Abstract
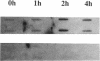
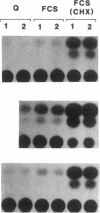
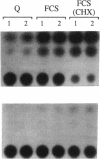
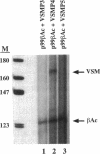
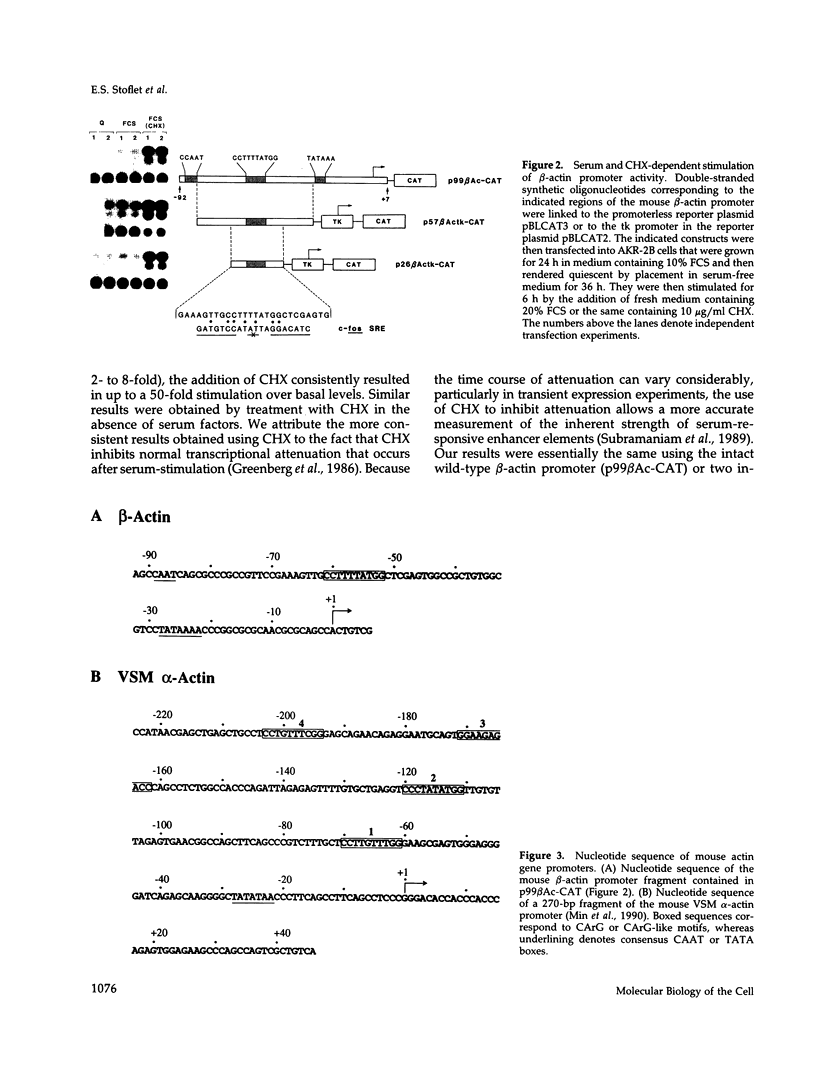
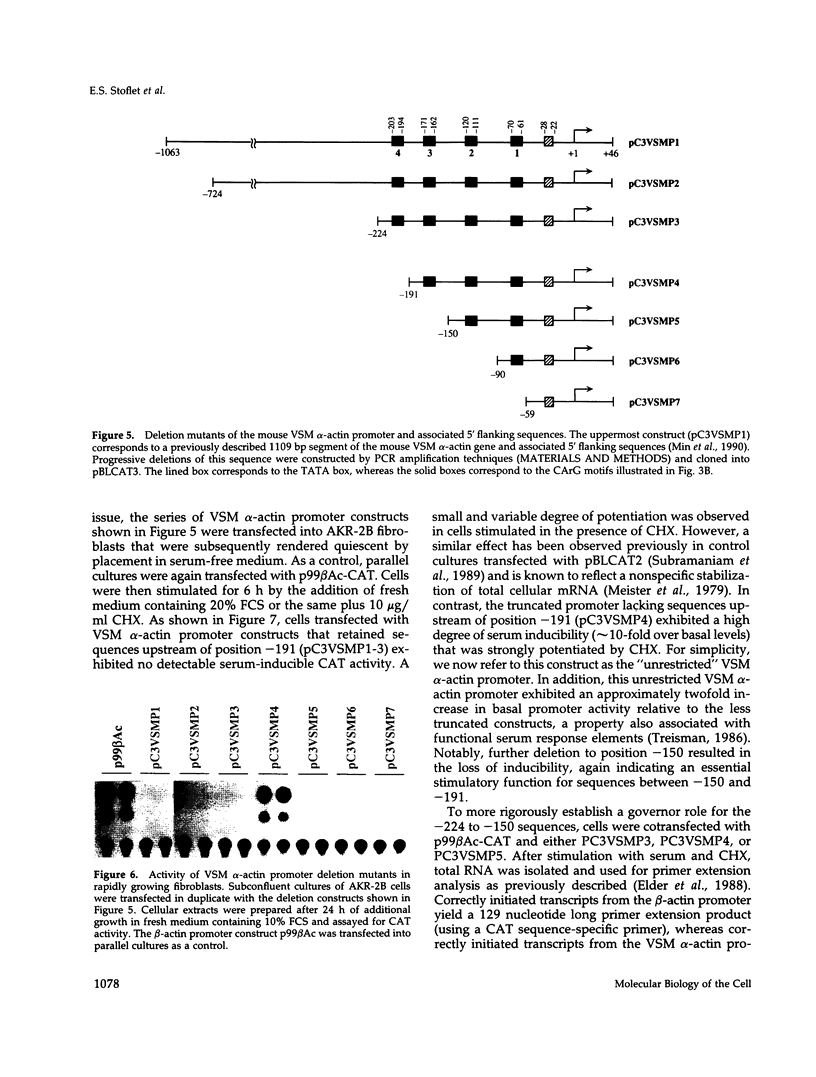
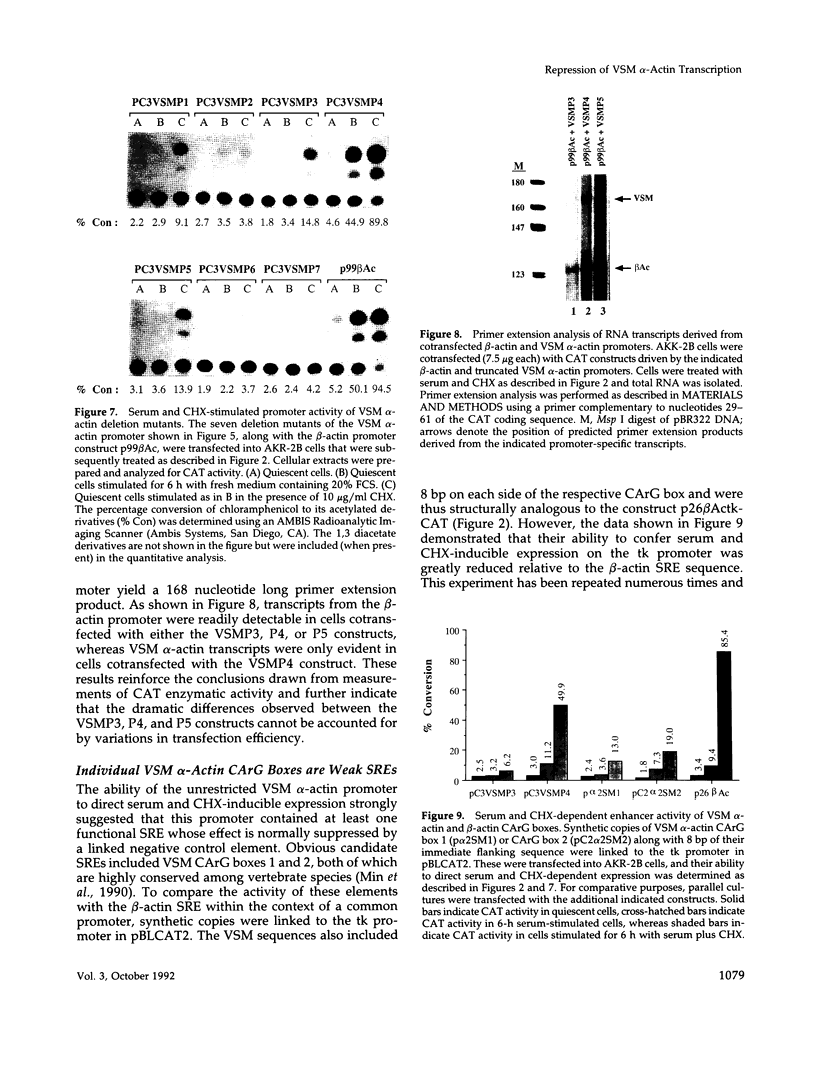
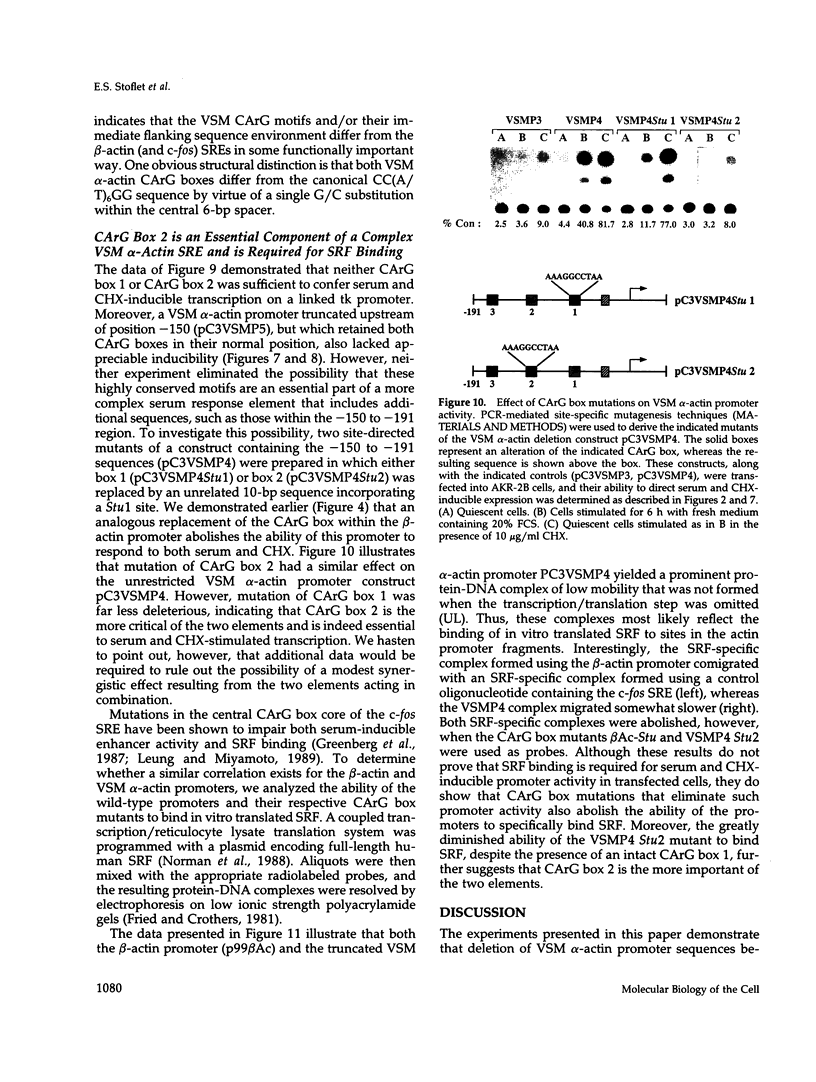
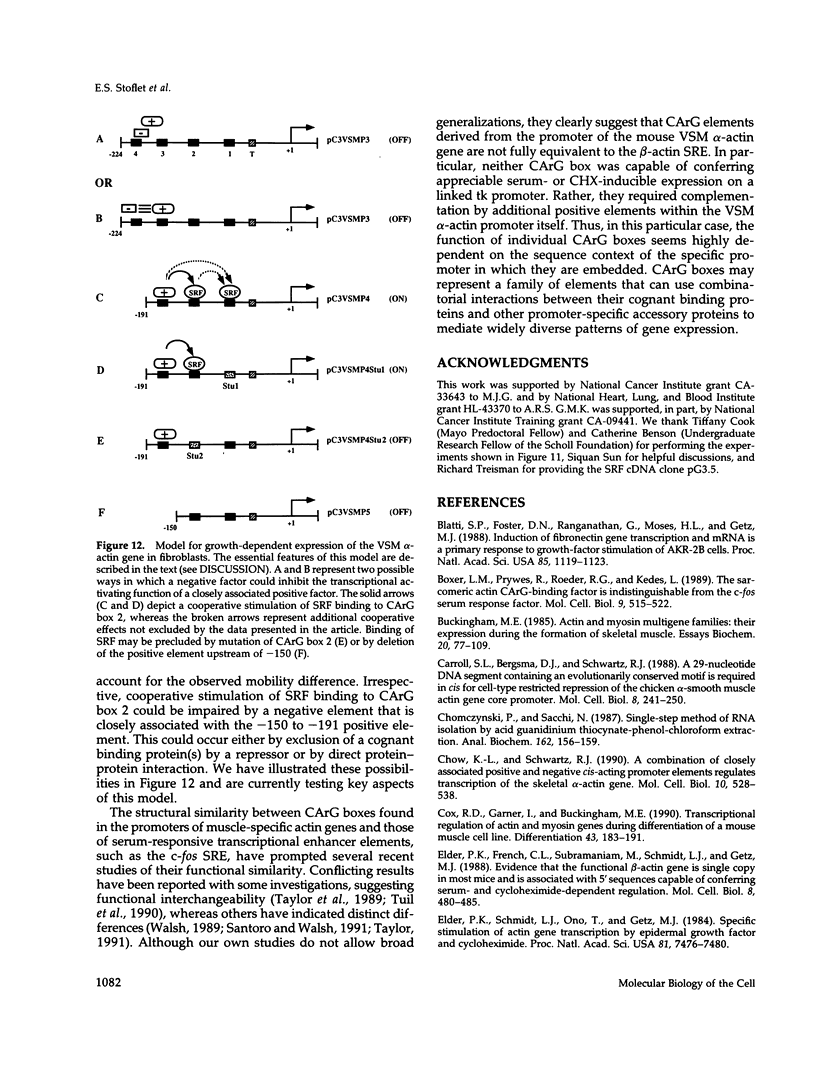
Treatment of AKR-2B mouse fibroblasts with serum growth factors or inhibitors of protein synthesis, such as cycloheximide, results in a stimulation of cytoskeletal beta-actin transcription but has no effect on transcription of muscle-specific isotypes, such as the vascular smooth muscle (VSM) alpha-actin gene. Deletion mapping and site-specific mutagenesis studies demonstrated that a single "CArG" element of the general form CC(A/T)6GG was necessary and possibly sufficient to impart serum and cycloheximide-inducibility to the beta-actin promoter. Although the VSM alpha-actin promoter exhibits at least three similar sequence elements, it remained refractory to serum and cycloheximide induction. However, deletion of a 33 base pair sequence between -191 and -224 relative to the transcription start site resulted in the transcriptional activation of this muscle-specific promoter in rapidly growing or serum-stimulated fibroblasts. Although the activity of this truncated promoter was potentiated by cycloheximide in a manner indistinguishable from that of the beta-actin promoter, this was dependent on a more complex array of interacting elements. These included at least one CArG box and a putative upstream activating element closely associated with the -191 to -224 inhibitory sequences. These results demonstrate that the expression of a muscle-specific actin gene in fibroblasts is suppressed by a cis-acting negative control element and that in the absence of this element, the promoter is responsive to growth factor-induced signal transduction pathways.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatti S. P., Foster D. N., Ranganathan G., Moses H. L., Getz M. J. Induction of fibronectin gene transcription and mRNA is a primary response to growth-factor stimulation of AKR-2B cells. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1119–1123. doi: 10.1073/pnas.85.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. M., Prywes R., Roeder R. G., Kedes L. The sarcomeric actin CArG-binding factor is indistinguishable from the c-fos serum response factor. Mol Cell Biol. 1989 Feb;9(2):515–522. doi: 10.1128/mcb.9.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M. E. Actin and myosin multigene families: their expression during the formation of skeletal muscle. Essays Biochem. 1985;20:77–109. [PubMed] [Google Scholar]
- Carroll S. L., Bergsma D. J., Schwartz R. J. A 29-nucleotide DNA segment containing an evolutionarily conserved motif is required in cis for cell-type-restricted repression of the chicken alpha-smooth muscle actin gene core promoter. Mol Cell Biol. 1988 Jan;8(1):241–250. doi: 10.1128/mcb.8.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chow K. L., Schwartz R. J. A combination of closely associated positive and negative cis-acting promoter elements regulates transcription of the skeletal alpha-actin gene. Mol Cell Biol. 1990 Feb;10(2):528–538. doi: 10.1128/mcb.10.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. D., Garner I., Buckingham M. E. Transcriptional regulation of actin and myosin genes during differentiation of a mouse muscle cell line. Differentiation. 1990 Jun;43(3):183–191. doi: 10.1111/j.1432-0436.1990.tb00445.x. [DOI] [PubMed] [Google Scholar]
- Elder P. K., French C. L., Subramaniam M., Schmidt L. J., Getz M. J. Evidence that the functional beta-actin gene is single copy in most mice and is associated with 5' sequences capable of conferring serum- and cycloheximide-dependent regulation. Mol Cell Biol. 1988 Jan;8(1):480–485. doi: 10.1128/mcb.8.1.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder P. K., Schmidt L. J., Ono T., Getz M. J. Specific stimulation of actin gene transcription by epidermal growth factor and cycloheximide. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7476–7480. doi: 10.1073/pnas.81.23.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsholtz H. P., Mangalam H. J., Potter E., Albert V. R., Supowit S., Evans R. M., Rosenfeld M. G. Two different cis-active elements transfer the transcriptional effects of both EGF and phorbol esters. Science. 1986 Dec 19;234(4783):1552–1557. doi: 10.1126/science.3491428. [DOI] [PubMed] [Google Scholar]
- Foster D. N., Min B., Foster L. K., Stoflet E. S., Sun S., Getz M. J., Strauch A. R. Positive and negative cis-acting regulatory elements mediate expression of the mouse vascular smooth muscle alpha-actin gene. J Biol Chem. 1992 Jun 15;267(17):11995–12003. [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Siegfried Z., Ziff E. B. Mutation of the c-fos gene dyad symmetry element inhibits serum inducibility of transcription in vivo and the nuclear regulatory factor binding in vitro. Mol Cell Biol. 1987 Mar;7(3):1217–1225. doi: 10.1128/mcb.7.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989 Apr 15;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. C., Chow K. L., Fang P., Schwartz R. J. Activation of skeletal alpha-actin gene transcription: the cooperative formation of serum response factor-binding complexes over positive cis-acting promoter serum response elements displaces a negative-acting nuclear factor enriched in replicating myoblasts and nonmyogenic cells. Mol Cell Biol. 1991 Oct;11(10):5090–5100. doi: 10.1128/mcb.11.10.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung S., Miyamoto N. G. Point mutational analysis of the human c-fos serum response factor binding site. Nucleic Acids Res. 1989 Feb 11;17(3):1177–1195. doi: 10.1093/nar/17.3.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister R. K., Hulman S. E., Johnson L. F. Rapid changes in poly (A)(+) mRNA content in growth stimulated fibroblasts following perturbations in protein synthesis. J Cell Physiol. 1979 Sep;100(3):531–538. doi: 10.1002/jcp.1041000315. [DOI] [PubMed] [Google Scholar]
- Min B. H., Foster D. N., Strauch A. R. The 5'-flanking region of the mouse vascular smooth muscle alpha-actin gene contains evolutionarily conserved sequence motifs within a functional promoter. J Biol Chem. 1990 Sep 25;265(27):16667–16675. [PubMed] [Google Scholar]
- Minty A., Kedes L. Upstream regions of the human cardiac actin gene that modulate its transcription in muscle cells: presence of an evolutionarily conserved repeated motif. Mol Cell Biol. 1986 Jun;6(6):2125–2136. doi: 10.1128/mcb.6.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohun T. J., Chambers A. E., Towers N., Taylor M. V. Expression of genes encoding the transcription factor SRF during early development of Xenopus laevis: identification of a CArG box-binding activity as SRF. EMBO J. 1991 Apr;10(4):933–940. doi: 10.1002/j.1460-2075.1991.tb08027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S. Y., Gunning P., Liu S. H., Leavitt J., Kedes L. Regulation of the human beta-actin promoter by upstream and intron domains. Nucleic Acids Res. 1989 Jan 25;17(2):601–615. doi: 10.1093/nar/17.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman C., Runswick M., Pollock R., Treisman R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988 Dec 23;55(6):989–1003. doi: 10.1016/0092-8674(88)90244-9. [DOI] [PubMed] [Google Scholar]
- Olson E. N. MyoD family: a paradigm for development? Genes Dev. 1990 Sep;4(9):1454–1461. doi: 10.1101/gad.4.9.1454. [DOI] [PubMed] [Google Scholar]
- Pollock R., Treisman R. Human SRF-related proteins: DNA-binding properties and potential regulatory targets. Genes Dev. 1991 Dec;5(12A):2327–2341. doi: 10.1101/gad.5.12a.2327. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro I. M., Walsh K. Natural and synthetic DNA elements with the CArG motif differ in expression and protein-binding properties. Mol Cell Biol. 1991 Dec;11(12):6296–6305. doi: 10.1128/mcb.11.12.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam M., Schmidt L. J., Crutchfield C. E., 3rd, Getz M. J. Negative regulation of serum-responsive enhancer elements. Nature. 1989 Jul 6;340(6228):64–66. doi: 10.1038/340064a0. [DOI] [PubMed] [Google Scholar]
- Taylor M. V. A family of muscle gene promoter element (CArG) binding activities in Xenopus embryos: CArG/SRE discrimination and distribution during myogenesis. Nucleic Acids Res. 1991 May 25;19(10):2669–2675. doi: 10.1093/nar/19.10.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M., Treisman R., Garrett N., Mohun T. Muscle-specific (CArG) and serum-responsive (SRE) promoter elements are functionally interchangeable in Xenopus embryos and mouse fibroblasts. Development. 1989 May;106(1):67–78. doi: 10.1242/dev.106.1.67. [DOI] [PubMed] [Google Scholar]
- Treisman R. Identification and purification of a polypeptide that binds to the c-fos serum response element. EMBO J. 1987 Sep;6(9):2711–2717. doi: 10.1002/j.1460-2075.1987.tb02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Tuil D., Clergue N., Montarras D., Pinset C., Kahn A., Phan-Dinh-Tuy F. CC Ar GG boxes, cis-acting elements with a dual specificity. Muscle-specific transcriptional activation and serum responsiveness. J Mol Biol. 1990 Jun 20;213(4):677–686. doi: 10.1016/S0022-2836(05)80255-4. [DOI] [PubMed] [Google Scholar]
- Walsh K. Cross-binding of factors to functionally different promoter elements in c-fos and skeletal actin genes. Mol Cell Biol. 1989 May;9(5):2191–2201. doi: 10.1128/mcb.9.5.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]