Abstract
In species with bi-parental care, individuals must partition energy between parental effort and mating effort. Typically, female songbirds invest more than males in reproductive activities such as egg-laying and incubation, but males invest more in secondary sexual traits used in attracting mates. Animals that breed more than once within a season must also allocate time and energy between first and subsequent breeding attempts and between current and future breeding seasons. To investigate strategies of reproductive investment by males and females and the consequences of such strategies, we manipulated the size of broods of Eastern Bluebirds Sialia sialis. Pairs with enlarged first broods were less likely to produce a second clutch or took longer to initiate one than pairs with reduced broods. After rearing enlarged broods, females were less likely than males to survive to the following year. Although plumage coloration is a sexually selected trait in Eastern Bluebirds that is influenced by nutritional stress, we did not detect an effect of brood-size manipulation on female coloration. Past research, however, demonstrates that, in males, plumage colour is negatively affected by increasing brood size. We suggest that there are sex-specific strategies of reproductive investment in Eastern Bluebirds, and that researchers should incorporate measures of residual reproductive value in studies of life-history evolution.
Keywords: brood manipulation, life-history evolution, multiple clutches, survival, trade-offs
A central theme in life-history theory is that investment in current reproduction should be offset by reduction in residual reproductive value (Williams 1966). Furthermore, individuals can allocate energy either to mating effort or to parental effort (Trivers 1972), so that current investment in parental effort is expected to affect future energy available for mating effort (Stearns 1992), including production of ornamental traits (Norris et al. 2004). In monogamous species with bi-parental care, both sexes benefit from increased care of offspring, but males have more to gain through investment in mating effort (Trivers 1972), especially given that extra-pair fertilizations are common in socially monogamous birds (Westneat et al. 1990).
Because of differential investment in egg production, incubation and production of sexual displays (Andersson 1994, Nager et al. 2001), males and females may respond differently to manipulations aimed at increasing parental care. When parental effort is experimentally increased in species with bi-parental care, females typically invest more in parental care (Sanz et al. 2000, Velando & Alonso-Alvarez 2003) and suffer higher mortality (Verhulst 1998) than males. In Collared Flycatchers Ficedula albicollis, for example, experimentally increasing parental effort caused females to reduce the size of future clutch sizes (Gustafsson & Pärt 1990) and caused males to experience a reduction in ornamentation (Gustafsson et al. 1995) that was likely to decrease their future reproductive success. In the Great Tit Parus major, experimental removal of second clutches increased the survival rates of females, but not those of males (Verhulst 1998).
Many species of birds rear more than one brood within a breeding season and so face the challenge of allocating time and energy between first and second broods in a manner that maximizes reproductive success (Williams 1966, Roff 1992, Stearns 1992). A number of studies have shown that when the size of first broods is experimentally increased, females are less likely to initiate subsequent broods or may delay initiation (Slagsvold 1984, Tinbergen & Daan 1990, Verhulst & Hut 1996). Male and female optima for the timing and number of subsequent clutches might be expected to differ, although these parameters are probably under female control.
The energetic and time constraints associated with multiple breeding efforts can carry over into other important life-history phenomena. Plumage colour can result either from pigments deposited in feathers or from the light interacting with the microstructures of feathers. Although many studies have shown that pigment-based colouration of birds commonly functions as an honest indicator of quality (reviewed in Hill 2006), recent studies indicate that structural colouration can also serve as a condition-dependent signal of individual quality (McGraw et al. 2002, Johnsen et al. 2003, Hill et al. 2005) and suggest that environmental perturbations could interfere with the precise arrangement of tissues at a nanostructure scale, thus affecting colouration (Shawkey et al. 2003, 2005).
Few studies have experimentally tested the effects of specific environmental variables on expression of female plumage colouration. The experimental studies to date suggest that patterns of condition dependency in female plumage traits mirror patterns of condition dependency in the equivalent male traits (reviewed in Amundsen & Pärn 2006). Indeed, female Common Eiders Somateria mollissima that experience greater mass loss or show decreased immunosuppression (or both) during the breeding season produce plumage with reduced ornamentation during the following moult (Hansson et al. 2006).
Eastern Bluebirds Sialia sialis are good subjects for a study of the reproductive investment strategies of males and females because they readily use nestboxes, breed repeatedly in the same location and tolerate considerable disturbance at the nest. In the southern part of their range, Eastern Bluebirds lay small clutches of eggs (mode = 4), and hatching is synchronous. Most pairs rear two broods per season (Gowaty & Plissner 1998), individuals are not migratory (L. Siefferman unpubl. data), and there is no difference in the annual survival of males and females (Plissner & Gowaty 1996). Eastern Bluebirds are socially monogamous, but extra-pair paternity is common, accounting for 40% of offspring in one population (Gowaty & Karlin 1984). Male Bluebirds assist in nest defence and feed nestlings and fledglings (Pinkowski 1978). After the female initiates a second clutch, the male becomes the primary provider of post-fledgling care of the first brood (L. Siefferman pers. obs.), and the interval between clutches is longer after rearing larger broods (Pinkowski 1977).
Males exhibit non-iridescent structural blue colouration over the back, head, wings and tail, and females show the same basic colour pattern but are substantially duller in colour, being greyish-blue where males are blue. Sexual selection is probably responsible for driving the elaboration of structural plumage colouration in male and female Eastern Bluebirds. Females and males that express brighter and more saturated UV-blue plumage pair earlier, feed chicks more often and have higher reproductive success (Siefferman & Hill 2003, 2005a). Plumage colouration mediates male–male competitions for breeding territories (Siefferman & Hill 2005b). Experimental manipulations of food availability suggest that structurally based plumage colouration is a condition-dependent trait in female Eastern Bluebirds (Siefferman & Hill 2005a). An annual moult occurs after the breeding season (late summer to early autumn), and there is no difference between the sexes in the time of onset or length of moult (Gowaty & Plissner 1998).
In this study we experimentally manipulated the size of Eastern Bluebird broods to investigate the effects on (1) the likelihood and timing of subsequent broods, (2) the characteristics of subsequent clutches (clutch size, hatching success, fledging success), (3) the number of nestlings reared over the season, (4) the survival of male and female parents, and (5) the relative plumage ornamentation of females in the subsequent year. In a previous study we showed that both females and males increase provisioning rates when given experimentally enlarged broods and considered the effects of brood-size manipulation on ornamental colouration in males (Siefferman & Hill 2005c). Although studies have investigated the likelihood of producing second broods in relation to manipulation of brood size, few studies have reported survival of adults or tested the effects of reproductive effort on female ornamentation.
METHODS
We studied a colour-marked population of Eastern Bluebirds on an 8-km2 study site from March to August 1999–2002 in Lee County, Alabama, USA (32°35′N, 82°28′W). All Bluebirds in this study population nested in wooden boxes that were erected and maintained by the authors. Adults were captured with mist-nets in early spring before eggs hatched. Each bird was marked with a unique combination of three coloured leg rings and one metal US Fish and Wildlife Service ring.
In 2000 and 2001, we enlarged or reduced the size of broods. We paired nests with 3–5 eggs and a common hatch date. Within each of 44 pairs of nests, we randomly selected one nest to have an enlarged brood and the other to have a reduced brood. We cross-fostered nestlings on the second day after hatching; enlarged broods were given two additional nestlings and reduced nests had two nestlings removed (mean ± sd chicks per nest; reduced broods = 2.2 ± 0.5, enlarged broods = 5.6 ± 0.6). The mean age at which nestlings fledged in this population was 17 days (L. Siefferman unpubl. data). The brood-size manipulation influenced parental feeding rates such that pairs that reared enlarged broods provisioned broods twice as often as pairs that reared reduced broods, but the proportional share of feedings by males and females was equal and did not vary with brood size (Siefferman & Hill 2005c).
We recorded whether each pair produced a further clutch following the manipulated brood. Pairs that initiated laying their first clutches after May 15 were excluded from analyses of second breeding attempts as there might not have been enough time remaining in the season to produce a second brood (L. Siefferman pers. obs.). The interval to initiation of the second clutch was estimated as the number of days from the date on which the first brood hatched to the date at which the first egg was laid in the next clutch by that female. Clutch size, brood size and the number of nestlings that fledged from the nests that followed brood-size manipulations were also recorded.
Over-winter survival of parents was assessed by whether or not individuals returned to the study site the following year. Because return rates in consecutive years are the product of true survival, site fidelity, breeding propensity and detection rate, one must interpret return rates carefully. Bluebirds are dependent on nestboxes and show strong fidelity to specific boxes, which makes the probability of detecting individuals high (Keyser et al. 2004). Eastern Bluebirds have high breeding-site fidelity, with most individuals returning to the same nest-site in subsequent years (Gowaty & Plissner 1998). As a result of an intensive capture/re-sighting protocol, the identity of the nesting adults in each year was known and the entire breeding population was censused each year. Because the study area was large, covering 8 km2 with a linear distance of 20 km between some parts of the study area, rates of adult nest-site fidelity could be estimated. During our 8-year study of this population, only three of 302 adult birds moved more than 500 m between breeding seasons and no birds moved more than 1.5 km. Furthermore, in 8 years only one adult bred in one year, disappeared in the following year and reappeared to breed in a third year. These observations support the notion that a very high proportion of surviving adults return to their breeding site of the previous year and thus we assumed that return rates are a good proxy for survival.
Because of sample size restriction, no pairs were monitored as controls during 2000 and 2001, although we did collect data from unmanipulated broods in 1999. The results of brood-size manipulation on adult survival are complex, so in post-hoc analyses we used 1999 as a control for the 2000 and 2001 manipulations.
At the time of capture, feather samples were collected from females for colour analysis. We measured plumage reflectance from eight rump feathers of each individual with an Ocean Optics S2000 spectrometer, a deuterium tungsten halogen light source (range 250–880 nm) and a Labsphere white standard following the methods of Siefferman and Hill (2003). We collected rump feathers because the colouration of this body region is known to be influenced by nutritional stress (Siefferman & Hill 2005a) and because colouration in this body region correlates strongly with that in other blue body regions (L. Siefferman unpubl. data). Reflectance data were summarized by calculating three standard descriptors of reflectance spectra. Brightness was estimated as the summed reflectance from 300 to 700 nm. UV chroma was estimated as the proportion of the total reflectance of the entire spectrum made up by total reflectance in the ultraviolet range (∫300–400/∫300–700 nm). Hue was calculated as the wavelength (nm) corresponding to maximal reflectance (λmax). Past research on this species indicated that the more ornamented birds have higher brightness, greater UV chroma and lower hue (peak reflectance at shorter wavelengths).
Statistical analysis
Over the course of 2 years, we manipulated the brood sizes of 88 nests of Eastern Bluebirds, but sample sizes for measures differ because some nests were lost to predators. We failed to capture two females and four males, and did not obtain fledgling mass for all manipulated nests. Birds that lost nests to predation were not included in the analyses. No birds had their broods manipulated in more than one year. We combined the data from 2000 and 2001 because analysis of variance indicated no effects of year on within-season re-nesting parameters or return rates (P > 0.10 in all cases). However, there was a significant effect of year on plumage colouration (P < 0.001 for all three colour parameters), and those data were standardized for year (mean = 0, sd = ±1). We tested for normality using Shapiro–Wilk tests, and used parametric tests when data were normally distributed. When data were not normally distributed, non-parametric tests were used. SPSS (version 11.5 Chicago, IL, USA) software was used to analyse the data, and all statistical tests were two-tailed (with the exception of one-tailed Fisher exact tests). Prior to the experiment, treatment group was not related to clutch size (Mann–Whitney U43,39 = 807, P = 0.58).
RESULTS
Second nesting attempts
There was a significant effect of the brood-size manipulation on the likelihood of repeat nesting. Eighty-two per cent of pairs with reduced broods produced a second clutch (23 of 28) whereas only 54% of pairs with enlarged broods produced a second clutch (15 of 28; Fisher exact test, P = 0.02). Of the pairs that produced a second clutch following the experiment, those that reared an enlarged brood were slower to re-nest than those that reared a reduced brood (Table 1). However, there was no effect of brood manipulation on clutch size, brood size or average fledgling mass of second clutches, and the number of offspring fledged from second nests did not differ between treatment groups (Table 1).
Table 1.
Effects of brood manipulation on interclutch interval, second brood characteristics (unmanipulated), overall seasonal fecundity and between-year differences in standardized female plumage. Means are given with se and samples sizes in parentheses.
| Response to treatment | Reduced* | Enlarged† | Statistic | P |
|---|---|---|---|---|
| Interclutch interval (days) | 28.3 ± 1.2 (23) | 37.1 ± 3.2 (15) | t = 3.0 | 0.005 |
| Clutch size of 2nd nest | 4.26 ± 0.13 (23) | 4.27 ± 0.18 (15) | U = 170 | 0.75 |
| Brood size of 2nd nest | 2.74 ± 0.36 (23) | 2.73 ± 0.47 (15) | U = 169 | 0.98 |
| No. of nestlings fledged from 2nd nest | 2.01 ± 0.38 (23) | 1.60 ± 0.50 (15) | U = 143 | 0.38 |
| Total nestlings reared | 5.86 ± 0.42 (44) | 7.56 ± 0.35 (39) | t = 3.1 | 0.003 |
| Change in female plumage brightness | − 0.32 ± 0.37 (13) | − 0.12 ± 0.36 (8) | t = 0.4 | 0.73 |
| Change in female plumage UV chroma | 0.11 ± 0.31 (13) | − 0.12 ± 0.31 (8) | t = 0.5 | 0.63 |
| Change in female plumage hue | − 0.17 ± 0.31 (13) | 0.16 ± 0.50 (8) | t = 0.6 | 0.57 |
Two chicks removed.
Two chicks added.
Annual fledgling success
To determine whether the brood-size manipulation affected the overall number of nestlings that fledged in the year of the manipulation, we calculated the total number of nestlings that lived to day 14 post-hatch from pairs that reared enlarged and reduced broods. Pairs that reared enlarged broods fledged more nestlings during the breeding season than did pairs that reared reduced broods (Table 1), indicating that the brood-size manipulation influenced the annual workload of the parents.
Parental return rates
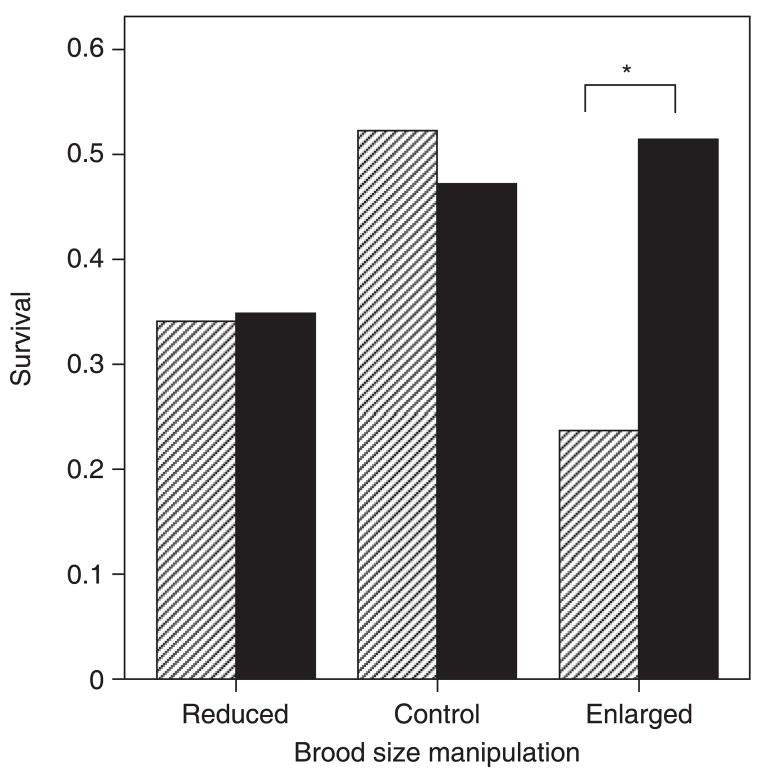
We compared the return rate of parents that reared enlarged broods with that of parents that reared reduced broods to determine whether the brood-size manipulation affected the likelihood that parents would survive to the following breeding season. Thirty-five per cent of males (n = 15) and 34% of females (n = 15) that reared reduced broods returned to breed in the next breeding season, whereas 51% of males (n = 19) and 24% of females (n = 9) that reared enlarged broods returned. To determine whether return rates were influenced by sex, treatment, the number of nests or number of nestlings reared, we used binomial regression. In this model, in which independent variables included parental sex and brood-size manipulation (treatment), we tested for a significant interaction between the sex and treatment, and covariates included the number of young reared and number of broods produced. We found a nearly significant interaction between sex and treatment (Wald statistic = 3.5, P = 0.06, Fig. 1). The binomial regression also showed that, overall, males were more likely to survive than females (Wald statistic = 6.2, P = 0.01), and there was a tendency for treatment to influence parental survival such that parents that reared enlarged broods were more likely to survive (Wald statistic = 3.5, P = 0.06). There was no significant effect of the number of nestlings reared (Wald statistic = 1.1, P = 0.29) or the number of clutches produced (Wald statistic = 0.6, P = 0.45) on return rate.
Figure 1.
Survival rates of male and female Eastern Bluebirds according to experimental treatment and sex (solid bars, males; hatched bars, females); *P < 0.05.
Experimental treatment did not affect male or female return rates (Fig. 1). A comparison of the return rates of males and females that reared enlarged broods indicated that females were less likely to survive than males (Fisher exact test, P = 0.01, Fig. 1). Among birds that raised reduced broods, however, there was no difference in the survival of males and females to the year following the manipulation (P = 0.56, Fig. 1).
The added costs of producing and incubating second clutches may have contributed to increased mortality rates of females that reared enlarged broods. Binomial regression was used to determine whether the return rates of males and females that reared enlarged broods were influenced by whether they produced a second brood. Producing a second brood influenced neither female (Wald statistic = 1.6, P = 0.21) nor male (Wald statistic = 1.5, P = 0.23) return rates.
During the 1999 breeding season, brood sizes were not manipulated. In 1999, we ringed 42 adult females and 36 adult males, and we recaptured 22 females (52%) and 17 males (47%) in 2000. A comparison of the return rates of control males and females showed no significant sex difference in survival (Fisher exact test, P = 0.82, Fig. 1). Moreover, a comparison of adults that reared enlarged and reduced broods in 2000 and 2001 with adults that reared control broods in 1999 demonstrated a significant interaction between sex and treatment on survival (multinomial regression: χ2 = 11.0, P = 0.05, Fig. 1). The treatment influenced survival of females but not males (Pearson’s χ2, females: P = 0.03, males: P = 0.30). Post-hoc analysis of female survival suggested that females that reared enlarged broods suffered greater mortality than control females (Fisher exact test, P = 0.008, Fig. 1). Moreover, there was a non-significant tendency for females that reared reduced broods to suffer greater mortality than control females (Fisher exact test, P = 0.07).
Female plumage colouration
We assessed whether the change in plumage colour from the year of the manipulation to the year following the manipulation (colour in year following manipulation – colour during manipulation year) differed between the females that reared enlarged and reduced broods. There was no effect of brood-size manipulation on change in plumage colouration between the surviving females that had reared enlarged or reduced broods (Table 1). Moreover, although the sample sizes were small, the 95% confidence intervals suggested that the experiment did not influence female colouration (95% CLs: brightness, − 1.35 to 0.96; UV chroma, − 0.74 to 1.19; hue − 1.47 to 0.83).
DISCUSSION
When the size of broods of Eastern Bluebirds was increased, there was evidence that increasing reproductive effort led to differential mortality of males and females. After rearing enlarged broods, females were less likely to survive than males. In fact, comparison of female survival after rearing manipulated broods with survival after rearing broods of natural sizes suggested that both increasing and decreasing brood size reduced female, but not male, survival. In contrast to males, which showed a significant reduction in plumage colouration in the year following brood enlargement (Siefferman & Hill 2005c), surviving females did not experience a reduction in plumage ornamentation after rearing enlarged broods. Brood-size manipulation influenced parental feeding rates; pairs that reared enlarged broods provisioned broods more often than pairs that reared reduced broods and the proportional share of maternal and paternal provisioning did not vary with brood size (Siefferman & Hill 2005c). By forcing parents to invest more in the first brood, we revealed a within-season trade-off of time or energy allocation; females that reared enlarged broods were less likely to lay subsequent clutches, and those that did lay second clutches were slower to do so.
Males may be less willing to risk increased mortality as a consequence of increased parental effort than females because their paternity is not assured. Moreover, males may balance allocation of time and energy for parental care with time and energy expended in pursuit of extra-pair copulations (Trivers 1972, Magrath & Komdeur 2003). The differential survival of females and males after rearing enlarged broods suggests that costs or benefits of clutch size differ between the sexes. In studies of other species of songbirds that experimentally reduced the contribution to nestling care by one partner, females compensated more than males for reduced help and showed greater deterioration of body condition as a consequence (Sanz et al. 2000, Velando & Alonso-Alvarez 2003). Particularly in species in which extra-pair paternity is common, females may be more willing than males to invest more heavily in enlarged broods (Westneat et al. 1990). Future work should test the costs and benefits to allocating time and energy towards parental care vs. extra-pair paternity in male Eastern Bluebirds.
It appears that females compensate for the extra energy or time invested in raising enlarged broods by either not laying a second clutch or by delaying the initiation of the second clutch. Slowing down or halting further reproduction in a season following a demanding first brood could be the result of increased duration of post-fledging care of larger broods, a depletion of the energy reserves of parents or a combination of both (Verhulst & Hut 1996). However, it is also possible that parents were less likely to produce a subsequent brood because of strategic adjustments based on the number of young produced (Verhulst & Tinbergen 1997). Unfortunately, our data did not allow us to differentiate directly between these alternative hypotheses. Although the likelihood and timing of producing a subsequent brood was affected by brood manipulation, the subsequent clutch size, brood size and fledgling success of second clutches were not affected. This suggests that females produce second clutches only when they have adequate energy and time reserves and/or adequate food resources and habitat quality to lay, incubate and provision a second clutch (Meijer & Drent 1999).
Differential return rates of females and males after rearing enlarged broods suggested that females pay larger mortality costs than males. The return rates of females that reared enlarged broods, however, were not influenced by whether they produced a second brood. It is likely that only females in exceptional body condition or with high-quality territories attempted to lay second clutches and that those females could do so without jeopardizing survival. It may be that females in lower body condition or with lower quality habitats would not have produced multiple clutches even under control conditions (Den Boer-Hazenwinkel 1987, Winkel & Winkel 1995). Our data on differential mortality of males and females corroborate the observations of Verhulst (1998), in which experimental removal of second clutches in years of low food availability improved female, but not male, survival in Great Tits. However, our estimates of survival could be biased if females that reared reduced broods experienced greater between-season breeding philopatry than females that reared enlarged broods. Dispersal of adult Eastern Bluebirds is more likely to occur after total nest failure (Gowaty & Plissner 1998). If females that reared smaller broods were more likely to disperse than females that reared larger broods, then our measure of female survival is conservative. Increased dispersal after rearing reduced broods, however, could explain the tendency of females to experience lower return rates than control females. Indeed, compared with controls, females showed reduced return rates after rearing enlarged or reduced broods. Males did not show a concurrent effect, suggesting that females may be more sensitive to brood manipulation than males.
Differential mortality between sexes as a consequence of brood-size manipulation suggests a sexual conflict with regard to optimal brood size decisions; it appears to be more profitable for males to rear larger broods than females. However, even though males were more likely than females to survive after rearing enlarged broods, the experimental manipulation had consequences for the future ornamentation of males that we did not detect in females. Males that reared enlarged broods suffered a reduction in the brightness of blue/UV structural plumage colouration (Siefferman & Hill 2005c). The effect of brood-size manipulation on the change in female plumage colouration between the treatment groups was not statistically significant. Although the sampling of surviving males was higher than surviving females, we could be relatively confident that there was no biologically relevant effect on female colouration. First, our confidence intervals suggest that even a much larger sample size would have yielded similar results, and secondly, an aviary-based experiment demonstrated an effect of nutritional stress on rump colouration with a smaller sample of females (Siefferman & Hill 2005a). It does appear, however, that any effect of the brood-size manipulation on female plumage was much weaker than that found in males (Siefferman & Hill 2005b). Structural colouration in male Bluebirds appears to play an important role in pairing and territory acquisition (Siefferman & Hill 2003, Siefferman & Hill 2005b), and males that returned with less colourful plumage tended to nest later (Siefferman & Hill 2005b). Thus, although males were more likely to survive than females after rearing enlarged broods, male Bluebirds did experience residual effects of rearing enlarged broods, as it affected their ability to secure mates in future years (Siefferman & Hill 2005b). In Eastern Bluebirds, females that display structural colouration with greater UV chroma and shorter wavelength hue provided more maternal care and experienced greater reproductive success (Siefferman & Hill 2005a), suggesting that colouration in females also plays a role in sexual selection. Moreover, food deprivation in females reduced these aspects of the structural colouration (Siefferman & Hill 2005a). It is curious, then, that food deprivation reduced structural colouration in females in a previous experiment but that brood-size manipulation did not influence female plumage colour in this study. This discrepancy may be the consequence of the overall high mortality rates of females that reared enlarged broods coupled with the possibility that only high-quality females returned. The low-quality females that could have had their plumage negatively affected by the brood enlargements may have died or been unable to acquire nestboxes.
An alternative explanation for the sex-specific effects of brood-size manipulation on survival and change in plumage colouration could be effects or interactions of the treatment on the moult schedules of males and females. In the Blue Tit Cyanistes caeruleus, males began moulting earlier than females and were less able to provision larger broods during moult, and both sexes suffered the consequences of this moult–breeding overlap (Svensson & Nilsson 1997). However, male and female Bluebirds begin moulting at the same time (Gowaty & Plissner 1998, our pers. obs.). Irrespective of the causes, the sex-specific effects of the brood-size manipulation on return rates and ornamentation suggest that male and female Bluebirds differ in strategy and consequences of manipulated reproductive investment.
Our study demonstrates that, in the Eastern Bluebird, there are trade-offs between investment in first broods and investment in second broods within the same season as well as residual reproductive investment in future years. Male and female Bluebirds appear to differ in the strategies by which they invest in reproduction, emphasizing the importance of comprehensive measures of residual reproductive value in studies of life-history evolution.
Acknowledgments
We are grateful to E. Gering for help with fieldwork. Comments from D. Broussard, J. McGlothlin, K. Navara, R. Nager, J. K. Nooker, S. Verhulst and anonymous reviewers greatly improved this manuscript. This research was conducted according to an animal use permit from Auburn University, ringing permits to G.E.H., and complies with the laws of the USA. The research was funded by NSF grants IBN 9722171, IBN 0235778 and DEB 0077804 to G.E.H., a CDC grant 5 R01 CI000226-03 and an NSF-NIH grant R01-AI49724 to G.E.H., and grants from the American Ornithologists’ Union, the Animal Behavior Society, the American Museum of Natural History and the Birmingham Audubon Society to L.S.
References
- Amundsen T, Pärn H. Female colouration: review of functional and nonfunctional hypotheses. In: Hill GE, McGraw KJ, editors. Bird Colouration, Vol. 1: Mechanisms and Measurements. Cambridge, MA: Harvard University Press; 2006. pp. 280–348. [Google Scholar]
- Andersson M. Sexual Selection in Animals. Princeton, NJ: Princeton University Press; 1994. [Google Scholar]
- Den Boer-Hazenwinkel J. On the costs of reproduction: parental survival and reproduction of second clutches in the Great Tit. Ardea. 1987;75:99–110. [Google Scholar]
- Gowaty PA, Karlin AA. Multiple paternity and paternity in single broods of monogamous Eastern Bluebirds (Sialia sialis) Behav Ecol Sociobiol. 1984;15:91–95. [Google Scholar]
- Gowaty PA, Plissner J. Eastern Bluebird, Sialia sialis. In: Poole A, Gill F, editors. The Birds of North America. Vol. 381. Philadelphia, PA: Academy of Natural Sciences; Washington, DC: American Ornithologists’ Union; 1998. [Google Scholar]
- Gustafsson L, Pärt T. Acceleration of senescence in the collared flycatcher Ficedula albicollis by reproductive costs. Nature. 1990;347:279–281. [Google Scholar]
- Gustafsson L, Qvarnström A, Sheldon BC. Trade-off between life-history traits and a secondary sexual character in male collared flycatchers. Nature. 1995;375:311–313. [Google Scholar]
- Hansson SA, Folstad I, Erikstad KE. White plumage reflects individual quality in female Eiders. Anim Behav. 2006;71:337–343. [Google Scholar]
- Hill GE. Environmental regulation of ornamental colouration. In: Hill GE, McGraw KJ, editors. Bird Colouration, Vol. I: Mechanisms and Measurements. Cambridge, MA: Harvard University Press; 2006. pp. 507–560. [Google Scholar]
- Hill GE, Doucet SM, Buchholz R. The effect of coccidial infection on iridescent plumage colouration in wild turkeys. Anim Behav. 2005;69:387–394. [Google Scholar]
- Johnsen A, Delhey K, Andersson S, Kempenaers B. Plumage colour in nestling blue tits: sexual dichromatism, condition dependence and genetic effects. Proc R Soc Lond B. 2003;270:1263–1270. doi: 10.1098/rspb.2003.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser AJ, Keyser MT, Promislow DEL. Life-History variation and demography of Western Bluebirds (Sialia mexicanus) in Oregon. Auk. 2004;121:118–133. [Google Scholar]
- Magrath MJL, Komdeur J. Is male care compromised by additional mating opportunity? Trends Ecol Evol. 2003;18:424–430. [Google Scholar]
- McGraw KJ, Mackillop EA, Dale J, Hauber M. Different colours reveal different information: how nutritional stress affects the expression of melanin- and structurally based ornamental plumage. J Exp Biol. 2002;20:3747–3755. doi: 10.1242/jeb.205.23.3747. [DOI] [PubMed] [Google Scholar]
- Meijer T, Drent R. Re-examination of the capital versus income dichotomy breeding in birds. Ibis. 1999;141:399–414. [Google Scholar]
- Nager RG, Monaghan P, Houston DC. The cost of egg production: increased egg production reduces future fitness in gulls. J Avian Biol. 2001;32:159–166. [Google Scholar]
- Norris DR, Marra PP, Montgomerie R, Kyser TK, Ratcliffe LM. Reproductive effort, molting latitude, and feather colour in a migratory songbird. Science. 2004;306:2249–2250. doi: 10.1126/science.1103542. [DOI] [PubMed] [Google Scholar]
- Pinkowski B. Breeding adaptations in the Eastern Bluebird. Condor. 1977;79:289–302. [Google Scholar]
- Pinkowski B. Feeding of nestling and fledgling Eastern Bluebirds. Wilson Bull. 1978;90:84–98. [Google Scholar]
- Plissner JH, Gowaty PA. Patterns of natal dispersal, turnover and dispersal costs in Eastern Bluebirds. Anim Behav. 1996;51:1307–1322. [Google Scholar]
- Roff DA. The Evolution of Life Histories; Theory and Analysis. New York: Chapman & Hall; 1992. [Google Scholar]
- Sanz JJ, Kranenbarg S, Tinbergen JM. Differential response by males and females to manipulation of partner contribution in the Great Tit (Parus major) J Anim Ecol. 2000;69:74–84. [Google Scholar]
- Shawkey MD, Estes AM, Siefferman L, Hill GE. Nanostructure predicts intraspecific variation in structural plumage colour. Proc R Soc Lond B. 2003;270:1455–1460. doi: 10.1098/rspb.2003.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawkey MD, Estes AM, Siefferman L, Hill GE. The anatomical basis for sexual dichromatism in non-iridescent ultraviolet-blue colouration of feathers. Biol J Linn Soc. 2005;84:259–271. [Google Scholar]
- Siefferman L, Hill GE. Structural and melanin plumage colouration indicate parental effort and reproductive success in male Eastern Bluebirds. Behav Ecol. 2003;14:855–861. [Google Scholar]
- Siefferman L, Hill GE. Evidence for sexual selection on structural plumage colouration in female Eastern Bluebirds (Sialia sialis) Evolution. 2005a;59:1819–1828. [PubMed] [Google Scholar]
- Siefferman L, Hill GE. UV-blue structural colouration and competition for nest boxes in male Eastern Bluebirds. Anim Behav. 2005b;69:67–72. [Google Scholar]
- Siefferman L, Hill GE. Male Eastern Bluebirds trade future ornamentation for current reproductive investment. Biol Lett. 2005c;1:208–211. doi: 10.1098/rsbl.2004.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagsvold T. Clutch size variation of birds in relation to nest predation: on the cost of reproduction. J Anim Ecol. 1984;53:945–953. [Google Scholar]
- Stearns SC. The Evolution of Life Histories. Oxford: Oxford University Press; 1992. [Google Scholar]
- Svensson E, Nilsson JA. The trade-off between molt and parental care: a sexual conflict in the blue tit? Behav Ecol. 1997;8:92–98. [Google Scholar]
- Tinbergen JM, Daan S. Family planning in the Great Tit (Parus major): optimal clutch size as integration of parental and offspring fitness. Behaviour. 1990;114:161–190. [Google Scholar]
- Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual Selection and the Descent of Man. Chicago: Adeline Press; 1972. pp. 136–179. [Google Scholar]
- Velando A, Alonso-Alvarez C. Differential body condition regulation by males and females in response to experimental manipulations of brood size and parental effort in the blue-footed booby. J Anim Ecol. 2003;72:846–856. [Google Scholar]
- Verhulst S. Multiple breeding in the Great Tit. II The cost of rearing a second clutch. Funct Ecol. 1998;12:132–140. [Google Scholar]
- Verhulst S, Hut RA. Post-fledgling care, multiple breeding and the costs of reproduction in the Great Tit. Anim Behav. 1996;51:957–966. [Google Scholar]
- Verhulst S, Tinbergen JM. Clutch size and parental effort in the Great Tit Parus major. Ardea. 1997;85:111–126. [Google Scholar]
- Westneat DF, Sherman PW, Morton ML. Ecology and evolution of extra-pair copulations in birds. In: Power DM, editor. Current Ornithology. Vol. 7. New York: Plenum Press; 1990. pp. 331–369. [Google Scholar]
- Williams GC. Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am Nat. 1966;100:687–690. [Google Scholar]
- Winkel W, Winkel D. Kosten und Nutzen von Zweitbruten bei der Tannenmeise (Parus ater) J Ornithol. 1995;136:29–36. [Google Scholar]