Abstract
A consensual, componential model of emotions conceptualises them as experiential, physiological, and behavioural responses to personally meaningful stimuli. The present review examines this model in terms of whether different types of emotion-evocative stimuli are associated with discrete and invariant patterns of responding in each response system, how such responses are structured, and if such responses converge across different response systems. Across response systems, the bulk of the available evidence favours the idea that measures of emotional responding reflect dimensions rather than discrete states. In addition, experiential, physiological, and behavioural response systems are associated with unique sources of variance, which in turn limits the magnitude of convergence across measures. Accordingly, the authors suggest that there is no “gold standard” measure of emotional responding. Rather, experiential, physiological, and behavioural measures are all relevant to understanding emotion and cannot be assumed to be interchangeable.
Keywords: Emotion, Measurement, Self-report, Autonomic nervous system, Startle modulation, Central nervous system, Behaviour, Specificity
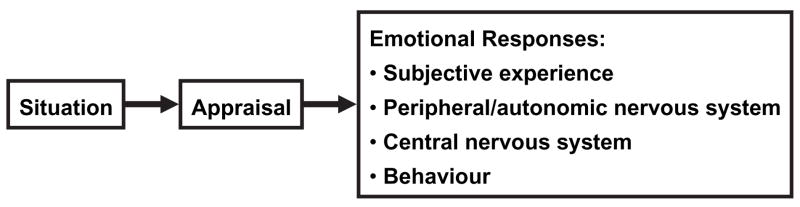
From an intuitive layperson perspective, it should be easy to determine whether someone is experiencing a particular emotion. However, scientific evidence suggests that measuring a person’s emotional state is one of the most vexing problems in affective science. To organise our review of research relevant to this question, we take as our starting point a consensual, componential model of emotion (see Figure 1). In this model, an emotional response begins with appraisal of the personal significance of an event (Lazarus, 1991; Scherer, 1984; Smith & Ellsworth, 1985), which in turn gives rise to an emotional response involving subjective experience, physiology, and behaviour (Frijda, 1988; Gross, 2007; Lang, 1988; Larsen & Prizmic-Larsen, 2006) The present review examines whether emotion-evocative stimuli are associated with discrete patterns of responding in each system, how such responses seem to be structured, and if such responses converge (i.e., are co-ordinated or correlated) with one another.
Figure 1.
A consensual component model of emotional responding.
Because the literatures that are relevant to the questions examined here are extensive, the present review must be selective. In our review, we concentrate on studies involving non-clinical human adult samples rather than children, animals, or clinical populations. We focus on the response components depicted in Figure 1 rather than on cognitive antecedents and correlates of emotion. To further constrain the scope of our review, we focus on emotional states rather than emotion-related traits such as extraversion and neuroticism (see Matthews & Gilliland, 1999; Robinson & Neighbors, 2006; Rusting, 1998, for relevant reviews). Finally, we focus our review on the most commonly used measures for each response system.
Throughout our review, we examine findings from both dimensional and discrete perspectives. According to the dimensional perspective, there are a few fundamental dimensions that organise emotional responses. The most commonly assumed dimensions are valence, arousal (sometimes referred to as activation), and approach–avoidance (Davidson, 1999; Lang, Bradley, & Cuthbert, 1997; Russell & Barrett, 1999; Schneirla, 1959; Watson, Wiese, Vaidya, & Tellegen, 1999). The valence dimension contrasts states of pleasure (e.g., happy) with states of displeasure (e.g., sad), and the arousal dimension contrasts states of low arousal (e.g., quiet) with states of high arousal (e.g., surprised). Approach motivation is characterised by tendencies to approach stimuli (e.g., as would likely be facilitated by excitement), whereas avoidance motivation is characterised by tendencies to avoid stimuli (e.g., as would likely be facilitated by anxiety).
Researchers disagree to some extent about which dimensional scheme should be used and how different dimensions relate to each other. For example, some theorists state that positive and negative emotions are inversely related (Russell, 1980), but others favour the view that positive and negative emotions are relatively independent of each other (Larsen, McGraw, & Cacioppo, 2001; Tellegen, Watson, & Clark, 1999). In addition, some argue that approach and avoidance are more or less synonymous with positive and negative emotional states, respectively (Watson et al., 1999). However, as we outline below, some emotional states such as anger pose problems for this view, in that they suggest a dissociation of valence and approach–avoidance (Harmon-Jones & Allen, 1998). More generally, our review will make it clear that different measures of emotion are particularly sensitive to different dimensions; thus, for different measures different dimensional schemes are most appropriate. Although dimensional frameworks disagree in some of their specifics, they agree that emotional states can be organised in terms of a limited number of underlying dimensions.
In contrast, the discrete emotions perspective contends that each emotion (e.g., anger, sadness, contempt) corresponds to a unique profile in experience, physiology, and behaviour (Ekman, 1999; Panksepp, 2007). It is possible to reconcile dimensional and discrete perspectives to some extent by proposing that each discrete emotion represents a combination of several dimensions (Haidt & Keltner, 1999; Smith & Ellsworth, 1985). For example, anger could be characterised by negative valence, high arousal, and approach motivation, whereas fear could be characterised by negative valence, high arousal, and avoidance motivation. Despite these considerations, dimensional and discrete perspectives differ in how they conceptualise and describe emotional states (Barrett, 2006b). For this reason, we contrast such perspectives in our review.
To guide the reader, Table 1 presents an overview of the measures reviewed for each response system depicted in the consensual model of Figure 1. Table 1 also summarises our conclusions concerning the aspects of emotional state best captured by each measure. We begin by reviewing self-report measures of emotion.
TABLE 1.
Overview of response systems, measures, and emotional states to which they are sensitive
| Response system | Measure | Sensitivity |
|---|---|---|
| Subjective experience | Self-report | Valence and arousal |
| Peripheral physiology (ANS) | Autonomic nervous system (ANS) measures | Valence and arousal |
| Affect-modulated startle | Startle response magnitude | Valence, particularly at high levels of arousal |
| Central physiology (CNS) | EEG | Approach and avoidance |
| fMRI, PET | Approach and avoidance | |
| Behaviour | Vocal characteristics: Amplitude, pitch | Arousal |
| Facial behaviour: Observer ratings | Valence; some emotion specificity | |
| Facial behaviour: EMG | Valence | |
| Whole body behaviour: Observer ratings | Some emotion specificity |
SELF-REPORT MEASURES OF EMOTION
In our view, the validity of self-reports of emotion is too often seen as an all-or-none phenomenon. Here, we follow Robinson and Clore (2002), who concluded that the degree to which self-reports are valid varies by the type of self-report (see also Robinson & Sedikides, in press). Specifically, self-reports of current emotional experiences are likely to be more valid than are self-reports of emotion made somewhat distant in time from the relevant experience (Robinson & Clore, 2002). In a very interesting study, for example, Barrett, Robin, Pietromonaco, and Eyssell (1998) asked men and women to report on their emotional traits “in general” as well as on their emotional reactions to events in daily life. Sex differences in emotional traits were prominent and large, whereas sex differences in daily experience were quite meagre and inconsistent, suggesting that trait reports of emotion are more biased (in this case by gender stereotypes) than reports made directly after an event. Conceptually similar findings have been reported when asking individuals to estimate their past or likely future responses to emotional events (e.g., Mitchell, Thompson, Peterson, & Cronk, 1997) On the basis of such evidence for bias, Robinson and Clore concluded that self-reports of one’s current experience (“online”) are likely to be more valid than self-reports concerning past, future, or trait-related experiences of emotion.
However, there are concerns that even “online” reports of emotion can be biased among certain groups of individuals. For example, it is thought that individuals high in social desirability may be less willing and/or capable of reporting negative emotional states (Paulhus & Reid, 1991; Welte & Russell, 1993). Although this suggestion has proven somewhat controversial (Shedler, Mayman, & Manis, 1993; Taylor, Lerner, Sherman, Sage, & McDowell, 2003), there are still concerns that individuals high in social desirability may give less valid reports of their emotions (Paulhus & John, 1998). A second relevant individual-difference variable is alexithymia. It has been suggested that individuals high in alexithymia react to emotional stimuli, but are less capable of conceptualising their emotional experiences in a manner conducive to self-report (Lane, Ahern, Schwartz, & Kaszniak, 1997). In sum, there are individual differences in awareness of and willingness to report on emotional states that potentially compromise even online reports of emotional experience.
Finally, one purpose of our review is to compare dimensional and discrete perspectives of emotional responding. In the domain of self-reported emotional states, it is quite clear that dimensions such as valence and arousal (Russell & Barrett, 1999) or tendencies toward approach and avoidance (Watson et al., 1999) capture the lion’s share of variance. Indeed, the dimensional nature of self-reported emotional responses is so substantial that it has been suggested that the dimensional correlates of self-reported emotion be examined first before there is any legitimate claim to emotion specificity (Watson, 2000).
Summary
Self-reports of emotion are likely to be more valid to the extent that they relate to currently experienced emotions. Even in this case, though, there are concerns that not all individuals are aware of and/or capable of reporting on their momentary emotional states. Finally, Table 1 follows from our review of this literature in suggesting that dimensional frameworks, relative to discrete ones, better capture this measure of emotion.
AUTONOMIC MEASURES OF EMOTION
The autonomic nervous system (ANS) is a general-purpose physiological system responsible for modulating peripheral functions (Öhman, Hamm, & Hugdahl, 2000). This system consists of sympathetic and parasympathetic branches, which are generally associated with activation and relaxation, respectively. Because of the general-purpose nature of the ANS, its activity is not exclusively a function of emotional responding, but rather encompasses a wide variety of other functions related to digestion, homeostasis, effort, attention, and so forth (Berntson & Cacioppo, 2000). This is an important point because it is often not clear whether activity in the ANS reflects emotional processes or, perhaps instead, other functions subserved by the ANS (Obrist, Webb, Sutterer, & Howard, 1970; Stemmler, 2004).
The most commonly assessed indices of ANS activation are based on electrodermal (i.e., sweat gland) or cardiovascular (i.e., blood circulatory system) responses. Electrodermal responding is typically quantified in terms of skin conductance level (SCL) or short-duration skin conductance responses (SCRs). The most commonly used cardiovascular measures include heart rate (HR), blood pressure (BP), total peripheral resistance (TPR), cardiac output (CO), pre-ejection period (PEP), and heart rate variability (HRV). Each of these measures varies in terms of whether it primarily reflects sympathetic activity, parasympathetic activity, or both. For example, SCL and PEP predominantly reflect sympathetic activity, HR and BP reflect a combination of sympathetic and parasympathetic activity, and HRV has been closely linked to parasympathetic activity (Cacioppo, Berntson, Larsen, Poehlmann, & Ito, 2000).
James (1884) was among the first psychologists to suggest that different emotional states (e.g., sadness, anger, fear) involve specific patterns of ANS activation. James’s speculations have been central to many important theories of emotion (Ellsworth, 1994; Lang, 1994), though Ellsworth cautions that it would be a mistake to equate James’s theory with peripheral ANS responding considered alone. Nonetheless, much of the research inspired by James’s theory of emotion has focused on ANS measures. One reason for the continued scientific interest in autonomic specificity is that people generally believe that their emotions involve discrete patterns of ANS activation (such as the presumed link between anxiety and increased heart rate: Scherer & Wallbott, 1994). However, the validity of such beliefs is suspect because perceptions of ANS responses are generally not predictive of actual ANS responses (Mauss, Wilhelm, & Gross, 2004; Pennebaker, 1982).
Furthermore, although some evidence for autonomic specificity has been reported (Christie & Friedman, 2004; Ekman, Levenson, & Friesen, 1983; Stemmler, Heldmann, Pauls, & Scherer, 2001), a recent meta-analysis has characterised such effects as inconsistent (Cacioppo et al., 2000). In this meta-analysis, only a small set of the 37 ANS measures reviewed reliably differentiated discrete emotions and replicable findings were specific to particular comparisons (e.g., finger temperature decreases less in anger than in fear, but finger temperature does not differentiate other discrete emotions). Also, although there were mean differences in some ANS responses across emotions, results were highly inconsistent across studies. By contrast, different induction methods (e.g., directed facial expressions versus film clips) have much more reliable effects on ANS measures than different emotions, again highlighting the paucity of support for the autonomic specificity hypothesis (Cacioppo et al., 2000).
Given these considerations, it may be best to view ANS responding in terms of broader dimensions such as arousal (Cacioppo et al., 2000; Duffy, 1962; Malmo, 1959). In support of this point, Peter Lang and colleagues have shown, in a number of studies (e.g., Bradley & Lang, 2000b; Lang, Greenwald, Bradley, & Hamm, 1993), that SCL increases systematically and linearly according to the rated arousal of emotional stimuli (e.g., slides). Moreover, the same studies have found that relations between stimulus arousal and SCL activity are independent of stimulus valence, emotion induction method, and, indeed, which specific emotion is targeted by the induction. Such findings are consistent with theories contending that ANS activity indexes the arousal level of the emotional state rather than its discrete emotional basis (Arnold, 1960; Cannon, 1931; Duffy, 1962).
However, not all measures of ANS responding map onto a single dimension. According to the principle of “directional fractionation” (Lacey, 1967), different measures of ANS activity can operate independently or even in opposition to each other. For example, HR decreases can co-occur with increases in sympathetic activity as assessed by other ANS measures (Bradley & Lang, 2000b; Lang et al., 1997; Libby, Lacey, & Lacey, 1973). To explain such fractionation of the ANS system, at least one additional dimension must be taken into consideration (Cacioppo et al., 2000; Russell & Barrett, 1999). Konorski, and later Lang (Konorski, 1967; Lang, Bradley, & Cuthbert, 1990; Lang et al., 1997) proposed appetitive and aversive systems as the second important dimension of ANS responding; others have proposed a similar valence dimension (Cacioppo et al., 2000; Russell & Barrett, 1999). For example, Cacioppo and colleagues’ meta-analysis (2000) revealed that blood pressure, cardiac output, heart rate, and skin conductance response duration respond to emotional valence.
Although individual ANS measures appear responsive to dimensions rather than discrete emotional states, the joint consideration of multiple ANS measures may support a greater degree of autonomic specificity (Cacioppo et al., 2000; Stemmler, 2004). For example, Stemmler reports that anger and fear, despite being matched in terms of valence and arousal, could be differentiated by a combination of cardiovascular and respiratory measures. Similarly, Kreibig, Wilhelm, Roth, and Gross (2007) found that eleven ANS measures, jointly considered, differentiated responses to fear-inducing versus sadness-inducing film clips (matched on valence and arousal) with 85% accuracy. Thus, combinations of multiple ANS measures may yield better predictions of discrete emotional states. However, data of this type often capitalise on sample-specific findings and should be viewed as tentative in the absence of replications.
Recall, also, that ANS measures serve multiple masters including perceived and actual task demands, coping appraisals, and motor behaviour (Obrist et al., 1970; Stemmler, 2004). For this reason, it may be problematic to view any ANS pattern as a straightforward reflection of the emotional state of the individual. Such considerations are particularly problematic for views emphasising an invariant, unmediated influence of emotion on physiological responding (Panksepp, 1999; Tompkins, 1995). By contrast, if one views emotions as inextricably linked to task demands, coping, and motor behaviour (Ekman, 1999; Larsen, Berntson, Poehlmann, Ito, & Cacioppo, in press; Levenson, 2003; Stemmler, 1989), then it is less of a concern that ANS activity responds to both emotional states and non-emotional factors.
Summary
The idea that discrete emotions have distinct autonomic signatures has not faired well in the literature. Instead, relevant studies often point to relationships among dimensions, particularly those of valence and arousal, and ANS responses. It is possible that considering patterns of multiple ANS measures will lead to autonomic specificity in the future, but more work is needed before coming to firm conclusions. Table 1 thus reinforces our central conclusion that ANS measures primarily respond to dimensional aspects of emotional states.
STARTLE RESPONSE MAGNITUDE AS A MEASURE OF EMOTION
Startle in response to a sudden, intense stimulus is a universal reflex that involves multiple motor actions, including tensing of the neck and back muscles and an eye blink (Landis & Hunt, 1939). The startle response serves a protective function, guarding against potential bodily injury (particularly of the eye) and serving as a behavioural interrupt that is thought to facilitate vigilance in relation to a possible threat (Graham, 1979). In support of this hypothesis, the amygdala, which is a brain structure centrally involved in vigilance and threat detection (Whalen, 1998), plays a key role in modulating the startle response in threatening contexts (Davis, 1989; Koch & Schnitzler, 1997). Because the startle response thus lies at the intersection of several response systems (ANS, CNS, and behaviour), we describe it in a separate section.
The most robust component of the behavioural cascade that constitutes the startle reflex is the eye blink. Therefore, the amplitude of the eye blink is usually used to index startle magnitude among human participants. Such procedures involve an electromyogram (EMG) measurement in which muscle activity is assessed from electrodes placed over the orbicularis oculi muscle, just beneath the lower eyelid. The most commonly used startle-eliciting stimulus is the so-called “startle probe”, a brief (50 ms) burst of white noise within the 95–110 decibel range.
Building to some extent on the work of Davis (1989), Lang (1995) made a strong case for the utility of startle amplitude as a measure of emotion. The logic here is that when the avoidance system is activated by a negative emotional state, then defensive responses (including the startle reflex) should be primed and thus increased relative to during neutral states. Conversely, higher levels of approach motivation likely inhibit tendencies toward a defensive orientation and should thus be associated with a lesser startle response magnitude relative to neutral states. Lang (1995) maps approach and avoidance onto positive and negative emotional states and thus hypothesises an inverse linear relationship between the valence of a person’s emotional state and the startle response magnitude.
Lang’s (1995) hypothesis has been strongly supported. Multiple studies have shown that when startle probes are delivered in the context of pictures and sounds that vary in valence, the magnitude of the startle response is larger in the context of unpleasant stimuli and smaller in the context of pleasant stimuli, both relative to neutral stimuli (Bradley, Cuthbert, & Lang, 1993; Bradley & Lang, 2000a; Vrana, Spence, & Lang, 1988). Such effects have been linked to emotional valence rather than to discrete emotional states (Lang, 1995). Convergent support for the startle response as a measure of emotional valence comes from the clinical literature. Phobic individuals should exhibit greater negative emotion and thus larger startle responses to phobic stimuli, and this result has been reported (Cook & Turpin, 1997). Conversely, individuals meeting criteria for psychopathy are thought to be deficient in threat processing. Consistent with this idea, such individuals, relative to non-psychopathic individuals, have been shown to exhibit smaller startle responses to threatening stimuli (Patrick, 1994).
Two important points qualify the general formulation that startle indexes emotional valence. First, it has been shown that startle magnitude is only sensitive to valence in the context of high-arousal stimuli (Cuthbert, Bradley, & Lang, 1996; Lang, 1995). Second, the startle appears to be particularly useful for understanding reactivity to perceived stimuli such as emotional pictures relative to other induction methods such as conditioning or imagery (Mallan & Lipp, 2007; Miller, Patrick, & Levenston, 2002; Sabatinelli, Bradley, & Lang, 2001). Within emotion-perception tasks, though, several potential confounds have been ruled out, including stimulus novelty, attentional factors, and sensory modality (Bradley, Cuthbert, & Lang, 1990; Bradley et al., 1993; Hawk & Cook, 1997; Lang et al., 1990 Lang et al., 1997).
Summary
Together, the results summarised here suggest that the startle response is a marker of the valence dimension of emotional states. Specifically, as summarised in Table 1, the startle response is reliably larger in the context of high-arousal negative stimuli and reliably smaller in the context of high-arousal positive stimuli. At the same time, the measure does not appear to assess discrete emotional states.
BRAIN STATES AS A MEASURE OF EMOTION
Following early theorising by Cannon (1931) and Bard (1928), many investigators have proposed that the physiological correlates of discrete emotions are likely to be found in the brain rather than in peripheral physiological responses (Buck, 1999; Izard, 2007; Panksepp, 2007). Researchers have taken up this challenge using EEG and neuroimaging methods. Because these methods produce very different types of data, we review EEG and imaging results separately.
Electroencephalography (EEG)
Although the temporal resolution of EEG is excellent, its spatial resolution is limited (Dale & Sereno, 1993). Thus, EEG measures typically contrast activation in fairly large regions of the brain, often anterior (i.e., front of brain) versus posterior (i.e., back of brain) in combination with the distinction between left-sided and right-sided hemispheric activation. The most common EEG measure of this type is alpha power (8–13 Hz band), which is thought to be inversely related to regional cortical activation (Allen, Urry, Hitt, & Coan, 2004). In our review, we focus on what is termed “frontal asymmetry”, which contrasts alpha power in the left frontal region with alpha power in the right frontal region, as this asymmetry-based measure has been particularly important to the emotion literature (Davidson, 1999).
Early studies of frontal asymmetry linked it to emotional valence. For example, Tomarken, Davidson, and Henriques (1990) found that greater left-sided activation at baseline predicted more intense experiences of positive than negative emotion, using a trait measure of emotional experience (although only among those individuals with stable EEG asymmetry profiles over time). Along similar lines, Davidson, Ekman, Saron, Senulis, and Friesen (1990) found that the induction of positive emotions by film clips led to greater left-sided frontal activation subsequent to the induction. These data suggest that frontal asymmetry assesses, or at least predisposes people to, pleasant emotional experiences (Davidson, 1999).
Subsequent studies, however, have provided convincing evidence that the frontal EEG asymmetry measure reflects the relative balance of approach versus avoidance motivation to a greater extent than it reflects emotional valence (Davidson, 1999). For example, Sutton and Davidson (1997) found that greater left-sided activation predicted dispositional tendencies toward approach, whereas greater right-sided asymmetry predicted dispositional tendencies toward avoidance. In contrast, the frontal asymmetry measure did not predict dispositional tendencies toward positive or negative emotions, suggesting an association of frontal asymmetry with approach–avoidance rather than with valence.
Other sources of data converge on a similar model of frontal asymmetry. Of particular importance are studies that link anger, an unpleasant but approach-related emotion, to greater left-hemispheric activation (Harmon-Jones & Allen, 1998; Harmon-Jones, Lueck, Fearn, & Harmon-Jones, 2006). Also, tendencies toward worry, thought to be approach-motivated in the sense of being linked to problem solving, have been linked to relatively greater left-frontal EEG activity (Heller, Schmidtke, Nitschke, Koven, & Miller, 2002). Thus, the emerging consensus appears to be that frontal EEG asymmetry primarily reflects levels of approach motivation (left hemisphere) versus avoidance motivation (right hemisphere).
Neuroimaging studies
Neuroimaging studies, using fMRI (functional magnetic resonance imaging) or PET (positron emission tomography) technologies, can locate activation in far more specific brain regions than EEG. For this reason, it has been proposed that neuroimaging methods may be better suited than EEG to reveal emotion specificity in the brain (Panksepp, 1998). fMRI measures the uptake of oxygen in the blood (the “blood oxygenation level dependent” or BOLD signal; Detre & Floyd, 2000). PET assesses metabolic activity in the brain through the injection of a radioactive isotope the concentrations of which can be measured by a positron-emitting radioisotope (Volkow, Rosen, & Farde, 1997). In both technologies, the assumption is that a greater signal reflects greater blood flow to a particular brain region, which in turn is thought to reflect activation of that region. For the sake of convenience, then, we refer to both sources of data in terms of the “activation” of the relevant brain region.
At the outset, it must be mentioned that any complex reaction such as an emotional state is likely to involve circuits rather than any brain region considered in isolation (Kagan, 2007; LeDoux, 2000; Storbeck, Robinson, & McCourt, 2006). However, particular brain regions may play a relatively greater or lesser role within larger circuits; thus localisation studies are meaningful in identifying the key regions involved. Our review here follows from two meta-analyses examining whether fear, disgust, sadness, and happiness can be linked to activation in particular brain regions (Murphy, Nimmo-Smith, & Lawrence, 2003; Phan, Wager, Taylor, & Liberzon, 2002). The majority of the reviewed studies were included in both meta-analyses, but the two meta-analyses differed somewhat in their analytic approach and, indeed, in their conclusions, as documented next.
The strongest apparent relation in both meta-analyses is between fear stimuli and amygdala activation (Murphy et al., 2003; Phan et al., 2002). However, there are reasons to resist the idea that amygdala activation is a straightforward reflection of fear. The amygdala is particularly responsive to fearful images relative to other fear-induction methods, and may thus be more closely tied to emotional perception than emotional experience (Wager et al., 2008). Moreover, the amygdala primarily responds to uncertainty and ambiguity, even relative to expected and unambiguous fearful stimuli (LeDoux, 1996; Pessoa, Padmala, & Ungerleider, 2005; Whalen, 1998). Additionally, other data have linked amygdala activation to negative emotions more generally (Cahill et al., 1996) and even to reward processing and positive emotional states (Canli, 2004; Murray, 2007). Finally, it has been shown that individuals with bilateral damage to the amygdala can experience negative emotions, including fear (Anderson & Phelps, 2001 Anderson & Phelps, 2002). The preponderance of evidence thus suggests that the amygdala primarily responds to unexpected inputs of motivational significance rather than the experience of fear or processing of fear-related stimuli per se (Barrett, 2006b; Berridge, 1999; Holland & Gallagher, 1999).
Both meta-analyses agree that disgust stimuli tend to be associated with insula activation. However, the meta-analysis of Phan et al. (2002) found that a wide variety of negative emotion inductions activated the insula as well. Thus, the idea that there is a specific link between insula activation and disgust appears problematic. Furthermore, the insula supports many psychological functions, including processing of taste information, implicit learning, procedural memory, and motor performance (e.g., Frank, O’Reilly, & Curran, 2006; Keele, Ivry, Mayr, Hazeltine, & Heuer, 2003; Kiefer & Orr, 1992). For these reasons, it is difficult to endorse the simple view that insula activation can be equated with disgust.
Considering sadness, Phan et al. (2002) reported that 60% of the studies they reviewed found activation in the medial prefrontal cortex (mPFC), but Murphy et al. (2003) reported the strongest localisation pattern in the supracallosal anterior cingulate cortex (ACC; with about 50% of studies manipulating sadness showing this effect). This may not be an important discrepancy because the supracallosal ACC is well connected to areas of the mPFC, and thus an ACC–mPFC circuit may be involved in sadness. However, Barrett (2006a) raised an important concern about such studies, namely that they typically relied on induction methods involving high cognitive demand such as recalling a past event that caused sadness. This is an important potential confound because Phan et al. reported that cognitively demanding emotion inductions activate rostral portions of the ACC to a greater extent than passive emotional processing tasks do. This presents a concern for claiming a 1-to-1 correspondence of sadness to activation of an ACC–mPFC circuit.
The neural correlates of anger and happiness have been even less robust than those discussed above (Murphy et al., 2003; Phan et al., 2002). Furthermore, for the correlates reported, there are potential confounds such as those pertaining to the induction method used (Barrett, 2006a; Wager et al., 2008). In addition, there are concerns that some of the studies reviewed in the two meta-analyses used methods that have limited spatiotemporal resolution. Thus, although there has been some progress in understanding the neural correlates of fear, disgust, and potentially sadness, the discrete-emotions perspective has yet to produce strong, replicable findings.
At the same time, meta-analyses provide support for a dimensional perspective on emotion and brain activity. Consistent with the EEG data reported above, approach-related emotional states appear to be left-lateralised in the brain (Murphy et al., 2003; Wager, Phan, Liberzon, & Taylor, 2003). In addition to these lateralised patterns, Wager et al. (2003) found systematic relations between approach-motivated states and anterior and rostral portions of the medial prefrontal cortex (PFC) as well as the nucleus accumbens. Wager et al. (2003) also found systematic relations between avoidance-motivated states and the amygdala (especially its lateral and basolateral nuclei) and the ACC. Thus, there is increasing evidence that emotional states related to approach and avoidance involve localisable brain circuits (Barrett & Wager, 2006; Wager et al., 2008).
Summary
EEG and neuroimaging studies converge in concluding that relative left-hemisphere activation is reflective of approach-related states, whereas relative right-hemisphere activation is reflective of avoidance-related states. Specific brain regions, too, appear to be linked to states of approach and avoidance, as reviewed in the section on neuroimaging studies. Table 1 thus concludes that CNS measures appear to be sensitive to the dimensions of approach and avoidance. That said, because emotional states are complex and likely to involve circuits, neuroimaging methods that examine interrelated activity among multiple brain regions may hold more promise for understanding whether and how emotional specificity is instantiated in the brain.
BEHAVIOUR AS A MEASURE OF EMOTION
Darwin (1965) suggested that emotions serve an evolved communicative function and thus should prime behaviours that reveal one’s emotional state to others (see Ekman, 1992, for a related view). Another set of theories links emotional states to action dispositions, such as the primed tendency toward flight in the case of fear (Frijda, 1986; Lang et al., 1997). According to these theories, it should be possible to infer a person’s emotional state from vocal characteristics, facial displays, and whole-body behaviours. We next review progress in this area of research. Because the term “expression” implies that emotions naturally trigger a given behaviour, we refer to “behaviour” or “movement” rather than “expression”.
Vocal characteristics
People often report that they infer the emotional states of others from vocal characteristics (Planalp, 1998). Scientific studies have examined this intuition most commonly by decomposing the acoustic waveform of speech and then assessing whether such acoustic properties are associated with the emotional state of the speaker (Juslin & Scherer, 2005). In our review, we concentrate on the most common measures, namely voice amplitude (i.e., loudness) and pitch (also known as fundamental frequency or F0). Although advances in the digital analysis of sound waveforms have made it increasingly feasible to measure other vocal characteristics such as minute changes of vocal-fold vibration (see Bachorowski & Owren, 1995; Protopapas & Lieberman, 1997, for reviews), work of this complex type is just beginning and much remains to be learned (Juslin & Scherer, 2005).
The most consistent association reported in the literature is between arousal and vocal pitch, such that higher levels of arousal have been linked to higher-pitched vocal samples (Bachorowski, 1999; Kappas, Hess, & Scherer, 1991; Pittam, Gallois, & Callan, 1990). For example, Scherer, Banse, Wallbott, and Goldbeck (1991) examined the acoustic features of emotional nonsense sentences spoken by actors. When the actors were depicting high-arousal emotions such as fear, joy, and anger, pitch was higher than when they were depicting lower-arousal emotions such as sadness. Similar findings have been reported in studies of vocal characteristics following success or failure feedback and in the context of naturalistic studies of emotion and vocal responses (Bachorowski & Owren, 1995).
Based on results of this type, Bachorowski and Owen (1995) suggested that vocal pitch can be used to assess the level of emotional arousal currently experienced by the individual. On the other hand, it has been more difficult to find vocal characteristics that are sensitive to valence (Bachorowski, 1999; Leinonen, Hiltunen, Linnankoski, & Laakso, 1997; Protopapas & Lieber-man, 1997). For example, anger and joy are similar in emotional arousal, but different in valence, yet both emotions have been linked to comparable vocal pitch and vocal amplitude (Johnstone & Scherer, 2000).
In the most comprehensive study that we know of, Banse and Scherer (1996) examined relations between 14 induced emotions and 29 acoustic variables. The authors found that a combination of ten acoustic properties differentiated discrete emotions to a greater extent than could be attributed to valence and arousal alone. For example, elation was characterised by medium low frequency (LF) energy and an increase of pitch over time, whereas anger was characterised by low LF energy and a decrease of pitch over time. However, these links were complex and multivariate in nature, involving post hoc comparisons that were novel to the literature and in some cases perhaps not theoretically motivated. Thus, replications are crucial to having greater confidence in the findings reported in this study.
Facial behaviour
Darwin (1965) reasoned that facial displays are closely tied to the likely behaviour of the organism (e.g., biting in the case of anger, which would result in exposed teeth). Darwin further contended that such emotion–behaviour links reflect biologically evolved mechanisms, in that they subserve survival-related actions and communication functions. Ekman built on Darwin’s analysis and showed that prototypic facial behaviours of at least six “basic” emotions (anger, fear, disgust, happiness, sadness, and surprise) could be recognised cross-culturally (Ekman & Friesen, 1971; Ekman, Sorenson, & Friesen, 1969; Fridlund, Ekman, & Oster, 1987; Izard, 1971). It is a different question—and one more pertinent to our review—to consider whether people spontaneously display such prototypic facial behaviours when in a particular emotional state.
Observer ratings
To examine the latter question, we review emotion-induction studies that have sought to link an induced emotional state to facial behaviours displayed during or immediately after the induction. Many of the relevant studies have quantified facial behaviour using componential coding. In most componential coding systems, trained coders detect facial muscle movements—or “facial actions”—using reliable scoring protocols (see Cohn & Ekman, 2005; Ekman & Friesen, 1978, for a comprehensive review). The most widely used componential coding system is the Facial Action Coding System (FACS; Ekman & Friesen, 1978; Ekman, Friesen, & Hager, 2002). The FACS is an anatomically based, comprehensive measurement system that assesses 44 different muscle movements (e.g., raising of the brows, tightening of the lips). As such, it measures all possible combinations of movements that are observable in the face rather than just movements that have been theoretically postulated. Other coding schemes seek to streamline the coding efforts by focusing on facial muscle contractions that are thought to have emotional significance (e.g., Izard, 1971; Kring & Sloan, 2007).
Facial behaviours appear to reliably indicate the valence of a person’s emotional state (Russell, 1994). For example, Duchenne (“non-social”) smiles—involving wrinkling of the muscles around the eyes—have often been linked to experiences of positive emotion (Ekman, Davidson, & Friesen, 1990; Frank, Ekman, & Friesen, 1993; Hess, Banse, & Kappas, 1995; Keltner & Bonanno, 1997). By contrast, negative emotion inductions are often associated with a visible facial behaviour in which the eyebrows are lowered and brought closer together (Kring & Sloan, 2007). In a recent study using a more molar facial action coding system, Mauss, Levenson, McCarter, Wilhelm, and Gross (2005) found strikingly large correlations between valence and the person’s facial behaviours, rs >.80.
The case for the emotion specificity of facial behaviour has been more problematic and, indeed, very few studies of this type have been reported. In one such study, Rosenberg and Ekman (1994) exposed participants to disgust- and fear-inducing film clips. Following each film clip, participants rated their experience of eight discrete emotions. Subsequently, videotaped facial behaviour was scored in terms of the same eight discrete emotions. The researchers then determined whether discrete experiences and facial behaviours co-occurred beyond chance level. This was the case, but such relationships were also weak and not very robust in nature (see Bonanno & Keltner, 2004, for additional results of this type).
Other results, though, present challenges for the entire enterprise of treating facial behaviours as a reflection of the person’s emotional state, regardless of. whether a dimensional or discrete perspective is adopted. For example, Schneider and Josephs (1991) found that children smiled more after failure feedback than after success feedback, clearly a problem for the assumption that smiles reflect positive emotional states. In addition, several studies have found that associations between positive emotional states and facial smiles are stronger—and perhaps exclusive to—contexts in which an audience is present (Fernandez-Dols & Ruiz-Belda, 1995; Fridlund, 1991; Kraut & Johnston, 1979). Such results comport with Darwin’s (1965) analysis of the communicative function of facial behaviour. They also suggest that it may often be hazardous to assume that exhibited facial behaviour provides a “direct readout” of a person’s emotional state.
Electromyography (EMG)
Facial behaviours potentially indicative of emotion can also be assessed with facial EMG, which involves measuring electrical potential from facial muscles via the placement of electrodes on the face. The two most frequently targeted muscle groups are the corrugator supercilii (associated with furrowing of the eyebrows) and the zygomatic muscle (associated with raising of the corners of the lips). Results from this literature have converged on the utility of these measures for assessing the valence of a person’s emotional state, but are generally viewed as limited in understanding discrete emotional reactions (Cacioppo, Berntson, Klein, & Poehlmann, 1997; Larsen et al., in press; but see Vrana, 1993). Corrugator muscle activity decreases linearly with the pleasantness of affective stimuli—responding to stimuli across the full valence spectrum, while zygomatic muscle activity increases linearly with the pleasantness of affective stimuli—responding to pleasant stimuli (see Bradley & Lang, 2000b; Lang et al., 1993; Larsen, Norris, & Cacioppo, 2003, for reviews). Cacioppo et al. suggested that facial EMG activity reflects implicit evaluation processes (Dimberg, Thunberg, & Elmehed, 2000), but more work of this type is warranted before coming to firm conclusions (Larsen et al., 2003).
Whole-body behaviour
Darwin (1965) presented the idea that bodily behaviours are biologically evolved to communicate one’s emotional state to conspecifics. Although research on bodily expressions of emotion is relatively sparse (Adolphs, 2002; Van den Stock, Righart, & de Gelder, 2007), the research that does exist points to the idea that at least certain emotional states may have distinct bodily behaviour signatures. In particular, pride and embarrassment have been linked to expansive and diminutive body postures, respectively. Stepper and Strack (1993) found that participants experienced greater pride if an elevated posture had been implicitly manipulated beforehand. Results from Tracy and Robins’ research programme confirm the link between pride and an expansive body posture (Tracy & Matsumoto, in press; Tracy & Robins, 2004; Tracy, Robins, & Lagattuta, 2005). Conversely, Keltner and Buswell (1997) have found that embarrassment is reflected in bodily postures associated with minimising one’s spatial presence, a result consistent with ethological data on dominance–submission and resulting behavioural postures (Mazur, 2005).
Although embarrassment and pride have been linked to distinct body postures, they have not been linked to distinct facial behaviours (Keltner & Buswell, 1997; Tracy & Robins, 2004). App, McIntosh, and Reed (2007) presented a social-functional analysis in which they provided a rationale for why some emotions are primarily associated with facial behaviours, whereas other emotions are primarily associated with whole-body behaviours. They suggested that some emotions, namely anger, fear, disgust, happiness, and sadness, primarily serve individual-level adaptive functions and should therefore be linked to facial behaviours rather than whole-body behaviours, which are potentially disruptive of an individual’s interactions with the environment. On the other hand, the authors suggested that emotions such as embarrassment, guilt, pride, and shame are centrally linked to a person’s position within a social status hierarchy. These emotions, then, should be more systematically associated with behaviours that signal to larger groups of individuals one’s current emotional state (i.e., whole-body behaviours). Functional analyses of this type appear promising for understanding links between emotions and behaviour, and more research is encouraged.
Summary
The assessment of vocal characteristics appears to be especially useful in understanding levels of emotional arousal, with higher levels of pitch and amplitude associated with higher levels of arousal (Table 1). By contrast, attempts to link emotional valence or discrete emotions to vocal characteristics have been met with mixed success at best, although more sophisticated methods may be capable of doing so in the future. Thus, we conclude that vocal characteristics are primarily reflective of the dimension of emotional arousal.
By contrast, facial behaviours appear to be particularly sensitive to the valence of a person’s emotional state (Table 1). An important caveat, though, is that a number of factors such as gender, culture, expressiveness, and the inferred presence of an audience, likely moderate relations between emotional states and facial behaviours. This may be true to such an extent that the absence of changes in facial behaviour should not be equated with the absence of an emotion, and vice versa.
Body posture has not received a great deal of attention as a measure of emotion. Yet, studies that have been conducted suggest that pride and embarrassment are associated with expansive versus diminutive postures, respectively (Table 1). App and colleagues’ analysis suggests that such links may be specific to social-status-related emotions (Table 1). If this proves to be the case, body posture measures might be unique among the measures that we reviewed in supporting a discrete emotional perspective.
GENERAL DISCUSSION
Having reviewed measures of the main components of emotional responding and their sensitivity to different aspects of emotional state, we now comment on two more general questions that cut across our review. The first question is whether dimensional or discrete approaches better capture the structure of emotional responses. The second question is whether multiple measures of emotion converge, as is suggested by the consensual model of Figure 1.
Measures of emotional responding: Dimensional or discrete?
Emotions have been conceptualised in both dimensional and discrete terms. Dimensional perspectives argue that emotional states are organised by underlying factors such as valence, arousal, and motivational state (Barrett & Russell, 1999; Watson et al., 1999). Discrete emotion perspectives, by contrast, suggest that each emotion (e.g., anger, sadness, happiness) has unique experiential, physiological, and behavioural correlates.
Our review tended to support the dimensional perspective. For example, we reviewed evidence for the idea that emotion specificity has been difficult to establish in the domains of ANS activity, affect-modulated startle responses, and vocal characteristics. Even in relation to measures of emotion that are associated with a greater degree of specificity, such as facial behaviour, dimensional frameworks appear to have substantial explanatory value. Thus, one conclusion of our review is that dimensions appear to capture the lion’s share of variance of emotional responses.
Dimensional and discrete perspectives can be reconciled to some extent by conceptualising discrete emotions in terms of combinations of multiple dimensions (e.g., anger = negative valence, high arousal, and high approach motivation) that appear discrete because they are salient (Carver, 2004; Haidt & Keltner, 1999). If discrete emotions are defined in this manner, there is no necessary antagonism between the two perspectives (Haidt & Keltner, 1999). However, to the extent that dimensional perspectives are sufficient for capturing the essence of particular emotional states, such perspectives should be favoured because they are more parsimonious (Watson, 2000). In addition, the available data are incompatible with the notion that discrete emotional states are categorically different from one another, that is, that they are “natural kinds” (cf. Barrett, 2006a).
Of course, some data differentiate emotional states beyond the three factors of valence, arousal, and approach–avoidance (App et al., 2007; Banse & Scherer, 1996; DeSteno, Petty, Wegener, & Rucker, 2000; Lerner, Dahl, Hariri, & Taylor, 2007; Rosenberg & Ekman, 1994). It may be that investigations using more sophisticated methods (e.g., ANS approaches that take into account combinations of variables or fMRI approaches that examine activity in brain circuits rather than specific brain regions), will support the discrete emotions perspective beyond what has been shown so far.
To what extent do different measures of emotion converge?
Our review focused on each measure of emotion individually. Thus, an important remaining question is the extent to which different measures of emotion converge in understanding a person’s emotional state. The idea that the components of emotion should converge is consistent with theories invoking the idea of “affect programmes”. When such programmes are activated, according to these theories, there should be convergent outputs in emotional experience, physiology, and behaviour (see Figure 1 for such a model).
This model has not been well supported in studies that have examined convergence of response systems. Correlations among multiple measures of emotion are moderate at best, small in typical studies, and inconsistent across studies (e.g., Cacioppo et al., 2000; Lang, 1988; Mauss et al., 2004). Psychometric factors could play some role in the lack of convergence typically observed. For example, any one measure of emotion is likely associated with variance unique to it, in turn rendering high levels of convergence difficult to find. Also, most prior studies have assessed coherence in terms of between-individual correlations, thus measuring whether individuals who respond strongly in one component also respond strongly in another. It has been noted that such between-individual analyses might not be the best test of response coherence but that within-individual associations of measures across time more closely denote response-system coherence as implied by the theories of emotion outlined above (Buck, 1980; Lacey, 1967; Stemmler, 1992).
Recent studies have addressed some of these psychometric limitations by using reliable and valid measures and by using within-subject designs (Mauss et al., 2005; Reisenzein, 2000; Ruch, 1995). These studies have found higher levels of convergence than prior studies, but the relevant correlations were still low to moderate in strength (e.g., Mauss et al., 2005). In sum, psychometric issues do not appear sufficient in understanding the low levels of convergence observed in studies of this type.
The typical lack of strong convergence among multiple measures of emotion has three important implications. First, it appears that the construct of “emotion” cannot be captured with any one measure considered alone (Lang, 1988; Mandler, 1975; Rachman, 1978). In other words, emotions are multiply determined rather than characterised by a one-dimensional process such as that depicted in Figure 1. Practically speaking, then, the more measures of emotion that are obtained and the better they are tailored to the particular context and research question, the more one will likely learn from a particular study (cf. Larsen & Prizmic-Larsen, 2006). Second, dissociations among different measures of emotion may be relatively normal rather than necessarily reflective of a dysregulated system. In this context, research that examines the mechanisms that mediate and explain particular response-system dissociations will be particularly useful. Third, there are likely to be moderator variables that affect convergence across measures of emotion (Fridlund, Schwartz, & Fowler, 1984; Lacey, Bateman, & Vanlehn, 1953; Picard, Vyzas, & Healey, 2001). If this is the case, then a more idiographic approach would be necessary to understand the nature of emotional response coherence (Malmo, Shagrass, & Davis, 1950).
CONCLUSIONS
The present review examined whether emotional states are associated with specific and invariant patterns of experience, physiology, and behaviour. We suggest that measures of emotional responding appear to be structured along dimensions (e.g., valence, arousal) rather than discrete emotional states (e.g., sadness, fear, anger). Additionally, different measures of emotion appear sensitive to different dimensional aspects of state (e.g., facial EMG is sensitive to valence, whereas skin conductance is sensitive to arousal) and are not strongly related to one another. Practically speaking, then, there is no “gold standard” measure of emotional responding. For theories of emotion, this means that there is no “thing” that defines emotion, but rather that emotions are constituted by multiple, situationally and individually variable processes.
Contributor Information
Iris B. Mauss, University of Denver, Denver, CO, USA
Michael D. Robinson, North Dakota State University, Fargo, ND, USA
References
- Adolphs R. Recognizing emotion from facial expressions: Psychological and neurological mechanisms. Behavioral and Cognitive Neuroscience Reviews. 2002;1(1):21–62. doi: 10.1177/1534582302001001003. [DOI] [PubMed] [Google Scholar]
- Allen JJB, Urry HL, Hitt SK, Coan JA. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology. 2004;41(2):269–280. doi: 10.1111/j.1469-8986.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411(6835):305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Is the human amygdala critical for the subjective experience of emotion? Evidence of intact dispositional affect in patients with amygdala lesions. Journal of Cognitive Neuroscience. 2002;14(5):709–720. doi: 10.1162/08989290260138618. [DOI] [PubMed] [Google Scholar]
- App B, McIntosh D, Reed C. A social-functional approach to emotion communication: “How” depends on “why”; Poster presented at the annual meeting of the Society for Personality and Social Psychology.2007. [Google Scholar]
- Arnold MB. Emotion and personality. New York: Columbia University Press; 1960. [Google Scholar]
- Bachorowski JA. Vocal expression and perception of emotion. Current Directions in Psychological Science. 1999;8(2):53–57. [Google Scholar]
- Bachorowski JA, Owren MJ. Vocal expression of emotion: Acoustic properties of speech are associated with emotional intensity and context. Psychological Science. 1995;6(4):219–224. [Google Scholar]
- Banse R, Scherer KR. Acoustic profiles in vocal emotion expression. Journal of Personality and Social Psychology. 1996;70(3):614–636. doi: 10.1037//0022-3514.70.3.614. [DOI] [PubMed] [Google Scholar]
- Bard P. A diencephalic mechanism for the expression of rage with special reference to the sympathetic nervous system. American Journal of Physiology. 1928;84:490–515. [Google Scholar]
- Barrett LF. Are emotions natural kinds? Perspectives in Psychological Science. 2006a;1:28–58. doi: 10.1111/j.1745-6916.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Barrett LF. Solving the emotion paradox: Categorization and the experience of emotion. Personality and Social Psychology Review. 2006b;10(1):20–46. doi: 10.1207/s15327957pspr1001_2. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Robin L, Pietromonaco PR, Eyssell KM. Are women the “more emotional” sex? Evidence from emotional experiences in social context. Cognition and Emotion. 1998;12(4):555–578. [Google Scholar]
- Barrett LF, Russell JA. The structure of current affect: Controversies and emerging consensus. Current Directions in Psychological Science. 1999;8(1):10–14. [Google Scholar]
- Barrett LF, Wager TD. The structure of emotion: Evidence from neuroimaging studies. Current Directions in Psychological Science. 2006;15(2):79–83. [Google Scholar]
- Berntson GG, Cacioppo JT. From homeostasis to allodynamic regulation. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 2. New York: Cambridge University Press; 2000. pp. 459–481. [Google Scholar]
- Berridge KC. Pleasure, pain, desire, and dread: Hidden core processes of emotion. In: Kahneman D, Diener E, Schwarz N, editors. Well-being: The foundations of hedonic psychology. New York: Russell Sage Foundation; 1999. pp. 525–557. [Google Scholar]
- Bonanno GA, Keltner D. The coherence of emotion systems: Comparing “on-line” measures of appraisal and facial expressions, and self-report [Brief Report] Cognition and Emotion. 2004;18(3):431–444. [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Startle reflex modification: Emotion or attention? Psychophysiology. 1990;27(5):513–522. doi: 10.1111/j.1469-8986.1990.tb01966.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Pictures as prepulse: Attention and emotion in startle modification. Psychophysiology. 1993;30(5):541–545. doi: 10.1111/j.1469-8986.1993.tb02079.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective reactions to acoustic stimuli. Psychophysiology. 2000a;37(2):204–215. [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: Behavior, feeling, and physiology. In: Lane RD, Nadel L, editors. Cognitive neuroscience of emotion. New York: Oxford University Press; 2000b. pp. 242–276. [Google Scholar]
- Buck R. Nonverbal behavior and the theory of emotion: The facial feedback hypothesis. Journal of Personality and Social Psychology. 1980;38(5):811–824. doi: 10.1037//0022-3514.38.5.811. [DOI] [PubMed] [Google Scholar]
- Buck R. The biological affects: A typology. Psychological Review. 1999;106(2):301–336. doi: 10.1037/0033-295x.106.2.301. [DOI] [PubMed] [Google Scholar]
- Cacioppo J, Berntson GG, Klein DJ, Poehlmann KM. The psychophysiology of emotion across the lifespan. Annual Review of Gerontology and Geriatrics. 1997;17:27–74. [Google Scholar]
- Cacioppo JT, Berntson GG, Larsen JT, Poehlmann KM, Ito TA. The psychophysiology of emotion. In: Lewis M, Haviland-Jones JM, editors. The handbook of emotion. New York: Guildford Press; 2000. [Google Scholar]
- Cahill L, Haier R, Fallon J, Akire M, Tang C, Keator D, et al. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proceedings of the National Academy of Sciences. 1996;93:8016–8321. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T. Functional brain mapping of extraversion and neuroticism: Learning from individual differences in emotion processing. Journal of Personality. 2004;72(6):1105–1132. doi: 10.1111/j.1467-6494.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- Cannon WB. Again the James–Lange and the thalamic theories of emotion. Psychological Review. 1931;38(4):281–295. [Google Scholar]
- Carver CS. Self-regulation of action and affect. In: Baumeister RF, Vohs KD, editors. Handbook of self-regulation: Research, theory, and applications. New York: Guilford Press; 2004. pp. 13–39. [Google Scholar]
- Christie IC, Friedman BH. Autonomic specificity of discrete emotion and dimensions of affective space: A multivariate approach. International Journal of Psychophysiology. 2004;51(2):143–153. doi: 10.1016/j.ijpsycho.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Cohn JF, Ekman P. Measuring facial action. In: Harrigan JA, Rosenthal R, Scherer KR, editors. The new handbook of methods in nonverbal behavior research. New York: Oxford University Press; 2005. pp. 9–64. [Google Scholar]
- Cook E, III, Turpin G. Differentiating orienting, startle, and defense responses: The role of affect and its implications for psychopathology. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and orienting: Sensory and motivational processes. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 1997. pp. 137–164. [Google Scholar]
- Cuthbert BN, Bradley MM, Lang PJ. Probing picture perception: Activation and emotion. Psychophysiology. 1996;33(2):103–111. doi: 10.1111/j.1469-8986.1996.tb02114.x. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. Journal of Cognitive Neuroscience. 1993;5(2):162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Darwin C. The expression of the emotions in man and animals. Chicago: The University of Chicago Press; 1965. (Original work published 1872) [Google Scholar]
- Davidson RJ. Neuropsychological perspectives on affective styles and their cognitive consequences. In: Dalgleish T, Power MJ, editors. Handbook of cognition and emotion. New York: Wiley; 1999. pp. 103–123. [Google Scholar]
- Davidson RJ, Ekman P, Saron CD, Senulis JA, Friesen WV. Approach–withdrawal and cerebral asymmetry: Emotional expression and brain physiology I. Journal of Personality and Social Psychology. 1990;58(2):330–341. [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear-potentiated startle. Annals of the New York Academy of Sciences. 1989;563:165–183. doi: 10.1111/j.1749-6632.1989.tb42197.x. [DOI] [PubMed] [Google Scholar]
- DeSteno D, Petty RE, Wegener DT, Rucker DD. Beyond valence in the perception of likelihood: The role of emotion specificity. Journal of Personality and Social Psychology. 2000;78(3):397–416. doi: 10.1037//0022-3514.78.3.397. [DOI] [PubMed] [Google Scholar]
- Detre JA, Floyd TF. Functional MRI and its applications to the clinical neurosciences. Neuroscientist. 2000;7:64–79. doi: 10.1177/107385840100700110. [DOI] [PubMed] [Google Scholar]
- Dimberg U, Thunberg M, Elmehed K. Unconscious facial reactions to emotional facial expressions. Psychological Science. 2000;11(1):86–89. doi: 10.1111/1467-9280.00221. [DOI] [PubMed] [Google Scholar]
- Duffy E. Activation and behavior. New York: Wiley; 1962. [Google Scholar]
- Ekman P. Facial expressions of emotion: New findings, new questions. Psychological Science. 1992;3:34–38. [Google Scholar]
- Ekman P. Basic emotions. In: Dalgleish T, Power MJ, editors. Handbook of cognition and emotion. New York: Wiley; 1999. pp. 45–60. [Google Scholar]
- Ekman P, Davidson RJ, Friesen WV. The Duchenne smile: Emotional expression and brain physiology II. Journal of Personality and Social Psychology. 1990;58(2):342–353. [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Constants across cultures in the face and emotion. Journal of Personality and Social Psychology. 1971;17(2):124–129. doi: 10.1037/h0030377. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Facial action coding system: A technique for the measurement of facial movement. Palo Alto, CA: Consulting Psychologists Press; 1978. [Google Scholar]
- Ekman P, Friesen WV, Hager JC. The facial action coding system. Salt Lake City, UT: Research Nexus eBook; 2002. [Google Scholar]
- Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221(4616):1208–1210. doi: 10.1126/science.6612338. [DOI] [PubMed] [Google Scholar]
- Ekman P, Sorenson ER, Friesen WV. Pan-cultural elements in facial displays of emotion. Science. 1969;164(3875):86–88. doi: 10.1126/science.164.3875.86. [DOI] [PubMed] [Google Scholar]
- Ellsworth PC. William James and emotion: Is a century of fame worth a century of misunderstanding? Psychological Review. 1994;101(2):222–229. doi: 10.1037/0033-295x.101.2.222. [DOI] [PubMed] [Google Scholar]
- Fernandez-Dols JM, Ruiz-Belda MA. Everyday conceptions of emotion: An introduction to the psychology, anthropology and linguistics of emotion. Vol. 81. New York: Kluwer Academic/Plenum Publishers; 1995. Expression of emotion versus expressions of emotions: Everyday conceptions of spontaneous facial behavior. [Google Scholar]
- Frank MG, Ekman P, Friesen WV. Behavioral markers and recognizability of the smile of enjoyment. Journal of Personality and Social Psychology. 1993;64(1):83–93. doi: 10.1037//0022-3514.64.1.83. [DOI] [PubMed] [Google Scholar]
- Frank MJ, O’Reilly RC, Curran T. When memory fails, intuition reigns: Midazolam enhances implicit inference in humans. Psychological Science. 2006;17(8):700–707. doi: 10.1111/j.1467-9280.2006.01769.x. [DOI] [PubMed] [Google Scholar]
- Fridlund AJ. Sociality of solitary smiling: Potentiation by an implicit audience. Journal of Personality and Social Psychology. 1991;60(2):229–240. [Google Scholar]
- Fridlund AJ, Ekman P, Oster H. Facial expressions of emotion. In: Siegman AW, Feldstein S, editors. Nonverbal behavior and communication. 2. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1987. pp. 143–223. [Google Scholar]
- Fridlund AJ, Schwartz GE, Fowler SC. Pattern recognition of self-reported emotional state from multiple-site facial EMG activity during affective imagery. Psycho-physiology. 1984;21(6):622–637. doi: 10.1111/j.1469-8986.1984.tb00249.x. [DOI] [PubMed] [Google Scholar]
- Frijda NH. The emotions. Cambridge, UK: Cambridge University Press; 1986. [Google Scholar]
- Frijda NH. The laws of emotion. American Psychologist. 1988;43(5):349–358. doi: 10.1037//0003-066x.43.5.349. [DOI] [PubMed] [Google Scholar]
- Graham FK. Distinguishing among orienting, defense, and startle reflexes. In: Kimmel HD, Olst EHV, Orlebeke JF, editors. The orienting reflex in humans. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1979. [Google Scholar]
- Gross JJ. Handbook of emotion regulation. New York: Guilford Press; 2007. [Google Scholar]
- Haidt J, Keltner D. Culture and facial expression: Open-ended methods find more expressions and a gradient of recognition. Cognition and Emotion. 1999;13(3):225–266. [Google Scholar]
- Harmon-Jones E, Allen JJB. Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality and Social Psychology. 1998;74(5):1310–1316. doi: 10.1037//0022-3514.74.5.1310. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Lueck L, Fearn M, Harmon-Jones C. The effect of personal relevance and approach-related action expectation on relative left frontal cortical activity. Psychological Science. 2006;17(5):434–440. doi: 10.1111/j.1467-9280.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- Hawk LW, Cook EW., III Affective modulation of tactile startle. Psychophysiology. 1997;34(1):23–31. doi: 10.1111/j.1469-8986.1997.tb02412.x. [DOI] [PubMed] [Google Scholar]
- Heller W, Schmidtke JI, Nitschke JB, Koven NS, Miller GA. States, traits, and symptoms: Investigating the neural correlates of emotion, personality, and psycho-pathology. In: Cervone D, Mischel W, editors. Advances in personality science. New York: Guilford Press; 2002. pp. 106–126. [Google Scholar]
- Hess U, Banse R, Kappas A. The intensity of facial expression is determined by underlying affective state and social situation. Journal of Personality and Social Psychology. 1995;69(2):280–288. [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends in Cognitive Sciences. 1999;3(2):65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Izard CE. The face of emotion. East Norwalk, CT: Appleton-Century-Crofts; 1971. [Google Scholar]
- Izard CE. Levels of emotion and levels of consciousness. Behavioral and Brain Sciences. 2007;30(1):96–98. [Google Scholar]
- James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- Johnstone T, Scherer KR. Vocal communication of emotion. In: Lewis M, Haviland-Jones JM, editors. Handbook of emotions. New York: Guilford Press; 2000. pp. 220–235. [Google Scholar]
- Juslin PN, Scherer KR. Vocal expression of affect. In: Harrigan JA, Rosenthal R, Scherer KR, editors. The new handbook of methods in nonverbal behavior research. New York: Oxford University Press; 2005. pp. 65–135. [Google Scholar]
- Kagan J. A trio of concerns. Perspectives on Psychological Science. 2007;2(4):361–376. doi: 10.1111/j.1745-6916.2007.00049.x. [DOI] [PubMed] [Google Scholar]
- Kappas A, Hess U, Scherer KR. Voice and emotion. In: Feldman RS, Rime B, editors. Fundamentals of nonverbal behavior. Cambridge, UK: Cambridge University Press; 1991. pp. 200–238. [Google Scholar]
- Keele SW, Ivry R, Mayr U, Hazeltine E, Heuer H. The cognitive and neural architecture of sequence representation. Psychological Review. 2003;110(2):316–339. doi: 10.1037/0033-295x.110.2.316. [DOI] [PubMed] [Google Scholar]
- Keltner D, Bonanno GA. A study of laughter and dissociation: Distinct correlates of laughter and smiling during bereavement. Journal of Personality and Social Psychology. 1997;73(4):687–702. doi: 10.1037//0022-3514.73.4.687. [DOI] [PubMed] [Google Scholar]
- Keltner D, Buswell BN. Embarrassment: Its distinct form and appeasement functions. Psychological Bulletin. 1997;122(3):250–270. doi: 10.1037/0033-2909.122.3.250. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Orr MR. Taste avoidance, but not aversion, learning in rats lacking gustatory cortex. Behavioral Neuroscience. 1992;106(1):140–146. doi: 10.1037//0735-7044.106.1.140. [DOI] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats–Circuits mediating evocation, inhibition and potentiation. Behavioural Brain Research. 1997;89(1–2):35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Konorski J. Integrative activity of the brain. Chicago: University of Chicago Press; 1967. [Google Scholar]
- Kraut RE, Johnston RE. Social and emotional messages of smiling: An ethological approach. Journal of Personality and Social Psychology. 1979;37(9):1539–1553. [Google Scholar]
- Kreibig SD, Wilhelm FH, Roth WT, Gross JJ. Cardiovascular, electrodermal, and respiratory response patterns to fear- and sadness-inducing films. Psychophysiology. 2007;44(5):787–806. doi: 10.1111/j.1469-8986.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- Kring AM, Sloan DM. The facial expression coding system (FACES): Development, validation, and utility. Psychological Assessment. 2007;19(2):210–224. doi: 10.1037/1040-3590.19.2.210. [DOI] [PubMed] [Google Scholar]
- Lacey J. Somatic response patterning and stress: Some revisions of activation theory. In: Appley MH, Trumbull R, editors. Psychological stress: Issues in research. New York: Appleton-Century-Crofts; 1967. [Google Scholar]
- Lacey JI, Bateman DE, Vanlehn R. Autonomic response specificity: An experimental study. Psychosomatic Medicine. 1953;15:8–21. doi: 10.1097/00006842-195301000-00002. [DOI] [PubMed] [Google Scholar]
- Landis C, Hunt W. The startle pattern. Oxford, UK: Farrar & Rinehart; 1939. [Google Scholar]
- Lane RD, Ahern GL, Schwartz GE, Kaszniak AW. Is alexithymia the emotional equivalent of blindsight? Biological Psychiatry. 1997;42(9):834–844. doi: 10.1016/s0006-3223(97)00050-4. [DOI] [PubMed] [Google Scholar]
- Lang PJ. What are the data of emotion? In: Hamilton V, Bower GH, Frijda NH, editors. Cognitive perspectives on emotion and motivation. New York: Kluwer Academic/Plenum Publishers; 1988. pp. 173–191. [Google Scholar]
- Lang PJ. The varieties of emotional experience: A meditation on James–Lange theory. Psychological Review. 1994;101(2):211–221. doi: 10.1037/0033-295x.101.2.211. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50(5):372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97(3):377–395. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation, and action. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and orienting: Sensory and motivational processes. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 1997. pp. 97–135. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Larsen JT, Berntson GG, Poehlmann KM, Ito TA, Cacioppo JT. The psychophysiology of emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. The handbook of emotions. 3. New York: Guilford Press; in press. [Google Scholar]
- Larsen JT, McGraw AP, Cacioppo JT. Can people feel happy and sad at the same time? Journal of Personality and Social Psychology. 2001;81(4):684–696. [PubMed] [Google Scholar]
- Larsen JT, Norris CJ, Cacioppo JT. Effects of positive and negative affect on electromyographic activity over zygomaticus major and corrugator supercilii. Psychophysiol-ogy. 2003;40(5):776–785. doi: 10.1111/1469-8986.00078. [DOI] [PubMed] [Google Scholar]
- Larsen RJ, Prizmic-Larsen Z. Measuring emotions: Implications of a multimethod perspective. In: Eid M, Diener E, editors. Handbook of multimethod measurement in psychology. Washington, DC: American Psychological Association; 2006. pp. 337–351. [Google Scholar]
- Lazarus RS. Emotion and adaptation. Oxford, UK: Oxford University Press; 1991. [Google Scholar]
- LeDoux JE. The emotional brain: The mysterious underpinnings of emotional life. New York: Simon & Schuster; 1996. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leinonen L, Hiltunen T, Linnankoski I, Laakso ML. Expression of emotional-motivational connotations with a one-word utterance. Journal of the Acoustical Society of America. 1997;102(3):1853–1863. doi: 10.1121/1.420109. [DOI] [PubMed] [Google Scholar]
- Lerner JS, Dahl RE, Hariri AR, Taylor SE. Facial expressions of emotion reveal neuroendocrine and cardiovascular stress responses. Biological Psychiatry. 2007;61(2):253–260. doi: 10.1016/j.biopsych.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Levenson RW. Blood, sweat, and fears: The autonomic architecture of emotion. In: Ekman P, Campos JJ, Davidson RJ, de Waal FBM, editors. Emotions inside out: 130 years after Darwin’s: The expression of the emotions in man and animals. New York: New York University Press; 2003. pp. 348–366. [DOI] [PubMed] [Google Scholar]
- Libby WL, Lacey BC, Lacey JI. Pupillary and cardiac activity during visual attention. Psychophysiology. 1973;10(3):270–294. doi: 10.1111/j.1469-8986.1973.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Mallan KM, Lipp OV. Does emotion modulate the blink reflex in human conditioning? Startle potentiation during pleasant and unpleasant cues in the picture–picture paradigm. Psychophysiology. 2007;44(5):737–748. doi: 10.1111/j.1469-8986.2007.00541.x. [DOI] [PubMed] [Google Scholar]
- Malmo RB. Activation: A neuropsychological dimension. Psychological Review. 1959;66(6):367–386. doi: 10.1037/h0047858. [DOI] [PubMed] [Google Scholar]
- Malmo RB, Shagrass C, Davis FH. Symptom specificity and bodily reactions during psychiatric interview. Psychosomatic Medicine. 1950;12:362–376. doi: 10.1097/00006842-195011000-00006. [DOI] [PubMed] [Google Scholar]
- Mandler G. Mind and emotion. New York: Wiley; 1975. [Google Scholar]
- Matthews G, Gilliland K. The personality theories of H. J. Eysenck and J. A. Gray: A comparative review. Personality and Individual Differences. 1999;26(4):583–626. [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5(2):175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Wilhelm FH, Gross JJ. Is there less to social anxiety than meets the eye? Emotion experience, expression, and bodily responding. Cognition and Emotion. 2004;18(5):631–662. [Google Scholar]
- Mazur A. Biosociology of dominance and deference. Lanham, MD: Rowman & Littlefield; 2005. [Google Scholar]
- Miller MW, Patrick CJ, Levenston GK. Affective imagery and the startle response: Probing mechanisms of modulation during pleasant scenes, personal experiences and discrete negative emotions. Psychophysiology. 2002;39(4):519–529. doi: 10.1017/s0048577202394095. [DOI] [PubMed] [Google Scholar]
- Mitchell TR, Thompson L, Peterson E, Cronk R. Temporal adjustments in the evaluation of events: The “rosy view”. Journal of Experimental Social Psychology. 1997;33(4):421–448. doi: 10.1006/jesp.1997.1333. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: A meta-analysis. Cognitive, Affective & Behavioral Neuroscience. 2003;3(3):207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Murray E. The amygdala, reward and emotion. Trends in Cognitive Sciences. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Obrist PA, Webb RA, Sutterer JR, Howard JL. The cardiac–somatic relationship: Some reformulations. Psychophysiology. 1970;6(5):569–587. doi: 10.1111/j.1469-8986.1970.tb02246.x. [DOI] [PubMed] [Google Scholar]
- Öhman A, Hamm A, Hugdahl K. Cognition and the autonomic nervous system: Orienting, anticipation, and conditioning. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 2. New York: Cambridge University Press; 2000. pp. 533–575. [Google Scholar]
- Panksepp J. Affective neuroscience: The foundations of human and animal emotions. New York: Oxford University Press; 1998. [Google Scholar]
- Panksepp J. Emotions as viewed by psychoanalysis and neuroscience: An exercise in consilience. Neuro-Psychoanalysis. 1999;1(1):15–38. [Google Scholar]
- Panksepp J. Neurologizing the psychology of affects: How appraisal-based constructivism and basic emotion theory can coexist. Perspectives on Psychological Science. 2007;2(3):281–295. doi: 10.1111/j.1745-6916.2007.00045.x. [DOI] [PubMed] [Google Scholar]
- Patrick CJ. Emotion and psychopathy: Startling new insights. Psychophysiology. 1994;31(4):319–330. doi: 10.1111/j.1469-8986.1994.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Paulhus DL, John OP. Egoistic and moralistic biases in self-perception: The interplay of self-deceptive styles with basic traits and motives. Journal of Personality. 1998;66(6):1025–1060. [Google Scholar]
- Paulhus DL, Reid DB. Enhancement and denial in socially desirable responding. Journal of Personality and Social Psychology. 1991;60(2):307–317. [Google Scholar]
- Pennebaker JW. Physical symptoms associated with blood pressure. Psychophysiology. 1982;19(2):201–210. doi: 10.1111/j.1469-8986.1982.tb02547.x. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, Ungerleider LG. Quantitative prediction of perceptual decisions during near-threshold fear detection. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(15):5612–5617. doi: 10.1073/pnas.0500566102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuoroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Picard RW, Vyzas E, Healey J. Toward machine emotional intelligence: Analysis of affective physiological state. IEEE Transactions Pattern Analysis and Machine Intelligence. 2001;23(10):1172–1191. [Google Scholar]
- Pittam J, Gallois C, Callan V. The long-term spectrum and perceived emotion. Speech Communication. 1990;9(3):177–187. [Google Scholar]
- Planalp S. Communicating emotion in everyday life: Cues, channels, and processes. In: Andersen PA, Guerrero LK, editors. Handbook of communication and emotion: Research, theory, applications, and contexts. San Diego, CA: Academic Press; 1998. pp. 29–48. [Google Scholar]
- Protopapas A, Lieberman P. Fundamental frequency of phonation and perceived emotional stress. Journal of the Acoustical Society of America. 1997;101(4 Pt 1):2267–2277. doi: 10.1121/1.418247. [DOI] [PubMed] [Google Scholar]
- Rachman S. Human fears: A three systems analysis. Scandinavian Journal of Behaviour Therapy. 1978;7(4):237–245. [Google Scholar]
- Reisenzein R. Exploring the strength of association between the components of emotion syndromes: The case of surprise. Cognition and Emotion. 2000;14(1):1–38. [Google Scholar]
- Robinson MD, Clore GL. Episodic and semantic knowledge in emotional self-report: Evidence for two judgment processes. Journal of Personality & Social Psychology. 2002;83(1):198–215. [PubMed] [Google Scholar]
- Robinson MD, Neighbors C. Catching the mind in action: Implicit methods in personality research and assessment. In: Eid M, Diener E, editors. Handbook of multimethod measurement in psychology. Washington, DC: American Psychological Association; 2006. pp. 115–125. [Google Scholar]
- Robinson MD, Sedikides C. Traits and the self: Toward an integration. In: Corr PJ, Matthews G, editors. Handbook of personality. Cambridge, UK: Cambridge University Press; in press. [Google Scholar]
- Rosenberg EL, Ekman P. Coherence between expressive and experiential systems in emotion. Cognition and Emotion. 1994;8(3):201–229. [Google Scholar]
- Ruch W. Will the real relationship between facial expression and affective experience please stand up: The case of exhilaration. Cognition and Emotion. 1995;9(1):33–58. [Google Scholar]
- Russell JA. A circumplex model of affect. Journal of Personality and Social Psychology. 1980;39(6):1161–1178. [Google Scholar]
- Russell JA. Is there universal recognition of emotion from facial expressions? A review of the cross-cultural studies. Psychological Bulletin. 1994;115(1):102–141. doi: 10.1037/0033-2909.115.1.102. [DOI] [PubMed] [Google Scholar]
- Russell JA, Barrett LF. Core affect, prototypical emotional episodes, and other things called emotion: Dissecting the elephant. Journal of Personality and Social Psychology. 1999;76(5):805–819. doi: 10.1037//0022-3514.76.5.805. [DOI] [PubMed] [Google Scholar]
- Rusting CL. Personality, mood, and cognitive processing of emotional information: Three conceptual frameworks. Psychological Bulletin. 1998;124(2):165–196. doi: 10.1037/0033-2909.124.2.165. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Lang PJ. Affective startle modulation in anticipation and perception. Psychophysiology. 2001;38(4):719–722. [PubMed] [Google Scholar]
- Scherer KR. Emotion as a multicomponent process: A model and some cross-cultural data. Review of Personality & Social Psychology. 1984;5:37–63. [Google Scholar]
- Scherer KR, Bance R, Wallbott HG, Goldbeck T. Vocal cues in emotion encoding and decoding. Motivation and Emotion. 1991;15:123–148. [Google Scholar]
- Scherer KR, Wallbott HG. Evidence for universality and cultural variation of differential emotion response patterning. Journal of Personality and Social Psychology. 1994;66:310–328. doi: 10.1037//0022-3514.66.2.310. [DOI] [PubMed] [Google Scholar]
- Schneider K, Josephs I. The expressive and communicative functions of preschool children’s smiles in an achievement-situation. Journal of Nonverbal Behavior. 1991;15(3):185–198. [Google Scholar]
- Schneirla TC. An evolutionary and developmental theory of biphasic processes underlying approach and withdrawal. In: Jones MR, editor. Nebraska symposium on motivation, 1959. Lincoln, NE: University of Nebraska Press; 1959. pp. 1–42. [Google Scholar]
- Shedler J, Mayman M, Manis M. The illusion of mental health. American Psychologist. 1993;48(11):1117–1131. doi: 10.1037//0003-066x.48.11.1117. [DOI] [PubMed] [Google Scholar]
- Smith CA, Ellsworth PC. Patterns of cognitive appraisal in emotion. Journal of Personality and Social Psychology. 1985;48(4):813–838. [PubMed] [Google Scholar]
- Stemmler G. The autonomic differentiation of emotions revisited: Convergent and discriminant validation. Psychophysiology. 1989;26(6):617–632. doi: 10.1111/j.1469-8986.1989.tb03163.x. [DOI] [PubMed] [Google Scholar]
- Stemmler G. Differential psychophysiology: Persons in situations. Berlin, Germany: Springer-Verlag; 1992. [Google Scholar]
- Stemmler G. Physiological processes during emotion. In: Philippot P, Feldman RS, editors. The regulation of emotion. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 2004. pp. 33–70. [Google Scholar]
- Stemmler G, Heldmann M, Pauls CA, Scherer T. Constraints for emotion specificity in fear and anger: The context counts. Psychophysiology. 2001;38(2):275–291. [PubMed] [Google Scholar]
- Stepper S, Strack F. Proprioceptive determinants of emotional and nonemotional feelings. Journal of Personality and Social Psychology. 1993;64:211–220. [Google Scholar]
- Storbeck J, Robinson MD, McCourt ME. Semantic processing precedes affect retrieval: The neurological case for cognitive primacy in visual processing. Review of General Psychology. 2006;10(1):41–55. [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8(3):204–210. [Google Scholar]
- Taylor SE, Lerner JS, Sherman DK, Sage RM, McDowell NK. Are self-enhancing cognitions associated with healthy or unhealthy biological profiles? Journal of Personality and Social Psychology. 2003;85(4):605–615. doi: 10.1037/0022-3514.85.4.605. [DOI] [PubMed] [Google Scholar]
- Tellegen A, Watson D, Clark LA. On the dimensional and hierarchical structure of affect. Psychological Science. 1999;10(4):297–303. [Google Scholar]
- Tomarken AJ, Davidson RJ, Henriques JB. Resting frontal brain asymmetry predicts affective responses to films. Journal of Personality and Social Psychology. 1990;59:791–801. doi: 10.1037//0022-3514.59.4.791. [DOI] [PubMed] [Google Scholar]
- Tompkins SS. Exploring affect. Cambridge, UK: Cambridge University Press; 1995. [Google Scholar]
- Tracy JL, Matsumoto D. More than a thrill: Cross cultural evidence for spontaneous displays of pride in response to athletic success. Proceedings of the National Academy of Sciences. in press. [Google Scholar]
- Tracy JL, Robins RW. Show your pride: Evidence for a discrete emotion expression. Psychological Science. 2004;15(3):194–197. doi: 10.1111/j.0956-7976.2004.01503008.x. [DOI] [PubMed] [Google Scholar]
- Tracy JL, Robins RW, Lagattuta KH. Can children recognize pride? Emotion. 2005;5(3):251–257. doi: 10.1037/1528-3542.5.3.251. [DOI] [PubMed] [Google Scholar]
- Van den Stock J, Righart R, de Gelder B. Body expressions influence recognition of emotions in the face and voice. Emotion. 2007;7(3):487–494. doi: 10.1037/1528-3542.7.3.487. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Rosen B, Farde L. Imaging the living human brain: Magnetic resonance imaging and positron emission tomography. Proceedings of National Academy of Sciences of the United States of America. 1997;94:2787–2788. doi: 10.1073/pnas.94.7.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana SR. The psychophysiology of disgust: Differentiating negative emotional contexts with facial EMG. Psychophysiology. 1993;30(3):279–286. doi: 10.1111/j.1469-8986.1993.tb03354.x. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: A new measure of emotion? Journal of Abnormal Psychology. 1988;97(4):487–491. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]
- Wager TD, Barrett LF, Bliss-Moreau E, Lindquist K, Duncan S, Kober H, et al. The neuroimaging of emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of emotions. New York: Guilford Press; 2008. pp. 249–271. [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: A meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Watson D. Mood and temperament. New York: Guildford Press; 2000. [Google Scholar]
- Watson D, Wiese D, Vaidya J, Tellegen A. The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology. 1999;76(5):820–838. [Google Scholar]
- Welte JW, Russell M. Influence of socially desirable responding in a study of stress and substance abuse. Alcoholism: Clinical and Experimental Research. 1993;17(4):758–761. doi: 10.1111/j.1530-0277.1993.tb00836.x. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science. 1998;7(6):177–188. [Google Scholar]