Abstract
Purpose
The goal of this study was to investigate the therapeutic potential of a novel immunotherapy strategy resulting in immunity to localized or metastatic HPV 16-transformed murine tumors.
Experimental design
Animals bearing E7-expressing tumors were co-immunized by lymph node injection with E7 49-57 antigen and TLR3-ligand (synthetic dsRNA). Immune responses were measured by flow cytometry and anti-tumor efficacy was evaluated by tumor size and survival. In situ cytotoxicity assays and identification of tumor-infiltrating lymphocytes and T regulatory cells were used to assess the mechanisms of treatment resistance in bulky disease. Chemotherapy with cyclophosphamide was explored to augment immunotherapy in late-stage disease.
Results
In therapeutic and prophylactic settings, immunization resulted in a considerable expansion of E7 49-57 antigen-specific T lymphocytes in the range of 1/10 CD8+ T cells. The resulting immunity was effective in suppressing disease progression and mortality in a pulmonary metastatic disease model. Therapeutic immunization resulted in control of isolated tumors up to a certain volume, and correlated with anti-tumor immune responses measured in blood. In situ analysis showed that within bulky tumors, T cell function was affected by negative regulatory mechanisms linked to an increase in T regulatory cells and could be overcome by cyclophosphamide treatment in conjunction with immunization.
Conclusions
This study highlights a novel cancer immunotherapy platform with potential for translatability to the clinic and suggests its potential usefulness for controlling metastatic disease, solid tumors of limited size, or larger tumors when combined with cytotoxic agents that reduce the number of tumor-infiltrating T regulatory cells.
Keywords: T Cells, Peptides, Tumor Immunity, Vaccination, HPV
Human papillomavirus (HPV)-associated tumors remain a significant healthcare problem worldwide, as the second leading cause of cancer mortality in women (1, 2), and the number one cause of cancer-related death in women in developing countries (3, 4). Although effective prophylactic HPV vaccines have recently been developed targeting the late (L1 and L2) genes (5), they are ineffective at eliminating pre-existing infection or HPV-related tumors whose cellular transformation and progression depend upon expression of the E6 and E7 early proteins (2). Therefore, a therapeutic vaccine triggering T cell immunity specific to the E6 and E7 early proteins of HPV 16 and 18 strains - those responsible for the pathogenesis of the majority of cervical cancers (6) – could offer a promising option to prevent the progression of HPV infection to cervical cancer, or the progression of early stage tumors to invasive disease (2, 7).
Recent advances in lymph node-targeted active immunotherapy, in our hands and others, have resulted in greatly improved CD8+ T cell responses against a variety of tumor antigens (8 - 10). Using a novel intra-lymphatic immunization approach consisting of the administration of a HPV 16 tumor-associated antigen (TAA) E7 49-57 peptide and a toll-like receptor 3 (TLR3) ligand (a synthetic double-stranded RNA molecule, polyinosinic-polycytidylic acid (pI:C)) as an adjuvant, we tested the potency and limitations of this approach in a well characterized HPV murine tumor model. The model utilized a highly malignant subclone (C3.43) of HPV 16-transformed B6 mouse embryo cells (11), transplanted into immune competent C57BL/6 mice, that was applicable for the evaluation of individual and metastatic tumors. This immunization approach brings together two distinguishing and potentially synergistic elements: i) targeted lymph node vaccination that has previously been tested in preclinical models and safety clinical trials (12, 13) and ii) the use of the pI:C adjuvant, previously demonstrated to greatly amplify immune responses, including MHC class I-restricted T cell immunity (14, 15).
Herein, we show that immunization yielded a dramatic expansion of E7 antigen-specific T lymphocytes. We demonstrate the applicability of this immunization platform to effectively treat solid tumors of limited size or rapidly progressing pulmonary metastatic disease, following prophylactic or therapeutic immunization. In addition, we define hurdles associated with active immunotherapy of bulky tumors and highlight a means to overcome those by targeting T regulatory cells (Tregs) using combination therapy with cyclophosphamide (CTX).
Materials and Methods
Mouse strains and tumor cell lines
Pathogen-free 8-10 week old female C57BL/6 mice were purchased from Jackson laboratory (Bar Harbor, ME). All animal studies were reviewed and approved by an IACUC institutional review committee. The HPV 16-expressing C3.43 tumor cell line was sub-cloned from C3 cells (11) generated from B6 mouse embryo cells transformed with an activated-ras oncogene and the complete HPV 16 genome. C3.43 cells were cultured in IMDM supplemented with 10% fetal bovine serum, 2mM L-glutamine, 50 mM 2-mercaptoethanol, and 1% penicillin/streptomycin.
Immunization
HPV 16 E7 49-57 (H-2Db) CTL epitope peptide (RAHYNIVTF) (11) was reconstituted in PBS at a final concentration of 0.5 mg/mL or 0.05 mg/mL and mixed with an equal volume of 0.5 mg/mL pI:C adjuvant (Sigma-Aldrich) in PBS. Mice received 25 μL per bilateral inguinal lymph node (IN) or 50 μL per subcutaneous (SC) injection of the E7 peptide + pI:C mixture. Each group of immunized mice, regardless of injection route, received 4 vaccinations on day 1, 4, 15, and 18, beginning at various time intervals prior to or following tumor challenge. Lymph node immunizations were done as described previously (10). Briefly, mice were anesthetized and an incision of 0.5-1 cm in length was made in the inguinal fold, exposing the inguinal lymph node, and a volume of 25 μL of each vaccine preparation was injected IN and the incision was closed with skin sutures.
C3.43 tumor challenge
For prophylactic vaccination studies, mice were immunized IN (day 1, 4, 15, and 18) and challenged SC with 105 C3.43 cells in the right flank on day 40. Mice were then re-challenged SC with 105 C3.43 cells on day 120 in the left flank. For therapeutic tumor studies, mice were SC challenged with 105 C3.43 cells in the right flank (day 0) and immunized IN with the E7 + pI:C vaccine (described above) beginning on day 7, 14, 20, or 28. Tumor growth was monitored twice a week with digital callipers and the volume was calculated using the formula: π × ((Length) × (Width)2 / 6).
Ex vivo analysis of antigen-specific T cells by tetramer
The antigen-specific CD8+ T cell response in immunized animals was measured by co-staining mononuclear cells isolated from blood after density centrifugation (Lymphocyte Mammal, Cedarlane Labs) with H-2Db E7 49-57 (RAHYNIVTF)-PE MHC tetramer (Beckman Coulter) and FITC conjugated rat anti-mouse CD8a (Ly-2) monoclonal antibody (BD Biosciences). Tetramer assay was conducted 7 days following the completion of the vaccination regimen (described above). Data were collected using a BD FACS Calibur flow cytometer and analyzed using Cell Quest software by gating on the lymphocyte population, collecting 2×104 CD8+ events for each sample, and calculating the percent of tetramer+ cells within the CD8+ T cell population.
Frequency and functional analysis of antigen-specific and Treg tumor-infiltrating lymphocytes (TILs)
Tumors were removed from sacrificed animals, homogenized with a scalpel, mixed with 0.1% collagenase buffer in PBS, and placed in a 37°C shaker for 1 hour. Each sample was filtered through a 40 μM filter (BD Biosciences) and TILs were enriched by density centrifugation. Antigen specific tumor-infiltrating CD8+ T cells were quantified by tetramer analysis (described above) and T-cell functionality was assessed by flow cytometry measuring the production of intracellular IFN-γ following E7 49-57 antigen stimulation. Briefly, TILs were stimulated / stained with E7 49-57 tetramer-PE, co-stained with FITC conjugated rat anti-mouse CD8a (Ly-2) monoclonal antibody, fixed / permeabilized (Cytofix/Cytoperm, BD Biosciences) and stained with anti-IFN-γ-APC antibody (BD Biosciences) prior to washing and flow cytometric analysis. The frequency of CD4+/CD25+/Fox P3hi tumor-infiltrating Tregs was measured by co-staining with intracellular Fox P3 and cell surface CD4 and CD25 antibodies (mouse reg t cell kit, E Bioscience).
In vivo analysis of T cell response
Specific targets were prepared from splenocytes isolated from syngeneic mice by density centrifugation and resuspended in HL-1 serum free medium (Cambrex) at a concentration of 107 cells/ml. Target cells were pulsed with 10-6M HPV 16 E7 49-57 (RAHYNIVTF) peptide or 10-6M MART-1 melan A 26-35 (ELAGIGILTV) control peptide for 1 hour (37°C, 5% CO2), washed and resuspended in PBS, and then labelled with 1.5μM CFSE (high) and 0.3μM CFSE(low) respectively, according to the manufacturer's instructions (CellTrace™ CFSE Cell Proliferation Kit, Molecular Probes). Immunized or control tumor-bearing mice were intravenously injected in the tail vein with an equal ratio of 107 cells of each CFSE(high) and CFSE(low) labelled target cell populations. At 18 hours, mononuclear cells from tumor and spleen were isolated by density centrifugation and the frequency of CFSE positive cells analysed by flow cytometry, gating on the lymphocyte population and measuring the percentage of CFSE positive cells in the FL-1 fluorescent channel. A decrease in the percentage of CFSE(high) (HPV 16 E7 49-57) target cells relative to CFSE(low) (MART-1 Melan A 26-35) control cells indicated specific lysis in vivo. The formula used to calculate the % specific lysis is as follows [(1 - % CFSEhigh / % CFSElow) from immunized mice - (1 - % CFSEhigh / %CFSElow) from naïve mice] × 100.
Combination therapy with CTX
A dose of 30 mg/kg CTX (Sigma) in C57BL/6 mice was found (by dose titration experiments) to be non cyto-reductive, non toxic and immune modulating (data not shown). For adjunctive therapy studies, mice were SC challenged with 105 C3.43 cells in the right flank on day 0, treated with 2 injections of 30 mg/kg CTX on day 14 and 18, and vaccinated IN with E7 + pI:C or controls on day 20, 24, 34, and 38. A second therapeutic cycle was administered beginning on day 45.
Pulmonary metastasis model
Mice were challenged intravenously in the tail vein with 5 × 105 C3.43 cells and then vaccinated IN with E7 + pI:C or controls beginning on day 1, 8, or 15, post tumor challenge. Animal health was monitored daily, moribund mice were humanely euthanized, and pulmonary tissue removed and analyzed visually and or by standard H&E staining to assess tumor load (Supplemental Fig 3).
Statistical Analysis
Statistical Analysis was performed using a two-tailed Student's t-test assuming equal variances. For survival experiments the log rank test was used. Differences were considered statistically significant for p < 0.05.
Results
Intra-lymph node immunization with E7 peptide and pI:C elicits protective immunity against HPV-transformed tumor challenge
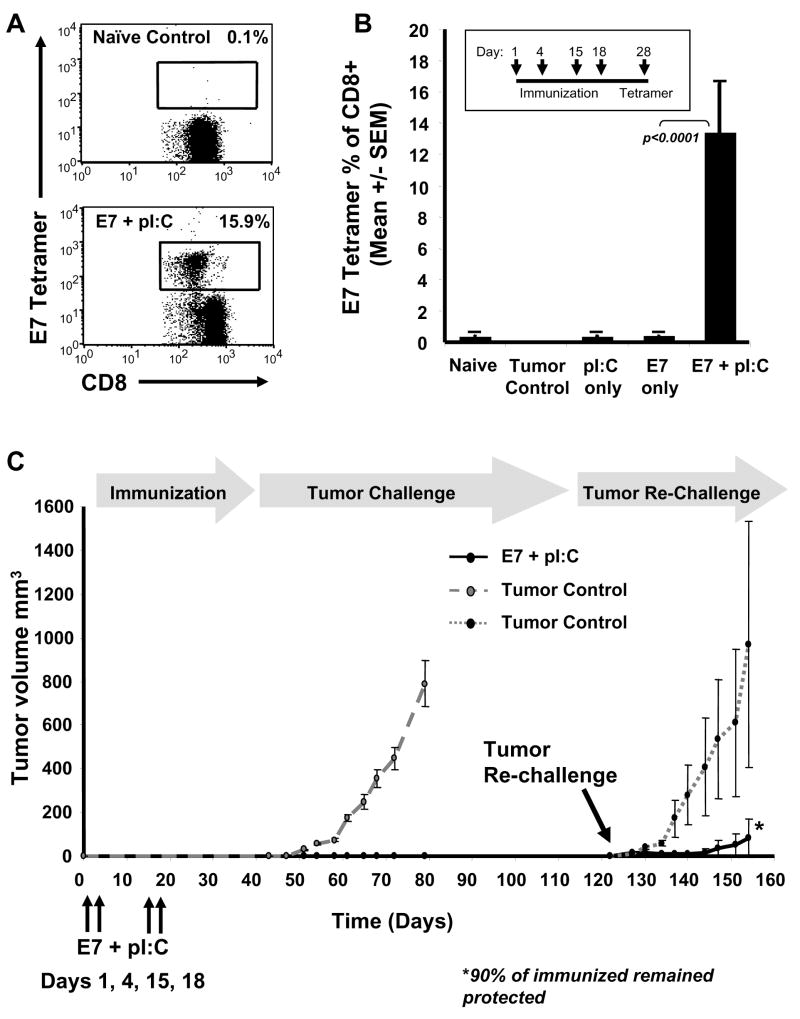
Direct lymph node injection of the HPV 16 E7 49-57 (RAHYNIVTF) epitope peptide in combination with pI:C dsRNA adjuvant resulted in robust antigen-specific T cell responses (in the range of 15% of the total circulating CD8+ T cells) in mice as measured by tetramer analysis (Fig. 1A,B). In contrast, E7 peptide or pI:C alone were not effective at inducing specific immunity (Fig. 1B). To assess the prophylactic nature of this vaccination approach, immunized mice were inoculated SC (day 40) with 105 HPV 16-transformed E7-expressing C3.43 cells and no evidence of tumor growth was detected up to 120 days. In addition, 90% of the mice remained protected following a second tumor challenge on day 120, with tumor progression significantly delayed in those few animals that did develop tumors (Fig. 1C).
Fig. 1.
Intra-lymph node immunization with E7 peptide and pI:C elicits robust immunity protective against tumor challenge. Flow cytometry dot plots (A) comparing the tetramer response from an immunized (E7 + pI:C) and naïve control mouse representative of data shown in (B). The results are expressed as the frequency of E7 tetramer+ CD8+ T cells relative to the total CD8+ T cell population measured in peripheral blood 10 days after the completion of the immunization protocol. Intra-lymphatic vaccination with E7 49-57 HPV antigen and pI:C resulted in substantial E7-specific CD8+ T cell responses, whereas pI:C or E7 49-57 peptide alone had no significant impact on immune response when compared to tumor control or naïve mice (B). The mean E7 tetramer response +/- SEM for each group is shown (n=10). HPV 16 E7 49-57 antigen-specific immune response correlated with tumor protection (C). Immunization of mice with E7 49-57 peptide and pI:C (n=20) resulted in complete protection from subcutaneous challenge with 105 HPV transformed C3.43 tumor cells as compared to tumor control mice (n=20) and 90% protection following a tumor re-challenge compared to second group of tumor control mice (n=3).
Therapeutic intra-lymph node immunization with E7 peptide and pI:C mediates immunological regression of solid tumor
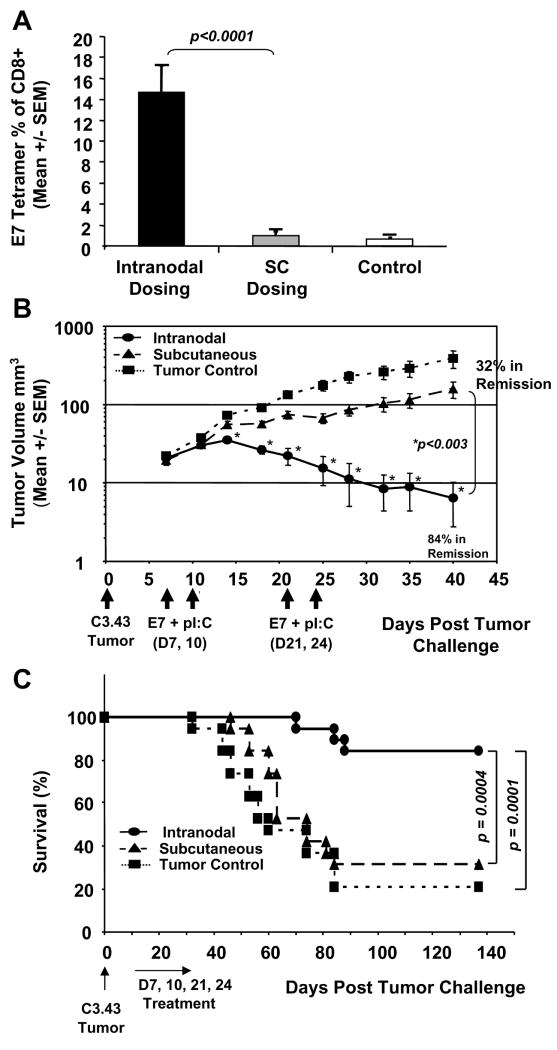
Next, we evaluated this approach against established C3.43 tumors to assess the efficacy of intra-lymph node immunotherapy. When tumors were clinically evident and palpable, mice were immunized with E7 peptide and pI:C in lymph nodes, or by SC injection with an equivalent amount of vaccine on day 7, 10, 21, and 24. Tetramer results (day 31) indicated that only mice immunized via lymph node injection generated statistically significant (p < 0.0001) E7 49-57 specific immune responses, with an average of 14.5% compared to SC dosed and unimmunized control mice (Fig. 2A), with average responses of 1.0% and 0.7%, respectively. As shown in Figure 2B, tumors in mice immunized by lymph node injection began to regress on day 15, coinciding with elevated immunity, and resulting in 84% of animals in complete tumor remission by day 40. This response was significantly superior to that of animals dosed SC (p < 0.003) whose tumor progression was delayed compared to tumor controls. Nevertheless, this apparently modest response in mice dosed SC resulted in 32% of animals in complete remission (Fig. 2B). Untreated tumor-bearing mice displayed background levels of E7 tetramer staining (Fig. 2A), potentially reflecting exposure to endogenous tumor antigen, although their tumors progressed exponentially without regression, as expected (Fig. 2B). Complementing these findings, intranodal immunization ultimately translated to significantly enhanced long-term survival compared to SC immunized (p = 0.0004) or tumor control (p = 0.0001) mice (Fig. 2C). In an independent study, intranodal vaccination with E7 peptide or pI:C adjuvant alone offered little, if any, therapeutic benefit when compared to the combination of E7 peptide + pI:C (Supplemental Fig. 1A and B). Therefore, a key prerequisite to achieve a substantial amplification of immunity (Fig 1B, 2A), tumor regression (Fig. 2B, Supplemental Fig. 1A) and survival (Fig. 2C, Supplemental Fig. 1B) was the direct intra-lymph node administration of E7 peptide and pI:C adjuvant, which could not be reproduced by E7 peptide alone, pI:C alone, or SC administration of the vaccine.
Fig. 2.
Intra-lymph node immunization with E7 peptide and pI:C mediates immunological regression of solid tumor. In a therapeutic model of HPV 16, the anti-tumor efficacy of intranodal versus subcutaneous (SC) dosing was compared. C57BL/6 mice were challenged SC with 105 C3.43 HPV tumor cells on day 0 and then immunized with a mixture of E7 49-57 peptide and pI:C in each bilateral inguinal lymph node (n=19) or an equivalent amount of vaccine SC (n=19) on day 7, 10, 21, and 24. The immune response was measured by E7 49-57 tetramer staining on day 31 from peripheral blood (A) and tumor size for each group was calculated and compared to untreated tumor challenged control (n=19) mice (B). Lymph node immunized mice generated statistically significant E7 49-57 specific immune responses with an average of 14.5% tetramer positive CD8+ T cells (A) compared to SC dosed mice (p < 0.0001). In addition, tumors in mice immunized in the lymph node began to regress on day 15 resulting in 84% of animals in remission at day 40 (B). This response was significantly superior to animals dosed SC (p < 0.003) whose tumor progression was only delayed compared to tumor controls but resulted in 32% of animals in disease remission. Untreated tumor control mice displayed background levels of E7 tetramer staining (A) and their tumors progressed exponentially without regression as expected (B). Log-rank statistical tests confirmed that survival in the E7 + pI:C group was significantly prolonged when compared to animals immunized SC (p = 0.0004) or when compared to tumor controls (p = 0.0001) (C).
We then showed that immune regression of established tumors depends on both the magnitude of immune response and the tumor size upon initiation of vaccination. The results summarized in Table 1 demonstrate that immunization early in the course of disease resulted in a much higher percentage of complete remissions (69%). In addition, a post-hoc analysis of measured immune response versus tumor outcome in earlier stage disease showed a clear correlation between the frequency of specific T cells induced following day 7 immunization and tumor regression (Supplemental Fig. 2A, B). When immunization was initiated later in the course of disease when tumors were considerably larger, the apparent impact of immunotherapy on tumor progression was minimal, despite the induction of substantial immune responses (Table 1).
Table 1.
Immune regression of established tumors depends on both the magnitude of immune response and tumor size at the initiation of vaccination.
|
*Initiation of Immunotherapy (Day post-challenge) |
†Tumor Volume at initiation of Immunotherapy (mm3) |
‡E7 Tetramer Response (% of CD8) |
Disease Outcome (%) |
|---|---|---|---|
| Day 7 (n=83) | 11.8+/-2.0 | 19.0+/-2.2 p = 0.01 | In Remission (69%, n = 57) p < 0.001 |
| 11.1 +/-2.3 | 9.5 +/- 2.2 | Progressing (31%, n=26) |
|
| Day 14 (n=40) | 24.9 +/- 5.8 p = 0.2 |
19.3+/-2.9 | In Remission (8%, n=3) p = 0.03 |
| 39.2 +/- 3.5 | 19.7+/-2.7 | Progressing (92%, n = 37) |
|
| Day 20 (n=40) | 26.0 +/- 14.3 p = 0.048 |
18.2+/-8.1 | In Remission (8%, n = 3) p = 0.02 |
| 137+/-15.3 | 21.6+/-2.7 | Progressing (92%, n = 37) |
|
| Day 28 (n=10) | N/A | N/A | In Remission (0%) |
| 238.9 +/- 64.0 | 31.1 +/-3.9 | Progressing (100%, n = 10) |
All mice received lymph node-targeted immunotherapy consisting of four injections of E7 49-57 peptide + pI:C on day 1, 4, 15 and 18 starting at indicated time intervals following C3.43 tumor challenge.
Maximum tumor size at the initiation of immunotherapy that completely regressed for each treatment group, day 7, 14, and 20, was 37.8, 81.3, and 112.1 mm3 respectively.
Immune response was evaluated by E7 49-57 tetramer assay 10 days following the last vaccination for each group.
CD4+/CD25+/FoxP3HI Tregs correlate with impaired function of TILs within advanced tumors and are reduced following CTX treatment
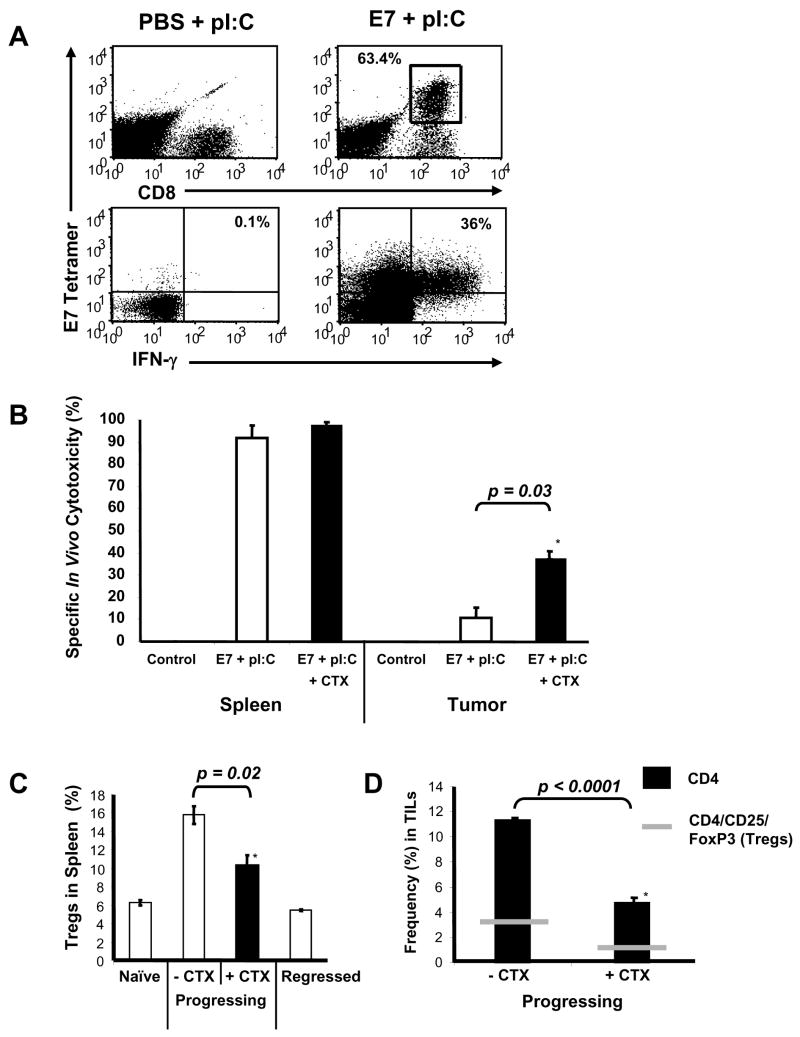
These results led us to hypothesize that either the tumor-specific T cells generated by this approach may lack sufficient homing signals to migrate into established tumors of larger size, or alternatively, that immune checkpoints within the tumor microenvironment may be limiting the effector function of the T cells despite effective local recruitment. To address this question, mice were immunized on day 20, 24, 34, and 38 following inoculation with C3.43 tumors, TILs were isolated from the tumors of E7 peptide + pI:C or PBS + pI:C immunized mice (as control), and their phenotype and functional status were assessed by flow cytometry. In Figure 3A (upper-right dot plots), TILs co-stained with E7 tetramer and anti-CD8 identified a large population of E7-specific T cells, clearly demonstrating their ability to infiltrate tumors following E7 peptide + pI:C immunization. However, E7-specific TILs were not detected in tumors isolated from PBS + pI:C immunized mice, showing the scarcity of endogenous T cells reactive against this epitope in the absence of immunization (Fig. 3A, upper-left dot plot). Furthermore, the functional status of these HPV-16 antigen-specific TILs was evaluated ex vivo by E7 49-57 peptide stimulation, resulting in intracellular IFN-γ production measured by flow cytometry. In the lower panels of Figure 3A, a significant proportion of E7-specific TILs (∼ 36%) could produce IFN-γ cytokine upon ex vivo peptide-stimulation, demonstrating a latent functional competency of the TILs. These data confirmed that E7-specific T cells resulting from intra-lymphatic immunization did migrate to established tumors and were functionally competent when tested ex vivo, but did not formally demonstrate their in situ functionality upon antigen engagement. To measure the TILs' function in situ, we used an in vivo CFSE cytotoxicity assay. To that aim, mice were inoculated with C3.43 tumors (day 0), immunized with E7 peptide + pI:C or PBS + pI:C control vaccine (day 20, 24, 34, and 38), and then challenged IV with an equal ratio of CFSEhigh (HPV 16 E7 49-57 labelled) and CFSElow (MART-1/Melan A 26-35 labelled) syngeneic splenocytes. Mononuclear cells from tumor and spleen were isolated 18 hours later and target-specific lysis was analyzed by flow cytometry. Interestingly, E7 + pI:C immunized mice with progressing tumors effectively cleared the HPV 16 E7 49-57 pulsed target cells in spleen, but not effectively within tumors (Figure 3B). These results led us to hypothesize that immune checkpoints, such as the one represented by CD4+/CD25+/FoxP3HI Tregs, likely to be present in high frequency in established tumors, may be responsible for this T cell suppressive effect (16). To assess this, we analyzed the CD4+/CD25+/FoxP3HI Treg frequency by flow cytometry in spleens of immunized mice whose tumors were progressing compared to naïve mice or mice whose tumors had regressed (Fig. 3C). Mice with progressing tumors had approximately 3-fold more Tregs in spleen than naïve or cured animals. In addition, a high frequency of tumor-infiltrating CD4+ T cells and CD4+/CD25+/FoxP3HI Tregs could be detected in mice with progressing disease, suggesting a potential explanation for the reduced efficacy of active immunotherapy in a more advanced disease setting (Fig. 3D, left bar). Previous studies have reported that CTX treatment can augment tumor vaccine immunity by reducing the frequency and abrogating the activity of Tregs in vivo (17 - 19). When immunized mice with progressive tumors were treated with a single intraperitoneal injection of 100 mg/kg CTX, the frequency of Tregs in spleen (Fig. 3C) and the number of CD4+ T cells as well as CD4+/CD25+/FoxP3HI Tregs in tumor (Fig. 3D, right bar) were significantly decreased. When directly tested in the in vivo CFSE cytotoxicity model, CTX treatment resulted in enhanced killing of E7 49-57 peptide-labelled syngeneic target cells in tumors of immunized mice (p = 0.03) and had no adverse effect on specific target lysis in spleen (Fig. 3B). These data provide a rationale for combining chemotherapy with immunotherapy for the treatment of late-stage tumors that may be only marginally affected by vaccination alone (Table 1).
Fig. 3.
CD4+/CD25+/FoxP3HI Tregs impair in situ function of TILs in advanced tumors and are reduced following CTX treatment. Mice were inoculated with 105 HPV-16 transformed C3.43 tumor cells on day 0 and immunized with E7 49-57 HPV peptide and pI:C in bilateral inguinal lymph nodes on day 20, 24, 34, and 38 (n=3). Immunization control mice received PBS + pI:C in bilateral inguinal lymph nodes on day 20, 24, 34, and 38 (n=3). (A) Flow cytometry dot plots comparing the frequency (top panel) and the functional ability to produce IFN-γ (bottom panel) of E7 49-57 antigen-specific TILs from representative E7 + pI:C immunized or tumor control (PBS + pI:C) mice. (B) Impaired in situ function of TILs measured by in vivo cytotoxicity assay. E7 + pI:C immunized mice with progressing tumors cleared greater than 90% of HPV 16 E7 49-57 labelled target cells in spleen whereas TILs from the same mice had little cytotoxic effect on target cells within established tumors. CTX treatment (100 mg/kg, IP) in a second group of E7 + pI:C immunized mice (n=3) with progressive disease resulted in enhanced killing of specific target cells in tumors (p = 0.03) and had no adverse effect on target cell lysis in spleen. (C) Immunized mice bearing HPV-16 transformed tumors (n=3) displayed approximately three fold higher numbers of CD4+/CD25+/FoxP3+ Tregs in spleen compared to naïve mice (n=3) or immunized mice whose tumors completely regressed (n=3). The level of CD4+/CD25+/FoxP3+ Tregs could be reduced in the spleen (C, n=3) and the levels of CD4+ and CD4+/CD25+/FoxP3+ cells could be reduced in tumor (D) of mice with progressive disease by a single intraperitoneal injection of 100 mg/kg CTX (n=3 mice / group).
Adjunctive therapy with CTX enables immunotherapy in an advanced disease setting
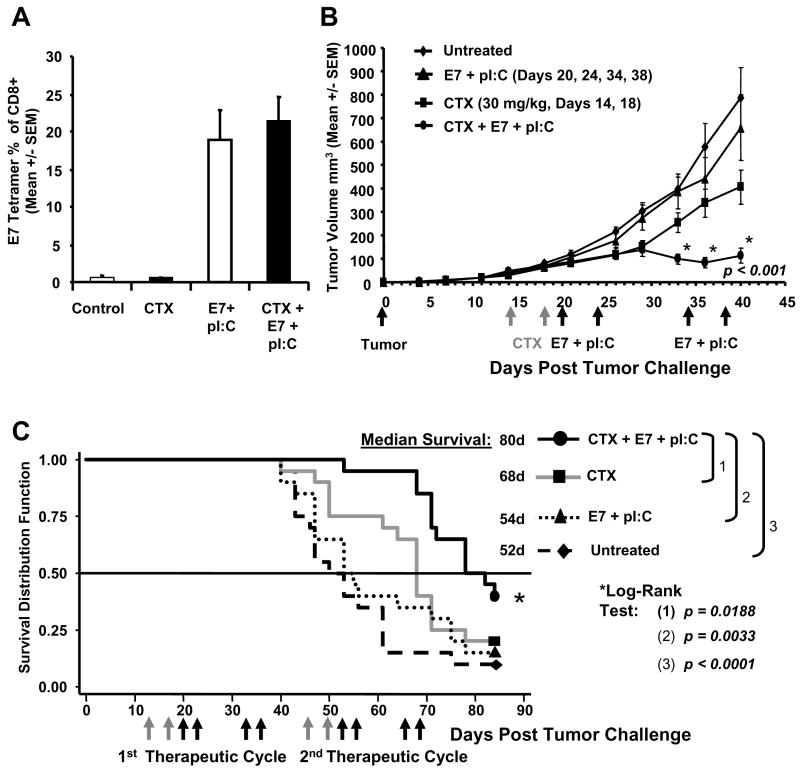
To test this hypothesis and determine if combination therapy with CTX would enable active immunotherapy in a more advanced disease setting, mice were inoculated SC with 105 C3.43 cells, and treated with either CTX (30 mg/kg) alone on day 14 and 18, immunized with E7 + pI:C alone on day 20, 24, 34, and 38, or treated with CTX and then immunotherapy. The immune response, measured by E7 49-57 tetramer staining on day 45 from peripheral blood, showed that the immunized-only group (E7 + pI:C) displayed HPV-specific immune responses in the range of 20%, with no observed inhibition of immune response in animals treated with CTX + immunotherapy (Fig. 4A). Furthermore, CTX + immunotherapy induced significant tumor regression (p < 0.001) compared to immunotherapy alone, dose-matched chemotherapy alone, or untreated tumor controls (Fig. 4B). In an attempt to maximize the potency of the immunization regimen assisted by CTX and to generate a more robust response in a relatively advanced tumor setting, a second treatment cycle was initiated and Kaplan-Meier estimates of the survival function were obtained for each of the four treatment conditions (Fig. 4C). Based on log-rank analysis, the survival in the CTX + immunotherapy group was significantly longer than the survival in the control group (p < 0.0001), the CTX only group (p = 0.0188) and the immunotherapy (E7 + pI:C) only group (p = 0.0033). The median survival time in the CTX + immunotherapy group was also longer (80 days) compared to the CTX only group (68 days) and the immunotherapy only group (54 days) in a setting associated with rapid tumor progression and mortality (median survival of control tumor-bearing mice was 52 days). Furthermore, it was not surprising that CTX alone resulted in some delayed tumor progression compared to untreated tumor control mice (Fig. 4B, p = 0.01) although, as discussed above, this effect did not translate to a significant survival benefit (Fig. 4C). These findings demonstrate that the sequential administration of CTX and intra-lymphatic HPV vaccination significantly improved the disease outcome in later stage disease.
Fig. 4.
Adjunctive therapy with CTX enables immunotherapy in a more advanced disease setting. Mice were inoculated with 105 HPV-16 transformed C3.43 tumor cells on day 0, treated with CTX on Day 14 and 18 (n=20), immunized with E7 49-57 HPV peptide and pI:C in bilateral inguinal lymph nodes on day 20, 24, 34, and 38 (n=20), or treated with CTX then E7 + pI:C immunotherapy (n=20). Immune response (A) and tumor progression (B) was compared to untreated tumor control mice (n=20). The immune response following immunotherapy was measured by E7 49-57 tetramer staining on day 45 from peripheral blood. The immunized only group (E7 + pI:C) displayed HPV specific immune responses in the range of 20% with no observed inhibition of immune response in animals treated with CTX + E7 + pI:C which generated a similar tetramer response. The naïve control (n=5) and CTX control groups generated background levels of tetramer staining (A). CTX + E7 + pI:C induced significant tumor regression (p < 0.001) compared to E7 + pI:C immunotherapy alone, CTX alone, and untreated tumor controls (B). Evaluation of adjunctive therapy on animal survival (C). A second therapeutic cycle was administered with animals receiving CTX on day 46 and 50 (n=20), lymph node immunization with E7 + pI:C on day 52, 56, 65, and 69 (n=20), or treated with CTX + E7 + pI:C (n=20). Grey arrows indicate CTX treatment and black arrows indicate immunization days. Log-Rank statistical tests confirmed that survival in the CTX + E7 + pI:C group was significantly longer than survival in the control group (p < 0.0001, n=20), the CTX only group (p = 0.0188) and the E7 + pI:C immunotherapy only group (p = 0.0033).
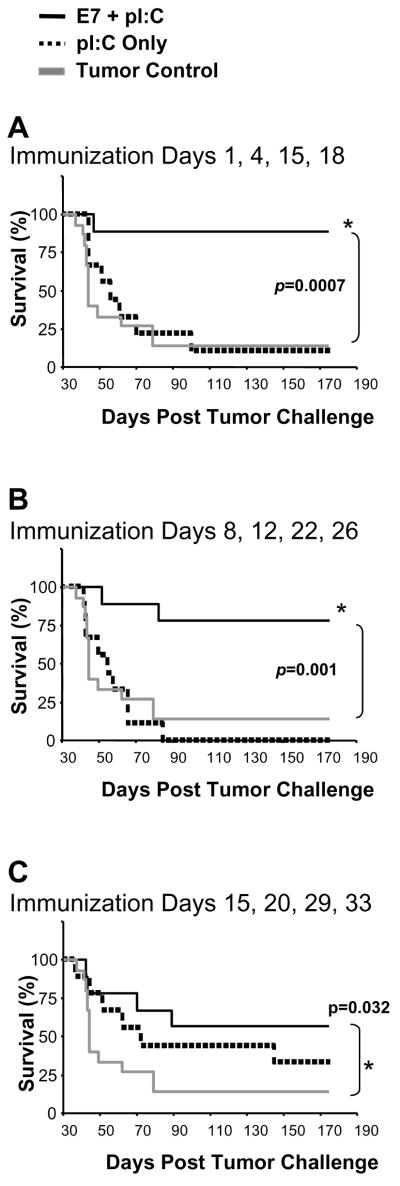
Effective control of rapidly progressing metastatic pulmonary tumor by intra-lymph node immunization with E7 peptide and pI:C
To test the potency of lymph node-targeted active immunotherapy in a setting of rapidly progressing metastatic disease, we injected mice intravenously with 5 × 105 C3.43 cells and evaluated the disease outcome following E7 + pI:C vaccination. We compared three vaccine time courses beginning on days 1, 8, or 15 post-tumor challenge to assess the therapeutic benefit of our immunotherapy method. When mice were immunized by lymph node injection with E7 + pI:C beginning on day 1 (Fig. 5A), day 8 (Fig. 5B), or day 15 (Fig. 5C), a significant beneficial impact on disease progression could be measured in all cases. More specifically, 89%, 78%, and 57% respectively, of the immunized mice were still alive at day 175 post-tumor challenge. The survival outcome of E7 + pI:C immunized mice was significantly superior to that of unimmunized tumor control and pI:C adjuvant-injected mice for the day 1 (Fig. 5A) and 8 (Fig. 5B) treatment regimens, with p-values equal to 0.0007 and 0.001 respectively. For mice that received immunotherapy with E7 + pI:C on day 15 following tumor injection (Fig. 5C), the survival outcome was significantly better than that of tumor control mice (p = 0.032) and more favorable, although not statistically different, from the pI:C adjuvant injected group which showed a subtle beneficial impact on disease progression. This result may be due to the immune modulating effect of pI:C via activation of TLR3 downstream pathways on immune cells or ‘somatic’ cells. In addition, tissue histology (Supplemental Fig. 3) showed no residual tumors in animals effectively treated with E7 + pI:C, while the control mice and the pI:C injected mice developed multiple lung tumors during the same time interval, explaining their deteriorated clinical status. In addition, systematic histological analysis of pulmonary tissue at day 8 after tumor challenge of naïve mice (the start of effective therapeutic immunization) clearly demonstrated the presence of multiple tumors of limited size within the lung parenchyma (Supplemental Fig. 3, top panel). This provided a more quantitative perspective on the disease burden manageable by this immunization strategy.
Fig. 5.
Control of pulmonary metastases by intra-lymph node immunization with E7 peptide and pI:C. Mice were injected intravenously with 5 × 105 C3.43 tumor cells and then immunized with one of the following intranodal E7 + pI:C vaccine time courses: day 1, 4, 15, and 20 (A); day 8, 12, 22, and 26 (B); or day 15, 20, 29, and 33 (C). Survival curves for each group (A, B, C) are shown and the outcome for E7 + pI:C immunized mice (n = 9) was compared to pI:C only (n = 9) and untreated tumor control mice (n=9). Log-Rank statistical tests confirmed that survival in the E7 + pI:C group for each vaccine time course (A, B and C) was significantly longer than survival in the tumor control group (p = to 0.0007, 0.001, and 0.032 respectively) and the pI:C only group when immunization began on day 1 (A, p = 0.006) and day 8 (B, p = 0.004) but not day 15 (C, p = 0.06).
In summary, active immunotherapy was highly effective at inducing clearance of pulmonary metastatic tumors and preventing overt disease and mortality in this rapidly progressing tumor setting.
Discussion
Cancers associated with ‘non-self’ tumor antigens linked to the pathogenesis of the disease, such as HPV antigen-expressing tumors, remain particularly appealing therapeutic opportunities due to the increased likelihood of eliciting effective immunity. Nevertheless, despite promising evidence obtained preclinically, some encouraging data in the clinic and efforts to design next generation approaches (2), there are still no approved therapeutic cancer vaccines and, in the particular case of HPV tumors, there are no investigational agents in late-stage clinical development.
Herein, we provided evidence supporting the use of a novel immunization platform leading to a robust induction of T cells against a relevant TAA (HPV 16 E7), with the resulting T cell repertoire effectively dominated by functional peptide-specific CD8+ T cells, recognizing a murine epitope (E7 49-57). More specifically, co-exposure of T cells within the lymph node microenvironment to antigen and a synthetic TLR3 ligand (pI:C) as a prototype adjuvant (15) yielded a high frequency of specific CD8+ MHC class-I restricted T cells (in the range of 1/10 CD8+ T cells), otherwise achievable in mice only by infection with select viruses or by genetic manipulation. This builds on the previously reported findings that intra-lymph node administration of non-replicating immunizing vectors such as peptides and plasmids, which typically have limited pharmacokinetic and pharmacodynamic effects if delivered using conventional parenteral means, greatly improves on the magnitude of immune response (8-10, 13) and points to potential improvements for other protein or peptide antigen-based immunization platforms (20, 21). In addition, use of intra-lymph node injection with synthetic, non-replicating vectors offers a straightforward means to achieve a substantial expansion of TAA-specific T cells, rather than using more complex immunization platforms such as liposomes, viral and bacterial vectors, or cell-based approaches (22-32) that may be associated with significant translational challenges and safety or manufacturing issues. Furthermore, even when a substantial magnitude of immunity is generated against a TAA, it is not clear whether there are intrinsic limitations to active immunotherapy associated with specific clinical settings. Thus, the question arises: Could improved cancer immunotherapy regimens be effective alone or would they require combination with traditional treatment approaches (e.g., chemotherapy) to be efficacious in a range of disease settings?
To address this question, we utilized an HPV tumor model that served the purpose of exploring key aspects of the translatability of the lymph node immunization technology described above, although certain inherent limitations of the model should be noted, such as the relevance of the select mouse HPV epitope to man. In this model, lymph node immunization with E7 + pI:C prevented tumor formation in a prophylactic setting and was associated with persistent immune memory (Fig. 1). In addition, immunotherapy, initiated when tumors were palpable yet limited in size (7 days after tumor challenge), translated into a high rate of tumor regression and survival with anti-tumor efficacy dependent on the co-administration of both the antigen and the adjuvant (Fig. 2 and Supplemental Fig. 1). By retrospectively stratifying treated mice into two groups - those that displayed an objective tumor response and those whose tumors continued to progress - a significant difference in epitope-specific CD8+ T cell response emerged, illustrating the critical importance of generating a substantial tumor antigen-specific immune response as a prerequisite for tumor regression (Table 1 and Supplemental Fig. 2). A systematic analysis of the efficacy of therapeutic vaccination at later time points following tumor challenge (days 14, 20, and 28) showed that, despite achieving immune responses of similar magnitude, the impact on tumor progression was quite limited when tumors at the initiation of vaccination were larger (Table 1).
To address this, we evaluated potential mechanisms responsible for treatment resistance in bulky disease. Using a CFSE in vivo cytotoxicity assay, we directly showed that tumor-specific T cells in the spleen cleared antigen-expressing target cells rapidly but were unable to do so within the tumor of the same animal, despite their local recruitment in substantial numbers and their ex vivo functionality. These data highlight the importance of the tumor microenvironment in regulating the activity of tumor-specific T cells and was confirmed by the following observations: i) CD4+/CD25+/Fox-P3 Tregs were present within tumors; ii) there was a correlation between the number of local Tregs and progressing or regressing tumor status; and iii) upon co-treatment with CTX, an alkylating agent known to interfere with Tregs (17-19), their number in spleen and tumor was significantly diminished (Fig. 3). Integrating tumor vaccines with standard chemotherapeutic drugs is a highly attractive approach due to the wide use of cytotoxic chemotherapy in the treatment of most malignancies, and thus we combined CTX with immunotherapy in an attempt to treat late-stage cancer. Chemoimmunotherapy with CTX was accompanied by enhancement of intra-tumoral activity of specific T cells, significant tumor growth suppression, and increased survival of treated mice in late-stage cancer following a second therapeutic immunization cycle (Fig. 4). This strategy was supported by a recent study showing that continuous immunization against a select tumor antigen resulted in sustained and elevated immunity (10). Although we have not formally generated evidence ruling out other immune escape mechanisms within tumors, the enabling effect of CTX relative to vaccination strongly suggested that this combination approach rendered E7-expressing tumor cells susceptible to immune-mediated clearance by changing the tumor milieu, resulting in a lower frequency of Tregs. In synergy with this interpretation, there is an accumulating body of evidence in support of the effect of CTX on Tregs and its significant immune modulating activity in relation to cancer vaccines (33, 34). Our data also suggests that the immune microenvironments in tumor and secondary lymphoid organs are quite different, specifically in regard to the Tregs' impact on CD8+ T cell function in tumor versus spleen, and we suggest the following possible explanations: i) Treg cells within the tumor site are more active in suppressing the anti-tumor effector cells; ii) Tregs within the tumor microenvironment act in concert with other immune suppressive mechanisms that are not active in spleen, as in the excess production of suppressive cytokines such as IL-10, TGFβ and others; and/or iii) infiltrating anti-tumor effector T cells are more susceptible to the effect of Treg cells. While these possibilities are not mutually exclusive and alternate mechanisms may also be at work, the results shown here do clearly support the use of CTX to augment immunotherapy in late-stage disease, with potential translational value.
Finally, we tested the applicability of this immunization platform in a setting of rapidly progressing pulmonary metastatic disease. Mice infused intravenously with HPV-transformed C3.43 cells rapidly developed multiple lung tumors (evident by microscopy as early as 8 days after challenge), progressed expeditiously, and became moribund within several weeks. Initiating therapeutic lymph-node vaccination within two weeks after tumor challenge resulted not only in robust induction of immunity, but also prevented disease progression to full-blown clinical manifestation as compared to untreated controls (Fig. 5). This was mirrored by the lack of residual tumors assessed macroscopically and microscopically, supporting an immune-mediated clearance of tumor cells and progressing tumor lesions within the lung parenchyma (Supplemental Fig. 3).
In conclusion, innovative immunotherapy approaches hold great promise for the treatment of cancer by harnessing a patient's immune system to eradicate their malignant neoplasms. Our findings describe a straightforward method to achieve substantial anti-tumor immunity and point to disease scenarios where immunotherapy may have the greatest potential, whether as a stand-alone therapy or combined with traditional treatments. The results of our study emphasize four key complementary aspects important to the development and translation of effective active cancer immunotherapies: i) selection of relevant antigens to which patients can rapidly mount substantial immune responses; ii) use of strongly immunogenic vaccination approaches, such as targeted lymph node vaccination, that result in high-magnitude, functional anti-tumor T cell responses; iii) selection of appropriate disease settings for preclinical and clinical testing that maximize the therapeutic potential; and iv) use of adjunctive therapy to overcome immune-suppressive mechanisms associated with larger tumors.
Supplementary Material
Fig. 1. Combinatorial intranodal delivery of tumor antigenic peptide and adjuvant is required for therapeutic efficacy. Intra-lymphatic vaccination with E7 49-57 HPV antigen and pI:C adjuvant resulted in significant tumor regression (A) and survival (B). Log-Rank statistical tests confirmed that survival in the E7 + pI:C group was significantly prolonged when compared to the control group (p = 0.0007) and the E7 peptide only group (p = 0.01) but was not significantly different from the pI:C only group (p = 0.1) although tumor progression was profoundly delayed (A, p < 0.02). N = 10 mice / group.
Fig. 2. Disease outcome correlates with elevated tumor-specific immune responses in mice receiving immunotherapy beginning 7 days after tumor challenge. Tumor volume results complied from five independent therapeutic tumor studies, comparing E7 + pI:C immunized mice (n=83) segregated based on disease outcome, show 69% (n=57) with regressing tumors compared to 31% (n=26) of mice with progressing disease (A). Mice with regressing tumors (n=57) generated a significantly higher E7 tetramer response (19.0 +/- 2.2, p = 0.01) compared to progressing mice (9.5 +/- 2.2, n=26) (B).
Fig. 3. Photographs of whole lung preparations and H&E staining of pulmonary tissue sections, demonstrating recovery from metastatic disease. As early as day 8 (top panel), metastatic tumor masses of various sizes are detected within the pulmonary tissue following i.v. injection of 5 × 105 C3.43 tumor cells into non-immunized mice. H&E sections of pulmonary tissue and photographs of lungs isolated on day 47 following tumor challenge from tumor control mice or mice injected beginning on day 8 with pI:C only show multiple tumors. In contrast, no residual tumors were observed in pulmonary tissue isolated on day 47 from animals effectively treated on day 8 by E7 + pI:C immunization (photomicrographs representative of 3 mice sampled from each experimental group).
Acknowledgments
This study was partially supported by NIH grant CA74397. W. M. Kast holds the Walter A. Richter Cancer Research Chair.
The Authors would like to thank Tessa Laurell Roper for editorial review of the manuscript.
Footnotes
Statement of Translational Relevance: Despite progress in cancer therapy, treatment of oncological diseases by translating novel immunotherapeutic approaches remains an important goal. Herein, we evaluated in a preclinical murine model several key parameters of an investigational platform technology translatable to the clinic. We show that immunization by direct intra-lymph node administration with a model HPV 16 E7 49-57 peptide and TLR3 adjuvant yielded a considerable expansion of functional HPV antigen-specific T cells, resulting in the eradication or effective control of localized and metastatic tumors. In addition, we bring evidence in support of combination chemoimmunotherapy in localized, advanced disease to counteract intra-tumoral immune suppressive mechanisms. Overall, our findings emphasize the translatability of active immunotherapy by lymphatic immunization and shed light on disease settings most amenable to cancer immunotherapy.
References
- 1.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 2.Hung C, Ma B, Monie A, Tsen S, Wu T. Therapeutic human papillomavirus vaccines: current clinical trials and future directions. Expert Opin Biol Ther. 2008;8:421–39. doi: 10.1517/14712598.8.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 4.Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 5.Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–51. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 6.Padilla-Paz LA. Human papillomavirus vaccine: history, immunology, current status, and future prospects. Clin Obstet Gynecol. 2005;48:226–40. doi: 10.1097/01.grf.0000151585.16357.e6. [DOI] [PubMed] [Google Scholar]
- 7.Tsukui T, Hildesheim A, Schiffman MH, et al. Interleukin 2 production in vitro by peripheral lymphocytes in response to human papillomavirus-derived peptides: correlation with cervical pathology. Cancer Res. 1996;56:3967–74. [PubMed] [Google Scholar]
- 8.Maloy KJ, Erdmann I, Basch V, et al. Intralymphatic immunization enhances DNA vaccination. PNAS. 2001;98:3299–303. doi: 10.1073/pnas.051630798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Beust BR, Johansen P, Smith KA, Bot A, Storni T, Kundig TM. Improving the therapeutic index of CpG oligodeoxynucleotides by intralymphatic administration. Eur J Immunol. 2005;35:1869–76. doi: 10.1002/eji.200526124. [DOI] [PubMed] [Google Scholar]
- 10.Smith KA, Tam VL, Wong RM, et al. Enhancing DNA vaccination by sequential injection of lymph nodes with plasmid vectors and peptides. Vaccine. 2009;27:2603–15. doi: 10.1016/j.vaccine.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 11.Feltkamp MC, Smits HL, Vierboom MP, et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242–9. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 12.Kundig TM, Bachmann MF, DiPaolo C, et al. Fibroblasts as efficient antigen-presenting cells in lymphoid organs. Science. 1995;268:1343–7. doi: 10.1126/science.7761853. [DOI] [PubMed] [Google Scholar]
- 13.Weber J, Boswell W, Smith J, et al. Phase 1 Trial of intranodal injection of a Melan-A/MART-1 DNA plasmid vaccine in patients with stage IV melanoma. J Immunother. 2008;31:215–23. doi: 10.1097/CJI.0b013e3181611420. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Smith D, Bot S, Dellamary L, Bloom A, Bot A. Noncoding RNA danger motifs bridge innate and adaptive immunity and are potent adjuvants for vaccination. J Clin Invest. 2002;110:1175–84. doi: 10.1172/JCI15536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nature Medicine. 2007;13:552–9. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 16.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–74. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machiels JPH, Reilly RT, Emens LA, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–97. [PubMed] [Google Scholar]
- 18.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–44. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 19.Taieb J, Chaput N, Schartz N, et al. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J Immunol. 2006;176:2722–9. doi: 10.4049/jimmunol.176.5.2722. [DOI] [PubMed] [Google Scholar]
- 20.Vambutas A, DeVoti J, Nouri M, et al. Therapeutic vaccination with papillomavirus E6 and E7 long peptides results in the control of both established virus-induced lesions and latently infected sites in a pre-clinical cottontail rabbit papillomavirus model. Vaccine. 2005;23:5271–80. doi: 10.1016/j.vaccine.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 21.Thompson HS, Davies ML, Holding FP, et al. Phase I safety and antigenicity of TA-GW: a recombinant HPV6 L2E7 vaccine for the treatment of genital warts. Vaccine. 1999;17:40–9. doi: 10.1016/s0264-410x(98)00146-7. [DOI] [PubMed] [Google Scholar]
- 22.Daftarian P, Mansour M, Benoit AC, et al. Eradication of established HPV 16-expressing tumors by a single administration of a vaccine composed of a liposome-encapsulated CTL-T help fusion peptide in a water-in-oil emulsion. Vaccine. 2006;24:5235–44. doi: 10.1016/j.vaccine.2006.03.079. [DOI] [PubMed] [Google Scholar]
- 23.Souders NC, Sewell DA, Pan ZK, et al. Listeria-based vaccines can overcome tolerance by expanding low avidity CD8+ T cells capable of eradicating a solid tumor in a transgenic mouse model of cancer. Cancer Immun. 2007;7:2. [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh CJ, Kim TW, Hung CF, et al. Enhancement of vaccinia vaccine potency by linkage of tumor antigen gene to gene encoding calreticulin. Vaccine. 2004;22:3993–4001. doi: 10.1016/j.vaccine.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 25.Lamikanra A, Pan ZK, Isaacs SN, Wu TC, Paterson YJ. Regression of established human papillomavirus type 16 (HPV-16) immortalized tumors in vivo by vaccinia viruses expressing different forms of HPV-16 E7 correlates with enhanced CD8(+) T-cell responses that home to the tumor site. Virol. 2001;75:9654–64. doi: 10.1128/JVI.75.20.9654-9664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–6. [PubMed] [Google Scholar]
- 27.Brandsma JL, Shlyankevich M, Zhang L, et al. Vaccination of rabbits with an adenovirus vector expressing the papillomavirus E2 protein leads to clearance of papillomas and infection. J Virol. 2004;78:116–23. doi: 10.1128/JVI.78.1.116-123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Gutierrez JG, Elpek KG, Montes de Oca-Luna R, Shirwan H, Sam Zhou H, McMasters KM. Vaccination with an adenoviral vector expressing calreticulin-human papillomavirus 16 E7 fusion protein eradicates E7 expressing established tumors in mice. Cancer Immunol Immunother. 2007;56:997–1007. doi: 10.1007/s00262-006-0247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tillman BW, Hayes TL, DeGruijl TD, Douglas JT, Curiel DT. Adenoviral vectors targeted to CD40 enhance the efficacy of dendritic cell-based vaccination against human papillomavirus 16-induced tumor cells in a murine model. Cancer Res. 2000;60:5456–63. [PubMed] [Google Scholar]
- 30.Wang TL, Ling M, Shih IM, et al. Intramuscular administration of E7-transfected dendritic cells generates the most potent E7-specific anti-tumor immunity. Gen Ther. 2000;7:726–733. doi: 10.1038/sj.gt.3301160. [DOI] [PubMed] [Google Scholar]
- 31.Huang CH, Peng S, He L, et al. Cancer immunotherapy using a DNA vaccine encoding a single-chain trimer of MHC class I linked to an HPV-16 E6 immunodominant CTL epitope. Gene Ther. 2005;12:1180–6. doi: 10.1038/sj.gt.3302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang EY, Chen CH, Ji H, et al. Antigen-specific cancer immunotherapy using a GM-CSF secreting allogeneic tumor cell-based vaccine. Int J Cancer. 2000;86:725–30. doi: 10.1002/(sici)1097-0215(20000601)86:5<725::aid-ijc19>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 33.Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005;65:8059–64. doi: 10.1158/0008-5472.CAN-05-1797. [DOI] [PubMed] [Google Scholar]
- 34.Wada S, Yoshimura K, Hipkiss EL, et al. Cyclophosphamide augments antitumor immunity: Studies in an autochthonous prostate cancer model. Cancer Res. 2009;69:4309–18. doi: 10.1158/0008-5472.CAN-08-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1. Combinatorial intranodal delivery of tumor antigenic peptide and adjuvant is required for therapeutic efficacy. Intra-lymphatic vaccination with E7 49-57 HPV antigen and pI:C adjuvant resulted in significant tumor regression (A) and survival (B). Log-Rank statistical tests confirmed that survival in the E7 + pI:C group was significantly prolonged when compared to the control group (p = 0.0007) and the E7 peptide only group (p = 0.01) but was not significantly different from the pI:C only group (p = 0.1) although tumor progression was profoundly delayed (A, p < 0.02). N = 10 mice / group.
Fig. 2. Disease outcome correlates with elevated tumor-specific immune responses in mice receiving immunotherapy beginning 7 days after tumor challenge. Tumor volume results complied from five independent therapeutic tumor studies, comparing E7 + pI:C immunized mice (n=83) segregated based on disease outcome, show 69% (n=57) with regressing tumors compared to 31% (n=26) of mice with progressing disease (A). Mice with regressing tumors (n=57) generated a significantly higher E7 tetramer response (19.0 +/- 2.2, p = 0.01) compared to progressing mice (9.5 +/- 2.2, n=26) (B).
Fig. 3. Photographs of whole lung preparations and H&E staining of pulmonary tissue sections, demonstrating recovery from metastatic disease. As early as day 8 (top panel), metastatic tumor masses of various sizes are detected within the pulmonary tissue following i.v. injection of 5 × 105 C3.43 tumor cells into non-immunized mice. H&E sections of pulmonary tissue and photographs of lungs isolated on day 47 following tumor challenge from tumor control mice or mice injected beginning on day 8 with pI:C only show multiple tumors. In contrast, no residual tumors were observed in pulmonary tissue isolated on day 47 from animals effectively treated on day 8 by E7 + pI:C immunization (photomicrographs representative of 3 mice sampled from each experimental group).