Abstract
Parkinson’s disease (PD) is a neurological disorder characterized by the progressive impairment of motor skills in patients. Growing evidence suggests that abnormal redox-active metal accumulation, caused by dysregulation, plays a central role in the neuropathology of PD. Redox-active metals (e.g. Fe and Cu) catalyze essential reactions for brain function. However, these metals can also participate in the generation of highly toxic free radicals that can cause oxidative damage to cells and ultimately lead to the death of dopamine-containing neurons. The emergence of redox-active metals as key players in the pathogenesis of PD strongly suggests that metal-chelators could be beneficial in the treatment of this condition. This mini-review summarizes major recent developments on natural, synthetic iron chelating compounds and hydrogen peroxide-triggered prochelators as potential candidates for PD treatment.
Keywords: Parkinson’s disease, iron, chelator, polyphenol, fenton reaction, free radicals
1. INTRODUCTION
Parkinson’s disease (PD) is a major progressive neurological disorder that affects about 1% of the population older than 50 years [1, 2]. PD is the second most common neurodegenerative disorder after Alzheimer’s disease. Unlike Alzheimer’s disease (AD), which affects the memory and behavior centers of the brain, PD is characterized by a progressive lack of control over voluntary movement. PD symptoms include: tremors at rest, slow movements (bradykinesia), muscle stiffness, gait changes, loss of balance, “freezing” of facial muscles or limbs, voice changes, and muscle pains (myalgia). Broadly speaking, the neuropathogenesis of PD is characterized by a preferential loss of dopaminergic neurons (dopamine making and releasing neurons) in the substantia nigra pars compacta (SNpc), located in the midbrain [2]. The degeneration and subsequent death of dopaminergic neurons, the darkly colored neurons that make up the SNpc, result in a loss of neurotransmitter dopamine, which has critical functions in the central nervous system (CNS) such as motor coordination, balance, emotions, and pleasure [3]. Behaviorally, it has been reported that people diagnosed with depression were about 3 times more likely to develop PD than people not diagnosed with depression [4]. Additionally, it has been reported that the treatment with dopamine-restoring drugs caused sudden hypersexual and compulsive gambling behaviors in some PD patients [5].
The prevalence of PD is rising worldwide as the average lifespan has increased. Yet, despite extensive research on the basic and clinical aspects of PD since it was first described in 1817, PD remains a progressive and incurable condition with a still poorly understood pathogenesis [6,7]. At present, therapies for treating PD do not restore or prevent dopaminergic neuron loss [8].
Current PD treatments can offer partial relief to the developing symptoms of the disease. For instance, the popular drug levodopa counteracts PD effects by supplying the brain with a dopamine precursor that can pass through the blood-brain barrier, and once inside the brain, it can be converted into dopamine by the surviving dopaminergic neurons. The loss of dopamine in the brain can also be slowed down with MAO-B (monoamine oxidase B) inhibitors, an enzyme that degrades dopamine into dihydroxyphenyl acetic acid (DOPAC) and hydrogen peroxide. Another common strategy for treating PD is the use of dopamine receptor agonists like bromocriptine (Parlodel), apomorphine (Apokyn), pramipexole (Mirapex), and ropinirole (Requip). For the treatment of tremors, novel anti-cholinergic drugs, which work by decreasing the activity of the neurotransmitter acetylcholine, have been developed. A comprehensive review of current drug therapies for PD has been published elsewhere [9]. In addition to drugs, severely afflicted PD patients who do not get adequate relief from medications can show moderate improvement with more invasive procedures like pallidotomy and deep brain stimulation. For a detailed and critical review of current and prospective therapies for PD, the reader is referred to a recent article by Pillay et al. [10].
Numerous hypotheses for the etiology of PD have been formulated. Thus far, research has failed to conclusively link any specific factor to the genesis of PD. However, it is generally accepted that a combination of genetic and environmental factors converging on mitochondrial defects, oxidative stress, aberrant protein aggregation and mutations in the parkin protein, account for most cases of PD [11, 12]. At the cellular level, several biochemical studies have suggested that factors like free radical species, mitochondrial complex I (NADH-ubiquinone oxidoreductase) deficiencies, excitotoxicity, and inflammation are implicated in the dysfunction and death of the pathogenetic cell in PD [2, 7]. More recently, protein misfolding and aggregation, as well as signal-mediated apoptosis, have been identified as factors in nigral neuron death [13, 14]. Interestingly, almost all of these factors could be triggered by cellular oxidative stress, since the dysfunction of mitochondrial complex I can lead to the generation of reactive oxygen species (ROS) [7]. In a self-feeding cycle, ROS can damage complex I by thiol oxidation or tyrosine nitration [15]. Supporting this mechanism, severely decreased levels of reduced glutathione (GSH) and lowered complex I activities have been detected in the SNpc of PD patients [16, 17]. Given that GSH is one of the major cellular antioxidants, and a cellular redox regulator, its depletion strongly indicates the oxidative stress status of SNpc neurons. In fact, the depletion of GSH in mammalian cells has already been used as an index of increased oxidative stress [18, 19]. To sum up, although the trigger factor remains elusive, the role of oxidative stress in the death of SNpc dopaminergic neurons is now accepted as the leading hypothesis for the progression of PD [2, 8]. Hence, neuroprotective agents that could reduce oxidative stress in the cell hold a great potential for the treatment of PD [7].
2. IRON AS A PROMOTOR OF RADICALS IN THE PD BRAIN
High oxygen consumption and high levels of polyunsaturated fatty acids, coupled with the non-regenerative nature of neurons, make the brain highly vulnerable to oxidative damage [20]. Although the nature of the ROS responsible for cell death and neurodegeneration in PD remains unclear, a growing body of evidence suggests the involvement of the extremely reactive hydroxyl radical (•OH) [1, 11, 21, 22]. Eq. (1) shows how hydroxyl radicals can be generated in cells via the reaction of hydrogen peroxide (H2O2) with redox active metals (e.g. Fe, Cu and Mn). Hydroxyl radicals produced through the Fenton reaction (eq. 1) have an estimated half-life of 1 nanosecond and can be highly toxic to biomolecules. Furthermore, cellular reductants such as ascorbic acid can reduce the oxidized metal (eq. 2), which can react again in the Fenton reaction and produce more hydroxyl radicals. Thus the reaction can be considered catalytic when excess H2O2 is available.
| (1) |
| (2) |
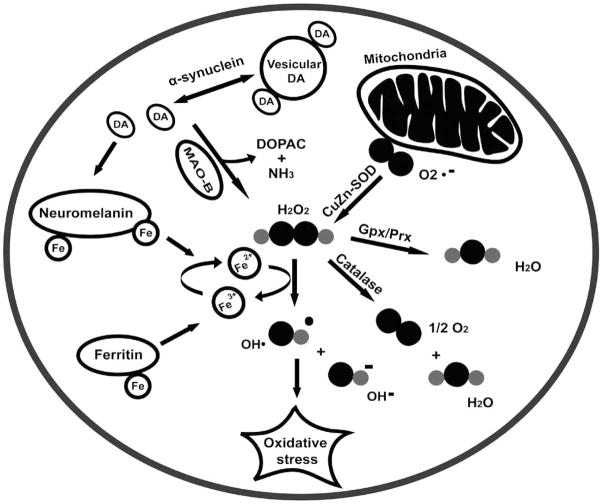
Iron accumulation and oxidative stress have been linked to histological changes in PD-affected brains. In the SNpc of diseased brains, PD is distinctively manifested as proteinaceous clusters called Lewy bodies, which are composed of ubiquitin, tyrosine hydroxylase, iron regulatory protein 2 (IRP-2) and α-synuclein fibrils [23]. Similar protein aggregates are also common to other neurological disorders like AD and Huntington’s disease (HD) [24]. What makes PD different is that the Parkinsonian brain appears to have a higher iron concentration than brains affected with AD or HD [28]. Thus, along with Lewy bodies, a distinctive feature of Parkinsonism in the brain is the accumulation of iron in the SNpc. The link between iron accumulation and the subsequent, or concurrent, biochemical/physiological events believed to take place during the pathogenesis of PD has been comprehensively reviewed by other authors [23, 25, 26]. Briefly, in SNpc neurons, enzymes MAO-B and SOD (superoxide dismutase) can produce hydrogen peroxide that can react with loosely bound iron (or copper), and generate hydroxyl radicals. In turn, higher levels of free radicals can cause the misfolding of α-synuclein protein, a seed protein in Lewy bodies, and the release of iron from neuromelanin, an iron sink believed to be produced by dopamine oxidation. Thus, a decrease in the production or activity of peroxidases such as glutathione peroxidase, peroxiredoxins or catalase can be upstream factors in iron-generated oxidative stress [25, 30]. Furthermore, loosely bound iron can also affect the function of iron regulatory proteins (IRP-1 and IRP-2) with the final result of decreasing the storage of iron by ferritin and increasing its cellular import [25, 28, 29]. The condensed oxidative stress hypothesis [10, 23, 25, 27] of domaninergic neuron death is shown in Fig. (1).
Fig. (1).
Oxidative stress hypothesis of Parkinson’s disease. DA, dopamine; MAO-B, monoamine oxidase B; DOPAC, dihydroxyphenyl acetic acid; CuZn-SOD, copper and zinc-containing superoxide dismutase; O2.−, superoxide anion; H2O2, hydrogen peroxide; OH•, hydroxyl free radical; OH−, hydroxyl ion; Gpx, glutathione peroxidase; Prx, peroxiredoxin. (Modified from references [10, 23, 25, 27]).
3. SYNTHETIC IRON CHELATING COMPOUNDS AS POTENTIAL DRUGS TREATING PARKINSON’S DISEASE
Many lines of evidence on the link between metal dyshomeostasis and neurodegenerative disorders such as AD and PD suggest that blood-brain barrier permeable metals chelators can be potential therapeutic agents in the treatment of these diseases [30,31]. In recent years, a few research groups have reported the design and synthesis of new iron chelators that could potentially be used in novel chelation therapies for the treatment of PD and other neurodegenerative diseases [22]. In vitro, and in some cases, in vivo studies have demonstrated that these iron chelators can significantly reduce neuronal death caused by oxidative stress in PD models. This section will review the structures and bioactivities of some novel synthetic iron chelators.
3.1. Desferrioxamine
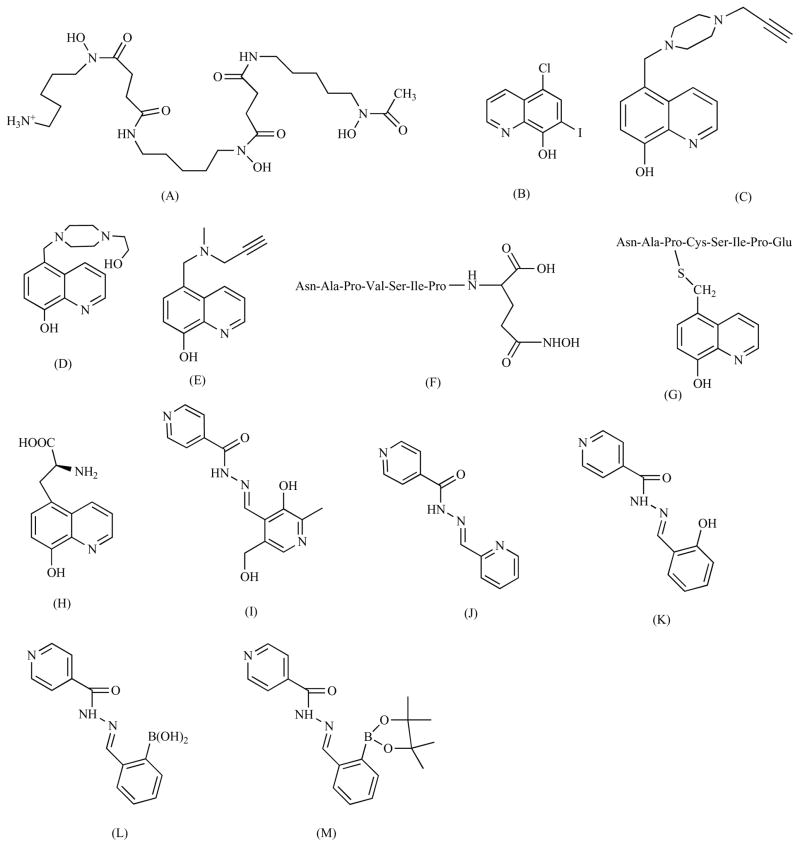
Desferrioxamine (also known as Desferal, Fig. 2A) is a hexadentate ligand with a higher binding affinity for Fe3+ than Fe2+ [32]. For decades, desferrioxamine has been successfully used to treat iron overload diseases [33]. In a study using rat PD model and 6-hydroxydopamine (6-OHDA) as the trigger of iron-catalyzed radical damage, it was found that desferrioxamine could protect up to 60% of the dopaminergic neurons from death [34]. Using a rat model as well, Xie et al. reported neuroprotective activities of the chelator desferrioxamine when the concentration of iron in the substantia nigra was increased [35]. Additionally, desferroxamine has been shown to inhibit iron-promoted radical damage in a dopaminergic cell line [36]. The importance of iron chelation in the neuroprotective activities of this chelator was further demonstrated by Youdim et al. who observed that neuroprotective effects were dependent on the concentration of desferrioxamine and not the PD symptoms-inducing toxin 6-OHDA [37]. Despite these results, the size and hydrophilic character of desferrioxamine renders it unable to cross the blood-brain barrier when administered orally [38].
Fig. (2).
Structures of synthetic iron chelators. Desferrioxamine (A), clioquinol (B), HLA20 (C), VK-28 (D), M30 (E), M98 (F), M99 (G), M10 (H), PIH (pyridoxal isonicotinoyl hydrazone) (I), PCIH (2-pyridylcarboxaldehyde isonicotinoyl hydrazone) (J), SIH(salicylaldehyde isonicotinoyl hydrazone) (K), prochelators SIH-B (2-boronobenzaldehyde isonicotinoyl hydrazone) (L) and BSIH (pinacol ester form of SIH-B) (M).
3.2. 8-Hydroxyquinolines
Clioquinol (5-chloro-7-iodo-8-hydroxyquinone, Fig. 2B) is a small lipophilic iron chelator that has been studied in phase II clinical trials for moderate AD cases [39]. In an in vivo study, Andersen et al. showed that clioquinol can reduce the concentration of iron in the substantia nigra of mice by 30% [40]. Significantly, the radical damage caused by neurotoxin MPTP (1-methyl-4-phenyl-1,2,3,6-tetra-pyridine) was largely decreased when the mice were pre-treated with clioquinol [38]. As with many chelators, clioquinol is not iron selective, and this may produce unintended effects. Yassin et al. reported that clioquinol can not only reduce the trace metal content in mice brains but also the levels of S-adenosylmethionine, which can be interpreted as a sign of vitamin B12 deficiency [41]. Unlike desferrioxamine, clioquinol can cross the brain-blood barrier, and thus can be administered orally.
Due to the neurotoxicity of halogenatedhydroxyquinolines [42], a new generation of hydroxyquinolines that overcomes this problem has been developed. HLA20 (Fig. 2C) is a nonchlorinated cell permeable 8-hydroxyquinoline analog firstly synthesized by Fridkin et al. [41]. In a 6-OHDA assay, HLA20 is reported to have protected over 60% of P19 cells (mouse embryonal carcinoma cell line) against oxidative damage [43]. EPR and spectrophotometric studies done by the Fridkin group showed that HLA20 can be a strong Fe3+ chelator and radical scavenger. In addition, Fridkin et al. showed that HLA20 can moderately inhibit the activity of enzyme MAO-B [41].
VK-28 (5-[4-(2-hydroxyethyl) piperazine-1-ylmethyl]-quinoline-8-ol, Fig. 2D) is another promising novel iron chelator [35,44]. As described by Ben-Shachar et al, VK-28 can penetrate the mitochondrial membrane and inhibit lipid peroxidation. In rats injected with VK-28, a 68 % protection of dopaminergic neurons against 6-OHDA-induced oxidative damage was observed [42]. Furthermore, as the authors pointed out, the large difference between the chelator and the neurotoxin (1:200) indicates that the neuroprotective effects are due to iron-chelation and not to direct interference with the toxin [42]. In a recent study, mice injected with lactacystin, a proteasome inhibitor, showed improved behavioral performance when injected with VK-28 [45].
M30 [5-(N-methyl-N-propargyaminomethyl)-8-hydroxyquinoline, Fig. 2E) is both an iron chelator and a MAO-B inhibitor [46]. Like HLA20, M30 belongs to a category of neuroprotective drugs that target different etiologies of a neurological disorder at the same time [47]. In vivo and in vitro assays have shown that M30 can prevent neurodegeneration induced by MTTP, and simultaneously increase the concentration of neurotransmitters dopamine, serotonin and noradrenaline [48,49]. Structurally, M30 and HLA20 also share a propargylamine moiety, which inhibits the MAO enzymes, induces the expression of antiapoptotic proteins Bcl-2 and Bcl-xl, and decreases the permeability of the mitochondrial membrane transition pore [43]. Comparative studies further revealed that M30 is a more potent MAO inhibitor than HLA20, with an IC50 over three orders of magnitude lower than that of HLA20; and unlike HLA20, M30 is a strong inhibitor of both MAO-A and MAO-B enzymes [50]. Furthermore, in a lactacystine-induced neuron death assay in mice, M30 was shown to be as effective as VK-28 at restoring the behavioral performance of mice [43].
Another novel approach to design neuronal iron chelators is the addition of neuroprotective peptide NAPVSIPQ (NAP) to an iron-chelating moiety. Preliminary work by Firdkin et al. has shown that chelators M98 and M99 (Figs. 2F and 2G) can inhibit lipid peroxidation in rat brain homogenates [49]. In neuroblastoma cells (SH-SY5Y), both chelators inhibited 6-OHDA-induced toxicity [51]. On the other hand, in PC12 cells, M98 was demonstrated to be a more potent inhibitor of 6-OHDA-induced cell death than M99 [49]. The same group also developed chelator M10 (Fig. 2H), which uses an 8-hydroxyquinoline iron-chelating moiety bonded to the brain selective neutral amino acid carrier also used by L-Dopa [52]. M10 was shown to be an inhibitor of Fe2+/ascorbate-initiated lipid peroxidation in rat brain mitochondrial homogenates [50]. Additionally, EPR studies showed that M10 can be a potent hydroxyl radical scavenger. Lastly, in a 6-OHDA assay, M10 displayed a protective activity in PC12 cells comparable to that of the chelator M30 [39].
3.3. Aroylhydrazones
Still in the early stages of animal studies, PCIH (2-pyridylcarboxaldehyde isonicotinoyl hydrazone) and its analogs, are novel aroylhydrazones (Fig. 2J), which hold promise as a new generation of lipophilic iron chelators [53]. PCIH and its analogs were synthesized from pyridoxal isocotinoyl hydrazone (PIH) (Fig. 2I) through a 1-step Schiff base condensation between 2-pyridylcarboxaldehyde and a number of acid hydrazides [51]. Initial studies have revealed that PCIH may not be a very selective or strong iron chelator, as it was observed that PCIH forms a more stable complex with Cu2+ [54]. Interestingly, the iron-mediated oxidation of PCIH gave rise to the diacylhydrazine chelator series, which are more efficient Fe chelators [55]. Based on potentiometric studies and the previously observed removal of iron from rabbit reticulocytes by PCIH, Richardson et al. proposed that a neutrally charged hydrazone can infiltrate the membrane; and once there, it can be oxidized to a hydrazine by iron, forming a neutral complex that can diffuse out of the membrane [51, 53].
3.4. Novel H2O2-Triggered Prochelators
The use of metal chelators can potentially have deleterious consequences as iron, copper, and other metals, are essential for numerous biological processes. Hence, prochelators, molecules that can be converted into chelators when an external circumstance (such as oxidative stress) is met, could be used to remove iron only when it has the potential of becoming toxic. With this approach in mind, SIH-B (2-borobenzalhdehyde isocotinoyl hydrazone) (Fig. 2L), BSIH (pinacol ester form of SIH-B) (Fig. 2M), and a few other derivatives have been developed recently [56–58] using tridentate chelator salicylaldehyde isocotinoyl hydrazone (SIH) (Fig. 2K) as their parent molecule. For instance, prochelator SIH-B does not bind iron (or copper) strongly, but when treated with H2O2, it can be converted into SIH at physiological pH [56]. This H2O2-sensed conversion is critical because the active chelator SIH can subsequently sequester iron and copper. This way, as it was demonstrated by a 2-deoxyribose degradation assay, H2O2 can act as a trigger for the transformation of prochelator SIH-B into the active chelator SIH, which in turn will form an inert complex with iron or copper and inhibit the formation of hydroxyl radicals through the Fenton reactions [56]. The H2O2-sensed “anti-Fenton” reactivity is an appealing characteristic that can be used to develop novel Fenton reaction-attenuating agents that can become active during oxidative stress without disturbing metal homeostasis. Such a strategy may offer promising candidates for potential therapeutics to treat PD and other neurological diseases. Experiments to test the activity of this compound in vivo are currently underway.
4. PLANT POLYPHENOLS AS MULTIFUNCTIONAL NEUROPROTECTIVE AGENTS
Polyphenols are a bioactive group of phytochemicals whose habitual consumption has been linked to reduced risks of cancer and cardiovascular diseases [59–61]. The benefits of a diet rich in polyphenols have been extended to brain protection as well [62,63]. With its high glucose and oxygen consumption, the brain has a higher need for neutralizing or removing toxic radicals. The importance of oxidative stress in the brain was highlighted in a 2004 review by Butterfield et al. claiming that 1 in 3 proteins or enzymes in the cell is dysfunctional due to oxidative damage [64]. Thus, it has been proposed that bioavailable antioxidants could prevent the development of neurodegenerative diseases [60, 61]. In some polyphenols, antioxidant activities can be attributed to strong iron-binding affinities that can inhibit the gene-ration of Fenton radicals, rather than to radical scavenging [65, 66]. Significantly, both free polyphenols and poly-phenol-metal complexes have been reported to have antioxidant properties [67]. More to the point, polyphenols have also been reported to be successful at affecting metal homeostasis in vivo. For instance, Gao et al. showed that polyphenols baicalin and quercetin can reduce liver damage caused by iron overload in mice [68]. This section will summarize the major recent developments on plant polyphenols that chelate iron and could potentially act as neuroprotective agents against PD.
4.1. Green Tea Polyphenols
High consumption of green tea, as shown in a widely publicized cross-sectional Japanese study, appears to prevent the progressive loss of cognitive skills long associated with AD, a neurological disorder linked to metal dysregulation [69]. Green tea has a high content of catechins [70] (a subclass of polyphenols), with epigallocatechin gallate (EGCG) (Fig. 3A) estimated to account for two-thirds of catechin content [71]. As antioxidants, an in vitro study found, tea catechins could inhibit the activities of cytochrome p450 2E1, Glutathione-S-Transferase, as well as lipid peroxidation [72]. Raza et al. found that catechins can partly inhibit Fenton reaction radical damage at low concentrations, whereas at high concentration the same catechins can act as prooxidants [70]. Similarly, Yu et al. reported that EGCG can act as a prooxidant in the presence of copper ions [73]. There is some evidence that EGCG is able to chelate iron. In an AD study in cell cultures, the administration of EGCG was identified as responsible for reducing the intracellular levels of APP protein, a redox-active metalloprotein [74]. Using mice treated with MTP, Mandel et al. showed that (−)-epigallocatechin-3-gallate and VK-28 can prevent the accumulation of iron in the SNpc [75]. However, in a rat study using 6-OHDA as the precursor of PD symptoms, Dexter et al. found that the animals treated with catechin-rich grape seeds and cocoa were not protected against dopaminergic neuron loss [76]. Interestingly, in a PC12 cell model of PD, it was found that EGCG and (−)-epicatechin gallate can protect the cells against 6-OHDA-induced death. Clearly, more studies are needed to assess the neuroprotective activities of green tea catechins. As pointed out by Pan et al. in a review, green tea polyphenols can protect the cell in a variety of ways that include (3)H-dopamine and (3)H-methyl-4-phenylpyridine uptake inhibition, catechol-O-methyltransferase activity reduction, radical scavenging, and iron chelation [77]. In addition, catechins can also help neurorescue efforts within the cell by down-regulating proapoptotic genes and promoting neurite outgrowth [78].
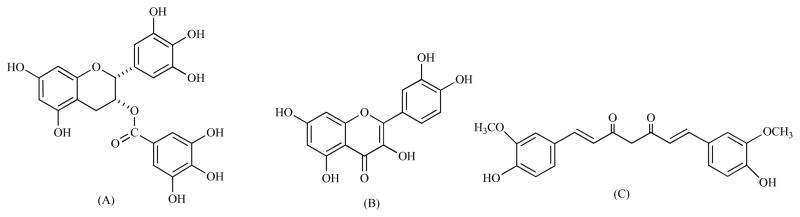
Fig. (3).
Structures of three natural polyphenols with iron binding properties. EGCG (epigallocatechin gallate) (A), Quercetin (B), Curcumin (C).
4.2. Other Polyphenols
Some polyphenols can be strong and bioavailable iron chelators. Our work on cranberry polyphenols revealed that flavonol quercetin (Fig. 3B) can completely inhibit Fenton chemistry in vitro under physiologically relevant conditions [63]. In a comparative experiment, we found that quercetin can compete with well-known cellular iron chelators ATP and citrate. The calculated apparent binding constant for the 1:1 quercetin:Fe2+ complex was found to be order of 106 M−1, suggesting that quercetin could be a strong enough chelator to affect iron homeostasis in vivo. Experiments by Gao et al. on mice [66] and more recently Bonanou et al. on HeLa and ASM cells have demonstrated that quercetin can remove intracellular iron [79]. Comparing quercetin to vitamin C, Lee et al. found that quercetin can also protect PC12 cells against hydrogen peroxide-induced oxidative stress, leading the researcher to conclude that quercetin can protect neuronal cells against oxidative-stress neurotoxicity more efficiently than vitamin C [80]. Electrospray ionization mass spectrometry (ESI-MS) studies performed by us and others suggest that many polyphenols analogous to quercetin can form complexes with both iron and copper at near physiological pH [63, 64, 81]. Polyphenols catechin, quercetin, chrysin, puerarin, naringenin, and genestein were found to protect mesencephalic dopamine neurons from apoptosis due to oxidative stress induced by MPP+, a Parkinsonism-inducing toxin [82]. In addition to this, polyphenols found in traditional Chinese medicinal plants also appear to have neuroprotective effects. In a 2000 review on traditional Chinese medicine (TCM) plants, Hostettmann et al. found that almost all the enzyme-inhibiting compounds identified in the TCM plants are phenolics, implying that these phenolic moieties were binding to the “action sites” through hydrogen bonding [83]. In another extensive review on TCM, Chen et al. showed that a number of polyphenols, such as the ones found in herbs pannax ginseng and ginkgo biloba, can protect dopamine neurons from neurotoxins MTPT and 6-OHDA in in vivo and in vitro assays [82]. Metal chelation is one of the possible mechanisms responsible for these bio-effects, the authors suggested [84]. Work in this laboratory has shown that certain bioactive polyphenols in the Asian medicinal plant Scutellaria baicalensis Georgi, are strong iron-chelators that can inhibit the Fenton reaction under physiologically relevant conditions [85].
Curcumin (Fig. 3C) is another polyphenol recently under a lot of scrutiny for its long acknowledged medicinal effects. A major bioactive polyphenolic compound, curcumin is found in the ancient Indian spice turmeric. In vitro studies have shown that curcumin can decrease the concentration of non-transferrin-bound Fe3+ from thalassemic plasma, although not as effectively as desferrioxamine [86]. The authors of this study additionally proposed that the beta-diketo moiety of curcumin is the iron-binding motif on this molecule [84]. A semi-empirical molecular orbital calculation reported by Ishihara et al. suggested that curcumin can form a 1:1 equimolar complex with ferric chloride [87]. More evidence that curcumin can act as an iron chelator was provided by Jiao et al. who observed that mice whose diets included curcumin had lower levels of iron-storage protein ferritin in the liver [88]. Studies on PC12 cells have shown that curcumin can increase cell viability against MPP+ (1-methyl-4-phenylpridinium) ions [89]. In rats injected with 6-OHDA, polyphenols curcumin and naringenin had protective effects on the dopamine levels in the striata, whereas polyphenols quercetin and fistein did not induce similar effects [90].
5. CONCLUDING REMARKS AND PERSPECTIVES
Whether it is a primary factor, a secondary factor or an exacerbating factor, numerous studies have consistently shown that iron dysregulation and accumulation in the PD brain appears to be central to the progression of this condition. Due to its redox nature, iron can react with endogenous hydrogen peroxide to produce hydroxyl radicals, which are highly harmful to the cell. Thus, an iron chelating compound that selectively targets excess iron in the brain merits more research as a possible therapeutic agent for PD. Preliminary work on synthetic iron chelators has shown that chelating compounds moieties like 8-hydroxyquinoline and salicylaldehyde isonicotinoyl hydrazone can be modified to make the molecules bifunctional, and thus increase their neuroprotective activity. For instance, compounds like M30 and HLA20 can not only chelate iron but also inhibit the activity of enzyme MAO-B. Other compounds like SIH-B can become chelators in the presence of hydrogen peroxide, making them selective to the conditions within dysfunctional dopaminergic neurons. Iron chelation may also explain the widely accepted good benefits of natural polyphenols on the brain. As a large number of articles have pointed out, polyphenols can act as multifunctional antioxidant agents capable of affecting metal homeostasis in the body. Polyphenols EGCG, curcumin and quercetin, for example, can form Fenton reaction-inert complexes with iron, and in the case of quercetin and curcumin, remove iron from cells. Moreover, some natural polyphenols have been shown to act as neuroprotective agents in PD models. Since the cause of PD has not been clearly identified, results with Parkinsonism-inducing toxins must be judged carefully. Taken as a whole, the promising results observed with iron chelators warrant additional in vivo research to establish more clearly that iron chelation is the mechanism responsible for preventing the death of dopaminergic neurons. As with any potential new drug, factors like size, lipophilicity, bioavailability, toxicity, selectivity, and renal clearance must be always taken into account before submitting the compound to clinical trials.
Acknowledgments
We thank NCCAM/NIH, National Parkinson Foundation, American Parkinson Disease Association and the UMass Cranberry Research Program for partially financial support. This project was made possible by Grant Number 5 R21 AT002743-02 from the National Center for Complementary and Alternative Medicine (NCCAM). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, or the National Institutes of Health.
References
- 1.Ebadi M, Sharma SK. Peroxynitrite and mitochondrial dysfunction in the pathogenesis of Parkinson’s disease. Antioxid Redox Signal. 2003;5:319–335. doi: 10.1089/152308603322110896. [DOI] [PubMed] [Google Scholar]
- 2.Schapira AH, Olanow CW. Neuroprotection in Parkinson disease: mysteries, myths, and misconceptions. JAMA. 2004;291:358–364. doi: 10.1001/jama.291.3.358. [DOI] [PubMed] [Google Scholar]
- 3.Schapira AHV, Bezard E, Brotchie J, Calon GL, Ferger B, Hengerer B, Hirsch E, Jenner P, Le Novère N, Obeso JA, Schwarzschild MA, Spampinato U, Davidai G. Novel Pharmacological Targets for the Treatment of Parkinson’s Disease. Nat Drug Discov Rev. 2006;10:845–854. doi: 10.1038/nrd2087. [DOI] [PubMed] [Google Scholar]
- 4.Schuurman AG, van den Akker M, Ensinck KT, Metsemakers JF, Knottnerus JA, Leentjens AF, Buntinx F. Increased risk of Parkinson’s disease after depression: a retrospective cohort study. Neurology. 2002;58(10):1501–1504. doi: 10.1212/wnl.58.10.1501. [DOI] [PubMed] [Google Scholar]
- 5.Voon V, Fox SH. Medication-related impulse control and repetitive behaviors in Parkinson disease. Arch Neurol. 2007;8:1089–1096. doi: 10.1001/archneur.64.8.1089. [DOI] [PubMed] [Google Scholar]
- 6.Siderowf A, Stern M. Update on Parkinson disease. Ann Intern Med. 2003;138:651–658. doi: 10.7326/0003-4819-138-8-200304150-00013. [DOI] [PubMed] [Google Scholar]
- 7.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 8.Kedar NP. Can we prevent Parkinson’s and Alzheimer’s disease? J Postgrad Med. 2003;49:236–245. [PubMed] [Google Scholar]
- 9.Hermanowicz N. Drug therapy for Parkinson’s disease. Semin Neurol. 2007;27:97–105. doi: 10.1055/s-2007-971177. [DOI] [PubMed] [Google Scholar]
- 10.Singh N, Pillary V, Choonara YE. Advances in the treatment of Parkinson’s disease. Prog Neurobiol. 2007;81:29–44. doi: 10.1016/j.pneurobio.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Betarbet R, Sherer TB, Di Monte DA, Greenamyre JT. Mechanistic approaches to Parkinson’s disease pathogenesis. Brain Pathol. 2002;12:499–510. doi: 10.1111/j.1750-3639.2002.tb00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poorkaj P, Nutt JG, James D, Gancher S, Bird TD, Steinbart E, Schellenberg GD, Payami H. Parkin mutation analysis in clinic patients with early-onset Parkinson’s disease. Am J Med Genet Part A. 2004;129A:44–50. doi: 10.1002/ajmg.a.30157. [DOI] [PubMed] [Google Scholar]
- 13.McNaught KSP, Olanow CW. Proteolytic stress: A unifying concept for the etiopathogenesis of Parkinson’s disease. Ann Neurol. 2003;53:S73–S86. doi: 10.1002/ana.10512. [DOI] [PubMed] [Google Scholar]
- 14.Tatton WG, Chalmers-Redman R, Brown D, Tatton N. Apoptosis in Parkinson’s disease: signals for neuronal degradation. Ann Neurol. 2003;53 Suppl 3:S61–70. doi: 10.1002/ana.10489. discussion S70–62. [DOI] [PubMed] [Google Scholar]
- 15.Jha N, Jurma O, Lalli G, Liu Y, Pettus EH, Greenamyre JT, Liu RM, Forman HJ, Andersen JK. Glutathione depletion in PC12 results in selective inhibition of mitochondrial complex I activity. Implications for Parkinson’s disease. J Biol Chem. 2000;275:26096–26101. doi: 10.1074/jbc.M000120200. [DOI] [PubMed] [Google Scholar]
- 16.Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoyagid F, Jenner P, Marsden CD. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 17.Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 18.Jain A, Martensson J, Stole E, Auld PA, Meister A. Glutathione deficiency leads to mitochondrial damage in brain. Proc Natl Acad Sci USA. 1991;88:1913–1917. doi: 10.1073/pnas.88.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martensson J, Jain A, Stole E, Frayer W, Auld PA, Meister A. Inhibition of glutathione synthesis in the newborn rat: a model for endogenously produced oxidative stress. Proc Natl Acad Sci USA. 1991;88:9360–9364. doi: 10.1073/pnas.88.20.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Floyd RA. Oxidative damage to behavior during aging. Science. 1991;254:1597. doi: 10.1126/science.1684251. [DOI] [PubMed] [Google Scholar]
- 21.Carreras MC, Franco MC, Peralta JG, Poderoso JJ. Nitric oxide, complex I, and the modulation of mitochondrial reactive species in biology and disease. Mol Aspects Med. 2004;25:125–139. doi: 10.1016/j.mam.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Park SU, Ferrer JV, Javitch JA, Kuhn DM. Peroxynitrite inactivates the human dopamine transporter by modification of cysteine 342: potential mechanism of neurotoxicity in dopamine neurons. J Neurosci. 2002;22:4399–4405. doi: 10.1523/JNEUROSCI.22-11-04399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Götz ME, Double K, Gerlach M, Youdim MB, Riederer P. The relevance of irn in the pathogenesis of Parkinson’s disease. Ann NY Acad Sci. 2004;1012:193–208. doi: 10.1196/annals.1306.017. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto M, Rockenstein E, Crews L, Masliah E. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer’s and Parkinson’s diseases. Neuromol Med. 2003;4:21–36. doi: 10.1385/NMM:4:1-2:21. [DOI] [PubMed] [Google Scholar]
- 25.Kaur D, Andersen J. Does cellular iron dysregulation play a causative role in parkinson’s disease? Ageing Res Rev. 2004;3:317–343. doi: 10.1016/j.arr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Berg D, Gerlach M, Youdim MB, Double KL, Zecca L, Riederer P, Becker G. Brain iron pathways and their relevance to Parkinson’s disease. J Neurochem. 2001;79:225–236. doi: 10.1046/j.1471-4159.2001.00608.x. [DOI] [PubMed] [Google Scholar]
- 27.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 28.Que EL, Domaille DW, Chang CJ. Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem Rev. 2008;108:1517–1549. doi: 10.1021/cr078203u. [DOI] [PubMed] [Google Scholar]
- 29.Rouault TA, Cooperman S. Brain Iron Metabolism. Semin Pediatr Neurol. 2006;13:142–148. doi: 10.1016/j.spen.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Angel I, Bar A, Horovitz T, Taler G, Krakovsky M, Resnitsky D, Rosenberg G, Striem S, Friedman JE, Kozak A. Metal ion chelation in neurodegenerative disorders. Drug Dev Res. 2002;56:300–309. [Google Scholar]
- 31.Richardson DR. Novel chelators for central nervous system disorders that involve alterations in the metabolism of iron and other metal ions. Ann NY Acad Sci. 2004;1012:326–341. doi: 10.1196/annals.1306.026. [DOI] [PubMed] [Google Scholar]
- 32.Molina-Holgado F, Hider RC, Gaeta A, Williams R, Francis P. Metals ions and neurodegeneration. Biometals. 2007;20:639–654. doi: 10.1007/s10534-006-9033-z. [DOI] [PubMed] [Google Scholar]
- 33.Mandel S, Amit T, Bar-Am O, Youdim MB. Iron dysregulation in Alzheimer’s disease: multimodal brain permeable iron chelating drugs, possessing neuroprotective-neurorescue and amyloid precursor protein-processing regulatory activities as therapeutic agents. Prog Neurobiol. 2007;82:348–360. doi: 10.1016/j.pneurobio.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Ben-Shachar D, Eshel G, Finberg JP, Youdim MB. The iron chelator desferrioxamine (Desferal) retards 6-hydroxydopamine-induced degeneration of nigrostriatal dopamine neurons. J Neurochem. 1991;56:1441–1444. doi: 10.1111/j.1471-4159.1991.tb11444.x. [DOI] [PubMed] [Google Scholar]
- 35.Jiang H, Luan Z, Wang J, Xie J. Neuroprotective effects of iron chelator Desferal on dopaminergic neurons in the substantia nigra of rats with iron-overload. Neurochem Int. 2006;49:605–609. doi: 10.1016/j.neuint.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Sangchot P, Sharma S, Chetsawang B, Porter J, Govitrapong P, Ebadi M. Deferoxamine attenuates iron-induced oxidative stress and prevents mitochondrial aggregation and alpha-synuclein translocation in SK-N-SH cells in culture. Dev Neurosci. 2002;24:143–153. doi: 10.1159/000065700. [DOI] [PubMed] [Google Scholar]
- 37.Youdim MB, Stephenson G, Ben Shachar D. Ironing iron out in Parkinson’s disease and other neurodegenerative diseases with iron chelators: a lesson from 6-hydroxydopamine and iron chelators, desferal and VK-28. Ann NY Acad Sci. 2004;1012:306–325. doi: 10.1196/annals.1306.025. [DOI] [PubMed] [Google Scholar]
- 38.Aouad F, Florence A, Zhang Y, Collins F, Henry C, Ward RJ, Crichton R. Evaluation of new iron chelators and their therapeutic potential. Inorg Chim Acta. 2002;339:470–480. [Google Scholar]
- 39.Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li QX, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi RE, Masters CL. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol. 2003;60:1685–1691. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- 40.Kaur D, Yantiri F, Rajagopalan S, Kumar J, Mo JQ, Boonplueang R, Viswanath V, Jacobs R, Yang L, Beal MF, DiMonte D, Volitaskis I, Ellerby L, Cherny RA, Bush AI, Andersen JK. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson’s disease. Neuron. 2003;37:899–909. doi: 10.1016/s0896-6273(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 41.Yassin MS, Ekblom J, Xilinas M, Gottfries CG, Oreland L. Changes in uptake of vitamin B(12) and trace metals in brains of mice treated with clioquinol. J Neurol Sci. 2000;173:40–44. doi: 10.1016/s0022-510x(99)00297-x. [DOI] [PubMed] [Google Scholar]
- 42.Tsubaki T, Honma Y, Hoshi M. Neurological syndrome associated with clioquinol. Lancet. 1971;1:696–697. doi: 10.1016/s0140-6736(71)92699-7. [DOI] [PubMed] [Google Scholar]
- 43.Zheng H, Weiner LM, Bar-Am O, Epsztejn S, Cabantchik ZI, Warshawsky A, Youdim MB, Fridkin M. Design, synthesis, and evaluation of novel bifunctional iron-chelators as potential agents for neuroprotection in Alzheimer’s, Parkinson’s, and other neurodegenerative diseases. Bioorg Med Chem. 2005;13:773–783. doi: 10.1016/j.bmc.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 44.Shachar DB, Kahana N, Kampel V, Warshawsky A, Youdim MB. Neuroprotection by a novel brain permeable iron chelator, VK-28, against 6-hydroxydopamine lession in rats. Neuropharmacology. 2004;46:254–263. doi: 10.1016/j.neuropharm.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Zhu W, Xie W, Pan T, Xu P, Fridkin M, Zheng H, Jankovic J, Youdim MB, Le W. Prevention and restoration of lactacystin-induced nigrostriatal dopamine neuron degeneration by novel brain-permeable iron chelators. FASEB J. 2007;21:3835–3844. doi: 10.1096/fj.07-8386com. [DOI] [PubMed] [Google Scholar]
- 46.Youdim MB, Fridkin M, Zheng H. Bifunctional drug derivatives of MAO-B inhibitor rasagiline and iron chelator VK-28 as a more effective approach to treatment of brain ageing and ageing neurodegenerative diseases. Mech Ageing Dev. 2005;126:317–326. doi: 10.1016/j.mad.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 47.Van der Schyf CJ, Gal S, Geldenhuys WJ, Youdim MB. Multifunctional neuroprotective drugs targeting monoamine oxidase inhibition, iron chelation, adenosine receptors, and cholinergic and glutamatergic action for neurodegenerative diseases. Expert Opin Investig Drugs. 2006;15:873–886. doi: 10.1517/13543784.15.8.873. [DOI] [PubMed] [Google Scholar]
- 48.Gal S, Zheng H, Fridkin M, Youdim MB. Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases. In vivo selective brain monoamine oxidase inhibition and prevention of MPTP-induced striatal dopamine depletion. J Neurochem. 2005;95:79–88. doi: 10.1111/j.1471-4159.2005.03341.x. [DOI] [PubMed] [Google Scholar]
- 49.Gal S, Fridkin M, Amit T, Zheng H, Youdim MB. M30, a novel multifunctional neuroprotective drug with potent iron chelating and brain selective monoamine oxidase-ab inhibitory activity for Parkinson’s disease. J Neural Transm Suppl. 2006;70:447–456. doi: 10.1007/978-3-211-45295-0_68. [DOI] [PubMed] [Google Scholar]
- 50.Zheng H, Gal S, Weiner LM, Bar-Am O, Warshawsky A, Fridkin M, Youdim MB. Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases: in vitro studies on antioxidant activity, prevention of lipid peroxide formation and monoamine oxidase inhibition. J Neurochem. 2005;95:68–78. doi: 10.1111/j.1471-4159.2005.03340.x. [DOI] [PubMed] [Google Scholar]
- 51.Zheng H, Blat D, Fridkin M. Novel neuroprotective neuotropic NAP analogs targeting metal toxicity and oxidative stress: potential candidates for the control of neurodegenerative diseases. J Neur Trams. 2006;71:163–172. doi: 10.1007/978-3-211-33328-0_18. [DOI] [PubMed] [Google Scholar]
- 52.Zheng H, Youdim MB, Weiner LM, Fridkin M. Novel potential neuroprotective agents with both iron chelating and amino acid-based derivatives targeting central nervous system neurons. Biochem Pharmacol. 2005;70:1642–1652. doi: 10.1016/j.bcp.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Richardson DR. Novel chelators for central nervous system disorders that involve alterations in the metabolism of iron and other metal ions. Ann NY Acad Sci. 2004;1012:326–341. doi: 10.1196/annals.1306.026. [DOI] [PubMed] [Google Scholar]
- 54.Armstrong CM, Bernhardt PV, Chin P, Richardson DR. Structural variations and formation constants of first-row transition metal complexes of biologically active aroylhydrazones. Eur J Inorg Chem 2003. 2003:1145–1156. [Google Scholar]
- 55.Bernhardt PV, Chin P, Richardson DR. Unprecedented oxidation of a biologically active aroylhydrazone chelator catalysed by iron (III): serendipitous identification of diacylhydrazine ligands with high iron chelation efficacy. J Biol Inorg Chem. 2001;6:801–809. doi: 10.1007/s007750100258. [DOI] [PubMed] [Google Scholar]
- 56.Wei Y, Guo M. Hydrogen peroxide triggered prochelator activation, subsequent metal chelation, and attenuation of the Fenton reaction. Angew Chem Int Ed. 2007;46:4722–4725. doi: 10.1002/anie.200604859. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 119:4806–4809. [Google Scholar]
- 57.Charkoudian LK, Pham DM, Franz KJ. A pro-chelator triggered by hydrogen peroxide inhibits iron-promoted hydroxyl radical formation. J Am Chem Soc. 2006;128:12424–12425. doi: 10.1021/ja064806w. [DOI] [PubMed] [Google Scholar]
- 58.Charkoudian LK, Pham DM, Kwon AM, Vangeloff AD, Franz KJ. Modifications of boronic ester pro-chelators triggered by hydrogen peroxide tune reactivity to inhibit metal-promoted oxidative stress. Dalton Trans. 2007:5031–5042. doi: 10.1039/b705199a. [DOI] [PubMed] [Google Scholar]
- 59.Neto CC. Cranberry and its phytochemicals: a review of in vitro anticancer studies. J Nutr. 2007;137:186S–193S. doi: 10.1093/jn/137.1.186S. [DOI] [PubMed] [Google Scholar]
- 60.Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 61.Scalbert A, Manach C, Morand C, Remesy C, Jimenez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 62.Schmitt-Schillig S, Schaffer S, Weber CC, Eckert GP, Muller WE. Flavonoids and the aging brain. J Physiol Pharmacol. 2005;56 Suppl 1:23–36. [PubMed] [Google Scholar]
- 63.Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol. 2006;545:51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 64.Poon HF, Calabrese V, Scapagnini G, Butterfield DA. Free radicals and brain aging. Clin Geriatr Med. 2004;20:329–359. doi: 10.1016/j.cger.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Guo M, Perez C, Wei Y, Rapoza E, Su G, Bou-Abdallah F, Chasteen ND. Iron-binding properties of plant phenolics and cranberry’s bio-effects. Dalton Trans. 2007:4951–4961. doi: 10.1039/b705136k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopes GKB, Schulman HM, Hermes-Lima M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim Biophys Acta. 1999;1472:142–152. doi: 10.1016/s0304-4165(99)00117-8. [DOI] [PubMed] [Google Scholar]
- 67.Malesev D, Kuntic V. Investigation of metal-flavonoid chelates and the determination of flavonoids via metal-flavonoid complexing reactions. J Serb Chem Soc. 2007;72:921–939. [Google Scholar]
- 68.Zhang Y, Li H, Zhao Y, Gao Z. Dietary supplementation of baicalin and quercetin attenuates iron overload induced mouse liver injury. Eur J Pharmacol. 2006;535:263–269. doi: 10.1016/j.ejphar.2006.01.067. [DOI] [PubMed] [Google Scholar]
- 69.Kuriyama S, Hozawa A, Ohmori K, Shimazu T, Matsui T, Ebihara S, Awata S, Nagatomi R, Arai H, Tsuji I. Green tea consumption and cognitive function: A cross-sectional study from the Tsurugaya Project 1. Am J Clin Nutr. 2006;83:355–361. doi: 10.1093/ajcn/83.2.355. [DOI] [PubMed] [Google Scholar]
- 70.Reto M, Figueira ME, Filipe HM, Almeida CM. Chemical composition of green tea (Camellia sinensis) infusions commercialized in Portugal. Plant Foods Hum Nutr. 2007;62:139–144. doi: 10.1007/s11130-007-0054-8. [DOI] [PubMed] [Google Scholar]
- 71.Hague T, Andrews PLR, Barker J, Naughton DP. Dietary chelators as antioxidant enzyme mimetics: Implications for dietary intervention in neurodegenerative diseases. Behav Pharmacol. 2006;17:425–430. doi: 10.1097/00008877-200609000-00008. [DOI] [PubMed] [Google Scholar]
- 72.Raza H, John A. In vitro protection of reactive oxygen species-induced degradation of lipids, proteins and 2-deoxyribose by tea catechins. Food Chem, Toxicol. 2007;45:1814–1820. doi: 10.1016/j.fct.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 73.Yu H, Yin J, Shen S. Effects of epi-gallocatechin gallate on PC-3 cell cytoplasmic membrane in the presence of Cu2+ Food Chem. 2005;95:108–115. [Google Scholar]
- 74.Reznichenko L, Amit T, Zheng H, Avramovich-Tirosh Y, Youdim MB, Weinreb O, Mandel S. Reduction of iron-regulated amyloid precursor protein and beta-amyloid peptide by (−)-epigallocatechin-3-gallate in cell cultures: implications for iron chelation in Alzheimer’s disease. J Neurochem. 2006;2:527–536. doi: 10.1111/j.1471-4159.2006.03770.x. [DOI] [PubMed] [Google Scholar]
- 75.Mandel S, Maor G, Youdim MBH. Iron and a-synuclein in the substantia nigra of MPTP-treated mice. Effect of neuroprotective drugs R-apomorphine and green tea polyphenol (−)-epigallocatechin-3-gallate. J Mol Neurosci. 2004;24:401–416. doi: 10.1385/JMN:24:3:401. [DOI] [PubMed] [Google Scholar]
- 76.Datla KP, Zbarsky V, Rai D, Parkar S, Osakabe N, Aruoma OI, Dexter DT. Short-term supplementation with plant extracts rich in flavonoids protect nigrostriatal dopaminergic neurons in a rat model of Parkinson’s disease. J Am Coll Nut. 2007;26:341–349. doi: 10.1080/07315724.2007.10719621. [DOI] [PubMed] [Google Scholar]
- 77.Pan T, Jankovic J, Le W. Potential therapeutic properties of green tea polyphenols in Parkinson’s disease. Drug Aging. 2003;20:711–721. doi: 10.2165/00002512-200320100-00001. [DOI] [PubMed] [Google Scholar]
- 78.Mandel S, Weinreb O, Reznichenko L, Kalfon L, Amit T. Green tea catechins as brain-permeable, non toxic iron chelators to iron out iron from the brain. J Neural Transm Suppl. 2006;71:249–257. doi: 10.1007/978-3-211-33328-0_26. [DOI] [PubMed] [Google Scholar]
- 79.Triantafyllou A, Liakos P, Tsakalof A, Chachami G, Paraskeva E, Molyvdas PA, Georgatsou E, Simos G, Bonanou S. The flavonoid quercetin induces hypoxia-inducible factor-1a (HIF-1a) and inhibits cell proliferation by depleting intracellular iron. Free Radical Res. 2007;41:342–356. doi: 10.1080/10715760601055324. [DOI] [PubMed] [Google Scholar]
- 80.Heo HJ, Lee CY. Protective Effects of Quercetin and Vitamin C against Oxidative Stress-Induced Neurodegeneration. J Agric Food Chem. 2004;52:7514–7517. doi: 10.1021/jf049243r. [DOI] [PubMed] [Google Scholar]
- 81.Fernandez MT, Mira ML, Florencio MH, Jennings KR. Iron and copper chelation by flavonoids: an electrospray mass spectrometry study. J Inorg Biochem. 2002;92:105–111. doi: 10.1016/s0162-0134(02)00511-1. [DOI] [PubMed] [Google Scholar]
- 82.Mercer LD, Kelly BL, Horne MK, Beart PM. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis: investigations in primary rat mesencephalic cultures. Biochem Pharmacol. 2005;69:339–345. doi: 10.1016/j.bcp.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 83.Tan RX, Meng JC, Hostettmann K. Phytochemical investigation of some traditional Chinese medicines and endophyte cultures. Pharm Biol. 2000;38:25–32. doi: 10.1076/phbi.38.6.25.5955. [DOI] [PubMed] [Google Scholar]
- 84.Chen L, Wang Y, Wei L, Shi M, Chan Y. Chinese herbs and herbal extracts for neuroprotection of dopaminergic neurons and potential therapeutic treatment of Parkinson’s disease. CNS & Neurological Disorders: Drug Targets. 2007;6:273–281. doi: 10.2174/187152707781387288. [DOI] [PubMed] [Google Scholar]
- 85.Perez CA, Wei Y, Guo M. Iron-binding and anti-Fenton properties of baicalein and baicalin. 2008 doi: 10.1016/j.jinorgbio.2008.11.003. manuscript in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Srichairatanakool S, Thephinlap C, Phisalaphong C, Porter JB, Fucharoen S. Curcumin contributes to in vitro removal of non-transferrin bound iron by deferiprone and desferrioxamine in thalassemic plasma. Med Chem. 2007;3:469–474. doi: 10.2174/157340607781745447. [DOI] [PubMed] [Google Scholar]
- 87.Ishihara M, Sakagami H. Re-evaluation of cytotoxicity and iron chelation activity of three b-diketones by semiempirical molecular orbital method. In Vivo. 2005;19:119–123. [PubMed] [Google Scholar]
- 88.Jiao Y, Wilkinson J, Pietsch EC, Buss JL, Wang W, Planalp R, Torti FM, Torti SV. Iron chelation in the biological activity of curcumin. Free Radical Biol Med. 2006;40:1152–1160. doi: 10.1016/j.freeradbiomed.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 89.Chen J, Tang XQ, Zhi JL, Cui Y, Yu HM, Tang EH, Sun SN, Feng JQ, Chen PX. Curcumin protects PC12 cells against 1-methyl-4-phenylpyridinium ion-induced apoptosis by bcl-2-mitochondria-ROS-iNOS pathway. Apoptosis. 2006;11:943–953. doi: 10.1007/s10495-006-6715-5. [DOI] [PubMed] [Google Scholar]
- 90.Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radical Res. 2005;39:1119–1125. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]