Abstract
Genetic studies have identified a high bone mass of phenotype in both human and mouse when canonical Wnt signaling is increased. Secreted frizzled related protein1 (sFRP1) is one of several Wnt antagonists and among the loss-of-function mouse models in which 32-week old mice exhibit a high bone mass phenotype. Here we show that impact fracture healing is enhanced in this mouse model of increased Wnt signaling at a physiologic level in young (8 week) sFRP1−/− mice which do not yet exhibit significant increase in BMD. The loss of sFRP1 function in vivo improves fracture repair by promoting early bone union without adverse effects on the quality of bone tissue reflected by increased mechanical strength. We observe a dramatic reduction of the cartilage callous, increased intramembranous bone formation with bone bridging by 14 days, and early bone remodeling during the 28 day fracture repair process in the sFRP1−/− mice. Our molecular analyses of gene markers indicate that the effect of sFRP1 loss-of-function during fracture repair is to accelerate bone healing after formation of the initial hematoma by directing mesenchymal stem cells into the osteoblast lineage via the canonical pathway. Further evidence to support this conclusion is the observation of maximal sFRP1 levels in the cartilaginous callus of a WT mouse, hence in sFRP1−/− mouse progenitor cells are shifted directly into osteoblast lineage. Thus, developing an antagonist to specifically inhibit sFRP1 represents a safe target for stimulating fracture repair and bone formation in metabolic bone disorders, osteoporosis and aging.
Keywords: Canonical Wnt signaling, sFRP1, Fracture repair, Mechanical strength, Mesenchymal stem cells, Accelerated osteogenesis
INTRODUCTION
The stages of fracture repair from the initial formation of a thrombus callus to the induction of endochondral bone formation and the longer process of bone remodeling until the final healing of the fracture site into lamellar bone have been well described (Gerstenfeld and Einhorn, 2006; Rozen et al, 2007). The basic signaling pathways which govern skeletal regeneration on a cellular level have come under increasing scrutiny in an effort to understand osseous tissue response to injury (Flick et al, 2003; Tsiridis et al, 2007; Lehmann et al, 2005). While the vast majority of fractures heal uneventfully, the deleterious effects associated with non-union are well recognized (Starr, 2008). Further, fractures result in significant morbidity on both an individual and a collective basis, particularly in light of the increasing frequency of fractures associated with osteoporosis (Dawson-Hughes et al, 2008; Tosteson et al, 2008). Indeed, older individuals, more at risk for falls, have reduced bone formation rates and patients requiring medications that interfere with the normal rate of bone repair, would greatly benefit from interventions that would accelerate and enhance the bone formation stages.
Fracture repair has been demonstrated to recapitulate growth plate biology in important ways, with many of the signaling pathways present in the growth plate, also being demonstrated in fracture repair (Vortkamp et al, 1998). Among these signaling cascades, PTH/PTHrP, BMP, and WNT signaling have been studied for their multiple effects on embryonic development and their roles in fracture repair (Day and Yang, 2008; Kakar et al, 2007; Tsuji et al, 2006; Baron and Rawadi, 2007b; Macsai et al, 2008). Elucidating these pathways has lead to an understanding of the principle signals and opportunities to develop pharmacologic components for interventions aimed at enhancing fracture repair. For example, the osteogenic BMP2 is currently used clinically to locally augment fracture repair (Tsuji et al, 2006; Khosla et al, 2008). Parathyroid hormone (PTH) has been shown to promote fracture healing in mice (Tsiridis et al, 2007; Kakar et al, 2007), but currently PTH is approved only for treating severe osteoporosis (Whitfield et al, 2002). The canonical Wnt signaling pathway is well established as promoting bone formation in the adult skeleton in humans and animal models (Bodine and Komm, 2006). Mechanisms for enhancing fracture repair in rodents that used intermittent parathyroid hormone treatment have implicated Wnt signaling to explain the effect of these treatments in enhancing the biochemical and mechanical effects in experimental models of fracture repair (Kakar et al, 2007). Wnt signaling is also related to the bone enhancing effects of BMP2 (Chen et al, 2007; Nakashima et al, 2005; Fischer et al, 2002). Thus multiple osteogenic signals converge for normal bone development and fracture repair (Lian et al, 2006).
Current studies are focused on pharmacologic approaches to promote enhanced Wnt signaling for increasing skeletal mass (Bodine, 2008; Baron and Rawadi, 2007a; Gregory et al, 2006; Katoh and Katoh, 2007). Wnt signaling is highly complex, and a large number of intra-and extra- cellular proteins and receptors in various combinations have been demonstrated to cause an equally wide range of responses. Both canonical, and non-canonical pathways influence the skeleton (Tu et al, 2007; Bodine and Komm, 2006) and can be elucidated in the face of a spectrum of Wnt signaling combinations, suggesting that regulation of Wnt signaling is both dynamic and contextual related to the cell type, the specific Wnt ligand and the interactions of Wnt nuclear transducers with other coregulators on target genes (Katoh and Katoh, 2007). The canonical β-catenin pathway operates through the LRP5/Frizzled receptor complex to block the kinase activity of GSK3β, which stabilizes β-catenin in the cytosol for translocation to the nucleus (Logan and Nusse, 2004; Forde and Dale, 2007). Intracellular beta catenin signaling has been demonstrated to have important regulatory effects on embryonic skeletal growth with high β-catenin promoting osteogenesis and low β-catenin cellular levels favoring chondrogenesis (Hill et al, 2005; Day et al, 2005). Wnt proteins have been shown to express in a distinct pattern in the developing growth plate, suggesting a specific role for each different Wnt molecule in endochondral bone formation, and also during fracture repair, where endochondral bone formation is recapitulated (Zhong et al, 2006; Macsai et al, 2008; Hadjiargyrou et al, 2002).
Wnt signaling occurs extracellularly and lends itself to the development of small molecule therapies aimed at enhancing fracture repair through manipulation of beta-catenin signaling. Recently, enhanced fracture healing was demonstrated in a transgenic murine model which constitutively expressed high levels of activated beta-catenin in an osteoblastic tissue specific manner (Chen et al, 2007). This enhancement highlighted the potential of stimulating fracture repair by increasing intracellular stabilized beta catenin. However, the Wnt pathway is tightly regulated by a number of inhibitory molecules including Wnt-inhibitory factor, Chibby, SOST/sclerostin, Dickopfs (DKK), and various secreted frizzled-related proteins (sFRPs), that antagonize WNT signaling to regulate activity of the pathway in a physiologic manner. Decreasing levels of these inhibitors is a viable way to enhance bone mass and promote bone healing (Baron and Rawadi, 2007a). It remains to be elucidated which of these molecules might be useful as a target for pharmacologic intervention and under what context would modifying their cellular levels lead to enhanced fracture repair.
We hypothesized that inhibiting the activity of sFRP1, which sequesters Wnt ligands, would stimulate fracture repair based on studies of the sFRP1−/− mouse phenotype (Bodine et al, 2004). These mice exhibit increased bone mineral density and reduced apoptosis, indicating effects on the skeleton in the absence of sFRP1. Of further significance, our previous work indicated a role for sFRP1 in chondrocyte and osteoblast differentiation (Gaur et al, 2006; Bodine et al, 2007), which are both critical stages of the fracture repair process. sFRP1 is known to modulate Wnt signaling by competing with extracellular Wnt for binding to the Frizzled receptor and further antagonizing Wnt signaling by binding directly to Wnt proteins (Hausler et al, 2004; Bodine et al, 2004). Because sFRP1−/− mouse exhibited a high bone mass phenotype compared to WT in adult mice without compromising normal growth and development, the extracellular nature of these interactions makes sFRP1 an attractive target for pharmacologic intervention to stimulate Wnt in the specific context of fracture repair. To examine this potential role of sFRP1, we used a mouse model deficient in sFRP1 to determine the influence of physiological enhancement of Wnt signaling in the absence of this Wnt antagonist during repair of a closed, traumatic tibial mid-shaft fracture. Our studies show an increased rate of bone formation with reduced chondrogenesis at the early stages of repair, thereby accelerating bony fusion.
MATERIAL AND METHODS
Animals
The sFRP1−/− mouse was generated by Lexicon Genetics, Inc. as previously described. The LacZ gene was inserted into exon 1 of the sFRP1 gene. WT and homozygous knockout mice lines were maintained at the University of Massachusetts under IACUC approved protocols. Standard genotyping was carried out as described previously.
Fracture Technique
Fractures were generated in the mid-shaft of the tibia in 8-weeks-old male WT and homozygous knockout animals. Institutional approval was obtained and all procedures were undertaken in accordance with approved IACUC methods. Animals were administered general anesthesia using IP injections of ketamine and xylazine. A midline skin incision over the knee joint was utilized to gain access to the proximal tibial metaphysis, and a pilot hole was made using a 25 gauge needle at a position just medial to the patella tendon. A 30 gauge wire was introduced into the tibial canal and cut at the level of the bone proximally. Wounds were closed, and the tibia was held in a fixed position while a drop weight from a standard height was used to deliver a fixed traumatic injury to the mid portion of the tibia, generating a fracture via three point bending, and ensuring that fractures were generated using a traumatic method with reproducible energy of injury.
Radiography
All animals were examined pre and post fracture using live fluoroscopy with an inverted Xiscan 1000 fluoroscope. Pre-fracture imaging was used to confirm correct positioning of the stabilizing wire, and post fracture imaging was used to confirm correct fracture location and configuration. Additionally, standard radiographs were obtained in all animals post fracture with a high resolution MX-20 (Faxitron Xray, IL) on mammography film. Additionally, animals were radiographed to document fracture repair at 7, 14, 21, and 28 days post-fracture.
Histology
The fractured legs were collected at the time of sacrifice and fixed in 4% paraformaldehyde. The samples were demineralized in 18% EDTA and processed for paraffin embedding. The sections were prepared at 10μm thickness and stained with Toluidine Blue to visualize the cartilage tissue on the section. For detection of osteoclasts and bone remodeling, Tartrate-resistant Acid Phosphatase (TRAP) staining was performed.
Quantitative Real Time PCR
The mice were sacrificed on day 14 post fracture to scrape the tissue from the fracture site in TRIzol reagent, avoiding any old bone tissue. For comparison to non-fractured bone, we dissected the trabecular bone of tibia from 10-week old WT and sFRP1−/− mice to be more analogous to the new bone deposited in the fracture site. The tissue was ground using a Polytron homogenizer and total RNA was isolated as per the manufacturer’s instructions (Invitrogen, CA). Any potential DNA contamination was removed by RNase-free DNase treatment. The reverse transcription reaction was performed on 1 μg of total RNA using the First Strand Synthesis Kit and random hexamer primers (Invitrogen, CA). Relative transcript levels were measured by real time PCR in 25 μl reaction volume on 96-well plate using ABI PRISM 7000 sequence detection system (Applied Biosystems, CA), following the recommended protocol for SYBR-Green (Applied Biosystems). Transcript levels were normalized with 18S ribosomal RNA levels using primers from Applied Biosystems and SYBR-Green master mix (Applied Biosystems, CA). The primers used for amplification are described in Table 1.
Table 1.
List of the primers used for quantitative Real-time PCR.
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Wnt1 | CAGGGTTCATAGCGATCCAT | CAAAGAGGGAGGGAGGTAGG |
| Wnt5a | TCCTATGAGAGCGCACGCAT | CAGCTTGCCCCGGCTGTTGA |
| Wnt5b | GAGGAGCAGGGCCGAGC | ACAGCTTGCCCTGGCCGGTTGA |
| Wnt7a | CAAGGCCAGTACCACCGGGA | TCCACGTGGACGGCCTC |
| LEF1 | AGTGCAGCTATCAACCAGAT | TTCATAGTATTTGGCCTGCT |
| TNFα | mm00443258_m1 (Applied Biosystems) | |
| sFRP1 | GCCACAACGTGGGCTACAA | ACCTCTGCCATGGTCTCGTG |
| Col2a1 | CTGGAATGTCCTCTGCGA | TGAGGCAGTCTGGGTCTTCAC |
| Sox9 | GAGGCCACGGAACAGACTCA | CAGCGCCTTGAAGATAGCATT |
| Col10a1 | CCTGCAGCAAAGGAAAACTC | TGTGGTAGTGGTGGAGGACA |
| MMP9 | mm00600163_m1 (Applied Biosystems) | |
| TRAP | mm00441908_m1 (Applied Biosystems) | |
| VEGF | ACTGGACCCTGGCTTTACTG | GGCAGTAGCTTCGCTGGTAG |
Mechanical Testing
The healing tibiae were harvested from WT and sFRP1−/− at day 14 and 28 post fracture and subjected to torsion test to failure using 55MT MicroTorsion testers (Instron, MA). Both proximal and distal ends of tibiae were potted in 1cm2 aluminum casing using polymethylmethacrylate (PMMA). An angular displacement was applied at the rate of 1°/sec until failure, keeping the direction of twist same as internal rotation of the distal tibia. The torsional stiffness was calculated as the slope of the torque-angular displacement.
Statistical Methods
Student’s T-test was performed to analyze the significance of the data for gene expression and mechanical strength testing.
RESULTS
Early Union of Bone Fracture in absence of sFRP1
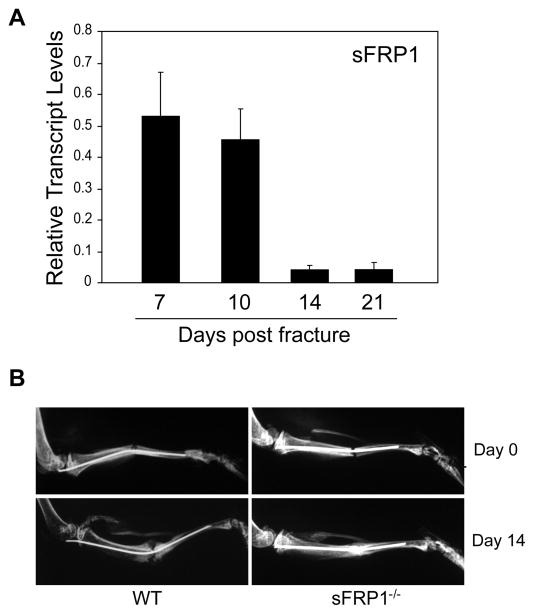
We have selected the sFRP1−/− mouse to test the hypothesis that Wnt signaling enhanced at physiologic levels are sufficient for promoting anabolic effects on bone fracture repair in 8-weeks old mice which do not yet exhibit a high bone mass phenotype (Bodine et al, 2004). However, in the sFRP1−/− mouse bone tissue as early as 4-weeks of age show increased expression of TCF1 and Runx2 (Gaur et al, 2005). Since Wnt signaling has been shown to be active during fracture healing (Chen et al, 2007; Zhong et al, 2006), we first determined the expression of the sFRP1, a secreted antagonist of Wnt signaling during fracture healing in the WT mouse. sFRP1 was expressed at peak levels in the early post-fracture stages at cartilaginous callus formation (day 7 and day 10; Fig. 1A). This level was ~20-folds higher than at the bone formation stages (day 14 an day 21; Fig 1A), suggesting an early function of sFRP1 for inhibition of Wnt signaling at the onset of callus formation. Fractures were generated in sFRP1−/−mice with same force as used for the WT mice. We determined the extent of fracture healing in tibial bone by radiography (Fig. 1B). The mid-tibia impact fracture at day 0 (upper panel) is equivalent for wild type and sFRP1−/−. By post-operative day 14, the sFRP1−/− mice demonstrated a higher radio-opacity at the fracture site compared to wild type (Fig. 1B, lower panel). These findings suggested an early bone union in sFRP1−/− mouse.
Figure 1. sFRP1 plays a critical role in early fracture repair.
(A) Higher expression of sFRP1 during early stages of fracture healing in WT mouse. The tissue was scraped from the fracture site from WT mice (n=3) and analyzed for sFRP1 expression. (B) Radiographic analysis of healing tibiae shows improved healing by reduced callus size and union of bone ends in absence of sFRP1. The upper panel shows the tibiae on the fracture day and the lower panel shows callus formation and healing 14 days post fracture.
Absence of sFRP1 leads to Increased Wnt Signaling during Fracture Repair
Modifications in Wnt signaling have been correlated with regulation of Wnt ligands as well as their target genes. To determine the effect of loss of sFRP1 on Wnt signaling during fracture repair, we harvested callus tissue at post-fracture day 14 (when bony union occurs in the sFRP1−/− mouse, but not in WT) for analysis of Wnt signaling components and their known target genes (Fig. 2). Wnt1 and Wnt7a, which function through canonical Wnt pathway, along with LEF1, a classical target of canonical Wnt signaling, were upregulated during fracture repair in the sFRP1−/− mice (Fig. 2A, upper panels). However, the established non-canonical Wnt5a/5b ligands exhibited decreased expression in the sFRP1−/− mice. These significant differences were consistent with downregulation in TNFα, a target of non-canonical Wnt signaling in sFRP1−/− mice (Fig. 2B, upper panels). These findings indicate that in the absence of sFRP1, canonical Wnt signaling is generally upregulated, while non-canonical Wnt signaling is downregulated.
Figure 2. Quantitative real time PCR showing upregulation of canonical Wnt signaling pathway at fracture site in sFRP1−/− 14 days post fracture.
The tissue was either scraped from the fracture site or metaphysis tissue was collected from intact bone from WT and sFRP1−/− (n=3 mice per group), processed for RNA and analyzed for the expression levels of components of Wnt signaling pathways in WT (□) and sFRP1−/− (■) mouse. (A) Expression pattern of Wnt ligands driving canonical pathway (Wnt1 and Wnt7a), and LEF1, a target gene of canonical in WT and sFRP1−/− animals. The upper panels show the expression in fracture bone, while lower panels show expression in intact, unfractured bone. (B) Expression profile of Wnt ligands required for initiation of non-canonical pathways (Wnt5a and Wnt5b) and a target gene, TNFα in WT and sFRP1−/− samples. The upper panels represent fractured bone profile, while lower panel show intact, unfractured bone profile. Relative transcript levels are shown with ± SEM. An asterisk mark (*) represents the p-value <0.02.
To ascertain that induced canonical Wnt signaling observed in the sFRP1−/− mouse at day 14 of fracture repair is unique to regenerating fracture tissues, we analyzed metaphyseal bone from long bones of 10 week-old WT and sFRP1−/− mice, to be more similar to the dynamic bone tissue present at the fracture site. The metaphysis tissues in wild type and sFRP1−/− mouse do not show any statistically significant difference in the expression levels of canonical (Wnt1 and Wnt7a) and non-canonical Wnt proteins (Wnt5a and Wnt 5b) as well as their respective target genes, LEF1 and TNFα. (Fig. 2A and B, lower panels). Furthermore, our observations suggest that sFRP1 contributes to antagonizing the beneficial effects of Wnt signaling during the fracture repair process.
Decreased Chondroid and Increased Osseous Activity Results in Accelerated Fracture Healing in the Absence of sFRP1
Endochondral bone formation begins with cartilage formation in the callus and the periosteal reaction on either side of the fracture site. To further analyze the positive effect of sFRP1 inhibition on fracture repair, the tissue composition and organization of the fracture site during the regeneration process from 1 to 4 weeks was examined comparing WT to the sFRP1−/− mouse. At post-fracture day 7, the relative cartilage area in sFRP1−/− and wild type animals shows no significant difference (Fig. 3A, day 7), indicating that induction of EBF occurred in a similar manner. However, early intramembranous bone formation could be seen in sFRP1−/− mice by visualization of newly formed woven bone with relatively mature osteocytes compared to WT (Figure 3B, i–iv). The TRAP activity reflecting bone remodeling by osteoclasts was also observed in sFRP1−/− whereas WT remained negative for TRAP activity (Fig. 3B, v–viii), suggesting an early start of bone remodeling in the absence of sFRP1.
Figure 3. Histologic analyses showing improved fracture healing by reduced callus cartilage and early bone formation in sFRP1−/− mouse.
The samples were processed for paraffin sections and stained for visualization of cellular structures and enzymatic activity. (A) Toluidine Blue stained sections from WT and sFRP1−/− healing bone samples at day 7 post-fracture, day 11 post-fracture, day 14 post-fracture and day 21 post-fracture. (B) Toluidine Blue and TRAP stained sections of day 7 post-fracture bone samples at higher magnifications, showing relatively mature osteocytes in the newly formed woven bone in sFRP1−/− (ii, iv) as compared to WT (i, iii). The lower panels show TRAP staining for osteoclast activity, visible in sFRP1−/− (vi, viii) but absent in WT (v, vii).
Changes in the callus size could be identified by day 11 post-fracture. The relative cartilage area at the fracture site in knockout mice was significantly less compared with wild type, and this difference continued to increase as fracture repair progressed (Fig. 3A, day 11). The chondroid tissue and callus size continued to increase in size from day 11 to 14 in the WT mouse, with small areas of new bone formation was observed in multiple mouse fracture samples. However, in the sFRP1−/− mouse, the callus size did not increase further after day 11, but rather the amount of chondroid tissue was replaced by osseous tissue. This resulted in far greater bone volume in the sFRP1−/− mouse compared to WT (Fig. 3A, day 11 and day 14). This combination of less chondroid formation and greater osseous replacement allowed for the faster bone bridging observed in the day 14 sFRP1−/− animals.
By day 21, both WT and sFRP1−/− showed bridging of the fracture site with osseous tissue. However, Toluidine blue staining of the wild type did reveal the presence of small amounts of cartilage, while in the sFRP1−/− group the transition from chondroid to osseous tissue was already complete. The sFRP1−/− animals demonstrated faster integration of new bone at the fracture ends with the original bone (Figure 3A, day 21). To conclude, the absence of sFRP1−/− results in a callus with less chondroid and continued increase in osseous tissue over a 3 week bone repair period. Thus, an acceleration of osseous regeneration leads to faster bridging of bone across the fracture site.
Our histological analyses revealed an equivalent callus size between WT and sFRP1−/− mice on day 7, but in sFRP1−/− fractures, the callus composition changed from the WT which increased in size and cartilage tissue with time. This suggested an early commitment of proliferating mesenchymal cells to the osteoblast lineage in the initial callus of the sFRP1−/− group. To address the mechanism of accelerated fracture repair, we performed gene expression analysis of chondroid and bone turnover markers to determine the effect of sFRP1 deletion in the callus tissues harvested 14 days post fracture. The proliferation markers for chondrocytes, Collagen type II and Sox9, were significantly lower in sFRP1−/− mice compared to WT mice (2–2.5 fold; Figure 4). No difference was observed in Collagen type X expression between WT and sFRP1−/− mice, consistent with cartilage on day 4. We further evaluated the transcript levels of bone remodeling markers to examine the extent of new bone formation at the injury site. All the markers MMP9, TRAP and VEGF were upregulated in sFRP1−/− mouse, suggesting active bone remodeling in absence of sFRP1. We did not examine the osteogenic markers since the tissue collected from the fracture site was enriched in cartilage tissue and contained minimal bone tissues.
Figure 4. Reduced chondrogenic proliferation and increased bone remodeling in mouse with induced Wnt signaling.
The healing tissue was scraped from WT and sFRP1−/− mice, total RNA was isolated, and analyzed for chondrogenic markers and bone remodeling markers. Relative transcript levels are presented ± SEM as error bars, WT as open box (□) and sFRP1−/− as filled box (■). * represents p-value < 0.01.
Increased Strength of Healing Bones in Presence of Induced Wnt Signaling
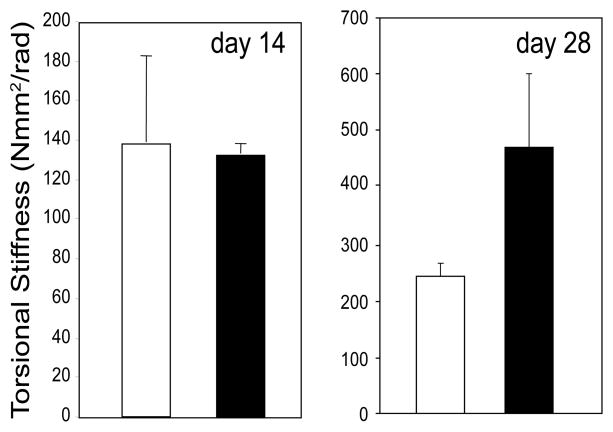
Our observations suggest a small callus size and faster repair in sFRP1−/− mouse. However, it is critical that the bone quality is not compromised in order to accelerate the fracture healing. To examine the strength of fractured bones after repair, we performed torsion testing to analyze the torsional stiffness of the healing/healed bones from WT and sFRP1−/− mice. We demonstrate that by day 14 post fracture, even though the callus size is larger in WT which also adds to the mechanical strength of the healing bones, there is no difference in the torsional stiffness of WT and sFRP1−/− samples (Figure 5, left panel). However, by 28 days post fracture, we observed a 1.9 fold increase in torsional stiffness in sFRP1−/− samples as compared to WT, which is approaching significance with p-value less than 0.2 (Figure 5, right panel). These observations suggest that deletion of sFRP1 and subsequent activation of Wnt signaling can provide reduced callus size and faster as well as improved fracture repair in mice.
Figure 5. Torsion testing of healing/healed bone samples shows improved mechanical strength in absence of sFRP1.
Torsional stiffness of the WT (☐) and sFRP1−/− (■) fractured bone samples (n=4) on day 14 post-fracture (left panel) and day 28 post fracture (right panel).
DISCUSSION
While other studies have recognized the potential for accelerating fracture healing by increasing Wnt signaling through modification of β-catenin levels or by antagonizing the inhibitory components of the Frizzled receptor complex (e.g. DKK1) (Chen et al, 2007; Gregory et al, 2006), a key finding of our studies is that loss of function of one of the secreted frizzled related protein antagonists (sFRP1) has a significant effect in accelerating fracture repair in a normal physiologic manner. The major effect of loss of sFRP1 activity is at the early stages of fracture repair enhancing osteogenesis and accelerating normal bone formation and matrix mineralization. Furthermore, at the late stages, the fracture repair tissue is mechanically sound. These are important considerations for developing the appropriate target to enhance canonical Wnt signaling for therapeutic application, particularly because increased Wnt signaling is associated with various cancers. However, no tumors examining multiple organs ever had been identified in sFRP1−/− mouse up to 1.5 years age (Trevant et al, 2008).
Fracture repair follows an ordered sequence of events analogous to endochondral bone formation and the molecular signaling pathways that involve cytokines and growth factors, influencing each of these stages are being characterized (Cho et al, 2002; Flick et al, 2003; Geiger et al, 2005; Einhorn et al, 1995; Kon et al, 2001; Tsuji et al, 2006). Our results indicate that increased canonical Wnt signaling in absence of sFRP1 contributes to regulation of several stages of fracture repair. The immediate biological response to a break in bone tissue is formation of the hematoma and inflammation during the first few days which is accompanied by secretion of numerous cytokines (IL1, IL6, TNFα from the inflammatory cells), as well as a release of growth factors (e.g., TGFβ1, FGF) from aggregated platelets. A vascular network is also established promoting the fibrin clots (Einhorn et al, 1995; Kon et al, 2001; Street et al, 2002). This initial response appears to be unaltered in the absence of Wnt signaling as the fracture callus in both the WT and sFRP1 null mouse is approximately a similar size through day 7. The increase in local growth factors from the hematoma leads to the recruitment mesenchymal stem cells (Rozen et al, 2007). One of these is TGFβ1 which upregulates the production of an extracellular matrix consisting of collagen, fibrin, and proteoglycans, thus contributing to the cartilage callus. It is at this stage (day 7–10) we observe peak levels of sFRP1 during fracture repair in WT mice. Several studies have noted that TGFβ receptors are downregulated during osteoblast differentiation and this is also observed during the fracture repair process (Centrella et al, 1995; Cho et al, 2002).
The second stage of fracture repair, formation of granulation tissue, is characterized by proliferating mesenchymal stem cells (MSCs) which differentiate into osteoblasts for intramembranous bone formation arising from underlying cortical bone distal to the fracture break. At the same time, MSCs are differentiating into cartilage tissue at the very edges of the broken bone (Gerstenfeld et al, 2003). Wnt signaling is tightly regulated at this stage by high levels of sFRP1 observed in wild type mouse. At this stage, day 7 in our studies, that enhanced Wnt signaling in sFRP1−/− mouse fracture tissue has a profound effect on formation of the intramembranous bone at the fracture site. The characteristics of the callus itself have changed, continuing to increase in size and cartilage tissue content in the WT mouse fractures, but not in the sFRP1−/− mouse. Between days 11 and 14 the control mice have not only increased the amount of granulation tissue, but the representation of soft callus to hard callus is far greater than observed in the sFRP1−/− mouse. Thus, the enhanced Wnt signaling appears to have diverted MSCs into the osteogenic lineage by day 11 as a result of the absence of sFRP1 which are observed at the highest expression level in the cartilage callus of the WT mouse. This interpretation is further supported by quantitation of the chondrogenic areas which is significantly decreased at both time points day 11 and 14 in the sFRP1−/− mouse. Our conclusion is consistent with the molecular marker analyses that demonstrate increased canonical Wnt signaling. The levels of activated canonical Wnt/β-catenin signaling are well established to influence chondrogenesis (low β-catenin) (Day et al, 2005; Hill et al, 2005) and through a direct interaction between Sox9 and β-catenin (Akiyama et al, 2004). Since the chondrogenic stage is important for rapid stabilization of the fractured bone, canonical Wnt signaling must be maintained at a modest level and is suppressed in part by high sFRP1 levels. Thus, absence of sFRP1 has a physiologic advantage as an extracellular therapeutic target for increasing fracture repair at early stages.
A novel finding of the consequences of enhanced Wnt signaling in our studies is the significant increase in VEGF expression at day 14 reflecting increased remodeling of the newly formed woven bone to lamellar intramembranous bone. During the initial stages in formation of the hematoma, establishing vascularity is a key event in subsequent healing of bone tissue (Komatsu and Hadjiargyrou, 2004). Several studies show that increased VEGF correlates with a better rate of fracture healing (Peng et al, 2005; Geiger et al, 2005). Early induction of Wnt signaling indirectly may increase VEGF through Runx2, since canonical Wnt signaling increases Runx2 expression which is an activator of VEGF (Zelzer et al, 2001; Gaur et al, 2005). The result of these accelerated processes is bone bridging of the fracture by 14 days.
Regulation of Wnt signaling by sFRP1 has been reported to inhibit osteoclast formation in vitro (Hausler et al, 2004), while other studies showed that sFRP1 expression is downregulated upon osteoclast differentiation (Trevant et al, 2008). Our observation of early onset of TRAP activity as well as increased expression of TRAP mRNA in absence of sFRP1, indicate that osteoclast activities and bone remodeling are enhanced by increased Wnt signaling. The bone remodeling is necessary to convert the increased woven bone that is deposited in the fracture repair tissue to lamellar bone. The increased mechanical strength we observe by day 28 in sFRP1−/− mouse demonstrates that this sequence has occurred to a greater extent than WT mouse.
The recruitment of mesenchymal cells directly into the osteogenic lineage is consistent with well established mechanisms of activated canonical Wnt signaling in bone. Confirmation that canonical Wnt signaling predominates is validated by the significant increase in LEF1 and a decrease in TNFα, a target of non-canonical Wnt signaling. We also find an increase in Wnt factors that regulate canonical signaling (Wnt1 and Wnt7a) and a decrease in the non-canonical Wnt5a and Wnt5b ligands which are involved in chondrogenesis. Notably, The canonical Wnt3a can promote chondrocyte dedifferentiation (Hwang et al, 2005). Both Wnt1 and Wnt7a have been shown to regulate chondrogenesis (Rudnicki and Brown, 1997; Fischer et al, 2002; Jackson et al, 2005). Studies have shown Wnt signaling is inhibited in the final stages of bone formation (Eijken et al, 2008; Matsuzaki et al, 2006; Rawadi et al, 2003; Li et al, 2005; Kahler et al, 2006). Therefore, our observations support a concept that in the absence of sFRP1, osteogenesis is favored over chondrogenesis, yet physiologic regulation of Wnt signaling is maintained in cells that differentiated into mature osteoblasts.
A striking advantage of directing a therapeutic target to sFRP1 is that it has partial redundant functions. The process of bone repair continues to undergo normal bone remodeling and mineralization of the newly formed woven bone. Although it has been reported that sFRP1 is detected in osteoclasts (Hausler et al, 2004; Trevant et al, 2008), it is non-essential for the normal remodeling of woven bone. This is consistent with the high bone mass phenotype observed in the sFRP1−/− mouse and is in striking contrast to other mouse models where β-catenin levels have been upregulated to a non-physiologic level of canonical Wnt signaling which compromises bone quality (Glass et al, 2005; Day et al, 2005; Hill et al, 2005). Stimulating canonical Wnt signaling within a physiologic range is an important consideration in developing a therapeutic target for enhancing bone formation not only during fracture repair but for metabolic bone diseases when modifying Wnt signaling. The enhanced fracture healing we observe in 8-weel old sFRP1−/− mice which do not exhibit a high bone mass at this age (earliest reported 32 weeks (Bodine et al, 2004)) further suggests the concept that enhancing Wnt signaling for a short period of time can be effective in stimulating MSC recruitment into the osteogenic lineage.
As both PTH and sFRP1 inhibition enhance fracture repair, it is of interest to note the differences between the effects of each events of the fracture repair process. In mice with closed femoral fractures, daily PTH treatment resulted in a larger initial callus and a 3-fold increase in chondrogenesis relative to osteogenesis. The increased volume of tissue that progressed through endochondral bone formation (Kakar et al, 2007) resulted in enhancement of bone tissue during fracture healing. Notably, PTH increased canonical Wnt signaling with a 10–20 fold peak from day 7 to 14 days post-fracture. This is the same period of effectiveness we find early bone formation and bridging in the sFRP1−/− mice. However, in contrast to PTH, increased Wnt signaling reduces the chondrogenic tissue volume in favor of bone formation during the early fracture stages. Thus, depending on the clinical circumstances of the fracture, options are available for accelerated bone bridging via direct modification of Wnt signaling or a need to also increase bone volume via PTH effects.
Acknowledgments
Contract Grant Sponsor: NIH
Contract Grant Number: R01 AR039588, R37 DE012528, and P30 DK32520. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
We thank L. Gerstenfeld and T. Einhorn (Department of Biomedical Engineering, Boston University) for advice in setting up the fracture model. We thank Zachary Mason for assistance with mechanical testing performed at Boston University. We thank Judy Rask and Marta DeSourdis for assistance in manuscript preparation.
References
- Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de CB. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007a;148:2635–2643. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- Baron R, Rawadi G. Wnt signaling and the regulation of bone mass. Curr Osteoporos Rep. 2007b;5:73–80. doi: 10.1007/s11914-007-0006-0. [DOI] [PubMed] [Google Scholar]
- Bodine PV. Wnt signaling control of bone cell apoptosis. Cell Res. 2008;18:248–253. doi: 10.1038/cr.2008.13. [DOI] [PubMed] [Google Scholar]
- Bodine PV, Komm BS. Wnt signaling and osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:33–39. doi: 10.1007/s11154-006-9002-4. [DOI] [PubMed] [Google Scholar]
- Bodine PV, Seestaller-Wehr L, Kharode YP, Bex FJ, Komm BS. Bone anabolic effects of parathyroid hormone are blunted by deletion of the Wnt antagonist secreted frizzled-related protein-1. J Cell Physiol. 2007;210:352–357. doi: 10.1002/jcp.20834. [DOI] [PubMed] [Google Scholar]
- Bodine PV, Zhao W, Kharode YP, Bex FJ, Lambert AJ, Goad MB, Gaur T, Stein GS, Lian JB, Komm BS. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18:1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- Centrella M, Casinghino S, Kim J, Pham T, Rosen V, Wozney J, McCarthy TL. Independent changes in type I and type II receptors for transforming growth factor beta induced by bone morphogenetic protein 2 parallel expression of the osteoblast phenotype. Mol Cell Biol. 1995;15:3273–3281. doi: 10.1128/mcb.15.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Whetstone HC, Lin AC, Nadesan P, Wei Q, Poon R, Alman BA. Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med. 2007;4:e249. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Tosteson AN, Melton LJ, III, Baim S, Favus MJ, Khosla S, Lindsay RL. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19:449–458. doi: 10.1007/s00198-008-0559-5. [DOI] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Day TF, Yang Y. Wnt and hedgehog signaling pathways in bone development. J Bone Joint Surg Am. 2008;90(Suppl 1):19–24. doi: 10.2106/JBJS.G.01174. [DOI] [PubMed] [Google Scholar]
- Eijken M, Meijer IM, Westbroek I, Koedam M, Chiba H, Uitterlinden AG, Pols HA, Van Leeuwen JP. Wnt signaling acts and is regulated in a human osteoblast differentiation dependent manner. J Cell Biochem. 2008;104:568–579. doi: 10.1002/jcb.21651. [DOI] [PubMed] [Google Scholar]
- Einhorn TA, Majeska RJ, Rush EB, Levine PM, Horowitz MC. The expression of cytokine activity by fracture callus. J Bone Miner Res. 1995;10:1272–1281. doi: 10.1002/jbmr.5650100818. [DOI] [PubMed] [Google Scholar]
- Fischer L, Boland G, Tuan RS. Wnt signaling during BMP-2 stimulation of mesenchymal chondrogenesis. J Cell Biochem. 2002;84:816–831. doi: 10.1002/jcb.10091. [DOI] [PubMed] [Google Scholar]
- Flick LM, Weaver JM, Ulrich-Vinther M, Abuzzahab F, Zhang X, Dougall WC, Anderson D, O’Keefe RJ, Schwarz EM. Effects of receptor activator of NFkappaB (RANK) signaling blockade on fracture healing. J Orthop Res. 2003;21:676–684. doi: 10.1016/S0736-0266(03)00011-1. [DOI] [PubMed] [Google Scholar]
- Forde JE, Dale TC. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell Mol Life Sci. 2007;64:1930–1944. doi: 10.1007/s00018-007-7045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PVN, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Canonical WNT signaling promotes osteogenesis by directly stimulating RUNX2 gene expression. J Biol Chem. 2005;280:33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- Gaur T, Lengner CJ, Hussain S, Trevant B, Ayers D, Stein JL, Bodine PVN, Komm BS, Stein GS, Lian JB. Secreted frizzled protein 1 regulates Wnt signaling for BMP2 induced chondrocyte differentiation. J Cell Physiol. 2006;208:87–96. doi: 10.1002/jcp.20637. [DOI] [PubMed] [Google Scholar]
- Geiger F, Bertram H, Berger I, Lorenz H, Wall O, Eckhardt C, Simank HG, Richter W. Vascular endothelial growth factor gene-activated matrix (VEGF165-GAM) enhances osteogenesis and angiogenesis in large segmental bone defects. J Bone Miner Res. 2005;20:2028–2035. doi: 10.1359/JBMR.050701. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Einhorn TA. Fracture healing: the biology of bone repair and regeneration. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Washington, DC: American Society for Bone and Mineral Research; 2006. pp. 42–48. [Google Scholar]
- Glass DA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Gregory CA, Green A, Lee N, Rao A, Gunn W. The promise of canonical Wnt signaling modulators in enhancing bone repair. Drug News Perspect. 2006;19:445–452. doi: 10.1358/dnp.19.8.1043960. [DOI] [PubMed] [Google Scholar]
- Hadjiargyrou M, Lombardo F, Zhao S, Ahrens W, Joo J, Ahn H, Jurman M, White DW, Rubin CT. Transcriptional profiling of bone regeneration. Insight into the molecular complexity of wound repair. J Biol Chem. 2002;277:30177–30182. doi: 10.1074/jbc.M203171200. [DOI] [PubMed] [Google Scholar]
- Hausler KD, Horwood NJ, Chuman Y, Fisher JL, Ellis J, Martin TJ, Rubin JS, Gillespie MT. Secreted frizzled-related protein-1 inhibits RANKL-dependent osteoclast formation. J Bone Miner Res. 2004;19:1873–1881. doi: 10.1359/JBMR.040807. [DOI] [PubMed] [Google Scholar]
- Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Hwang SG, Yu SS, Lee SW, Chun JS. Wnt-3a regulates chondrocyte differentiation via c-Jun/AP-1 pathway. FEBS Lett. 2005;579:4837–4842. doi: 10.1016/j.febslet.2005.07.067. [DOI] [PubMed] [Google Scholar]
- Jackson A, Vayssiere B, Garcia T, Newell W, Baron R, Roman-Roman S, Rawadi G. Gene array analysis of Wnt-regulated genes in C3H10T1/2 cells. Bone. 2005;36:585–598. doi: 10.1016/j.bone.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Kahler RA, Galindo M, Lian J, Stein GS, van Wijnen AJ, Westendorf JJ. Lymphocyte enhancer-binding factor 1 (Lef1) inhibits terminal differentiation of osteoblasts. J Cell Biochem. 2006;97:969–983. doi: 10.1002/jcb.20702. [DOI] [PubMed] [Google Scholar]
- Kakar S, Einhorn TA, Vora S, Miara LJ, Hon G, Wigner NA, Toben D, Jacobsen KA, Al-Sebaei MO, Song M, Trackman PC, Morgan EF, Gerstenfeld LC, Barnes GL. Enhanced chondrogenesis and Wnt signaling in PTH-treated fractures. J Bone Miner Res. 2007;22:1903–1912. doi: 10.1359/jbmr.070724. [DOI] [PubMed] [Google Scholar]
- Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13:4042–4045. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- Khosla S, Westendorf JJ, Oursler MJ. Building bone to reverse osteoporosis and repair fractures. J Clin Invest. 2008;118:421–428. doi: 10.1172/JCI33612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu DE, Hadjiargyrou M. Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone. 2004;34:680–688. doi: 10.1016/j.bone.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Kon T, Cho TJ, Aizawa T, Yamazaki M, Nooh N, Graves D, Gerstenfeld LC, Einhorn TA. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res. 2001;16:1004–1014. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- Lehmann W, Edgar CM, Wang K, Cho TJ, Barnes GL, Kakar S, Graves DT, Rueger JM, Gerstenfeld LC, Einhorn TA. Tumor necrosis factor alpha (TNF-alpha) coordinately regulates the expression of specific matrix metalloproteinases (MMPS) and angiogenic factors during fracture healing. Bone. 2005;36:300–310. doi: 10.1016/j.bone.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Li X, Liu P, Liu W, Maye P, Zhang J, Zhang Y, Hurley M, Guo C, Boskey A, Sun L, Harris SE, Rowe DW, Ke HZ, Wu D. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet. 2005;37:945–952. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, Young DW. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Macsai CE, Foster BK, Xian CJ. Roles of Wnt signalling in bone growth, remodelling, skeletal disorders and fracture repair. J Cell Physiol. 2008;215:578–587. doi: 10.1002/jcp.21342. [DOI] [PubMed] [Google Scholar]
- Matsuzaki E, Takahashi-Yanaga F, Miwa Y, Hirata M, Watanabe Y, Sato N, Morimoto S, Hirofuji T, Maeda K, Sasaguri T. Differentiation-inducing factor-1 alters canonical Wnt signaling and suppresses alkaline phosphatase expression in osteoblast-like cell lines. J Bone Miner Res. 2006;21:1307–1316. doi: 10.1359/jbmr.060512. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Katagiri T, Tamura M. Cross-talk between Wnt and bone morphogenetic protein 2 (BMP-2) signaling in differentiation pathway of C2C12 myoblasts. J Biol Chem. 2005;280:37660–37668. doi: 10.1074/jbc.M504612200. [DOI] [PubMed] [Google Scholar]
- Peng H, Usas A, Olshanski A, Ho AM, Gearhart B, Cooper GM, Huard J. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res. 2005;20:2017–2027. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- Rawadi G, Vayssiere B, Dunn F, Baron R, Roman-Roman S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res. 2003;18:1842–1853. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- Rozen N, Lewinson D, Bick T, Meretyk S, Soudry M. Role of bone regeneration and turnover modulators in control of fracture. Crit Rev Eukaryot Gene Expr. 2007;17:197–213. doi: 10.1615/critreveukargeneexpr.v17.i3.30. [DOI] [PubMed] [Google Scholar]
- Rudnicki JA, Brown AM. Inhibition of chondrogenesis by Wnt gene expression in vivo and in vitro. Dev Biol. 1997;185:104–118. doi: 10.1006/dbio.1997.8536. [DOI] [PubMed] [Google Scholar]
- Starr AJ. Fracture repair: successful advances, persistent problems, and the psychological burden of trauma. J Bone Joint Surg Am. 2008;90(Suppl 1):132–137. doi: 10.2106/JBJS.G.01217. [DOI] [PubMed] [Google Scholar]
- Street J, Bao M, Deguzman L, Bunting S, Peale FV, Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van BN, Redmond HP, Carano RA, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosteson AN, Melton LJ, III, Dawson-Hughes B, Baim S, Favus MJ, Khosla S, Lindsay RL. Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int. 2008;19:437–447. doi: 10.1007/s00198-007-0550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevant B, Gaur T, Hussain S, Symons J, Komm BS, Bodine PV, Stein GS, Lian JB. Expression of secreted frizzled related protein 1, a Wnt antagonist, in brain, kidney, and skeleton is dispensable for normal embryonic development. J Cell Physiol. 2008;217:113–126. doi: 10.1002/jcp.21482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiridis E, Morgan EF, Bancroft JM, Song M, Kain M, Gerstenfeld L, Einhorn TA, Bouxsein ML, Tornetta P., III Effects of OP-1 and PTH in a new experimental model for the study of metaphyseal bone healing. J Orthop Res. 2007;25:1193–1203. doi: 10.1002/jor.20420. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- Tu X, Joeng KS, Nakayama KI, Nakayama K, Rajagopal J, Carroll TJ, McMahon AP, Long F. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev Cell. 2007;12:113–127. doi: 10.1016/j.devcel.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vortkamp A, Pathi S, Peretti GM, Caruso EM, Zaleske DJ, Tabin CJ. Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mech Dev. 1998;71:65–76. doi: 10.1016/s0925-4773(97)00203-7. [DOI] [PubMed] [Google Scholar]
- Whitfield JF, Morley P, Willick GE. Parathyroid hormone, its fragments and their analogs for the treatment of osteoporosis. Treat Endocrinol. 2002;1:175–190. doi: 10.2165/00024677-200201030-00005. [DOI] [PubMed] [Google Scholar]
- Zelzer E, Glotzer DJ, Hartmann C, Thomas D, Fukai N, Soker S, Olsen BR. Tissue specific regulation of VEGF expression during bone development requires Cbfa1/Runx2. Mech Dev. 2001;106:97–106. doi: 10.1016/s0925-4773(01)00428-2. [DOI] [PubMed] [Google Scholar]
- Zhong N, Gersch RP, Hadjiargyrou M. Wnt signaling activation during bone regeneration and the role of Dishevelled in chondrocyte proliferation and differentiation. Bone. 2006;39:5–16. doi: 10.1016/j.bone.2005.12.008. [DOI] [PubMed] [Google Scholar]