycled as a part of outer segment renewal. The RPE cell contributes to enrich photoreceptor cells in DHA by taking up this fatty acid from the bloodstream through the choriocapillaris (40). Since DHA is the initial precursor of NPD1 synthesis, we explored the hypothesis that part of the DHA arriving during the photoreceptor renewal may be used for NPD1 synthesis. Free DHA accumulates in RPE cells and media, 6 h after the onset of phagocytosis, and oxidative stress results in further increases of free DHA (35). This approach has identified a remarkable phagocytosis-dependent NPD1 synthesis in the presence of oxidative stress. Rod outer segment tips are the biologically relevant ligand for the retinal pigment epithelium. When RPE cells undergoing photoreceptor phagocytosis were subjected to oxidative stress, accumulation of NPD1 was observed (no accumulation was observed). This NPD1 increase was several times higher than that observed in RPE cells that phagocytized microspheres, amounting to a 36.6-fold NPD1 increase for the outer segment-treated cells after oxidative stress. Control cells showed only a 15-fold increase after oxidative stress (Figure 3, C). This enhanced synthesis of NPD1 after outer segment phagocytosis is concomitant with outer segment-induced attenuation of oxidative stress-mediated apoptosis (Figure 3, A and B). Although ARPE-19 cells also phagocytized, the biologically inert polystyrene microspheres during these studies, NPD1 content was not affected in RPE cells or in the incubation media (Figure 3, C). Moreover, although oxidative stress did stimulate NPD1 accumulation, this was also not affected by microsphere phagocytosis. These results correlate with the observed lack of cytoprotection offered by microsphere phagocytosis (Figure 3, A and B). In addition, outer segment-mediated retinal pigment epithelium protection against oxidative stress, with concurrent NPD1 synthesis, takes place in low passage primary human RPE cells, prepared from human eyes supplied by the National Development and Research Institutes, Inc. (Bazan NG, et al., unpublished studies).
Unlike non-specific, non-biological ligand microsphere phagocytosis, outer segments also have been reported to trigger early-response gene induction in the retinal pigment epithelium (43), including COX-2 (44) and peroxisome proliferator-activated receptor gamma (PPARγ) expression (45). Whether any of these events are related to the NPD1 survival signaling described here remains to be ascertained.
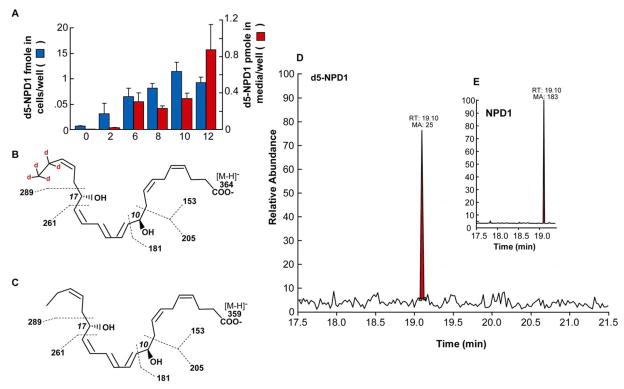
We simultaneously measured free DHA pool size by LC-MS/MS, and found that it increases as a function of time of exposure to oxidative stress in ARPE-19 cells (35). Free DHA in cells showed a moderate increase after 6 h when cells were subjected only to outer segment phagocytosis (10.5-fold increase). Oxidative stress, however, strongly enhanced free DHA accumulation in a time-dependent fashion, peaking at 16 h. Interestingly, although there was an overall 10-fold increase, outer segment phagocytosis kept the DHA pool size at a constant 2.4-fold increased level. This implies that NPD1 synthesis reflects an event other than enhanced, overall availability of free DHA upon phagocytosis. There is a correlation between increases in free DHA pool size and increases in NPD1 synthesis. Outer segment phagocytosis stimulates NPD1 synthesis at 3–6 h in cells and accumulation in media after 16 h (Figure 3, C), while free DHA increases earlier and keeps accumulating up to 16 h. These enhancements in DHA and NPD1 pool size are much larger when outer segment phagocytosis takes place on RPE cells exposed to oxidative stress. Interestingly, microsphere phagocytosis does not cause enhanced changes in DHA (35) and NPD1 (Figure 3, C). Thus, a very specific DHA pool may be the precursor for NPD1. The studies illustrated in Figure 4 demonstrate a correlation between enhanced free DHA and increased NPD1 content in ARPE-19 cells undergoing oxidative stress. ARPE-19 cells incubated with 2H5-DHA show that 2H5-NPD1 is formed. This approach allows us to follow DHA conversion specifically because the deuterium is on the methylene carbons 21 and 22, which are not metabolically altered. Also, the products are heavier than the same non-deuterated molecule (by a mass unit of 1) and can be detected by tandem mass spectrometry (35). Figure 4 illustrates the characterization of 2H5-NPD1 (negative molecular ion m/z 364.2), as well as endogenous non-deuterated NPD1 (negative molecular ion m/z 359.2). These observations support the notion that as free DHA accumulates in the ARPE-19 cells during photoreceptor phagocytosis, it is a substrate for NPD1 synthesis.
Figure 4.
Deuterated DHA, added to the ARPE-19 cell medium, is incorporated and converted to deuterated NPD1. (A) Time course of synthesis of d5-NPD1 in ARPE-19 cells and medium, after 100nM [2H5] DHA was added to cells at the onset of oxidative stress. Results are the average ± SEM (n=3). (B) Molecular structure of [2H5] NPD1, showing molecular ion m/z 364.15; the localization of deuterium at carbon 21 and 22 is also indicated. Dashed lines show typical product ion breakpoints at MS collision cell. (C) Molecular structure of endogenous NPD1, with molecular and product ions as indicated in B. (D and E) Typical TIC curve of detection for [2H5] NPD1 and endogenous NPD1 (E) in ARPE-19 cells treated as described in A. (Published with permission from Mukherjee, et al. Photoreceptor outer segment phagocytosis attenuates oxidative stress-induced apoptosis with concomitant neuroprotectin D1 synthesis. Proc Natl Acad Sci USA. 2007;104:13158–63. Copyright 2007 National Academy of Sciences, U.S.A.).
AA is also a precursor of bioactive lipids, including prostaglandins and lipoxygenase products, which have been correlated with photoreceptor phagocytosis (46, 47). That AA is released under the present experimental conditions (data not shown), led us to explore some of the AA cascade members. We found that lipoxin A4, 12(S) HETE, and 15(S) HETE were unchanged during outer segment phagocytosis (35), thereby suggesting that NPD1 is selectively synthesized during this fundamental event that underlies photoreceptor cell renewal essential to sustaining its integrity.
Intercellular Trafficking of DHA: Significance for Omega-3 Conservation
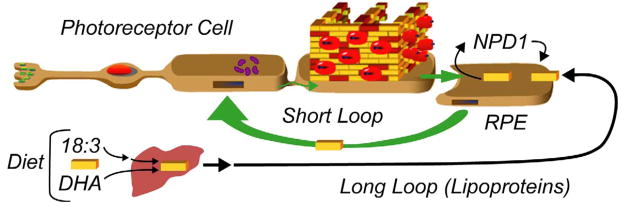
A conceptual advancement in our understanding of DHA conservation in the central nervous system came from the studies supporting the suggestion that specific intercellular trafficking assures the retention/conservation of DHA (31, 48). Figure 5 illustrates this concept. The liver takes up DHA and linolenic acid (18:3, n-3) that are supplied by the diet, and elongates and desaturates the linolenic acid to DHA. Linolenic acid-acid derived DHA and DHA directly available to the liver are then esterified into phospholipids, secreted as lipoproteins (49), and delivered to the retinal pigment epithelium (RPE) through the choriocapillaris, which is located beneath the retinal pigment epithelial cells. The molecular details of this delivery are not understood. Is a receptor-like mechanism present? It is remarkable that lipoproteins with DHA-acylated phospholipids selectively deliver DHA to the RPE and brain, and to a lesser extent, to the testes and other tissues. This strongly implies specialized DHA uptake by the CNS. The short loop recycles DHA from the RPE to the outer segment, via the inner segment. DHA in the RPE (delivered by the long loop or taken up during shedding from the tip of the photoreceptor and phagocytosis) travels back through the inter-photoreceptor matrix to the inner segment where phospholipids, including those containing DHA, are synthesized. The connecting cilium enables DHA to return to the outer segment, as part of phospholipids, for biogenesis of disc membranes (41). Figure 5 represents DHA-phospholipids as piles of yellow bricks, in which rhodopsin (illustrated as red apple-like structures) and other proteins are embedded. The RPE cell is depicted as biosynthesizing NPD1, which in turn is released and, in an autocrine fashion, elicits its action through a cell surface receptor (unpublished data).
Figure 5.
Extracellular trafficking of DHA and neuroprotectin D1 synthesis in retinal pigment epithelial cells. The liver-to-photoreceptor long loop is the transport of DHA through the blood stream by lipoproteins (49). Yellow bricks depict DHA. Short loop retrieval of DHA through interphotoreceptor matrix after intermittent photoreceptor outer segment renewal. Wall in outer segment illustrates disc membranes; the red “fruit-like” structure represents rhodopsin molecules. (Modified and published with permission from Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29:263–71).
It has been shown that astrocytes mediate bloodstream uptake of DHA, and that these cells are intimately related with neurons, mainly at synapses (40). It is therefore conceivable that brain DHA conservation mechanisms may involve astrocyte/neuronal relationships.
Aging and Alzheimer’s Disease
Deficiency in DHA is associated with cognitive decline (37) and has also been implied in Alzheimer’s disease (AD). Pro-inflammatory gene expression patterns in 4-week-old human brain cells that have been exposed to Aβ42, DHA, and NPD1 disclose the following: that inducible pro-inflammatory cytokines IL-1β and chemokine exodus protein 1 (CEX-1), prostaglandin synthase COX-2, the tumor necrosis factor alpha (TNF-α)–inducible proinflammatory element B94 (50, 51), and TNF-α are upregulated by Aβ42 and downregulated by DHA or NPD1 (Table 1). The expression of these pro-inflammatory genes is upregulated in the brains of AD patients (50).
Table 1.
NPD1 Induces an Anti-Apoptotic/Anti-Inflammatory Gene-Expression Program in Human Neural Cells
| Gene | Function |
Aβ42 | DHA | NPD1 | ||
|---|---|---|---|---|---|---|
| Apoptosis |
Inflammation |
|||||
| Anti- | Pro- | |||||
| Cytokine IL-1β | ✓ | ✓ | ✓ | + 4.1 | * | * |
| CEX-1 (Chemokine Exodus Protein 1) | ✓ | ✓ | ✓ | + 57 | * | * |
| Blf (A1) | ✓ | – | – | * | + 3.9 | + 6.7 |
| Bcl-2 | ✓ | – | – | * | + 2.5 | + 3.3 |
| Bcl-xL | ✓ | – | – | * | + 1.5 | + 2.4 |
| Bax | – | ✓ | – | + 3.2 | * | * |
| Bik | – | ✓ | – | + 2.8 | * | * |
| COX-2 | – | – | ✓ | + 5.4 | * | * |
| B94 (TNF α-inducible pro-apoptotic protein) | – | – | ✓ | + 4.2 | * | * |
| TNF α | – | – | ✓ | + 4.8 | * | * |
= no significant change (<2.0 fold over control and/or p > 0.05, AN OVA).
HN cells (cc-2599, Cambree Corporation, in primary culture as described in reference 55) were treated with Aβ42, DHA, or NPD1. Increases for Aβ42-, DHA-, or NPD1 -treated HN cells are expressed as fold increases over untreated, age-matched controls. CEX-1 (GenBank U64197) is a marker for inflammatory and oxidative stress responses, and B94 (GenBank M92357) is a TNF-α–inducible proinflammatory element. Genes are classified according to major functions, although many have multiple cellular roles. Analytical criteria were gene changes of at least 2-fold over control and P less than 0.05 (ANOVA) in Aβ42-stressed HN cells. NS, no significant change. (Modified table is published with permission from Lukiw WJ, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest 2005;115:2774–83).
Since Bcl-2 protein family members are a target of NPD1 (34), these proteins were also studied in human brain cells treated with Aβ42 (25 μM) and DHA or NPD1 (each 50 nM ambient). Overall, Aβ42 markedly enhanced a complex proapoptotic gene-expression program that includes the proapoptotic Bax and Bik proteins. These proteins clearly participate in apoptosis signaling in other cells (52–54). DHA and NPD1 each showed enhanced expression of Bcl-xl, Bcl-2, and Bfl-1(A1)—antiapoptotic members of the Bcl-2 gene family—and relative downregulation of Bax and Bik (Table 1). Bax and Bik were upregulated in Aβ42-treated cells 3.2- and 2.8-fold, respectively, over age-matched control cells, and did not change with DHA or NPD1 (Table 1). The antiapoptotic Bcl-2 family member Bfl-1(A1) was upregulated by DHA and NPD1 to about 4- and 6-fold over controls, respectively; Bfl-1(A1) reached the highest significance of any upregulated gene in NPD1-treated HN cells (Table 1) (37). Subtraction of DHA from NPD1 DNA-array signals revealed an additional 56 genes, which were upregulated 2-fold or greater over controls (37).
To explore the possible significance of DHA-derived NPD1, the levels of NPD1 were quantified in AD hippocampal cornu ammonis region 1 (CA1), a brain region involved in memory and targeted by neuropathology in the early stages of AD (51, 54, 55). According to the plaque and tangle count, all but one of the AD brain samples analyzed were from AD patients at a moderate stage of disease development (37). While there were no significant differences in the age or postmortem sampling interval between the AD and control groups, and no differences in the RNA yields or spectral quality (37), unesterified DHA pool sizes in the control groups were 2-fold higher than in AD CA1; NPD1 levels in AD were on average about one-twentieth of those in age-matched controls (37). Depending on brain region and stage of disease development, the population of neurons remaining in AD brain has been estimated to range from 59% (56), to 77% (57), to 89% (58) of age-matched controls for the same region. Thus, the loss of 11–41% of neurons is insufficient to account for the observed 20-fold reduction in the NPD1 content in AD CA1 when compared with age-matched controls. These observations indicate that, despite modestly decreased availability of unesterified DHA, NPD1 levels were markedly reduced in AD CA1, perhaps as the result of lipid peroxidation. As a result, NPD1’s neuroprotective bioactivity during brain cell degeneration may have been lost. In these same human CA1 hippocampal samples, the levels of expression were examined for a cytosolic PLA2 (cPLA2) (GenBank D38178; encoding an 82.5-kDa, calcium-dependent cPLA2) and 15-LOX (GenBank M23892; encoding 15-lipoxygenase), 2 key enzymes in the mobilization of DHA and NPD1 biosynthesis. In AD CA1, when compared with age-matched controls, cPLA2 abundance was increased, while 15-LOX was decreased almost 2-fold. Decreased abundance of NPD1 in AD CA1 may be explained, at least in part, by a disruption in the expression and regulation of the PLA2 and/or 15-LOX enzymes necessary for NPD1 biosynthesis (37).
These observations support the notion that NPD1 promotes brain cell survival via the induction of antiapoptotic and neuroprotective gene-expression programs that suppress Aβ42induced neurotoxicity.
Conclusions
NPD1, a stereospecific DHA-derived mediator endogenously synthesized by cells from the nervous system and other cells, induces signaling pathways that promote homeostatic regulation, activate anti-inflammatory signaling, and foster cell survival. One target of this mediator is the Bcl-2 family of proteins, a pre-mitochondrial apoptotic signaling event induced under conditions of oxidative stress. As a consequence of NPD1-regulation of these families of proteins, effector caspase-3 activation and DNA degradation are attenuated. NPD1 also potently counteracts cytokine-triggered pro-inflammatory COX-2 gene induction, another factor in cell damage. In ischemia-reperfusion-injured hippocampi and in neural progenitor cells stimulated by IL-1β, COX-2 expression is related to nuclear factor kappa B (NFκB) activation. NPD1 inhibits NFκB and COX-2 induction under those conditions (32). NPD1’s neuroprotective bioactivity in brain ischemia-reperfusion includes decreased infarct size and inhibition of polymorphonuclear leukocyte infiltration (32). Pro-inflammatory injury of the retinal pigment epithelium is involved in age-related macular degeneration and in the pathoangiogenesis component of the wet form of this disorder. The active DHA supply to the brain and retina from the liver through the blood stream is necessary, particularly during postnatal development. DHA supply also is pivotal when homeostasis is disrupted, especially during aging. The polyunsaturated fatty acyl chains of membrane phospholipids are decreased as a consequence of lipid peroxidation in aging, retinal degenerations, and neurodegenerations such as AD (59, 60). In ischemia, neurotrauma, and seizures, loss of brain DHA occurs due to phospholipase A2-activated cleavage of DHA-containing phospholipids (3–5). It is conceivable that DHA gives rise to other bioactive mediators, as highlighted by the initial identification of stereospecific DHA derivatives (32). Further understanding of the signals that modulate synthesis of NPD1, and of other DHA-derived mediators, may be valuable for developing novel therapeutic approached for stroke, neurotrauma, and neurodegenerative diseases. NPD1 and its cellular target(s) may allow for the design of novel therapeutic approaches and DHA-delivery systems for managing retina and brain cytoprotection and, in turn, enhance neural cell survival in several of these diseases. Moreover, dietary supplementation approaches (doses and timing of administration) incorporating the omega-3 essential fatty DHA need to be properly defined to effectively maintain homeostasis, prevent diseases, and slow down the initiation and progression of neurodegenerations.
Acknowledgments
Sources of support: National Institutes of Health, National Center for Research Resources grant P20 RR016816; National Institutes of Health, National Eye Institute grant R01 EY005121; and National Institute of Neurological Disorder and Stroke grant R01 NS046741.
Footnotes
Conflict of interest: NGB is a consultant for Resolvyx Pharmaceuticals (Bedford, MA).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simopoulos AP, Leaf A, Salem N., Jr Workshop on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. J Am Coll Nutr. 1999;18:487–89. doi: 10.1080/07315724.1999.10718888. [DOI] [PubMed] [Google Scholar]
- 2.Bazan NG. Supply of n-3 polyunsaturated fatty acids and their significance in the central nervous system. In: Wurtman RJ, Wurtman JJ, editors. Nutrition and the brain. Vol. 8. New York: Raven Press, Ltd; 1990. pp. 1–24. [Google Scholar]
- 3.Bazan NG. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J Lipid Res. 2003;44:2221–33. doi: 10.1194/jlr.R300013-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Horrocks LA, Farooqui AA. NMDA receptor-stimulated release of arachidonic acid: mechanisms for the Bazan effect. In: Municio AM, Miras-Portugal MT, editors. Cell signal transduction, second messengers, and protein phosphorylation in health and disease. New York: Plenum Press; 1994. pp. 113–28. [Google Scholar]
- 5.Sun GY, Xu J, Jensen MD, Simonyi A. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J Lipid Res. 2004;45:205–13. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Birkle DL, Bazan NG. Effect of K+ depolarization on the synthesis of prostaglandins and hydroxyeicosatetra(5,8,11,14)enoic acids (HETE) in the rat retina. Evidence for esterification of 12-HETE in lipids. Biochim Biophys Acta. 1984;795:564–73. doi: 10.1016/0005-2760(84)90187-5. [DOI] [PubMed] [Google Scholar]
- 7.Reddy TS, Bazan NG. Synthesis of arachidonoyl coenzyme A and docosahexaenoyl coenzyme A in synaptic plasma membranes of cerebrum, cerebellum and brain stem of rat brain. J Neurosci Res. 1985;13:381–90. doi: 10.1002/jnr.490130305. [DOI] [PubMed] [Google Scholar]
- 8.Reddy TS, Bazan NG. Long chain acyl CoA synthetase in microsomes from rat brain gray matter and white matter. Invest Ophthalmol Vis Sci. 1985;10:377–86. doi: 10.1007/BF00964606. [DOI] [PubMed] [Google Scholar]
- 9.Roberts LJ, 2nd, Montine TJ, Markesbery WR, et al. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 1998;273:13605–12. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- 10.Musiek ES, Brooks JD, Joo M, et al. Electrophilic cyclopentenone neuroprostanes are anti-inflammatory mediators formed from the peroxidation of the {omega}-3 polyunsaturated fatty acid docosahexaenoic acid. J Biol Chem. 2008;283:19927–35. doi: 10.1074/jbc.M803625200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez M. Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr. 1992;120:S129–38. doi: 10.1016/s0022-3476(05)81247-8. [DOI] [PubMed] [Google Scholar]
- 12.Diau GY, Hsieh AT, Sarkadi-Nagy EA, Wijendran V, Nathanielsz PW, Brenna JT. The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system. BMC Med. 2005;3:11. doi: 10.1186/1741-7015-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greiner RC, Winter J, Nathanielsz PW, Brenna JT. Brain docosahexaenoate accretion in fetal baboons: bioequivalence of dietary alpha-linolenic and docosahexaenoic acids. Pediatr Res. 1997;42:826–34. doi: 10.1203/00006450-199712000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Aveldano MI, Bazan NG. Acyl groups, molecular species and labeling by 14C glycerol and 3H-arachidonic acid of vertebrate retina glycerolipids. In: Bazan NG, Brenner RR, Giusto NM, editors. Adv Exp Biosynthesis of Lipids. Vol. 83. New York: Plenum Press; 1977. pp. 397–404. [DOI] [PubMed] [Google Scholar]
- 15.Litman BJ, Niu SL, Polozova A, Mitchell DC. The role of docosahexaenoic acid containing phospholipids in modulating G protein-coupled signaling pathways: visual transduction. J Mol Neurosci. 2001;16:237–42. doi: 10.1385/JMN:16:2-3:237. [DOI] [PubMed] [Google Scholar]
- 16.Salem N, Jr, Kim HY, Yergey JA. Docoshexaenoic acid: membrane function and metabolism. In: Simopoulos AP, Kifer RR, Martin R, editors. The Health Effects of Polyunsaturated Fatty Acids in Seafoods. New York: Academic Press; 1986. pp. 263–317. [Google Scholar]
- 17.Catalan J, Moriguchi T, Slotnick B, Murthy M, Greiner RS, Salem N., Jr Cognitive deficits in docosahexaenoic acid-deficient rats. Behav Neurosci. 2002;116:1022–31. doi: 10.1037//0735-7044.116.6.1022. [DOI] [PubMed] [Google Scholar]
- 18.Moriguchi T, Salem N., Jr Recovery of brain docosahexaenoate leads to recovery of spatial task performance. J Neurochem. 2003;87:297–309. doi: 10.1046/j.1471-4159.2003.01966.x. [DOI] [PubMed] [Google Scholar]
- 19.Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–59. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 20.Anderson RE, Maude MB, Bok D. Low docosahexaenoic acid levels in rod outer segment membranes of mice with rds/peripherin and P216L peripherin mutations. Invest Ophthalmol Vis Sci. 2001;42:1715–20. [PubMed] [Google Scholar]
- 21.Anderson RE, Maude MB, McClellan M, Matthes MT, Yasumura D, LaVail MM. Low docosahexaenoic acid levels in rod outer segments of rats with P23H and S334ter rhodopsin mutations. Mol Vis. 2002;8:351–58. [PubMed] [Google Scholar]
- 22.Bicknell IR, Darrow R, Barsalou L, Fliesler SJ, Organisciak DT. Alterations in retinal rod outer segment fatty acids and light-damage susceptibility in P23H rats. Mol Vis. 2002;8:333–40. [PubMed] [Google Scholar]
- 23.Organisciak DT, Darrow RM, Jiang YL, Blanks JC. Retinal light damage in rats with altered levels of rod outer segment docosahexaenoate. Invest Ophthalmol Vis Sci. 1996;37:2243–57. [PubMed] [Google Scholar]
- 24.Stinson AM, Wiegand RD, Anderson RE. Recycling of docosahexaenoic acid in rat retinas during n-3 fatty acid deficiency. J Lipid Res. 1991;32:2009–17. [PubMed] [Google Scholar]
- 25.Wheeler TG, Benolken RM, Anderson RE. Visual membranes: specificity of fatty acid precursors for the electrical response to illumination. Science. 1975;188:1312–14. doi: 10.1126/science.1145197. [DOI] [PubMed] [Google Scholar]
- 26.Kim HY, Akbar M, Lau A, Edsall L. Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3). Role of phosphatidylserine in antiapoptotic effect. J Biol Chem. 2000;275:35215–23. doi: 10.1074/jbc.M004446200. [DOI] [PubMed] [Google Scholar]
- 27.Neuringer M, Connor WE, Lin DS, Barstad L, Luck S. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc Natl Acad Sci USA. 1986;83:4021–25. doi: 10.1073/pnas.83.11.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weisinger HS, Armitage JA, Jeffrey BG, et al. Retinal sensitivity loss in third-generation n-3 PUFA-deficient rats. Lipids. 2002;37:759–65. doi: 10.1007/s11745-002-0958-3. [DOI] [PubMed] [Google Scholar]
- 29.Neuringer M, Connor WE, Van Petten C, Barstad L. Dietary omega-3 fatty acid deficiency and visual loss in infant rhesus monkeys. J Clin Invest. 1984;73:272–76. doi: 10.1172/JCI111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bazan NG, Birkle DL, Reddy TS. Docosahexaenoic acid (22:6, n-3) is metabolized to lipoxygenase reaction products in the retina. Biochem Biophys Res Commun. 1984;125:741–47. doi: 10.1016/0006-291x(84)90601-6. [DOI] [PubMed] [Google Scholar]
- 31.Bazan NG, Birkle DL, Reddy TS. Biochemical and nutritional aspects of the metabolism of polyunsaturated fatty acids and phospholipids in experimental models of retinal degeneration. In: LaVail MM, et al., editors. Retinal degeneration: experimental and clinical studies. Alan R. Liss, Inc; 1985. pp. 159–87. [Google Scholar]
- 32.Marcheselli VL, Hong S, Lukiw WJ, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–17. doi: 10.1074/jbc.M305841200. Erratum in: J Biol Chem 2003;278:51974. [DOI] [PubMed] [Google Scholar]
- 33.Belayev L, Marcheselli VL, Khoutorova L, et al. Docosahexaenoic acid complexed to albumin elicits high-grade ischemic neuroprotection. Stroke. 2005;36:118–23. doi: 10.1161/01.STR.0000149620.74770.2e. [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci USA. 2004;101:8491–6. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukherjee PK, Marcheselli VL, de Rivero Vaccari JC, Gordon WC, Jackson FE, Bazan NG. Photoreceptor outer segment phagocytosis attenuates oxidative stress-induced apoptosis with concomitant neuroprotectin D1 synthesis. Proc Natl Acad Sci USA. 2007;104:13158–63. doi: 10.1073/pnas.0705963104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci USA. 2007;104:13152–7. doi: 10.1073/pnas.0705949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lukiw WJ, Cui JG, Marcheselli VL, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–83. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1992;33:1–17. [PubMed] [Google Scholar]
- 39.Bazan NG. Homeostatic regulation of photoreceptor cell integrity: significance of the potent mediator neuroprotectin D1 biosynthesized from docosahexaenoic acid: the Proctor Lecture. Invest Ophthalmol Vis Sci. 2007;48:4866–81. doi: 10.1167/iovs.07-0918. [DOI] [PubMed] [Google Scholar]
- 40.Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29:263–71. doi: 10.1016/j.tins.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez de Turco EB, Gordon WC, Bazan NG. Light stimulates in vivo inositol lipid turnover in frog retinal pigment epithelial cells at the onset of shedding and phagocytosis of photoreceptor membranes. Exp Eye Res. 1992;55:719–25. doi: 10.1016/0014-4835(92)90176-s. [DOI] [PubMed] [Google Scholar]
- 42.Chen H, Anderson RE. Metabolism in frog retinal pigment epithelium of docosahexaenoic and arachidonic acids derived from rod outer segment membranes. Exp Eye Res. 1993;57:369–77. doi: 10.1006/exer.1993.1136. [DOI] [PubMed] [Google Scholar]
- 43.Ershov AV, Lukiw WJ, Bazan NG. Selective transcription factor induction in retinal pigment epithelial cells during photoreceptor phagocytosis. J Biol Chem. 1996;271:28458–62. doi: 10.1074/jbc.271.45.28458. [DOI] [PubMed] [Google Scholar]
- 44.Ershov AV, Bazan NG. Induction of cyclooxygenase-2 gene expression in retinal pigment epithelium cells by photoreceptor rod outer segment phagocytosis and growth factors. J Neurosci Res. 1999;58:254–61. [PubMed] [Google Scholar]
- 45.Ershov AV, Bazan NG. Photoreceptor phagocytosis selectively activates PPARgamma expression in retinal pigment epithelial cells. J Neurosci Res. 2000;60:328–37. doi: 10.1002/(SICI)1097-4547(20000501)60:3<328::AID-JNR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 46.Ershov AV, Parkins N, Lukiw WJ, Bazan NG. Modulation of early response gene expression by prostaglandins in cultured rat retinal pigment epithelium cells. Curr Eye Res. 2000;21:968–74. doi: 10.1076/ceyr.21.6.968.6987. [DOI] [PubMed] [Google Scholar]
- 47.Bazan NG, Bazan HE, Birkle DL, Rossowska M. Synthesis of leukotrienes in frog retina and retinal pigment epithelium. J Neurosci Res. 1987;18:591–96. doi: 10.1002/jnr.490180412. [DOI] [PubMed] [Google Scholar]
- 48.Bazan NG, Reddy TS, Redmond TM, Wiggert B, Chader GJ. Endogenous fatty acids are covalently and noncovalently bound to interphotoreceptor retinoid-binding protein in the monkey retina. J Biol Chem. 1985;260:13677–80. [PubMed] [Google Scholar]
- 49.Bazan NG, Rodriguez de Turco EB. Alterations in plasma lipoproteins and DHA transport in progressive rod-cone degeneration (PRCD). In: Kato S, et al., editors. Retinal degeneration and regeneration, proceedings of an international symposium in Kanazawa; Japan. July 8–9, 1995; Amsterdam, NY; Kugler Publications. 1996. pp. 89–97. [Google Scholar]
- 50.Colangelo V, Schurr J, Ball MJ, Pelaez RP, Bazan NG, Lukiw WJ. Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J Neurosci Res. 2002;70:462–73. doi: 10.1002/jnr.10351. [DOI] [PubMed] [Google Scholar]
- 51.Liao YF, Wang BJ, Cheng HT, Kuo LH, Wolfe MS. Tumor necrosis factor-alpha, interleukin-1beta, and interferon-gamma stimulate gamma-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J Biol Chem. 2004;279:49523–32. doi: 10.1074/jbc.M402034200. [DOI] [PubMed] [Google Scholar]
- 52.Akhtar RS, Ness JM, Roth KA. Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim Biophys Acta. 2004;1644:189–203. doi: 10.1016/j.bbamcr.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 53.Metcalfe AD, Hunter HR, Bloor DJ, et al. Expression of 11 members of the BCL-2 family of apoptosis regulatory molecules during human preimplantation embryo development and fragmentation. Mol Reprod Dev. 2004;68:35–50. doi: 10.1002/mrd.20055. [DOI] [PubMed] [Google Scholar]
- 54.Kim HJ, Chae SC, Lee DK, et al. Selective neuronal degeneration induced by soluble oligomeric amyloid beta protein. FASEB J. 2003;17:118–20. doi: 10.1096/fj.01-0987fje. [DOI] [PubMed] [Google Scholar]
- 55.Bahr BA, Hoffman KB, Yang AJ, Hess US, Glabe CG, Lynch G. Amyloid beta protein is internalized selectively by hippocampal field CA1 and causes neurons to accumulate amyloidogenic carboxyterminal fragments of the amyloid precursor protein. J Comp Neurol. 1998;397:139–47. [PubMed] [Google Scholar]
- 56.Terry RD. Cortical morphometry in Alzheimer’s disease. In: Katzman R, editor. Biological aspects of Alzheimer’s disease (Banbury report) Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1983. pp. 95–8. [Google Scholar]
- 57.Crapper DR, Dalton AJ, Skoptiz M, Scott JW, Hachinski VC. Alzheimer degeneration in Down’s syndrome. Electrophysiologic alterations and histopathologic findings. Arch Neurol. 1975;32:618–23. doi: 10.1001/archneur.1975.00490510074006. [DOI] [PubMed] [Google Scholar]
- 58.Pelvig DP, Pakkenberg H, Regeur L, Oster S, Pakkenberg B. Neocortical glial cell numbers in Alzheimer’s disease. A stereological study. Dement Geriatr Cogn Disord. 2003;16:212–19. doi: 10.1159/000072805. [DOI] [PubMed] [Google Scholar]
- 59.Nourooz-Zadeh J, Liu EHC, Yhlen B, Änggård EE, Halliwell B. F4-isoprostanes as specific marker of docosahexaenoic acid peroxidation in Alzheimer’s disease. J Neurochem. 1999;72:734–40. doi: 10.1046/j.1471-4159.1999.0720734.x. [DOI] [PubMed] [Google Scholar]
- 60.Scott BL, Bazan NG. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci USA. 1989;86:2903–7. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]